SUMMARY
Cocaine’s behavioral-stimulant effects derive from potentiation of synaptic signaling by dopamine and serotonin leading to transcriptional alterations in postsynaptic cells. We report that a signaling cascade involving nitric oxide (NO) and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) mediates cocaine’s transcriptional and behavioral actions. Lower, behavioral-stimulant doses enhance the cAMP response element-binding (CREB) signaling system, while higher, neurotoxic doses stimulate the p53 cytotoxic system. The drug CGP3466B, which potently and selectively blocks GAPDH nitrosylation and GAPDH-Siah binding, prevents these actions as well as behavioral effects of cocaine providing a strategy for anticocaine therapy.
INTRODUCTION
Behavioral-stimulant effects of cocaine are thought to be initiated by potentiation of the synaptic actions of dopamine and, possibly, serotonin associated with inhibition of neurotransmitter transport (Amara and Kuhar, 1993; Amara and Sonders, 1998; Thomas et al., 2008). Nuclear events triggered by cocaine, including chromatin remodeling (Levine et al., 2011; Maze et al., 2010; Taniguchi et al., 2012), have been linked to behavioral actions with reports of increased signaling via transcription factors such as CREB and ΔfosB (McClung and Nestler, 2003; Robison and Nestler, 2011) leading to augmented expression of transcriptional targets such as BDNF (Graham et al., 2007; Im et al., 2010; McGinty et al., 2010) and immediate-early genes such as c-fos and Arc (Larson et al., 2010). Mechanisms connecting neurotransmitter-receptor interactions to these transcriptional systems upon cocaine treatment have not been well characterized.
A signaling system initiated by nitric oxide (NO) mediates diverse physiologic and pathophysiologic events in blood vessels, inflammatory tissues, and in neuronal systems with notable behavioral alterations attendant upon deletion of neuronal NO synthase (nNOS) (Tricoire and Vitalis, 2012; Zhou and Zhu, 2009). The cascade commences with activation of NO-elicited nitrosylation of glyceraldehyde-3-phosphate dehydrogenase (GAPDH) with nuclear translocation of GAPDH, together with the ubiquitin E3 ligase Siah1. With cytotoxic stresses nuclear GAPDH activates the histone acetylating enzymes p300/CBP leading to acetylation and activation of p53 and enhancement of cell death transcriptional targets such as PUMA and Bax (Sen et al., 2008; Zhou and Zhu, 2009). With physiologic stimuli, such as the neurotrophic factors BDNF (brain-derived neurotrophic factor) and NGF (nerve growth factor), the nuclear complex of nitrosylated GAPDH, linked to Siah1 and the histone-methylating enzyme SUV39H1, triggers degradation of SUV39H1 via the ubiquitin E3 ligase activity of Siah1 (Sen and Snyder, 2011). This facilitates acetylation of histone H3 leading to CREB binding to DNA with enhanced expression of CREB-regulated genes such as c-fos and BDNF and associated augmentation of nerve outgrowth.
In the present study, we report a signaling cascade wherein low, behavioral-stimulant doses of cocaine trigger formation of the nitrosylated GAPDH/Siah1 complex leading to augmented expression of CREB genes, whereas higher, neurotoxic doses activate the NO/GAPDH/p53 system. CGP3466B, a very potent inhibitor of GAPDH nitrosylation and GAPDH-Siah binding, prevents both stimulant and neurotoxic actions of cocaine.
RESULTS
Nitrosylated GAPDH Contributes to Both Behavioral and Neurotoxic Effects of Cocaine
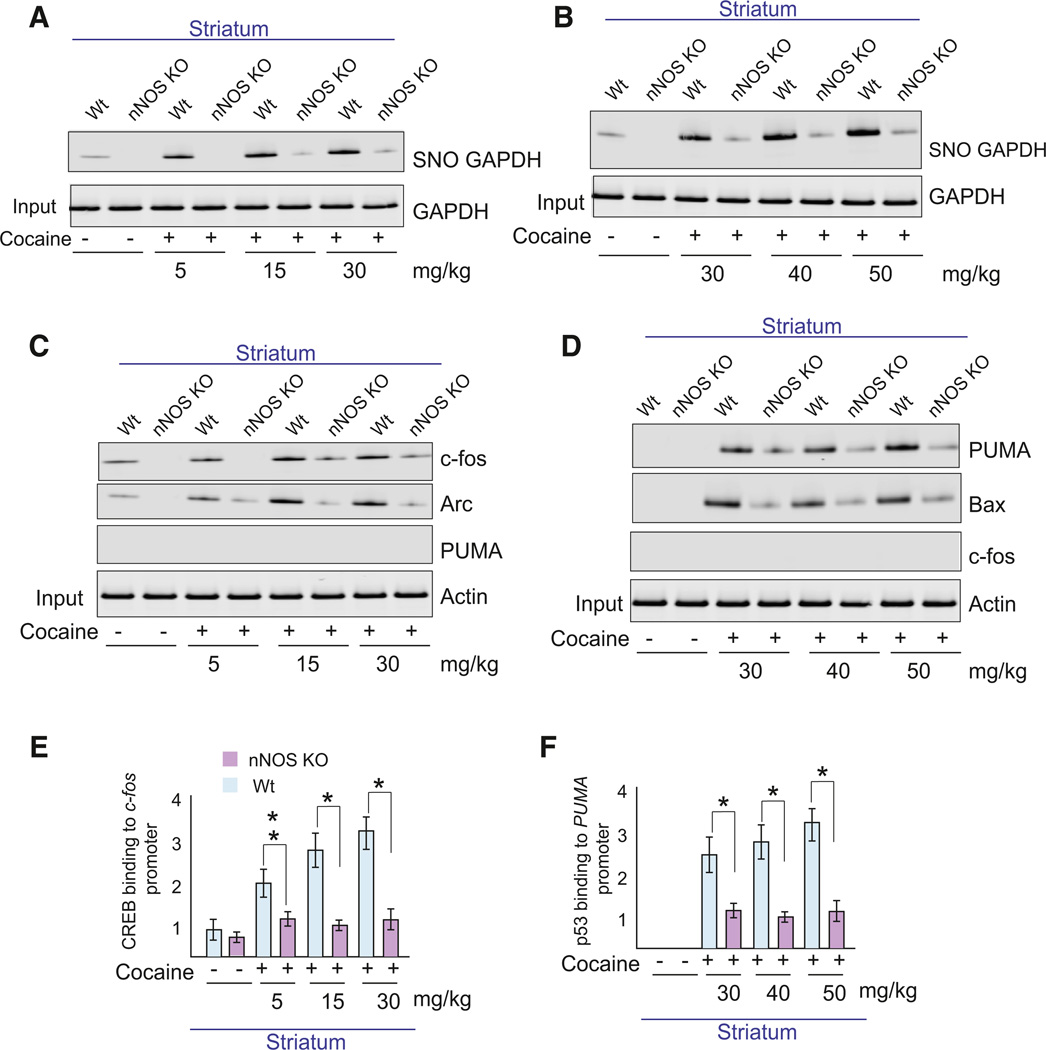
To determine a role for the NO-GAPDH cascade in cocaine actions, we treated mice with single behavioral-stimulant doses of cocaine (5–30 mg/kg; Figure 1A) or a neurotoxic regimen of 5 doses of cocaine (30–50 mg/kg), monitoring nitrosylation (Figure 1B) of GAPDH (SNO-GAPDH) in mice with targeted deletion of nNOS. Both stimulant and neurotoxic treatment protocols of cocaine augment levels of nitrosylated GAPDH, with the neurotoxic protocol eliciting a larger effect. All these influences of cocaine are virtually abolished in nNOS knockout mice, establishing that cocaine impacts this signaling pathway.
Figure 1. Neuronal NO Mediates Cocaine’s Enhancement of p53 and CREB Gene Transcription.
(A and B) Levels of nitrosylated GAPDH (SNO-GAPDH) in mice receiving behavioral-stimulant doses (A) or neurotoxic doses (B) of cocaine in both wild-type and nNOS knockout (nNOS KO) mice. (C and D) Differential expression of c-fos, BDNF, Arc, PUMA, and Bax proteins with behavioral doses (C) or neurotoxic doses (D) of cocaine. (E and F) Quantitative ChIP analysis of binding of CREB and p53 to c-fos and PUMA promoters in striatum following cocaine treatment. Data were normalized by total input and presented as bound/input. *p < 0.01, n = 3, one-way ANOVA, mean ± SEM. See also Figure S1.
To discriminate effects of dose and dosing scheduled, we administered single 5–40 mg/kg doses as well as multiple treatments with these doses. Single doses of cocaine (5–30 mg/kg) induce CREB binding to the c-fos promoter (see Figure S1A available online) with increases in c-fos protein levels (Figure S1B). By contrast, treatment with 40 mg/kg cocaine, either in single or multiple doses, induces p53 binding to the PUMA promoter (Figure S1C), with attendant increases in PUMA protein levels (Figure S1B). Administering 30 mg/kg cocaine for 5 days induces PUMA (Figure S1B, red dotted box), while single doses of 30 mg/kg cocaine induce c-fos level (Figure S1B, blue dotted box). Accordingly, single doses of cocaine (5–30 mg/kg) were used to study behavioral effects of cocaine and 40 mg/kg cocaine was employed as a neurotoxic dose. Mice receiving 30 mg/kg cocaine for 5 consecutive days were also used to study cocaine-associated cell death.
Earlier, we reported that NO-GAPDH signaling initiates a cascade leading to nuclear transcription of p53 and CREB targets (Sen et al., 2008; Sen and Snyder, 2011). The stimulant cocaine regimen augments levels of c-fos and the immediateearly gene Arc but not PUMA, and these effects are lost in nNOS knockout mice (Figure 1C). By contrast, the neurotoxic cocaine regimen enhances levels of PUMA and Bax but not c-fos with these actions absent in nNOS mutants (Figure 1D).
Utilizing ChIP assays, we monitored CREB binding to the c-fos promoter with stimulant doses of cocaine (Figure 1E) and p53 binding to the PUMA promoter with the neurotoxic regimen (Figure 1F). CREB-c-fos promoter binding is markedly increased by stimulant doses of cocaine, effects abolished in nNOS knockouts (Figure 1E). The neurotoxic cocaine regimen greatly increases p53 binding to the PUMA promoter, effects which are lost in nNOS knockouts (Figure 1F).
Nitrosylation of GAPDH Is Required for Cocaine Actions In Vivo
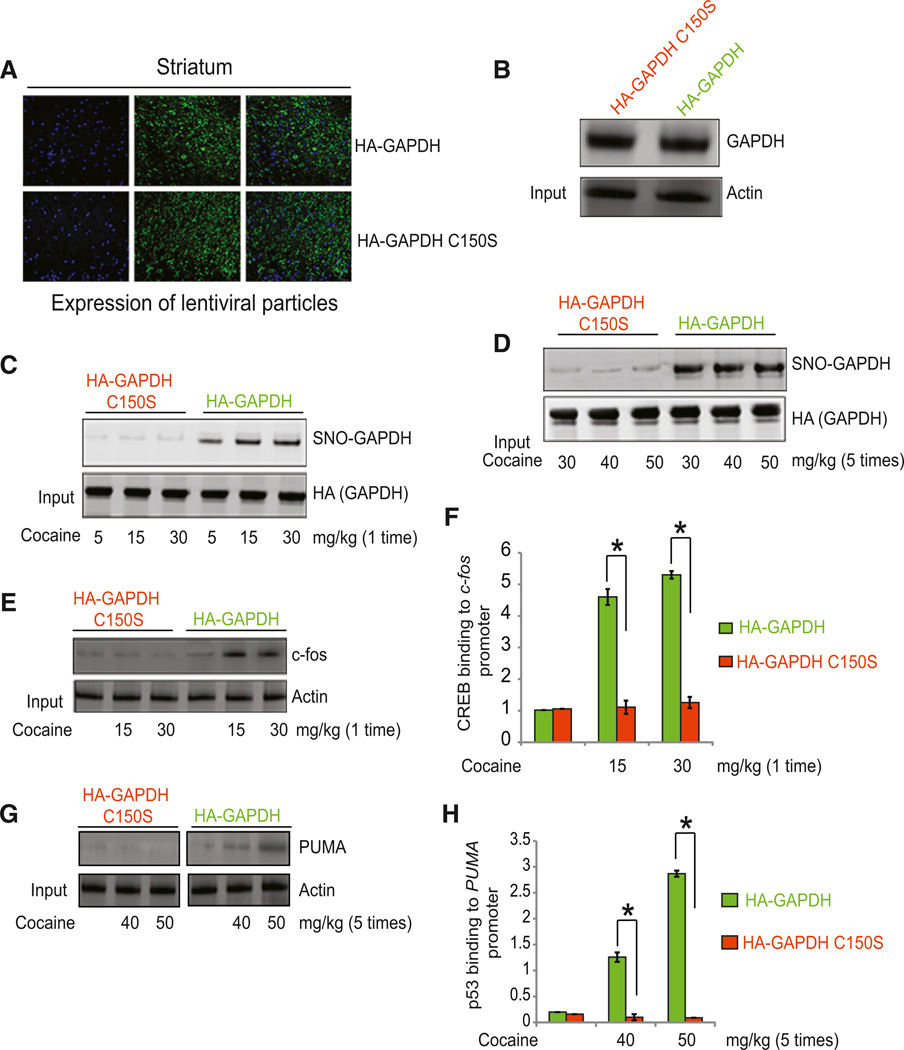
Loss of cocaine actions in nNOS knockout mice establishes a role for neuronal NO under these influences. To assess more directly whether effects of cocaine are specifically mediated by GAPDH nitrosylation, we administered lentiviral particles of wild-type GAPDH and C150S mutant of GAPDH—which cannot be nitrosylated—into the striatum of mice brain. Expression levels of both constructs are the same (Figures 2A and 2B). After overexpression of these constructs, we treated mice with single behavioral-stimulant doses of cocaine (5–30 mg/kg) or a neurotoxic regimen of 5 doses of cocaine (30–50 mg/kg) and monitored nitrosylation of GAPDH. Nitrosylation of wild-type but not C150S mutant GAPDH is observed after treatment with both behavioral and neurotoxic doses of cocaine (Figures 2C and 2D). As expected, CREB binding to the c-fos promoter and levels of c-fos are increased in mice overexpressing wild-type GAPDH but not GAPDH C150S (Figures 2E and 2F). Similarly, with neurotoxic doses of cocaine, p53 binding to the PUMA promoter and PUMA protein levels are enhanced in mice overexpressing wild-type GAPDH (Figures 2G and 2H). This suggests that nitrosylation of GAPDH mediates both behavioral and neurotoxic doses of cocaine significantly.
Figure 2. Effect of GAPDH or C150S GAPDH on Behavioral and Neurotoxic Actions of Cocaine.
(A) Confocal microscopic analysis of the intrastriatal injection of lentiviral particles of HAGAPDH and HA-C150S GAPDH in mice brain. (B) Western blot analysis to detect overexpression of HA-GAPDH and HA-C150S GAPDH in brain. (C) Level of nitrosylation of GAPDH (SNO-GAPDH) in striatum of mice receiving single injections of cocaine (5–30 mg/kg). (D) Level of nitrosylation of GAPDH (SNO-GAPDH) in striatum of mice receiving cocaine (30–50 mg/kg for 5 days). (E and F) Levels of c-fos (E) and CREB binding to c-fos promoter (F) were measured in mice overexpressing HA-GAPDH or HA-C150S GAPDH in striatum. (G and H) PUMA protein level (G) and p53 binding to PUMA promoter (H) were measured in mice overexpressing GAPDH or GAPDH-C150S in striatum. *p < 0.01, n = 3, one-way ANOVA, mean ± SEM.
Effects of CGP3466B on Behavioral Effects of Cocaine
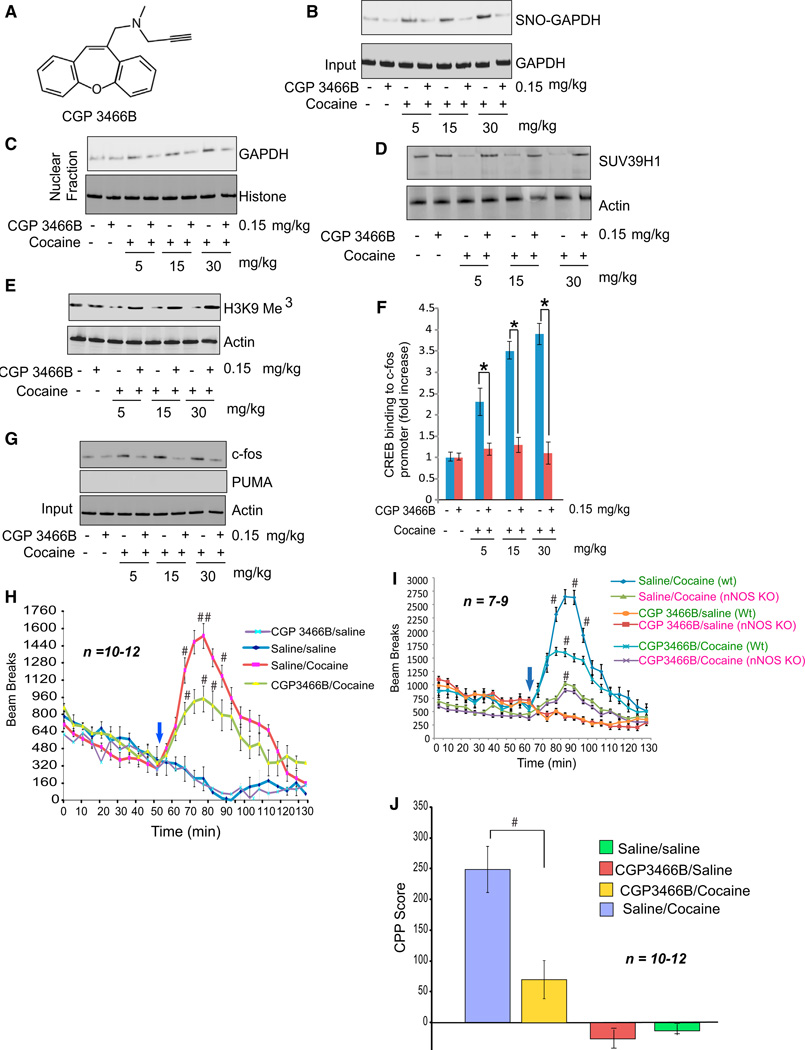
We sought pharmacologic means to interrupt the cocaine-NO-GAPDH signaling system in intact animals. The monoamine oxidase inhibitor deprenyl is known to be neuroprotective (The Parkinson Study Group, 1989; Hara et al., 2006; Sagot et al., 2000; Waldmeier et al., 2000). Derivatives of deprenyl, such as CGP3466B (Figure 3A), lack monoamine oxidase inhibitory activity but retain neuroprotective effects (Hara et al., 2006). CGP3466B prevents the nitrosylation of GAPDH with extraordinary potency, acting in subnanomolar concentrations, with resultant inhibition of GAPDH binding to Siah1 and nuclear translocation of GAPDH. Behavioral-stimulant doses of cocaine (5–30 mg/kg) increase GAPDH nitrosylation in the corpus striatum— the site of highest dopamine levels in the brain—in a dose-dependent fashion (Figure 3B). Administration of 0.15 mg/kg CGP3466B almost completely blocks the stimulation of GAPDH nitrosylation in striatum elicited by cocaine (Figure S2A). CGP3466B also prevents the cocaine-elicited nuclear translocation of GAPDH (Figure 3C).
Figure 3. CGP3466B Prevents Behavioral Actions of Cocaine.
(A) Structure of CGP3466B. (B–C) CGP3466B decreases nitrosylation level of GAPDH (B) and nuclear translocation of GAPDH (C) in striatum of cocaine-treated mice. (D and E) CGP3466B prevented cocaine-elicited degradation of SUV349H1 (D) and H3K9 trimethylation (E) in striatum. (F and G) Analysis of CREB binding to c-fos promoter (F) and c-fos and PUMA levels (G) in mice receiving cocaine with or without CGP3466B. (H) Locomotor sensitization of mice monitored in open field test upon treatment with cocaine and CGP3466B. #p < 0.05, n = 10–12, two-way ANOVA, mean ± SEM. (I) Locomotor sensitization of wild-type versus nNOS knockout mice upon treatment with cocaine and CGP3466B, monitored via open-field test. #p < 0.05, n = 7–9, two-way ANOVA, mean ± SEM. (J) Conditioned place preference performance in mice receiving cocaine with or without CGP3466B. #p < 0.05, n = 10–12, two-way ANOVA, mean ± SEM. See also Figures S2 and S3.
Behavorial actions of cocaine are thought to involve augmented synaptic actions of dopamine upon D1 receptors and are typically blocked by the D1 antagonist SCH23390 (Noda and Nabeshima, 2004). Stimulation of GAPDH nitrosylation both by stimulant (Figure S2B) and neurotoxic (Figure S2C) cocaine regimens is blocked by SCH23390 administration. Thus, the NO-GAPDH signaling pathway, like behavioral influences of cocaine, is mediated by dopamine signaling through D1 receptors.
With physiologic stimuli, such as the neurotrophic factors, BDNF and NGF, the nuclear complex of nitrosylated GAPDH linked to Siah1 and the histone methylating enzyme SUV39H1 triggers degradation of SUV39H1 via the ubiquitin E3 ligase activity of Siah1 (Sen and Snyder, 2011). This facilitates enhanced expression of CREB regulated genes such as c-fos. Behavioral-stimulant doses of cocaine enhance nuclear degradation of SUV39H1 (Figure 3D), with attendant decreased histone methylation (Figure 3E) and increased transcriptional activation of CREB (Figure 3F) as well as augmented expression of c-fos (Figure 3G). Administration of CGP3466B blocks degradation of SUV39H1 and stimulation of c-fos expression (Figures 3D–3G). CGP3466B has no adverse effects on central or peripheral movement (Figure S3A) or body weight (Figure S3B) of mice.
We investigated whether the NO-GAPDH-CREB system mediates behavioral-stimulant effects of cocaine. CGP3466B was administered at 0.15 mg/kg IP once a day for 5 days with cocaine injected on the fifth day at the same time as the last dose of CGP3466B. We monitored locomotor stimulation by cocaine in an open field model (Figures 3H, S3C, and S3D). CGP3466B (0.15 mg/kg) substantially reduces locomotor stimulation by cocaine while having no effect on basal locomotor activity. Cocaine induced locomotor stimulation is virtually abolished in nNOS KO mice, consistent with participation by NO in cocaine’s actions (Figure 3I). Administering CGP3466B to nNOS knockout mice treated with cocaine does not further decrease locomotor stimulation, implicating nitrosylation of GAPDH in the behavioral responses (Figure 3I). To evaluate behavioral preference for cocaine, we employed the conditioned place preference paradigm, with cocaine preference reduced by CGP3466B treatment (Figure 3J). In this experiment, mice received CGP3466B during the preconditioning stage of the test. When mice were conditioned to the chamber in the absence of CGP3466B, treatment with single dose of CGP3466B only on the day of testing failed to alter conditional place preference (Figure S1D).
Effects of MAO-B Inhibitors on Behavioral Actions of Cocaine
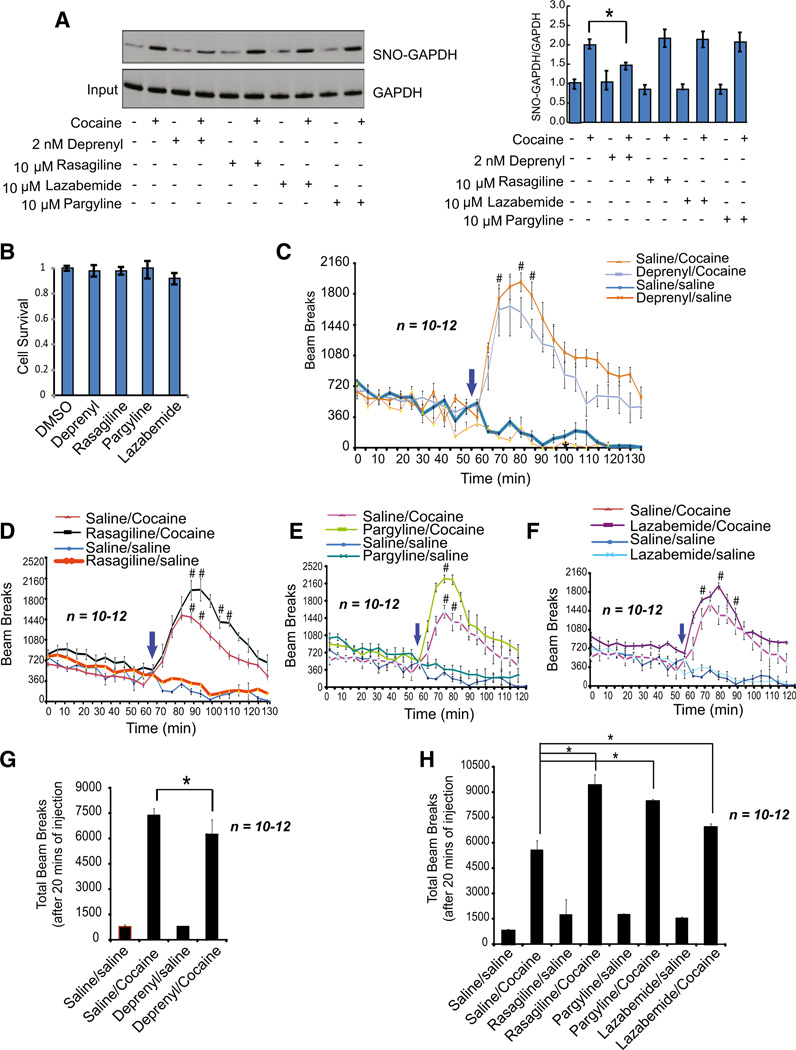
As reported earlier (Hara et al., 2006), the MAO-B inhibitor deprenyl, like CGP3466B, potently inhibits GAPDH nitrosylation both in vitro and in the brains of mice treated with the neurotoxic agent 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP). In cortical cultures, 2 nM deprenyl, like CGP3466B, prevents cocaine-induced nitrosylation of GAPDH (Figure 4A). Other monoamine oxidase inhibitors such as rasagiline, lazabemide, and pargyline do not prevent GAPDH nitrosylation. None of these MAO-B inhibitors effects cell death in primary cortical neurons (Figure 4B). We wondered whether deprenyl, by inhibiting GAPDH nitrosylation, would, like CGP3466B, decrease behavioral effects of cocaine (Figure 4C). However, monoamine oxi-dase inhibitors prevent the destruction of dopamine, and of themselves, elicit locomotor stimulation (Figures 4D–4F). To discriminate the NO-GAPDH actions from the effects of monoamine oxidase inhibition, we compared locomotor influences of deprenyl with the other monoamine oxidase inhibitors (Figures 4G and 4H). Deprenyl significantly decreases cocaine’s locomotor activation, though to a lesser extent than CGP3466B. By contrast, rasagiline, pargyline, and lazabemide, when combined with cocaine, elicit greater locomotor stimulation than cocaine alone. Thus, deprenyl, as well as CGP3466B, appears to reduce locomotor stimulant effects of cocaine by its inhibition of GAPDH nitrosylation.
Figure 4. Effect of Monoamine Oxidase-B (MAO-B) Inhibitors on Cocaine-Induced Locomotor Sensitization of Mice.
(A) Analysis of nitrosylation of GAPDH in primary cortical neuronal cells treated with the MAO-B inhibitors deprenyl, rasagiline, lazabemide, or pargyline. (B) Treatment with deprenyl, rasagiline, lazabemide, or pargyline has no effect on cell survival of primary cortical neurons. (C–F) Analysis of locomotor sensitization by open-field test upon treatment with deprenyl (C), rasagiline (D), pargyline (E), or lazabemide (F) with or without cocaine. #p < 0.05, n= 10–12, two-way ANOVA, mean ± SEM. (G and H) Total beam breaks were measured in mice treated with cocaine alone or with cocaine anddeprenyl (G), rasagiline, lazabemide or pargyline (H). *p < 0.01, n = 10–12, one-way ANOVA, mean ± SEM.
Effects of CGP3466B on Neurotoxic Effects of Cocaine
These experiments establish that the NO-GAPDH signaling pathway mediates behavioral-stimulant effects of cocaine. We wondered whether this cascade also participates in neurotoxic influences of cocaine. Accordingly, we explored actions of higher doses of cocaine (30–50 mg/kg) administered daily for 5 days with or without CGP3466B. These higher doses of cocaine elicit robust nitrosylation of GAPDH in the striatum, effects abolished by CGP3466B (Figure 5A). Similarly, nuclear translocation of GAPDH at these doses of cocaine is prevented by CGP3466B (Figure 5B).
Figure 5. CGP3466B Prevents Neurotoxic Effects of Cocaine In Vivo.
(A and B) Nitrosylation levels of GAPDH (A) and nuclear translocation of GAPDH (B) in striatum were decreased in mice treated with cocaine and CGP3466B compared to cocaine treated mice. (C) Acetylation of GAPDH and p53, as well as p300-GAPDH association in mice treated with cocaine with or without treatment by CGP3466B. (D and E) Analysis of p53 binding to PUMA promoter (D) and c-fos and PUMA levels (E) in mice treated with cocaine with or without CGP3466B. *p < 0.01, n = 3, one-way ANOVA, mean ± SEM. (F) Analysis of cell death by TUNEL assay in striatum induced by cocaine with or without chronic treatment with CGP3466B in mice. *p < 0.01, n = 9, one-way ANOVA, mean ± SEM. (G) Balance and coordination of motor activity evaluated by rotarod analysis test in mice receiving cocaine with or without CGP3466B. #p < 0.05, ##p < 0.01, n = 9, two-way ANOVA, mean ± SEM. See also Figure S4.
Earlier, we reported that cytotoxic insults, via the NO-GAPDH pathway, lead to binding of GAPDH to the protein acetylating enzyme p300, which in turn acetylates GAPDH and activates the transcriptional activity of p53, resulting in induction of PUMA (Sen et al., 2008). Neurotoxic doses of cocaine augment binding of p300-GAPDH and acetylation of both GAPDH and p53 in the striatum of intact animals (Figures 5C and S4A). Activation of p53 leads to increases in p53 binding to the PUMA promoter (Figure 5D) and augmentation of PUMA levels (Figure 5E). However, in contrast to low doses of cocaine, which activate c-fos but not PUMA, at high cocaine doses c-fos is undetectable, while PUMA expression is increased. CGP3466B blocks activation of p53 (Figures 5C and 5D) and induction of PUMA level (Figure 5E).
To evaluate cocaine neurotoxicity, we monitored apoptosis utilizing the TUNEL procedure (Figure 5F). Cellular apoptosis is increased by cocaine at 30 mg/kg with substantially greater effects at 50 mg/kg. Treatment with CGP3466B markedly reduces apoptosis.
As a behavioral index of neurotoxic insults of cocaine, we examined motor coordination in the rotarod test (Figure 5G). At 50 mg/kg cocaine disrupts performance, substantially lowering the latency for falling off the rod. Cotreatment with CGP3466B markedly improves performance, increasing latency 2.5-to 3-fold. By contrast, the MAO-B inhibitors deprenyl, rasagiline, pargyline, and lazabemide do not rescue cocaine induced cell death (Figure S4B) and do not improve rotarod performance (Figure S4C). Moreover, under basal conditions in the absence of cocaine, these drugs do not affect rotarod performance. The failure of deprenyl to elicit protective effects in these experimental conditions may reflect its monoamine oxidase inhibitory actions potentiating the disruptive influences of cocaine.
DISCUSSION
In summary, our study establishes that cocaine signals by stimulating the NOGAPDH signaling cascade involving nuclear translocation of GAPDH to alter transcriptional events. At lower, behavioralstimulant doses cocaine predominantly signals through CREB, thence the c-fos promoter, activating transcription of genes such as BDNF and Arc. By contrast, high, neurotoxic doses preponderantly elicit p53 mediated transcription of prodeath genes such as PUMA and Bax. Earlier, we differentiated molecular mechanisms for cytotoxic versus neurotrophic signaling by the NO-GAPDH system (Sen et al., 2008; Sen and Snyder, 2011). Following cytotoxic insults nuclear GAPDH activates acetylation by p300/CBP of p53 leading to prodeath gene stimulation (Sen et al., 2008). On the other hand, physiologic stimulation by neurotrophic agents such as BDNF and NGF elicits nuclear association of GAPDH with Siah1 in a complex with the histone methylating enzyme SUV39H1 (Sen and Snyder, 2011). In this complex Siah1, via its ubiquitin E3 ligase activity, elicits degradation of SUV39H1. The resultant decreased methylation of histone H3K9 leads to augmented acetylation of this histone and increased transcription by CREB leading to enhanced neuronal outgrowth (Sen and Snyder, 2011). We presume that the behavioral-stimulant and neurotoxic actions of cocaine reflect the signaling systems linked to CREB and p53, respectively.
We employed CGP3466B as a tool to inhibit GAPDH nitrosylation potently and selectively and thereby disrupt the signaling cascade. CGP3466B prevents transcriptional signaling of cocaine and reverses both the behavioral stimuli and the neurotoxic actions of cocaine. Deprenyl, which like CGP3466B, blocks GAPDH nitrosylation, also reduces behavioral-stimulant effects of cocaine. Its actions contrast with those of other monoamine oxidase inhibitors that do not affect GAPDH nitrosylation and which, instead, augment cocaine’s stimulant influences.
Our studies may have therapeutic implications. In this context it is notable that pretreatment with CGP3466B reduces conditioned placed preference, a measure of cocaine seeking behavior. Individuals ingesting high doses of cocaine often experience life-threatening brain damage. The ability of CGP3466B to decrease the neurotoxic effects of cocaine implies that drugs such as CGP3466B may be of value in treating cocaine overdose. CGP3466B and related drugs display neuroprotective actions in numerous animal models including Parkinson’s disease (Hara et al., 2006; LeWitt, 2004; Olanow et al., 2006; Waldmeier et al., 2000) motorneuron disease (Sagot et al., 2000), muscular dystrophy (Erb et al., 2009; Meinen et al., 2011), and amyotrophic lateral sclerosis (Leigh et al., 2004; Miller et al., 2007).
EXPERIMENTAL PROCEDURES
Unless otherwise noted, all data are representative of three individual experiments.
Drugs
Cocaine HCl (Sigma-Aldrich) was dissolved in physiological saline. CGP3466B was obtained from Tocris Bioscience and dissolved to 10 mM in water and 100 mM in DMSO (vehicle). Deprenyl (soluble in PBS), Rasagiline (soluble in PBS), Lazabemide (soluble in DMSO), SCH32290 (soluble in PBS), and Pargyline (soluble in PBS) were obtained from Sigma. Antibodies purchased from commercial: c-fos (Santa Cruz, 1:150 dilution), Arc (Santa Cruz, 1:250 dilution), and PUMA (Santa Cruz, 1:250 dilution), CREB (Santa Cruz, 2 µg per 100–500 µg of total protein), and p53 (Santa Cruz, 2 µg per 100–500 µg of total protein).
Sample Preparation from Cocaine-Injected Mice
C57BL/6 mice and nNOS knockout (Jackson) mice were injected once per day (intraperitoneally [i.p.]) with saline or cocaine (0–50 mg/kg) before rapid isolation of striatum 1 hr after injection.. Striatum lysates were used to measure nitrosylation of GAPDH by biotin switch assay. Striatum lysates were also used to do western blot hybridization to measure c-fos, Arc, PUMA levels, and ChIP assay to measure CREB and p53 binding to c-fos and PUMA promoter respectively.
Behavioral Testing
Mice
Male wild-type mice with C57BL/6 genetic background from Jackson laboratories at 10 weeks of age were used for all behavioral testing. All animals were handled according to the Johns Hopkins University School of Medicine Animal Care and Use guidelines.
Open-Field Test
Open-field assessments were conducted as described (Levine et al., 2011; Maze et al., 2010) with brief modifications. The open field is a square arena with the dimensions 47 cm × 47 cm with a 38.1 cm high clear plastic wall. Activity chambers were computer interfaced for data sampling. Sixteen infrared photobeams in each direction (16 × 16), 2.77 cm spacing, were used to record movement. The San Diego PAS software recorded the total number of beam breaks, as well as the beam break’s location in the center and peripheral areas.
Mice were divided in 5 groups (n = 10–12 each). Each group of mice were injected (i.p.) with either CGP3466B or the MAO-B inhibitors deprenyl, rasagiline, lazabemide, and pargyline for 5 days. Mice were allowed to acclimate to the apparatus for 1 hr. After 60 min, mice received saline or cocaine (30 mg /kg of cocaine i.p.), and were returned to the open-field apparatus for a subsequent 75 min in which their activity was recorded. All data was measured in 5 min intervals and aggregated as necessary. Beam breaks or distance traveled for 20 min following cocaine/saline intraperitoneal injection after the first stage of testing were used to determine immediate peak locomotor response from cocaine injection. In between testing of mice, the arena was wiped with Vimoba cleaning solution. Mice were tested at the same time every day in order to minimize any variation due to circadian rhythm changes.
Rotarod Performance
The test was performed as described previously (Xi et al., 2011) with minor modifications. A four-station mouse rotarod device (AccuScan Instruments) was used to study the effects of CGP3466B, Deprenyl, Rasagiline, Lazabemide and Pargyline on cocaine induced operant locomotion in mice. The speed of rotation of the rotarod was increased from 4 to 32 rpm over 7 min.
Mice were divided into 5 groups (n = 8). For 5 days prior to testing mice received one dose of deprenyl (0.25 mg/kg), rasagiline (2.5 mg/kg), pargyline (50 mg/kg), lazabemide (50 mg/kg), or saline followed by a high dose of cocaine (50 mg/kg). Mice were placed on the rotarod at a starting speed of 4 rpm, and the speed of the rotarod was increased to 32 rpm over the course of 7 min. This was repeated for a total of three trials in a day, and the latency at which the mice fell off the rotarod was recorded. Mice were tested at the same time every day in order to minimize any variation due to circadian rhythm changes.
Conditioned Place Preference
The test was performed as described previously (Levine et al., 2011; Maze et al., 2010; Xi et al., 2011) with minor modifications. Two groups of wild-type mice were used to study the effect of CGP 3466B (0.15 mg/kg) on cocaine- (7 mg/kg) induced conditioned place preference or aversion. A three-chamber place preference apparatus was used. This apparatus consisted of two large side compartments and one small central compartment, which separated the large compartments. Two of the large compartments had different visual and tactile cues. One of the large compartments contained a green wire mesh floor. The second large compartment contained a cream-colored rubber floor. The small central compartment had a smooth clear Plexiglas floor. A white opaque wall encircled the apparatus in order to block out any other visual cues in the behavioral testing area.
Wild mice were divided into two groups. During the preconditioning phase (day 1), mice were placed in the small central compartment and were allowed to freely explore the entire apparatus for 15 min. Experimental software (Any-Maze Video Tracking System v. 4.72) measured the amount of time each mouse spent in each of the three compartments. Mice that did not show a strong preference for either of the large chambers on day 1 were advanced to the next stage of testing.
For the next 10 days (days 2–11), mice entered the conditioning stage of testing with one session per day. On days 2, 4, 6, 8, and 10, one of the groups received an injection of saline and were then confined into the cream-colored rubber floor compartment for 15 min immediately following injection. The second group received an injection of CGP 3466B (0.15 mg/kg) exactly 2 hr prior to testing and then received a second injection of saline before being confined to the same cream-colored rubber floor compartment for 15 min. The compartment was cleaned with Vimoba in between mice. On days 3, 5, 7, 9, and 11, this same protocol was followed, substituting the saline injection with cocaine (7 mg/kg) before both groups were confined to the green-mesh floor chamber for 15 min. The treatment group continued to receive an intraperitoneal injection of CGP3466B (0.15 mg/kg) exactly 2 hr before receiving the cocaine conditioning injection. Only one session of conditioning was conducted each day. On the probe trial day (24 hr after the last conditioning session), mice were given an injection of saline then were allowed to freely explore the three compartments for 15 min the time spent in each of the compartments was recorded by computer software. CPP score was calculated as time spent in cocaine chamber minus the time spent in saline/CGP3466B treated chamber.
Supplementary Material
ACKNOWLEDGMENTS
We thank Roxanne Barrow, Bindu Paul, Adele Snowman, and other members of the Snyder lab for their help and support throughout this project. This work was supported by USPHS grant DA-000266.
Footnotes
SUPPLEMENTAL INFORMATION
Supplemental Information includes four figures and Supplemental Experimental Procedures and can be found with this article online at http://dx.doi.org/10.1016/j.neuron.2013.03.021.
REFERENCES
- Amara SG, Kuhar MJ. Neurotransmitter transporters: recent progress. Annu. Rev. Neurosci. 1993;16:73–93. doi: 10.1146/annurev.ne.16.030193.000445. [DOI] [PubMed] [Google Scholar]
- Amara SG, Sonders MS. Neurotransmitter transporters as molecular targets for addictive drugs. Drug Alcohol Depend. 1998;51:87–96. doi: 10.1016/s0376-8716(98)00068-4. [DOI] [PubMed] [Google Scholar]
- Erb M, Meinen S, Barzaghi P, Sumanovski LT, Courdier-Früh I, Rüegg MA, Meier T. Omigapil ameliorates the pathology of muscle dystrophy caused by laminin-alpha2 deficiency. J. Pharmacol. Exp. Ther. 2009;331:787–795. doi: 10.1124/jpet.109.160754. [DOI] [PubMed] [Google Scholar]
- Graham DL, Edwards S, Bachtell RK, DiLeone RJ, Rios M, Self DW. Dynamic BDNF activity in nucleus accumbens with cocaine use increases self-administration and relapse. Nat. Neurosci. 2007;10:1029–1037. doi: 10.1038/nn1929. [DOI] [PubMed] [Google Scholar]
- Hara MR, Thomas B, Cascio MB, Bae BI, Hester LD, Dawson VL, Dawson TM, Sawa A, Snyder SH. Neuroprotection by pharmacologic blockade of the GAPDH death cascade. Proc. Natl. Acad. Sci. USA. 2006;103:3887–3889. doi: 10.1073/pnas.0511321103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Im HI, Hollander JA, Bali P, Kenny PJ. MeCP2 controls BDNF expression and cocaine intake through homeostatic interactions with microRNA-212. Nat. Neurosci. 2010;13:1120–1127. doi: 10.1038/nn.2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larson EB, Akkentli F, Edwards S, Graham DL, Simmons DL, Alibhai IN, Nestler EJ, Self DW. Striatal regulation of DFosB, FosB, and cFos during cocaine self-administration and withdrawal. J. Neurochem. 2010;115:112–122. doi: 10.1111/j.1471-4159.2010.06907.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leigh PN, Swash M, Iwasaki Y, Ludolph A, Meininger V, Miller RG, Mitsumoto H, Shaw P, Tashiro K, Van Den Berg L. Amyotrophic lateral sclerosis: a consensus viewpoint on designing and implementing a clinical trial. Amyotroph. Lateral Scler. Other Motor Neuron Disord. 2004;5:84–98. doi: 10.1080/14660820410020187. [DOI] [PubMed] [Google Scholar]
- Levine A, Huang Y, Drisaldi B, Griffin EA, Jr, Pollak DD, Xu S, Yin D, Schaffran C, Kandel DB, Kandel ER. Molecular mechanism for a gateway drug: epigenetic changes initiated by nicotine prime gene expression by cocaine. Sci. Transl. Med. 2011;3:107–109. doi: 10.1126/scitranslmed.3003062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeWitt PA. Clinical trials of neuroprotection for Parkinson’s disease. Neurology. 2004;63(7, Suppl 2):S23–S31. doi: 10.1212/wnl.63.7_suppl_2.s23. [DOI] [PubMed] [Google Scholar]
- Maze I, Covington HE, 3rd, Dietz DM, LaPlant Q, Renthal W, Russo SJ, Mechanic M, Mouzon E, Neve RL, Haggarty SJ, et al. Essential role of the histone methyltransferase G9a in cocaine-induced plasticity. Science. 2010;327:213–216. doi: 10.1126/science.1179438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClung CA, Nestler EJ. Regulation of gene expression and cocaine reward by CREB and DeltaFosB. Nat. Neurosci. 2003;6:1208–1215. doi: 10.1038/nn1143. [DOI] [PubMed] [Google Scholar]
- McGinty JF, Whitfield TW, Jr, Berglind WJ. Brain-derived neurotrophic factor and cocaine addiction. Brain Res. 2010;1314:183–193. doi: 10.1016/j.brainres.2009.08.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meinen S, Lin S, Thurnherr R, Erb M, Meier T, Rüegg MA. Apoptosis inhibitors and mini-agrin have additive benefits in congenital muscular dystrophy mice. EMBO Mol. Med. 2011;3:465–479. doi: 10.1002/emmm.201100151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller R, Bradley W, Cudkowicz M, Hubble J, Meininger V, Mitsumoto H, Moore D, Pohlmann H, Sauer D, Silani V, et al. TCH346 Study Group. Phase II/III randomized trial of TCH346 in patients with ALS. Neurology. 2007;69:776–784. doi: 10.1212/01.wnl.0000269676.07319.09. [DOI] [PubMed] [Google Scholar]
- Noda Y, Nabeshima T. Involvement of signal tranSD1uction cascade via dopamine-D1 receptors in phencyclidine dependence. Ann. N Y Acad. Sci. 2004;1025:62–68. doi: 10.1196/annals.1316.008. [DOI] [PubMed] [Google Scholar]
- Nott A, Watson PM, Robinson JD, Crepaldi L, Riccio A. S-Nitrosylation of histone deacetylase 2 induces chromatin remodelling in neurons. Nature. 2008;455:411–415. doi: 10.1038/nature07238. [DOI] [PubMed] [Google Scholar]
- Olanow CW, Schapira AH, LeWitt PA, Kieburtz K, Sauer D, Olivieri G, Pohlmann H, Hubble J. TCH346 as a neuroprotective drug in Parkinson’s disease: a double-blind, randomised, controlled trial. Lancet Neurol. 2006;5:1013–1020. doi: 10.1016/S1474-4422(06)70602-0. [DOI] [PubMed] [Google Scholar]
- Robison AJ, Nestler EJ. Transcriptional and epigenetic mechanisms of addiction. Nat. Rev. Neurosci. 2011;12:623–637. doi: 10.1038/nrn3111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagot Y, Toni N, Perrelet D, Lurot S, King B, Rixner H, Mattenberger L, Waldmeier PC, Kato AC. An orally active anti-apoptotic molecule (CGP 3466B) preserves mitochondria and enhances survival in an animal model of motoneuron disease. Br. J. Pharmacol. 2000;131:721–728. doi: 10.1038/sj.bjp.0703633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sen N, Snyder SH. Neurotrophin-mediated degradation of histone methyltransferase by S-nitrosylation cascade regulates neuronal differentiation. Proc. Natl. Acad. Sci. USA. 2011;108:20178–20183. doi: 10.1073/pnas.1117820108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sen N, Hara MR, Kornberg MD, Cascio MB, Bae BI, Shahani N, Thomas B, Dawson TM, Dawson VL, Snyder SH, Sawa A. Nitric oxide-induced nuclear GAPDH activates p300/CBP and mediates apoptosis. Nat. Cell Biol. 2008;10:866–873. doi: 10.1038/ncb1747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taniguchi M, Carreira MB, Smith LN, Zirlin BC, Neve RL, Cowan CW. Histone deacetylase 5 limits cocaine reward through cAMPinduced nuclear import. Neuron. 2012;73:108–120. doi: 10.1016/j.neuron.2011.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The Parkinson Study Group. Effect of deprenyl on the progression of disability in early Parkinson’s disease. N. Engl. J. Med. 1989;321:1364–1371. doi: 10.1056/NEJM198911163212004. [DOI] [PubMed] [Google Scholar]
- Thomas MJ, Kalivas PW, Shaham Y. Neuroplasticity in the mesolimbic dopamine system and cocaine addiction. Br. J. Pharmacol. 2008;154:327–342. doi: 10.1038/bjp.2008.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tricoire L, Vitalis T. Neuronal nitric oxide synthase expressing neurons: a journey from birth to neuronal circuits. Front. Neural Circuits. 2012;6:82. doi: 10.3389/fncir.2012.00082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldmeier PC, Spooren WP, Hengerer B. CGP 3466 protects dopaminergic neurons in lesion models of Parkinson’s disease. Naunyn Schmiedebergs Arch. Pharmacol. 2000;362:526–537. doi: 10.1007/s002100000300. [DOI] [PubMed] [Google Scholar]
- Xi ZX, Peng XQ, Li X, Song R, Zhang HY, Liu QR, Yang HJ, Bi GH, Li J, Gardner EL. Brain cannabinoid CB2 receptors modulate cocaine’s actions in mice. Nat. Neurosci. 2011;14:1160–1166. doi: 10.1038/nn.2874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou L, Zhu DY. Neuronal nitric oxide synthase: structure, subcellular localization, regulation, and clinical implications. Nitric Oxide. 2009;20:223–230. doi: 10.1016/j.niox.2009.03.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.