Abstract
Histone deacetylase (HDAC) inhibitors have shown preclinical efficacy in solid tumors, including ovarian cancers. Our group has published that the HDAC inhibitor, romidepsin (FK228) suppresses ovarian cancer cell growth at nanomolar concentrations in vitro. HDAC inhibitors appear to be even more effective when used in combination with other anti-tumor agents. However, it remains unclear which anti-tumor agents are best suited for combination therapy. A recent report suggested that aspirin (acetylsalicylic acid, ASA) is synergistic with HDAC inhibitors in ovarian cancer cells. ASA is a relatively selective inhibitor of cyclooxygenase-1 (COX-1) and has anti-proliferative effects in ovarian cancer cells. The goal of this study was to investigate the impact of ASA on the activity of the HDAC inhibitor, FK228 in COX-1 positive (OVCAR-3) and COX-1 negative (SKOV-3) human ovarian cancer cell lines. The growth inhibitory effects of FK228 were enhanced by ASA in COX-1 positive ovarian cancer cells. In contrast, ASA had no influence on the results of FK228 treatment in COX-1 negative ovarian cancer cells. Upregulation of the cell cycle control protein p21 was induced robustly by FK228 in both cell lines. In the COX-1 positive cells, p21 expression was augmented by the addition of ASA to FK228 treatment. Furthermore, COX-1 siRNA attenuated the effects of combined ASA and FK228 on the levels of p21 expression and the amount of growth inhibition. The additional increase in p21 by ASA in FK228-treated cells was not observed at the promoter or transcriptional levels. However, a significant delay in p21 protein degradation in the presence of ASA and FK228 in COX-1 positive cells was associated with inhibition of proteasome activity. Our study provides a potential rationale for combining ASA with HDAC inhibitors in a subset of ovarian cancers.
Keywords: Ovarian cancer, romidepsin, aspirin, p21, HDAC inhibitors, COX inhibitors, cell cycle control
INTRODUCTION
Ovarian cancer is the most common cause of death from cancer of the female reproductive tract and is the fifth cause of cancer death in women in the United States.1 Most women are diagnosed with metastatic disease that often requires treatment with radical surgery followed by cytotoxic chemotherapy.2, 3 To date, there are no reliable methods to detect early stage disease and there is still no identifiable precursor to high grade serous epithelial ovarian tumors, the most common type of ovarian cancer.4 New diagnostic markers, targets for therapy and therapeutic options are needed to reduce the morbidity and mortality observed with advanced stage ovarian cancer.
Histone deacetylase (HDAC) inhibitors are a promising class of anti-neoplastic drugs, because of their multiple effects on proliferation, cell cycle arrest, apoptosis, differentiation, angiogenesis and DNA damage.5–7 A potential advantage of HDAC inhibitors is a preference for targeting tumor cells over normal cells that provides a potentially less toxic alternative to standard chemotherapy.7 HDACs, particularly class I isoenzymes, are highly expressed in ovarian cancers.8, 9 Our group has shown that the class I selective HDAC inhibitor, romidepsin (FK228) is effective in reducing ovarian cancer cell proliferation at nanomolar concentrations compared with pan-HDAC inhibitors suberoylanilide hydroxamic acid (SAHA) (micromolar) and sodium butyrate (millimolar) in ovarian cancer cells.8 FK228 is currently in phase I-II clinical investigation in hematopoietic malignancies and solid tumors and was recently approved by the FDA for the treatment of T cell cutaneous lymphoma.10 It is increasingly evident that HDAC inhibitors are more effective when combined with other anti-tumor agents.6, 7 A phase II trial of the HDAC inhibitior vorinostat as a single agent in the treatment of ovarian cancer was disappointing.11Thus, an area of ongoing research is determining a rational approach for determining the best anti-tumor drugs for combination therapy with HDAC inhibitors.
A recent study suggests aspirin (acetylsalicylic acid, ASA) sensitizes ovarian cancer cells to HDAC inhibitors.12 ASA is a relatively selective cyclooxygenase (COX)-1 inhibitor13 and may play a role in the prevention of ovarian cancer, although there are conflicting results in the literature14 In preclinical studies, ASA is known to suppress the growth of COX-1 expressing cells in models of ovarian cancer.15–18 To our knowledge, ASA has not been examined in combination with the potent HDAC inhibitor FK228 in COX-1 expressing ovarian cancer cells. In the present study, we present new evidence that ASA enhances the effects of FK228 in COX-1 expressing ovarian cancer cells by augmenting p21 expression, in part via protein stabilization and proteasome inhibition.
MATERIALS AND METHODS
Reagents
Romidepsin (FK228) was kindly provided by Gloucester Pharmaceuticals (Cambridge, MA). Aspirin (ASA), penicillin G/streptomycin and cycloheximide were purchased from Sigma (St. Louis, MO). All liquid culture media were acquired from Invitrogen Life Technologies (Grand Island, NY). Pre-designed siRNAs for non-targeting Control, COX-1 and DharmaFECT 1 were purchased from Dharmacon (Lafayette, CO).
Cell culture
The human ovarian cancer cell lines OVCAR-3 (COX-1 positive) and SKOV-3 (COX-1 negative)15 were obtained from the American Type Culture Collection (Manassas, VA). Both cell lines are well-characterized as part of the NCI-60 Cancer Panel19, 20. Cells (approximately 5 × 104 cells/ml) were cultured at 37°C in a water-saturated atmosphere of 95% air and 5% CO2 in 24- or 6-well plates with appropriate media: McCoy’s 5A medium with penicillin/streptomycin and 10% FBS for SKOV-3 cells and RPMI medium with penicillin/streptomycin and 10% FBS for OVCAR-3 cells. After allowing cellular attachment to the plates cells were treated with ASA, FK228 and the combination and details are described in Results.
Cell proliferation assays
The cell proliferation assays were performed using the cleavage of 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) to a colored product. After incubation in a 24-well plate, each well was washed twice with phenol red-free media and MTT solution (1mg/ml of phenol red-free media:PBS = 4:1) was added. The plates were incubated for 3 h with protection from light. The MTT solution was removed and 500 µl of isopropanol was added. The plates were placed on a shaker for 10 min at room temperature to thoroughly dissolve the MTT color product. Optical density was measured at 595 nm using a microplate reader (Bio-Rad, Hercules, CA). Values were normalized to the untreated controls. We used CalcuSyn software (Biosoft, Cambridge, UK) to evaluate the effects of the drug combination of ASA and FK228 and analyzed interactions between fixed ratios of each drug based on the Combination Index (CI) method of Chou and Talaly.21 The drug combinations were then defined as synergistic (CI < 0.9), additive (CI = 0.9–1.1) or antagonistic (CI > 1.1).
Flow cytometry
Flow cytometry was performed as previously published.22 Briefly, ovarian cancer cells were seeded at equal densities in 6-well plates and maintained in culture for 24 h. Cells were then treated in triplicate with ASA, FK228, the combination or media alone as a control for 48 h. Adherent and nonadherent cells were harvested with cold phosphate-buffered saline (PBS) and stained with propidium iodide [50 mg/ml in 0.1% (w/v) sodium citrate, 0.1% (v/v) triton X-100] overnight. After overnight incubation, samples were analyzed using a FACScan flow cytometer (BD Biosciences) and the percentage of cells in G0/G1; G2/M and S phases quantified utilizing FloJo software (Tree Star Inc., Ashland, OR). The percentage of cells with sub-diploid DNA content was also measured as a read-out of cell apoptosis. Results were analyzed as a percentage of the control, untreated cells.
Western blot
Whole-cell lysates were prepared, fractionated on SDS-polyacrylamide gels and transferred to nitrocellulose membranes according to established procedures. Blocking of nonspecific proteins was performed by incubation of the membranes with 5% nonfat dry milk in Tris-buffered saline Tween-20 (TBST containing 10 mM Tris, 150 mM phosphate buffered saline, 0.05% Tween 20, pH 8.0) for 2 h at room temperature. The following primary antibodies were used: COX-1, COX-2, HDAC2, HDAC4 and HDAC6 (Santa Cruz Biotechnology, Santa Cruz, CA); HDAC1 (Imgenex, San Diego, CA); HDAC3 (Novus Biologicals, Littleton, CO); and p21 (Cell Signaling Technology, Beverly, MA). After overnight incubation at 4°C, the membranes were washed 3 times with TBST for 10 min, followed by incubation for 1 h with horseradish peroxidase conjugated secondary antibody according to each primary antibody. The membranes were then rinsed 3 times with TBST for 10 min, and the bands were visualized by enhanced chemiluminescence detection kits from GE Healthcare (Piscataway, NJ). After membrane stripping for 10 min with methanol containing 3% H2O2, β-actin (Santa Cruz Biotechnology, Santa Cruz, CA) was detected in order to serve as an internal loading control of cell lysates.
Transient transfection and luciferase assays
Ovarian cancer cells were seeded to approximately 50% confluence in 24-well plates were washed once with fresh media without additives and were transiently transfected with p21 promoter subcloned into pGL2-basic luciferase reporter vector (a gift from Dr. Xiao-Fan Wang, Duke University Medical Center, Durham, NC) for 3 h at 37°C using Lipofectamine™ (Invitrogen Life Technologies, Grand Island, NY). Transfected cells were treated as outlined in Results and incubated for 16h. After rinsing cells with ice-cold PBS and adding lysis buffer (Promega, Madison, WI), cell lysates were used for determination of luciferase activity using a microplate luminometer. Luciferase activity, expressed as relative light units, was normalized to measured protein levels.
RT-PCR
Total RNA was isolated using TRIzol® reagent (Invitrogen Life Technologies, Grand Island, NY). The RT reaction conditions, using random primers with reverse transcriptase M-MLV (Invitrogen), were at 42°C for 60 min followed by 94°C for 10 min. Specific primers for p21 and GAPDH were obtained from Integrated DNA Technologies (Coralville, IA) and designed as follows: 5’-ATG TCA GAA CCG GCT GGG GAT-3’ (sense) and 5’-CGC TCC CAG GCG AAG TCA C-3’ (antisense) for p21 and 5’-TCA CCA TCT TCC AGG AGC GAG A-3’ (sense) and 5’-CAG GGA TGA CCT TGC CCA CAG-3’ (antisense) for GAPDH. GAPDH was used as a loading control. PCR was performed with Taq DNA polymerase (Invitrogen Life Technologies, Grand Island, NY) under the following conditions: denaturation at 94°C for 1 min, annealing at 58°C for 1 min and extension at 74°C for 1 min with 30 cycles. Amplified PCR products were analyzed by electrophoresis in 2% agarose gels containing 1 µg ethidium bromide/ml. The fluorescent images were photographed under UV light.
Cell-based proteasome assay
After 24h and 48h culture in 24-well plates, cells were incubated with the Promega Proteasome-Glo™ Chymotrypsin-Like Cell-Based Assay Reagent (Promega, Madison, WI) for 10 min according to manufacturer’s instruction. Luciferase activity was determined using a microplate luminometer.
Gene silencing with siRNA
The expression level of COX-1 was selectively knocked down by using pre-designed siRNAs (ON-TARGET plus Duplex PTGS1) obtained from Dharmacon (Lafayette, CO). A non-targeting siRNA (NT1) was used as a negative control. OVCAR-3 cells were transiently transfected with 10 nM siRNA (final concentration of maximum knock-down) for 72 h using the transfection reagent. Cells were treated as described in Results and cell proliferation assay was performed at 48 h treatment. Western blot was performed to validate the silencing of protein expression.
Statistics
Each experiment was repeated at least 3 times. Data were analyzed by the paired Student’s t-test and one-way analysis of variance (ANOVA) as appropriate. If statistical significance (p ≤ 0.05) was determined by ANOVA, the data were further analyzed by Tukey’s pairwise comparisons to detect specific differences between treatments.
RESULTS
The growth inhibitory effects of FK228 are augmented by ASA in COX-1 positive ovarian cancer cells
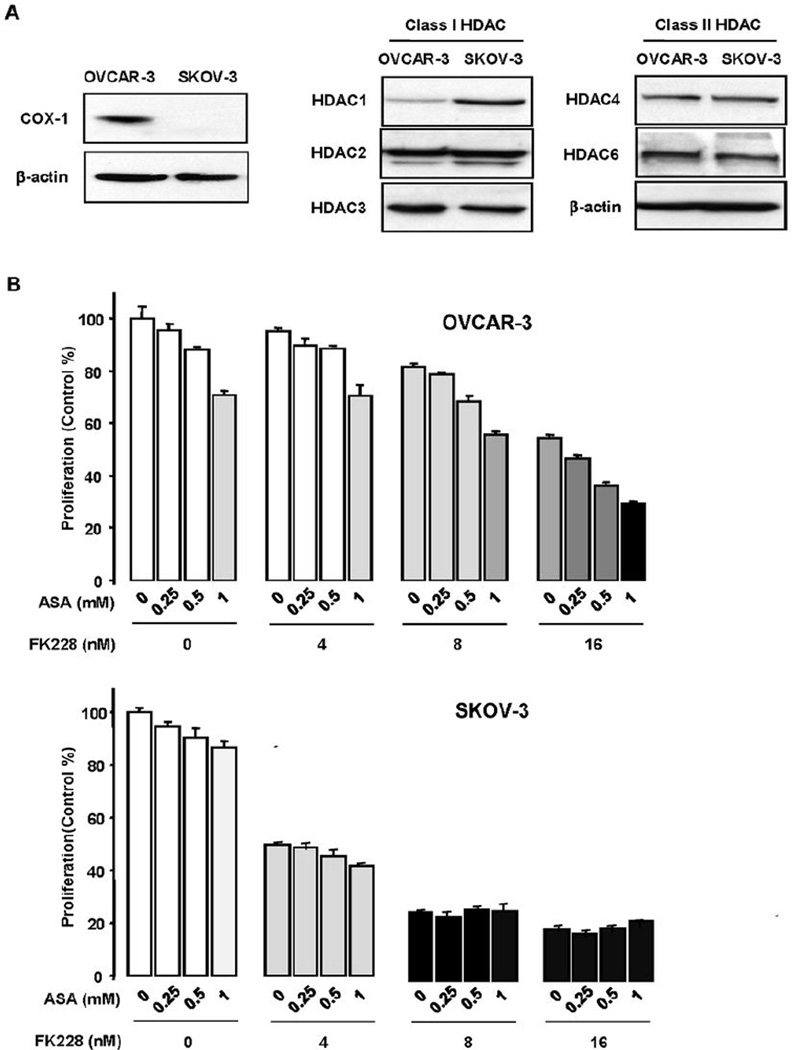
We first confirmed that OVCAR-3 cells constitutively express COX-1 protein and SKOV-3 cells do not (Fig. 1A), in line with prior reports.15 Neither cell line expressed COX-2 at baseline.15 HDAC isoforms (HDACs 1, 2, 3, 4 and 6) were similarly expressed in both cell lines, with a slightly higher level of HDAC1 expression in SKOV-3 cells (Fig. 1A). Both SKOV-3 and OVCAR-3 cells were sensitive to FK228 (Fig. 1B).8 Consistent with a previously published data,16 ASA reduced cell proliferation in OVCAR-3 cells in a dose-dependent manner, but had no effect on SKOV-3 cell proliferation (Fig. 1B). ASA enhanced the ability of FK228 to inhibit OVCAR-3 cell growth in a manner that appeared to be additive, with a CI of 1.04 (Fig. 1B). On the other hand, ASA had no significant impact on cell proliferation or on the observed FK228-induced suppression of cell growth in SKOV-3 cells (Fig. 1B).
Figure 1. Aspirin enhances the growth inhibitory effects of FK228 in COX-1 expressing OVCAR-3 ovarian cancer cells.
(A) COX-1 and HDAC protein expression in the ovarian cancer cell lines OVCAR-3 and SKOV-3 by Western blot. (B) Combined effects of ASA and FK228 on cell proliferation in OVCAR-3 and SKOV-3 ovarian cancer cells. Cells were treated for 48 h with ASA alone (0.25, 0.5 and 1 mM), FK228 alone (4, 8 and 16 nM) and the combination in a dose-dependent manner. Experiments were performed in triplicate and all data are shown as mean ± SE. Different letters indicate significance (p ≤ 0.05) with ANOVA and Tukey’s pairwise analyses.
FK228-induced p21 expression is intensified in the presence of ASA in COX-1 positive ovarian cancer cells
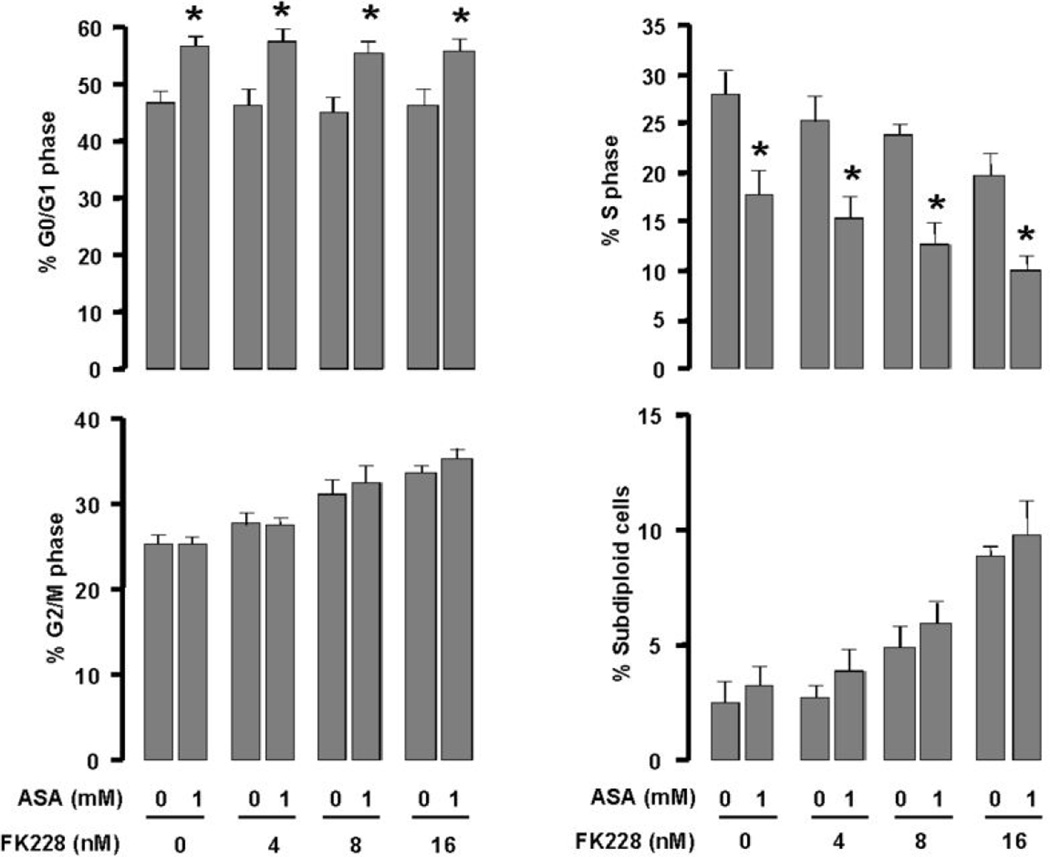
Flow cytometry and cell cycle analysis were performed to evaluate the effects of ASA, FK228 and the combination of drugs on the percentage of cells in each phase of the cell cycle in OVCAR-3 cells. Each drug alone and in combination induced a G0/G1 arrest (Fig 2). In addition, the percentage of cells in the S phase decreased with ASA, FK228 and the combination. Furthermore, the percentage of sub-diploid cells, suggestive of apoptosis, was increased by ASA and FK228 combined in a dose-dependent manner.
Figure 2. ASA, FK228 and the combination of drugs induce a G0/G1 cell cycle arrest and increase sub-diploid DNA content in OVCAR-3 ovarian cancer cells.
Cells were treated for 48 h with ASA (1 mM), FK228 (4, 8 and 16 nM) or the combination. Flow cytometry assays were performed to determine the percentage of cells in each phase of the cell cycle and the percentage of cells with sub-diploid DNA content as a measure of apoptosis. Experiments were performed in triplicate and all data are shown as mean ± SE. Asterisks indicate significance (p ≤ 0.05) using the paired Student’s t-test.
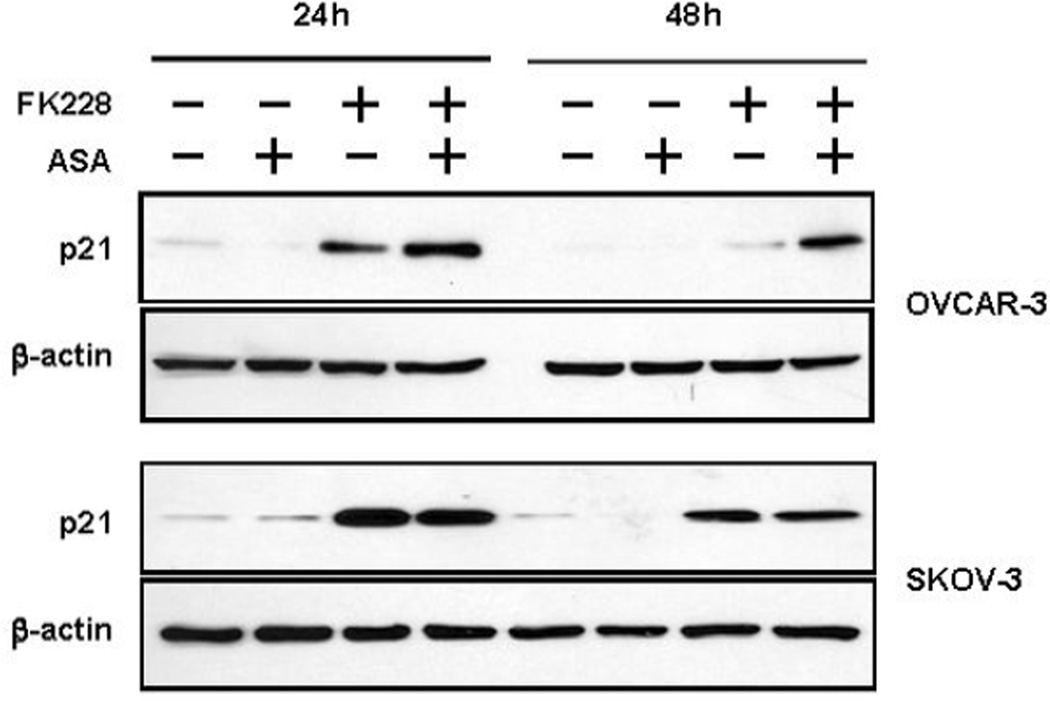
A likely mechanism for the decrease in S phase and cell cycle arrest is the induction of cyclin dependent kinase inhibitor p21, based on the functional role of p21 and the fact that it is a known target of ASA and HDAC inhibitors.5, 23 Furthermore, our group has previously shown that p21 is induced by FK228 in ovarian cancer cells in a p53-independent manner.8 We measured p21 expression by Western blot analysis in OVCAR-3 and SKOV-3 cells after 24 and 48 h treatment with ASA, FK228 or both drugs combined. In OVCAR-3 cells, ASA enhanced and sustained the expression of p21-induction by FK228 (Fig. 3). On the other hand, ASA had no effect on FK228-induced p21 protein in SKOV-3 cells, which displayed robust induction of p21 in response to FK228 alone.
Figure 3. ASA increases and prolongs FK228-induced p21 expression in OVCAR-3 ovarian cancer cells.
OVCAR-3 and SKOV-3 cells were treated with FK228 (8 nM), ASA (1 mM) or both for 24 and 48 h. The expression levels of p21 were measured by Western blot.
Silencing COX-1 with siRNA partially attenuates the effects of ASA on FK228-induced p21 protein expression and suppression of ovarian cancer cell growth
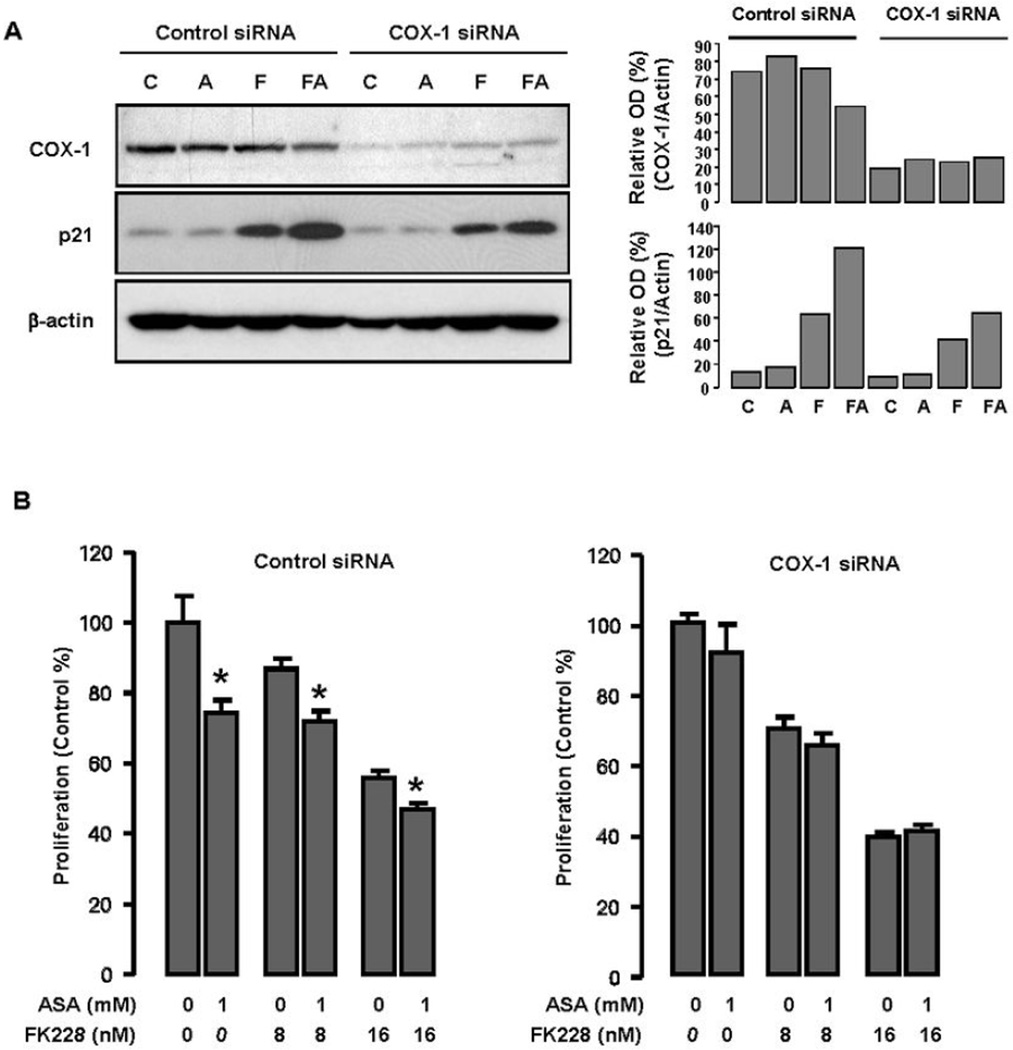
Because ASA is a relatively selective COX-1 inhibitor13 and suppresses COX-1 expressing ovarian cancer cell growth,15–18 COX-1 appears to be a therapeutic target for ASA in COX-1 expressing ovarian cancer cells. Therefore, to evaluate the influence of COX-1 on p21 expression, we knocked down gene expression of COX-1 in OVCAR-3 ovarian cancer cells by using COX-1 siRNA and non-targeting siRNA as a negative control. Although COX-1 was not completely silenced, levels of p21 protein expression were significantly reduced with COX-1 siRNA treatment (Fig. 4A). In addition, COX-1 silencing prevented further suppression of cell growth in cells treated with both ASA and FK228 (Fig. 4B).
Figure 4. Silencing COX-1 partially reverses the growth inhibitory effects of ASA on FK228-induced p21 expression and growth inhibition in OVCAR-3 ovarian cancer cells.
(A) Effects of COX-1 siRNA on p21 induced by ASA and FK228. Gene silencing with siRNA strategies was performed as described in Materials and Methods, with control samples being incubated with non-targeted siRNA sequences. After 72h transfection, OVCAR-3 cells were treated with FK228 (8 nM) alone and the combination of ASA (1 mM) for 24 h. Western blots were performed using antibodies for COX-1, p21 and a loading control β-actin. These figures represent data from duplicate experiments. C = control; A = ASA; F = FK228; FA = FK228+ASA. (B) Effects of COX-1 siRNA on cell growth suppressed by ASA and FK228. After 72h transfection, OVCAR-3 cells were treated with FK228 (8 and 16 nM) alone and the combination of ASA (1 mM) for 48 h and the rate of cell proliferation was measured using MTT assays. Experiments were performed in triplicate and all data are shown as mean ± SE. Asterisks indicate significance (p ≤ 0.05) with the paired Student’s t-test after ASA treatment.
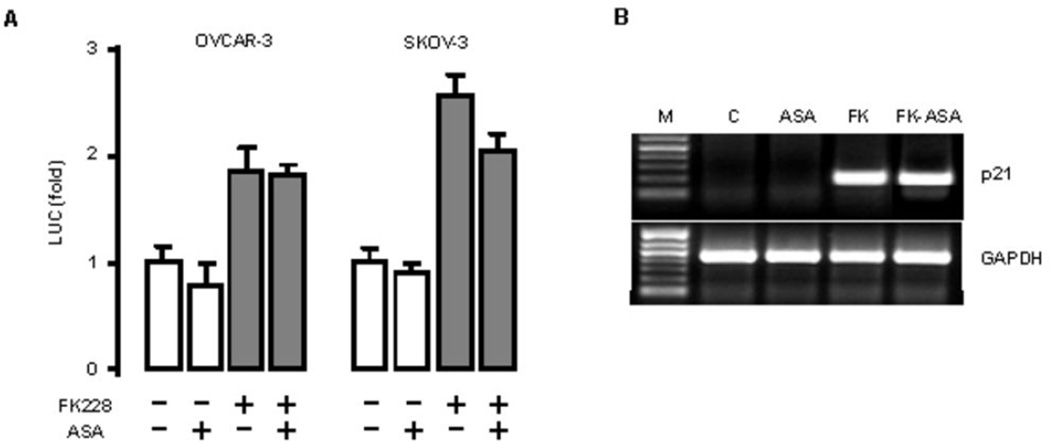
ASA has no effect on FK228-induced p21 promoter activity and p21 mRNA in ovarian cancer cells
It is well-known that HDAC inhibitors activate p21 expression at the transcriptional level5. In order to clarify how ASA enhanced and prolonged FK228-induced p21 expression, we first evaluated the promoter activity of p21. We found that full-length p21 promoter activity was increased approximately 2-fold by FK228, but was not affected by ASA (Fig. 5A). Then, we examined the effects of ASA and FK228 on the levels mRNA expression of p21 and found p21 levels were significantly upregulated by FK228, with no further increase observed in the presence of ASA (Fig. 5B).
Figure 5. ASA is not associated with FK228-induced p21 expression at the promoter or transcriptional levels in ovarian cancer cells.
(A) The effect of ASA on FK228-induced p21 promoter activity. Cells were transfected for 3 h with the p21 promoter (500 ng/ml). Where indicated, cells were incubated with FK228 (8 nM), ASA (1 mM) or both overnight. The luciferase activity was normalized to total protein concentrations and expressed as a fold change in comparison to control. A gray bar indicates significant increases (p ≤ 0.05) when calculated by ANOVA and Tukey’s pairwise comparisons. (B) Effects of ASA on FK228-induced p21 mRNA. OVCAR-3 cells were incubated with FK228 (FK, 8 nM), ASA (1 mM) or both for 24 h. After isolating total RNA, RT-PCR was performed using specific primers for p21 and GAPDH as a loading control with 30 cycles. A representative result is shown. The first lane is the molecular marker (M).
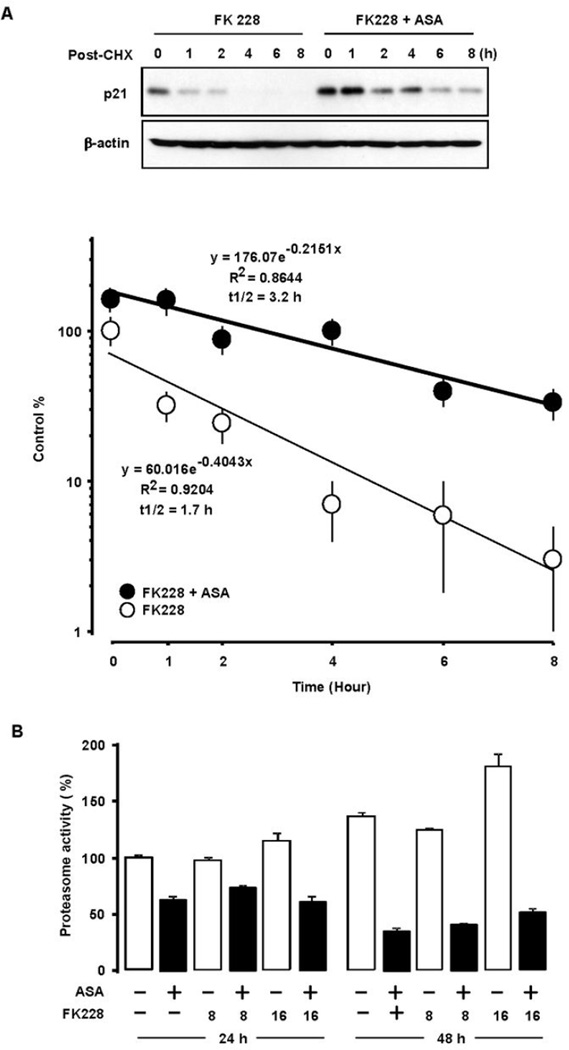
ASA prolongs the half-life of FK228-induced p21 protein and inhibits proteasome activity in ovarian cancer cells
Because we did not observe differences at the promoter or transcriptional levels by adding ASA to FK228 treatment, we asked if protein stabilization could contribute to the observed enhanced and prolonged induction of p21 with combination treatment. Protein degradation studies were performed using the protein synthesis inhibitor cycloheximide. After 24 h incubation with FK228 alone or combined with ASA, cycloheximide was added to block protein synthesis. We observed that ASA increased FK228-induced p21 protein at the 24 h time point compared with FK228 alone (Fig. 6A). In cells treated with FK228 alone, p21 was rapidly degraded with only trace levels of expression after 4 h. ASA maintained FK228-induced p21 protein and extended p21 expression levels beyond 8 h. The observed half-life of FK228-induced p21 protein was 1.7 h. Combining ASA with FK228 almost doubled the half-life of p21 to 3.2 h. Since p21 stability is associated with proteasomemediated degradation,24, 25 proteasome assays were performed. ASA inhibited proteasome activity (Fig. 6B) at the 24 h and 48 h time points regardless of FK228 treatment in OVCAR-3 cells.
Figure 6. ASA increases FK228-induced p21 protein stability.
(A) OVCAR-3 cells were incubated with FK228 (8 nM) alone and the combination of ASA (1 mM) for 24 h and added with cycloheximide (5 µM) for 1, 2, 4, 6 and 8 h. The signal intensities were quantified by densitometry using Quantity One (Biorad, Hercules, CA). Absolute values were obtained after determining the average density of each spot, the background with minimum value and the interquartile ranges. Experiments were performed in triplicate and a representative result is shown. (B) The effect of ASA on proteasome activity. OVCAR-3 cells were treated for 24 and 48 h with ASA (1 mM), FK228 (8 and 16 nM) or the combination. Cell-based proteasome assays were performed in triplicate and all data are shown as mean ± SE. Black bars indicate significance (p ≤ 0.05) with the paired Student’s t-test.
DISCUSSION
In this study, we asked whether combining a relatively selective COX-1 inhibitor ASA with the potent HDAC inhibitor FK228 could enhance the effects of FK228 in COX-1 positive ovarian cancer cells. We found that ASA influences FK228-inhibited cell growth in COX-1 positive cells in an additive manner. The combination of ASA and FK228 leads to a G0/G1 cell cycle arrest, reduces the S phase and increases sub-diploid DNA content indicative of apoptosis. Furthermore, ASA prolongs the half-life of p21 protein induced by FK228, most likely by blocking its degradation through inhibition of proteasome activity. Silencing COX-1 with siRNA reduces relative levels of p21 expression and growth inhibition when ASA and FK228 are combined, suggesting that the COX-1 inhibition influences these results.
Although controversy exists, there is a possible inverse relationship between the use of aspirin and the risk for developing ovarian cancer.14 ASA is a known relatively selective inhibitor of COX-1.13 Moreover, COX-1 is highly expressed in subsets of human and mouse ovarian cancers,15–18 implying that COX-1 could be a target for therapy. Our finding that ASA suppresses cell growth in COX-1 positive human OVCAR-3 ovarian cancer cells is consistent with previously published results demonstrating ASA inhibits ovarian cancer progression in mouse, human16 and avian26 models of ovarian cancer. In contrast, ASA produces minimal anti-proliferative effect in COX-1 negative SKOV-3 cells.
The data presented here differ from a recently published report, in which the HDAC inhibitors, SAHA and sodium butyrate, each studied in combination with the ASA appear to synergistically induce cell death in A2780 ovarian cancer cells.12 In this previously published report, the authors propose that the effects of ASA are independent of COX inhibition and are instead due to the acetyl and salicyl moieties of ASA interacting cooperatively interact with HDAC inhibitors. However, the status of COX and HDAC protein expression in the A2780 cells was not reported. Furthermore, the doses of ASA used were 1–4mM15–18 and the doses of HDAC inhibitors were in the micromolar to millimolar range (higher than the doses studied by our group).
Anti-tumor agents that induce downstream targets of p53 such as p21 are important for the treatment of tumors with non-functional or mutant p53. The expression of wild-type p53 is not required for HDAC inhibitor-induced apoptosis and HDAC inhibitors induce p21 independent of p53.5 The molecular background of OVCAR-3 and SKOV-3 ovarian cancer cells is well-established.19 OVCAR-3 is p53 mutated and SKOV-3 is p53 null. SKOV-3 has additional mutations in p16 and PIK3CA genes.19, 20 Both cell lines induce p21 after exposure to FK228, but only OVCAR-3 cells induce higher levels of p21 in the presence of FK228 and ASA. It is not clear if this difference in response is due to differences in the other known molecular features of each cell line, but based on our results, it appears that COX-1 inhibition contributes to the observed prolongation and enhancement of p21 expression.
Although the exact mechanisms are unknown, our results suggest the upregulation and stabilization of p21 induced by ASA and FK228 may be cell-type specific and independent of p53. At the concentrations studied, ASA did not appear to directly induce p21, which has been shown in other reports to occur through inhibition of nuclear factor kappa B.27 The mechanisms by which ASA further enhances FK228-induced p21 do not appear to be at the promoter or transcriptional levels. On the other hand, the degradation half-life of p21 protein is prolonged by the combination of ASA and FK228 through stabilization of the p21 protein, most likely through inhibition of proteasome activity.24, 25
Taken together, these results support the possibility that ASA could enhance the chemotherapeutic effects of the potent HDAC inhibitor, FK228 in subtypes of ovarian cancer. Our results taken together with previous observations warrant further investigation of ASA and similar drugs that prolong p21 expression in combination with HDAC inhibitors as a therapeutic strategy for the treatment of ovarian cancer.
Acknowledgements
We thank Dr. Xiao-Fan Wang (Duke University Medical Center, Durham, NC) for the p21 promoter constructs. We thank Lynne Black (Vanderbilt University, Nashville, TN) for her editorial assistance.
Grant support: This research was supported in part by funding through the Meharry-Vanderbilt-TSU Cancer Partnership NIH 2U54CA091408 (D.S.S. & D.K.), the Research Centers in Minority Institutions NIH G12RR03032 (D.S.S. & D.K.) and Gloucester Pharmaceuticals, Inc. Research Grant (D.K.).
Abbreviations Used
- ASA
Aspirin
- COX-1
Cyclooxygenase-1
- COX-2
Cyclooxygenase-2
- HDAC
Histone deacetylase
- LUC
Luciferase
- FK228
Romidepsin
- RT-PCR
Reverse transcription polymerase chain reaction
REFERENCES
- 1.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer Statistics 2009. CA Cancer J Clin. 2009 doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 2.Chobanian N, Dietrich CS., 3rd Ovarian cancer. Surg Clin North Am. 2008;88:285–299. doi: 10.1016/j.suc.2007.12.002. vi. [DOI] [PubMed] [Google Scholar]
- 3.Cannistra SA. Cancer of the ovary. N Engl J Med. 2004;351:2519–2529. doi: 10.1056/NEJMra041842. [DOI] [PubMed] [Google Scholar]
- 4.Landen CN, Jr, Birrer MJ, Sood AK. Early events in the pathogenesis of epithelial ovarian cancer. J Clin Oncol. 2008;26:995–1005. doi: 10.1200/JCO.2006.07.9970. [DOI] [PubMed] [Google Scholar]
- 5.Bolden JE, Peart MJ, Johnstone RW. Anticancer activities of histone deacetylase inhibitors. Nat Rev Drug Discov. 2006;5:769–784. doi: 10.1038/nrd2133. [DOI] [PubMed] [Google Scholar]
- 6.Frew AJ, Johnstone RW, Bolden JE. Enhancing the apoptotic and therapeutic effects of HDAC inhibitors. Cancer Lett. 2009 doi: 10.1016/j.canlet.2009.02.042. [DOI] [PubMed] [Google Scholar]
- 7.Haberland M, Montgomery RL, Olson EN. The many roles of histone deacetylases in development and physiology: implications for disease and therapy. Nat Rev Genet. 2009;10:32–42. doi: 10.1038/nrg2485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Khabele D, Son DS, Parl AK, Goldberg GL, Augenlicht LH, Mariadason JM, et al. Drug-induced inactivation or gene silencing of class I histone deacetylases suppresses ovarian cancer cell growth: implications for therapy. Cancer Biol Ther. 2007;6:795–801. doi: 10.4161/cbt.6.5.4007. [DOI] [PubMed] [Google Scholar]
- 9.Weichert W, Denkert C, Noske A, Darb-Esfahani S, Dietel M, Kalloger SE, et al. Expression of class I histone deacetylases indicates poor prognosis in endometrioid subtypes of ovarian and endometrial carcinomas. Neoplasia. 2008;10:1021–1027. doi: 10.1593/neo.08474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lech-Maranda E, Robak E, Korycka A, Robak T. Depsipeptide (FK228) as a novel histone deacetylase inhibitor: mechanism of action and anticancer activity. Mini Rev Med Chem. 2007;7:1062–1069. doi: 10.2174/138955707782110178. [DOI] [PubMed] [Google Scholar]
- 11.Modesitt SC, Sill M, Hoffman JS, Bender DP. A phase II study of vorinostat in the treatment of persistent or recurrent epithelial ovarian or primary peritoneal carcinoma: a Gynecologic Oncology Group study. Gynecol Oncol. 2008;109:182–186. doi: 10.1016/j.ygyno.2008.01.009. [DOI] [PubMed] [Google Scholar]
- 12.Sonnemann J, Huls I, Sigler M, Palani CD, Hong le TT, Volker U, et al. Histone deacetylase inhibitors and aspirin interact synergistically to induce cell death in ovarian cancer cells. Oncol Rep. 2008;20:219–224. [PubMed] [Google Scholar]
- 13.Mitchell JA, Akarasereenont P, Thiemermann C, Flower RJ, Vane JR. Selectivity of nonsteroidal antiinflammatory drugs as inhibitors of constitutive and inducible cyclooxygenase. Proc Natl Acad Sci U S A. 1993;90:11693–11697. doi: 10.1073/pnas.90.24.11693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Prizment AE, Folsom AR, Anderson KE. Nonsteroidal anti-inflammatory drugs and risk for ovarian and endometrial cancers in the Iowa Women's Health Study. Cancer Epidemiol Biomarkers Prev. 2010;19:435–442. doi: 10.1158/1055-9965.EPI-09-0976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gupta RA, Tejada LV, Tong BJ, Das SK, Morrow JD, Dey SK, et al. Cyclooxygenase-1 is overexpressed and promotes angiogenic growth factor production in ovarian cancer. Cancer Res. 2003;63:906–911. [PubMed] [Google Scholar]
- 16.Daikoku T, Tranguch S, Chakrabarty A, Wang D, Khabele D, Orsulic S, et al. Extracellular signal-regulated kinase is a target of cyclooxygenase-1-peroxisome proliferatoractivated receptor-delta signaling in epithelial ovarian cancer. Cancer Res. 2007;67:5285–5292. doi: 10.1158/0008-5472.CAN-07-0828. [DOI] [PubMed] [Google Scholar]
- 17.Daikoku T, Tranguch S, Trofimova IN, Dinulescu DM, Jacks T, Nikitin AY, et al. Cyclooxygenase-1 is overexpressed in multiple genetically engineered mouse models of epithelial ovarian cancer. Cancer Res. 2006;66:2527–2531. doi: 10.1158/0008-5472.CAN-05-4063. [DOI] [PubMed] [Google Scholar]
- 18.Daikoku T, Wang D, Tranguch S, Morrow JD, Orsulic S, DuBois RN, et al. Cyclooxygenase-1 is a potential target for prevention and treatment of ovarian epithelial cancer. Cancer Res. 2005;65:3735–3744. doi: 10.1158/0008-5472.CAN-04-3814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ikediobi ON, Davies H, Bignell G, Edkins S, Stevens C, O'Meara S, et al. Mutation analysis of 24 known cancer genes in the NCI-60 cell line set. Mol Cancer Ther. 2006;5:2606–2612. doi: 10.1158/1535-7163.MCT-06-0433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.DTP N. NCI-60 DTP Human Tumor Cell Line Screen. NCI/NIH. 2010 [Google Scholar]
- 21.Chou TC, Talalay P. Quantitative analysis of dose-effect relationships: the combined effects of multiple drugs or enzyme inhibitors. Adv Enzyme Regul. 1984;22:27–55. doi: 10.1016/0065-2571(84)90007-4. [DOI] [PubMed] [Google Scholar]
- 22.Khabele D, Lopez-Jones M, Yang W, Arango D, Gross SJ, Augenlicht LH, et al. Tumor necrosis factor-alpha related gene response to Epothilone B in ovarian cancer. Gynecol Oncol. 2004;93:19–26. doi: 10.1016/j.ygyno.2003.11.058. [DOI] [PubMed] [Google Scholar]
- 23.Ocker M, Schneider-Stock R. Histone deacetylase inhibitors: signalling towards p21cip1/waf1. Int J Biochem Cell Biol. 2007;39:1367–1374. doi: 10.1016/j.biocel.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 24.Bloom J, Amador V, Bartolini F, DeMartino G, Pagano M. Proteasome-mediated degradation of p21 via N-terminal ubiquitinylation. Cell. 2003;115:71–82. doi: 10.1016/s0092-8674(03)00755-4. [DOI] [PubMed] [Google Scholar]
- 25.Dikshit P, Chatterjee M, Goswami A, Mishra A, Jana NR. Aspirin induces apoptosis through the inhibition of proteasome function. J Biol Chem. 2006;281:29228–29235. doi: 10.1074/jbc.M602629200. [DOI] [PubMed] [Google Scholar]
- 26.Urick ME, Giles JR, Johnson PA. Dietary aspirin decreases the stage of ovarian cancer in the hen. Gynecol Oncol. 2009;112:166–170. doi: 10.1016/j.ygyno.2008.09.032. [DOI] [PubMed] [Google Scholar]
- 27.Rundall BK, Denlinger CE, Jones DR. Combined histone deacetylase and NF-kappaB inhibition sensitizes non-small cell lung cancer to cell death. Surgery. 2004;136:416–425. doi: 10.1016/j.surg.2004.05.018. [DOI] [PubMed] [Google Scholar]