Abstract
Environmental enrichment can modulate mild and chronic stress, responses to anxiogenic stimuli as well as drug vulnerability in a number of animal models. The current study was designed to examine the impact of postnatal environmental enrichment on selectively bred 4th generation high (HAn) and low anxiety (LAn) male rats. After weaning, animals were placed in isolated, social and enriched environments (e.g., toys, wheels, ropes, changed weekly). We measured anxiety-like behavior (ALB) on the elevated plus maze (EPM; trial 1 at PND 46, trial 2 at PND 63), amphetamine (0.5 mg/kg, IP)-induced locomotor behavior, basal and post anxiogenic stimuli changes in (1) plasma corticosterone, (2) blood pressure and (3) core body temperature. Initially, animals showed consistent trait differences on EPM with HAn showing more ALB but after 40 days in select housing, HAn rats reared in an enriched environment (EE) showed less ALB and diminished AMPH-induced activity compared to HAn animals housed in isolated (IE) and social environments (SE). In the physiological tests, animals housed in EE showed elevated adrenocortical responses to forced novel object exposure but decreased body temperature and blood pressure changes after an air puff stressor. All animals reared in EE and SE had elevated BDNF-positive cells in the central amygdala (CeA), CA1 and CA2 hippocampal regions and the caudate putamen, but these differences were most pronounced in HAn rats for CeA, CA1 and CA2. Overall, these findings suggest that environmental enrichment offers benefits for trait anxiety rats including a reduction in behavioral and physiological responses to anxiogenic stimuli and amphetamine sensitivity, and these responses correlate with changes in BDNF expression in the central amygdala, hippocampus and the caudate putamen.
Keywords: Environmental enrichment, elevated plus maze, heart rate, body temperature, BDNF, central amygdala
1. Introduction
Anxiety disorders are prevalent and have been studied extensively in clinical settings (Mojabai, Olfson and Mechanic, 2002; Ressler and Mayber, 2007; Young et al., 2001). Many people suffering from anxiety often also present with substance use (Merikangas, Dierker and Szatmari, 1998) with an estimated 17.71% of people meeting criteria for both a 12-month substance use disorder and anxiety disorder (Grant et al. 2004). An often-used method for studying anxiety in an animal model is the exploitation of selective breeding to produce animals that display a specific anxiety profile. Inbred lines of high anxiety-like behavior (HAB) rats show similar profiles to anxious clinical populations, with increased adrenocortical response (Landgraf et al., 1999) and greater activation in brain areas implicated in anxiety (Hasler et al., 2004; Salomé et al., 2004). Individuals abusing psychostimulant drugs such as cocaine also show compromised adrenocortical responses, indicating that dysregulation of the hypothalamic-pituitary-adrenal (HPA) axis implicated in anxiety disorders, may contribute to aspects of addiction as well (Contoreggi et al., 2003). Rats phenotyped as high responders (e.g., rats that self-administer amphetamine and sucrose more readily) show a more robust corticosterone (CORT) response to novelty exposure (Cain, Saucier and Bardo, 2005; Piazza et al., 1999). Further, in Lewis and Fischer 344 inbred rat strains that show varying vulnerabilities to psychostimulant drugs of abuse, baseline CORT levels in a novel environment are positively correlated with amphetamine (AMPH) locomotor activity (Miserendino, Haile and Kosten, 2003).
While adrenocortical responses are an index of HPA axis activity (for review, see Lovallo, 2006), with persistent stress elevating plasma CORT levels (Brennan et al., 2000), physiological changes such as body temperature and heart rate have also been shown to fluctuate in rodents following mild environmental stressors such as odors, air puffs and strobe lights (Harkin et al., 2002). Psychological stressors can also interact with these measures, with early work by Long and colleagues (1990) showing that “cage-switch stress” (moving a rat to an empty cage previously occupied by another rat) increased body temperature on average 1.21°C. More recent work has also shown increases in body temperature (0.93°C) and heart rate in rats following a brief air puff (Harkin et al., 2002), and stress-induced hyperthermia (Olivier et al., 2003). Still other mild stressors such as novel odors or strobe lights can elevate body temperature, basal heart rate and increase locomotor activity in rodents (Harkin et al., 2002). Differences in physiological measures have been noted in animals exhibiting anxiety like behavior in social defeat paradigms (Bhatnagar et al., 2006) and in rats exposed to acute and repeated social stress paradigms, with tachycardia responses showing adaptation to repeated stress (Chen and Herbert, 1995). Inhibition of cellular activity with gamma aminobutyric acid (GABA) in the dorsomedial hypothalamus attenuates the adrenocorticotropic hormone (ACTH), tachycardia and blood pressure spikes while GABA stimulation in the paraventricular nucleus only affected ACTH levels following the air stress (Stotz-Potter, Morin and DiMicco, 1996).
The enriched environment (EE), social housing cages equipped with toys that provide sensorimotor stimulation, has been implicated in restoring stress-induced learning deficits and depressive-like behavior (Cui et al., 2006). Enrichment can also modify the brain and adrenocortical response to stress (Diamond, 2001) and sensitivity to psychostimulant drugs of abuse (Bardo et al., 2001) in a number of animal models. Further, higher basal levels of ACTH have been found in males reared in isolated environments versus those reared in group housing. In addition following stress exposure those in isolation also exhibited an increase in CORT and ACTH compared to their group housed counterparts (Weiss et al., 2004). There are also a few studies examining the impact of EE on the physiological responses to stress that show EE can reverse elevated heart rate, systolic blood pressure and hyperthermia (Lawson et al., 2000; Sharp, Azar and Lawson, 2005).
Neurotrophin expression, particularly brain derived neurotrophic factor (BDNF) expression, has been implicated in the benefits associated with environmental enrichment (Ickes et al., 2000; Rossi et al., 2006). Furthermore, BDNF expression is thought to influence synaptic modifications that may underlie the neuroplasticity necessary for stress resilience since BDNF knock out mice are stressed (for review, see Chourbaji et al., 2008), and BDNF heterozygous mice only show a partial recovery in exploratory behavior and dendritic spine proliferation from enriched environments compared to their wild-type counterparts (Zhu et al., 2009).
The current study was designed to determine how EE might influence basal and mild stress-induced physiological responses (e.g., CORT, heart rate, blood pressure and core body temperature) in selective outbred animals phenotyped as high (HAn) and low anxiety (LAn) (i.e., unrelated mating pairs). In addition, we set out to assess any changes in anxiety response on the elevated plus maze and amphetamine-induced locomotion in selectively bred animals following EE relative to their counterparts housed in social and isolated environments. Finally, we measured brain derived neurotrophin factor (BDNF) protein levels in hippocampus, central amygdala and caudate putamen to assess any changes in levels depending on trait anxiety or postnatal housing experience.
2. Experimental Procedures
2.1 Experimental Subjects
Sixty male Long Evans rats were used in this study. All animals were acquired from the fourth generation of high anxiety (HAn) or low anxiety (LAn) unrelated same-phenotype pairings. These trait anxiety lines were bred at the University of Massachusetts Boston taking care to not cross sibling pairs. HAn or LAn status was determined using percent open arm (OA) time and OA entries in the elevated plus maze (EPM) with LAn animals showing less anxiety-like behavior (upper quartile) in the apparatus than their HAn counterparts (lower quartile). Animals were maintained in a temperature and humidity controlled environment on a 12 h light-dark cycle (lights on at 800 h); food and water was available ad libitum except during testing procedures. All protocols received approval from the University of Massachusetts IACUC and closely followed the applicable portions of the National Institute of Health Guide for the Care and Use of Laboratory Animals (NIH Publications No. 80–23; Revised 1996).
2.2 Postnatal Housing Environments and Timing of Testing
On average postnatal day (PND) 23, animals were weaned and not more than two male littermates were used to establish the trait and postnatal housing environment groups. A total of 30 LAn males were placed in either an isolated environment (IE, n=10), social environment (SE n=10), or enriched environment (EE, n=10). The same groups were established using 30 HAn males (n=10 per group). The IE consisted of one rat housed in a standard Plexiglas cage (17 × 24 × 20 cm) with contact bedding. The SE was constructed of a large Plexiglas rectangular environment (24 × 40 × 81 cm) with normal contact bedding housing ten same-phenotyped animals. The EE consisted of a large wire metal cage with a smooth metal floor (94 × 94 × 51) equipped with contact bedding. Various objects consisting of plastic and wood toys (e.g. Lego blocks, buckets, rattles, wheels, hides) as well as objects to promote movement such as ropes, hanging ladders and hanging chains were included and rearranged and/or changed weekly with new objects being introduced or others removed. Animals remained in IE, SE and EE for a minimum of 40 days and then began a schedule of behavioral and physiological tests with allowance for rest periods between tests (Fig. 1).
Fig. 1.
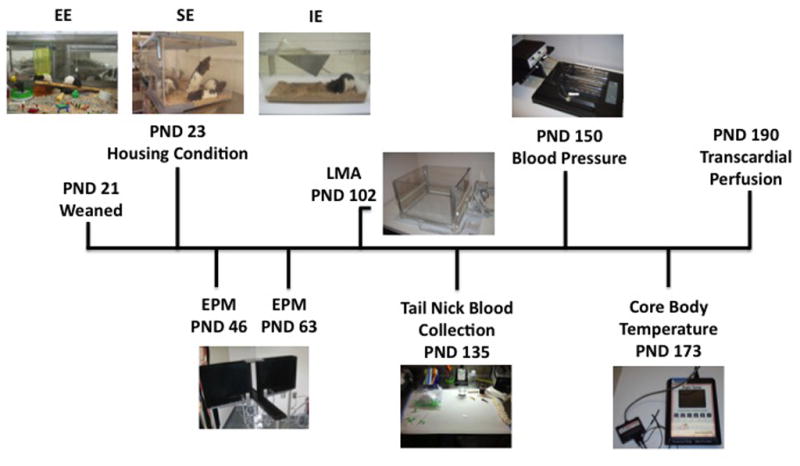
Diagram of the timeline for placement of HAn and LAn lines in select housing condition, schedule of behavioral and physiological tests through study termination and brain harvesting. Abbreviations: EPM, elevated plus maze; LMA, locomotor activity; BP, blood pressure; T, core body temperature.
2.3 Elevated plus maze
A plus-shaped maze constructed of black Plexiglas (Med Associates, VT) with two opposing arms equipped with just a floor, and two opposing arms with floors and high enclosed walls, was used to measure anxiety response. Animals were placed at the juncture of the maze and allowed to remain in the maze for 5 min on PND 46 and 40 days post-housing on PND 63. We recorded the following behaviors: percent time on open arms, percent open arm entries, percent closed arm time and percent closed arm entries. Data are represented as group means ±S.E.M.
2.4 Locomotor activity
Animals were placed in a locomotor activity (LMA) system (Med Associates, St. Albans VT) for monitoring over 1.5 h. On PND 102, all animals were first habituated to the chamber for 30 min and then removed and administered D-amphetamine (AMPH, 0.5 mg/kg IP) and then returned to the chamber for 60 min post-injection recording (distance traveled (cm) and vertical counts).
2.5 Tail artery blood draw
Rats were transported at 0900 in individual transport cages, with access to food and water, in groups of 8 from the colony to the lab on PND 155. A 15 min habituation period was observed upon arrival. Rats were allowed to freely move during procedure, with the additional option of burrowing into a mesh glove. An initial incision was made 5–7cm from the distal end of the tail using a razor blade. 300μl of blood was collected into disposable pre-heparinized capillary tubes (RAM Scientific, NY) and immediately following collection the blood sample was kept on wet ice. The tail nick incision site was cleaned with warm saline solution and povidone-iodine was applied before returning the rat to the transport cage. This procedure was repeated for the post-stressor blood draw that followed 30 min after initial stressor (forced novel environment + novel object exposure) was introduced. Whenever possible, the original incision site was used for collection. All samples were centrifuged at 4°C 2500G for a minimum of 10 min. Plasma was collected via micropipette and stored at −80°C.
2.6 Determination of plasma corticosterone
Plasma aliquots were assayed for corticosterone using the Coat-A-Count(r) Rat Corticosterone RIA Kit (Siemens Medical Solutions Diagnostics, Los Angeles, CA). The assay detection limit was 5.7 ng/ml, and average intra-assay variability (between duplicates) was 2.25%. In order to control for initial to post-stressor variability, we also calculated percent change from the initial measure (i.e., “baseline”) in a separate analysis.
2.7 Non-invasive blood pressure
Determination of blood pressure was made using the CODA non-invasive tail-cuff blood pressure system (Kent Scientific, Torrington CT). Animals were habituated to an appropriately sized restraint tube for 3 consecutive days for 15 min each day with cuff systems in place (PND 150). On the fourth consecutive day, animals were again secured and blood pressure (diastolic (DAP) and systolic arterial pressure (SAP)) data were collected each minute for 15 min to determine initial blood pressure that is set as the “baseline” measure. Following this first data collection, animals were subjected to a stressor (10 sec continuous air puff) and DAP and SAP data were collected for an additional 15 min.
2.8 Core body temperature
Core body temperature (T) was measured using the Right Temp Warming System (Kent Scientific, Torrington, CT) equipped with appropriate rat probes. On PND 173, probes were inserted rectally to record an initial T (°C) that we refer to as “baseline”. Animals were then lightly restrained and exposed to a continuous air puff-stressor for 10 sec. Another T recording was made 30 min post-stressor. Additionally, prior to all data collection animals were exposed to the temperature acquisition protocol to ensure the rectal probe did not act as an additional stressor.
2.9 Transcardial perfusion
At the termination of all testing, animals were administered a lethal dose of Nembutal (50 mg/kg/ml IP). Animals were first perfused with 0.9% isotonic saline followed by 4% paraformaldehyde. Brains were removed and stored in 4% paraformaldehyde until blocking. Blocks of tissue were then stored for 24 hrs first in 20% sucrose-4% paraformaldehyde and then moved to 10% sucrose-4% paraformaldehyde. 30 μm sections were taken of the medial hippocampus and central amygdala and stored in 4% paraformaldehyde briefly prior to immunohistochemistry.
2.10 Immunohistochemistry: Brain-derived neurotrophic factor
30 μm sections were washed for 1h in NaPBS followed by incubation, first for 1 h at RT in anti-brain derived neurotrophic factor in NaPBS-0.4% Triton-X (diluted 1:10,000; Millipore Billerica MA) then at 4°C for 72 h. The sections were rinsed for 1 h in NaPBS then incubated in biotin-goat anti-rabbit in NaPBS-0.4% Triton-X (1:600; Vector Labs, Burlingame CA) for 1 h at RT. The sections were rinsed in NaPBS and incubated for 1 h at RT in avidin-biotin solution (45:45:10,000; Vector Labs, Burlingame CA) followed by rinses in NaPBS and TBS. Finally, the sections were developed in diaminobenzidine-peroxidase solution (DAB Kit; Vector Labs, Burlingame CA) to visualize BDNF IR cells.
The sections were imaged at 20× magnification using a SPOT Flex Monochrome microscopy camera and SPOT 5.0 software (Diagnostic Instruments, Sterling Heights MI). An observer blind to treatment groups made manual BDNF-IR cell counts of 3 sections bilaterally (6 measurements total, averaged per animal) within the area of interest using the atlas of Paxinos and Watson (2004); next, after setting a threshold to minimize the background “noise” while keeping the BDNF-positive neurons visible, counts were automated using ImageJ software (NIH). We were able to validate the automated counts with the manual counts for representative animals from each brain region. All BDNF-IR data are expressed as the number (mean ± S.E.M.) of cells expressing BDNF-IR in a 2502μm box placed over the targeted brain region.
2.11 Data analysis
In order to determine any effects of trait anxiety, housing and potential interactions, we performed two-way ANOVA for each of the behavioral and physiological assays (2 Factors: Trait, Housing). For locomotor activity, we performed a repeated measures three-way ANOVA (3 Factors: Trait, Housing, Time). Planned Bonferroni comparisons were carried out to determine any pair-wise differences. For immunohistochemical analyses, we took coronal sections at three anatomical levels for each of the brain areas (CA1, CA2 and CA3 of the hippocampus, CeA and CPu) and analyzed each bilaterally; thus six data points were averaged for each animal. GraphPad Prism (v. 10) and SPSS for Mac were used for statistical analyses and graphing of data. Alpha was set at p<0.05 for all significance levels.
3. Results
Figure 1 illustrates the timeline for the study from weaning, placement into select housing conditions, EPM tests, and the subsequent behavioral and physiological tests through study termination and tissue harvesting.
3.1 Elevated plus maze
3.1.1 Percent Time on Open Arms (PND 46)
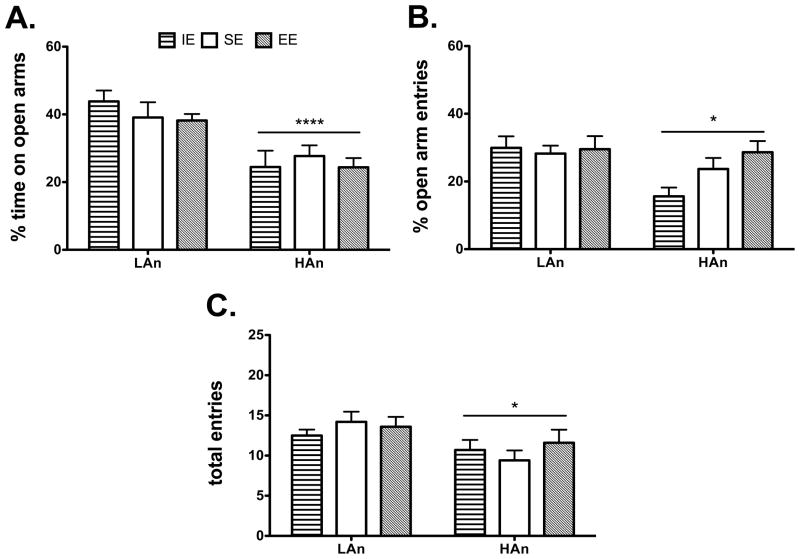
We did an initial EPM test (PND 46) of LAn/HAn animals after only about 20 days in the select housing conditions and did not observe any significant differences for the main effect of Housing [F(2,54)=2.1605, p>0.05, n.s.] or for the interaction [F(2,54)=2.250, p>0.5, n.s.] but did observe significant Trait differences for %OA time [F(1,54)=26.53, p<0.0001], with HAn animals showing more anxiety-like behavior compared to LAn (i.e., less %OA time) (Fig. 2A).
Fig. 2.
A–C. At PND 46, 4th generation selective outbred animals tested on the elevated plus maze exhibit differences in anxiety-like behavior. (A) Percent time on open arms differed between anxiety lines with HAn animals showing less %OA time. (B) Open arm entries also differed between HAn (greater %OA entries) and LAn lines. (C) HAn animals made significantly fewer entries into all arms of the maze overall compared to LAn rats (*p<0.05, ***p<0.0001, Trait effect).
3.1.2 Percent Open Arm Entries (PND 46)
Testing at PND 46 did not indicate any main effect of Housing [F(2,54)=0.3501, p>0.05, n.s.] or Housing × Trait interaction for %OA [F(2,54)=0.6734, p>0.05, n.s.]. HAn animals had significantly more % OA entries than their LAn counterparts [F(2,54)=6.515, p<0.05] (Fig. 2B).
3.1.3 Total Entries (PND 46)
ANOVA for total entries revealed a significant group difference with HAn animals making fewer entries in all arms of the maze at PND 46 [F(2,54)=7.892, p<0.001] (Fig. 2C).
3.1.4 Percent Time on Open Arms (PND 63)
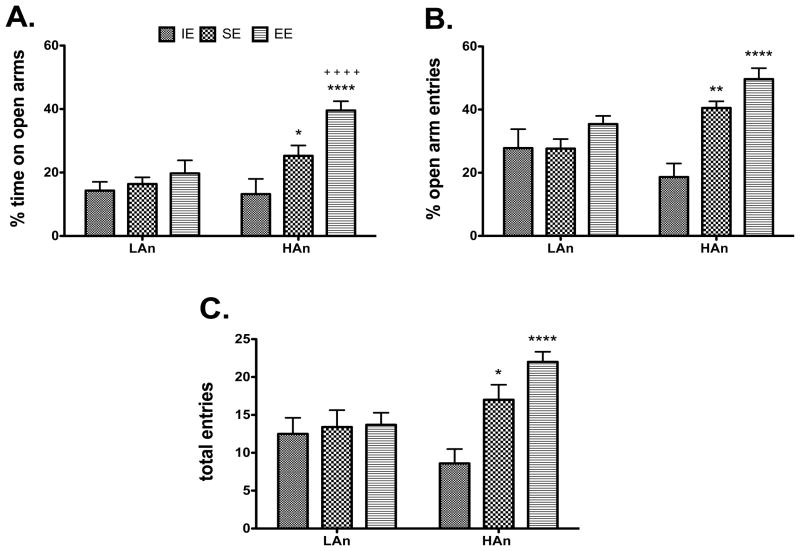
Animals were placed in EE, SE and IE environments for a minimum of 40 days and then tested again on the EPM (where possible, all litters were tested on the same day or over 2–3 days). Two-way ANOVA revealed significant main effects of Housing [F(2,53)=3.850, p<0.05] and Trait [F(1,53)=17.65, p<0.001] as well as a significant Housing × Trait interaction [F(2,53)=4.80, p<0.05]. Post hoc tests revealed HAn EE rats differed from HAn IE (p<0.0001) and SE (p<0.05) rats, with HAn EE making many more open arm entries (Fig. 3A).
Fig. 3.
A–C. Anxiety-like behavior on the elevated plus maze is altered following 40-day social and enriched housing environment. (A) High anxiety (HAn) animals housed in enrichment showed less anxiety-like behavior, as indicated by increased time spent on open arms of the EPM. *p<0.05 EE compared to SE, same trait; ***p<0.001 EE relative to IE, same trait; ++++p<0.0001 HAn EE versus LAn EE. (B) HAn animals from EE (****p<0.0001) and SE (**p<0.01) had more percent entries into the open arms compared to IE. (C) HAn rats from SE and EE environments made more total entries (open and closed arms) than HAn animals from IE. *p<0.05 SE relative to IE; ****p<0.0001 EE compared to IE.
3.1.5 Percent Open Arm Entries (PND 63)
Analysis of the total arm entries revealed a marginally significant Trait effect [F(1,53)=3.716, p=0.0593]. Significant Housing [F(2,53)=12.90, p<0.0001] and Housing × Trait effects [F(2,53)=5.891, p<0.01] were also found. Subsequent analyses showed HAn rats reared in SE (p<0.01) and EE (p<0.0001) had many more entries than those reared in IE, same trait (Fig. 3B).
3.1.6 Total Entries (PND 63)
ANOVA found a significant Housing effect [F(2,54)=7.700, p<0.01] and a Trait effect that approached significance [F(1,54)=3.007, p=0.0886] as well as an interaction [F(2,54)=5.337, p<0.01]. Rats from SE (p<0.05) and EE (p<0.0001) differed from those reared in IE (Fig. 3C).
3.2 Locomotor activity
Animals were brought to the testing room and allowed a 30-min habituation to the testing chamber. After, each rat was given an amphetamine injection (0.50 mg/kg, IP) and returned to the activity monitors for an additional 60 min. We analyzed the block of data (distance traveled (cm) or rearing bouts) for habituation (30 min) and post-AMPH (60 min) time-points using a repeated measures three-way ANOVA (Trait, Housing, Time, repeated measures factor) and ran post-hoc tests when there was a main effect of Housing and an interaction effect that included the Housing factor.
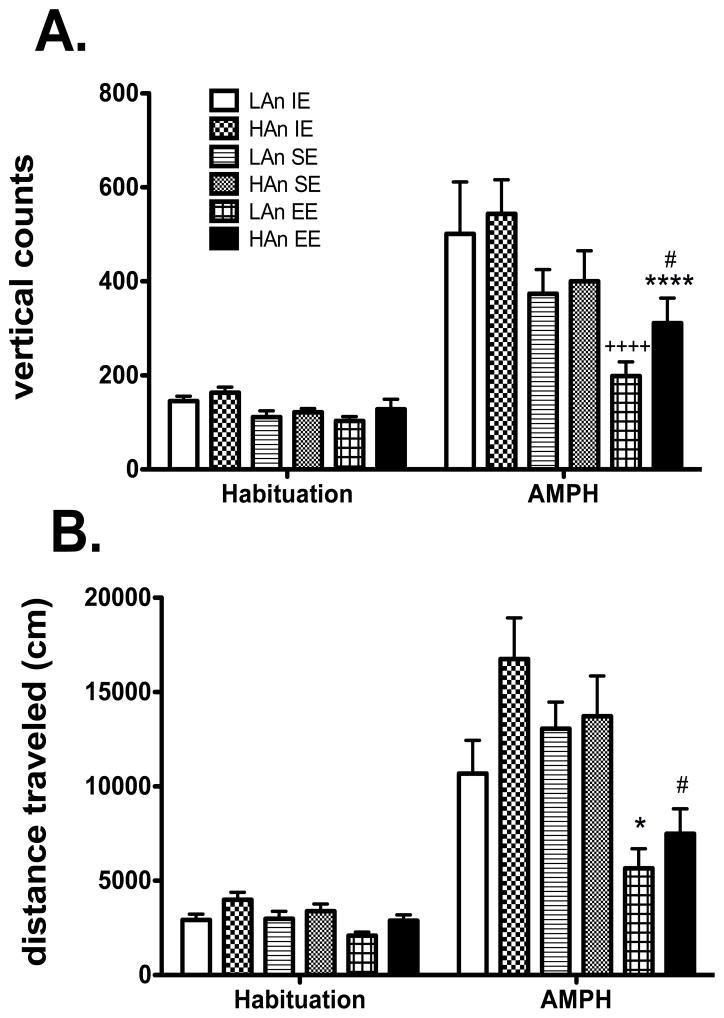
3.2.1 Rearing Bouts
ANOVA indicated main effects of Housing [F(2,108)=8.972, p<0.0001], Trait [F(1,108)=6.589, (p<0.05), Time [F(1,108)=86.614, p<0.000], as well as a Trait × Housing interaction [F(2,108)=5.363, p<0.01] (Fig. 4A). Animals reared in HAn EE differed significantly from those from LAn EE (p<0.05); HAn EE differed from HAn IE (p<0.000), LAn EE differed from LAn IE (p<0.0001) and EE rats were also marginally different from those from SE (p=0.052) for the AMPH 60-min time block.
Fig. 4.
A–B. Changes in locomotor behavior in EE housed animals. (A) In terms of rearing bouts, HAn and LAn rats from EE differed significantly from those housed in IE environments after AMPH administration ****p<0.0001, HAn comparison, ++++p<0.0001, LAn comparison; HAn EE made more rears than LAn EE #p<0.05. (B) For distance traveled, EE rats showed decreased AMPH-induced activity relative to other groups (#p<0.05 LAn EE relative to LAn SE); (**p<0.01 HAn EE compared to HAn IE).
3.2.2 Distance traveled (cm)
Significant effects of Housing [F(2,108)=13.762, p<0.0001] and Trait [F(1,108)=6.589, p<0.05] were obtained for distance traveled. We also observed interaction effects for Housing × Time [F(2,108)=8.452, p<0.0001] and Trait × Housing [F(1,54)=4.284, p<0.05] with EE reared rats showing less AMPH-induced locomotion compared to SE for LAn (p<0.05), relative to IE for HAn (p<0.01). Moreover, HAn (8041 cm (±91)) animals showed greater AMPH hyper-locomotor activity compared to LAn rats (6233 cm (±64)) (Fig. 4B).
3.3 Plasma adrenocortical response
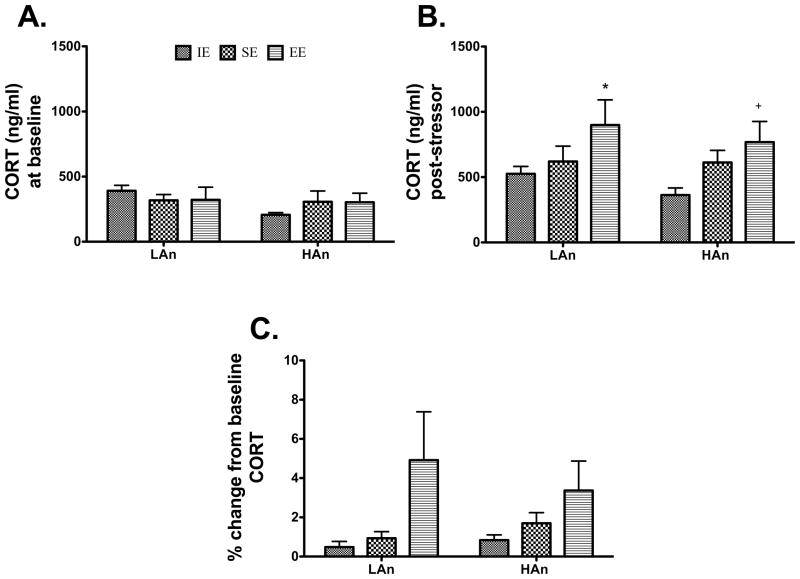
3.3.1 Baseline
No significant differences were observed for baseline plasma corticosterone (CORT) levels for Trait or Housing environment factors (Fig. 5A).
Fig. 5.
A–B. Housing environment impacts the adrenocortical response to a mild stressor. (A) We observed no baseline differences for CORT for trait or housing factors. (B) EE housed rats showed a greater CORT response to a mild stressor as compared to all other housing groups (*p<0.05, LAn EE relative to SE and IE, same trait; +p<0.05, HAn EE compared to SE and IE, same trait). (C) Animals from EE also showed the most significant changes from baseline compared to other groups (*p<0.05).
3.3.2 Post-stressor
Following a 30-min forced novel environment exposure, a markedly different profile for the animals reared in enriched environments emerged. EE-housed animals consistently showed higher CORT levels after stress. This was supported by a significant Housing effect [F(2,41)=4.872, p<0.05] with EE animals showing the greatest CORT responses (Fig. 5B).
3.3.3 Percent change from baseline
The delta was calculated for each Housing by Trait condition. A marked effect of Housing was observed [F(2,41)=4.403, p<0.05] with EE reared animals showing a greater percent change from baseline CORT responses (p<0.05) (Fig. 5C).
3.4 Hemodynamic responses and core body temperature
3.4.1 Systolic arterial pressure (SAP)
Analysis of the baseline SAP data indicated a Housing × Trait interaction [F(2,54)=3.697, p<0.05] with HAn IE having the highest SAP (relative to SE and EE, p<0.05). Post-stressor, we found a significant interaction effect (Housing × Trait) [F(2,54)=3.219, p<0.05], again with HAn IE animals showing the greatest post-stressor SAP. For percent change from baseline SAP, a significant difference was observed for the Housing condition [F(2, 52)=4.827, p<0.05] with Trait approaching significance [F(1, 52)=3.013, p=0.0885]. Animals reared in IE had the greatest change in SAP (Table 1).
Table 1.
Basal and post-stressor hemodynamic and core body temperature responses in low and high anxiety rats reared in isolated (IE), social (SE) and enriched environments (EE).
| Baseline | Post-stressor | % Change from baseline | ||||
|---|---|---|---|---|---|---|
| LAn | HAn | LAn | HAn | LAn | HAn | |
| IE | ||||||
| Systolic (mm Hg) | 119±8 | 139±6a | 131±5 | 154±7b | 14±7 | 16±4 |
| Diastolic (mm Hg) | 100±3 | 109±5 | 114±4 | 119±7 | 15±3 | 10±7 |
| Mean arterial pressure (mm Hg) | 106±4 | 119±5 | 120±4 | 124±5 | 14±3 | 6±4 |
| Core body T (°C) | 36±0.2 | 37±0.4 | 37±0.2 | 38±0.2 | 3±1 | 2±0.5 |
| SE | ||||||
| Systolic (mm Hg) | 135±5 | 121±6 | 144±6 | 121±8 | 7±4 | 1±5 |
| Diastolic (mm Hg) | 108±4 | 103±5 | 113±4 | 103±6 | 5±5 | 0±4 |
| Mean arterial pressure (mm Hg) | 117±4 | 109±5 | 123±4 | 109±6 | 6±4 | 0±4 |
| Core body T (°C) | 36±0.3 | 36±0.3 | 37±0.2 | 37±0.3 | 3±1 | 2±0.5 |
| EE | ||||||
| Systolic (mm Hg) | 116±7 | 123±6 | 123±5 | 132±8 | 7±5 | 9±8 |
| Diastolic (mm Hg) | 103±6 | 102±7 | 102±4 | 101±6c | 2±7 | 3±8 |
| Mean arterial pressure (mm Hg) | 107±6 | 109±4 | 109±6 | 111±6 | 4±6 | 5±7 |
| Core body T (°C) | 36±0.3 | 36±0.3 | 36±0.3 | 36±0.2 | 2±1 | 2±0.5 |
Values are expressed as group means ±S.E.M. LAn, low anxiety; HAn, high anxiety; IE, impoverished environment; SE, social environment; EE, enriched environment’ MAP, mean arterial pressure.
p<0.05 significant differences at baseline between HAn IE and SE and EE groups;
p<0.05,
p<0.05 significant differences at post-stressor between HAn IE and SE and EE groups;
p<0.05 significant differences at post-stressor between HAn EE and IE groups.
3.4.2 Diastolic arterial pressure (DAP)
Analysis of the baseline DAP data indicated no significant differences. For post-stressor and percent change from baseline, we observed a significant Housing effect [F(2,54)=4.158, p<0.05] with EE-housed rats having lower DAP (p<0.05) and percent change from baseline DAP [F(2, 52)=4.827, p<0.05]. In addition, for post-stressor, the Trait effect approached significance [F(1,52)=3.013, p<0.0885] (Table 1).
3.4.3 Mean arterial pressure (MAP)
Mean arterial pressure was calculated (Eqn. 1) at baseline, post-stressor and percent change from baseline was also determined. ANOVA indicated no significant differences for baseline, a Housing effect that was marginally significant for post-stressor [F(2,54)=2.686, p<0.0772] and no significant differences for percent change from baseline.
| Eqn. 1 |
where MAP, mean arterial pressure; P, pressure; dias, diastolic; sys, systolic.
3.5 Core body temperature
The two-way ANOVA for baseline did not reveal any significant differences, however, after an air puff to the face, a significant effect of Housing was revealed [F(2,51)=2.964, p<0.05] with EE animals showing a decrease in T post-stressor relative to IE (p<0.01). No significant differences were observed for percent change from baseline data (Table 1).
3.6 Brain derived neurotrophic factor (BDNF) immunohistochemistry
3.6.1 Central Amygdala
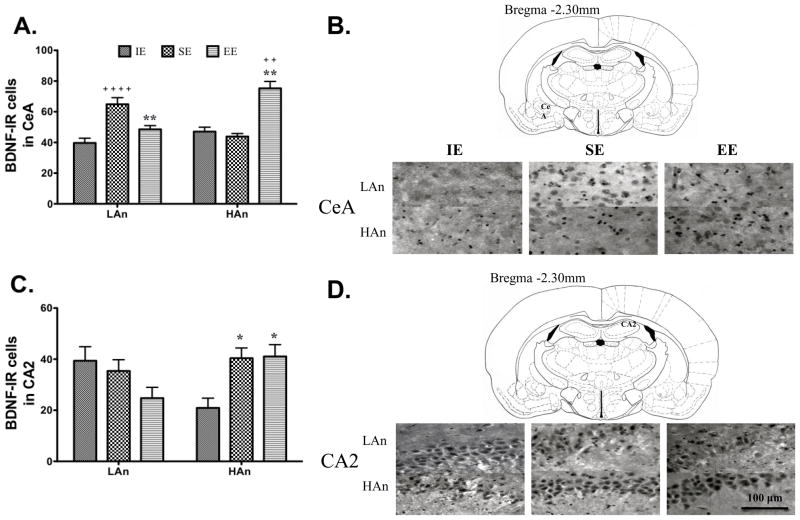
The two-way ANOVA revealed a significant Housing effect [F(2,78)=15.74, p<0.0001] and a significant Housing × Trait interaction [F(2,78)=25.88, p<0.0001]. Post hoc tests indicated that the SE and EE groups differed from IE (p<0.05) and HAn EE had greater BDNF positive cells than IE (p<0.0001) and LAn SE greater than IE (p<0.0001) (Fig. 6A).
Fig. 6.
A–D. The average number of BDNF immunoreactive (IR) cells varies as a function of trait anxiety level and postnatal housing condition. (A) Average number of BDNF positive cells in the central amygdala (CeA) was increased in HAn animals housed in EE relative to IE (++p<0.01) and SE (**p<0.01). For LAn, the SE housed animals had more BDNF-positive cells than EE and IE groups (**p≪0.01, SE vs. EE), (++++p<0.0001, SE vs. IE). (B) Line drawing showing CeA (top) and representative BDNF-IR of trait anxiety animals from each housing condition. BDNF-IR was also increased for HAn SE and EE animals in the (C) CA2 (*p<0.05, SE and EE relative to IE). (D) Schematic of coronal section at bregma −2.30mm where the CA2 region of the hippocampus was identified (top) with representative BDNF-IR for each Trait × Housing group. Scale bar is 100 μm for all images.
3.6.2 Hippocampus
A significant interaction effect (Housing × Trait) was found for the CA1 [F(2,84)=3.88, p<0.05] and CA2 [F(2,72)=7.93, p<0.001] regions of the hippocampus. Post hoc tests indicated SE and EE rats had a greater number of BDNF-IR cells compared to HAn animals reared in IE (p<0.05). Analysis of the CA3 revealed a significant effect of Housing [F(2,66)=4.40, p<0.05] with SE greater than IE (p<0.05) (Fig. 6B–E). No significant effects were found for the dentate gyrus (not shown).
3.6.3 Caudate putamen (CPu)
Data analysis of BDNF immunoreactivity in the CPu revealed a main effect of Housing [F(2,95)=5.213, p<0.01] with IE greater than SE and EE (Fig. 6F).
4. Discussion
The present study was designed to determine the impact of the housing environment on the behavioral and physiological responses to stress in animals with either low or high anxiety. We measured behavior on the elevated plus maze (EPM), basal and air puff stress-induced physiological responses (i.e., blood pressure and body temperature), stress induced CORT levels and AMPH-induced locomotion in Long Evans rats phenotyped as high and low anxiety. We found that while the high anxiety profile was apparent at PND 46, after only 21 days in select housing conditions we observed no housing effects. LAn rats consistently showed less anxiety-like behavior regardless of housing condition. We re-tested the animals on the EPM nearly three weeks later and this re-trial resulted in similar trait differences as well as housing differences. Recent work shows that hooded and Wistar rats can be re-tested successfully in EPM with a minimum inter-trial interval of 3 (Ademec and Shallow, 2000) or 4 (Schneider et al, 2011) weeks and thus, our current findings of sustained anxiogenic effects in the EPM across the two trials are consistent. Housing differences were the most salient in all other physiological tests. That is, regardless of anxiety-like phenotype expressed using EPM, stress-induced adrenocortical, hemodynamic and core body temperature responses were consistently attenuated by EE in many instances and by SE in others.
4.1 Environmental enrichment reduces psychogenic responses in highly anxious rats
In contrast to low percent open arm time and entries observed in HAn rats at PND 46, HAn EE animals showed a reversal of this anxiety-like behavior (i.e., increased time/entries on open arm) and decreased total activity. This latter finding suggests that while LAn animals housed in EE are more active in general, they also show increased preference for the open arms. Thus, there are specific qualities of the EPM to which the animals are responding (Mallo et al., 2007) that may interact with the age of the animal (Branchi et al. 2011) as well as the strain (). There is increasing evidence to support intervention in the postnatal environment as a model manipulation to reverse the impact of early adverse experiences such a prenatal stress (Laviola et al., 2004; Morley-Fletcher et al., 2003), maternal separation (Francis et al., 2002) and prenatal cocaine insult (Neugebauer et al., 2004) as well as prenatal alcohol exposure (Hannigan, Berman and Zajac, 1993). Recently, several lines of work implicate EE in the ability to reduce the stress profile as indicated by blunted adrenocortical responses and decreased anxiety-like behavior in the elevated plus maze in normal mice Benaroya-Milshtein et al., 2004), but also in highly anxious models including BALB/c mice (surgically-manipulated for high anxiety) and other strains of high anxiety rats (for review, see Fox, Merali and Harrison, 2006).
4.2 Environmental enrichment reduces amphetamine hyperactivity in high and low anxiety rats
EE impacted stimulant vulnerability for the HAn rats as well since this group had a diminished AMPH-induced locomotor response. The protective effects of EE on drug vulnerability have been reported previously for cocaine (Magalhaes et al., 2004), morphine (Alexander, Coambs and Hadaway, 1978) and more recently, for amphetamine (Bardo et al., 2001; Ravenelle et al., 2013) and are in support of the current findings. Unique to the present work is the demonstration that EE reduced amphetamine sensitivity in both trait anxiety lines. In the LAn animals, EE reduced rearing bouts, an index of exploration, to a greater degree than in HAn while it reduced locomotor behavior more in the HAn line. This behavioral difference between HAn and LAn in response to EE is interesting in that in their review, Simpson and Kelly (2011) suggest that EE increases adaptation and promotes exploration when anxiety is reduced. Therefore, our findings implicate EE in differentially conferring protection against amphetamine sensitivity based on trait anxiety. There is evidence that enrichment can reduce a distinct behavioral profile, animals that are high responders to novelty (Piazza et al., 1999) show increased drug vulnerability (Gingras and Cools, 1997) and enrichment modulates this effect.
4.3 Environmental enrichment reduces some physiological responses to acute stress
Basal corticosterone levels did not vary depending on trait or housing condition. After a forced novel environment + novel object exposure, however, marked differences emerged with EE housed animals, regardless of trait, showing elevated CORT concentrations over their SE and IE counterparts. There are inconsistencies in reporting the adrenocortical response in rodents following EE housing with some indicating no difference (Pham et al., 1999), increases (Moncek et al., 2004) or decreases (Belz et al., 2004). Our findings do not provide support for changes in the adrenocortical response as the mechanism contributing to the benefits observed in the EE highly anxious rats, though in earlier work (Ravenelle et al., 2013) we did report EE animals from high anxiety lines had lower CORT responses to acute stress. This discrepancy may be due in part to the timing of blood draw (PND 155 currently, PND 126 in Ravenelle et al. (2013) and methodological differences. In the current study, we used tail artery blood draw for baseline and post-stressor measurements while in our earlier work we collected terminal trunk blood. Francis and colleagues (2002) also found that EE reversed the behavioral indices of stress without altering corticotropin-releasing factor mRNA levels. The authors suggest that there is an alternative mechanism(s) that does not involve direct reversal of neural mechanisms that may have been altered by early life events, but may instead occur at another developmental period to offset this early damage. In our study, the animals were placed in EE at weaning and we began testing at adolescence, which represents a critical period of neural development and the rats also received constant stimulation in EE, variables known to influence neural and behavioral outcomes (Pereira et al., 2008). In fact, it has been long reported that developmental milestones such as adolescence are marked by highly plastic neural systems in which a number of organisms are sensitive to enrichment (Branchi et al., 2011). This plasticity may be the level of the compensatory changes that promote the stress adaptability of the animals housed in enrichment.
EE did protect against hyperthermia and blood pressure spikes in highly anxious rats following an air puff stressor. While there were no basal differences in core body temperature, after a mild stressor rats from EE showed reduced core body temperature compared to those from IE and the interaction with trait effect highlights that this was most pronounced for the HAn line. Research on social stress shows increases in core body temperature 30 min after stressor, with some adaptability after repeated stress and a test of novel restraint (Bhatnagar et al., 2006), suggesting that the EE environment for the current animals may have induced adaptability in the autonomic system regulating body temperature. Furthermore, rats from EE and SE also showed a significant decrease in diastolic arterial pressure (DAP) to the air puff stressor relative to IE animals. Social enrichment effects on physiological measures were also found in male Sprague-Dawley rats (Sharp, Azar and Lawson, 2005) and spontaneously hypertensive male rats (Lawson, Churchill and Churchill, 2000; Sharp, Azar and Lawson, 2005) with animals housed in social groups showing dampened heart rate and blood pressure. In a model of repeated stress, authors Chen and Herbert (1995) reported decreased c-fos expression in the paraventricular nucleus, an area with functional correlates to cardiac and adrenocortical responses, and medial amygdala that paralleled the more rapid return to baseline for heart rate.
Social housing can also have unintended stressful effects depending on the sex of the animals and the parameters of the housing, causing increases in cardiovascular and anxiety-like behavioral measures (Brown and Grunberg, 1995). Socially housed male animals show high levels of anxiety, correspondingly high levels of cardiovascular and behavioral anxiety relative to their female counterparts. This distinction is removed when animals are not only group housed but also receive enrichment (Sharp, Azar and Lawson, 2005), a finding that also supports our results in the EE males from the HAn line.
4.4 Environmental enrichment effects on behavioral and physiological stress responses parallel changes in BDNF protein levels
It is well established that environmental enrichment confers persistent stress-reducing benefits to a number of animal species with normative and highly anxious profiles (for review, see [Fox, Merali and Harrison, 2006). Several proposed mechanisms for the beneficial effects of EE have been highlighted including hippocampal neurogenesis (Brown et al., 2003), alterations in levels of neurotrophins and their receptors (Pham et al., 1999), adaptive changes along the adrenocortical axis Moncek et al., 2004), as well as morphological changes in the cortex (Johansson and Belichenko, 2002). In the current work, we add to the evidence implicating changes in neurotrophin levels in paralleling high anxiety-like behavioral reversal following EE. We found that EE resulted in increased brain derived neurotrophin (BDNF) expression in the central amygdala, and the CA2 region of the hippocampus. Interestingly, SE elevated BDNF levels in CA2 and CA3 of the HAn animals, that may be accompanied by synaptic and dendritic modifications as has been described elsewhere (Johansson and Belichenko, 2002). The central amygdala is a limbic structure with functional connections to fear and anxiety circuits, and neuroplasticity in medial and central regions have been reported in models of stress adaptation (see McEwen (2007) for review). In the HAn rats, SE conferred changes in BDNF induction levels, perhaps due to the stress that males experienced in the social environment with conspecifics. Chourbaji and colleagues (Chourbaji et al., 2008) found that mice with a partial deletion of the BDNF gene that show anxiety-like behavior recovered from these deficits if they were group housed with enrichment, however, if they were simply group-housed they did not recover. Finally, an increase in BDNF positive cells was also found in the CPu of animals housed in IE, and neurotrophin changes in this striatal region are implicated in neuroplasticity necessary for psychostimulant drug activation (McGinity et al. 2011). Since we administered only a single dose of amphetamine and did not explore sensitization or reward parameters, we chose the CPu as opposed to the nucleus accumbens as BDNF changes have been noted in CPu after a single AMPH injection (McGinity et al. 2011). Thus, our findings further support that plasticity-linked changes in the CPu are critical for psychostimulant drug sensitivity, and that isolated housing and anxiety may contribute to the neural adaptation that creates this vulnerability (Miserendino, Haile and Kosten, 2003).
4.5 Conclusions
In summary, our current work implicates that the EE experience beginning at weaning is adequate to modulate the behavioral, autonomic stress response and amphetamine sensitivity in Long Evans rats and that these enrichment effects are more robust than those related to selectively breeding for trait anxiety. Since the current screening and breeding for trait anxiety relies solely on phenotyping on the single elevated plus maze test and outbreeding, it is arguable that the animals are not truly trait anxious. However, we do find increased extreme trait anxiety responses across generations (Ravenelle et al., 2013) and similar trait-specific responses in another test of anxiety, the open field (unpublished findings). This modulation by EE is associated with the induction of BDNF in the central amygdala, hippocampus and the CPu of rats housed in EE as well as some from SE. The current findings may offer insight into possible cellular mechanisms contributing to stress reactivity and adaptability common in trait anxiety. Our results are encouraging, in that components of EE may be an important intervention to alter the devastating effects of clinical anxiety as significant clinical gains have been seen with sensorimotor enrichment in autistic children (Woo and Leon, 2013) and with exercise therapy to improve physiological and psychological aspects of anxiety (for review, see Ströhle, 2009).
Highlights.
Outbred high anxiety (HAn) rats show anxiety-like behavior on elevated plus maze
Enriched environment (EE) reduces anxiety and amphetamine response in HAn/LAn rats
EE lowers hemodynamic and temperature responses to air puff stressor
Amygdala BDNF-positive cells are elevated in HAn rats reared in EE and socially (SE)
Hippocampal and striatal BDNF cells are increased in HAn EE and SE rats
Acknowledgments
The authors gratefully acknowledge Elizabeth Boates and Mitzi Sweeney for assistance with animal maintenance and husbandry, technical help and comments on the manuscript. STD was supported by Award Number P20MD002290 from the National Institute on Minority Health and Health Disparities (Celia Moore, Ph.D., P.I.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute on Minority Health and Health Disparities or the National Institutes of Health. JJB and EMB were supported by NIH grant 593 R01DA25674.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ademac R, Shallow T. Effects of baseline anxiety on response to kindling of the right medial amygdala. Physiol Behav. 2004;70:67–80. doi: 10.1016/s0031-9384(00)00247-x. [DOI] [PubMed] [Google Scholar]
- Alexander BK, Coambs RB, Hadaway PF. The effect of housing and gender on morphine self-administration in rats. Psychopharmacology. 1978;58:175–179. doi: 10.1007/BF00426903. [DOI] [PubMed] [Google Scholar]
- Bardo MT, Klebaur JE, Valone JM, Deaton C. Environmental enrichment decreases intravenous self-administration of amphetamine in female and male rats. Psychopharmacology. 2001;155:278–284. doi: 10.1007/s002130100720. [DOI] [PubMed] [Google Scholar]
- Belz EE, Kennell JS, Czambel K, Rubin RT, Rhodes ME. Environmental enrichment lowers stress-responsive hormones in singly housed male and female rats. Pharmacol Biochem Behav. 2003;76:481–486. doi: 10.1016/j.pbb.2003.09.005. [DOI] [PubMed] [Google Scholar]
- Benaroya-Milshtein N, Hollander N, Apter A, Kukulansky T, Raz N, Wilf A, Yaniv I, Pick CG. Environmental enrichment in mice decreases anxiety, attenuates stress responses and enhances natural killer cell activity. Eur J Neurosci. 2004;20:1341–1347. doi: 10.1111/j.1460-9568.2004.03587.x. [DOI] [PubMed] [Google Scholar]
- Bhatnagar S, Vining C, Iyer V, Kinni V. Changes in hypothalamic-pituitary-adrenal function, body temperature, body weight and food intake with repeated social stress exposure in rats. J Neuroendocrinol. 2006;18:13–24. doi: 10.1111/j.1365-2826.2005.01375.x. [DOI] [PubMed] [Google Scholar]
- Branchi I, Karpova NN, D’Andrea I, Castrén E, Alleva E. Epigenetic modifications induced by early enrichment are associated with changes in timing of induction of BDNF expression. Neurosci Lett. 2011;495:168–172. doi: 10.1016/j.neulet.2011.03.038. [DOI] [PubMed] [Google Scholar]
- Brennan FX, Ottenweller JE, Seifu Y, Zhu G, Servatius RJ. Persistent stress-induced elevations of urinary corticosterone in rats. Physiol Behav. 2000;71:441–6. doi: 10.1016/s0031-9384(00)00365-6. [DOI] [PubMed] [Google Scholar]
- Brown J, Cooper-Kuhn C, Kempermann G, Van Praag H, Winkler J, Gage F, Kuhn H. Enriched environment and physical activity stimulate hippocampal but not olfactory bulb neurogenesis. Eur J Neurosci. 2003;17:2042–2046. doi: 10.1046/j.1460-9568.2003.02647.x. [DOI] [PubMed] [Google Scholar]
- Brown KJ, Grunberg NE. Effects of housing on male and female rats: crowding stresses males but calms females. Physiol Behav. 1995;58:1085–1089. doi: 10.1016/0031-9384(95)02043-8. [DOI] [PubMed] [Google Scholar]
- Cain ME, Saucier DA, Bardo MT. Novelty seeking and drug use: Contribution of an animal model. Exp Clin Psychopharm. 2005;13:367–375. doi: 10.1037/1064-1297.13.4.367. [DOI] [PubMed] [Google Scholar]
- Chen X, Herbert J. Regional changes in c-fos expression in the basal forebrain and brainstem during adaptation to repeated stress: Correlations with cardiovascular, hypothermic and endocrine responses. Neuroscience. 1995;64:675–685. doi: 10.1016/0306-4522(94)00532-a. [DOI] [PubMed] [Google Scholar]
- Chourbaji S, Brandwein C, Vogt MA, Dormann C, Hellweg R, Gass P. Nature vs. nurture: Can enrichment rescue the behavioral phenotype of BDNF heterozygous mice? Behav Brain Res. 2008;192:254–258. doi: 10.1016/j.bbr.2008.04.015. [DOI] [PubMed] [Google Scholar]
- Contoreggi C, Herning RI, Na P, Gold PW, Chrousos G, Negro PJ, et al. Stress hormone responses to corticotropin-releasing hormone in substance abusers without severe comorbid psychiatric disease. Biol Psychiat. 2003;54:873–888. doi: 10.1016/s0006-3223(03)00167-7. [DOI] [PubMed] [Google Scholar]
- Cui M, Yang Y, Zhang J, Han H, Wenpei M, Hongbin L, et al. Enriched environment experience overcomes the memory deficits and depressive-like behavior induced by early life stress. Neurosci Lett. 2006;404:208–212. doi: 10.1016/j.neulet.2006.05.048. [DOI] [PubMed] [Google Scholar]
- Diamond MC. Response of the brain to enrichment. An Acad Bras Ciênc. 2001;73:211–220. doi: 10.1590/s0001-37652001000200006. [DOI] [PubMed] [Google Scholar]
- Fox CF, Merali Z, Harrison C. Therapeutic and protective effect of environmental enrichment against psychogenic and neurogenic stress. Behav Brain Res. 2006;175:1–8. doi: 10.1016/j.bbr.2006.08.016. [DOI] [PubMed] [Google Scholar]
- Francis DD, Diorio J, Plotsky PM, Meaney MJ. Environmental enrichment reverses the effects of maternal separation on stress reactivity. J Neurosci. 2002;22:7840–7843. doi: 10.1523/JNEUROSCI.22-18-07840.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gingras MA, Cools AR. No major differences in locomotor responses to dexamphetamine in high and low responders to novelty: a study of Wistar rats. Pharmacol Biochem Behav. 1997;57:857–862. doi: 10.1016/s0091-3057(96)00320-6. [DOI] [PubMed] [Google Scholar]
- Grant BF, Stinson FS, Dawson DA, Chou SP, Dufour MC, Compton W, et al. Prevalence and co-occurrence of substance use disorders and independent mood and anxiety disorders: results from the National Epidemiologic Survey on Alcohol and Related Conditions. Arch Gen Psychiat. 2004;61:807–816. doi: 10.1001/archpsyc.61.8.807. [DOI] [PubMed] [Google Scholar]
- Hannigan JH, Berman RF, Zajac CS. Environmental enrichment and the behavioural effects of prenatal exposure to alcohol in rats. Neurotoxicol Teratol. 1993;15:261–266. doi: 10.1016/0892-0362(93)90007-b. [DOI] [PubMed] [Google Scholar]
- Harkin A, Connor TJ, O’Donnell JM, Kelly JP. Physiological and behavioral responses to stress: What does a rat find stressful? Lab Animal. 2002;31:42–50. doi: 10.1038/5000150. [DOI] [PubMed] [Google Scholar]
- Hasler G, Fromm S, Ruben PA, Luckenbaugh DA, Drevets WC, Grillon C. Cerebral blood flow in immediate and sustained anxiety. J Neurosci. 2007;27:6313–6319. doi: 10.1523/JNEUROSCI.5369-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ickes BR, Pham TM, Sanders LA, Albeck DS, Mohammed AH, Granholm A–C. Long-term environmental enrichment leads to regional increases in neurotrophin levels in rat brain. Exp Neurol. 2000;164:45–52. doi: 10.1006/exnr.2000.7415. [DOI] [PubMed] [Google Scholar]
- Johansson BB, Belichenko P. Neuronal plasticity in dendritic spines: Effect of environmental enrichment on intact and postischemic rat brain. J Cereb Blood Flow. 2002;22:89–96. doi: 10.1097/00004647-200201000-00011. [DOI] [PubMed] [Google Scholar]
- Landgraf R, Wigger A, Holsboer F, Neumann ID. Hyper-reactive hypothalamo-pituitary-adrenocortical axis in rats bred for high anxiety-related behaviour. J Neuroendocrinol. 1999;11:405–407. doi: 10.1046/j.1365-2826.1999.00342.x. [DOI] [PubMed] [Google Scholar]
- Laviola G, Rea M, Morley-Fletcher S, Di Carlo S, Bacosi A, De Simone R, Bertini M, Pacifici R. Beneficial effects of enriched environment on adolescent rats from stressed pregnancies. 2004;20:1655–1664. doi: 10.1111/j.1460-9568.2004.03597.x. [DOI] [PubMed] [Google Scholar]
- Lawson DM, Churchill M, Churchill PC. The effects of housing enrichment on cardiovascular parameters in spontaneously hypertensive rats. Contemp Top Lab Anim. 2000;39:9–13. [PubMed] [Google Scholar]
- Long NC, Vander AJ, Kluger MJ. Stress-induced rise of body temperature in rats is the same in warm and cool environments. Physiol Behav 1990. 1990;47:773–775. doi: 10.1016/0031-9384(90)90093-j. [DOI] [PubMed] [Google Scholar]
- Lovallo WR. Cortisol secretion patterns in addiction and addiction risk. Int J Psychophysiol. 2006;59:195–202. doi: 10.1016/j.ijpsycho.2005.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magalhaes A, Summavielle T, Tavares MA, de Sousa L. Effects of postnatal cocaine exposure and environmental enrichment on rat behavior in a forced swim test. Ann NY Acad Sci. 2004;1025:619–629. doi: 10.1196/annals.1316.077. [DOI] [PubMed] [Google Scholar]
- Mällo T, Alttoa A, Kõiv K, Tõnissaar M, Eller M, Harro J. Rats with persistently low or high exploratory activity: Behaviour in tests of anxiety and depression, and extracellular levels of dopamine. Behav Brain Res. 2007;177:269–281. doi: 10.1016/j.bbr.2006.11.022. [DOI] [PubMed] [Google Scholar]
- Merikangas KR, Dierker LC, Szatmari P. Psychopathology among offspring of parents with substance abuse and/or anxiety disorders: a high-risk study. J Child Psychol Psych. 1998;39:711–720. [PubMed] [Google Scholar]
- Miserendino MJ, Haile CN, Kosten TA. Strain differences in response to escapable and inescapable novel environments and their ability to predict amphetamine-induced locomotor activity. Psychopharmacology. 2003;167:281–290. doi: 10.1007/s00213-003-1411-4. [DOI] [PubMed] [Google Scholar]
- Mojtabai R, Olfson M, Mechanic D. Perceived need and help-seeking in adults with mood, anxiety, or substance use disorders. Arch Gen Psychiat. 2002;59:77–84. doi: 10.1001/archpsyc.59.1.77. [DOI] [PubMed] [Google Scholar]
- Moncek F, Duncko R, Johansson BB, Jezova D. Effect of environmental enrichment on stress related systems in rats. J Neuroendocrinol. 2004;16:423–431. doi: 10.1111/j.1365-2826.2004.01173.x. [DOI] [PubMed] [Google Scholar]
- Morley-Fletcher S, Rea M, Maccari S, Laviola G. Environmental enrichment during adolescence reverses the effects of prenatal stress on play behaviour and HPA axis reactivity in rats. Eur J Neurosci. 2003;18:3367–3374. doi: 10.1111/j.1460-9568.2003.03070.x. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Physiology and neurobiology of stress and adaptation: central role of the brain. Physiol Rev. 2007;87:873–904. doi: 10.1152/physrev.00041.2006. [DOI] [PubMed] [Google Scholar]
- McGinity JF, Bache AJ, Coleman NT, Sun W-L. The role of BDNF/TrkB signaling in acute amphetamine-induced locomotor activity and opioid peptide gene expression in the rat dorsal striatum. Front Syst Neurosci. 2011;5:60–75. doi: 10.3389/fnsys.2011.00060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neugebauer NM, Cunningham ST, Zhu J, Bryant RI, Middleton LS, Dwoskin LP. Environmental enrichment on behavior and dopamine transporter function in medial prefrontal cortex in adult rats prenatally treated with cocaine. Dev Brain Res. 2004;153:213–223. doi: 10.1016/j.devbrainres.2004.09.001. [DOI] [PubMed] [Google Scholar]
- Olivier B, Zethof T, Pattij T, Van Boogaert M, Van Oorschot R, Leahy C, et al. Stress-induced hyperthermia and anxiety: pharmacological validation. Eur J Pharmacol. 2003;463:117–132. doi: 10.1016/s0014-2999(03)01326-8. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 4. San Diego CA: Academic Press; 2004. [DOI] [PubMed] [Google Scholar]
- Pereira LO, Strapasson ACP, Nabinger PM, Achaval M, Netto CA. Early enriched housing results in partial recovery of memory deficits in female, but no in male, rats after neonatal hypoxia-ischemia. Brain Res. 2008;1218:257–266. doi: 10.1016/j.brainres.2008.04.010. [DOI] [PubMed] [Google Scholar]
- Pham TM, Ickes B, Albeck D, Söderström S, Granholm AC, Mohammed A. Changes in brain nerve growth factor levels and nerve growth factor receptors in rats exposed to environmental enrichment for one year. Neuroscience. 1999;94:279–286. doi: 10.1016/s0306-4522(99)00316-4. [DOI] [PubMed] [Google Scholar]
- Piazza PV, Deminière JM, Le Moal M, Simon H. Factors that predict individual vulnerability to amphetamine self-administration. Science. 1989;245:1511–1513. doi: 10.1126/science.2781295. [DOI] [PubMed] [Google Scholar]
- Ravenelle B, Byrnes EM, Byrnes JJ, McInnis C, Park JH, Donaldson ST. Environmental enrichment effects on the neurobehavioral profile of selective outbred trait anxiety rats. Behav Brain Res. 2013;252:49–57. doi: 10.1016/j.bbr.2013.05.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ressler KJ, Mayberg HS. Targeting abnormal neural circuits in mood and anxiety disorders: from the laboratory to the clinic. Nat Neurosci. 2007;10:1116–1124. doi: 10.1038/nn1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi E, Angelucci A, Costantin L, Braschi C, Mazzantini M, Babbini F, Fabbri ME, Tessarollo L, Maffei L, Berardi N, Caleo M. Brain-derived neurotrophin factor (BDNF) is required for the enhancement of hippocampal neurogenesis following environmental enrichment. Eur J Neurosci. 2006;24:1850–1856. doi: 10.1111/j.1460-9568.2006.05059.x. [DOI] [PubMed] [Google Scholar]
- Salomé N, Salchner P, Viltart O, Sequeira H, Wigger A, Landgraf R, Singewald N. Neurobiological correlates of high (HAB) versus low anxiety-related behavior (LAB): differential Fos expression in HAB and LAB rats. Biological Psychiat. 2004;55:715–723. doi: 10.1016/j.biopsych.2003.10.021. [DOI] [PubMed] [Google Scholar]
- Sharp J, Azar T, Lawson D. Effects of cage enrichment program on heart rate, blood pressure, and activity of male Sprague-Dawley and spontaneously-hyperactive rats monitored by radiotelemetry. Contemp Top Lab Anim Sci. 2005;44:32–40. [PubMed] [Google Scholar]
- Ströhle A. Physical activity, exercise, depression and anxiety disorders. J Neural Transm. 2009;116:777–784. doi: 10.1007/s00702-008-0092-x. [DOI] [PubMed] [Google Scholar]
- Stotz-Potter EH, Morin SM, DiMicco JA. Effect of microinjection of muscimol into the dorsomedial or paraventricular nucleus on air stress-induced Neuroendocrine and cardiovascular changes in rats. Brain Res. 1996;742:219–224. doi: 10.1016/s0006-8993(96)01011-6. [DOI] [PubMed] [Google Scholar]
- Weiss IC, Pryce CR, Jongen-Rêlo AL, Nanz-Bahr NI, Feldon J. Effect of social isolation on stress-related behavioural and neuroendocrine state in the rat. Behav Brain Res. 2004;152:279–295. doi: 10.1016/j.bbr.2003.10.015. [DOI] [PubMed] [Google Scholar]
- Young AS, Klap R, Sherbourne CD, Wells KB. The quality of care for depressive and anxiety disorders in the United States. Arch Gen Psychiat. 2001;58:55–61. doi: 10.1001/archpsyc.58.1.55. [DOI] [PubMed] [Google Scholar]
- Zhu SW, Codita A, Bogdanovic N, Hjerling-Leffler J, Ernfors P, Winblad B, Dickins DW, Mohammed AH. Influence of environmental manipulation on exploratory behaviour in male BDNF knockout mice. Behav Brain Res. 2009;197:339–346. doi: 10.1016/j.bbr.2008.09.032. [DOI] [PubMed] [Google Scholar]