Abstract
During the wet season of 2010, yellow fever virus (YFV) was detected in field-collected mosquitoes in the Kédougou region in southeastern Senegal. During this outbreak, we studied the association of the abundance of YFV-infected mosquitoes and land cover features to try and understand the dynamics of YFV transmission within the region. In total, 41,234 mosquito females were collected and tested for virus infection in 5,152 pools. YFV was detected in 67 pools; species including Aedes furcifer (52.2% of the infected pools), Ae. luteocephalus (31.3% of the infected pools), Ae. taylori (6.0% of the infected pools) and six other species (10.4% of the infected pools) captured in September (13.4%), October (70.1%), and November (16.4%). Spatially, YFV was detected from mosquitoes collected in all land cover classes but mainly, forest canopies (49.2%). Human infection is likely mediated by Ae. furcifer, the only species found infected with YFV within villages. Villages containing YFV-infected mosquitoes were significantly closer to large forests (> 2 ha) than villages in which no infected mosquitoes were detected.
Introduction
Yellow fever virus (YFV; Flaviviridae: Flavivirus) infects primates (humans and monkeys) in tropical areas of Africa and Latin America and causes hemorrhagic fever in these hosts.1–3 YFV is transmitted to vertebrates mainly by the bite of infected mosquitoes. Despite the availability of a safe and efficacious vaccine, there are still an estimated 200,000 annual cases of yellow fever, causing 30,000 deaths.4 The number of reported cases of yellow fever within countries and the number of countries reporting cases is rising in Africa. Changes in the epidemiological pattern of YFV transmission in East Africa are supported by the recent increase of the frequency of yellow fever outbreaks in Sudan, Kenya, Uganda, and Ethiopia between 2003 and 20135–8 (after nearly 40 years of silence). Outbreaks were also recorded from 2007 to 2009 in West Africa in areas that had not confirmed cases in decades.9–11
After the yellow fever epidemic reported in 1965 in Diourbel, Senegal,12 a surveillance program of annual mosquito collections for virus isolation was set up in 1972 in Kédougou in southeastern Senegal to study the epidemiology and ecology of YFV and vectors. The main findings of this longitudinal study include identification of YFV vectors, discovery of the sylvatic cycle in this region, and characterization of the temporal pattern of YFV detection.
Furthermore, this program had important public health implications, because YFV emergences in Kédougou were generally followed by other outbreaks in western Senegal13–16 and other West African countries. Thus, the Kédougou surveillance was considered as a YFV forecasting tool in West Africa.17
Despite these significant advances, the mechanisms of emergence and maintenance of YFV remain poorly understood. An important question is whether the periodic YFV appearances and disappearances represent cycles of permanent enzootic transmission or reintroduction from other parts of West Africa.
In 2009, a tripartite research program designed to monitor the dynamics and mechanisms of arthropod-borne virus (arbovirus) infection in mosquito vectors, non-human primates, and humans in southeastern Senegal was initiated. The environmental factors that influence the abundance, distribution, and infection of mosquito vectors that participate in the sylvatic cycles of arboviruses, including chikungunya (CHIKV), dengue virus 2, and YFV, were investigated in this area starting in June of 2009. We recently reported the distribution and abundance of adult mosquitoes potentially involved in the enzootic cycle of CHIKV as well as their levels of infection in the five most abundant land cover classes (forest, savannah, agriculture, barren, and village) found in an area of 1,650 km2 around the town of Kédougou.18 Potential vectors were collected in each of the land cover classes, but Aedes furcifer was the only species that occurred in all land cover classes and also entered villages to feed on humans. This species was considered to be the most important bridge vector between sylvatic amplification hosts and human populations during the 2009 CHIKV outbreak. During the 2010/2011 rainy season, an amplification of YFV occurred in the same area. Here, we describe the spatial and temporal patterns of this YFV amplification.
Materials and Methods
Study area.
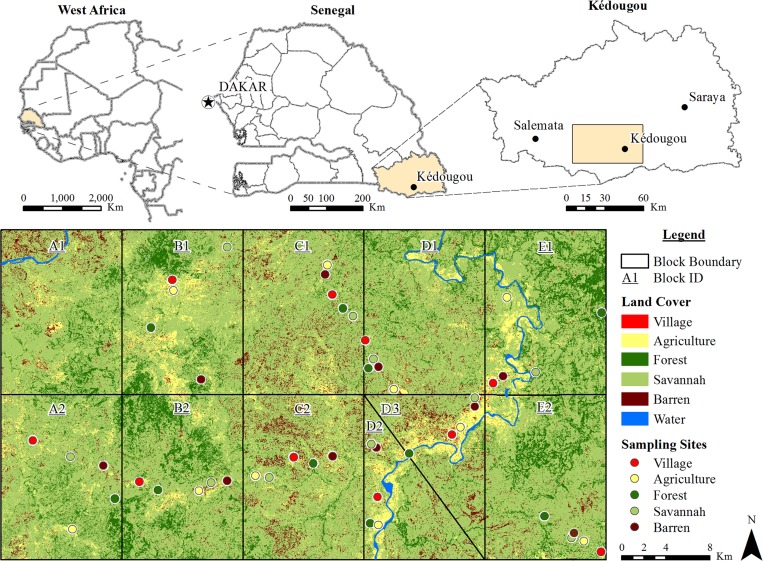
The study was undertaken in the Kédougou region, southeastern Senegal (12°33′ N, 12°11′ W) at the border with both Mali and Guinea (Figure 1). The area is hilly and contrasts with the low flat plain that constitutes the rest of Senegal. The Kédougou region receives 1,200–1,300 mm annual rainfall, with a single rainy season from May and June to October and November. Mean daily temperatures vary from 33°C to 39.5°C during the year. Kédougou is in a transition zone between the dry tropical forest and the savannah belt. Relics of forests, gallery forests along rivers, and savannahs constitute the natural vegetation. Deforestation for cultivation, gold mining, and human habitation has greatly reduced the forested area.
Figure 1.
Study area. The rectangle in the upper right map corresponds to 1,650 km2 divided in the 10 blocks (A2, B1, B2, C1, C2, D1, D2, D3, E1, and E2) below. Data were collected in each of the five land cover classes indicated by colored circles (agriculture, barren, village [indoor and outdoor], savannah, and forest [canopy and ground]) in the 10 blocks. The diagonal line separates blocks D2 and D3, and they replace block A1, which was abandoned because of inaccessibility.
The human population of the region is ca. 80,000, and 55% of the population is under the age of 20 years. It is primarily rural area (84%) with a low density of inhabitants (four inhabitants per 1 km2) mostly living in small, dispersed villages averaging 60 inhabitants. The economy depends on agriculture and cattle farming along with hunting, gathering, and harvesting wood for crafts, which result in human contact with forests.
Mosquito sampling.
The mosquito sampling protocol was extensively described by Diallo and others.18 Briefly, an area of 1,650 km2 (30 km in the north to south direction and 55 km in the east to west direction) of the Kédougou region (Figure 1) was divided into 10 blocks. In each block, the five major land cover classes were classified as forest, barren, savannah, agriculture, and village by remote sensing and geospatial analyses.18 Mosquitoes were sampled by human landing collections, the only effective method for collecting sylvatic Aedes and the most appropriate method for determining the risk of human infection. Collections were performed two times per month by teams of three collectors capturing specimens landing on their legs and working simultaneously in forest canopy, forest ground, savannah, agriculture, village indoor, village outdoor, and barren areas from 18:00 to 21:00 hours (based on previous data on host-seeking periodicity).19 In each village, mosquito sampling was conducted by six landing collectors per evening. Five houses were selected in the village following a transect going from one border to the opposite through the center (one house in the center, one house in each of the borders, and one house between each border and the center). Each sampling evening, one indoor collector and one outdoor collector were positioned in each house. The collectors were positioned extending in one direction from the center to the border in three houses on the first sampling night and the opposite direction on the second sampling night to avoid bias caused by possible vector confinement within the village. Captures occurred monthly for two consecutive nights per site from May of 2010 to March of 2011. Captured mosquitoes were frozen, sorted on a chill table using morphological keys20–26 into monospecific pools, and frozen in liquid nitrogen for viral detection processing.
Determination of parity.
For each land cover class and each sampling evening, the ovaries from a sample of a maximum of 10 specimens of the unengorged mosquito vectors (Ae. furcifer, Ae. taylori, and Ae. luteocephalus) were dissected on a slide containing distilled water. The degree of coiling of ovarian tracheoles was then observed to determine whether the female was parous or nulliparous.27
Detection of virus in mosquito pools.
Monospecific mosquito pools were homogenized in 2.5 mL Leibovitz 15 (GibcoBRL, Grand Island, NY) cell culture medium containing 20% fetal bovine serum (FBS) (GibcoBRL) and centrifuged for 20 minutes at 10,000 × g at 4°C. For the homogenate, 1 mL supernatant was inoculated into AP61 (Ae. pseudoscutellaris mosquito) or Vero African green kidney cells as described previously.28 Cells were incubated at 28°C (AP61) or 37°C (Vero), and cytopathic effects were recorded daily. Within 10 days, slides were prepared for immunofluorescence assay (IFA) against seven pools of immune ascitic fluids specific for most of the African mosquito-borne arboviruses. Viruses were identified by complement fixation and seroneutralization tests by intracerebral inoculation into newborn mice, which was approved by the University of Texas Medical Branch Institutional Animal Care and Use Committee.
For the real-time reverse transcriptase polymerase chain reaction (RT-PCR) assay, 100 μL supernatant were used for RNA extraction with the QiaAmp Viral RNA Extraction Kit (Qiagen, Heiden, Germany) according to the manufacturer's protocol. RNA was amplified using an ABI Prism 7000 SDS Real-Time apparatus (Applied Biosystems, Foster City, CA) using the QuantiTect kit (Qiagen, Hilden, Germany). The 25 μL reaction volume contained 1 μL extracted RNA, 2× QuantiTect Probe, RT-Master Mix, 10 μM each primer, and the probe. The primer and probe sequences used for YFV included the primers RP-YFV (GTCRRTTCTCTGCTAATCGCTCA) and FP-YFV (ATTGAGGTGYATTGGTCTGC) and the probe P-YFV (FAM-AGTTGCTAGGCAATAAA-BBQ). The following thermal profile was used: a single cycle of reverse transcription for 10 minutes at 50°C and 15 minutes at 95°C for reverse transcriptase inactivation and DNA polymerase activation followed by 40 amplification cycles of 15 seconds at 95°C and 1 minute at 60°C (annealing extension step).
Data analysis.
For analysis of the distribution of vector species among land cover classes, the landing rate (average per site of female mosquitoes per person per evening) was used as a measure of absolute abundance. Abundance data were log-transformed (log10 [n + 1]) and analyzed using analysis of variance (ANOVA) followed by a Tukey post-hoc test. The pooled infection rate program (PooledInfRate, version 3.0; Center for Disease Control and Prevention, Fort Collins, CO; http://www.cdc.gov/ncidod/dvbid/westnile/software.htm) was used to calculate minimum field infection rates per 1,000 mosquitoes tested and the 95% confidence intervals for the species found positive for YFV. The entomologic inoculation rate defined as the number of infected mosquito bites per human per evening, month, or transmission season (September to November) was also calculated. Differences of frequencies between groups were tested by the χ2 test. All analyses were conducted using R.29
Results
In total, 41,234 adult female mosquitoes, comprising 58 species within five genera, were collected (Table 1), predominantly Ae. furcifer (22.3%), Ae. vittatus (16.0%), Ae. luteocephalus (14.6%), and Ae. dalzieli (13.9%). Ae. taylori (4.2%) and Ae. africanus (3.6%) composed a relatively small portion of the collection.
Table 1.
Mosquitoes collected and YFV infection in different sampling sites in Kédougou in 2010
| Species | LR | EIR | MIR | LL | UL | No. of pools | No. of positive pools | No. of mosquitoes collected | No. of positive sites | No. of positive months | No. of positive landscapes |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Infected mosquitoes | |||||||||||
| Ae. furcifer | 3 | 0.0112 | 3.74 | 2.54 | 5.05 | 633 | 35 | 9,216 | 25 | 3 | 6 |
| Ae. luteocephalus | 1.96 | 0.0069 | 3.50 | 2.00 | 4.99 | 396 | 21 | 6,010 | 14 | 3 | 4 |
| An. funestus | 2 | 0.0032 | 1.58 | 0.00 | 4.75 | 228 | 1 | 637 | 1 | 1 | 1 |
| Ae. taylori | 0.57 | 0.0012 | 2.10 | 0.05 | 4.57 | 238 | 4 | 1,726 | 4 | 2 | 2 |
| Ae. africanus | 0.47 | 0.0006 | 1.37 | 0.00 | 3.26 | 97 | 2 | 1,465 | 2 | 1 | 1 |
| Ae. centropunctatus | 0.06 | 0.0003 | 5.68 | 0.00 | 16.79 | 72 | 1 | 179 | 1 | 1 | 1 |
| Ae. dalzieli | 1.8 | 0.0003 | 0.17 | 0.00 | 0.52 | 436 | 1 | 5,716 | 1 | 1 | 1 |
| Ae. mcintoshi | 0.04 | 0.0003 | 7.63 | 0.00 | 23.06 | 53 | 1 | 134 | 1 | 1 | 1 |
| Ae. vittatus | 2.12 | 0.0003 | 0.15 | 0.00 | 0.45 | 458 | 1 | 6,609 | 1 | 1 | 1 |
| Non-infected mosquitoes | |||||||||||
| Ae. aegypti | 395 | ||||||||||
| Ae. argenteopunctatus | 1,117 | ||||||||||
| Ae. bromeliae | 2 | ||||||||||
| Ae. cozi | 4 | ||||||||||
| Ae. cumminsii | 111 | ||||||||||
| Ae. fowleri | 52 | ||||||||||
| Ae. freetownnensis | 2 | ||||||||||
| Ae. hirsutus | 63 | ||||||||||
| Ae. longipalpis | 1 | ||||||||||
| Ae. metallicus | 189 | ||||||||||
| Ae. minutus | 719 | ||||||||||
| Ae. mixtus | 5 | ||||||||||
| Ae. ochraceus | 55 | ||||||||||
| Ae. opok | 4 | ||||||||||
| Ae. sudanensis | 2 | ||||||||||
| Ae. unilineatus | 85 | ||||||||||
| Ae. vexans | 12 | ||||||||||
| An. brohieri | 12 | ||||||||||
| An. brunnipes | 1 | ||||||||||
| An. coustani | 2,773 | ||||||||||
| An. domicola | 46 | ||||||||||
| An. flavicosta | 91 | ||||||||||
| An. gambiae | 111 | ||||||||||
| An. hancocki | 121 | ||||||||||
| An. nili | 368 | ||||||||||
| An. pharoensis | 16 | ||||||||||
| An. pretoriensis | 2 | ||||||||||
| An. rufipes | 60 | ||||||||||
| An. squamosus | 11 | ||||||||||
| An. wellcomei | 117 | ||||||||||
| An. ziemanni | 10 | ||||||||||
| Cx. annulioris | 39 | ||||||||||
| Cx. antennatus | 21 | ||||||||||
| Cx. cinerellus | 1 | ||||||||||
| Cx. cinereus | 3 | ||||||||||
| Cx. decens | 5 | ||||||||||
| Cx. ethiopicus | 6 | ||||||||||
| Cx. macfiei | 1 | ||||||||||
| Cx. neavei | 24 | ||||||||||
| Cx. nebulosus | 6 | ||||||||||
| Cx. neoafricanus | 122 | ||||||||||
| Cx. perfuscus | 97 | ||||||||||
| Cx. poicilipes | 134 | ||||||||||
| Cx. quinquefasciatus | 408 | ||||||||||
| Cx. tritaeniorhynchus | 4 | ||||||||||
| Cx.bitaeniorhynchus | 64 | ||||||||||
| Er. quinquevittatus | 11 | ||||||||||
| Ma. africana | 367 | ||||||||||
| Ma. uniformis | 1,672 | ||||||||||
| Total | 41,234 | ||||||||||
An. = Anopheles; Cx. = Culex; EIR = entomologic inoculation rate (number of infected mosquito bites per person per evening); LL = lower limit of the infection rate; LR = landing rate (number of mosquitoes captured per person per evening); Ma. = Mansonia; MIR = infection rate (estimated number of positive mosquitoes per 1,000 mosquitoes tested); UL = upper limit of infection rate.
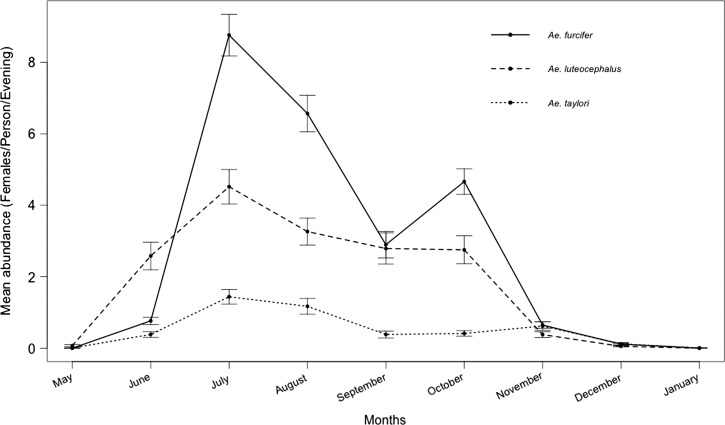
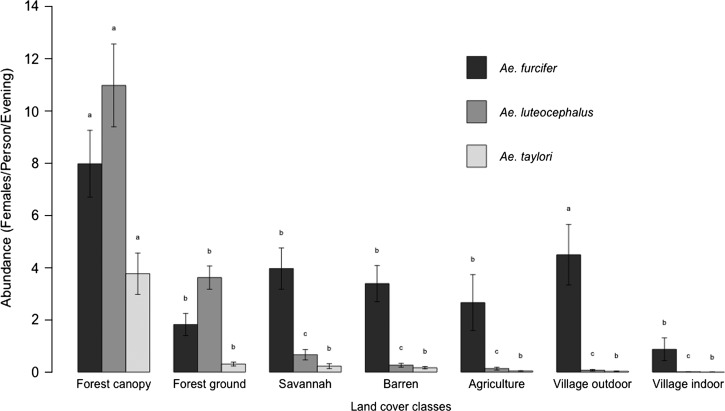
Seasonally, the highest landing rates were observed in July, August, and October for Ae. furcifer and July, August, and November for Ae. taylori (Figure 2). Ae. luteocephalus showed high landing rates from June to October, with a peak in July (Figure 2 ). Ae. furcifer was present in all land cover classes, whereas Ae. luteocephalus and Ae. taylori were collected mainly in the forest canopy and ground and totally absent indoors within villages. Ae. furcifer, Ae. luteocephalus, and Ae. taylori all showed the highest landing rates in the forest canopy (Figure 3 ), but notably, Ae. furcifer also showed high landing rates in villages.
Figure 2.
Seasonal patterns of potential YFV mosquito vectors in different land cover classes near Kédougou, Senegal in 2010.
Figure 3.
Patterns of potential YFV mosquito vectors in different land cover classes near Kédougou, Senegal in 2010. Error bars represent the SEMs; for each species, the means with the same letter are statistically comparable. The mean with the letter a is significantly higher, followed by b and c.
Most mosquitoes (more than 70%) that were dissected were parous for all species (Table 2). The monthly parous rates of each species were significantly different (P < 0.05). All species had comparable parous rates in the different land cover classes (P > 0.1).
Table 2.
Seasonal and temporal dynamics of parous rates for potential YFV vectors in Kédougou from 2010 to 2011
| Ae. furcifer parity rates (total dissected) | Ae. luteocephalus parity rates (total dissected) | Ae. taylori parity rates (total dissected) | |
|---|---|---|---|
| Month | |||
| May | na | 25 (8) | 0 (1) |
| June | 16.7 (66) | 23.7 (114) | 17.5 (40) |
| July | 90.0 (30) | 96.7 (30) | 73.3 (30) |
| August | 99.5 (192) | 95.7 (116) | 100.0 (42) |
| September | 72.4 (163) | 78.9 (95) | 83.3 (30) |
| October | 96.6 (174) | 97.8 (91) | 100.0 (23) |
| November | 97.5 (118) | 100.0 (55) | 98.7 (78) |
| December | 100.0 (37) | 100.0 (22) | 100.0 (37) |
| January | 100.0 (1) | na | 100.0 (1) |
| Land cover classes | |||
| Forest canopy | 83.4 (277) | 78.4 (319) | 82.6 (235) |
| Forest ground | 88.1 (67) | 76.1 (142) | 70.8 (24) |
| Savannah | 91.2 (136) | 72.0 (25) | 100.0 (9) |
| Barren | 81.6 (114) | 78.3 (23) | 100.0 (12) |
| Agriculture | 88.2 (76) | 75.0 (16) | na |
| Village | 84.7 (111) | 71.4 (7) | 100.0 (3) |
na = not applicable.
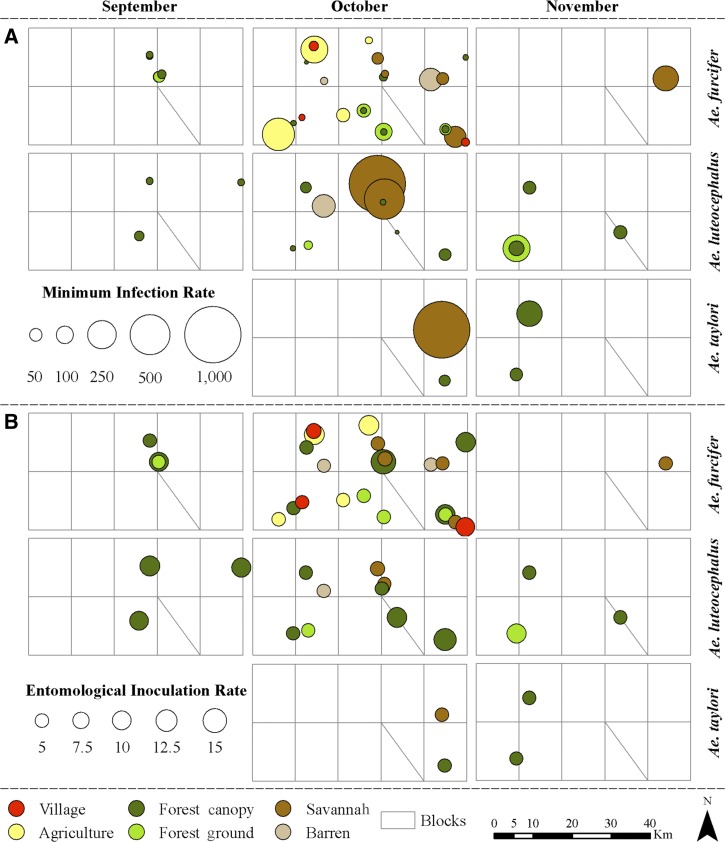
Overall, 67 of 5,152 pools tested were positive for YFV (Tables 1, 3, and 4). The infected pools were distributed as follows: Ae. furcifer (35 pools or 52.2% of the infected pools), Ae. luteocephalus (21 pools or 31.3% of the infected pools), Ae. taylori (4 pools or 6.0% of the infected pools), Ae. africanus (2 pools or 3% of the infected pools), and Ae. dalzieli, Ae. centropunctatus, Ae. mcintoshi, Ae. vittatus, and An. funestus (1 pool each or 1.5% each of the infected pools) captured in September (9 pools or 13.4% of the infected pools), October (47 pools or 70.1% of the infected pools), and November (11 pools or 16.4% of the infected pools). Mean minimum infection rates among species differed significantly (χ2 = 43.4, degrees of freedom [df] = 8, P < 0.0001). Only infection rates of Ae. furcifer, Ae. luteocephalus, and Ae. taylori showed temporal and spatial variations (Figure 4 ). The highest infection rates were observed in October for Ae. furcifer and Ae. taylori and November for Ae. luteocephalus. The difference in monthly minimum infection rates was statistically significant only for Ae. luteocephalus (χ2 = 12.94, df = 2, P = 0.002).
Table 3.
Mosquitoes collected and YFV infection of potential vectors among land cover classes in Kédougou in 2010.
| No. collected | No. pools | No. positive pools | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sept | Oct | Nov | Total | Sept | Oct | Nov | Total | Sept | Oct | Nov | Total | |
| Ae. furcifer | ||||||||||||
| Forest canopy | 361 | 799 | 148 | 1,308 | 18 | 35 | 18 | 71 | 3 | 10 | 0 | 13 |
| Forest ground | 50 | 188 | 5 | 243 | 7 | 17 | 3 | 27 | 1 | 3 | 0 | 4 |
| Savannah | 58 | 226 | 30 | 314 | 12 | 21 | 6 | 39 | 0 | 4 | 1 | 5 |
| Barren | 87 | 166 | 27 | 280 | 11 | 19 | 11 | 41 | 0 | 2 | 0 | 2 |
| Agriculture | 48 | 193 | 31 | 272 | 10 | 20 | 6 | 36 | 0 | 6 | 0 | 6 |
| Village | 104 | 344 | 28 | 476 | 11 | 25 | 9 | 45 | 0 | 5 | 0 | 5 |
| Total sites | 708 | 1,916 | 269 | 2,893 | 69 | 137 | 53 | 259 | 4 | 30 | 1 | 35 |
| Ae. luteocephalus | ||||||||||||
| Forest canopy | 537 | 788 | 139 | 1,464 | 22 | 37 | 17 | 76 | 4 | 7 | 4 | 15 |
| Forest ground | 120 | 317 | 20 | 457 | 9 | 20 | 8 | 37 | 0 | 1 | 2 | 3 |
| Savannah | 21 | 13 | 0 | 34 | 10 | 6 | 16 | 0 | 2 | 0 | 2 | |
| Barren | 20 | 18 | 0 | 38 | 6 | 6 | 12 | 0 | 1 | 0 | 1 | |
| Agriculture | 2 | 3 | 0 | 5 | 2 | 2 | 4 | 0 | 0 | 0 | 0 | |
| Village | 2 | 9 | 1 | 12 | 3 | 4 | 1 | 8 | 0 | 0 | 0 | 0 |
| Total sites | 702 | 1,148 | 160 | 2,010 | 52 | 75 | 26 | 153 | 4 | 11 | 6 | 21 |
| Ae. taylori | ||||||||||||
| Forest canopy | 87 | 149 | 229 | 465 | 10 | 17 | 20 | 47 | 0 | 1 | 2 | 3 |
| Forest ground | 6 | 7 | 9 | 22 | 2 | 5 | 4 | 11 | 0 | 0 | 0 | 0 |
| Savannah | 5 | 1 | 6 | 5 | 1 | 6 | 0 | 1 | 0 | 1 | ||
| Barren | 3 | 3 | 21 | 27 | 1 | 3 | 7 | 11 | 0 | 0 | 0 | 0 |
| Agriculture | 4 | 4 | 3 | 3 | 0 | 0 | 0 | 0 | ||||
| Village | 1 | 4 | 1 | 6 | 1 | 2 | 1 | 4 | 0 | 0 | 0 | 0 |
| Total sites | 97 | 172 | 261 | 530 | 14 | 35 | 33 | 82 | 0 | 2 | 2 | 4 |
| Total main vectors | ||||||||||||
| Forest canopy | 985 | 1,736 | 516 | 3,237 | 50 | 89 | 55 | 194 | 7 | 18 | 6 | 31 |
| Forest ground | 176 | 512 | 34 | 722 | 18 | 42 | 15 | 75 | 1 | 4 | 2 | 7 |
| Savannah | 79 | 244 | 31 | 354 | 22 | 32 | 7 | 61 | 0 | 7 | 1 | 8 |
| Barren | 110 | 187 | 48 | 345 | 18 | 28 | 18 | 64 | 0 | 3 | 0 | 3 |
| Agriculture | 50 | 200 | 31 | 281 | 12 | 25 | 6 | 43 | 0 | 6 | 0 | 6 |
| Village | 107 | 357 | 30 | 494 | 15 | 31 | 11 | 57 | 0 | 5 | 0 | 5 |
Nov = November; Oct = October; Sept = September.
Table 4.
Landing, infection, and potential entomological inoculation rates of potential yellow fever vectors among land cover classes in Kédougou in 2010
| MIR | LR | EIR | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sept | Oct | Nov | Mean | Sept | Oct | Nov | Mean | Sept | Oct | Nov | Mean | |
| Ae. furcifer | ||||||||||||
| Forest canopy | 8.31 | 12.52 | 0 | 9.94 | 10.03 | 14.02 | 2.47 | 8.55 | 0.08 | 0.18 | 0 | 0.08 |
| Forest ground | 20.00 | 15.96 | 0 | 16.46 | 1.39 | 0.83 | 0.08 | 1.56 | 0.03 | 0.01 | 0 | 0.03 |
| Savannah | 0.00 | 17.70 | 33.33 | 15.92 | 1.61 | 0.97 | 0.5 | 2.01 | 0 | 0.02 | 0.02 | 0.03 |
| Barren | 0.00 | 12.05 | 0 | 7.14 | 2.42 | 1.45 | 0.45 | 1.79 | 0 | 0.02 | 0 | 0.01 |
| Agriculture | 0.00 | 31.09 | 0 | 22.06 | 1.33 | 0.80 | 0.52 | 1.74 | 0 | 0.02 | 0 | 0.04 |
| Village | 0.00 | 14.53 | 0 | 10.50 | 2.89 | 1.73 | 0.47 | 3.05 | 0 | 0.03 | 0 | 0.03 |
| Total sites | 5.65 | 15.66 | 3.72 | 12.10 | 3.28 | 5.32 | 0.75 | 3.09 | 0.02 | 0.08 | 0.00 | 0.04 |
| Ae. luteocephalus | ||||||||||||
| Forest canopy | 7.45 | 8.88 | 28.78 | 10.25 | 14.92 | 9.42 | 2.32 | 9.57 | 0.11 | 0.08 | 0.07 | 0.10 |
| Forest ground | 0 | 3.15 | 100.00 | 6.56 | 3.33 | 2.00 | 0.33 | 2.93 | 0 | 0.01 | 0.03 | 0.02 |
| Savannah | 0 | 153.85 | 0 | 58.82 | 0.58 | 0.35 | 0.22 | 0 | 0.05 | 0 | 0.01 | |
| Barren | 0 | 55.56 | 0 | 26.32 | 0.56 | 0.33 | 0.24 | 0 | 0.02 | 0 | 0.01 | |
| Agriculture | 0 | 0 | 0 | 0 | 0.06 | 0.03 | 0.03 | 0 | 0 | 0 | 0 | |
| Village | 0 | 0 | 0 | 0 | 0.06 | 0.03 | 0.02 | 0.08 | 0 | 0 | 0 | 0 |
| Total sites | 5.70 | 9.58 | 37.50 | 10.45 | 3.25 | 3.19 | 0.44 | 2.15 | 0.02 | 0.03 | 0.02 | 0.02 |
| Ae. taylori | ||||||||||||
| Forest canopy | 0 | 6.71 | 8.73 | 6.45 | 2.42 | 1.53 | 3.82 | 3.04 | 0 | 0.01 | 0.03 | 0.02 |
| Forest ground | 0 | 0 | 0 | 0 | 0.17 | 0.12 | 0.15 | 0.14 | 0 | 0 | 0 | 0 |
| Savannah | 0 | 200.00 | 0 | 166.67 | 0.08 | 0.02 | 0.04 | 0 | 0.02 | 0 | 0.01 | |
| Barren | 0 | 0 | 0 | 0 | 0.08 | 0.05 | 0.35 | 0.17 | 0 | 0 | 0 | 0 |
| Agriculture | 0 | 0 | 0 | 0 | 0.07 | 0.03 | 0 | 0 | 0 | 0 | ||
| Village | 0 | 0 | 0 | 0 | 0.03 | 0.07 | 0.02 | 0.04 | 0 | 0 | 0 | 0 |
| Total sites | 0.00 | 11.63 | 7.66 | 7.55 | 0.45 | 0.48 | 0.73 | 0.57 | 0.00 | 0.01 | 0.01 | 0.004 |
| Total vectors | ||||||||||||
| Forest canopy | 7.11 | 10.37 | 11.63 | 9.58 | 27.36 | 30.46 | 8.6 | 21.2 | 0.19 | 0.32 | 0.10 | 0.20 |
| Forest ground | 5.68 | 7.81 | 58.82 | 9.70 | 4.89 | 8.53 | 0.57 | 4.63 | 0.03 | 0.07 | 0.03 | 0.04 |
| Savannah | 0 | 28.69 | 32.26 | 22.60 | 2.19 | 4.07 | 0.52 | 2.27 | 0 | 0.12 | 0.02 | 0.05 |
| Barren | 0 | 16.04 | 0 | 8.70 | 3.06 | 3.12 | 0.8 | 2.21 | 0 | 0.05 | 0 | 0.02 |
| Agriculture | 0 | 30.00 | 0 | 21.35 | 1.39 | 3.33 | 0.52 | 1.8 | 0 | 0.10 | 0 | 0.04 |
| Village | 0 | 14.01 | 0 | 10.12 | 2.97 | 5.95 | 0.5 | 3.17 | 0 | 0.08 | 0 | 0.03 |
Figure 4.
Patterns of (A) minimum infection rates and (B) entomological inoculation rates of the main potential YFV mosquito vectors in different land cover classes near Kédougou, Senegal in 2010.
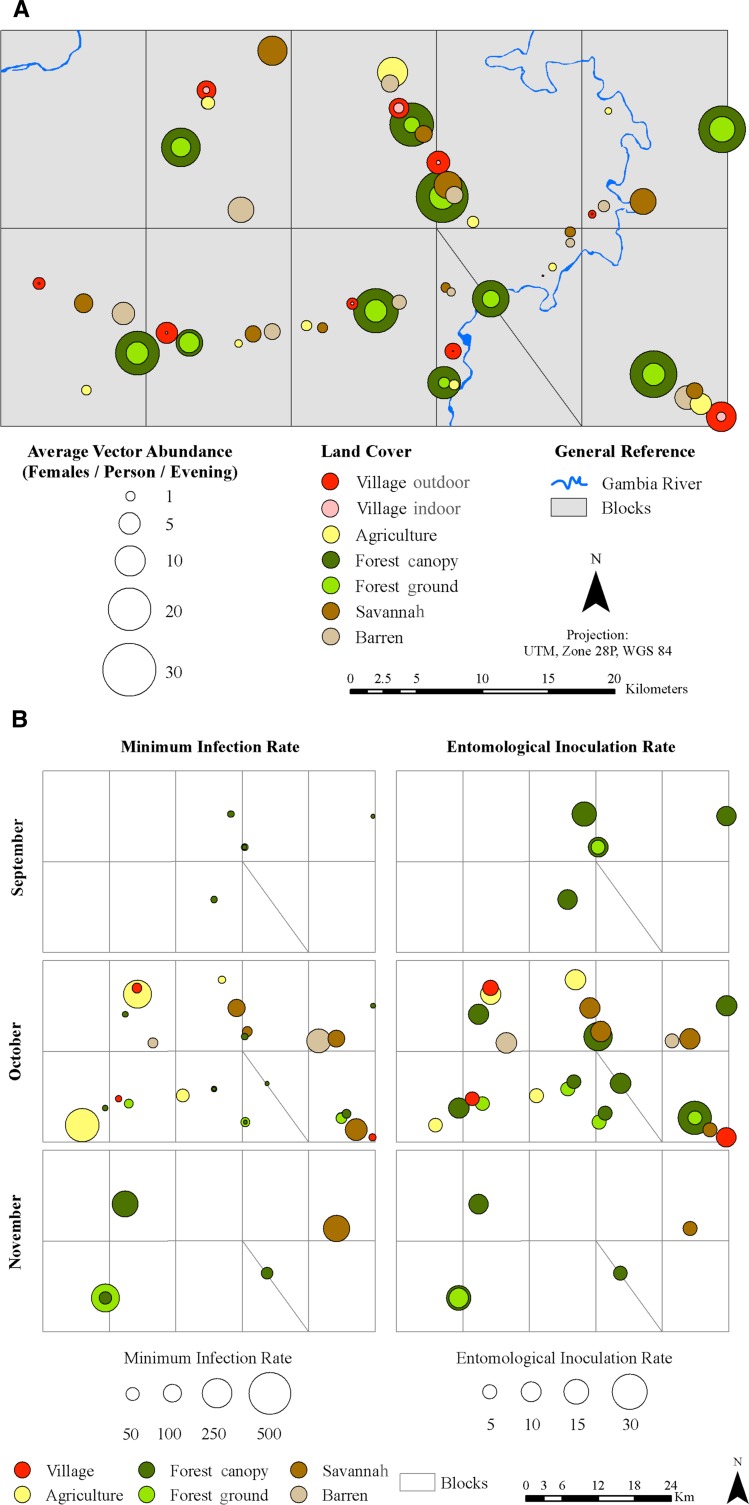
Spatially, YFV was detected from mosquitoes collected in all 10 blocks sampled (A2, B1, B2, C1, C2, D1, D2, D3, E1, and E2) and all land cover classes (Figures 4 and 5 and Table 3), including all 10 forest canopy sites (33 pools; 49.2%), 4 savannah sites (11 pools; 16.4%), 6 forest ground sites (7 pools; 10.4%), 4 agricultural sites (7 pools; 10.4%), 3 village sites (5 pools; 7.5%), and 2 barren sites (4 pools, 6.0%). There were no significant differences among sampling blocks (χ2 = 4.1, df = 9, P = 0.9); however, there was a significant association between land cover classes and the presence of YFV (χ2 = 25.1, df = 6, P = 0.003).
Figure 5.
(A) Patterns of average biting rates (females per person per evening) of the pooled main potential YFV mosquito vectors in different land cover classes near Kédougou, Senegal in 2010. (B) Patterns of minimum infection rates and entomological inoculation rates of the pooled main potential YFV mosquito vectors in different land cover classes near Kédougou, Senegal in 2010.
To assess the impact of proximity to forests on human exposure to YFV, villages were coded as either positive (produced at least one YFV-positive mosquito pool) or negative (produced no positive pools) for YFV. Distance and forest size data were log-transformed to render them normally distributed. Positive villages were significantly closer to large (> 2 ha) forests (mean distance = 445 m, SE = 289) than negative villages (mean distance = 1,418 m, SE = 189; t test, df = 8, t = −2.82, P = 0.02).
Differences of mean minimum infection rates among different land cover classes were not statistically significant for Ae. furcifer (χ2 = 4.2, df = 5, P = 0.5) and the whole mosquito set (χ2 = 8.0, df = 5, P = 0.2) but were significant for Ae. luteocephalus (χ2 = 9.2, df = 3, P = 0.03) and Ae. taylori (χ2 = 18.1, df = 1, P = 0.04), for which the highest mean infection rate occurred in the savannah (Figures 4 and 5B). The temporal pattern of vector infection rate differences was statistically comparable in the different land cover classes except for Ae. luteocephalus on the forest ground (χ2 = 10.6, df = 1, P = 0.001), where infection rate was significantly higher in November (Table 4).
Assuming that infected mosquitoes were capable of transmission, the highest mean entomological inoculation rate was generated by Ae. furcifer (3.6 infectious bites between September and November) followed by Ae. luteocephalus (1.8 infectious bites between September and November) and Ae. taylori (0.4 infectious bites between September and November) (Table 4). YFV infectivity was found in Ae. luteocephalus and Ae. furcifer in September (0.6 infectious bites each), all three species in October (2.4 infectious bites for Ae. furcifer, 0.9 infectious bites for Ae. luteocephalus, and 0.3 infectious bites for Ae. taylori), and Ae. luteocephalus (0.6 infectious bites) and Ae. taylori (0.3 infectious bites) in November (Figure 4). Spatially, the highest inoculation rates were observed in the forest canopy in September by Ae. luteocephalus (3.3 infectious bites) and the forest canopy in October by Ae. furcifer (5.4 infectious bites). Between September and November, results suggest that primates in the forest canopy might have received at least 7.9, 7.9, and 1.3 infectious bites from Ae. furcifer, Ae. luteocephalus, and Ae. taylori, respectively. This monkey may have received between 0 and 25.5 infectious bites from Ae. furcifer, between 0 and 15.3 infectious bites from Ae. luteocephalus, and between 0 and 5.2 infectious bites from Ae. taylori depending on the specific canopy site. When all three vectors are taken into account, the inoculation rate varied between 5.2 and 30.5 infectious bites depending on the canopy site. Only Ae. furcifer was implicated as potentially involved in the transmission within villages; a villager may have received at least 0.78 infectious bites from this species during the transmission season. This risk varied among villages between 0 and 9.4 infectious bites.
Discussion
Among the different land cover classes investigated (forest canopy, forest ground, savannah, barren, agriculture, village indoor, and village outdoor), the forest canopy was the only type for which all sites surveyed were positive for YFV. Moreover, several primate species infected by the virus in the Kédougou area and elsewhere in Africa feed and sleep in the forest canopy.17,30 Together, these observations suggest a sylvatic origin of the virus amplification.
The amplification was first detected in 4 of 10 forest canopies surveyed, reached its maximum in October when the virus was found in all 10 forest canopies, and was last detected in 4 forest canopies different from the ones in which the amplification was first detected. This scenario suggests two possible patterns of amplification. In the first scenario, the virus emerged in a subset of forest canopy sites, was amplified, and spread broadly across forest canopies in the region. It is important to note here that such canopies are not continuous but occur in discrete patches.31 Dissemination of YFV from initial sites of emergence could be the result of the movement of viremic vertebrates and/or infected mosquito vectors. This hypothesis is consistent with early studies suggesting that permanent enzootic foci of YFV do not exist in West Africa and that the virus is continually moving in the form of traveling epizootic waves.30,32 Furthermore, a study on the spread of YFV along major rivers in West Africa concluded that the virus is enzootic in the forest area and maintained in traveling sylvatic waves.33 The second possible scenario is that enzootic YFV was broadly distributed across forest canopies before its amplification but that sites differed in the dynamics of amplification because of some relevant factor, such as density of mosquito eggs, monkey density, or microclimate, such that the virus was detectable at some sites before other sites.
YFV was isolated only between September and November in this study, confirming its seasonal pattern of transmission, which has been described for other sylvatic arbovirus amplification observed in previous outbreaks in the region.17,18,34 Although mosquitoes were abundant in July and August of 2010, no positive pools were identified, indicating that the virus was either absent or present at extremely low prevalence in vectors before September of 2010.
Our study highlights that the highest risk of human exposure in this amplification peaked in October. October is the month when most of the sites, including villages and agricultural fields, contained YFV-infected mosquitoes. The virus was detected in mosquitoes in only 30% of villages surveyed, indicating spatial variation in human risk of infection by mosquito vectors within villages. These data are consistent with the results in the work by Cornet and others,30 which showed that the 1977 YFV amplification, showed by a collection of infected mosquitoes, was not uniform among villages. In contrast, Traore-Lamizana and others17 detected infected mosquitoes during the 1993 YFV amplification in all four villages investigated, although the sample size, in terms of numbers of villages, in this latter study was very small.
Importantly, we showed an association of YFV positivity for villages with proximity of villages to large forest. This key finding will allow for spatial delineation of the risk of people for exposure to sylvatic yellow fever and be useful for the most efficient targeting of control measures.
We isolated YFV mainly from Ae. furcifer, Ae. taylori, and Ae. luteocephus. This virus was also isolated only from these same three species in the 1993 YFV outbreak in southeastern Senegal,17 and they were also implicated as the main YFV vectors in the 1977 sylvatic outbreak in this region.19 It is noteworthy that these vectors reached very high parity rates just 3 months after the beginning of the rainy season, indicating high survival rates and a favorable environment for arbovirus amplification in all the land cover classes surveyed. The longer vectors live, the higher the probability that they will be able to transmit arboviruses. The amplification coincided with a decrease in vector parity in September. Apart from these main vectors, YFV has also been isolated from Ae. neoafricanus, Ae. vittatus, Ae. africanus, and Ae. metallicus in southeastern Senegal and Ae. opok, the Ae. tarsalis group, and Eretmapodites inornatus in other West African countries.35 Possible YFV vectors in East/Central Africa include Ae. simpsoni/bromeliae, Er. chrysogaster, Ae. dentatus, Ae. keniensis, Coquillettidia fuscopennata, Amblyomma variegatum, and Phlebotime species.36 Four species, Ae. dalzieli, Ae. centropunctatus, Ae. mcintoshi, and An. funestus, were associated with YFV for the first time in this investigation. These new associations are probably a consequence of the wider spatial and seasonal scope of our study compared with previous studies of YFV in the Kédougou area.
The frequency of YFV infection in Ae. furcifer and Ae. luteocephalus suggests that they should be considered the principal vectors for sylvatic YFV. However, some important differences were observed in their specific role in the cycle. Ae. furcifer was found to be infected at equal rates in all land cover classes investigated, whereas infected Ae. luteocephalus occurred mainly in forest and savannah. These distributions indicate that Ae. luteocephalus was probably only an epizootic vector responsible for the transmission of the virus among monkeys in the canopies, whereas Ae. furcifer was responsible for transmission within villages. Ae. furcifer acted also, like Ae. luteocephalus, as an epizootic vector in addition to its role as bridge vector, transporting the virus from the forest canopy to villages located within it flight range. This species was the only vector that commonly contacted humans within the villages and was found infected within villages during this amplification. This result is concordant with previous reports on outbreaks of sylvatic YFV, dengue virus 2, and CHIKV in southeastern Senegal17,37–39 and Burkina Faso.40 Despite its lower entomological inoculation rate, Ae. taylori likely also serves as an epizootic vector in the forest canopy.
Ae. aegypti has always been the main or sole incriminated vector in urban YFV epidemics in central and western Senegal,12–14 but it has never been found infected in southeastern Senegal.17,18,30,34 The lack of infectivity may be explained by the behavior of this species, which lives in domestic environments and is highly anthropohilic in central and western Senegal; however, in southeastern Senegal, it may live in domestic and sylvatic environments but is minimally attracted to humans, even in some previous 25-hour collections.18,19,41 The vector competence of the southeastern Senegalese population of Ae. aegypti for YFV has never been tested.
A limited study on the host feeding patterns of the three major YFV vectors discussed above in the Kédougou area showed that only Ae. taylori had fed on monkeys and that most Ae. luteocephalus and Ae. furcifer had fed, surprisingly, on birds.42 These three species also fed on other mammals, including cows, humans, and duikers. These feeding patterns and the fact that YFV was first detected in Ae. luteocephalus and Ae. furcifer in the amplification suggest the possibility of a secondary transmission cycle involving other vertebrates. Ae. luteocephalus and Ae. furcifer have been found infected with some arbovirus, like Bouboui and Chikungunya, that were also associated with several vertebrates species other than primates.35
In conclusion, the current study evidenced a widespread outbreak of epizootic YFV in southeastern Senegal, with predominant circulation in forest canopies and variable frequency in all other land cover classes, including villages. The study highlights the importance of Ae. luteocephalus as an epizootic vector of YFV and supports the role played by Ae. furcifer as an epizootic vector of YFV and an important bridge vector to humans in the area. In addition, later yellow fever outbreaks were reported in Sierra Leone, Cote d'Ivoire, Ghana, and Cameroon in 2011 and Gambia, Chad, Republic of Congo, and Sudan in 2012.43 These patterns support the usefulness of the Kédougou surveillance program as a forecasting tool for YFV elsewhere in Africa.17
ACKNOWLEDGMENTS
The authors thank Saliou Ba, Momar Tall, and Bidiel Fall for their technical assistance in the field and the population of Kédougou for their collaboration.
Footnotes
Financial support: This research was supported by National Institutes of Health Grant R01AI069145, National Center for Research Resources Grant 5P20RR016480-12, and National Institute of General Medical Sciences Grant 8 P20 GM103451-12 from the National Institutes of Health.
Authors' addresses: Diawo Diallo, Cheikh T. Diagne, Yamar Ba, and Mawlouth Diallo, Unité d'Entomologie Médicale, Institut Pasteur de Dakar, Dakar, Sénégal, E-mails: diawod@yahoo.com, cheikhdiagnea09z@yahoo.fr, ba@pasteur.sn, and diallo@pasteur.sn. Amadou A. Sall, Oumar Faye, and Ousmane Faye, Unité des Arbovirus et Virus de Fièvres Hémorragiques, Institut Pasteur de Dakar, Dakar, Sénégal, E-mails: asall@pasteur.sn, oumarfaye@pasteur.sn, and ofaye@pasteur.sn. Kathryn A. Hanley, Department of Biology, New Mexico State University, Las Cruces, NM, E-mail: khanley@nmsu.edu. Michaela Buenemann, Department of Geography, New Mexico State University, Las Cruces, NM, E-mail: elabuen@nmsu.edu. Scott C. Weaver, Institute for Human Infections and Immunity, Center for Tropical Diseases, and Department of Pathology, University of Texas Medical Branch, Galveston, TX, E-mail: sweaver@utmb.edu.
References
- 1.Barrett AD, Monath TP. Epidemiology and ecology of yellow fever virus. Adv Virus Res. 2003;61:291–315. doi: 10.1016/s0065-3527(03)61007-9. [DOI] [PubMed] [Google Scholar]
- 2.Monath TP. Yellow fever: an update. Lancet Infect Dis. 2001;1:11–20. doi: 10.1016/S1473-3099(01)00016-0. [DOI] [PubMed] [Google Scholar]
- 3.Robertson SE, Hull BP, Tomori O, Bele O, LeDuc JW, Esteves K. Yellow fever: a decade of reemergence. JAMA. 1996;276:1157–1162. [PubMed] [Google Scholar]
- 4.Mutebi JP, Barrett AD. The epidemiology of yellow fever in Africa. Microbes Infect. 2002;4:1459–1468. doi: 10.1016/s1286-4579(02)00028-x. [DOI] [PubMed] [Google Scholar]
- 5.Onyango CO, Ofula VO, Sang RC, Konongoi SL, Sow A, De Cock KM, Tukei PM, Okoth FA, Swanepoel R, Burt FJ, Waters NC, Coldren RL. Yellow fever outbreak, Imatong, southern Sudan. Emerg Infect Dis. 2004;10:1063–1068. doi: 10.3201/eid1006.030738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gould LH, Osman MS, Farnon EC, Griffith KS, Godsey MS, Karch S, Mulenda B, El Kholy A, Grandesso F, De Radiguès X, Brair M-E, Briand S, El Tayeb ESM, Hayes EB, Zeller H, Perea W. An outbreak of yellow fever with concurrent chikungunya virus transmission in South Kordofan, Sudan, 2005. Trans R Soc Trop Med Hyg. 2008;102:1247–1254. doi: 10.1016/j.trstmh.2008.04.014. [DOI] [PubMed] [Google Scholar]
- 7.Yuill TM, Woodall JP, Baekeland S. Latest outbreak news from ProMED-mail. Yellow fever outbreak—Darfur Sudan and Chad. Int J Infect Dis. 2013;17:476–478. [Google Scholar]
- 8.Reiter P, Cordellier R, Ouma JO, Cropp CB, Savage HM, Sanders EJ, Marfin AA, Tukei PM, Agata NN, Gitau LG, Rapuoda BA, Gubler DJ. First recorded outbreak of yellow fever in Kenya, 1992–1993. II. Entomologic investigations. Am J Trop Med Hyg. 1998;59:650–656. doi: 10.4269/ajtmh.1998.59.650. [DOI] [PubMed] [Google Scholar]
- 9.WHO Outbreak news. Yellow fever, Liberia. Wkly Epidemiol Rec. 2008;83:158. [PubMed] [Google Scholar]
- 10.WHO Outbreak news. Yellow fever, Togo—update. Wkly Epidemiol Rec. 2007;82:50. [PubMed] [Google Scholar]
- 11.WHO Outbreak news. Yellow fever, Republic of the Congo. Wkly Epidemiol Rec. 2009;84:161. [PubMed] [Google Scholar]
- 12.Chambon L, Wone I, Bres P, Cornet M, Ly C, Michel A, Lacan A, Robin Y, Henderson BE, Williams KH, Camain R, Lambert D, Rey M, Mar ID, Oudart JL, Causse G, Ba H, Martin M, Artus JC. An epidemic of yellow fever in Senegal in 1965. Bull World Health Organ. 1967;36:113–150. [PMC free article] [PubMed] [Google Scholar]
- 13.Thonnon J, Fontenille D, Tall A, Diallo M, Renaudineau Y, Baudez B, Raphenon G. Re-emergence of yellow fever in Senegal in 1995. Am J Trop Med Hyg. 1998;59:108–114. doi: 10.4269/ajtmh.1998.59.108. [DOI] [PubMed] [Google Scholar]
- 14.Thonnon J, Spiegel A, Diallo M, Sylla R, Fall A, Mondo M, Fontenille D. Yellow fever outbreak in Kaffrine, Senegal 1996: epidemiological and entomological findings. Trop Med Int Health. 1998;3:872–877. doi: 10.1046/j.1365-3156.1998.00317.x. [DOI] [PubMed] [Google Scholar]
- 15.Digoutte JP, Plassart H, Salaun JJ, Heme G, Ferrara L, Germain M. Three cases of yellow fever contracted in Senegal. Bull World Health Organ. 1981;59:759–766. [PMC free article] [PubMed] [Google Scholar]
- 16.WHO Yellow fever, Senegal—update. Wkly Epidemiol Rec. 2002;77:349. [PubMed] [Google Scholar]
- 17.Traore-Lamizana M, Fontenille D, Zeller HG, Mondo M, Diallo M, Adam F, Eyraud M, Maiga A, Digoutte JP. Surveillance for yellow fever virus in eastern Senegal during 1993. J Med Entomol. 1996;33:760–765. doi: 10.1093/jmedent/33.5.760. [DOI] [PubMed] [Google Scholar]
- 18.Diallo D, Sall AA, Buenemann M, Chen R, Faye O, Diagne CT, Ba Y, Dia I, Watts D, Weaver SC, Hanley KA, Diallo M. Landscape ecology of sylvatic chikungunya virus and mosquito vectors in southeastern Senegal. PLoS Negl Trop Dis. 2012;6:e1649. doi: 10.1371/journal.pntd.0001649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cornet M, Chateau R, Valade M, Dieng PY, Raymond H, Lorand A. Données bio-écologiques sur les vecteurs potentiels de virus amaril. Cah Orstom Ser Ent Med Parasitol. 1978;16:315–341. [Google Scholar]
- 20.Edwards FW. Mosquitoes of the Ethiopian Region: III Culicine Adults and Pupae. London, UK: British Museum of Natural History; 1941. [Google Scholar]
- 21.Ferrara L, Germain M, Hervy JP. Aedes (Diceromyia) furcifer (Edwards, 1913) et Aedes (Diceromyia) taylori (Edwards, 1936): le point sur la différentiation des adultes. Cah Orstom Ser Ent Med Parasitol. 1984;22:95–98. [Google Scholar]
- 22.Huang YM. Aedes (Stegomyia) bromeliae (Diptera: Culicidae), the yellow fever virus vector in East Africa. J Med Entomol. 1986;23:196–200. doi: 10.1093/jmedent/23.2.196. [DOI] [PubMed] [Google Scholar]
- 23.Huang YM, Ward RA. A pictoral key for the identification of the mosquitoes associated with yellow fever in Africa. Mosq Syst. 1981;13:138–149. [Google Scholar]
- 24.Jupp PG. Mosquitoes of Southern Africa: Culicinae and Toxorhynchitinae. Hartebeespoort, South Africa: Ekogilde cc Publishers; 1997. [Google Scholar]
- 25.Cornet M. Aedes (Stegomyia) cozi n.sp., une nouvelle espèce de Culicidae au Sénégal. Cah Orstom Ser Ent Med Parasitol. 1973;11:175–180. [Google Scholar]
- 26.Diagne N, Fontenille D, Konate L, Lamizana MT, Molez JF, Trape JF, Faye O. Les Anophèles du Sénégal: liste comment & et illustrée. Bull Soc Pathol Exot. 1994;87:1–9. [PubMed] [Google Scholar]
- 27.Detinova TS. Age-grouping methods in Diptera of medical importance with special reference to some vectors of malaria. Monogr Ser World Health Organ. 1962;47:13–191. [PubMed] [Google Scholar]
- 28.Digoutte JP, Calvo-Wilson MA, Mondo M, Traore-Lamizana M, Adam F. Continuous cell lines and immune ascitic fluid pools in arbovirus detection. Res Virol. 1992;143:417–422. doi: 10.1016/s0923-2516(06)80135-4. [DOI] [PubMed] [Google Scholar]
- 29.R Development Core Team . A language and environment for statistical computing. In: Team RDC, editor. R Foundation for Statistical Computing. Vienna, Austria: R Foundation; 2011. [Google Scholar]
- 30.Cornet M, Robin Y, Heme G, Adam C, Renaudet J, Valade M, Eyraud M. Une poussée épizootique de fièvre jaune selvatique au Sénégal oriental. Isolement du virus de lots de moustiques adultes males et femelles. Med Mal Infect. 1979;9:63–66. [Google Scholar]
- 31.Tappan G, Sall M, Wood E, Cushing M. Ecoregions and land cover trends in Senegal. J Arid Environ. 2004;59:427–462. [Google Scholar]
- 32.Chambon L, Digoutte JP, Cornet M, Robin Y. Recent data on the yellow fever epidemiologic situation in tropical Africa. Bull Soc Pathol Exot. 1971;64:673–683. [PubMed] [Google Scholar]
- 33.Gayral P, Cavier R. Current entomological and ecological data on yellow fever vectors in western Africa. Bull Soc Pathol Exot. 1971;64:701–708. [PubMed] [Google Scholar]
- 34.Cornet M, Robin Y, Chateau R, Heme G, Adam C, Valade M. Isolement d'arbovirus au Senegal oriental a partir de moustiques (1972–1977) et note sur l'epidemiologie des virus transmis par les Aedes, en particulier du virus amaril. Cah Orstom Ser Ent Med Parasitol. 1979;17:149–163. [Google Scholar]
- 35.Adam F, Digoutte JP. Virus d'Afrique (Base de Données). Centre Collaborateur OMS de Référence et de Recherche pour les Arbovirus et les Virus de Fièvres Hémorrhagiques (CRORA). African Viruses (Database). WHO Collaborating Centre for Arbovirus and Haemorrhagic Fever Reference. 2013. http://www.pasteur.fr/recherche/banques/CRORA/ Available at. Accessed April 2, 2013.
- 36.Ellis BR, Barrett AD. The enigma of yellow fever in East Africa. Rev Med Virol. 2008;18:331–346. doi: 10.1002/rmv.584. [DOI] [PubMed] [Google Scholar]
- 37.Traore-Lamizana M, Zeller H, Monlun E, Mondo M, Hervy JP, Adam F, Digoutte JP. Dengue 2 outbreak in southeastern Senegal during 1990: virus isolations from mosquitoes (Diptera: Culicidae) J Med Entomol. 1994;31:623–627. doi: 10.1093/jmedent/31.4.623. [DOI] [PubMed] [Google Scholar]
- 38.Diallo M, Ba Y, Sall AA, Diop OM, Ndione JA, Mondo M, Girault L, Mathiot C. Amplification of the sylvatic cycle of dengue virus type 2, Senegal, 1999–2000: entomologic findings and epidemiologic considerations. Emerg Infect Dis. 2003;9:362–367. doi: 10.3201/eid0903.020219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Monlun E, Zeller H, Le Guenno B, Traore-Lamizana M, Hervy JP, Adam F, Ferrara L, Fontenille D, Sylla R, Mondo M. Surveillance of the circulation of arbovirus of medical interest in the region of eastern Senegal. Bull Soc Pathol Exot. 1993;86:21–28. [PubMed] [Google Scholar]
- 40.Robert V, Lhuillier M, Meunier D, Sarthou JL, Monteny N, Digoutte JP, Cornet M, Germain M, Cordellier R. Yellow fever virus, dengue 2 and other arboviruses isolated from mosquitos, in Burkina Faso, from 1983 to 1986. Entomological and epidemiological considerations. Bull Soc Pathol Exot. 1993;86:90–100. [PubMed] [Google Scholar]
- 41.Diallo D, Diagne CT, Hanley KA, Sall AA, Buenemann M, Ba Y, Dia I, Weaver SC, Diallo M. Larval ecology of mosquitoes in sylvatic arbovirus foci in southeastern Senegal. Parasit Vectors. 2012;5:286. doi: 10.1186/1756-3305-5-286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Diallo D, Chen R, Diagne CT, Ba Y, Dia I, Sall AA, Weaver SC, Diallo M. Bloodfeeding patterns of sylvatic arbovirus vectors in southeastern Senegal. Trans R Soc Trop Med Hyg. 2013;107:200–203. doi: 10.1093/trstmh/trs095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.WHO Yellow fever in Africa and South America, 2011–2012. Wkly Epidemiol Rec. 2013;88:285–296. [PubMed] [Google Scholar]