Abstract
After a category 4 cyclone that caused extensive population displacement and damage to water and sanitation infrastructure in Fiji in March 2010, a typhoid vaccination campaign was conducted as part of the post-disaster response. During June–December 2010, 64,015 doses of typhoid Vi polysaccharide vaccine were administered to persons ≥ 2 years of age, primarily in cyclone-affected areas that were typhoid endemic. Annual typhoid fever incidence decreased during the post-campaign year (2011) relative to preceding years (2008–2009) in three subdivisions where a large proportion of the population was vaccinated (incidence rate ratios and 95% confidence intervals: 0.23, 0.13–0.41; 0.24, 0.14–0.41; 0.58, 0.40–0.86), and increased or remained unchanged in 12 subdivisions where little to no vaccination occurred. Vaccination played a role in reducing typhoid fever incidence in high-incidence areas after a disaster and should be considered in endemic settings, along with comprehensive control measures, as recommended by the World Health Organization.
Introduction
Typhoid fever (typhoid) causes an estimated 20 million cases and 200,000 deaths annually worldwide, primarily in south Asia and sub-Saharan Africa.1 Typhoid is caused by infection with the bacterium Salmonella enterica serovar Typhi (Typhi). The disease usually presents as a non-specific febrile illness, and laboratory confirmation depends on isolation of the organism by culture of blood, stool, or bone marrow. Provision of safe water, adequate sanitation, and good hygiene (WaSH) is the mainstay of typhoid prevention and control efforts.
Two typhoid vaccines, an injectable Vi polysaccharide (ViPS) and an oral, live-attenuated Ty21a strain of Typhi, are licensed in many countries for persons ≥ 2 years of age. The ViPS vaccine is ∼70% effective and is administered as one dose with immunity lasting 3 years, and the Ty21a vaccine is 53–78% effective and is administered as 3–4 doses with immunity lasting 5 years.2 In 2008, the World Health Organization (WHO) issued a revised position statement recommending the use of typhoid vaccines for controlling endemic and epidemic disease.2 However, there is no specific recommendation for typhoid vaccine use in post-disaster settings, and use in endemic settings remains limited. Recently, typhoid vaccination is increasingly being considered an important intervention in high incidence settings, because substantial time and resources are required to implement the long-term infrastructure and behavior changes needed to reduce disease burden, and antibiotic resistance of Typhi strains are emerging, which limits treatment options.3
Typhoid is endemic in the Republic of Fiji, a nation of 332 islands in the South Pacific Ocean with a population of 837,271 persons.4 In 2005, laboratory-confirmed typhoid incidence was reported to be 33 cases per 100,000 persons,5 and in 2006, a WHO study estimated that typhoid incidence in Fiji's Northern division was 136–1,052 typhoid cases per 100,000 persons (Vally H, unpublished data), which is categorized as “high” according to the WHO definition of ≥ 100 cases per 100,000 per year.1 At least 13 typhoid outbreaks in Fiji have been reported by local and international media since 2005 (WHO-Division of Pacific Technical Support [WHO-DPS], unpublished data). Laboratory-based typhoid surveillance began in 2001 and includes blood and stool culture, antimicrobial susceptibility testing, and weekly electronic reporting to the Fiji Centre for Communicable Disease Control (FCCDC). Based on WHO and United Nations Children's Fund (UNICEF) definitions, 98% of the population has access to improved drinking water sources, and 83% have access to improved sanitation facilities6; however, the definition of “improved drinking water sources” includes piped untreated surface water, which is a water source in many rural communities that could be subject to fecal contamination.
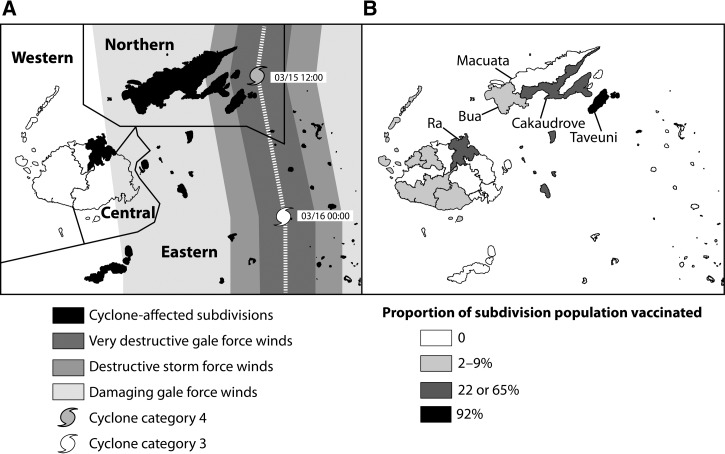
Cyclones are common during November to April each year in Fiji. On March 15, 2010, category 4 tropical cyclone Tomas struck Fiji, resulting in USD $43.6 million in damage. Over 17,000 people were evacuated to shelters, and a state of disaster was declared in the heavily affected Northern and Eastern divisions (Figure 1A).7,8 As part of the post-disaster response, the Fiji Ministry of Health (FMOH) and Fiji Health Sector Improvement Program (FHSIP) initiated a large typhoid vaccination program with funding from the Australian Government Overseas Aid program (Australian Aid) and technical support from WHO-DPS.9 During June through December 2010, 64,015 doses of the typhoid ViPS vaccine were administered to persons ≥ 2 years of age, primarily in cyclone-affected areas that were previously known to be endemic for typhoid; these targeted areas generally consisted of a fraction of a subdivision, with the exception of one subdivision (the island of Taveuni) where all areas were targeted. A small proportion of the vaccine was used in non-cyclone-affected villages experiencing outbreaks or otherwise considered to be at high risk for typhoid. In conjunction with the campaign, the FMOH also conducted limited-scale demonstrations on hand washing in communities where typhoid vaccine was provided and training of facilitators to conduct community-based training on safe water and sanitation practices.
Figure 1.
(A) Path of Cyclone Tomas and storm-affected areas, Fiji, 2010. Cyclone-affected subdivisions are indicated and include all Northern and Eastern subdivisions; one Western subdivision (Ra, indicated in panel B) was in the projected path of the cyclone, resulting in widespread evacuation of the population. Map is adapted from a bulletin of the International Federation of Red Cross and Red Crescent Societies.7 (B) The proportion of the population vaccinated against typhoid by subdivision, Fiji, 2010. Subdivisions discussed in this report are labeled and shaded based on proportion of the population vaccinated: high (black), mid-level (dark grey), low (light grey), or no vaccination (white).
To our knowledge, this is the first large-scale typhoid vaccine intervention conducted in a post-disaster setting and the first documented use of typhoid ViPS vaccine in the Pacific Islands region; the only other documented use of any typhoid vaccine in the region was a 1957 study that showed the effectiveness of an older generation acetone-inactivated vaccine in the Kingdom of Tonga.10 We conducted a retrospective evaluation to assess the coverage of the typhoid vaccination campaign, the epidemiology of typhoid in Fiji during 2008–2011, and the change in typhoid incidence before and after the campaign in subpopulations with varying levels of vaccination.
Methods
Study setting.
Fiji has four health system divisions (Northern, Western, Central, and Eastern), which are further divided into 22 subdivisions and 79 medical areas. Over 95% of the population lives in the Northern, Central, and Western divisions, and < 5% live in the Eastern division, which is made up exclusively of small islands. Each division is served by one divisional hospital, and smaller subdivisional hospitals and medical area health centers.
During June–December 2010, the typhoid vaccination campaign was conducted at fixed posts in 16 targeted medical areas that were located in 10 subdivisions across all four divisions. The number of campaign personnel used ranged from 10 to 50 per area, and included nurse vaccinators; village health workers, who worked as assistants and social mobilizers; and drivers. Three multi-terrain vehicles were procured specifically for the vaccination campaign and donated to the FMOH afterward for use in investigating typhoid cases and other outreach activities. Cold chain equipment and facilities from the Expanded Program on Immunization, FMOH were used during the campaign, and technical support was provided by FHSIP and WHO-DPS. During September–December 2010, the typhoid vaccination campaign and information on prevention of typhoid were publicized through daily television and radio advertisements, and distribution of brochures, posters, and bookmarks. Data on vaccine wastage and cost per dose administered were not available.
Assessment of the typhoid vaccination campaign coverage and the proportion of population vaccinated.
We obtained immunization registers and summary reports from WHO-DPS, FHSIP, and FMOH divisional and subdivisional hospitals, and medical area health centers. “Reported vaccination coverage” was calculated by dividing the number of vaccine doses reported to have been administered by the number of persons ≥ 2 years of age who were vaccinated among those targeted for the campaign. Because the campaign targeted specific medical areas and villages within subdivisions, which were the lowest reporting level for which typhoid surveillance data were available, we estimated the proportion of the population that received typhoid vaccine at the subdivision level by dividing the number of persons vaccinated in the subdivision by the respective 2010 subdivision population estimates from FMOH. We retrospectively categorized subdivisions by the proportion of subdivision population vaccinated: 1) “high vaccination” (one subdivision, 92% population vaccinated); 2) “mid-level vaccination” (two subdivisions, 22% or 65% population vaccinated); and 3) “low to no vaccination” (12 subdivisions, 0–9% of the population vaccinated).
Epidemiology of typhoid during 2008–2011.
Laboratory testing for typhoid in Fiji occurs through culture of patient blood and stool specimens at three divisional hospital laboratories (Northern, Western, and Central). Samples from patients at subdivisional hospitals and medical area health centers are sent to the divisional laboratories for culture confirmation; in the Eastern division, samples are sent to the Central division laboratory. We obtained data on culture-confirmed typhoid cases during January 2008 to December 2011 from the national typhoid laboratory surveillance database, managed by the FCCDC, and supplemented these with any additional blood cultures that were positive for Typhi in the records at divisional and subdivisional laboratories. We excluded all samples obtained through screening of asymptomatic case contacts to identify typhoid carriers. A confirmed typhoid case was defined as an infection in a person whose blood, stool, or urine specimen yielded Typhi (“positive culture”). Because detailed patient residence information was not reported, we analyzed confirmed typhoid cases by the division and subdivision of the health facility where the sample was collected. We calculated confirmed typhoid incidence rates for the national, divisional, and subdivisional levels during 2008–2011. For the years 2008–2010, we used the respective annual populations from the FMOH as denominators, and for 2011, we estimated populations based on average annual growth rates during 2008–2010.
Impact of the vaccination campaign on typhoid incidence.
To evaluate the change in typhoid incidence before and after the campaign, we calculated incidence rate ratios (IRRs) and 95% confidence intervals (CIs) for the year post-campaign (2011) versus the average of 2 years pre-campaign (2008–2009). For one subdivision (Nadi) where there were no cases in the pre-campaign period, we used a Monte Carlo simulation of the Fisher's exact test to determine whether there was a statistically significant difference in incidence between the pre- and post-campaign periods. The change in incidence across the subdivision categories of high, mid-level, and low to no vaccination was evaluated.
To account for variation in the number of blood cultures performed over time, we calculated the proportion of all blood cultures that yielded Typhi by subdivision using denominators we collected for individual laboratories. We restricted the analysis to five subdivisions with pre-campaign typhoid incidence > 90 cases per 100,000 per year (Taveuni, Cakaudrove, Ra, Macuata, and Bua). Because the campaigns in these subdivisions took place in August 2010, we restricted the analysis to blood cultures performed during the months of January–August of 2008–2011, to permit the inclusion of more years of pre-campaign data and to account for disease seasonality. We used log-binomial regression to estimate risk ratios (RRs) and 95% CIs for the proportion of blood cultures that were positive for Typhi during post-campaign (2011) versus pre-campaign (2008–2010) years.
To estimate cases of clinical illness compatible with typhoid that may have been missed by surveillance because of a lack of laboratory testing, we conducted a retrospective review of medical records of all hospital admissions among persons ≥ 2 years of age at four subdivisional hospitals in the Northern (Bua, Cakaudrove, Taveuni) and Western (Nadroga) divisions during pre-campaign (January–March 2009) and post-campaign (January–March 2011) time periods; patient visits meeting defined diagnostic criteria were selected for abstraction (Supplemental Table 1). We excluded cases with a clear alternative diagnosis and defined a suspected typhoid case-patient as a person ≥ 2 years of age with 3 or more days of fever and one or more of the following gastrointestinal symptoms: diarrhea, constipation, or abdominal pain. Because the medical records of some confirmed typhoid cases were unavailable, we aggregated suspected and confirmed cases (regardless of whether they met the suspected typhoid case definition) from our investigation with other confirmed cases reported to surveillance for the relevant time period and subdivision, and compared case counts for pre- and post-campaign periods.
The assessment was approved by the FMOH Ethics Board and Centers for Disease Control and Prevention (CDC) as public health surveillance and program evaluation activity.
Results
Assessment of the typhoid vaccination campaign coverage and the proportion of population vaccinated.
During June–December 2010, a total of 64,015 doses of ViPS vaccine were administered to a target population of 65,294 persons ≥ 2 years of age (Table 1). The mean reported administrative vaccination coverage was 98% (range 84–115%) in individual targeted areas (Table 1). The subdivisions with the largest target populations and the highest proportions of the total population vaccinated were in the Northern (Taveuni, Cakaudrove) and Western divisions (Ra); these subdivisions were designated as having “high vaccination” (Taveuni, 92% population vaccinated) or “mid-level vaccination” (Cakaudrove, 65% population vaccinated; Ra, 22% population vaccinated) (Table 1 and Figure 1B). Overall, 7% of the total Fijian population was vaccinated during the campaign (Table 1).
Table 1.
Reported coverage of the target population ≥ 2 years of age and the proportion of entire population vaccinated during the typhoid vaccination campaign, by division and subdivision—Fiji, June–December 2010
| Division (calculation) | Subdivision | Vaccinated (A) | Target (B) | Reported coverage of target population (A/B*100) | Population* (C) | Proportion of population vaccinated (A/C*100) |
|---|---|---|---|---|---|---|
| Northern | Macuata | − | − | − | 74,441 | − |
| Cakaudrove | 22,601 | 24,006 | 94% | 34,812 | 65% | |
| Bua† | 981 | 894 | 110% | 15,375 | 6% | |
| Taveuni | 15,022 | 14,982 | 100% | 16,292 | 92% | |
| Total | 38,604 | 39,882 | 97% | 140,920 | 27% | |
| Western | Lautoka/Yasawa | 1,733 | 1,729 | 100% | 104,525 | 2% |
| Nadi | − | − | − | 87,156 | − | |
| Ba | 2,947 | 3,311 | 89% | 58,538 | 5% | |
| Nadroga | 4,911 | 5,011 | 98% | 53,778 | 9% | |
| Tavua | − | − | − | 27,165 | − | |
| Ra | 6,696 | 5,817 | 115% | 29,968 | 22% | |
| Total | 16,287 | 15,868 | 103% | 361,130 | 5% | |
| Central | Suva | 4,531 | 4,756 | 95% | 206,379 | 2% |
| Serua/Namosi | 709 | 841 | 84% | 25,346 | 3% | |
| Rewa | − | − | − | 78,007 | − | |
| Tailevu | − | − | − | 21,370 | − | |
| Naitasiri | − | − | − | 19,682 | − | |
| Total | 5,240 | 5,597 | 94% | 350,784 | 1% | |
| Eastern | Lomaiviti | 3,884 | 3,947 | 98% | 17,349 | 22% |
| Kadavu | − | − | − | 10,327 | − | |
| Lakeba | − | − | − | 7,338 | − | |
| Lomaloma | − | − | − | 3,266 | − | |
| Rotuma | − | − | − | 1,910 | − | |
| Total | 3,884 | 3,947 | 98% | 40,190 | 10% | |
| Fiji | Total | 64,015 | 65,294 | 98% | 893,024 | 7% |
“–”designates no vaccination campaign.
2010 populations from Public Health Information System (PHIS), Fiji Ministry of Health.
The population targeted for vaccination in Bua was residents of Kubulau medical area (110% coverage). Although Kubulau is formally a part of Bua subdivision, the population has better access to the hospital in Cakuadrove, and is more likely to be recorded as “Cakaudrove” cases in laboratory-based surveillance.
Epidemiology of typhoid during 2008–2011.
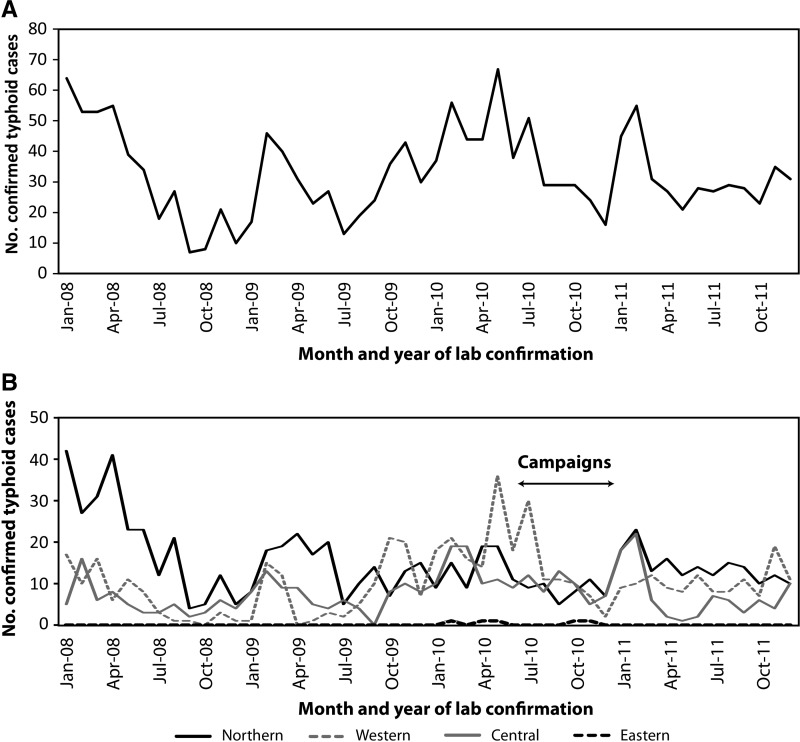
A total of 1,582 confirmed typhoid cases were reported during 2008–2011. Of the 1,560 confirmed cases with information on the source of the clinical specimen, 1,449 (93%) were confirmed by isolation of Typhi from blood, 108 (7%) from stool, and 3 from other sterile sites (< 1%). The median age of confirmed case-patients was 24 years (range < 1–95 years); 879 (56%) were male, and 1,496 (95%) were indigenous Fijian. Nationally, the typhoid incidence in 2008, 2009, and 2011 was 44, 40, and 42 cases per 100,000 persons per year, respectively; in 2010, an incidence of 52 cases per 100,000 persons per year was reported, which was 24% higher than the average of the other years (Table 2 ). In all years, there was a seasonal distribution of cases with highest incidence occurring during January through July (Figure 2A).
Table 2.
Annual confirmed typhoid incidence during January 1, 2008–December 31, 2011 and incidence risk ratios post-campaign (2011) versus pre-campaign (2008–2009 average), by division and subdivision—Fiji
| No. confirmed typhoid cases | Annual confirmed typhoid incidence (cases/100,000/year) | IRR (2011 vs. 2008–9) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Division | Subdivision | 2008 | 2009 | 2010 | 2011 | Total | 2008 | 2009 | 2010 | 2011 | Estimate | 95% CI |
| Northern | Taveuni* | 46 | 63 | 27 | 13 | 149 | 291.5 | 387.6 | 165.7 | 78.5 | 0.23 | 0.13–0.41 |
| Cakaudrove† | 101 | 15‡ | 34 | 34 | 184 | 289.2 | 43.7‡ | 97.7 | 97.8 | 0.58 | 0.40–0.86 | |
| Macuata§ | 70 | 67 | 41 | 68 | 246 | 96.0 | 92.8 | 55.1 | 90.4 | 0.96 | 0.72–1.28 | |
| Bua§ | 29 | 23 | 30 | 54 | 136 | 178.1 | 145.7 | 195.1 | 361.5 | 2.23 | 1.52–3.26 | |
| Total | 246 | 168 | 132 | 169 | 715 | 175.9 | 121.3 | 93.7 | 119.4 | 0.80 | 0.67–0.96 | |
| Western | Ra† | 58 | 56 | 68 | 14 | 196 | 190.5 | 208.0 | 226.9 | 46.8 | 0.24 | 0.14–0.41 |
| Lautoka/Yasawa§ | 13 | 28 | 44 | 33 | 118 | 13.7 | 29.3 | 42.1 | 30.1 | 1.40 | 0.88–2.21 | |
| Nadi§ | 0 | 0 | 10 | 37 | 47 | − | − | 11.5 | 41.6 | −¶ | −¶ | |
| Ba§ | 0 | 4 | 18 | 9 | 31 | − | 7.0 | 30.7 | 15.7 | 4.68 | 1.44–15.20 | |
| Nadroga§ | 3 | 4 | 47 | 28 | 82 | 5.7 | 7.6 | 87.4 | 51.7 | 7.78 | 3.40–17.82 | |
| Tavua§ | 3 | 5 | 7 | 3 | 18 | 10.2 | 16.9 | 25.8 | 11.5 | 0.85 | 0.22–3.19 | |
| Total | 77 | 97 | 194 | 124 | 492 | 21.8 | 27.8 | 53.7 | 33.9 | 1.37 | 1.09–1.72 | |
| Central | Suva§ | 63 | 73 | 110 | 58 | 304 | 31.5 | 36.1 | 53.3 | 27.7 | 0.82 | 0.60–1.11 |
| Serua/Namosi§ | 0 | 1 | 3 | 2 | 6 | 0.0 | 3.9 | 11.8 | 8.0 | 4.08 | 0.37–45.02 | |
| Rewa§ | 2 | 3 | 12 | 12 | 29 | 2.6 | 3.9 | 15.4 | 15.2 | 4.67 | 1.65–13.27 | |
| Tailevu§ | 0 | 2 | 4 | 7 | 13 | − | 9.0 | 18.7 | 33.6 | 7.53 | 1.57–36.26 | |
| Naitasiri§ | 1 | 5 | 4 | 8 | 18 | 4.9 | 19.5 | 20.3 | 40.1 | 3.08 | 1.07–8.88 | |
| Total | 66 | 84 | 133 | 87 | 370 | 19.1 | 23.8 | 37.9 | 24.5 | 1.14 | 0.88–1.49 | |
| Eastern | Total | 0 | 0 | 5 | 0 | 5 | − | − | − | − | − | − |
| Fiji | Total | 389 | 349 | 464 | 380 | 1,582 | 44.3 | 39.6 | 52.0 | 42.1 | 1.01 | 0.89–1.14 |
IRR = incidence rate ratio (bold IRRs are statistically significant results); 95% CI = 95% confidence interval; “–” = value not calculated.
High vaccination (92% of the population vaccinated).
Mid-level vaccination (65% or 22% of the population vaccinated).
Low to no vaccination (0–9% of the population vaccinated).
The number of blood cultures tested in Cakaudrove in 2009 was reduced, accounting for only 37% of the average for 2008, 2010, and 2011 (Table 3).
Statistically significant increase (P < 0.001) by Monte Carlo estimation of a Fisher exact test for Nadi.
Figure 2.
Number of confirmed typhoid fever cases reported to the laboratory surveillance system by month and year of laboratory confirmation—Fiji, January 1, 2008–December 31, 2011. (A) Nationwide. (B) By division.
At the divisional level during 2008–2011, the overall annual incidence of confirmed typhoid was highest in the Northern division (94–176 cases per 100,000 persons per year), followed by the Western (22–54 cases per 100,000 persons per year) and Central divisions (19–38 per 100,000 persons per year) (Table 2). Although most confirmed cases were reported from the Northern division during 2008–2009, from late 2009 through 2010, confirmed typhoid cases from the Central and Western divisions reached and exceeded, respectively, those from the Northern division (Table 2 and Figure 2B). Only five confirmed cases were reported in the Eastern division in 2010 (Table 2); this division was excluded from further analysis because of limited sample collection and case reporting.
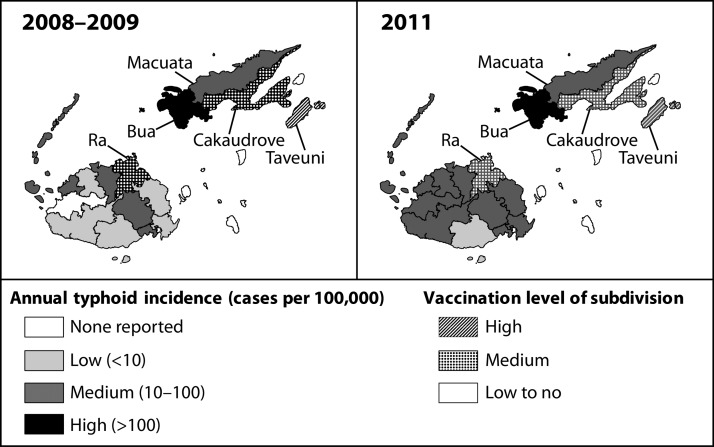
At the subdivision level during 2008–2009, the incidence was “high” in three Northern subdivisions (Taveuni, Cakaudrove, and Bua) and one Western subdivision (Ra); the average incidence in the remaining Northern subdivision (Macuata) was 94 cases per 100,000 per year (Table 2 and Figure 3). The increase in incidence in 2010 in the Western subdivision of Nadroga (87 cases per 100,000 persons) was attributed to an outbreak (Table 2).
Figure 3.
Map of annual confirmed typhoid fever incidence pre-campaign (January 1, 2008–December 31, 2009) and post-campaign (January 1–December 31, 2011), by subdivision—Fiji.
Impact of the vaccination campaign on typhoid incidence.
Nationwide, the average annual confirmed typhoid incidence during the pre-campaign years of 2008–2009, was the same as the post-campaign year of 2011 (42 cases per 100,000 persons per year) (Table 2). However, at the division level, there was a statistically significant decrease in typhoid incidence in the Northern division during the post-campaign year compared with pre-campaign years (IRR = 0.80, 95% CI 0.67–0.96), a statistically significant increase in the Western division (IRR = 1.37, 95% CI 1.09–1.72), and no significant change in the Central division (Table 2). At the subdivision level, there was a statistically significant decrease during the post-campaign year compared with pre-campaign years in the subdivision with high vaccination (Taveuni, IRR = 0.23, 95% CI 0.13–0.41), and statistically significant decreases in the subdivisions with mid-level vaccination (Ra, IRR = 0.24, 95% CI 0.14–0.41; Cakaudrove, IRR = 0.58, 95% CI 0.40–0.86) (Table 2 and Figure 3). In subdivisions with low to no vaccination, confirmed typhoid incidence in 2011 significantly increased in seven subdivisions (IRR range: 2.23–7.78), and did not significantly change in five others (Table 2 and Figure 3).
Among the subdivisions with a typhoid incidence of > 90 cases per 100,000 per year before the campaign, the proportion of cultures positive for Typhi during January–August 2011 compared with the same months during 2008–2010 decreased significantly in the subdivision with high vaccination (Taveuni, RR = 0.27, 95% CI 0.13–0.57) and one subdivision with mid-level vaccination (Ra, RR = 0.48, 95% CI 0.26–0.89); there was no statistically significant change in the other subdivision with mid-level vaccination (Cakaudrove, RR = 1.36, 95% CI 0.93–1.99). In the two subdivisions with low to no vaccination, the proportion of cultures positive for Typhi increased significantly (Macuata, RR = 1.56, 95% CI 1.12–2.16; Bua RR = 1.79, 95% CI 1.19–2.69) (Table 3).
Table 3.
Risk ratios (RRs) and 95% confidence intervals (CIs) for the proportion of cultures positive for Typhi post-campaign (2011) vs. pre-campaign (2008–2010) in subdivisions with high pre-campaign incidence—Fiji, January 1–August 31, 2008–2011
| No. cultures yielding Typhi | No. cultures | % Cultures yielding Typhi | RR (2011 vs. 2008–10) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Subdivision | 2008 | 2009 | 2010 | 2011 | 2008 | 2009 | 2010 | 2011 | 2008 | 2009 | 2010 | 2011 | Estimate | 95% CI |
| Taveuni* | 38 | 49 | 22 | 7 | 237 | 279 | 196 | 168 | 16 | 18 | 11 | 4 | 0.27 | 0.13–0.57 |
| Ra†‡ | 20 | 17 | 52 | 10 | 69 | 77 | 207 | 82 | 29 | 22 | 25 | 12 | 0.48 | 0.26–0.89 |
| Cakaudrove† | 72 | 5 | 23 | 24 | 196 | 50 | 241 | 86 | 37 | 10 | 10 | 28 | 1.36 | 0.93–1.99 |
| Macuata§ | 54 | 43 | 31 | 48 | 2,464 | 2,606 | 2,059 | 1,719 | 2 | 2 | 2 | 3 | 1.56 | 1.12–2.16 |
| Bua§¶ | 16 | 18 | 16 | 36 | 0 | 122 | 74 | 116 | − | 15 | 22 | 31 | 1.79 | 1.19–2.69 |
RR = risk ratio (bold RRs are statistically significant results); 95% CI = 95% confidence interval; “–” = value not calculated.
High vaccination (92% of the population vaccinated).
Mid-level vaccination (65% or 22% of the population vaccinated).
Low to no vaccination (0–9% of the population vaccinated).
Missing January–March 2008 data.
Missing January–August 2008 data.
In an inpatient medical records review at four subdivisional hospitals, case counts for suspected and confirmed typhoid decreased by 69% in 2011 compared with 2009 in the subdivision with high vaccination (Taveuni), increased by 15% in a subdivision with mid-level vaccination (Cakaudrove), and either increased by 93% or remained unchanged in the two subdivisions with low to no vaccination (Supplemental Tables 2 and 3).
Discussion
After substantial damage to water and sanitation infrastructure, a typhoid vaccination campaign was successfully conducted ∼6 months after a cyclone struck a typhoid-endemic area of Fiji. To our knowledge, this is the first documented use of a currently licensed typhoid vaccine in the Pacific Islands region and the first evaluation of the programmatic use of typhoid ViPS vaccine in a post-disaster setting. The campaign goal was to prevent major typhoid outbreaks; our evaluation found that typhoid incidence declined post-campaign in areas with high or mid-level vaccination, and increased or remained unchanged in areas with low or no vaccination. These changes in typhoid incidence occurred in the absence of major improvements to water and sanitation infrastructure.
The sharpest decline in typhoid incidence was observed in the one subdivision with high vaccination, Taveuni (92% vaccinated), where the entire population was targeted by the campaign. Taveuni, which was one of the subdivisions worst affected by the cyclone, had the greatest post-campaign decreases in typhoid incidence, proportion of blood cultures positive for Typhi, and number of hospitalized cases. The impact of vaccination might have been enhanced because Taveuni is an island, with more limited population exchange with unvaccinated areas than in other parts of the country.
In subdivisions with mid-level vaccination, Ra and Cakaudrove, residents lived both in areas that were targeted and not targeted during the vaccination campaign. In Ra subdivision (22% vaccinated), there were significant post-campaign decreases in incidence and in the proportion of blood cultures positive for Typhi. It is not known whether endemic typhoid in this subdivision was primarily limited to the targeted area, resulting in the campaign being particularly effective despite the relatively low proportion of the population that was vaccinated overall. A similar outcome has been observed in China where targeting of high incidence areas resulted in decreased typhoid incidence.11 In Cakaudrove subdivision (65% vaccinated), incidence decreased post-campaign, but the proportion of cultures positive for Typhi and the number of hospitalized cases was similar before and after the campaign. It is not possible for us to assess whether ongoing typhoid in Cakaudrove is occurring primarily within unvaccinated areas, or whether the local epidemiology or targeting strategy made the campaign less effective than in other subdivisions.
Confirmed annual typhoid incidence in Fiji during 2008–2009 and 2011 was unchanged (42 cases per 100,000 persons per year), and was slightly increased relative to the national incidence estimated in a 2004 and 2005 laboratory-based Salmonella surveillance project (4 and 33 cases per 100,000 persons per year, respectively).5 This is not surprising given that the campaign did not target the entire population. The highest confirmed typhoid incidence for 2008–2011 was observed in the Northern division, similar to the published study during 2004–2005.5 The increase in typhoid incidence in the Western division appears to have started in 2010, with six outbreaks reported during 2010–2013 (WHO-DPS, unpublished data). More than 95% of all confirmed typhoid cases in Fiji occur among indigenous Fijians, who account for only 56% of the population; the disproportionate concentration of typhoid among this group has previously been reported.5 Mass gatherings and communal ceremonies involving the sharing of kava, a local beverage, are believed to contribute to the spread of typhoid outbreaks, but a comprehensive evaluation of risk factors has not been performed.
The rationale for the campaign was that an increase in typhoid incidence might occur in Fiji after the extensive flooding, population displacement, and damage to water and sanitation systems.9 Flooding has been reported as a risk factor for Salmonella enterica serovar Paratyphi A infections,12 and typhoid outbreaks after cyclones and hurricanes have been documented in the literature.13–15 In the Pacific, unpublished reports from WHO and South Pacific Commission have linked typhoid outbreaks with other cyclones in Fiji and Samoa (Samuela J, unpublished data; Souares Y, unpublished data). Despite their common transmission routes, reports are relatively few compared with the extensive documentation of post-disaster outbreaks of non-specific diarrhea and cholera.16,17 It remains unknown whether the lack of documentation of typhoid outbreaks after disasters is caused by a difference in typhoid epidemiology or the difficulty in diagnosing typhoid.
Our evaluation has several limitations. First, we could not evaluate the role that the hygiene and educational interventions may have played in reducing typhoid incidence. Second, there was a potential for misclassification bias in subdivisions with mid-level vaccination because they contained residents both from areas that were targeted for vaccination and areas that were not targeted; individual vaccination status and area of residence were unknown. For this reason, the impact of the vaccination campaign on the targeted population may not have been accurately estimated. Third, the clinical case definition for suspected typhoid cases used in the medical records review was not specific and may have resulted in the inclusion of patients with other febrile illnesses, such as dengue or leptospirosis. Finally, the incidence rates presented underestimate true typhoid incidence in Fiji because of low testing rates (≤ 50% of suspected typhoid cases were tested), low test sensitivity (a single blood culture will only detect ≤ 50% of true typhoid infections) and limited representativeness of the surveillance system, especially in rural and remote areas (e.g., the Eastern Division). To enable a rigorous vaccine impact assessment in any future vaccination strategy, we recommend including a systematic evaluation component in the planning stage of the campaign.
After a cyclone in a typhoid-endemic area, a targeted typhoid vaccination campaign was feasible and was followed by a decrease in cases in some vaccinated areas from pre-cyclone levels. Further evaluation of typhoid vaccination in other post-disaster-endemic areas is needed to document the usefulness of vaccine in these settings. Recently, data have become available from typhoid vaccination programs in Thailand, China, Vietnam, and India, and large-scale vaccine demonstration projects in five Asian countries that have confirmed the effectiveness of vaccines for reducing typhoid in endemic, high-incidence settings.11,18–21 Typhoid vaccination can also be considered in other high-incidence settings, along with comprehensive typhoid control measures.2
Supplementary Material
ACKNOWLEDGMENTS
We acknowledge Australian Aid for funding the 2010 typhoid vaccination campaign and other typhoid-related interventions in Fiji, and Sanofi-Pasteur's donation of 10,000 vaccine doses. We thank the following individuals and their organizations for contributions to the evaluation, laboratory testing and support, and report writing: Dr. Josefa Koroivueta, Dr. Frances Bingwor, Dr. Eka Buadromo, Dr. Salanieta Saketa, Dr. Pem Namgyal, Dr. Dassi, Dr. Tiko, Dr. Jaoji Vulibeci, Dr. Ana Maisema, Dr. Setareki Sowane, Dr. Orisi Matatolu, Dr. Ifereimi Waqainabiete, Dr. Jamesa Tudravu, Dr. Shiromani, Dr. Chaochi, Dr. Waqa, Apaitia Vakacegu, Dr. Prem Singh, Uraia Rabuatoka, Josese Limaono, Jioji Rasila, Shalini Singh, and James Pruckler. This work could not have been completed without the assistance of WHO-DPS staff, Pam Borg, Emma Gibbons, Lynette Evans, who provided administrative and logistical support, and local staff specifically hired for project fieldwork and data entry: Dr. Kitione Rawali, Laisani Ravalita, Anaseini Naivalu, Unaseni Basalala, Samu Waqaniburotu, and Losalini Senicaucau. Electronic map files were graciously provided by Fiji Land Information Systems (FLIS), FMOH, and the United Nations Office for the Coordination of Humanitarian Affairs.
Disclaimer: The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention or other organizations.
Footnotes
Financial support: Funding was provided by the Centers for Disease Control and Prevention and World Health Organization.
Authors' addresses: Heather M. Scobie, Eric Mintz, Kathleen A. Wannemuehler, Terri B. Hyde, and Kashmira Date, Global Immunization Division, Epidemic Intelligence Service, and Division of Foodborne, Waterborne and Environmental Diseases, Centers for Disease Control and Prevention, Atlanta, GA, E-mails: vih8@cdc.gov, edm1@cdc.gov, kpw9@cdc.gov, tkh4@cdc.gov, and gln7@cdc.gov. Eric Nilles, Division of Pacific Technical Support, World Health Organization, Suva, Fiji, E-mail: nillese@wpro.who.int. Mike Kama and Akanisi Dawainavesi, Fiji Centre for Communicable Disease Control, Suva, Fiji, E-mails: mnkama02@gmail.com and dawainavesia@wpro.who.int. Jacob L. Kool, WHO Country Liaison Office, Port Vila, Vanuatu, E-mail: koolj@wpro.who.int. Sheetalpreet Singh, Health Information Unit, Ministry of Health, Suva, Fiji, E-mail: sheetal.nagra@gmail.com. Samuel Korovou, Ministry of Health, Labasa, Fiji, E-mail: skorokou@yahoo.uk. Kylie Jenkins, Fiji Health Sector Improvement Program, Ministry of Health, Suva, Fiji, E-mail: kylie.jenkins@fhssp.org.fj.
References
- 1.Crump JA, Luby SP, Mintz ED. The global burden of typhoid fever. Bull World Health Organ. 2004;82:346–353. [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization Typhoid vaccines: WHO position paper. Wkly Epidemiol Rec. 2008;83:49–59. [PubMed] [Google Scholar]
- 3.Crump JA, Mintz ED. Global trends in typhoid and paratyphoid fever. Clin Infect Dis. 2010;50:241–246. doi: 10.1086/649541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fiji Bureau of Statistics Fiji Statistics at a Glance. 2007. http://www.statsfiji.gov.fj/ Available at. Accessed July 8, 2011.
- 5.Dunn J, Pryor J, Saketa S, Delai W, Buadromo E, Kishore K, Naidu S, Greene S, Varma J, Chiller T. Laboratory-based Salmonella surveillance in Fiji, 2004–2005. Pac Health Dialog. 2005;12:53–59. [PubMed] [Google Scholar]
- 6.UNICEF . Fiji Statistics. 2010. http://www.unicef.org/infobycountry/fiji_statistics.html Available at. Accessed June 19, 2013. [Google Scholar]
- 7.International Federation of Red Cross and Red Crescent Societies . Fiji: Cyclone Tomas. 2010. http://www.pacificdisaster.net/pdnadmin/data/original/FJI_TC_Tomas_2010_IFRC_bulletin1.pdf Information Bulletin No. 1, March 17, 2010. GLIDE no. TC-2010-000054-FJI. Available at. Accessed August 16, 2013. [Google Scholar]
- 8.United Nations Office for the Coordination of Humanitarian Affairs . Fiji: Tropical Cyclone Tomas. 2010. http://reliefweb.int/sites/reliefweb.int/files/resources/470D59B7BE1686028525770300728D74-Full_Report.pdf Situation Report No. 6, April 12, 2010. Available at. Accessed August 16, 2013. [Google Scholar]
- 9.Jenkins Kylie. Post Cyclone Tomas Support to Typhoid Fever Control in Fiji: Report to AusAID: Fiji Health Sector Improvement Program. 2011. [Google Scholar]
- 10.Tapa S, Cvjetanovic B. Controlled field trial on the effectiveness of one and two doses of acetone-inactivated and dried typhoid vaccine. Bull World Health Organ. 1975;52:75–79. [PMC free article] [PubMed] [Google Scholar]
- 11.DeRoeck D, Ochiai RL, Yang J, Anh DD, Alag V, Clemens JD. Typhoid vaccination: the Asian experience. Expert Rev Vaccines. 2008;7:547–560. doi: 10.1586/14760584.7.5.547. [DOI] [PubMed] [Google Scholar]
- 12.Vollaard AM, Ali S, van Asten HA, Widjaja S, Visser LG, Surjadi C, van Dissel JT. Risk factors for typhoid and paratyphoid fever in Jakarta, Indonesia. JAMA. 2004;291:2607–2615. doi: 10.1001/jama.291.21.2607. [DOI] [PubMed] [Google Scholar]
- 13.Bissell RA. Delayed-impact infectious disease after a natural disaster. J Emerg Med. 1983;1:59–66. doi: 10.1016/0736-4679(83)90010-0. [DOI] [PubMed] [Google Scholar]
- 14.Toole MJ. In: Communicable disease and disease control. The Public Health Consequences of Disasters. Noji EK, editor. New York: Oxford University Press; 1997. pp. 83–100. [Google Scholar]
- 15.Polonsky J, Luquero F, Francois G, Rousseau C, Caleo G, Ciglenecki I, Delacre C, Siddiqui MR, Terzian M, Verhenne L, Porten K, Checchi F. Public health surveillance after the 2010 Haiti earthquake: the experience of medecins sans frontieres. PLoS Curr. 2013;7:5. doi: 10.1371/currents.dis.6aec18e84816c055b8c2a06456811c7a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kouadio IK, Aljunid S, Kamigaki T, Hammad K, Oshitani H. Infectious diseases following natural disasters: prevention and control measures. Expert Rev Anti Infect Ther. 2012;10:95–104. doi: 10.1586/eri.11.155. [DOI] [PubMed] [Google Scholar]
- 17.Spiegel PB, Le P, Ververs M-T, Salama P. Occurrence and overlap of natural disasters, complex emergencies and epidemics during the past decade (1995–2004) Confl Health. 2007;1:2. doi: 10.1186/1752-1505-1-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ochiai RL, Acosta CJ, Agtini M, Bhattacharya SK, Bhutta ZA, Do CG, Dong B, Chen X, Stanton B, Kaljee L, Nyamete A, Galindo CM, von Seidlein L, DeRoeck D, Jodar L, Clemens J. The use of typhoid vaccines in Asia: the DOMI experience. Clin Infect Dis. 2007;45:S34–S38. doi: 10.1086/518144. [DOI] [PubMed] [Google Scholar]
- 19.Ochiai RL, Acosta CJ, Danovaro-Holliday MC, Baiqing D, Bhattacharya SK, Agtini MD, Bhutta ZA, Canh do G, Ali M, Shin S, Wain J, Page AL, Albert MJ, Farrar J, Abu-Elyazeed R, Pang T, Galindo CM, von Seidlein L, Clemens JD. Domi Typhoid Study Group A study of typhoid fever in five Asian countries: disease burden and implications for controls. Bull World Health Organ. 2008;86:260–268. doi: 10.2471/BLT.06.039818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bodhidatta L, Taylor DN, Thisyakorn U, Echeverria P. Control of typhoid fever in Bangkok, Thailand, by annual immunization of schoolchildren with parenteral typhoid vaccine. Rev Infect Dis. 1987;9:841–845. doi: 10.1093/clinids/9.4.841. [DOI] [PubMed] [Google Scholar]
- 21.Yang HH, Wu CG, Xie GZ, Gu QW, Wang BR, Wang LY, Wang HF, Ding ZS, Yang Y, Tan WS, Wang WY, Wang XC, Qin M, Wang JH, Tang HA, Jiang XM, Li YH, Wang ML, Zhang SL, Li GL. Efficacy trial of Vi polysaccharide vaccine against typhoid fever in south-western China. Bull World Health Organ. 2001;79:625–631. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.