Abstract
Cases of melioidosis and glanders are rare in the United States, but the etiologic agents of each disease (Burkholderia pseudomallei and Burkholderia mallei, respectively) are classified as Tier 1 select agents because of concerns about their potential use as bioterrorism agents. A rapid, highly sensitive, and portable assay for clinical laboratories and field use is required. Our laboratory has further evaluated a latex agglutination assay for its ability to identify B. pseudomallei and B. mallei isolates. This assay uses a monoclonal antibody that specifically recognizes the capsular polysaccharide produced by B. pseudomallei and B. mallei, but is absent in closely related Burkholderia species. A total of 110 B. pseudomallei and B. mallei were tested, and 36 closely related Burkholderia species. The latex agglutination assay was positive for 109 of 110 (99.1% sensitivity) B. pseudomallei and B. mallei isolates tested.
The Gram-negative bacteria Burkholderia pseudomallei and Burkholderia mallei are the etiologic agents of melioidosis and glanders, respectively. Melioidosis typically causes disease in humans and is endemic to Southeast Asia and northern Australia, whereas glanders is a disease most commonly seen in horses, mules, and donkeys in the Middle East, Africa, and India. Both bacteria are of concern because of their potential use as bioterrorism agents. The rarity of both diseases in the United States and other countries where the diseases are not endemic could delay proper diagnosis by physicians and laboratory staff during a bioterrorism event caused by responders' unfamiliarity with the diseases. Diagnostic confirmation of both diseases relies on microbiological culture. However, B. pseudomallei is commonly dismissed as a culture contaminant, and along with B. mallei may be misidentified by standard identification methods including API 20NE and other automated bacterial identification systems. Therefore, rapid diagnostic tools for bacterial identification are essential to provide an effective response by public health authorities in the event of a bioterrorism incident. The goal of this study was to evaluate a rapid assay for the identification of B. pseudomallei and B. mallei.
Latex agglutination assays have been used successfully in Southeast Asia and northern Australia to identify B. pseudomallei isolates and closely related species.1 Assays such as these are based on the use of monoclonal antibodies (MAbs) that recognize an exopolysaccharide present on the cell surface of B. pseudomallei and B. mallei.2–4 Nonetheless, these assays are normally evaluated with limited strains isolated from endemic areas, and its use for strains isolated from all other countries has not been adequately evaluated.2–4
Our laboratory has evaluated a rapid latex agglutination assay developed by Mahidol University (Bangkok, Thailand) using an inclusivity panel of 110 geographically and genetically diverse B. pseudomallei and B. mallei isolates, stored at The Centers for Disease Control and Prevention (CDC), Atlanta, GA. We also evaluated the assay with an exclusivity panel of 36 closely related Burkholderia species, which included agents that have not been previously tested by this or similar antigen detection assays. We focused on the closest phylogenetic relatives of B. pseudomallei including other Burkholderia species that have been associated with human disease such as Burkholderia oklahomensis and Burkholderia gladioli. Burkholderia oklahomensis has been reported to cause infections associated with deep tissue wounds,5,6 whereas B. gladioli can cause a range of diseases from fatal foodborne illness,7 to sepsis in newborns,8 and lung infections in patients with cystic fibrosis.9 This latex agglutination assay could be valuable in correctly identifying select agents and excluding closely related Burkholderia species that cause similar disease in humans.
The antibody-latex suspension based on the 4B11 monoclonal antibody was prepared by Mahidol University as previously described.2–4 The assay was performed also as previously described with slight modification.1 Briefly, isolates were subcultured twice on trypticase soy agar (TSA) containing 5% sheep's blood and incubated for 18–24 hours at 37°C. Single colonies were picked and added to 10 μL of the latex suspension on a ringed glass microscope slide. The glass slide containing the latex suspension with the suspended colony was subjected to gentle rocking for 2 minutes after which time the reaction was recorded as either positive (agglutination) or negative (no agglutination) (Figure 1). Burkholderia pseudomallei K96243 was used as the positive control in all experiments and Burkholderia thailandensis E264 (American Type Culture Collection [ATCC] type strain 700388) was used as the negative control each time isolates were tested, and all tests were performed in triplicate.
Figure 1.
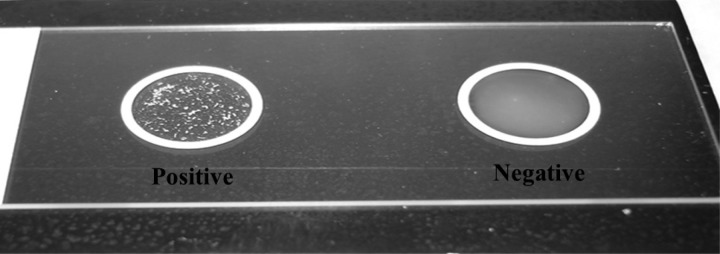
Burkholderia pseudomallei and Burkholderia thailandensis positive and negative reactions after incubation with the latex agglutination reagent.
Under our assay conditions, the latex agglutination test was positive on 109 of 110 (99.1% sensitivity) isolates tested on the inclusivity panel. This number included a total of 77 B. pseudomallei isolates, of which 76 (98.7% sensitivity) were positive and 33 B. mallei isolates of which all were positive (100% sensitivity) (Table 1). The B. pseudomallei isolate that tested negative in our assay, CDC2721686 (MSHR1655), was isolated from a patient with a chronic B. pseudomallei infection after being first diagnosed with melioidosis in 2000.10 This rare B. pseudomallei isolate was from the patient in an ongoing study consisting of 815 patients that were culture-positive for melioidosis in Darwin, Australia. Since 1989, this patient is the only survivor from this study to remain chronically colonized by B. pseudomallei. CDC2721686 (MSHR1655) was isolated 37 months after the initial melioidosis diagnosis and has undergone major genome-wide rearrangements resulting in a loss of function in many genes that are important in pathogenesis. Of particular interest to this study is the loss of function of wcbR, which encodes an essential fatty acid synthase required in capsular polysaccharide synthesis.11 We believe this would explain the inability of the latex agglutination assay to correctly identify this isolate. In addition to testing negative in our assay, when subjected to standard biochemical tests for the identification of B. pseudomallei, the isolate was non-motile, but otherwise normal under our assay conditions. When the latex agglutination assay was tested against an exclusivity panel of closely related Burkholderia species, 35 of 36 (97.2% specificity) yielded negative results (Table 2). The closely related Burkholderia that tested positive in our assay is a rare variant of B. thailandensis (CDC3015869, TX DOH) that has been previously described as containing B. pseudomallei capsule genes.12
Table 1.
Burkholderia pseudomallei and Burkholderia mallei inclusivity panel
| Species | Strain identifier | Location of origin | Result |
|---|---|---|---|
| Burkholderia pseudomallei | CDC2721620 | France | Positive |
| Burkholderia pseudomallei | CDC2721628 | Madagascar | Positive |
| Burkholderia pseudomallei | CDC2721639 | Kenya | Positive |
| Burkholderia pseudomallei | CDC0022138 | Thailand | Positive |
| Burkholderia pseudomallei | Bp92; CDC2721623 | Australia | Positive |
| Burkholderia pseudomallei | Human 88; PHLS 45 | Thailand | Positive |
| Burkholderia pseudomallei | Bp104; CDC2721624 | Australia | Positive |
| Burkholderia pseudomallei | CDC2721635; PHLS 36 | Singapore | Positive |
| Burkholderia pseudomallei | Bp73; Ln31348 | Malaysia | Positive |
| Burkholderia pseudomallei | PHLS 208 | Ecuador | Positive |
| Burkholderia pseudomallei | CDC2721102; F5013 | Georgia | Positive |
| Burkholderia pseudomallei | BpG9709; CDC0032026 | India | Positive |
| Burkholderia pseudomallei | PHLS 19; CDC2721625 | Singapore | Positive |
| Burkholderia pseudomallei | CDC2721676 | USA | Positive |
| Burkholderia pseudomallei | Bp2889; SID2889 | Bangladesh | Positive |
| Burkholderia pseudomallei | CDC2721630; 7605 | France | Positive |
| Burkholderia pseudomallei | Bp68; CDC2721641 | Fiji | Positive |
| Burkholderia pseudomallei | PHLS 17; CDC2721619 | Indonesia | Positive |
| Burkholderia pseudomallei | PHLS 38 | Singapore | Positive |
| Burkholderia pseudomallei | 1106a; CDC0022030 | Thailand | Positive |
| Burkholderia pseudomallei | Bp53; CDC2721633 | Thailand | Positive |
| Burkholderia pseudomallei | Bp24; CDC2721620 | France | Positive |
| Burkholderia pseudomallei | BpG9313; CDC0032029 | USA | Positive |
| Burkholderia pseudomallei | CDC2721162 | Australia | Positive |
| Burkholderia pseudomallei | CDC2721114; G6715 | USA (Ohio) | Positive |
| Burkholderia pseudomallei | CDC2721626 | Thailand | Positive |
| Burkholderia pseudomallei | CDC0032028 | USA (Ohio) | Positive |
| Burkholderia pseudomallei | CDC721096; 81A442 | USA (New York) | Positive |
| Burkholderia pseudomallei | CDC0032024 | Puerto Rico | Positive |
| Burkholderia pseudomallei | Thai NE Human 99 | Thailand | Positive |
| Burkholderia pseudomallei | CDC1029240 | USA (Oregon) | Positive |
| Burkholderia pseudomallei | CDC2721617 | Australia | Positive |
| Burkholderia pseudomallei | Bp14; CDC2721618 | Philippines | Positive |
| Burkholderia pseudomallei | BpH1442; CDC0032025 | USA (Delaware) | Positive |
| Burkholderia pseudomallei | MSHR640;CDC8724880 | Australia | Positive |
| Burkholderia pseudomallei | 465a; CDC8724601 | Australia | Positive |
| Burkholderia pseudomallei | MSHR99; CDC8724881 | Australia | Positive |
| Burkholderia pseudomallei | CDC1756207 | Australia | Positive |
| Burkholderia pseudomallei | CDC8724890 | Australia | Positive |
| Burkholderia pseudomallei | #711; CDC2721675 | USA (Washington) | Positive |
| Burkholderia pseudomallei | CDC2734678; 620 | Thailand | Positive |
| Burkholderia pseudomallei | CDC8724908 | Australia | Positive |
| Burkholderia pseudomallei | CDC8724883 | Australia | Positive |
| Burkholderia pseudomallei | CDC2734694; PM40 | Thailand | Positive |
| Burkholderia pseudomallei | PM26; CDC2734683 | Thailand | Positive |
| Burkholderia pseudomallei | PHLS 75 | Malaysia | Positive |
| Burkholderia pseudomallei | CDC8724901 | Australia | Positive |
| Burkholderia pseudomallei | PM115; CDC2734709 | Thailand | Positive |
| Burkholderia pseudomallei | CDC2721825 | Thailand | Positive |
| Burkholderia pseudomallei | Bp40 | Singapore | Positive |
| Burkholderia pseudomallei | CDC8724894 | Australia | Positive |
| Burkholderia pseudomallei | CDC2734661; SA923 | Thailand | Positive |
| Burkholderia pseudomallei | PHLS 79 | Malaysia | Positive |
| Burkholderia pseudomallei | BpH1689; CDC0032024 | USA (Florida) | Positive |
| Burkholderia pseudomallei | CDC2721184 | Ecuador | Positive |
| Burkholderia pseudomallei | CDC2721634 | Thailand | Positive |
| Burkholderia pseudomallei | CDC1756205 | Australia | Positive |
| Burkholderia pseudomallei | CDC8724905 | Australia | Positive |
| Burkholderia pseudomallei | CDC0022203 | Thailand | Positive |
| Burkholderia pseudomallei | CDC2721637 | Pakistan | Positive |
| Burkholderia pseudomallei | CDC8724896 | Thailand | Positive |
| Burkholderia pseudomallei | CDC8724889 | Australia | Positive |
| Burkholderia pseudomallei | CDC8724898 | Australia | Positive |
| Burkholderia pseudomallei | CDC2721686 | Australia | Negative |
| Burkholderia pseudomallei | CDC8724899 | Thailand | Positive |
| Burkholderia pseudomallei | CDC8724882 | Australia | Positive |
| Burkholderia pseudomallei | CDC8724900 | Australia | Positive |
| Burkholderia pseudomallei | CDC8724892 | Australia | Positive |
| Burkholderia pseudomallei | CDC8724893 | Australia | Positive |
| Burkholderia pseudomallei | CDC2721761 | Vietnam | Positive |
| Burkholderia pseudomallei | CDC8724885 | USA | Positive |
| Burkholderia pseudomallei | CDC0022358 | Thailand | Positive |
| Burkholderia pseudomallei | CDC8724877 | Australia | Positive |
| Burkholderia pseudomallei | CDC1756206 | Australia | Positive |
| Burkholderia pseudomallei | CDC8724895 | Australia | Positive |
| Burkholderia pseudomallei | CDC8724903 | Australia | Positive |
| Burkholderia pseudomallei | CDC8724878 | Australia | Positive |
| Burkholderia mallei | CDC2721277 | China | Positive |
| Burkholderia mallei | CDC2734821 | China | Positive |
| Burkholderia mallei | CDC2721278 | USA (New Mexico) | Positive |
| Burkholderia mallei | CDC0031066 | India | Positive |
| Burkholderia mallei | CDC2734315 | Turkey | Positive |
| Burkholderia mallei | CDC0031065 | Turkey | Positive |
| Burkholderia mallei | CDC2734302 | Turkey | Positive |
| Burkholderia mallei | CDC2734301 | Turkey | Positive |
| Burkholderia mallei | CDC0031304 | USA (Maryland) | Positive |
| Burkholderia mallei | CDC2721273 | Burma | Positive |
| Burkholderia mallei | KC 235; CDC2721274 | USA (Maryland) | Positive |
| Burkholderia mallei | KC0248; CDC4017733 | USA | Positive |
| Burkholderia mallei | CDC2721279 | USA (New York) | Positive |
| Burkholderia mallei | CDC2721280 | Iran | Positive |
| Burkholderia mallei | CDC8724847 | Unknown | Positive |
| Burkholderia mallei | CDC2734305 | India | Positive |
| Burkholderia mallei | CDC2734303; GB10 | India | Positive |
| Burkholderia mallei | CDC8724837 | Turkey | Positive |
| Burkholderia mallei | CDC8724838 | Turkey | Positive |
| Burkholderia mallei | CDC8724839 | Turkey | Positive |
| Burkholderia mallei | CDC8724841 | Turkey | Positive |
| Burkholderia mallei | CDC2734300 | Turkey | Positive |
| Burkholderia mallei | CDC2734301 | Turkey | Positive |
| Burkholderia mallei | CDC2734317 | India | Positive |
| Burkholderia mallei | CDC2721275 | China | Positive |
| Burkholderia mallei | CDC2734299 | Hungary | Positive |
| Burkholderia mallei | CDC2734311 | England | Positive |
| Burkholderia mallei | CDC0031063 | Hungary | Positive |
| Burkholderia mallei | CDC0031064 | India | Positive |
| Burkholderia mallei | CDC2721276 | USA | Positive |
| Burkholderia mallei | CDC2721648 | Burma | Positive |
| Burkholderia mallei | CDC2734312 | Turkey | Positive |
| Burkholderia mallei | CDC2721280 | Iran | Positive |
Table 2.
Burkholderia exclusivity panel
| Species | Strain identifier | Location of origin | Result |
|---|---|---|---|
| Burkholderia thailandensis | CDC3015869 | USA (Texas) | Positive |
| Burkholderia thailandensis | CDC2721621 | France | Negative |
| Burkholderia thailandensis | CDC2721627 | Thailand | Negative |
| Burkholderia thailandensis | CDC2721121 | USA (Louisiana) | Negative |
| Burkholderia thailandensis | CDC2721643 | Unknown | Negative |
| Burkholderia thailandensis | CDC2721701 | Thailand | Negative |
| Burkholderia thailandensis | CDC2721723 | Thailand | Negative |
| Burkholderia thailandensis | CDC2721744 | Malaysia | Negative |
| Burkholderia humptydooensis | CDC2721687 | Australia | Negative |
| Burkholderia oklahomensis | CDC4002358 | USA (Oklahoma) | Negative |
| Burkholderia oklahomensis | CDC4021865 | USA (Oklahoma) | Negative |
| Burkholderia oklahomensis | CDC4021866 | USA (Oklahoma) | Negative |
| Burkholderia vietnamiensis | CDC2734483 | Vietnam | Negative |
| Burkholderia pyrrocinia | ATCC 15958 | Unknown | Negative |
| Burkholderia caledonica | CDC8724197 | United Kingdom | Negative |
| Burkholderia caribensis | CDC8724200 | Martinique | Negative |
| Burkholderia ambifaria | CDC8724201 | USA (Wisconsin) | Negative |
| Burkholderia anthina | CDC8724199 | USA (Tennessee) | Negative |
| Burkholderia cocovenenans | CDC2734715 | Indonesia | Negative |
| Burkholderia ferrariae | CDC8724209 | Brazil | Negative |
| Burkholderia hydrophila | CDC2721759 | Thailand | Negative |
| Burkholderia fungorum | ATCC BAA-463 | Unknown | Negative |
| Burkholderia glathei | CDC2734719 | Germany | Negative |
| Burkholderia graminis | CDC2734716 | France | Negative |
| Burkholderia hospita | CDC8724207 | Belgium | Negative |
| Burkholderia kururiensis | CDC2734717 | China | Negative |
| Burkholderia nodosa | CDC8724205 | Brazil | Negative |
| Burkholderia phenazinium | ATCC 33666 | Unknown | Negative |
| Burkholderia phenoliruptrix | CDC8724203 | USA | Negative |
| Burkholderia phymatum | CDC8724208 | French Guiana | Negative |
| Burkholderia phytofirmans | CDC8724204 | Germany | Negative |
| Burkholderia sacchari | CDC8724202 | Brazil | Negative |
| Burkholderia silvatlantica | ATCC BAA-1244 | Brazil | Negative |
| Burkholderia rhizoxinica | DSM19002 | Germany | Negative |
| Burkholderia endofungorum | DSM19003 | Germany | Negative |
| Burkholderia gladioli | CDC3027208 | USA (California) | Negative |
Rapid diagnostic assays, such as the one we have evaluated, would have the most impact in clinical laboratories. This would allow for early identification of suspect isolates and thus on-site diagnosis instead of needing to submit samples to regional laboratories that would delay results. This assay does have several advantages over the current reference level testing. This assay is simple, does not require extra equipment, and can easily be performed. However, the extent to which this assay or similar antigen detection assays can be used on patient samples is yet to be determined.
Footnotes
Authors' addresses: Brea D. Duval, Mindy G. Elrod, Jay E. Gee, and Alex R. Hoffmaster, Centers for Disease Control and Prevention, Atlanta, GA, E-mails: BDuval@cdc.gov, MGElrod@cdc.gov, JGee1@cdc.gov, and AHoffmaster@cdc.gov. Narisara Chantratita, Sarunporn Tandhavanant, and Direk Limmathurotsakul, Mahidol University, Bangkok, Thailand, E-mails: Narisara@tropmedres.ac, Sarunporn@tropmedres.ac, and Direk@tropmedres.ac.
References
- 1.Amornchai P, Chierakul W, Wuthiekanun V, Mahakhunkijcharoen Y, Phetsouvanh R, Currie BJ, Newton PN, van Vinh Chau N, Wongratanacheewin S, Day NP, Peacock SJ. Accuracy of Burkholderia pseudomallei identification using the API 20NE system and a latex agglutination test. J Clin Microbiol. 2007;45:3774–3776. doi: 10.1128/JCM.00935-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anuntagool N, Naigowit P, Petkanchanapong V, Aramsri P, Panichakul T, Sirisinha S. Monoclonal antibody-based rapid identification of Burkholderia pseudomallei in blood culture fluid from patients with community-acquired septicaemia. J Med Microbiol. 2000;49:1075–1078. doi: 10.1099/0022-1317-49-12-1075. [DOI] [PubMed] [Google Scholar]
- 3.Samosornsuk N, Lulitanond A, Saenla N, Anuntagool N, Wongratanacheewin S, Sirisinha S. Short report: evaluation of a monoclonal antibody-based latex agglutination test for rapid diagnosis of septicemic melioidosis. Am J Trop Med Hyg. 1999;61:735–737. doi: 10.4269/ajtmh.1999.61.735. [DOI] [PubMed] [Google Scholar]
- 4.Wuthiekanun V, Anuntagool N, White NJ, Sirisinha S. Short report: a rapid method for the differentiation of Burkholderia pseudomallei and Burkholderia thailandensis. Am J Trop Med Hyg. 2002;66:759–761. doi: 10.4269/ajtmh.2002.66.759. [DOI] [PubMed] [Google Scholar]
- 5.McCormick JB, Weaver RE, Hayes PS, Boyce JM, Feldman RA. Wound infection by an indigenous Pseudomonas pseudomallei-like organism isolated from the soil: case report and epidemiologic study. J Infect Dis. 1977;135:103–107. doi: 10.1093/infdis/135.1.103. [DOI] [PubMed] [Google Scholar]
- 6.Nussbaum JJ, Hull DS, Carter MJ. Pseudomonas pseudomallei in an anopthalmic orbit. Arch Ophthalmol. 1980;98:1224–1225. doi: 10.1001/archopht.1980.01020040076008. [DOI] [PubMed] [Google Scholar]
- 7.Meng Z, Li Z, Jin J, Zhang Y, Liu X, Yiang X, Ren H. Studies on fermented corn flour poisoning in rural areas of China. I. Epidemiology, clinical manifestations, and pathology. Biomed Environ Sci. 1988;1:101–104. [PubMed] [Google Scholar]
- 8.Dursun A, Zenciroglu A, Karagol BS, Hakan N, Okumus N, Gol N, Tanir G. Burkholderia gladioli sepsis in newborns. Eur J Pediatr. 2012;171:1503–1509. doi: 10.1007/s00431-012-1756-y. [DOI] [PubMed] [Google Scholar]
- 9.Ross JP, Holland SM, Gill VJ, DeCarlo ES, Gallin JI. Severe Burkholderia (Pseudomonas) gladioli infection in chronic granulomatous disease: report of two successfully treated cases. Clin Infect Dis. 1995;21:1291–1293. doi: 10.1093/clinids/21.5.1291. [DOI] [PubMed] [Google Scholar]
- 10.Price EP, Sarovich DS, Mayo M, Tuanyok A, Drees KP, Kaestli M, Beckstrom-Sternberg SM, Babic-Sternberg JS, Kidd TJ, Bell SC, Keim P, Pearson T, Currie BJ. Within-host evolution of Burkholderia pseudomallei over a twelve-year chronic carriage infection. MBio. 2013;4:e00388. doi: 10.1128/mBio.00388-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cuccui J, Milne TS, Harmer N, George AJ, Harding SV, Dean RE, Scott AE, Sarkar-Tyson M, Wren BW, Titball RW, Prior JL. Characterization of the Burkholderia pseudomallei K96243 capsular polysaccharide I coding region. Infect Immun. 2012;80:1209–1221. doi: 10.1128/IAI.05805-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sim BM, Chantratita N, Ooi WF, Nandi T, Tewhey R, Wuthiekanun V, Thaipadungpanit J, Tumapa S, Ariyaratne P, Sung WK, Sem XH, Chua HH, Ramnarayanan K, Lin CH, Liu Y, Feil EJ, Glass MB, Tan G, Peacock SJ, Tan P. Genomic acquisition of a capsular polysaccharide virulence cluster by non-pathogenic Burkholderia isolates. Genome Biol. 2010;11:R89. doi: 10.1186/gb-2010-11-8-r89. [DOI] [PMC free article] [PubMed] [Google Scholar]


