Abstract
Plague is an often fatal, primarily flea-borne rodent-associated zoonosis caused by Yersinia pestis. We sought to identify risk factors for plague by comparing villages with and without a history of human plague cases within a model-defined plague focus in the West Nile Region of Uganda. Although rat (Rattus rattus) abundance was similar inside huts within case and control villages, contact rates between rats and humans (as measured by reported rat bites) and host-seeking flea loads were higher in case villages. In addition, compared with persons in control villages, persons in case villages more often reported sleeping on reed or straw mats, storing food in huts where persons sleep, owning dogs and allowing them into huts where persons sleep, storing garbage inside or near huts, and cooking in huts where persons sleep. Compared with persons in case villages, persons in control villages more commonly reported replacing thatch roofing, and growing coffee, tomatoes, onions, and melons in agricultural plots adjacent to their homesteads. Rodent and flea control practices, knowledge of plague, distance to clinics, and most care-seeking practices were similar between persons in case villages and persons in control villages. Our findings reinforce existing plague prevention recommendations and point to potentially advantageous local interventions.
Introduction
Plague, which is caused by Yersinia pestis, is a primarily flea-borne rodent-associated zoonosis that was the cause of three major historical pandemics that claimed millions of human lives.1 Although in modern times human plague cases still occur sporadically, improved sanitation has limited the scale of epidemics to focal outbreaks.2 Furthermore, advances in diagnostics and access to appropriate antibiotic therapy have reduced case-fatality rates.3 Despite the decrease in human plague cases, plague bacteria continue to circulate in enzootic hosts and their fleas within plague-endemic regions. Thus, the threat of human infections is still an appreciable concern in disease-endemic countries because of the high fatality rate of the pathogen for untreated cases and its epidemic potential.4
Humans are most at risk for exposure to plague bacteria during epizootics when rodent hosts die in large numbers, forcing their potentially infectious fleas to abandon their dying hosts.1 As rodent host numbers decrease with the progression of the epizootic, fleas will occasionally take a blood meal from humans, thus increasing the risk of human plague infections. Epizootics are most likely to occur when rodent and flea numbers are increased;5–7 thus, plague prevention strategies often focus on controlling flea vector and rodent host populations. In addition to the use of insecticides to reduce fleas on and off of hosts, prevention recommendations often include reducing food and harborage for rodents in the home environment.4,8,9 Furthermore, because of the rapid clinical progression of plague in humans, educating the public and health care providers of signs of plague and the need to seek care immediately is advised.9
In recent decades, most human plague cases have been reported from east and central Africa and Madagascar.10 During 2004–2009, the Democratic Republic of Congo accounted for 64% of the annual reported incidence of plague from the African region. All cases were reported from the Orientale Province, which borders the West Nile Region of northwestern Uganda.10 To aid in better targeting plague prevention resources, recent research efforts in Uganda have sought to define when and where humans are most at risk for plague in the far eastern edge of this plague focus.11–14
During August 1999–July 2011, a total of 2,409 suspect plague cases were reported from the West Nile Region of Uganda; most cases occurred during September–December, a time period that corresponds with the primary rainy season.13 Modeling of inter-annual variation showed that annual plague case counts were negatively associated with dry season rainfall (December–February) and positively associated with rainfall immediately preceding the plague season.13 Spatial risk modeling has demonstrated that in the West Nile Region, plague risk is higher above 1,300 meters above sea level than below this value. Furthermore, covariates included in these models suggested that localities that are generally wetter, but with discontinuous rainfall, pose an increased risk for plague compared with drier areas.11,12,14
Although existing spatial models performed well in broadly defining the plague focus, there were many villages within the focus where human plague cases had not been reported by clinics during approximately a decade of surveillance. Such an observation raised the question of whether these disparities in case counts among villages within the risk area were attributable to differences in access to care, care-seeking behavior or knowledge of plague, agricultural or food storage practices, rodent and vector control strategies, or fine-scale ecologic differences (e.g., differences in host and flea community structure). In this study, we sought to identify risk factors for plague by comparing each of these categories between villages of similar population size situated within the model-defined risk area that had or had not reported human plague cases.
Materials and Methods
Description of study site.
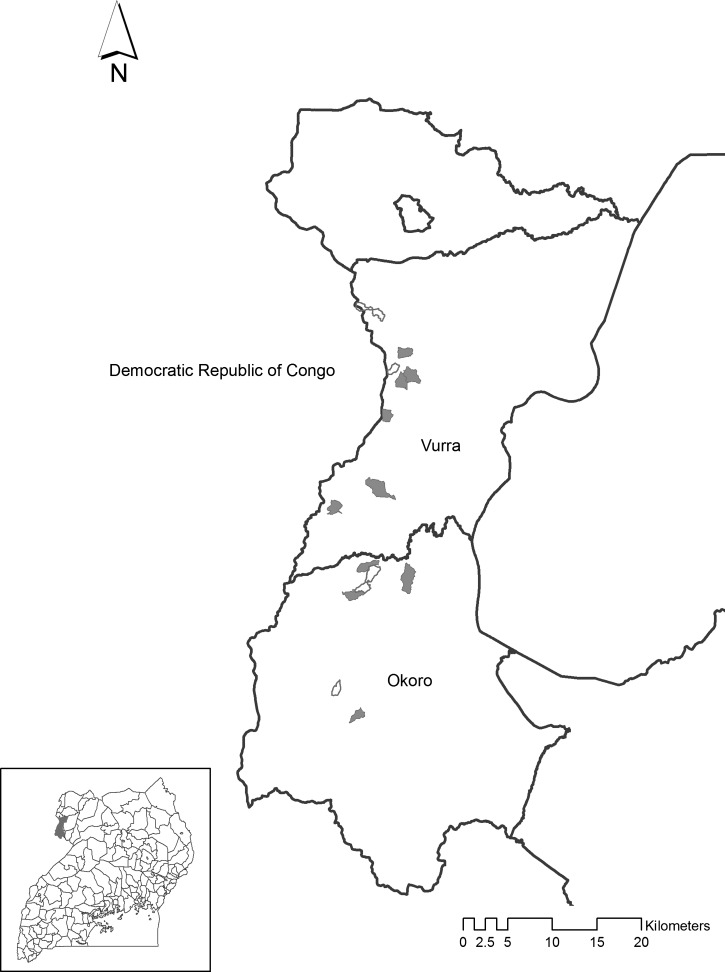
Our study was conducted in the plague-endemic counties of Vurra and Okorro, situated in Arua and Zombo Districts, respectively, within the West Nile Region of northwestern Uganda (Figure 1). Throughout the two districts, approximately 90% of the population resides in rural areas, with close to 60% of those persons living in Ugandan government–defined poverty; more than two-thirds rely on subsistence farming (i.e., use of traditional seed strains, livestock breeds, hand tools, and indigenous technical knowledge) to make a living.15 Villagers typically reside in homesteads comprised of extended families living in multiple earthen structures (huts) with thatch roofs that are surrounded by small agricultural plots or other vegetation.
Figure 1.
Locations of case (shaded) and control (unshaded) villages within Vurra and Okoro Counties, West Nile Region, Uganda. County locations within Uganda are shown in the inset.
Vurra and Okorro counties straddle the Rift Valley escarpment, resulting in markedly different ecologic conditions above and below the escarpment. Lower elevation sites are typically warmer and drier and have sandier soils than sites above the escarpment.11,13,16 Previous studies showed that human plague cases are more common above the escarpment than below.11,14 Correspondingly, flea species diversity is significantly higher above the escarpment within the plague focus, compared with lower elevation sites outside the focus, and this has been hypothesized to be important for enzootic maintenance of Y. pestis.17
Selection of case and control villages.
Ten case villages and five control villages were selected from within areas that were classified by geographic information system–based statistical models as posing an increased risk for plague.11 In other words, regardless of case or control status, based on remotely sensed landscape level features, all villages enrolled in the study were believed to be ecologically conducive for plague activity. Ascertainment of village plague case histories has been described.11,14 In brief, a retrospective review of clinic log books from 31 local health facilities was performed to compile a list of suspect plague cases by village during 1999–2007. Beginning in 2000 in Okoro County, a separate standardized reporting form was used and it captured more detailed information about environmental data often associated with plague cases (e.g., reported rodent die-offs). Standard criteria for a plague diagnosis in Uganda are sudden onset of fever, chills, malaise, headache, or prostration accompanied by painful regional lymphadenopathy (bubonic), hematemesis or hematochezia (septicemic), or coughs with hemoptysis (pneumonic). During the 2008–2009 plague season, cases were laboratory confirmed based on Y. pestis specific phage lysis of primary specimens or seroconversion.18 From these retrospective record reviews, a single database was compiled that contained suspect (diagnosed with plague at a health center and treated), probable (diagnosed at health center with plague, treated and also reported a rat fall in village of residence) and laboratory-confirmed cases. For each case, onset date, village of residence, and health center were recorded. A database of control villages was constructed as follows.11 To control for access to care and minimize the likelihood that plague had occurred in a particular village, we visited each of the seven clinics from which laboratory-confirmed cases were reported. From the clinic log books, we extracted the name of the first village to appear before or after the plague case that was not represented on the list of villages with a history of plague. For each case and control village, perimeters were mapped using handheld global positioning system units.
Nine of the ten case villages enrolled in our study reported at least one laboratory-confirmed case in 2008 and also reported at least four probable or suspect cases during 1999–2007. The one remaining case village that did not report a laboratory-confirmed case reported 73 suspected or probable cases during 1999–2007. None of the selected villages reported plague cases in 2011, the year before initiation of this study. Cases and controls were selected to have similar risk coverage and housing density. Specifically, because the previous plague risk model was based on gridded data and each village contained some gridded cells that were not considered risk, we calculated the proportion of gridded cells within each village that was classified as having increased risk.11 In addition, we digitized hut locations using WorldView imagery to approximate population size in each village. Before enrollment in the study, similarities between case and control villages with respect to the proportion of each village classified by the model as posing an increased plague risk and with respect to the number of huts in villages were confirmed by using Mann-Whitney U tests.
Sampling sessions.
Because previous work showed that human plague cases occur seasonally and are often associated with rainfall, we selected our sampling periods to occur within each of the four seasons.13 Session one (June 27–July 11, 2012) was conducted during the interval season that is generally cool with variable rainfall. Session two (September 25–October 9, 2012) was conducted during the cool, primary rainy season when most yearly rainfall occurs. Session 3 (January 7–21, 2013) was conducted during the hot, dry season. Session 4 (March 18–April 27, 2013) was conducted during the secondary rainy season, which is generally warm and rainy, but rainfall is typically less than during the primary rainy season.
Permission to work in these villages was obtained from the village chairman and individual homeowners before beginning the study. All protocols were reviewed by the Science and Ethics Committee of the Uganda Virus Research Institute, the Uganda National Council for Science and Technology, and the Institutional Animal Care and Use Committee (Protocol no. 12-008) of the Division of Vector-Borne Diseases of the U.S. Centers for Disease Control and Prevention. The study was determined exempt from human studies research by the Institutional Review Board of the U.S. Centers for Disease Control and Prevention (Protocol no. 6246.0).
Description of questionnaire.
A standardized questionnaire was administered in local languages at each household at the commencement of each trapping session by research team members who were fluent in these languages. Responses were provided by the self-identified head of household. Topics addressed in the survey included household demographics, sleeping practices, crops grown in plots adjacent to homesteads, home maintenance, livestock and pet ownership, rodent and flea control strategies, access to health care, and knowledge of plague.
Description of small mammal and flea collections.
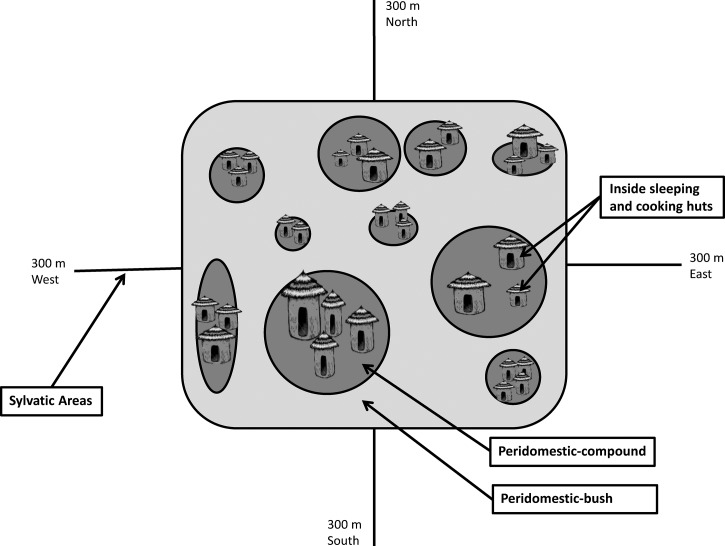
For each trapping session and each village, Sherman (model 3310A; H.B. Sherman Trap Company, Tallahassee, FL) and Tomahawk (model TLT102; Tomahawk Live Trap Company, Tomahawk, WI) traps were baited with equal portions of maize, ground nuts, and dried fish and all traps were operable from shortly before dusk to shortly after dawn for a single night per session. Traps were set in four locations designated as inside, peridomestic-compound, peridomestic-bush, and sylvatic (Figure 2). The numbers and locations of traps set per location and session are described below. Collectively, over the four trapping sessions, the numbers of trap nights for all villages combined were as follows: 4,800 inside, 3,600 each for peridomestic-compound and peridomestic-bush, and 7,200 for sylvatic locations.
Figure 2.
Schematic of small mammal and flea trapping locations, West Nile Region, Uganda.
Within each village for each session, 10 homesteads were randomly selected. Within each homestead, two Sherman and two Tomahawk traps each were placed inside one sleeping and one cooking hut. This yielded a total of 80 trap nights inside huts per village per session. A sleeping hut was defined as a hut lacking a fire pit or burning stove where family members sleep. A cooking hut was defined by the presence of a fire pit or burning stove used for cooking foods; residents may or may not also use such huts for sleeping. Also inside of each hut, two modified Kilonzo flea traps were placed in each hut to capture host-seeking fleas. The modified Kilonzo pan traps have been described.19 In brief, the trap consists of a shallow pan containing water and detergent with a flashlight suspended over the pan.
Six rodent traps (three Sherman and three Tomahawk traps) were placed within five meters of the sleeping and cooking huts, a setting referred to as peridomestic-compound and that typically represents bare soil. An additional three Sherman and three Tomahawk traps were placed just outside the homestead perimeter (within five meters of the homestead edge). Such areas contained some form of vegetation, generally crops or uncultivated vegetation, and define the peridomestic-bush setting. This arrangement yielded a total of 60 trap nights per peridomestic subcategory per village per trap session. Orienting from the center of the village, and defining the sylvatic region, one Tomahawk and one Sherman trap were placed every 20 meters for 300 meters from the edge of the village in each of the four cardinal directions. This arrangement yielded a total of 120 trap nights in sylvatic settings per village per session.
Upon collection, small mammals were anesthetized by using halothane, combed for ectoparasites, and identified to genus or species based on morphologic features (e.g., length of body, tail, ear, and hind foot, and weight).20 For hosts that could not be identified to species in the field based on morphologic characteristics, only genus level identification was provided. Finally, each individual received an ear tag with a unique identification number. Upon recovery from anesthesia, animals were released at the site of capture. Collected fleas were stored at ambient temperature in 70% ethanol and later identified to species according to published taxonomic keys.21–24
Statistical analysis.
Responses to survey questions were compared between cases and controls by using Fisher's exact tests. Because of the high number of households that were repeated in session four, and because most questions referred to on-going practices and were not expected to change among sessions, this session was not included in the analysis of questionnaire data. Analyses of small mammal and flea data included all four sessions because the recapture rate was extremely low (only seven animals were recaptured between sessions) and because seasonal variation in small mammal abundance is known to occur.25 Host diversity was estimated by using Simpson's index of diversity26 for each site for all sessions combined. This index is described as: 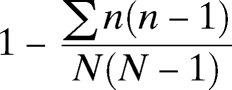 where n is the total number of hosts of a particular species or genus and N is the total number of hosts of all species. The Simpson's index of diversity ranges from 0 to 1; a greater value indicates greater diversity within the sample. Median numbers of hosts captured per site (e.g., per village and trap location within villages), total number of fleas collected, fleas per host, and host diversity were compared between cases and controls by using Wilcoxon rank-sum tests with chi-square approximations. Host and flea abundances between cooking and sleeping huts within the same homestead were compared by using Wilcoxon signed-rank tests. All results were considered significant if P < 0.05. All analyses were performed using JMP version 10 (SAS Institute, Cary, NC).
where n is the total number of hosts of a particular species or genus and N is the total number of hosts of all species. The Simpson's index of diversity ranges from 0 to 1; a greater value indicates greater diversity within the sample. Median numbers of hosts captured per site (e.g., per village and trap location within villages), total number of fleas collected, fleas per host, and host diversity were compared between cases and controls by using Wilcoxon rank-sum tests with chi-square approximations. Host and flea abundances between cooking and sleeping huts within the same homestead were compared by using Wilcoxon signed-rank tests. All results were considered significant if P < 0.05. All analyses were performed using JMP version 10 (SAS Institute, Cary, NC).
Results
Household demographics.
Of 450 attempted interviews (300 cases and 150 controls), 445 were completed (296 cases and 149 controls); for the remainder of households, residents were unavailable to respond to surveys. Comparisons of case and control villages did not show any statistically significant differences with respect to household demographics (i.e., numbers of household members by sex or age class) or numbers of huts within homesteads. All respondents self-identified as head of household and were comprised of 58% females (n = 258) who ranged in age from 13 to 72 years (median = 35 years) and 41% males (n = 183) who ranged in age from 15 to 79 years (median = 35 years). In four instances, the age and sex of the respondent was not indicated. Homesteads included in the survey had a median of 3 huts (range = 1–12 huts), and the median number of persons residing within homesteads was 6 (range = 1–30 persons).
Sleeping practices.
Among 296 responses from case villages and 149 responses from control villages, all respondents reported that a family member slept on at least one of three substrates: reed or straw mat, foam mattress, or stuffed mattress. Reed or straw mats were the most commonly reported sleeping substrate and such mats were used more frequently in case villages (88%) compared with control villages (77%) (χ2 = 10.18, P = 0.001, by Fisher's exact test). Cases and controls reported similar use of foam (47% of cases, 54% of controls) or stuffed mattresses (14% of cases, 19% of controls) (χ2 ≤ 2.68, P ≥ 0.10).
Crops grown in plots adjacent to homesteads and food storage.
Type of crops grown in plots adjacent to homesteads is shown in Table 1. Maize, cassava, and beans were among the most commonly reported crops grown near homesteads and frequencies were similar between homesteads situated in case or control villages. In contrast, compared with cases, controls more frequently reported growing coffee, tomatoes, onions, and melons (Table 1). Respondents from case villages more frequently reported storing crops or food in huts where persons slept (96%) compared with those from control villages (90%) (χ2 = 5.99, P = 0.03). Although less common than storing food in sleeping huts, householders from case villages were more likely to cook in huts where persons slept (11%) and to store food in a granary (22%) compared with those from control villages (4% and 11%, respectively) (χ2 ≥ 5.91–6.05, P < 0.02). Among the 71 respondents who reported storing food in a granary, 89% of cases (56 of 63) and 63% of controls (5 of 8) reported also storing food in sleeping huts.
Table 1.
Crops grown adjacent to case and control homesteads, West Nile Region, Uganda
| Crop | Total (n = 432) | Cases (n = 287) | Controls (n = 14) | P* | |||
|---|---|---|---|---|---|---|---|
| No. present | % | No. present | % | No. present | % | ||
| Maize | 327 | 75.69 | 217 | 75.61 | 110 | 75.86 | 1.00 |
| Cassava | 277 | 64.12 | 189 | 65.85 | 88 | 60.69 | 0.29 |
| Beans | 245 | 56.71 | 172 | 59.93 | 73 | 50.34 | 0.06 |
| Sorghum | 210 | 48.61 | 147 | 51.22 | 63 | 43.45 | 0.15 |
| Potatoes | 190 | 43.98 | 125 | 43.55 | 65 | 44.83 | 0.84 |
| Ground nuts | 161 | 37.27 | 109 | 37.98 | 52 | 35.86 | 0.75 |
| Banana | 163 | 37.73 | 108 | 37.63 | 55 | 37.93 | 1.00 |
| Pumpkin | 149 | 34.49 | 107 | 37.28 | 42 | 28.97 | 0.09 |
| Coffee | 112 | 25.93 | 63 | 21.95 | 49 | 33.79 | 0.01 |
| Millet | 100 | 23.15 | 69 | 24.04 | 31 | 21.38 | 0.63 |
| Tomatoes | 64 | 14.81 | 31 | 10.80 | 33 | 22.76 | < 0.01 |
| Onions | 53 | 12.27 | 25 | 8.71 | 28 | 19.31 | < 0.01 |
| Sweet potato or yam | 44 | 10.19 | 24 | 8.36 | 20 | 13.79 | 0.09 |
| Squash | 16 | 3.70 | 8 | 2.79 | 8 | 5.52 | 0.18 |
| Sugar cane | 11 | 2.55 | 7 | 2.44 | 4 | 2.76 | 1.00 |
| Tobacco | 7 | 1.62 | 7 | 2.44 | 0 | 0.00 | 0.10 |
| Melons | 5 | 1.16 | 0 | 0.00 | 5 | 3.45 | < 0.01 |
| Pineapples | 2 | 0.46 | 1 | 0.35 | 1 | 0.69 | 1.00 |
| Cotton | 1 | 0.23 | 0 | 0.00 | 1 | 0.69 | 0.34 |
By Fisher's exact two-tailed tests.
Livestock and pet ownership.
Livestock ownership was similar between households in case or control villages; 75% (234 of 296) of households in case villages and 75% (112 of 149) of households in control villages reported that they own livestock. Most households reported owning chickens and goats. Pigs, sheep, cattle, and guinea pigs were less common (Table 2). Comparing the types of livestock owned, we found that homesteads in case villages were similar to those in control villages. Cat ownership was similarly uncommon among households in case (3%) and control (6%) villages. However, case village households (17%) more often reported owning dogs than households in control villages (8%) (χ2 = 6.05, P = 0.018). In addition, 6 (12%) of 49 households in case villages that reported owning a dog indicated that they allowed the dog into huts at night where family members were sleeping; none of the 12 households with dogs in control villages reported practicing the same behavior.
Table 2.
Livestock ownership in case and control homesteads, West Nile Region, Uganda
| Type | % of cases (no.) | % of controls (no.) |
|---|---|---|
| Goats | 70 (207) | 64 (96) |
| Sheep | 21 (61) | 21 (31) |
| Pigs | 24 (72) | 28 (42) |
| Cattle | 15 (45) | 17 (26) |
| Chickens | 74 (218) | 70 (104) |
| Guinea pigs | 8 (23) | 5 (7) |
Home maintenance.
Smearing walls and floors of huts with mud was equally common among households in case and control villages (98% of households in case and control villages). When asked the reasons for mud smearing, most responses pertained to a sense of cleanliness and many specified that they believed it reduced fleas in huts. Significantly more households in control villages reported replacing roof thatch (75%) than those in case villages (63%) (χ2 ≥ 5.74, P ≤ 0.01). In response to the question, “Do you keep garbage (non-human waste) in or near your home,” 85% of householders residing in case villages (251 of 296) answered in the affirmative compared with 75% of controls (111 of 149) (χ2 = 6.93, P = 0.01). Among villagers responding yes to the previous question, most (66% of cases, 65% of controls) reported keeping garbage outside sleeping huts. Residents of case villages were more likely to report keeping garbage inside their sleeping huts (31% [77 of 251] compared with controls (19% [21 of 111]) (χ2 = 5.39, P = 0.02), whereas residents of control villages more commonly reported keeping garbage outside the homestead but within the village (12% [13 of 111]) compared with residents of case villages (5% [13 of 251]; χ2 = 4.92, P = 0.04). When asked how garbage is maintained before disposal, responses were similar between cases and controls. Most responses indicated that garbage is stored in the open (78% of cases and 81% of controls). Similar proportions of cases and controls reported leaving the garbage to decompose (63% of 251 cases and 65% of 111 controls). However, a significantly higher proportion of villagers in case villages reported burying garbage (35% [88 of 251]) compared with controls (21% [23 of 111]; χ2 = 7.82, P = 0.01). Relatively few reported burning garbage (15% of cases [38 of 251], 16% of controls [18 of 111]).
Rodent and flea control strategies.
Contact rates between humans and rats appeared to be higher within households situated in case villages compared with controls. In case villages, householders (24%) were more likely than controls (13%) to report that a family member had been bitten by a rat within the past three months (χ2 = 7.36, P = 0.004). Of the 89 households reporting rat bites, 84 (94%) reported that the bite(s) occurred while the injured person was sleeping.
Despite differences in human–rat contact rates between case and control villages, rodent and flea control strategies appeared to be similar between these categories. Approximately 90% of respondents reported practicing some form of rodent controls inside of their homes (88% of cases [n = 261], 89% of controls [n = 132]). Three-fourths (74% of cases [n = 220], 76% of controls [n = 114]) reported using poison (mostly indocid [indomethacin] or rat killer). Approximately 40% reported using traps (41% of cases [n = 120], 39% of controls [n = 58]). Despite these efforts, responses indicated that rodents remain a problem. In response to the question, “What does your household do with live rodents found in or around the house?” none of the respondents reported that they did not have rodents in their homes. Few (7% of cases, 6% of controls) reported doing nothing to control rodents. Nearly two-thirds (65% of cases and 66% of controls) reported killing rodents by using physical means. Nearly half of respondents (43% cases and 39% of controls) reported using chemical compounds to kill rodents. Fewer (9% of cases and 15% of controls) reported some other means of controlling rodents. Often these responses included cats or dogs killing rodents. We note that there were inconsistencies in the reported proportions of respondents using chemical versus physical means to control rodents in and around homes between the two questions asked pertaining to rodent control. Based on the two responses, we estimate that between half to three-fourths of respondents use poisons and approximately 40–65% use physical means to control rodents.
When asked, “How do you or your family members remove rodents, either dead or alive, when found in or around the house?” more than three-fourths of respondents reported using tools to remove rodents (82% of cases [n = 244] and 89% of controls [n = 132]). In contrast, few reported using bare hands (9% of cases [n = 26], 5% of controls [n = 8]) or covered hands (10% of cases [n = 28], 6% of controls [n = 9]) to remove rodents. Although households in case villages trended towards removing rodents with their hands, covered or not, more frequently than controls, the differences were not statistically significant. Nearly half of respondents reported disposing of carcasses by burial (48% for cases and controls [n = 143 and 71], respectively), and the remainder reported that they discard carcasses in the bush (27% of cases [n = 79], 32% of controls [n = 47]) or in pit latrines (25% of cases [n = 74], 22% of controls [n = 33]). Fewer than 5% of respondents reported burning carcasses, feeding them to cats or dogs, or discarding in a rubbish pit. Disposal methods between cases and controls were statistically similar.
Approximately half of households, and at similar frequencies between cases and controls, reported practicing some form of insect control (58% of case households, 51% of controls). When a method of insect control was indicated, most cited smearing mud on floors as the method practiced. Few households cited use of a commercial product to control fleas (4% of cases, 8% of controls). When a product was indicated, it was usually cypermethrin.
Health care-seeking and knowledge of plague.
Villagers in case and control categories reported similar access to transportation (bicycles) (57% of cases [n = 169]; 60% of controls [n = 90]) and reported similar distances from their homes to the health center they are most likely to visit when ill (median 3 km for cases [range = 1–10 km]; median 3 km for controls [range = 1–12 km]). When asked, “If you or a family member were ill and thought the illness was caused by plague, where would you go for treatment first?” most respondents reported seeking care at a health center (85% of cases [n = 252], 89% of controls [n = 132]). Approximately 10% of respondents said they would visit a regional hospital or visit a drug shop. Significantly more respondents from case villages (3% [n = 9]) than control villages (0%) reported seeking care first from a traditional healer (χ2 = 5.67, P = 0.01) (Table 3).
Table 3.
Health care–seeking behavior (“if you or a family member were ill and thought the illness was caused by plague, where would you go for treatment first?”), West Nile Region, Uganda
| Response | % of cases (no.) | % of controls (no.) |
|---|---|---|
| Would not seek treatment/treat self | 0.0 (0) | 0.7 (1) |
| Regional hospital | 8.1 (24) | 12.1 (18) |
| Local drug shop | 9.8 (29) | 8.1 (12) |
| Local health center | 85.1 (252) | 88.6 (132) |
| Traditional healer | 3.0 (9)* | 0 (0) |
| Don't know | 2.0 (6) | 2.0 (3) |
| Other | 1.4 (4) | 0.7 (1) |
Statistically significant difference between cases and controls (P = 0.05).
Responses to questions about knowledge of how persons get sick with plague and the symptoms associated with plague were similar between cases and controls. Approximately one-third of respondents cited fleas as the most common way of catching plague (33% of cases [n = 98]; 34% of controls [n = 51]). Approximately 15% responded that plague was caught by being dirty (13% of cases [n = 29], 18% of controls [n = 27]). Fewer than 5% of respondents cited mosquitoes, bad water, sick animals, or touching infected pets as a means of transmission. Most householders responded that they did not know (41% of cases [n = 121], 36% of controls [n = 54]). Considerably fewer households responded that they did not know the symptoms most suggestive of plague (26% of cases and controls). Responses to the question about symptoms most suggestive of plague were similar between cases and controls with painful swellings (63% of cases [n = 187], 64% of controls [n = 95]) and fever (43% of cases [n = 128] and 50% of controls [n = 75]) as the most commonly cited symptoms. Responses regarding plague treatment were similar between cases and controls; approximately three-fourths of householders responded that antibiotics could cure plague (77% of cases [n = 229] and 72% of controls [n = 107]).
Small mammals and fleas.
During 19,200 trap nights spanning each of the four trapping sessions (June 2012–April 2013), a total of 1,792 small mammals comprised of at least 15 species were captured (Table 4). Together, the four most abundant hosts made up 90% of the total capture: Rattus rattus (48%), Crocidura spp. (28%), Mastomys natalensis (9%), and Arvicanthis niloticus (6%). Collectively, these four are referred to as key host species. Nearly half (48%) of all hosts captured were captured inside huts. Furthermore, small mammal abundance was significantly higher inside cooking huts than in sleeping huts (Z = 10,666, P < 0.001). Thirty-one percent of hosts were captured in the sylvatic setting, and 18% and 3%, respectively, were captured in the peridomestic setting outside or inside the compound. Scaled per 100 trap nights, we found that small mammals were most abundant inside huts (17.92 hosts per 100 trap nights), followed by the peridomestic-bush (9.00 per 100 trap nights), sylvatic setting (7.73 per 100 trap nights), and least abundant in the peridomestic-compound setting (1.51 per 100 trap nights). Host diversity was low inside huts; R. rattus accounted for 95% of the total capture. In contrast, the four most common hosts captured in the peridomestic-compound, peridomestic-bush, and sylvatic settings accounted for only 93%, 87%, and 74% of captures, respectively. Host diversity, as measured with the Simpson's diversity index based on the hosts listed in Table 5 for all trap sites within a village combined, was similar between case (median = 0.67, range = 0.55–0.76) and control villages (median = 0.63, range = 0.50–0.70) (χ2 = 1.50, degrees of freedom [df] = 1, P = 0.22).
Table 4.
Small mammals by capture location for sessions 1–4 combined, West Nile Region, Uganda
| Host | Inside* | Peridomestic compound† | Peridomestic bush† | Sylvatic‡ | ||||
|---|---|---|---|---|---|---|---|---|
| No. (% of total) | No. per 100 trap nights | No. (% of total) | No. per 100 trap nights | No. (% of total) | No. per 100 trap nights | No. (% of total) | No. per 100 trap nights | |
| Rattus rattus | 814 (94.65) | 16.96 | 13 (24.08) | 0.36 | 16 (4.95) | 0.44 | 12 (2.16) | 0.17 |
| Crocidura spp. | 21 (2.44) | 0.44 | 28 (51.85) | 0.78 | 199 (61.61) | 5.53 | 254 (45.77) | 3.53 |
| Mastomys natalensis | 6 (0.70) | 0.13 | 5 (9.26) | 0.14 | 33 (10.22) | 0.92 | 110 (19.82) | 1.53 |
| Arvicanthis niloticus | 7 (0.81) | 0.15 | 4 (7.41) | 0.11 | 48 (14.86) | 1.33 | 45 (8.11) | 0.63 |
| Aethomys kaiseri | 0 (0.00) | 0.00 | 0 (0.00) | 0.00 | 11 (3.41) | 0.31 | 26 (4.68) | 0.36 |
| Tatera valida | 0 (0.00) | 0.00 | 0 (0.00) | 0.00 | 3 (0.94) | 0.08 | 25 (4.50) | 0.35 |
| Lophuromys sikapusi | 0 (0.00) | 0.00 | 0 (0.00) | 0.00 | 1 (0.31) | 0.03 | 19 (3.42) | 0.26 |
| Unidentified | 8 (0.93) | 0.16 | 1 (1.85) | 0.03 | 2 (0.61) | 0.06 | 9 (1.62) | 0.13 |
| Aethomys hindei | 0 (0.00) | 0.00 | 1 (1.85) | 0.03 | 2 (0.61) | 0.06 | 11 (1.98) | 0.15 |
| Taterillus emini | 0 (0.00) | 0.00 | 1 (1.85) | 0.03 | 1 (0.31) | 0.03 | 10 (1.80) | 0.14 |
| Lophuromys flavopunctatus | 0 (0.00) | 0.00 | 0 (0.00) | 0.00 | 1 (0.31) | 0.03 | 9 (1.62) | 0.13 |
| Mus spp. | 3 (0.35) | 0.06 | 1 (1.85) | 0.03 | 6 (1.86) | 0.18 | 9 (1.62) | 0.13 |
| Praomys jacksoni | 0 (0.00) | 0.00 | 0 (0.00) | 0.00 | 0 (0.00) | 0.00 | 9 (1.62) | 0.13 |
| Cricetomys gambianus | 1 (0.12) | 0.02 | 0 (0.00) | 0.00 | 0 (0.00) | 0.00 | 3 (0.55) | 0.04 |
| Dasymys imcomtus | 0 (0.00) | 0.00 | 0 (0.00) | 0.00 | 0 (0.00) | 0.00 | 3 (0.55) | 0.04 |
| Lemniscomys striatus | 0 (0.00) | 0.00 | 0 (0.00) | 0.00 | 0 (0.00) | 0.00 | 1 (0.18) | 0.01 |
| Total | 860 (100.00) | 17.92 | 54 (100.00) | 1.51 | 323 (100.00) | 9.00 | 555 (100.00) | 7.73 |
Based on 4,800 trap nights.
Based on 3,600 trap nights.
Based on 7,200 trap nights.
Table 5.
Fleas collected from pan traps and hosts during sessions 1–4, West Nile Region, Uganda*
| Host species | No. hosts or pan traps | No. fleas (no. fleas/host) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Xc | Dly | Ccab | Xb | St | Eg | Cb | Unk | Dlo | Cf | Xn | Tp | Total | ||
| Pan trap | 539 | 48 | 1 | 0 | 4 | 1 | 138 | 0 | 7 | 0 | 851 | 0 | 14 | 1,064 |
| Rattus rattus | 855 | 475 (0.56) | 34 (0.04) | 9 (0.01) | 74 (0.08) | 2 (0.00) | 0 (0.00) | 1 (0.00) | 7 (0.01) | 1 (0.00) | 4 (0.00) | 0 (0.00) | 1 (0.00) | 608 (0.71) |
| Crocidura spp | 502 | 132 (0.26) | 39 (0.08) | 14 (0.03) | 0 (0.00) | 70 (0.14) | 0 (0.00) | 1 (0.00) | 2 (0.00) | 0 (0.00) | 0 (0.00) | 1 (0.00) | 0 (0.00) | 259 (0.52) |
| Mastomys natalensis | 154 | 101 (0.66) | 71 (0.46) | 13 (0.08) | 3 (0.02) | 6 (0.04) | 0 (0.00) | 6 (0.04) | 0 (0.00) | 1 (0.01) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 201 (1.31) |
| Arvicanthis niloticus | 104 | 21 (0.20) | 93 (0.89) | 74 (0.71) | 21 (0.20) | 2 (0.02) | 24 (0.23) | 10 (0.10) | 1 (0.01) | 3 (0.03) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 249 (2.39) |
| Aethomys kaiseri | 37 | 6 (0.16) | 14 (0.38) | 2 (0.05) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 1 (0.03) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 23 (0.62) |
| Tatera valida | 28 | 1 (0.04) | 23 (0.82) | 1 (0.04) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 2 (0.07) | 0 (0.00) | 27 (0.96) |
| Lophuromys sikapusi | 20 | 1 (0.05) | 15 (0.75) | 23 (1.15) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 4 (0.20) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 43 (2.15) |
| Unidentified | 20 | 13 (0.65) | 8 (0.4) | 1 (0.05) | 0 (0.00) | 1 (0.05) | 0 (0.00) | 1 (0.05) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 24 (1.2) |
| Mus spp. | 19 | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 1 (0.05) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 1 (0.05) |
| Aethomys hindei | 14 | 13 (0.93) | 9 (0.64) | 3 (0.21) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 1 (0.08) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 26 (1.86) |
| Taterillus emini | 12 | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) |
| Lophuromys flavopunctatus | 10 | 0 (0.00) | 0 (0.00) | 6 (0.60) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 6 (0.60) |
| Praomys jacksoni | 9 | 0 (0.00) | 1 (0.11) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 1 (0.11) |
| Cricetomys gambianus | 4 | 1 (0.25) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 1 (0.25) |
| Dasymys incomtus | 3 | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) |
| Lemniscomys striatus | 1 | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) |
| Total hosts and host-associated fleas | 1792 | 764 (0.43) | 307 (0.17) | 146 (0.08) | 98 (0.05) | 82 (0.05) | 24 (0.01) | 23 (0.01) | 11 (0.01) | 6 (0.00) | 4 (0.00) | 3 (0.00) | 1 (0.00) | 1469 (0.82) |
Xc = Xenopsylla cheopis; Dly = Dinopsyllus lypusus; Ccab = Ctenphthalmus cabirus; Xb = Xenopsylla brasiliensis; St = Stivalius torvus; Eg = Echinophaga gallinacea; Cb = C. bacopus; Unk = unknown; Dlo = D. longifrons; Cf = Ctenocephalides felis; Xn = X. nubica; Tp = Tunga penetrans. Hosts are shown in descending order of abundance. Fleas are shown in decreasing number of abundance on hosts.
In sylvatic areas, total abundance of hosts per session and per trap site was significantly higher for case villages (median 7.1 per 100 trap nights (range = 0.8–20.0) than for controls (5.8 per 100 trap nights (range = 1.7–11.7) (χ2 = 5.03, df = 1, P = 0.02). Marginally higher numbers of A. niloticus were captured in case villages (median = 0.8 per 100 trap nights, range = 0–3.3) than in control villages (median = 0 per 100 trap nights [range = 0–2.5] χ2 = 4.05, df = 1, P = 0.04). Abundances of Crocidura spp. (median = 1.7 per 100 trap nights [range = 0–14.1]), M. natalensis (median = 0.83 [range = 0–9.1]), and R. rattus (median = 0 [range = 0–1.7]) were similar between cases and controls. When host abundance was compared within each of the four trap sessions, abundance trended higher in case villages than in control villages, but individual comparisons were not statistically significant.
Total host abundance and abundances of the four key hosts inside of huts and in the peridomestic settings were similar between case and control villages. Furthermore, we compared small mammal abundance inside of huts between those that did or did not report practices found to be significantly different between cases and controls (i.e., sleeping on reed or straw bedding, replacing roof thatch, owning dogs, having been bitten by rats, storing garbage inside huts, cooking inside huts where persons slept, storing food in granaries, and growing various crops) and did not detect any statistically significant differences.
A total of 1,469 fleas, comprised of at least 11 species were obtained from 1,792 hosts (Table 5). The five most commonly collected species (Xenopsylla cheopis, Dinopsyllus lypusus, Ctenphthalmus cabirus, X. brasiliensis, and Stivalius torvus) accounted for 95% of the total fleas collected from hosts. The first four species and at least one other species of Stivalius have been implicated as vectors of plague bacteria elsewhere.27–33 Among the four most commonly collected hosts, A. niloticus was the most heavily infested (average = 2.39 fleas per individual). Demonstrating the diversity of fleas hosted by A. niloticus, we found that 9 of the 11 flea species collected from all sources were recovered from this host; five of these flea species had a flea index > 0.10 flea per host. In contrast, overall flea indices for R. rattus, Crocidura spp., and M. natalensis ranged from 0.52 to 1.31. However, within any host species, the number of flea species for which the flea index was > 0.10 fleas per host was only 1 for R. rattus and 2 for Crocidura spp. and M. natalensis. Inside and outside huts, the proportion of hosts infested was similar between cases and controls. Likewise, when comparing flea loads of each of the four key vectors (X. cheopis and X. brasiliensis combined, Ctenophthalmus spp., and Dinopsyllus spp.) and each of the four key hosts (R. rattus, A. niloticus, Crocidura spp., and M. natalensis) inside and outside huts, infestation levels were similar between case and control villages. When we compared X. cheopis and X. brasiliensis infestation of R. rattus inside or outside homes, we found that flea loads were similar on this host between case and control villages. The same result was true for each pairwise combination of key vector and key host. Furthermore, no differences were observed between cases and controls for any of the four sessions compared individually.
During 2,400 pan trap nights spanning each of the four sessions, 1,064 fleas comprised of at least eight species were recovered from 1,200 huts, yielding an average of 0.89 fleas collected per hut. Ctenocephalides felis, a species rarely collected from small mammals (Table 5), accounted for 80% of the fleas collected in pan traps. In contrast, one of the fleas most commonly collected from hosts, X. cheopis, accounted for only 4.5% of pan trap collections.
Xenopsylla cheopis were more abundant in pan traps set in case homesteads (median = 0, range = 0–3 fleas per homestead) than in control homesteads (median = 0, range = 0–1 fleas per homestead) (χ2 = 5.76, df = 1, P = 0.02). Similarly, C. felis were more abundant in pan traps set in case homesteads (median = 0, range = 0–3) than in control homesteads (median = 0, range = 0–12) (χ2 = 7.06, df = 1, P = 0.01). Both species were more abundant in cooking huts than in sleeping huts (Z ≥ 219, P < 0.0001). Furthermore, both species were more abundant in pan traps in households that reported sleeping on reed or straw bedding (χ2 ≥ 3.94, df = 1, P ≤ 0.05). Abundance of host-seeking C. felis was significantly higher in households that did not report owning a dog than in those that reported dog ownership (χ2 = 15.87, df = 1, P < 0.0001); abundance of host-seeking X. cheopis did not differ between these categories.
Discussion
Owing largely to the low incidence and sporadic occurrence of human plague cases, there have been few case–control studies that identified risk factors for plague.34–38 We identified presumptive risk factors by comparing villages with and without a history of human plague cases within a model-defined plague focus.11 Several variables were found to differ between these villages, and we believe that these variables are worthy of further investigation within the context of plague epizootics or human plague cases. Specifically, we found that although rat abundance was similar inside huts within case and control villages, contact rates between rats and humans (as measured by reported rat bites) was higher in case villages. Furthermore, host-seeking flea loads were higher in case villages than in control villages. These findings are suggestive of microhabitat or human behavioral differences between case and control households that may be conducive to flea survival or breeding or to increased contact rates between humans and rats. Together, these findings support the prevailing assumption that most human plague cases in the West Nile Region are acquired in the home environment.38,39 Our study did not address explicitly why, despite similar numbers of rats per hut, rat bites and abundance of host-seeking rat fleas were higher in case villages than in control villages. Nonetheless, several potential risk factors were identified in our study that are well aligned with existing plague prevention recommendations.
In agreement with previous studies in east Africa that compared host abundance and flea loads on hosts in communities with or without a history of plague,37,38 we showed that host and on-host flea communities were generally similar between case and control locations. As in a previous study from the West Nile Region,38 host diversity was low in human habitations; the dominant host was R. rattus, a host that is commonly implicated in plague epizootics. We showed also that in case and control locations, host diversity increased with increasing distance from the home, but in case villages only, there was a trend toward increased host abundance in sylvatic areas. Increased host abundance has often been positively associated with rates of Y. pestis transmission.5,40 Thus, our observation of increased host abundances in sylvatic trap sites within case villages is consistent with the dominant hypothesis in east Africa that plague bacteria are maintained in enzootic cycles in sylvatic areas and occasionally spill over into commensal rat populations.33,38,41,42 The finding of similar host abundance inside of huts regardless of plague history could suggest that abundance is generally high enough within homes to pose a risk to inhabitants and that human behavior may significantly impact the risk of plague infection in this setting.43
In general, poor housing construction or maintenance, storage of food inside homes, and availability of harborage for rodents (e.g., thatch roofing, dense vegetation, or garbage around homes) promote rodent survival in the home environment.43–45 Thus, in an effort to reduce contact between rodents, their fleas, and humans, plague prevention strategies often focus on reducing food and harborage for rodents in this setting.4,8,9,35 Providing support for this recommendation, our study showed that homesteads in case villages more frequently reported storing food, and garbage (largely food scraps) inside huts where persons sleep, or cooking inside huts where persons sleep. Furthermore, roof thatch was replaced more commonly in control villages than in case villages. Rats commonly nest in the roof thatch. Thus, frequent replacement of roofing material may reduce rat abundance. Frequency of roof replacement was weakly and negatively correlated with rodent abundance in homes in Mozambique.44 Comparing small mammal abundance within homesteads that did or did not store food, garbage, or cook in huts where persons sleep, or those that did or did not replace roof thatch did not identify any significant differences. However, our questionnaire asked villagers if they commonly engage in these practices, but the responses given did not necessarily reflect practices at the time of our small mammal collections.
Use of granaries to store food is often recommended as a means of reducing rodents in huts and to prevent plague. However, their use has decreased considerably largely because of the risk of food theft.39,46 Interestingly, although granary usage was reportedly low, our study showed that it was more common in case villages than in control villages. It is unclear whether case villages simply have more stored food than control villages, thus justifying granary use, or if granaries, which are constructed of locally available materials and often are not rodent proof, may concentrate rodents in the home environment and contribute to plague risk.47 Although our study did not address the quantities of foods grown or stored, we did seek to identify the types of crops grown in fields adjacent to homesteads. Consistent with a previous study in the West Nile Region that focused on different villages than those included in the present study,36 we also showed that compared with cases, controls were more likely to grow coffee and melons. Future studies are needed to assess whether these crops are serving as a barrier that may disrupt contacts between sylvatic and commensal rodents, have some repellent qualities, or simply correlate with other protective behaviors that were not measured in this study.
Mirroring results reported from a previous survey in other villages in the West Nile Region,46 our study showed that most villagers recognize rodents in their homes to be a concern and are using available methods to reduce their abundances. However, based on survey responses and the abundance of rats trapped within homes, the existing methods are clearly inadequate for eliminating rodents entirely or, perhaps, even for reducing numbers in any significant way. In the absence of environmental modifications, such as improved home construction, better food storage methods, or implementation of an intensive rodent control program,4,8,44,46 significant reduction of rodent burdens inside of homes is unlikely. Given the limited economic resources available in these subsistence farming villages, such improvements are unlikely to become widespread in the foreseeable future.
In the interest of reducing plague incidence, effective strategies exist to control fleas in the home environment. Although flea control efforts directed specifically at reducing flea loads on rats are likely to be helpful in reducing human plague risk, these efforts might not result in widespread support among local residents because rat fleas (X. cheopis and X. brasiliensis) typically remain on their rat hosts or in the nests of these animals and, therefore, rarely infest persons living in these areas except during periods when plague epizootics occur or perhaps when large numbers of rats have been poisoned, either of which could cause rat fleas to seek new hosts. In general, residents are more likely to remain enthusiastic about flea control programs that reduce not only largely unnoticed rat fleas but the numbers of other biting or venomous arthropods, such as cat fleas, mosquitoes, or spiders, which are generally perceived as a problem in homes. For example, indoor residual spraying was shown to significantly reduce flea loads and infestation rates on rats for at least 100 days in the West Nile Region. In addition, the treatment is effective at reducing other biting insects and therefore has the benefit of reducing risk of other vector-borne diseases.19 Our study identified an interest by villagers in controlling fleas in the home environment; approximately half of respondents reported that they do something to control fleas. Most reported smearing mud on floors to control fleas. However, a study in Tanzania showed no association between mud plastering and plague occurrence or flea abundance.48
As suggested, sleeping on floors may put persons at greater risk of exposure to fleas, thus increasing plague risk.43 Similar to an earlier study in the West Nile Region,36 our study found that sleeping substrate could indirectly affect contact rates between humans and fleas. Specifically, we showed that persons in case villages were more likely to sleep on reed or straw mats compared with their counterparts in control villages. It is unclear if this relates simply to this substrate putting persons in closer contact with the ground than other substrates, or if these mats may be more likely than alternatives (e.g., foam or other stuffed mattresses) to harbor fleas because the organic content or dust in the mats could provide a suitable substrate for breeding of fleas.22,43 Although not mutually exclusive, the latter suggestion was supported by our observation that X. cheopis and C. felis were more abundant in households that reported sleeping on reed or straw bedding than in those that did not sleep on this material.
Previous studies have implicated dogs in the home environment to pose a risk for human exposure to plague bacteria because of their potential to carry infectious fleas into close contact with humans.32,34,35 In our study, compared with control villages, householders in case villages more frequently reported owning dogs. Perhaps seemingly counter-intuitive to our finding, we showed that abundance of host-seeking C. felis was significantly lower in homesteads that reported dog ownership. However, this is likely because dogs are a preferred host for C. felis,49 thus, presence of this host may reduce the abundance of host-seeking fleas inside huts. However, C. felis is an inefficient vector of Y. pestis and is an unlikely bridging vector from zoonotic hosts to humans because of its infrequent feeding on rodents.50,51 We did not find any significant differences in the abundance of X. cheopis, an efficient vector of Y. pestis that readily bites rodents and humans,30 between homesteads reporting dog ownership and those that did not report dog ownership. However, relative to C. felis, numbers of X. cheopis were low and are likely to increase during plague epizootics when rodent hosts die in large numbers, forcing their fleas to search for alternative hosts.
Our selection of control villages aimed to reduce the likelihood of finding differences between cases and controls in access to health clinics. Although control villages were selected because at least one person from each control village was seen in clinics, we had no prior knowledge of the frequency of care-seeking at clinics within case or control villages. Thus, we sought to rule out the possibility that plague cases were occurring at similar rates within these categories, but that controls were less likely to visit health clinics and might therefore be captured at lower rates within our surveillance system. Here, we showed that knowledge about plague, care-seeking behavior, distance to clinics, and most care-seeking practices were similar between cases and controls. However, in case villages compared with control villages, respondents more frequently reported they would go to a traditional healer first if they believed that their illness was caused by plague, although the proportion was still quite low (< 3%). Nonetheless, local involvement by traditional healers in referring plague cases to clinics could reduce the time from recognition of illness to treatment, which is likely to improve outcomes of infection. Although most those interviewed were knowledgeable of signs and symptoms of plague and how persons become infected, the finding that approximately 40% of respondents did not know how persons were likely to become infected and approximately 25% did not know the signs and symptoms of plague shows room for improvement in community education efforts.
Although we identified several variables that differed significantly between cases and controls, the magnitude of the differences were generally quite small. This finding therefore begs the question of whether any of these variables alone significantly increases plague risk. Our findings suggest that several factors likely need to be optimal for human risk of exposure to plague bacteria to increase substantially. Future studies are needed to determine if any of the risk factors identified in our study directly relate to rodent abundance or flea loads and to examine whether these strategies could be modified to reduce plague risk (e.g., replacing roof thatch, modifying food and garbage storage practices, instituting better practices to reduce fleas on and off hosts in the home setting). Furthermore, longitudinal monitoring of host and flea communities could identify changes that occur before epizootics that could ultimately be used as an early-warning to initiate more intensive prevention and control efforts. Finally, improved health education could reduce plague cases and fatality rates associated with plague cases. Our study finds support for existing plague recommendations, including reduction of food and harborage for rodents in the home environment, use of flea control for pets and inside of homes, continued efforts to educate the public and health care providers about plague signs and the importance of prompt treatment with appropriate antibiotic therapy.
ACKNOWLEDGMENTS
We thank the communities of the West Nile Region for their participation in the study; E. Kajik, V. Andama, C. Munguijakisa, M. Olowo, E. Tibo, F. Wunna, and M. Yafesi for assistance conducting interviews and collecting field specimens; and T. S. Asaku for logistical and administrative support.
Footnotes
Financial support: This study was supported in part by the United States Agency for International Development Emerging Pandemic Threat Program.
Authors' addresses: Rebecca J. Eisen, Katherine MacMillan, Emily Zielinski-Gutierrez, Christine B. Graham, Karen A. Boegler, Russell E. Enscore, and Kenneth L. Gage, Bacterial Diseases Branch, Division of Vector Borne Diseases, National Center for Emerging and Zoonotic Infectious Diseases, Centers for Disease Control and Prevention, Fort Collins, CO, E-mails: dyn2@cdc.gov, iky4@cdc.gov, ezb0@cdc.gov, hyb4@cdc.gov, kje5@cdc.gov, renscore@cdc.gov, and klg0@cdc.gov. Linda A. Atiku and Joseph T. Mpanga, Plague Section, Uganda Virus Research Institute, Entebbe, Uganda, E-mails: l.atikupraise@yahoo.com and joe1ug@msn.com.
References
- 1.Gage KL, Kosoy MY. Natural history of plague: perspectives from more than a century of research. Annu Rev Entomol. 2005;50:505–528. doi: 10.1146/annurev.ento.50.071803.130337. [DOI] [PubMed] [Google Scholar]
- 2.Tikhomirov E. Epidemiology and distribution of plague. In: Dennis DT, Gage KL, Gratz N, Poland JD, Tikhomirov E, editors. Plague Manual: Epidemiology, Distribution, Surveillance and Control. Geneva: World Health Organization; 1999. pp. 11–37. [Google Scholar]
- 3.Crook LD, Tempest B. Plague. A clinical review of 27 cases. Arch Intern Med. 1992;152:1253–1256. doi: 10.1001/archinte.152.6.1253. [DOI] [PubMed] [Google Scholar]
- 4.Duplantier JM. Surveillance and control of plague. In: Carniel E, Hinnebusch BJ, editors. Yersina: Systems Biology and Control. Norfolk, UK: Caister Academic Press; 2012. pp. 183–199. [Google Scholar]
- 5.Davis S, Begon M, De Bruyn L, Ageyev VS, Klassovskiy NL, Pole SB, Viljugrein H, Stenseth NC, Leirs H. Predictive thresholds for plague in Kazakhstan. Science. 2004;304:736–738. doi: 10.1126/science.1095854. [DOI] [PubMed] [Google Scholar]
- 6.Eisen RJ, Gage KL. Adaptive strategies of Yersinia pestis to persist during inter-epizootic and epizootic periods. Vet Res. 2009;40:1. doi: 10.1051/vetres:2008039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pham HV, Dang DT, Tran Minh NN, Nguyen ND, Nguyen TV. Correlates of environmental factors and human plague: an ecological study in Vietnam. Int J Epidemiol. 2009;38:1634–1641. doi: 10.1093/ije/dyp244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gratz NG. Control of plague transmission. Plague Manual: Epidemiology, Distribution, Surveillance and Control. Geneva: World Health Organization; 1999. pp. 97–134. [Google Scholar]
- 9.Gage KL. Plague Manual: Epidemiology, Distribution, Surveillance and Control. Geneva: World Health Organization; 1999. Plague surveillance; pp. 135–165. [Google Scholar]
- 10.World Health Organization Human plague: review of regional morbidity and mortality, 2004–2009. Wkly Epidemiol Rec. 2010;85:40–45. [PubMed] [Google Scholar]
- 11.Eisen RJ, Griffith KS, Borchert JN, MacMillan K, Apangu T, Owor N, Acayo S, Acidri R, Zielinski-Gutierrez E, Winters AM, Enscore RE, Schriefer ME, Beard CB, Gage KL, Mead PS. Assessing human risk of exposure to plague bacteria in northwestern Uganda based on remotely sensed predictors. Am J Trop Med Hyg. 2010;82:904–911. doi: 10.4269/ajtmh.2010.09-0737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.MacMillan K, Monaghan AJ, Apangu T, Griffith KS, Mead PS, Acayo S, Acidri R, Moore SM, Mpanga JT, Enscore RE, Gage KL, Eisen RJ. Climate predictors of the spatial distribution of human plague cases in the West Nile region of Uganda. Am J Trop Med Hyg. 2012;86:514–523. doi: 10.4269/ajtmh.2012.11-0569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moore SM, Monaghan A, Griffith KS, Apangu T, Mead PS, Eisen RJ. Improvement of disease prediction and modeling through the use of meteorological ensembles: human plague in Uganda. PLoS ONE. 2012;7:e44431. doi: 10.1371/journal.pone.0044431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Winters AM, Staples JE, Ogen-Odoi A, Mead PS, Griffith K, Owor N, Babi N, Enscore RE, Eisen L, Gage KL, Eisen RJ. Spatial risk models for human plague in the West Nile Region of Uganda. Am J Trop Med Hyg. 2009;80:1014–1022. [PubMed] [Google Scholar]
- 15.Lakwo A, Cwinyaai W, Abdallay O. West Nile Profiling. Nebbi, Uganda: Agency for Accelerated Regional Development; 2008. [Google Scholar]
- 16.Monaghan AJ, MacMillan K, Moore SM, Mead PS, Hayden MH, Eisen RJ. A regional climatography to support human plague modeling in the West Nile, Uganda. J Appl Meteorol Climatol. 2012;51:1201–1221. [Google Scholar]
- 17.Eisen RJ, Borchert JN, Mpanga JT, Atiku LA, MacMillan K, Boegler KA, Montenieri JA, Monaghan A, Gage KL. Flea diversity as an element for persistence of plague bacteria in an East African plague focus. PLoS ONE. 2012;7:e35598. doi: 10.1371/journal.pone.0035598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chu MC. Laboratory Manual of Plague Diagnostics. Atlanta, GA: Centers for Disease Control and Prevention and Geneva: World Health Organization; 2000. p. 129. [Google Scholar]
- 19.Borchert JN, Eisen RJ, Atiku LA, Delorey MJ, Mpanga JT, Babi N, Enscore RE, Gage KL. Efficacy of indoor residual spraying using lambda-cyhalothrin for controlling nontarget vector fleas (Siphonaptera) on commensal rats in a plague endemic region of northwestern Uganda. J Med Entomol. 2012;49:1027–1034. doi: 10.1603/me11230. [DOI] [PubMed] [Google Scholar]
- 20.Delany MJ. The Rodents of Uganda. Kettering, UK: The George Press; 1975. [Google Scholar]
- 21.Haselbarth E. Siphonaptera. In: Zumpt F, editor. The Arthropod Parasites of Vertebrates in Africa South of the Sahara (Ethiopia region) Johannesburg, South Africa: South African Institute of Medical Research; 1966. pp. 117–212. [Google Scholar]
- 22.Hopkins GH. Annotated and illustrated keys to the known fleas of east Africa. Ugandan J. 1947;11:133–191. [Google Scholar]
- 23.Hopkins GH, Rothschild M. An Illustrated Catalogue of the Rothschild Collection of Fleas (Siphonaptera) in the British Museum (Natural History): Hystrichopsyllidae. London: Ballantyne and Company; 1966. [Google Scholar]
- 24.Smit FG. Siphonaptera (Fleas) In: Smith KG, editor. Insects and other Arthropods of Medical Importance. London: British Museum of Natural History; 1973. pp. 325–371. [Google Scholar]
- 25.Nowak RM. Walker's Mammals of the World. Baltimore, MD: The Johns Hopkins University Press; 1990. [Google Scholar]
- 26.Simpson EH. Measurements of diversity. Nature. 1949;163:688. [Google Scholar]
- 27.Bacot AW, Martin CJ. Observations on the mechanism of the transmission of plague by fleas. J Hyg. 1914;13((Plague Suppl III)):423–439. [PMC free article] [PubMed] [Google Scholar]
- 28.Davis DHS, Heisch RB, McNeil D, Meyer KF. Serological survey of plague in rodents and other small mammals in Kenya. Trans R Soc Trop Med Hyg. 1968;62:838–861. doi: 10.1016/0035-9203(68)90013-8. [DOI] [PubMed] [Google Scholar]
- 29.Eisen RJ, Wilder AP, Bearden SW, Montenieri JA, Gage KL. Early-phase transmission of Yersinia pestis by unblocked Xenoopsylla cheopis (Siphonaptera: pulicidae) is as efficient as transmission by blocked fleas. J Med Entomol. 2007;44:678–682. doi: 10.1603/0022-2585(2007)44[678:etoypb]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 30.Gratz NG. Rodent reservoirs and flea vectors of natural foci of plague. In: Dennis DT, Gage KL, Gratz NG, Poland JD, Tikhomirov E, editors. Plauge Manual: Epidemiology, Distribution, Surveillance and Control. Geneva: World Health Organization; 1999. pp. 63–96. [Google Scholar]
- 31.Kilonzo BS. A survey of rodents and their flea ectoparasites in north-eastern Tanzania. E African J Med Res. 1976;3:117–125. [Google Scholar]
- 32.Pollitzer R. Plague. World Health Organization Monograph Series No. 22. Geneva: World Health Organization; 1954. [Google Scholar]
- 33.Velimirovic B, Zikmund V, Herman J. Plague in the Lake Edwards focus; the Democratic Republic of Congo, 1960–1966. Z Tropenmed Parasitol. 1969;20:373–387. [PubMed] [Google Scholar]
- 34.Gould LH, Pape J, Ettestad P, Griffith KS, Mead PS. Dog-associated risk factors for human plague. Zoonoses Publ Hlth. 2008;55:448–454. doi: 10.1111/j.1863-2378.2008.01132.x. [DOI] [PubMed] [Google Scholar]
- 35.Mann JM, Martone WJ, Boyce JM, Kaufmann AF, Barnes AM, Weber NS. Endemic human plague in New Mexico: risk factors associated with infection. J Infect Dis. 1979;140:397–401. doi: 10.1093/infdis/140.3.397. [DOI] [PubMed] [Google Scholar]
- 36.MacMillan K, Enscore RE, Ogen-Odoi A, Borchert JN, Babi N, Amatre G, Atiku LA, Mead PS, Gage KL, Eisen RJ. Landscape and residential variables associated with plague-endemic villages in the West Nile region of Uganda. Am J Trop Med Hyg. 2011;84:435–442. doi: 10.4269/ajtmh.2011.10-0571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Laudisoit A, Neerinckx S, Makundi RH, Leirs H, Krasnov BR. Are local plague endemicity and ecological characteristics of vectors and reservoirs related? A case study in north-east Tanzania. Curr Zool. 2009;55:200–211. [Google Scholar]
- 38.Amatre G, Babi N, Enscore RE, Ogen-Odoi A, Atiku LA, Akol A, Gage KL, Eisen RJ. Flea diversity and infestation prevalence on rodents in a plague-endemic region of Uganda. Am J Trop Med Hyg. 2009;81:718–724. doi: 10.4269/ajtmh.2009.09-0104. [DOI] [PubMed] [Google Scholar]
- 39.Orach SO. Plague Outbreaks: the Gender and Age Perspective in Okoro County, Nebbi District, Uganda. Nebbi, Uganda: Agency for Accelerated Regional Development; 2003. [Google Scholar]
- 40.Davis S, Trapman P, Leirs H, Begon M, Heesterbeek JAP. The abundance threshold for plague as a critical percolation phenomenon. Nature. 2008;454:634–637. doi: 10.1038/nature07053. [DOI] [PubMed] [Google Scholar]
- 41.Msangi AS. The surveillance of rodent populations in east Africa in relation to plague endemicity. Dar Salam University Sci J. 1975;1:8–20. [Google Scholar]
- 42.Njunwa KJ, Mwaiko GL, Kilonzo BS, Mhina JI. Seasonal patterns of rodents, fleas and plague status in the Western Usambara Mountains, Tanzania. Med Vet Entomol. 1989;3:17–22. doi: 10.1111/j.1365-2915.1989.tb00469.x. [DOI] [PubMed] [Google Scholar]
- 43.Kilonzo BS, Mvena ZSK, Machangu RS, Mbise TJ. Preliminary observations on factors responsible for long persistence and continued outbreaks of plague in Lushoto district, Tanzania. Acta Trop. 1997;68:215–227. doi: 10.1016/s0001-706x(97)00096-x. [DOI] [PubMed] [Google Scholar]
- 44.Belmain SR, Meyer AN, Penicela L, Xavier R, Jones SC, Zhai J, Robinson WH. Population management of rodent pests through intensive trapping inside rural households in Mozambique. In: Jones SC, Zhai J, Robinson WH, editors. Proceedings of the Fourth International Conference on Urban Pests. 2002. pp. 421–428. [Google Scholar]
- 45.Kingdon J. East African Mammals: An Atlas of Evolution in Africa: Hares and Rodents. London: The University of Chicago Press; 1974. [Google Scholar]
- 46.Eisen RJ, Enscore RE, Atiku LA, Zielinski-Gutierrez E, Mpanga JT, Kajik E, Andama V, Mungujakisa C, Tibo E, MacMillan K, Borchert JN, Gage KL. Evidence that rodent control strategies ought to be improved to enhance food security and reduce the risk of rodent-borne illnesses within subsistence farming villages in the plague-endemic West Nile region, Uganda. Int J Pest Manage. 2013;59:259–270. doi: 10.1080/09670874.2013.845321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hirst LF. The Conquest of Plague: A Study of the Evolution of Epidemiology. Oxford, UK: Carendon Press; 1953. [Google Scholar]
- 48.Laudisoit A, Leirs H, Makundi RH, Van Dongen S, Davis S, Neerinckx S, Deckers J, Libois R. Plague and the human flea, Tanzania. Emerg Infect Dis. 2007;13:687–693. doi: 10.3201/eid1305.061084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rust MK, Dryden MW. The biology, ecology, and management of the cat flea. Annu Rev Entomol. 1997;42:451–473. doi: 10.1146/annurev.ento.42.1.451. [DOI] [PubMed] [Google Scholar]
- 50.Eisen RJ, Borchert JN, Holmes JL, Amatre G, Van Wyk K, Enscore RE, Babi N, Atiku LA, Wilder AP, Vetter SM, Bearden SW, Montenieri JA, Gage KL. Early-phase transmission of Yersinia pestis by cat fleas (Ctenocephalides felis) and their potential role as vectors in a plague-endemic region of Uganda. Am J Trop Med Hyg. 2008;78:949–956. [PubMed] [Google Scholar]
- 51.Graham CB, Borchert JN, Black WC, Atiku LA, Mpanga JT, Boegler KA, Moore SM, Gage KL, Eisen RJ. Blood meal identification in off-host cat fleas (Ctenocephalides felis) from a plague-endemic region of Uganda. Am J Trop Med Hyg. 2013;88:381–389. doi: 10.4269/ajtmh.2012.12-0532. [DOI] [PMC free article] [PubMed] [Google Scholar]