Abstract
We conducted a cross-sectional survey of Trypanosoma cruzi infection of Triatoma infestans as well as dogs and cats in 327 households from a well-defined rural area in northeastern Argentina to test whether the household distribution of infection differed between local ethnic groups (Tobas and Creoles) and identify risk factors for host infection. Overall prevalence of infection of bugs (27.2%; 95% confidence interval = 25.3–29.3%), dogs (26.0%; 95% confidence interval = 23.3–30.1%), and cats examined (28.7%; 95% confidence interval = 20.2–39.0%) was similar. A multimodel inference approach showed that infection in dogs was associated strongly with the intensity and duration of local exposure to infected bugs and moderately with household ethnic background. Overall, Toba households were at a substantially greater risk of infection than Creole households. The strong heterogeneities in the distribution of bug, dog, and cat infections at household, village, and ethnic group levels may be used for targeted vector and disease control.
Introduction
Vector-borne transmission of Trypanosoma cruzi—the etiologic agent of Chagas disease—remains a major public health problem in Latin America.1 With its roots intimately tied to poverty, inappropriate housing, and neglected rural populations, suppression efforts in the southern cone countries of South America have made significant progress to reducing parasite transmission mediated by the main vector Triatoma infestans but not to the point of achieving sustained vector elimination.2,3 Domestic vector-borne transmission persists in the Gran Chaco—an ecoregion with dry (western) and humid (eastern) sections extending mainly over northern Argentina, southeastern Bolivia, and western Paraguay.3 The Gran Chaco coincides with the core of the geographical distribution of T. infestans. Local human populations (comprising mostly Creoles and numerous indigenous groups) are sparsely distributed in rural areas, living in a subsistence economy with scarce access to the limited health services available.4 Studies of Chagas disease in indigenous groups have been conducted typically at the population level,5–11 and they paid little or no attention to the household environment where vector-mediated transmission occurs.12,13
Two ethnic groups (Creoles and Tobas) coexist in some rural districts of the Argentinean Chaco. Several surveys reported high seroprevalence of T. cruzi in Tobas and other indigenous groups in the Argentine and Paraguayan Chaco, which sometimes exceeded the infection prevalence in local Creoles.5–11 A recent cross-sectional study conducted in Pampa del Indio showed that house infestations were significantly more frequent in Toba's domiciles.14 However, householders' ethnic background adjusted for other factors had ambiguous effects on infestation and relative bug abundance in a multimodel inference framework. How these infestation-related measures translate into parasite transmission metrics is an important research topic with few precedents.12,13,15,16
One strategy to assess risk on the household level is the use of domestic dogs as sentinels or surrogates of human infection, circumventing the serological screening of human populations. Dogs and cats are major domestic reservoir hosts of T. cruzi, particularly in the southern cone countries,3,13,15–20 where the occurrence of human incidence and prevalence of infection in bugs were strongly associated with the presence and number of infected dogs.13,20 Unlike T. cruzi-seropositive humans, infected dogs and cats are key infectious sources for triatomine bugs.19 The relative odds of dog infection vary with factors that mainly reflect local vector-borne transmission at the dog's house (i.e., infected bug abundance), other concurrent sources of infection, and cumulative exposure period (i.e., age).15,16,21 Vertical transmission and cases imported from elsewhere may be relevant transmission routes depending on the epidemiological context, whereas oral transmission was sometimes suspected.16,21
Based on the existing evidence, we tested whether Toba households and their dogs and cats were at greater risk of infection by T. cruzi than Creole households using a multimodel inference framework. We also sought to identify risk factors for host infection and investigated the demography of dog and cat populations according to householders' ethnic background to explain transmission patterns and mechanisms. Our study reveals various heterogeneities in the transmission of T. cruzi at household and ethnic group levels that are relevant for targeted vector and disease control.
Materials and Methods
Study area.
Fieldwork was conducted in a rural section of Pampa del Indio municipality, Province of Chaco, Argentina, in 2007 and 2008. The study area included 327 inhabited house compounds grouped in 13 villages.14 Houses typically had one room where people slept (domicile) and separate peridomestic structures (kitchens, storerooms, corrals, and chicken coops). Creoles and Tobas inhabited 81% and 16% of the study houses, respectively. In our study area, Tobas are organized in communities; therefore, the initial assignment of a household to Toba or Creole ethnic group was based on the community in which they were living and the appearance and language spoken by the head of the family who answered the questionnaire. A household census conducted in 2010 in cooperation with local health personnel confirmed all the initial assignments and only identified six households with a mixed ethnic background; in all cases, the male head of the family was Creole. The last community-wide insecticide spraying campaign conducted by vector control program staff before our baseline survey was carried out approximately in 1996; however, some insecticide treatments were applied by villagers or hospital staff in 2006.
Triatomine collection.
A baseline cross-sectional survey collected T. infestans in 39.8% of all inhabited house compounds using a dislodging spray in October and November of 2007 before spraying all houses with pyrethroid insecticides.14 Householders were explained the goals of the research and provided oral consent to access their premises. All collected triatomine bugs were placed in labeled plastic bags identified with the capture ecotope, transported to the field laboratory, and counted according to species and stage or sex. Feces from all live third-instar nymphs and larger stages of T. infestans were examined for T. cruzi infection by direct microscopic observation (MO) at 400× within 20 days of collection.
Dog and cat surveys.
We conducted three non-overlapping, cross-sectional, house-to-house surveys targeting all dogs and cats residing in seven contiguous villages (10 de Mayo, Campo Los Toros, El Salvaje, La Loma, Las Chuñas, Los Ciervos, and Santos Lugares; totaling 173 inhabited houses) in August, September, and December of 2008. Operational constraints restricted the number of study villages to seven. Selection of this subset was based on including spatially contiguous villages covering the observed range of house infestation and bug infection with T. cruzi (most Toba households; predominating in 10 de Mayo and Las Chuñas) and achieving a large sample of domestic animals (> 400).
Animal owners were interviewed with a standard demographic questionnaire for each dog and cat as described.15 Information on whether the dog ate raw viscera and drank fresh blood when owners slaughtered wild or domestic animals and whether the animal had permanent residence in the study villages or traveled with the owner was also requested. On subsequent visits to 146 houses in October and November of 2009, householders were informed of their animals' infection status; information on the fate of each dog or cat during the intervening period and apparent cause of death was requested.
All dogs and cats were considered eligible for the infection survey, and animals were handled as described.22 Failure to examine some of the animals was mainly because they were absent, ran away, or could not be handled. Only one householder refused having his animals examined for infection, because they were aggressive. Dogs and cats ages 4 months or older were diagnosed serologically, whereas younger animals (40 dogs and 3 cats) were examined only by xenodiagnosis, because maternally derived antibodies to T. cruzi could induce a false-positive serological result. Six dogs and one cat older than 4 months of age that could not be bled were examined only by xenodiagnosis. Animal care and use were performed according to guidelines issued by the Institutional Animal Care and Use Committee at the Faculty of Exact and Natural Sciences, which is based on the International Guiding Principles for Biomedical Research Involving Animals developed by the Council for International Organizations of Medical Sciences. All research activities were conducted according to protocols approved by the Dr. Carlos Barclay Independent Ethical Committee for Clinical Research from Buenos Aires, Argentina (Institutional Review Board number 00001678, National Institutes of Health registered, protocol number TW-01-004).
Serodiagnosis and xenodiagnosis.
Dog and cat sera were tested for antibodies to T. cruzi using an indirect hemagglutination assay (IHA) following the manufacturer's instructions (Wiener Laboratories S.A.I.C., Buenos Aires, Argentina) and an in-house enzyme-linked immunosorbent assay (ELISA) as described.22 Sera from 32 dogs and 14 cats with discordant results between IHA and ELISA were tested with an indirect immunofluorescence test (IFAT; Ififluor Parasitest Chagas, Laboratorio IFI, Buenos Aires, Argentina). Titers ≥ 1:16 (IHA), titers ≥ 1:32 (IFAT), or optical absorbance ≥ 0.17 (ELISA) were used as cutoff values. Non-reactive and reactive sera were clearly differentiated, with higher degrees of agreement between ELISA and IHA in dogs (copositivity = 100%, conegativity = 90%) than cats (copositivity = 94%, conegativity = 78%); sensitivities of ELISA and IHA were 100%, and specificity was 96% for both tests22–24; 16 dogs and 10 cats that were both seronegative and xenodiagnosis-negative were also negative by a polymerase chain reaction assay targeted to minicircle DNA.22 Seropositive refers to samples reactive by at least two different serologic tests.
Xenodiagnosis was performed using 20 uninfected, laboratory-reared fourth-instar nymphs of T. infestans exposed to the animal's belly for 20 minutes and examined as described.19,22 Infected means that animals had a positive xenodiagnosis and/or were seropositive to T. cruzi. The results of serodiagnosis and xenodiagnosis were combined to calculate the composite prevalence of T. cruzi infection.
Data analysis.
Agresti–Coull binomial 95% confidence intervals (95% CIs) were used for proportions; Wilson CIs were calculated when samples sizes were small (N ≤ 40).25 Lloyd's index of patchiness was used to assess the household aggregation of T. cruzi infection.
The annual force of infection (λ) in dogs was estimated retrospectively using a catalytic model, with recovery rate set to zero (susceptible-infected). This model assumes that the incidence of infection is time- and age-independent19; λ was estimated using non-linear least squares procedures using Matlab 6.3 (The MathWorks, Natick, MA) and the catalytic model λ = −ln(1 − pa)/a, where pa is the proportion of infected individuals within the age class with midpoint that is a. Ages at the time of community-wide insecticide spraying were reconstructed by subtracting 9 months from the reported ages of dogs and cats.
We used an information theoretic approach to identify the best-fitting model of host infection using the strategy outlined by Burnham and Anderson.26 For this purpose, we fitted a random intercept multiple logistic regression model to a global model that included all the explanatory variables using the lmer function implemented in the lme4 package in R (version 2.15.1).27 The random intercept (i.e., random effects) model allows for the fact that observations on host infection at household level are not independent,6,13,15,16 which the current study also shows. Predictors of dog infection were explicitly established a priori based on existing empirical evidence13,15,16,20 on the major role of local T. infestans-mediated transmission, which was modified by resting habits and sex, and current hypotheses on potential effects of ethnicity and exposure to oral infection to avoid overparameterization. Predictors were restricted in number to maintain a ratio of 15 observations per predictor.
The first global model fitted to host infection data (p) was
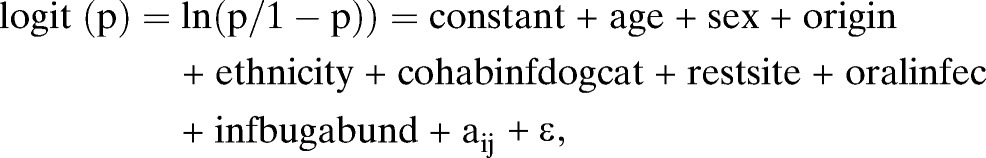 |
where age (in months)—a surrogate of length of exposure—was reconstructed to represent host age at baseline before control interventions. Sex was male or female, origin of dog was urban immigrant, rural immigrant, or native, ethnicity of the animal's household was Toba or Creole, and the number of other infected dogs or cats with which the animal cohabited (cohabinfdogcat) was split into three levels: 0, 1, or ≥ 2 infected dogs or cats. The animals' domestic resting habits were slept in domiciles, kitchens, and storerooms either indoors or against the outer walls of domiciles (restsite; with two levels) or not; whether the dog ate raw viscera or drank fresh blood when owners slaughtered wild or domestic animals or was used for hunting (oralinfec) was determined. The variable aij is a normally distributed random term with mean of zero and variance of σa2, and ε is an error term normally and independently distributed with mean of zero and variance of σ2. Reference levels were the lowest age group, males, urban immigrants, Creoles, not cohabiting with an infected animal, no domestic resting habit, and no exposure to oral infection.
The entomological predictors initially considered in the global model were the average abundance of T. infestans captured per 15 minutes-person per site in domiciles, kitchens, and storerooms (i.e., typical resting sites of dogs and cats) at the animal's house compound and infected bug abundance at these sites (categorized in three levels: no bugs, 1–9 bugs per 15 minutes-person, and ≥ 10 bugs per 15 minutes-person). These data were mainly derived from timed manual searches at site level before control interventions; a few bugs collected by householders were added to timed manual collections on the assumption that they would have been captured manually. Infestation data for one house that could not be inspected at baseline were derived from insecticide knockdown collections at the same house. Relative and infected bug abundances were highly correlated (Pearson's r = 0.85, P < 0.0001); therefore, both variables were subsumed into a new variable (infbugabund) categorized in four levels (no bugs collected, 0 infected bugs per 15 minutes-person, 1–9 infected bugs per 15 minutes-person, and ≥ 10 infected bugs per 15 minutes-person). The condition numbers for the explanatory variables for dog infection were < 7.2, indicating that the statistically significant correlation coefficients recorded between some of the predictors (r < 0.5) were not strong and would not cause multicolinearity problems.
The first global model included 292 dogs (from 139 households) born before the community-wide residual spraying with insecticides with no missing data in the study variables. Because of the large number of missing data for origin of dog and exposure to oral infection (both of which proved to be unimportant in the first global model), we excluded these variables and ran a second model that comprised 353 dogs and 153 houses. On a post-hoc basis, we investigated the effects on dog infection of the interaction between ethnic background and every other factor in the global models. These terms were added one by one to each model and tested separately to avoid convergence problems.
The number of cats born before the insecticide spraying campaign (57 cats from 49 houses) limited the number of predictors investigated to three (age of the cat, infected bug abundance, and ethnicity or household number of other infected dogs or cats), which were highly correlated.
The package MuMIn was used to obtain estimates of second-order Akaike's Information Criterion corrected for small samples (AICc), the difference between AICc and the lowest AICc-scored model (ΔAICc), and model probabilities (i.e., Akaike weights) for each of the possible models.26 The subset of models that was within two AICc from the best-fitting model was considered the top models.26 Because no single model had a superior Akaike weight, the relative importance (RI) of each explanatory variable in the model set was computed.26 Variables with high RI were identified as the main risk factors. The overall quality of the fitted logistic regression models was assessed by means of the Hosmer and Lemeshow test using the averaged coefficients, grouping the data in 10 equal-sized groups, and calculating the area under the receiver operating characteristic curve (ROC).
Results
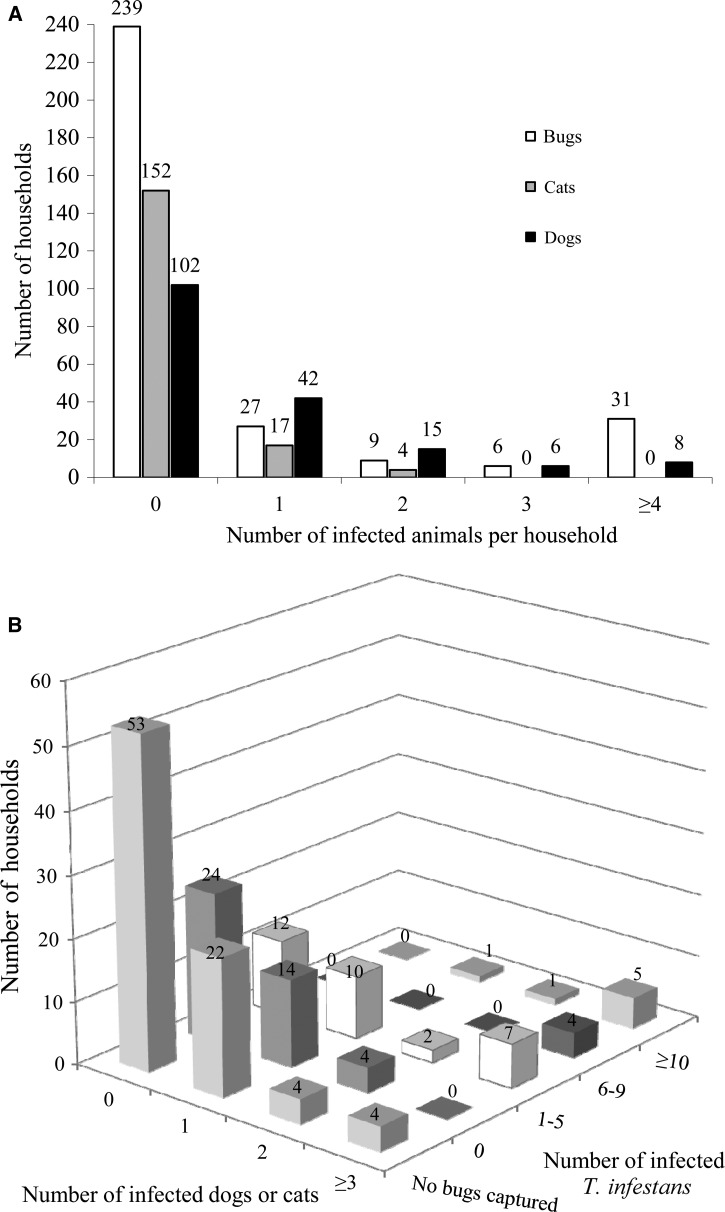
In total, 1,869 (84%) late-stage bugs were examined for infection. Of all animals registered, 481 dogs (92%) and 87 cats (76%) were examined for infection. The overall prevalence of infection was very similar among the bugs (27.2%; 95% CI = 25.3–29.3%), dogs (26.0%; 95% CI = 23.3–30.1%), and cats (28.7%; 95% CI = 20.2–39.0%) examined. Infected bugs were found in 48.7% of 150 T. infestans-positive houses. More households harbored at least one infected dog (41.0%; 95% CI = 34.0–48.5%) than at least one infected cat (12.1%; 95% CI = 8.0–17.9%) or one infected bug (23.4%; 95% CI = 19.0–28.4%). Most houses were either apparently uninfested or harbored uninfected bugs, and a few houses (7%) harbored more than five infected bugs (Figure 1). The frequency distributions of infected vectors and hosts were aggregated at the household level (Lloyd's index of patchiness; bugs, 18.8; dogs, 2.1; cats, 2.3). Infection in dogs and cats was marginally associated among 68 houses that harbored individuals examined from both species (Fisher's test, P = 0.07). Nearly all (96%) of the infected bugs were collected in houses with at least one infected dog. Infected bugs were three to eight times more likely to be found in houses harboring at least one infected dog or cat than houses without them.
Figure 1.
Household distribution of T. cruzi infection in dogs, cats, and T. infestans in Pampa del Indio in 2007 and 2008 for (A) each host and (B) host infection versus vector infection.
Household infection and ethnic group.
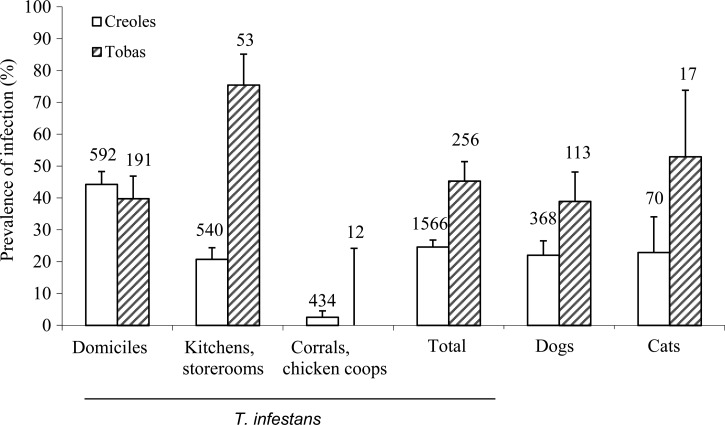
Overall bug infection prevalence was almost two times as high in Toba households (45.3%) as Creole households (24.6%; Fisher's test, P < 0.0001), but there were large variations between ecotopes within each ethnic group (Figure 2). Bug infection was similar in domiciles from both groups (39.8–44.3%), peaked in Toba kitchens and storerooms (75.5% versus 20.7%), and was rare in corrals and chicken coops (< 3%). Infection prevalence increased steadily with bug stage from 8.8% among third-instar nymphs, 14.8% in fourth-instar nymphs, 30.5% in fifth-instar nymphs, and 28.5% in adult males to 33.9% among adult females.
Figure 2.
Prevalence of T. cruzi infection by ethnic group in dogs, cats, and T. infestans in Pampa del Indio in 2007 and 2008. Numbers on top of bars are individuals examined for infection. Whiskers indicate the upper limit of the 95% CI. Household ethnic background was unknown for 47 bugs.
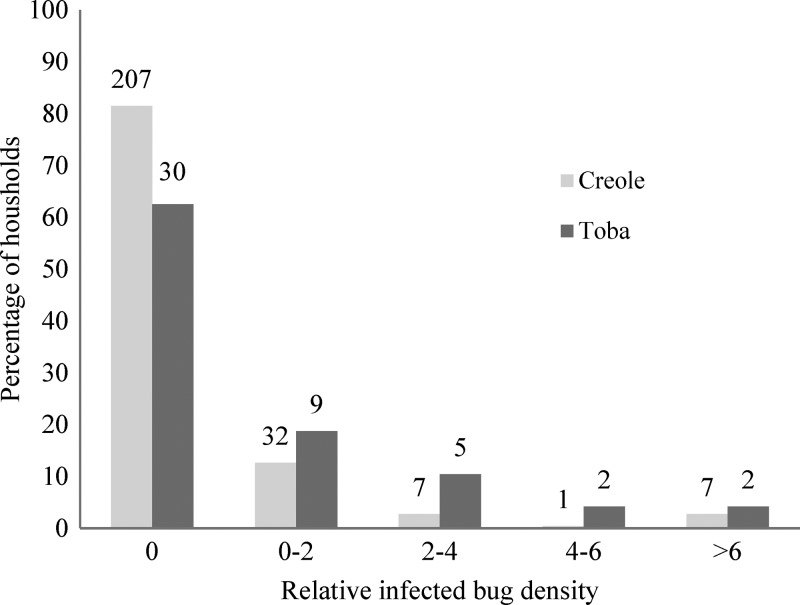
The percentage of domiciles with at least one infected bug was nearly two times more frequent among Tobas (37.5%) than Creoles (17.1%; Fisher's test, P = 0.003). The same trend was recorded in kitchens and storerooms (12.0% and 6.5%, respectively), but it was not statistically significant (Fisher's test, P = 0.2). Infected bug abundance per unit of catch effort in domiciles, kitchens, and storerooms was significantly larger in Toba than Creole households (Mann–Whitney, P < 0.005) (Figure 3).
Figure 3.
Distribution of infected bug abundance per unit of catch effort according to household ethnic group. Numbers on top of bars are numbers of households.
Host infection prevalence was almost twofold higher in Toba than Creole households in both dogs (38.9% and 22.0%; Fisher's test, P = 0.001) and cats (52.9% and 22.9%, P = 0.03) (Figure 2). Similarly, Toba households had two times as many infected dogs (1.3; SD = 1.7) and cats (0.3; SD = 0.6) per house than Creole households (0.6; SD = 0.9 and 0.1; SD = 0.3, respectively). Within each ethnic group, dogs and cats had rather similar infection prevalence.
Risk factors for host infection.
Univariate analyses showed that infection in dogs born before the community-wide insecticide spraying was associated significantly with age and origin of the dog, householders' ethnic background, the number of other infected dogs or cats in the house, and infected bug abundance in domiciles, kitchens, and storerooms (Table 1). Cat infection was associated significantly with household number of infected dogs or cats, ethnicity, and infected bug abundance.
Table 1.
Prevalence of T. cruzi infection according to potential risk factors in dogs and cats born before residual spraying with insecticides of all houses in Pampa del Indio in 2008
| Factor | Dogs | Cats | ||
|---|---|---|---|---|
| Percent infected (number examined) | Univariate odds ratio (95% CI) | Percent infected (number examined) | Univariate odds ratio (95% CI) | |
| Age (months) | – (371) | 1.0 (1.00–1.01) | – (57) | 1.0 (0.95–1.01) |
| Sex | ||||
| Male | 27.7 (282) | 1.0 | 30.8 (39) | 1.0 |
| Female | 38.2 (89) | 1.6 (1.0–2.7) | 44.4 (18) | 1.8 (0.6–5.7) |
| Domestic resting habit | ||||
| No | 33.3 (81) | 1.0 | n.d. | n.d. |
| Yes | 29.8 (272) | 0.8 (0.5–1.4) | n.d. | n.d. |
| Ethnic group | ||||
| Creole | 25.3 (293) | 1.0 | 27.1 (48) | 1.0 |
| Toba | 50.0 (82) | 2.9 (1.8–4.9)* | 77.8 (9) | 9.4 (1.7–51.4)† |
| Origin | ||||
| Urban immigrant | 18.7 (91) | 1.0 | 36.4 (11) | 1.0 |
| Rural immigrant | 19.1 (21) | 1.0 (0.3–3.4) | 100.0 (1) | – |
| Native | 37.1 (213) | 2.6 (1.4–4.7)* | 29.7 (37) | 0.7 (0.2–3.0) |
| Number of infected dogs or cats with which the dog cohabited | ||||
| 0 | 17.2 (204) | 1.0 | 19.2 (26) | 1.0 |
| 1 | 25.0 (84) | 1.6 (0.9–3.0) | 12.5 (16) | 0.6 (0.1–3.5) |
| ≥ 2 | 67.8 (87) | 10.2 (5.7–18.1)* | 86.7 (15) | 27.3 (4.6–161.8)* |
| Infected bug abundance in domiciles, kitchens, and storerooms | ||||
| Uninfested premises‡ | 21.6 (190) | 1.0 | 26.7 (30) | 1.0 |
| 0 | 18.3 (71) | 0.8 (0.4–1.6) | 11.1 (9) | 0.3 (0.0–3.2) |
| 1–9 | 46.5 (86) | 3.2 (1.8–5.5) | 57.1 (14) | 3.7 (1.0–13.9) |
| ≥ 10 | 79.2 (24) | 13.8 (4.9–39.2) | 75.0 (4) | 8.2 (0.7–91.2) |
| Oral infection risk | ||||
| No | 20.0 (15) | 1.0 | n.d | n.d. |
| Yes | 31.8 (277) | 1.8 (0.5–6.8) | n.d | n.d. |
n.d. = not done.
P < 0.001.
P < 0.01.
Data missing for 33 and 15 dogs inhabiting Creole and Toba households, respectively.
The multimodel inference framework identified only three models of dog infection that had strong support (ΔAICc ≤ 2), whereas the other 12 models had considerable support (Table 2). The predictors with high RI present in the top three models were age of the dog (RI = 1.00), the household number of infected dogs or cats with which the dog cohabited (RI = 0.94), and infected bug abundance (RI = 0.91). Ethnic background (RI = 0.57) had an intermediate RI. Domestic resting habit, origin, sex of dog, and exposure to oral infection were unimportant (RI < 0.35). The averaged logistic model fitted the data closely (χ2 = 5.7, degrees of freedom [df] = 8, P = 0.68), and the area under the ROC curve was 0.84. The same qualitative results were obtained with the second global model (AICc = 363.0, log likelihood = −172.2); the same predictors of dog infection with high RI were identified in the top models, and the RI for ethnicity (0.49) was also intermediate. However, model uncertainty declined, which was reflected in increasing Akaike weights for the top models. Model-averaged coefficients for the second global model are shown in Supplemental Table 1. Global models, including the interaction between ethnic background and age (RI = 0.54; regression coefficient ± SD = 0.019 ± 0.010), had a marginally better fit than the models with no interaction (AICc = 364.7; log likelihood = −170.3; likelihood ratio test, χ2 = 3.8, df = 1, P < 0.06), with ethnicity effects gaining more prominence (RI = 0.76). All interaction terms between ethnicity and other factors were unimportant.
Table 2.
Multimodel assessment of factors associated with T. cruzi infection in dogs born before residual spraying with insecticides of all houses in Pampa del Indio in 2008
| Model | df | Variables analyzed* | Model fit | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (1) Age | (2) Infected bug abundance | (3) Ethnicity | (4) Origin | (5) Cohabiting with infected dogs/cats | (6) Oral infection risk | (7) Sex | (8) Domestic resting habit | Log likelihood | ΔAICi† | ωi | |||
| 1 | 1235 | 9 | X | X | X | – | X | – | – | – | −132.0 | 0.00 | 0.14 |
| 2 | 125 | 8 | X | X | – | – | X | – | – | – | −133.6 | 1.05 | 0.08 |
| 3 | 12357 | 10 | X | X | X | – | X | – | X | – | −131.8 | 1.76 | 0.06 |
| 4 | 12358 | 10 | X | X | X | – | X | – | – | X | −132.0 | 2.09 | 0.05 |
| 5 | 12356 | 10 | X | X | X | – | X | X | – | – | −132.0 | 2.14 | 0.05 |
| 6 | 1257 | 9 | X | X | – | – | X | – | X | – | −133.1 | 2.17 | 0.05 |
| 7 | 12345 | 11 | X | X | X | X | X | – | – | – | −131.0 | 2.19 | 0.05 |
| 8 | 1245 | 10 | X | X | – | X | X | – | – | – | −132.3 | 2.74 | 0.04 |
| 9 | 1258 | 9 | X | X | – | – | X | – | – | X | −133.5 | 3.02 | 0.03 |
| 10 | 1256 | 9 | X | X | – | – | X | X | – | – | −133.5 | 3.07 | 0.03 |
| 11 | 12457 | 11 | X | X | – | X | X | – | X | – | −131.6 | 3.44 | 0.03 |
| 12 | 123457 | 12 | X | X | X | X | X | – | X | – | −130.6 | 3.67 | 0.02 |
| 13 | 123578 | 11 | X | X | X | – | X | – | X | X | −131.8 | 3.86 | 0.02 |
| 14 | 123567 | 11 | X | X | X | – | X | X | X | – | −131.8 | 3.92 | 0.02 |
| 15 | 12578 | 10 | X | X | – | – | X | – | X | X | −133.0 | 4.18 | 0.02 |
| RI | 1.00 | 0.91 | 0.57 | 0.32 | 0.94 | 0.26 | 0.34 | 0.27 | |||||
ΔAICci = AICci − AICcmin. ωi = exp(−1/2 ΔAICci)/Σ exp (−1/2 ΔAICci). X = variable included in the model; – = variable not included in the model.
Variables (detailed in the text): age, reconstructed age (months), infected bug abundance (four levels), ethnicity, ethnic background of the animal's household (Toba or Creole), origin (three levels), number of T. cruzi-infected dogs or cats with which the animal cohabited (three levels), oral infection risk, sex, and whether the dog rested in domiciles, kitchens, or storerooms (two levels).
Lowest AICc = 282.66.
In cats, both the household number of other infected dogs and cats and ethnic background had large RI (RI = 1.0) when included separately in the global model, whereas age and infected bug abundance were less important (RI < 0.25; not shown). The area under the ROC curve was 0.77.
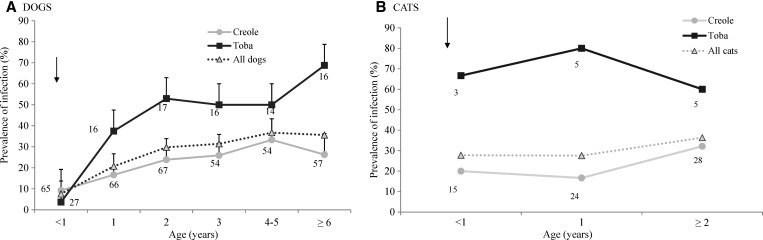
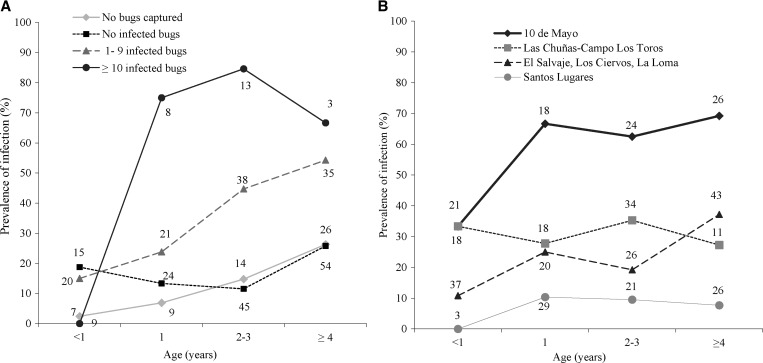
Dog infection prevalence increased with age (substantially more so in Toba households [Figure 4A], where it steadily increased from 3.7% in animals ages < 1 year to 68.8% in older animals). Infection in Creoles' dogs increased less steeply (from 9.2% to 26.3%) for the same age classes. The age-specific proportion of infected dogs predicted by the catalytic model departed from the observed data in a highly significantly fashion (χ2 = 34.7, df = 5, P < 0.0001), which also occurred for each separate ethnic group (data not shown).
Figure 4.
Age-specific composite prevalence of T. cruzi in dogs and cats according to household ethnic group in Pampa del Indio in 2008. (A) Dogs. (B) Cats. Twelve dogs and five cats of unknown age were excluded. Numbers close to data points represent the numbers of animals examined for infection. Arrows indicate the community-wide insecticide spraying campaign. Whiskers indicate the upper limit of the 95% CI.
Unlike dogs, in cats, the prevalence of T. cruzi infection increased slightly from 27.8% in animals < 1 year up to 36.4% in cats ages ≥ 2 years (Figure 4B). Cat infection in Toba households fluctuated between 60.0% and 80.0%, with no clear age-related trend among the few animals examined (N = 13), whereas in Creole households, infection increased twofold (from 16.7% to 32.1%) in cats ages ≥ 2 years.
We investigated variations in age–infection curves of dogs in relation to infected bug abundance and village (Figure 5). In households with either no infestation or no infected bugs detected, infection increased from 3% to 18% in dogs ages < 1 year to 26% in dogs ages ≥ 4 years, whereas with higher infected bug abundance, infection fluctuated between 67% and 85% in dogs ≥ 1 year of age (Figure 5A). Age–infection prevalence curves varied widely between clusters of adjacent villages, with a steeper curve in 10 de Mayo village (where Toba households comprised approximately 70% of all units) (Figure 5B). In other villages, age–infection curves were mostly flat or increased very slightly with age.
Figure 5.
Age-specific prevalence of T. cruzi in dogs in Pampa del Indio in 2007 and 2008 according to (A) the relative abundance of peridomestic infected T. infestans and (B) clusters of adjacent villages. Numbers close to data points represent the numbers of dogs examined for infection. Only dogs born before insecticide spraying are included, with ages reconstructed at the time of control interventions.
Post-intervention infections.
Infection prevalence was 7.5% in 94 dogs and 20.8% in 24 cats examined that had been born after the community-wide insecticide spraying. We could not clearly identify a single infection route for these animals. Seven infected dogs and three of five infected cats (all ages 3–7 months) lived in apparently uninfested houses at the time of the infection survey; two other cats lived in houses with infested domiciles or nearby peridomestic ecotopes. Six of the infected dogs were native, and only one dog had traveled outside the study area. One infected dog and one infected cat were born to T. cruzi-seropositive mothers; the remainder had missing data for this variable. Six of seven infected dogs were fed with raw viscera or blood from fresh kills of wild or domestic animals.
Demography of domestic animals.
Dogs were 4.6 times more abundant than cats; 96% of houses owned at least one dog, and 49% of houses owned at least one cat (Table 3). The percentage of dog-owning households among Creoles (98%) was significantly higher than in Toba households (88%; Fisher's test, P = 0.03). However, the mean number of dogs (3.0) and cats (0.7) per household did not differ significantly between ethnic groups (Mann–Whitney test; P > 0.3 for dogs and P > 0.4 for cats). The overall sex ratio in dogs was significantly skewed to males (χ2 = 64.1, df = 1, P < 0.0001) and differed substantially between Creole (male to female = 4.0) and Toba (male to female = 1.1) households. Among cats, the overall sex ratio was also biased to males (male to female = 1.8, χ2 = 4.3, df = 1, P < 0.05). Both host populations were very young, regardless of the household's ethnic background, with median ages of 24 months for dogs and 12 months for cats.
Table 3.
Demographic attributes of dog and cat populations from Creole and Toba households in Pampa del Indio in 2008
| Attribute | Dogs | Cats | ||||
|---|---|---|---|---|---|---|
| Creole | Toba | Total | Creole | Toba | Total | |
| Total number (animal-to-human ratio) | 410 (0.77) | 115 (0.59) | 525 (0.72) | 95 (0.18) | 19 (0.10) | 114 (0.16) |
| Households owning animals (%) | 98 | 88* | 96 | 50 | 41 | 49 |
| Mean number per household (SD) | 2.9 (1.7) | 3.4 (2.3) | 3.0 (1.8) | 0.7 (0.8) | 0.6 (0.8) | 0.7 (0.8) |
| Sex ratio (male per female) | 4.0 | 1.1 | 2.9† | 1.8 | 2.2 | 1.8* |
| Median age, months (first–third quartiles) | 24 (12–48) | 24 (12–48) | 24 (12–48) | 14 (12–36) | 12 (12–24) | 12 (12–30) |
| Domestic resting habits (%) | 47 | 55 | 48‡ | 66 | 71 | 67§ |
| Native (%) | 64 | 82† | 68¶ | 69 | 92 | 72⊥ |
| Born over previous 9 months (%) | 20 | 21 | 20 | 29 | 16 | 27 |
P < 0.05.
P < 0.001.
Data missing for 33 and 15 dogs inhabiting Creole and Toba households, respectively.
Data missing for seven and two cats inhabiting Creole and Toba households, respectively.
Data missing for 69 and 13 dogs inhabiting Creole and Toba households, respectively.
Data missing for 10 and 7 cats inhabiting Creole and Toba households, respectively.
Both dogs and cats were significantly more frequently born in the study area (native) in Toba (82–92%) than Creole (64–69%) households (Table 3). In consequence, the age at which dogs entered the house differed significantly between ethnic groups (χ2 = 16.6, df = 2, P < 0.001); more dogs were born at the house where they were listed among Toba (57%) than Creole (33%) households. Of all dogs and cats listed, 20–27% had been born during the 9-month period after the insecticide spraying campaign. A similar fraction of the dogs (77%) and cats (74%) enumerated initially was alive and present in the study villages 13–14 months later. Very few dogs were reported to have ever traveled with their owners in Toba (3%) and Creole (5%) households. More cats (67%) than dogs (48%) had domestic resting habits, but both hosts also slept in storerooms or kitchens (25–27%).
The main reported functions of dogs were hunting (45%), guardian or working with livestock (53%), and pet (2%). Tobas used dogs for hunting (52%) more frequently than Creoles (38%; Fisher's test, P = 0.01). Most dogs were fed raw viscera (84%) and allowed to drink blood from fresh kills (84%), regardless of ethnic group (Fisher's test, P = 0.08).
Discussion
We made four conclusions from our study. (1) Toba households were at a substantially greater risk of infection than Creole households. (2) T. cruzi infection in dogs was closely associated with explanatory variables that reflected vector-borne transmission at a household level. (3) There were strong heterogeneities in the distributions of bug, dog, and cat infections at household, village, and ethnic group levels. (4) The demography of dogs and cats differed substantially between ethnic groups in several respects relevant to parasite transmission.
Household ethnic background was a risk factor for dog and cat infection, with Toba households having significantly higher infected bug abundance and infection prevalence in bugs, dogs, and cats and more infected dogs or cats. In the multimodel analysis of dog infection, the RI of ethnic background was moderately high when the interaction between ethnicity and age of dog was included. The excess risk associated with ethnic background may be interpreted in terms of poverty-related factors (e.g., more precarious housing conditions),14 which directly affected both the presence and abundance of infected bugs. The excess risk in Toba households is expressed clearly in the increasing age–infection curve (Figure 4A) and the interaction between ethnicity and age of dog, indicating higher exposure during the preceding years.
These findings are consistent with the generalized notion that indigenous populations and other marginalized groups are more exposed to Chagas and other neglected tropical diseases.6,9,28 However, some marginalized Creole groups (e.g., in Campo Los Toros village) were indistinguishable from Toba households in terms of reduced livelihoods, house infestation, and vector or host infection patterns, pointing to the underlying socioeconomic roots of the risk differential.
Infection in dogs born before the community-wide insecticide spraying was associated positively and strongly with variables reflecting the intensity and duration of exposure to infected bugs at the household level, which is in agreement with other empirical studies and predictions of a mathematical model of transmission.15,16,19,21,29 Native dogs were more frequently infected than urban immigrant dogs, which was expected. The fact that rural immigrant dogs were less frequently infected than native dogs is most likely related to the latter being more common in Toba households, where they were more exposed to infection from an earlier age.
A novel finding of our study is that infection prevalence slightly increased with age for dogs residing in houses that were apparently uninfested or contained no infected bugs. This pattern may be explained by one or more of the following mechanisms: (1) misclassification of house infestation status caused by the limited sensitivity of the methods used to detect bugs and bug infection,14,30,31 perhaps combined with more efficient parasite transmission at low infected bug densities; (2) vertical transmission15,16; (3) undocumented changes in exposure to bugs in the recent past; and (4) other sources of infection not accounted for, including orally acquired infections, travel history to other infested houses or villages, and transmission mediated by sylvatic triatomine bugs (apparently of minor local significance).32
In support of undocumented changes in exposure to bugs (mechanism 3), differences between study villages in age-related trends in dog infection and the lack of fit of the catalytic model13,19 provide strong evidence that the risk of infection had not been spatially and temporally homogeneous over the previous 5–10 years. Domestic infestations also varied 10-fold between villages, despite the fact that the area was apparently homogeneous.14 These strong differences between villages in part may be explained by variations in the type and frequency of insecticide applications conducted incidentally by villagers and other support groups.
The link between T. cruzi infection in dogs and a putative oral transmission route (mechanism 4) had very little empirical support in the multimodel analysis. However, the finding of 2 of 44 dogs infected with T. cruzi III33—a genotype entirely restricted to local armadillos32,34—combined with the widespread habit of feeding dogs with raw viscera and fresh blood suggest the occurrence of events of oral transmission from sylvatic sources. One of the dogs infected with T. cruzi III was a 3-month-old pup born in an uninfested house after the insecticide spraying campaign that was reportedly fed with blood or raw viscera. Because its mother's infection status was unknown, either oral or vertical transmission may explain the occurrence of this sylvatic genotype.
The distribution of T. cruzi infection in dogs, cats, and bugs was aggregated at the household level, which constitutes a generalized pattern13,15,16,19 and supports the use of random effects logistic regression models. Another finding of our study was the frequent occurrence of infected bugs in kitchens and storerooms, which are frequent resting sites of dogs, cats, and chickens. Because these structures were within the walking range of triatomines,35 they may be a source of infected bugs that invade human habitations.
Both dogs and cats were very strongly associated with the occurrence of T. cruzi infection in T. infestans populations in the study area. Nearly all (96%) of the infected bugs were collected in houses with at least one infected dog, a fact that is consistent with the high infectiousness of infected dogs.19 Moreover, dog infections were more widely distributed at the household level than cat or bug infection. Molecular typing of T. cruzi stocks isolated from dogs, cats, and T. infestans showed that they all shared the same parasite discrete typing units (TcV and TcVI) and therefore, were components of the same transmission cycle.33,36 Infection in cats exceeded that in dogs consistently over the same age groups (Figure 4), perhaps because they had more frequent domestic resting habits than dogs. Unlike dogs, domestic cats do not fulfill the desirable attributes of a natural sentinel of parasite transmission,37 because they are much fewer and more difficult to handle than dogs.
The demography of local dog and cat populations presented several features common to resource-limited rural areas in northern Argentina, such as fast population turnover rates and a strongly biased sex ratio to males.15,16,21,38 However, it differed substantially between ethnic groups in several respects. The balanced sex ratio in dogs from Toba households suggests that they did not regulate dog numbers by culling female pups and therefore, had more dogs from an earlier age. These greater rates of recruitment of susceptible hosts may increase the basic reproduction number of T. cruzi in dogs, with more vertical infections and more native cases occurring in Toba households. In contrast, Creole households brought dogs and cats from outside the study area much more frequently than Tobas, which may increase the chance of introducing infected animals (depending on the sources).
Our study has both limitations and strengths. Although our initial assignment of ethnic background was based on the appearance and language spoken by the male head of the family who was interviewed rather than self-identification, census data collected subsequently corroborated the initial assignment. A thorough description of ethnic groups and a sociological analysis of the reasons or causes leading to the observed differences between ethnic groups are beyond the scope of the current study and warrant the use of appropriate ethnographic and social methods. To allow for the 9-month time lag between domestic animal surveys and the insecticide spraying campaign, risk factor analysis only included animals born before interventions. A consequence of this time lag combined with the fast annual turnover rates of dog populations (averaging 22.5%) is that the observed infection prevalence in dogs underestimated the expected prevalence at baseline (29.8%) roughly by 15%.38 Although the villages selected for domestic animal surveys were not chosen at random, the results may be representative of the entire area, which was suggested by the very slight differences (3%) in average bug infection prevalence between selected and excluded villages. Major strengths of our study are the large sample of households, bugs, and domestic animals examined with high coverage, the simultaneous use of validated serological and parasitological methods to minimize misclassification bias, and the stratified analysis of household-level data on infestation and bug infection combined with host infection and demographics.
Our findings have implications for vector and disease control. A direct consequence of the various heterogeneities involved in parasite transmission39 is that targeting high-risk households (or villages) that concentrate T. cruzi infection (here, about 25% of all houses) with enhanced vector surveillance and control actions will exert a strong impact on parasite transmission levels and prevention.40 Such households and villages are also immediate targets for human diagnosis and etiologic treatment with the available drugs. Proxy indicators of high-risk households include domestic bug abundance (infected or total), precarious housing quality, and lack of effective use of insecticides.
Our findings show that dogs and cats are key components of domestic and peridomestic transmission cycles in the humid Chaco and support the use of domestic dogs as sentinels of transmission.3,15,16,21 There are few management options for T. cruzi-infected dogs and cats in terms of community acceptance, ethics, and cost-effectiveness, and their relative merits are unclear. Dog and cat owners are not prone to simply dispense with infected animals that are fully asymptomatic. Insecticide-impregnated collars and vaccines are expected to reduce domestic transmission41,42 but still need to pass field trials and cost-effectiveness analysis.
Our study documents active transmission of T. cruzi in rural villages of the humid Argentinean Chaco almost 15 years after the Southern Cone Initiative was launched.2,3 Compared with other resource-limited rural communities in the dry Chaco,14–16 various transmission indices reflected the absence of both insecticide spraying campaigns and an effective vector surveillance and response system. This pattern was, in part, mitigated by non-systematic domestic applications of insecticide by householders and other support groups. Sustainable vector and disease control in the Gran Chaco demands an integrated strategy that addresses the multilevel heterogeneities recorded and the socioeconomic and cultural dimensions of the problem.
Supplementary Material
ACKNOWLEDGMENTS
The authors thank Jorge Nasir and the Chagas Control Program of Chaco for support in field operations; Francisco Petrocco, Laura Tomassone, Marina Leporace, Leonardo A. Lanati, and M. Laura Peresan Martínez for field and laboratory assistance; María del Pilar Fernández for statistical advice; and the villagers of Pampa del Indio for kindly welcoming us into their homes and cooperating with the investigation.
Disclaimer: The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
Financial support: This study was supported by awards from the International Development Research Center (EcoHealth Program), United Nations Children's Fund, the United Nations Development Programme, the World Bank and the World Health Organization Special Programme for Research and Training in Tropical Diseases, University of Buenos Aires (to R.E.G.), and the National Institute of Environmental Health Sciences (to U.K., R.E.G., and Joel Cohen) as well as National Institutes of Health/National Science Foundation Ecology of Infectious Disease Program Award R01 TW05836 funded by the Fogarty International Center. M.V.C., M.M.O., and R.E.G. are members of Consejo Nacional de Investigaciones Científicas y Técnicas Researcher's Career.
Authors' addresses: M. Victoria Cardinal, M. Marcela Orozco, Gustavo F. Enriquez, Leonardo A. Ceballos, María Sol Gaspe, Julián A. Alvarado-Otegui, Juan M. Gurevitz, and Ricardo E. Gürtler, Laboratory of Eco-Epidemiology, Department of Ecology, Genetics and Evolution, University of Buenos Aires, Buenos Aires, Argentina, E-mails: mvcardinal@ege.fcen.uba.ar, marcelaorozco.vet@gmail.com, gustavoenriquez@ege.fcen.uba.ar, leonefasto@ege.fcen.uba.ar, solgaspe@ege.fcen.uba.ar, lavizcachasp@hotmail.com, jmgurevitz@yahoo.com.ar, and gurtler@ege.fcen.uba.ar. Uriel Kitron. Department of Environmental Studies, Emory University, Atlanta, GA, E-mail: ukitron@emory.edu.
References
- 1.World Health Organization . Report of the Scientific Working Group on Chagas Disease. Geneva: World Health Organization; 2007. [Google Scholar]
- 2.Schofield CF, Jannin J, Salvatella R. The future of Chagas disease control. Trends Parasitol. 2006;22:583–588. doi: 10.1016/j.pt.2006.09.011. [DOI] [PubMed] [Google Scholar]
- 3.Gürtler RE, Kitron U, Cecere MC, Segura EL, Cohen JE. Sustainable vector control and management of Chagas disease in the Gran Chaco, Argentina. Proc Natl Acad Sci USA. 2007;104:16194–16199. doi: 10.1073/pnas.0700863104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gürtler RE. Eco-epidemiología regional de la transmisión vectorial: enfermedad de Chagas en el Gran Chaco. In: Silveira AC, editor. La enfermedad de Chagas a la puerta de los 100 años del conocimiento de una endemia americana ancestral. Buenos Aires, Argentina: Panamerican Health Organization—Fundación Mundo Sano; 2007. [Google Scholar]
- 5.Basombrío MA, Segovia A, Peralta Ramos M, Esteban E, Stumpf R, Jurgensen P, Winkler MA, Sayre K, Ferrer JF. Endemic Trypanosoma cruzi infection in Indian populations of the Gran Chaco territory of South America: performance of diagnostic assays and epidemiological features. Ann Trop Med Parasitol. 1999;93:41–48. doi: 10.1080/00034989958780. [DOI] [PubMed] [Google Scholar]
- 6.Alonso J, Fabre A, Galván M, Lucero R, Brusés B, Kuc A. La enfermedad de Chagas en poblaciones aborígenes del noreste de Argentina. Enf Emerg. 2009;11:115–118. [Google Scholar]
- 7.Sosa-Estani S, Dri L, Touris C, Abalde S, Dell'Arciprette A, Braunstein J. Transmisión vectorial y congénita del Trypanosoma cruzi en Las Lomitas, Formosa. Medicina (B Aires) 2009;69:424–430. [PubMed] [Google Scholar]
- 8.Chapman MD, Baggaley RC, Godfrey-Fausset PF, Malpas TJ, White G, Canese J, Miles MA. Trypanosoma cruzi from the Paraguayan Chaco – isoenzyme profiles of strains isolated at Makthlawaiya. J Protozool. 1984;31:482–486. doi: 10.1111/j.1550-7408.1984.tb02999.x. [DOI] [PubMed] [Google Scholar]
- 9.Biancardi MA, Moreno MC, Torres N, Pepe C, Altcheh J, Freilij H. Seroprevalencia de la enfermedad de Chagas en 17 parajes del ‘‘Monte Impenetrable’’ de la Provincia del Chaco. Medicina (B Aires) 2003;63:125–129. [PubMed] [Google Scholar]
- 10.Moretti E, Castro I, Franceschi C, Basso B. Chagas disease: serological and electrocardiographic studies in Wichi and Creole communities of Misión Nueva Pompeya, Chaco, Argentina. Mem Inst Oswaldo Cruz. 2010;105:621–626. doi: 10.1590/s0074-02762010000500004. [DOI] [PubMed] [Google Scholar]
- 11.Rojas de Arias A, Monzón MI, Velázquez de Saldívar G, Guillén E, Arrua NT. A seroepidemiologic survey of Chagas’ disease in two Paraguayan villages. Bull Pan Am Health Organ. 1984;18:164–171. [PubMed] [Google Scholar]
- 12.Mott KE, Mota EA, Sherlock I, Hoff R, Muniz TM, Oliveira TS, Draper CC. Trypanosoma cruzi infection in dogs and cats and household seroreactivity to T. cruzi in a rural community in northeast Brazil. Am J Trop Med Hyg. 1978;27:1123–1127. doi: 10.4269/ajtmh.1978.27.1123. [DOI] [PubMed] [Google Scholar]
- 13.Gürtler RE, Cecere MC, Lauricella MA, Petersen RM, Chuit R, Segura EL, Cohen JE. Incidence of Trypanosoma cruzi infection among children following domestic reinfestation after insecticide spraying in rural northwestern Argentina. Am J Trop Med Hyg. 2005;73:95–103. [PMC free article] [PubMed] [Google Scholar]
- 14.Gurevitz JM, Ceballos LA, Gaspe MS, Alvarado-Otegui JA, Enríquez GF, Kitron U, Gürtler RE. Factors affecting infestation by Triatoma infestans in a rural area of the humid Chaco in Argentina: a multi-model inference approach. PLoS Negl Trop Dis. 2011;5:e1349. doi: 10.1371/journal.pntd.0001349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cardinal MV, Lauricella MA, Marcet PL, Orozco MM, Kitron U, Gürtler RE. Impact of community-based vector control on house infestation and Trypanosoma cruzi infection in Triatoma infestans, dogs and cats in the Argentine Chaco. Acta Trop. 2007;103:201–211. doi: 10.1016/j.actatropica.2007.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cardinal MV, Castañera MB, Lauricella MA, Cecere MC, Ceballos LA, Vazquez-Prokopec GM, Kitron U, Gürtler RE. A prospective study of the effects of sustained vector surveillance on Trypanosoma cruzi infection of dogs and cats in rural northwestern Argentina. Am J Trop Med Hyg. 2006;75:753–761. [PMC free article] [PubMed] [Google Scholar]
- 17.Crisante G, Rojas A, Teixeira MMG, Añez N. Infected dogs as a risk factor in the transmission of human Trypanosoma cruzi infection in western Venezuela. Acta Trop. 2006;98:247–254. doi: 10.1016/j.actatropica.2006.05.006. [DOI] [PubMed] [Google Scholar]
- 18.Estrada-Franco JG, Bhatia V, Diaz-Albiter H, Ochoa-Garcia L, Barbosa A, Vazquez-Chagoyan JC, Martinez-Perez MA, Guzman-Bracho C, Garg N. Human Trypanosoma cruzi infection and seropositivity in dogs, Mexico. Emerg Infect Dis. 2006;12:624–630. doi: 10.3201/eid1204.050450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gürtler RE, Cecere MC, Lauricella MA, Cardinal MV, Kitron U, Cohen JE. Domestic dogs and cats as sources of Trypanosoma cruzi infection in rural northwestern Argentina. Parasitology. 2007;134:69–82. doi: 10.1017/S0031182006001259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gürtler RE, Cohen JE, Cecere MC, Chuit R, Segura EL. Influence of humans and domestic animals on the household prevalence of Trypanosoma cruzi in Triatoma infestans populations in northwest Argentina. Am J Trop Med Hyg. 1998;58:748–758. doi: 10.4269/ajtmh.1998.58.748. [DOI] [PubMed] [Google Scholar]
- 21.Castañera MB, Lauricella MA, Chuit R, Gürtler RE. Evaluation of dogs as sentinels of the transmission of Trypanosoma cruzi in a rural area of north-western Argentina. Ann Trop Med Parasitol. 1998;92:671–683. doi: 10.1080/00034983.1998.11813327. [DOI] [PubMed] [Google Scholar]
- 22.Enriquez GF, Cardinal MV, Orozco MM, Schijman AG, Gürtler RE. Detection of Trypanosoma cruzi infection in naturally infected dogs and cats using serological, parasitological and molecular methods. Acta Trop. 2013;126:211–217. doi: 10.1016/j.actatropica.2013.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lauricella MA, Castañera MB, Gürtler RE, Segura EL. Immunodiagnosis of Trypanosoma cruzi (Chagas disease) infection in naturally infected dogs. Mem Inst Oswaldo Cruz. 1998;93:501–507. doi: 10.1590/s0074-02761998000400016. [DOI] [PubMed] [Google Scholar]
- 24.Lauricella MA, Wisnivesky-Colli C, Gürtler R, Petersen R, Bujas M, Segura EL. Standardization of serological tests for detecting anti-Trypanosoma cruzi antibodies in dogs. Mem Inst Oswaldo Cruz. 1993;88:413–417. doi: 10.1590/s0074-02761993000300010. [DOI] [PubMed] [Google Scholar]
- 25.Brown LD, Cai TT, DasGupta A. Interval estimation for a binomial proportion. Stat Sci. 2001;16:101–117. [Google Scholar]
- 26.Burnham KP, Anderson DR. Model Selection and Multimodel Inference: A Practical Information-Theoretic Approach. New York, NY: Springer-Verlag; 2002. [Google Scholar]
- 27.R Development Core Team . R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing; 2012. [Google Scholar]
- 28.Ehrenberg JP, Ault SK. Neglected diseases of neglected populations: Thinking to reshape the determinants of health in Latin America and the Caribbean. BMC Public Health. 2005;5:119. doi: 10.1186/1471-2458-5-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cohen JE, Gürtler RE. Modeling household transmission of American trypanosomiasis. Science. 2001;293:694–698. doi: 10.1126/science.1060638. [DOI] [PubMed] [Google Scholar]
- 30.Gurevitz JM, Gaspe MS, Enriquez GF, Provecho YM, Kitron U, Gürtler RE. Intensified surveillance and insecticide-based control of the Chagas disease vector Triatoma infestans in the Argentinean Chaco. PLoS Negl Trop Dis. 2013;7:e2158. doi: 10.1371/journal.pntd.0002158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Marcet PL, Duffy T, Cardinal MV, Burgos JM, Lauricella MA, Levin MJ, Kitron U, Gürtler RE, Schijman AG. PCR-based identification of Trypanosoma cruzi lineages in feces of triatomine bugs from rural northwestern Argentina. Parasitology. 2006;132:57–65. doi: 10.1017/S0031182005008772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Alvarado-Otegui JA, Ceballos LA, Orozco MM, Enríquez G, Cardinal MV, Schijman AG, Kitron U, Gürtler RE. The sylvatic transmission cycle of Trypanosoma cruzi in the humid Chaco of Argentina. Acta Trop. 2012;124:79–86. doi: 10.1016/j.actatropica.2012.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Enriquez GF, Cardinal MV, Orozco MM, Lanati L, Schijman AG, Gürtler RE. Discrete typing units of Trypanosoma cruzi identified in rural dogs and cats in the humid Argentinean Chaco. Parasitology. 2012;140:1–6. doi: 10.1017/S003118201200159X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Orozco MM, Enriquez GF, Alvarado-Otegui JA, Cardinal MV, Schijman AG, Kitron U, Gürtler RE. New sylvatic hosts of Trypanosoma cruzi and their reservoir competence in the humid Chaco of Argentina: a longitudinal study. Am J Trop Med Hyg. 2013;88:872–882. doi: 10.4269/ajtmh.12-0519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Abrahan LB, Gorla DE, Catalá SS. Dispersal of Triatoma infestans and other Triatominae species in the arid Chaco of Argentina: flying, walking or passive carriage? The importance of walking females. Mem Inst Oswaldo Cruz. 2011;106:232–239. doi: 10.1590/s0074-02762011000200019. [DOI] [PubMed] [Google Scholar]
- 36.Maffey L, Cardinal MV, Lanati L, Lauricella MA, Schijman AG, Gürtler RE. Direct molecular identification of Trypanosoma cruzi discrete typing units in domestic and peridomestic Triatoma infestans and Triatoma sordida from the Argentine Chaco. Parasitology. 2012;139:1–10. doi: 10.1017/S0031182012000856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Anonymous . Animals as Sentinels of Environmental Health Hazards. Washington, DC: National Academy Press; 1991. [PubMed] [Google Scholar]
- 38.Gürtler RE, Kravetz FO, Petersen RM, Lauricella MA, Wisnivesky-Colli C. The prevalence of Trypanosoma cruzi and the demography of dog population after insecticidal spraying of houses: a predictive model. Ann Trop Med Parasitol. 1990;84:313–323. doi: 10.1080/00034983.1990.11812475. [DOI] [PubMed] [Google Scholar]
- 39.Woolhouse MEJ, Dye C, Etard JF, Smith T, Charlwood JD, Garnett GP, Hagan P, Hii JLK, Ndhlovu PD, Quinnell RJ, Watts CH, Chandiwana SK, Anderson RM. Heterogeneities in the transmission of infectious agents: implications for the design of control programs. Proc Natl Acad Sci USA. 1997;94:338–342. doi: 10.1073/pnas.94.1.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vazquez-Prokopec GM, Spillmann C, Zaidenberg M, Gürtler RE, Kitron U. Spatial heterogeneity and risk maps of community infestation by Triatoma infestans in rural northwestern Argentina. PLoS Negl Trop Dis. 2012;6:e1178. doi: 10.1371/journal.pntd.0001788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Reithinger R, Ceballos L, Stariolo R, Davies CR, Gürtler RE. Extinction of experimental Triatoma infestans populations following continuous exposure to dogs wearing deltamethrin-treated collars. Am J Trop Med Hyg. 2006;74:766–771. [PMC free article] [PubMed] [Google Scholar]
- 42.Basso B, Castro I, Introini V, Gil P, Truyens C, Moretti E. Vaccination with Trypanosoma rangeli reduces the infectiousness of dogs experimentally infected with Trypanosoma cruzi. Vaccine. 2007;25:3855–3858. doi: 10.1016/j.vaccine.2007.01.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.