Abstract
Chagas disease, caused by the protozoan parasite Trypanosoma cruzi, is a major cause of morbidity and mortality in Central and South America. Geographic variations in the sensitivity of serologic diagnostic assays to T. cruzi may reflect differences in T. cruzi exposure. We measured parasite-specific T-cell responses among seropositive individuals in two populations from South America with widely varying antibody titers against T. cruzi. Antibody titers among seropositive individuals were significantly lower in Arequipa, Peru compared with Santa Cruz, Bolivia. Similarly, the proportion of seropositive individuals with positive T-cell responses was lower in Peru than Bolivia, resulting in overall lower frequencies of interferon-γ (IFNγ)-secreting cells from Peruvian samples. However, the magnitude of the IFNγ response was similar among the IFNγ responders in both locations. These data indicate that immunological discrepancies based on geographic region are reflected in T-cell responses as well as antibody responses.
Introduction
The protozoan parasite Trypanosoma cruzi infects between 8 and 11 million people and causes human Chagas disease, which is endemic to most countries of Central and South America.1 Chagas disease is strongly associated with poverty and substandard housing, which facilitates close interaction of the triatomine vector and the human host. Infection is lifelong. Although the majority of infected individuals remains asymptomatic, 20–30% of infected persons develop cardiomyopathy and/or gastrointestinal megasyndrome. Despite the fact that parasites persist for the life of the host, the low numbers of circulating parasites during chronic infection have, to date, rendered parasitologic diagnosis ineffective, although polymerase chain reaction (PCR) methods for detecting parasites are improving and becoming standardized.2 Currently, diagnosis relies on serological detection of anti-T. cruzi antibodies using conventional serological tests, such as enzyme-linked immunoassays (ELISAs), immunofluorescence assays (IFAs), and Western blotting. These tests detect antibodies against whole-parasite lysates, trypomastigote-excreted/secreted antigens (TESAs), or recombinant proteins. The World Health Organization recommends positive results from two distinct serological assays to confirm a diagnosis of T. cruzi infection.1
No single serological test for Chagas disease has sufficient sensitivity and specificity to be used alone; a confirmed diagnosis relies on concordant results on at least two tests using different antigens and/or formats.3 There is evidence that the sensitivity of serological assays may differ depending on the geographic source of the specimens. Two different rapid diagnostic tests showed sensitivities of 87.5% and 90% in specimens from Bolivia compared with 30% and 54% in specimens from Peru.4 The specimens used for the rapid test evaluation were triply concordant by commercial ELISA, IFA, and radioimmunoprecipitation assay (RIPA) and therefore, may represent an overestimate of sensitivity of the evaluated tests.3 Lower sensitivity has also been observed for some assays in specimens from Panama5,6 and Mexico.7 These differences may be caused by variations in the T. cruzi strains inhabiting these different geographic ranges, because antigenic variation between strains or infectivity of the strains may alter the quality or quantity of the antibody response, leading to differential sensitivities to serodiagnostic tests.
In the comparison of rapid tests, specimens from either country that had false negative results had significantly lower antibody titers than those specimens with true positive results, and the distribution of antibody titers for specimens from Peru was significantly lower than the distribution for specimens from Bolivia, providing an explanation for the high rate of false negative results in Peru. Differences in sensitivity to T. cruzi serological assays by specimens from individuals living in different geographic regions likely represent a weaker adaptive immune response to the parasite. We hypothesize that the weaker adaptive immune responses in individuals with low antibody titers will be reflected in T-cell responses as well. In this study, we examined T-cell responses in individuals from Peru and Bolivia to test this hypothesis. Measuring interferon-γ (IFNγ) release from antigen-stimulated peripheral blood mononuclear cells (PBMCs), we observed that a lower proportion of seropositive individuals in Peru had cellular recall responses than individuals in Bolivia, but among IFNγ+ individuals, the frequency of antigen-responsive cells was similar.
Materials and Methods
Ethics statement.
Peruvian specimens were collected during four different studies, all with protocols approved by the Institutional Review Boards of Johns Hopkins University and Asociacion Benefica PRISMA. All Bolivian specimens were collected during a study with the protocol approved by the Institutional Review Boards of Johns Hopkins University and Hospital Universitario Japones. All participants provided written informed consent before specimen collection. In the case of children, informed consent was provided by the parent or guardian.
Study participants.
Peruvian specimens were collected from participants in four studies of Chagas disease conducted in or near Arequipa: 68 participants in a community study in LaJoya,8,9 17 participants in a study of urban screening in the city,10 14 participants in a community study in a periurban site,11 and 6 participants in a study of heart disease in an urban hospital (Kaplinski M, unpublished data). Six additional specimens were collected from healthy Peruvian volunteers. In Bolivia, specimen collection was performed as part of a study of the use of pupillometry to assess autonomic function in patients with Chagas disease (Halperin A, unpublished data). Table 1 shows participant demographic data. All samples for this study were collected between September of 2009 and September of 2010. Serum, plasma, and PBMC samples were all collected at the same time.
Table 1.
Population demographics (Peru versus Bolivia)
| Variables | Peru (n = 76)* | Bolivia (n = 112) | P value† |
|---|---|---|---|
| Sex | |||
| Male | 32 (42.1) | 44 (39.3) | |
| Female | 44 (57.9) | 68 (60.7) | 0.699 |
| Age (years) | |||
| ≤ 36 | 36 (47.4) | 14 (12.5) | |
| 37–49 | 16 (21.1) | 27 (24.1) | |
| 50–59 | 10 (13.2) | 41 (36.6) | |
| ≥ 60 | 14 (18.4) | 30 (26.8) | < 0.001 |
Peru sample restricted to those individuals with full serology data for all four tests.
Fisher's exact and Kwallis tests for comparison by country.
Parasites and parasite antigens.
T. cruzi trypomastigotes from five strains were cultured in Vero (green monkey kidney epithelial) cells in 162-cm2 tissue culture-treated flasks in a 37°C CO2 incubator. The strains used were Brazil (laboratory strain), Tc23 and Tc21 (isolated from the Arequipa/LaJoya region during the study), and DH29 and Viera Roq (Bolivian strains). Trypomastigotes were harvested from the culture supernatants and lysed by five to eight rounds of freezing and thawing. Cell debris was pelleted by centrifugation at 12,000 rpm in a microcentrifuge, and the supernatants were passed through a 0.22-μm filter. Protein levels were determined using a BCA assay (Pierce, Rockford, IL).
Serologic diagnosis.
One aliquot of each serum specimen was frozen without additives and shipped from the collection site in Santa Cruz or Arequipa to the Infectious Disease Laboratory (LID) at the Universidad Peruana Cayetano Heredia in Lima, Peru. An in-house IFA using standard methods and two commercially available ELISAs (Chagatek ELISA; bioMérieux, Marcy l'Etoile, France [based on epimastigote lysate antigen]; Chagatest Recombinante ELISA [recombinant proteins] and Wiener ELISA; Wiener Laboratories, Rosario, Argentina) were performed on all specimens. Using the manufacturer's instructions for the Chagatek plates, the cutoff was set at 0.100 optical density (OD) units above the mean absorbance of the two negative control specimens included on each plate. Absorbance (ABS) was defined as OD – cutoff. Any sample with a positive ABS value (ABS > 0) was defined as seropositive. For the Wiener plates, the cutoff was set at 0.300 OD units above the mean absorbance of three negative control specimens; specimens with OD values within the range of the cutoff ± 10% were considered inconclusive and rerun. If the OD still fell in this range, the final result was considered inconclusive. TESA in-house tests were also run on each sample. Specimens positive or negative by all three conventional serologic tests were considered to have confirmed results. Peruvian samples were also tested with an in-house ELISA using a locally isolated parasite strain. Briefly, the ELISA was performed in Immunolon 1B plates (Thermo Fisher Scientific, Pittsburgh, PA) coated for 18 hours with 1.5 μg/mL parasite antigen in bicarbonate buffer; then, serum was added at a 1/200 dilution followed by the conjugate 1/10,000 (goat anti-human immunoglobulin G (IgG); KPL, Gaithersburg, MD). The cutoff was calculated with the mean of negative control + 3 SDs (Arequipa epimastigote antigen ELISA [EAE]; LID, Lima, Peru).
PBMC isolation.
Blood was collected into one 8-mL cell preparation tube (CPT; BD, Franklin Lakes, NJ) containing sodium citrate, Ficoll, and a polyester plasma separator gel. Tubes were centrifuged 20 minutes, and PBMCs were removed from the Ficoll Buffy coat and washed two times in phosphate-buffered saline (PBS) containing 5% fetal bovine serum (FBS; Fisher Scientific, Pittsburgh, PA). PBMCs were cryopreserved in FBS containing 10% dimethyl sulfoxide (DMSO; Sigma Aldrich, St. Louis, MO) and shipped on LN2 to Centers for Disease Control and Prevention in Atlanta, GA for storage frozen in LN2 until analysis. Plasma was aliquoted to cryogenic vials and frozen at < −20°C.
Cell stimulation and ELISPOT.
PBMCs were thawed, and viability was assessed by trypan blue exclusion. Only samples with a cell viability of > 70% were examined by ELISPOT assay. Because of the variability in the numbers of cells obtained from each individual, the hierarchy of Tc21 > DH29 > Brazil > Tc23 > Viera Roq was chosen for the study to obtain responses from strains representing the different geographic regions as well as the Brazil strain, which has been previously used for in vitro stimulation of T-cell responses.
For assessment of T-cell responses to T. cruzi antigens, 4×105 PBMCs were stimulated with 25 μg/mL T. cruzi amastigote/trypomastigotes lysate for 16–20 hours in a 5% CO2 atmosphere at 37°C on ELISPOT plates (BD) coated with capture antibody for IFNγ.12 After washing off the cells, bound IFNγ was detected with a secondary biotinylated anti-human IFNγ antibody, which in turn, bound streptavidin conjugated to horseradish peroxidase (HRP). Colorimetric changes after the addition of HRP substrate and a chromogenic reagent will detect the presence of bound IFNγ secreted on antigenic stimulation, and the frequency of cytokine-secreting cells was determined using an ELISPOT plate reader (CTL Technologies, Cleveland, OH) and expressed as spot-forming units (SFUs).
Statistical analyses: normalization of IFNγ ELISPOT data.
Raw SFU data were log-transformed. Given differences in background ELISPOT responses in Bolivian versus Peruvian specimens, ELISPOT responses of a given specimen after stimulation with T. cruzi antigens were normalized to that specimen's paired media-only control. Fold change in ELISPOT response was then calculated using the formula [log (SFUT. cruzi) – log(SFUmedia) = fold change in ELISPOT response]. Individuals were further categorized as ELISPOT responders versus non-responders. Using ELISPOT response data from 30 healthy North American controls with no evidence of T. cruzi exposure, a cutoff of SFUs > 30 among media-only stimulated cells and a twofold increase in response after T. cruzi stimulation were used.
Participant demographics, T. cruzi serologic responses by Chagatek, and ELISPOT responses were compared by geographical region using the Wilcoxin rank sum test and χ2 test for continuous and categorical factors, respectively. Among specimens from Peru, differences in ELISPOT response by T. cruzi antigen were compared using the Kruskal–Wallis test and Wilcoxin rank sum test for assessment of ELISPOT response as continuous or categorical, respectively. The relationships of fold change in ELISPOT responses and T. cruzi antibody responses were compared by geographical region using Spearman's rank correlation and linear regression analysis adjusting for age. All analyses were performed using STATA 11 (College Station, TX). A P value < 0.05 is considered statistically significant.
Results
Study demographics and T. cruzi serology by geographic region.
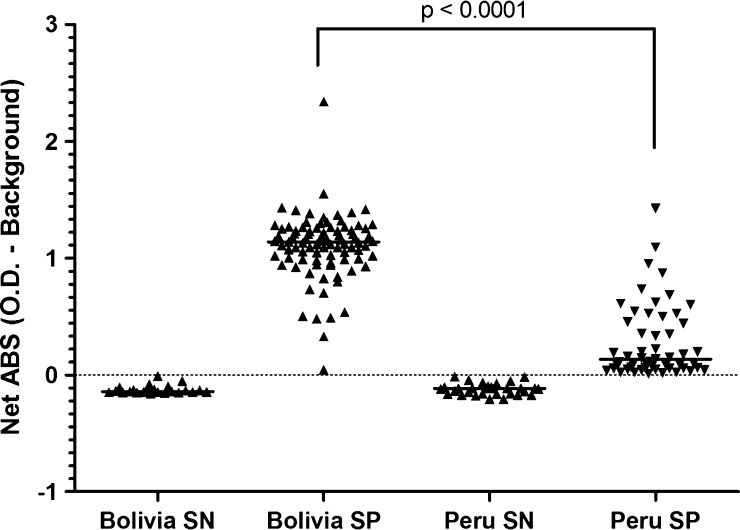
In total, 76 individuals from Arequipa, Peru and 112 individuals from Santa Cruz, Bolivia (referred to by their country of origin) were included in the current study. Individuals from Bolivia were more likely to be older than individuals from Peru (P < 0.01) (Table 1). Serum samples obtained by venipuncture after informed consent were assayed for T. cruzi-specific antibodies. Analysis was focused on comparisons of absorbance values among chronically infected seropositives between the two countries rather than the percent of people who were seropositive. As in previous evaluations, the distribution in absorbance values by Chagatek ELISA was significantly lower in specimens from Peru than Bolivia (0.194 versus 1.14; P < 0.01) (Figure 1).
Figure 1.
Differences in absorbance values of seropositive individuals in two different geographic regions. Sera from individuals in Bolivia (Santa Cruz) and Peru (Arequipa region) were tested using a Chagatek assay for detection of T. cruzi-specific antibodies. Seropositives were determined as described in Materials and Methods. SN = seronegative; SP = seropositive.
Comparison of parasite-specific T-cell responses between Bolivia and Peru.
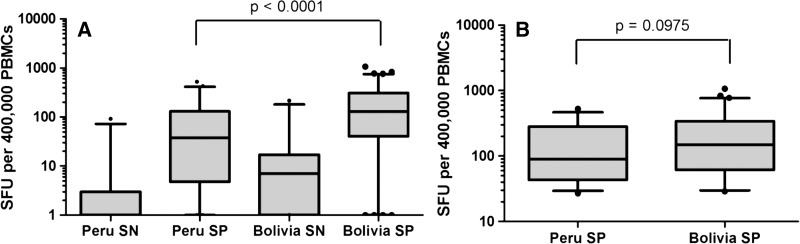
T. cruzi antigen-induced IFNγ responses were compared between specimens from Peru and Bolivia to determine if differences in antibody titers were reflected in T-cell responses. Among seropositives, median SFUs of parasite-stimulated IFNγ production were higher in individuals from Bolivia (median = 131.5, interquartile range = 41.0–328.5) compared with Peru (median = 40.0, interquartile range = 6.0–183.7; P < 0.01) (Figure 2A). Furthermore, response rates were higher for PBMC specimens from seropositive individuals from Bolivia than Peru (92.1% versus 66.7%; P < 0.01). When the analysis was restricted to those individuals seropositive by Chagatek assay who were defined as ELISPOT responders, the median number of SFUs was similar among specimens from Peru compared with Bolivia (P = 0.20) (Figure 2B). There were a few samples from Chagatek-seronegative individuals from each country that were IFNγ-positive (2 of 31 from Peru and 3 of 23 from Bolivia; data not shown), but these numbers did not differ by region. Additionally, two individuals from Peru with samples that tested Chagatek-negative but T cell-positive later tested positive by Wiener ELISA, Arequipa EAE, and the TESA blot (data not shown).
Figure 2.
Comparison of T. cruzi-specific T-cell responses by country stratified by Chagatek serologic status. PBMCs were stimulated with media alone or T. cruzi antigen (Tc21 strain) in an ELISPOT assay as described. Data are expressed as IFNγ SFUs induced by T. cruzi antigen. Data are displayed as a box and whiskers graph, with minimum and maximum values shown. (A) IFNγ responses from SN or SP individuals in Peru and Bolivia. (B) SFUs of only IFNγ responders.
IFNγ production among specimens from Peru by T. cruzi strain.
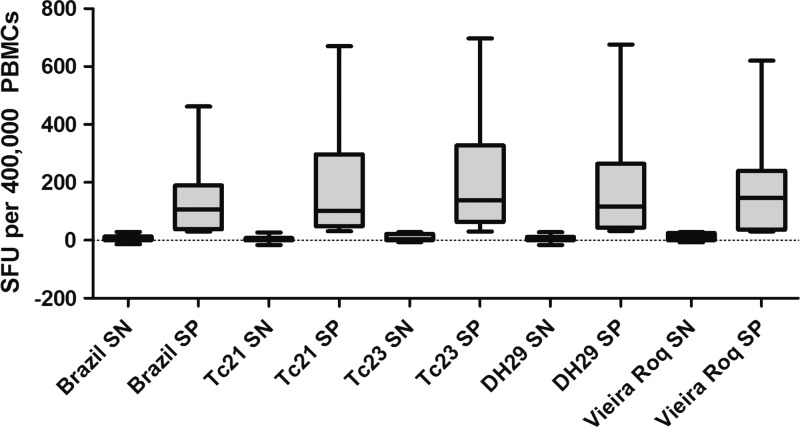
Cellular responses in Peruvian individuals to antigen obtained from parasites from Peru and Bolivia were examined. No statistically significant differences were observed in ELISPOT responses by T. cruzi strain (Figure 3 ). The percentage of patients responding to each T. cruzi strain ranged from 40% to 59.6% (Table 2 ). Among the samples from seropositive individuals producing IFNγ recall responses, over 80% responded to three or more of the parasite strains, and among these ELISPOT responders, similar frequencies of IFNγ-producing cells were observed (Table 2). In three samples, only one of five strains tested stimulated an IFNγ response, but in each case, the positive response fell just above the cutoff (SFU = 34, 36, and 37), indicating low frequencies of IFNγ-producing cells in those individuals. Two of 30 seronegative individuals produced IFNγ in response to antigen stimulation with three different strains (Table 2).
Figure 3.
IFNγ recall responses to various T. cruzi strains in Peruvians. PBMCs from Peruvian individuals were stimulated with whole-parasite lysate from each strain as shown. Data are displayed as a box and whiskers graph, with minimum and maximum values shown. P > 0.05 for all strains.
Table 2.
Analysis of Peruvian PBMCs stimulated with up to five different strain of T. cruzi
| T. cruzi strain | Number of samples tested | Number of seropositive samples tested | Number of seropositive samples positive for IFNg release (%) | Number of seronegative samples positive for IFNg release (%) |
|---|---|---|---|---|
| Tc21 | 82 | 52 | 31 (59.6) | 2* (6.7) |
| DH29 | 80 | 50 | 22 (44) | 1* (3.3) |
| Brazil | 70 | 45 | 18 (40) | 1* (3.3) |
| Tc23 | 59 | 34 | 19 (55.9) | 2* (6.7) |
| Vieira Roq | 50 | 30 | 15 (50) | 0 (0) |
In total, 2 of 30 seronegative samples tested positive against 3 antigens each: one against Brazil, Tc21, and Tc23, and one against Tc21, Tc23, and DH29.
Relationship between serologic and T-cell responses in Peru versus Bolivia.
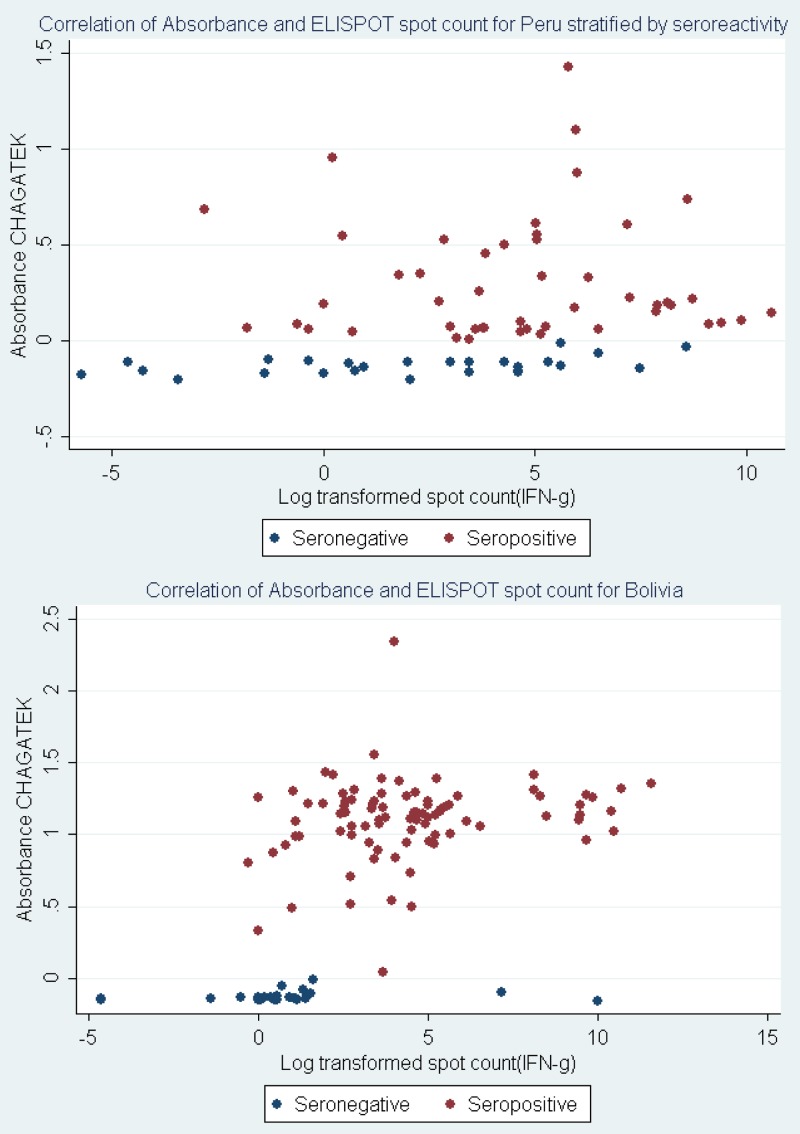
To better understand and compare the relationship of T-cell and serologic responses in individuals from Peru versus Bolivia, serologic absorbance (ABS) and fold change in ELISPOT response were plotted separately by T. cruzi serostatus. Among Bolivian specimens, there was a clear distinction in ELISPOT response by serostatus, with little to no overlap between groups (Figure 4). Conversely, among those individuals from Peru, there was a failure to cluster among seropositive and ELISPOT-positive responses (Figure 4). Linear regression analysis confirmed these observations. Changes in ELISPOT responses among those individuals from Bolivia were associated with significant changes in serologic responses (P < 0.01). Conversely, no such association was observed among specimens from Peru (P = 0.17). Similar patterns were observed among Peruvian samples when ELISPOT responses were compared with results from the Weiner ELISA or Arequipa EAE (data not shown).
Figure 4.
Scatterplot of T-cell responses and Chagatek absorbance values by country. Scatterplot analysis of absorbance values versus ELISPOT responses revealed two distinct patterns and simple linear regression to estimate the relationship between the two measures of T. cruzi exposure (controlling for age and sex). Among Bolivian specimens, a positive association was observed, where a 1 log unit change in ELISPOT responses resulted in an X-unit change in absorbance. Conversely, among Peruvian specimens, the change in absorbance as a function of Tc21 response was lower and marginally significant. Note that the y axes scales are different in the two graphs.
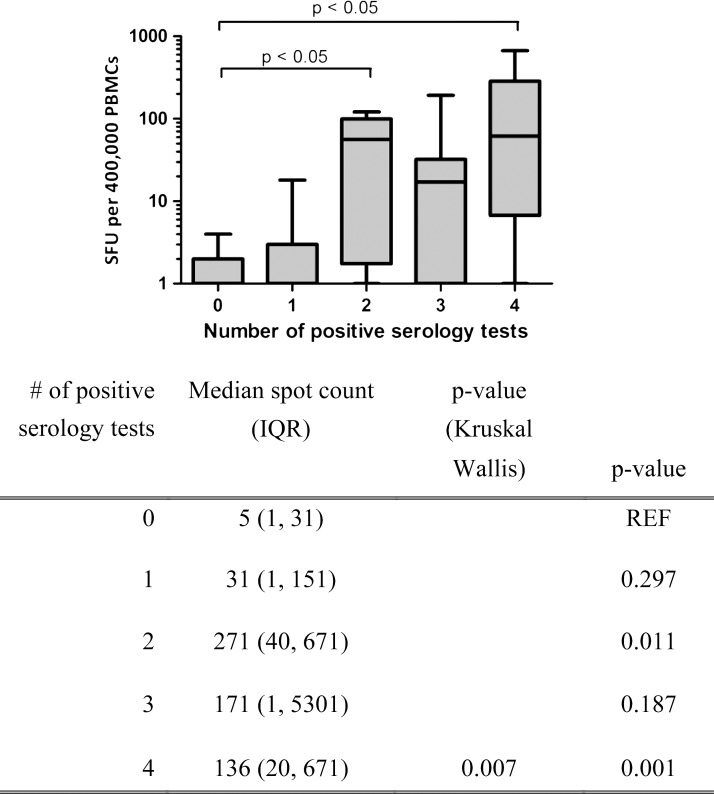
Sera from the Peruvian participants were further assayed for antibodies against T. cruzi using the commercial Weiner ELISA, an in-house ELISA using a T. cruzi isolate from Arequipa (Arequipa EAE), and TESA blot. When taken together with TESA blot analysis, there is a strong correlation between the frequency of T. cruzi-specific T cells and the number of positive serological tests (Figure 5).
Figure 5.
Median ELISPOT IFNγ spot count by number of positive serology tests for Peruvian samples. Upper panel shows IFNγ-producing cells stratified by number of positive serology tests for the same individual grouped by the number of positive tests. Data are displayed as a box and whiskers graph, with minimum and maximum values shown on a log-scale y axis. Lower panel shows median spot count and P values comparing the median spot count for one, two, three, or four serological tests with no positive serological tests (REF). IQR = interquartile range.
Discussion
Chagas disease presents as a spectrum of disorders, including an indeterminate lifelong infection with no clinical manifestations, varying degrees of cardiomyopathy, and gastrointestinal megasyndromes. The lack of a gold standard for T. cruzi diagnosis is a continuing problem. Serodiagnosis is the primary method for T. cruzi diagnostics, with the World Health Organization standard being a positive result on at least two different serologic assays. Efforts continue to develop alternative tests because of the difficulties associated with Chagas serology. Reports of false negatives by serological diagnostics as assessed by PCR positivity13–15 and cellular responses16 have surfaced but are rare, possibly because of the fact that seronegative samples are not uniformly tested for other measures of infection. Additionally, geographic differences in antibody titers, assay sensitivity, and assay positive predictive value for T. cruzi serological assays have been previously reported.4,7,17 Bolivia has the highest rates of T. cruzi infection and chagasic cardiomyopathy in the world, whereas Peru has much lower rates of infection.18,19 Direct comparison of antibody titers shows discrepancies between Peru and Bolivia, with specimens from seropositive individuals in Bolivia having significantly higher absorbance values than seropositive individuals in Peru. We sought to determine whether the differences in serotiters between individuals residing in Peru and Bolivia reflected an overall lower adaptive immune response in Peruvian patients that might be linked to local parasite strains.
The observed regional variation in antibody responses was reflected somewhat, although not completely, in T-cell responses. Among seropositive individuals, IFNγ responses from parasite-stimulated PBMCs were observed in 85.2% of Bolivian individuals, but only 58.8% of Peruvians. However, among Peruvian samples in which IFNγ responses are detected, the magnitude of the response is equivalent to the magnitude seen in Bolivian samples, showing that there is not a complete dampening of the immune response to T. cruzi in Peruvian individuals. Stimulation of PBMCs with T. cruzi strains from different geographical locations did not show any differences in IFNγ secretion, and PBMCs from Bolivian individuals responded as well to the Peruvian Tc21 strain as the laboratory strain Brazil (data not shown). These similarities in IFNγ induction by the various strains suggest that the different parasite populations exhibit significant shared or cross-reactive epitopes. In experimental models of T. cruzi infection, CD8+ T-cell responses vary in both magnitude and kinetics depending on the infecting strain, although the differences are more pronounced in acute infection than chronic infection.20 It is also possible that genetic differences in the study populations may account for the differences in the immune responses observed; these differences will need to be examined in future studies of geographic differences in immune responses to T. cruzi.
The lack of a 1:1 correlation between T-cell and antibody responses in T. cruzi-infected individuals has been previously reported.21 The absence of detectable T-cell responses in infected individuals may result from a failure to mount a T-cell response or a failure to maintain that response. The lack of initiation of a T-cell response to T. cruzi infection may result from a low infective dose of parasites or deficient signaling through Toll-like receptors during the early stages of infection.22,23 Deletion of the antigen-specific T-cell responses during chronic infection is well-documented but normally associated with high pathogen loads,24,25 making it less likely that T. cruzi-reactive T cells are deleted in these individuals who almost invariably have very low parasite loads in chronic infection. Experimental models of T. cruzi infection also show maintenance of anti-T. cruzi T-cell responses during chronic infection.20,26 The relatively high proportion of seropositive individuals with undetectable T-cell responses complicates efforts to use T-cell responses as possible diagnostics or test-of-cure assays.21
Some specimens from Chagatek-negative participants mounted T-cell responses (6.5% from Peru and 13% from Bolivia), although the two samples from Peru that tested seronegative by Chagatek but had positive T-cell reactivity later tested positive by the Wiener ELISA, Arequipa EAE, and TESA blot. T. cruzi-specific T-cell responses from seronegative individuals have previously been reported,16 although at a higher frequencies (35% of seronegatives in that study) than observed in our study. T-cell responses detected in the absence of antibody responses are seen in other infections as well, notably hepatitis C virus (HCV),27–29 herpes simplex virus (HSV),30 and human immunodeficiency virus (HIV)31 infections. In one study, 55% of seronegative intravenous drug users had T-cell responses to HCV antigens.27 The lack of antibody responses in individuals with T-cell responses may be because of a failure to seroconvert after rapid clearance of acute infection, seroreversion, or maintenance of T-cell responses by low-level antigen stimulation of T-cell responses without the generation of parasitemia or antibody responses.29
One limitation of this study is the difference in the patient populations in Peru and Bolivia. In Peru, patients were recruited from several ongoing community studies in Arequipa, where there is ongoing transmission. Because of the low prevalence of T. cruzi infection observed in these studies, we specifically recruited individuals who had previously tested positive for T. cruzi infection. Therefore, we were unable to specifically compare overall T. cruzi seroprevalence in Peru and Bolivian populations for this study. The Bolivian specimens came from a hospital-based cardiology study in Santa Cruz, in which patients were almost certainly infected many years before enrollment. There is currently no evidence for ongoing transmission of T. cruzi in Santa Cruz. It is, therefore, unlikely that higher antibody or T-cell responses in Bolivian samples are because of repeated exposure of immune cells to parasites from infected vectors.
T. cruzi exhibits a high degree of genetic heterogeneity that may result in quantitatively lower immune responses based on differences in antigen expression levels or patterns.20 No parasites were isolated or typed from the study participants, and currently, no definitive evidence exists to point at parasite differences in these two regions; however, other studies have shown preferential isolation of specific parasite types in individuals living in different geographic regions.32–34 In this study, the source of the antigen used for T-cell stimulation or antibody detection had little effect on the outcomes of that assay (i.e., sera and PBMCs from individuals from Arequipa did not respond more robustly to antigen from parasites isolated from Arequipa than other areas). This finding suggests that geographic differences in seroreactivity to diagnostic tests reflect physiological differences in antibody levels and T-cell responses that are quantitative and not qualitative. This finding is in contrast to previous data suggesting that diagnostic sensitivity can be increased by testing specimens against locally derived antigen.35 Enough cross-reactivity presumably exists between antigens found in different diagnostic tests and the various infecting strains to have only a limited effect on the quality of the assay. Interestingly, because Peruvian serum samples test positive against additional diagnostic assays, the correlation with positive ELISPOT responses increases, which is seen in Figure 5, whereas there is a tight correlation with a single positive test in the Bolivian samples. This result may indicate that there is a difference in the generation or maintenance in the immune response to T. cruzi in the participants from this Peruvian region, again indicating a quantitative but not qualitative difference in the immune response.
This study, therefore, suggests that individuals in Peru generate lower-magnitude immune responses or maintain them at a lower level than individuals in Bolivia. Additional examination of parasite populations in these regions is needed to understand the differences in the immune responses in these regions. Because of the quantitative difference in T. cruzi-specific immune responses seen in these different regions, validation of new diagnostic and screening tests for Chagas disease should include specimens from all countries endemic for T. cruzi to ensure that tests capture low-titer antibody responses.
ACKNOWLEDGMENTS
The authors acknowledge members of the Chagas Working Group: Katty Borrini, Jenny Ancca Juarez, Michelle Kaplinski, Juan Cornejo, Cesar Bocangel Jr., Cesar Bocangel Sr., Stephen Delgado, Alicia Hidron, Fernando Malaga-Chavez, Andy Catacora, Karina Oppe, Katelyn Levy, Miranda Hillyard, Megan Christenson, Lina Mollesaca-Riveros, Jose Ylla-Velásquez, Javier Quintanilla-Calderón, Jorge Apaza-Perez, Roger Quispe-Apaza, Danitza Pamo-Tito, and Oscar Carrion. We gratefully acknowledge the invaluable contributions of the Ministerio de Salud del Perú (MINSA), the Dirección General de Salud de las Personas (DGSP), the Estrategia Sanitaria Nacional de Prevención y Control de Enfermedades Metaxenicas y Otras Transmitidas por Vectores (ESNPCEMOTVS), the Dirección General de Salud Ambiental (DIGESA), the Gobierno Regional de Arequipa, the Gerencia Regional de Salud de Arequipa (GRSA), the Pan American Health Organization (PAHO/OPS), and the Canadian International Development Agency (CIDA).
Footnotes
Financial support: This work was funded by National Institutes of Health Tropical Medical Research Center Grant P50 AI074285 and National Institutes of Health Fogarty Scholars Program Grant R24 TW007988.
Authors' addresses: Diana L. Martin and Brook Goodhew, Centers for Disease Control and Prevention, Atlanta, GA, E-mails: hz3@cdc.gov and isd6@cdc.gov. Morgan Marks, Merck and Co., Whitehouse Station, NJ, E-mail: mmarkster@gmail.com. Gerson Galdos-Cardenas and Lisbeth Ferrufino, Hospital Universitario Japones, Santa Cruz, Bolivia, and Asociación Benéfica PRISMA, Lima, Peru, E-mails: gerson_galdos@yahoo.es and lisbeth_ferrufino@yahoo.es. Robert H. Gilman, Johns Hopkins University Bloomberg School of Public Health, Baltimore, MD, E-mail: gilmanabob@gmail.com. Gerardo Sanchez and Manuela Verastegui, Universidad Peruana Cayetano Heredia, Lima, Perú, E-mails: gjsg2@hotmail.com and mveraste@jhsph.edu. Patricia Escalante, Universidad Peruana Cayetano Heredia, Arequipa, Perú, E-mail: patriciaeem2008@gmail.com. Cesar Naquira, Arequipa Ministry of Health, Arequipa, Peru, E-mail: cesar.naquira@gmail.com. Michael Z. Levy, University of Pennsylvania, Philadelphia, PA, E-mail: mzlevy@mail.med.upenn.edu. Caryn Bern, University of California, San Francisco, CA, E-mail: Caryn.Bern2@ucsf.edu.
References
- 1.World Health Organization First WHO Report on Neglected Tropical Diseases: Working to Overcome the Global Impact of Neglected Tropical Disease. 2012. http://whqlibdoc.who.int/publications/2010/9789241564090_eng.pdf Available at. Accessed March 20, 2012.
- 2.Schijman AG, Bisio M, Orellana L, Sued M, Duffy T, Mejia Jaramillo AM, Cura C, Auter F, Veron V, Qvarnstrom Y, Deborggraeve S, Hijar G, Zulantay I, Lucero RH, Velazquez E, Tellez T, Sanchez Leon Z, Galvao L, Nolder D, Monje Rumi M, Levi JE, Ramirez JD, Zorrilla P, Flores M, Jercic MI, Crisante G, Anez N, De Castro AM, Gonzalez CI, Acosta Viana K, Yachelini P, Torrico F, Robello C, Diosque P, Triana Chavez O, Aznar C, Russomando G, Buscher P, Assal A, Guhl F, Sosa Estani S, DaSilva A, Britto C, Luquetti A, Ladzins J. International study to evaluate PCR methods for detection of Trypanosoma cruzi DNA in blood samples from Chagas disease patients. PLoS Negl Trop Dis. 2011;5:e931. doi: 10.1371/journal.pntd.0000931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Afonso AM, Ebell MH, Tarleton RL. A systematic review of high quality diagnostic tests for Chagas disease. PLoS Negl Trop Dis. 2012;6:e1881. doi: 10.1371/journal.pntd.0001881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Verani JR, Seitz A, Gilman RH, LaFuente C, Galdos-Cardenas G, Kawai V, de LaFuente E, Ferrufino L, Bowman NM, Pinedo-Cancino V, Levy MZ, Steurer F, Todd CW, Kirchhoff LV, Cabrera L, Verastegui M, Bern C. Geographic variation in the sensitivity of recombinant antigen-based rapid tests for chronic Trypanosoma cruzi infection. Am J Trop Med Hyg. 2009;80:410–415. [PubMed] [Google Scholar]
- 5.Umezawa ES, Luquetti AO, Levitus G, Ponce C, Ponce E, Henriquez D, Revollo S, Espinoza B, Sousa O, Khan B, da Silveira JF. Serodiagnosis of chronic and acute Chagas' disease with Trypanosoma cruzi recombinant proteins: results of a collaborative study in six Latin American countries. J Clin Microbiol. 2004;42:449–452. doi: 10.1128/JCM.42.1.449-452.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Caballero ZC, Sousa OE, Marques WP, Saez-Alquezar A, Umezawa ES. Evaluation of serological tests to identify Trypanosoma cruzi infection in humans and determine cross-reactivity with Trypanosoma rangeli and Leishmania spp. Clin Vaccine Immunol. 2007;14:1045–1049. doi: 10.1128/CVI.00127-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sosa-Estani S, Gamboa-Leon MR, Del Cid-Lemus J, Althabe F, Alger J, Almendares O, Cafferata ML, Chippaux JP, Dumonteil E, Gibbons L, Padilla-Raygoza N, Schneider D, Belizan JM, Buekens P, Working G. Use of a rapid test on umbilical cord blood to screen for Trypanosoma cruzi infection in pregnant women in Argentina, Bolivia, Honduras, and Mexico. Am J Trop Med Hyg. 2008;79:755–759. [PubMed] [Google Scholar]
- 8.Delgado S, Castillo Neyra R, Quispe Machaca VR, Ancca Juarez J, Chou Chu L, Verastegui MR, Moscoso Apaza GM, Bocangel CD, Tustin AW, Sterling CR, Comrie AC, Naquira C, Cornejo del Carpio JG, Gilman RH, Bern C, Levy MZ. A history of chagas disease transmission, control, and re-emergence in peri-rural La Joya, Peru. PLoS Negl Trop Dis. 2011;5:e970. doi: 10.1371/journal.pntd.0000970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tustin AW, Small DS, Delgado S, Castillo Neyra R, Verastegui MR, Ancca Juárez JM, Quispe Machaca VR, Gilman RH, Bern C, Levy MZ. Use of individual-level covariates to improve latent class analysis of Trypanosoma cruzi diagnostic tests. Epidemiol Method. 2012;1:33–54. doi: 10.1515/2161-962X.1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hunter GC, Borrini-Mayori K, Ancca Juarez J, Castillo Neyra R, Verastegui MR, Malaga Chavez FS, Cornejo del Carpio JG, Cordova Benzaquen E, Naquira C, Gilman RH, Bern C, Levy MZ. A field trial of alternative targeted screening strategies for Chagas disease in Arequipa, Peru. PLoS Negl Trop Dis. 2012;6:e1468. doi: 10.1371/journal.pntd.0001468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Levy MZ, Bowman NM, Kawai V, Plotkin JB, Waller LA, Cabrera L, Steurer F, Seitz AE, Pinedo-Cancino VV, Cornejo del Carpio JG, Cordova Benzaquen E, McKenzie FE, Maguire JH, Gilman RH, Bern C. Spatial patterns in discordant diagnostic test results for Chagas disease: links to transmission hotspots. Clin Infect Dis. 2009;48:1104–1106. doi: 10.1086/597464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Laucella SA, Postan M, Martin D, Hubby Fralish B, Albareda MC, Alvarez MG, Lococo B, Barbieri G, Viotti RJ, Tarleton RL. Frequency of interferon- gamma-producing T cells specific for Trypanosoma cruzi inversely correlates with disease severity in chronic human Chagas disease. J Infect Dis. 2004;189:909–918. doi: 10.1086/381682. [DOI] [PubMed] [Google Scholar]
- 13.Avila HA, Pereira JB, Thiemann O, De Paiva E, DeGrave W, Morel CM, Simpson L. Detection of Trypanosoma cruzi in blood specimens of chronic chagasic patients by polymerase chain reaction amplification of kinetoplast minicircle DNA: comparison with serology and xenodiagnosis. J Clin Microbiol. 1993;31:2421–2426. doi: 10.1128/jcm.31.9.2421-2426.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Salomone OA, Basquiera AL, Sembaj A, Aguerri AM, Reyes ME, Omelianuk M, Fernandez RA, Enders J, Palma A, Barral JM, Madoery RJ. Trypanosoma cruzi in persons without serologic evidence of disease, Argentina. Emerg Infect Dis. 2003;9:1558–1562. doi: 10.3201/eid0912.030008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Batista AM, Aguiar C, Almeida EA, Guariento ME, Wanderley JS, Costa SC. Evidence of Chagas disease in seronegative Brazilian patients with megaesophagus. Int J Infect Dis. 2010;14:e974–e977. doi: 10.1016/j.ijid.2010.05.017. [DOI] [PubMed] [Google Scholar]
- 16.Olivera GC, Albareda MC, Alvarez MG, De Rissio AM, Fichera LE, Cooley G, Yachelini P, Hrellac HA, Riboldi H, Laucella SA, Tarleton RL, Postan M. Trypanosoma cruzi-specific immune responses in subjects from endemic areas of Chagas disease of Argentina. Microbes Infect. 2010;12:359–363. doi: 10.1016/j.micinf.2010.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ponce C, Ponce E, Vinelli E, Montoya A, de Aguilar V, Gonzalez A, Zingales B, Rangel-Aldao R, Levin MJ, Esfandiari J, Umezawa ES, Luquetti AO, da Silveira JF. Validation of a rapid and reliable test for diagnosis of Chagas' disease by detection of Trypanosoma cruzi-specific antibodies in blood of donors and patients in Central America. J Clin Microbiol. 2005;43:5065–5068. doi: 10.1128/JCM.43.10.5065-5068.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Salud OPDL . Estimacion cuantitativa de la enfermedad de Chagas en las Americas. Montevideo, Uruguay: Organizacion Panamericana de la Salud; 2006. [Google Scholar]
- 19.Bern C, Montgomery SP. An estimate of the burden of Chagas disease in the United States. Clin Infect Dis. 2009;49:e52–e54. doi: 10.1086/605091. [DOI] [PubMed] [Google Scholar]
- 20.Martin DL, Weatherly DB, Laucella SA, Cabinian MA, Crim MT, Sullivan S, Heiges M, Craven SH, Rosenberg CS, Collins MH, Sette A, Postan M, Tarleton RL. CD8+ T-Cell responses to Trypanosoma cruzi are highly focused on strain-variant trans-sialidase epitopes. PLoS Pathog. 2006;2:e77. doi: 10.1371/journal.ppat.0020077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Laucella SA, Mazliah DP, Bertocchi G, Alvarez MG, Cooley G, Viotti R, Albareda MC, Lococo B, Postan M, Armenti A, Tarleton RL. Changes in Trypanosoma cruzi-specific immune responses after treatment: surrogate markers of treatment efficacy. Clin Infect Dis. 2009;49:1675–1684. doi: 10.1086/648072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bafica A, Santiago HC, Goldszmid R, Ropert C, Gazzinelli RT, Sher A. Cutting edge: TLR9 and TLR2 signaling together account for MyD88-dependent control of parasitemia in Trypanosoma cruzi infection. J Immunol. 2006;177:3515–3519. doi: 10.4049/jimmunol.177.6.3515. [DOI] [PubMed] [Google Scholar]
- 23.Padilla AM, Simpson LJ, Tarleton RL. Insufficient TLR activation contributes to the slow development of CD8+ T cell responses in Trypanosoma cruzi infection. J Immunol. 2009;183:1245–1252. doi: 10.4049/jimmunol.0901178. [DOI] [PubMed] [Google Scholar]
- 24.Wherry EJ, Ahmed R. Memory CD8 T-cell differentiation during viral infection. J Virol. 2004;78:5535–5545. doi: 10.1128/JVI.78.11.5535-5545.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wherry EJ, Blattman JN, Murali-Krishna K, van der Most R, Ahmed R. Viral persistence alters CD8 T-cell immunodominance and tissue distribution and results in distinct stages of functional impairment. J Virol. 2003;77:4911–4927. doi: 10.1128/JVI.77.8.4911-4927.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bustamante JM, Bixby LM, Tarleton RL. Drug-induced cure drives conversion to a stable and protective CD8+ T central memory response in chronic Chagas disease. Nat Med. 2008;14:542–550. doi: 10.1038/nm1744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Freeman AJ, Ffrench RA, Post JJ, Harvey CE, Gilmour SJ, White PA, Marinos G, van Beek I, Rawlinson WD, Lloyd AR. Prevalence of production of virus-specific interferon-gamma among seronegative hepatitis C-resistant subjects reporting injection drug use. J Infect Dis. 2004;190:1093–1097. doi: 10.1086/422605. [DOI] [PubMed] [Google Scholar]
- 28.Al-Sherbiny M, Osman A, Mohamed N, Shata MT, Abdel-Aziz F, Abdel-Hamid M, Abdelwahab SF, Mikhail N, Stoszek S, Ruggeri L, Folgori A, Nicosia A, Prince AM, Strickland GT. Exposure to hepatitis C virus induces cellular immune responses without detectable viremia or seroconversion. Am J Trop Med Hyg. 2005;73:44–49. [PubMed] [Google Scholar]
- 29.Zeremski M, Shu MA, Brown Q, Wu Y, Des Jarlais DC, Busch MP, Talal AH, Edlin BR. Hepatitis C virus-specific T-cell immune responses in seronegative injection drug users. J Viral Hepat. 2009;16:10–20. doi: 10.1111/j.1365-2893.2008.01016.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Posavad CM, Wald A, Hosken N, Huang ML, Koelle DM, Ashley RL, Corey L. T cell immunity to herpes simplex viruses in seronegative subjects: silent infection or acquired immunity? J Immunol. 2003;170:4380–4388. doi: 10.4049/jimmunol.170.8.4380. [DOI] [PubMed] [Google Scholar]
- 31.Kaul R, Dong T, Plummer FA, Kimani J, Rostron T, Kiama P, Njagi E, Irungu E, Farah B, Oyugi J, Chakraborty R, MacDonald KS, Bwayo JJ, McMichael A, Rowland-Jones SL. CD8(+) lymphocytes respond to different HIV epitopes in seronegative and infected subjects. J Clin Invest. 2001;107:1303–1310. doi: 10.1172/JCI12433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Macedo AM, Machado CR, Oliveira RP, Pena SD. Trypanosoma cruzi: genetic structure of populations and relevance of genetic variability to the pathogenesis of Chagas disease. Mem Inst Oswaldo Cruz. 2004;99:1–12. doi: 10.1590/s0074-02762004000100001. [DOI] [PubMed] [Google Scholar]
- 33.Manoel-Caetano Fda S, Silva AE. Implications of genetic variability of Trypanosoma cruzi for the pathogenesis of Chagas disease. Cad Saude Publica. 2007;23:2263–2274. doi: 10.1590/s0102-311x2007001000002. [DOI] [PubMed] [Google Scholar]
- 34.Zafra G, Mantilla JC, Jacome J, Macedo AM, Gonzalez CI. Direct analysis of genetic variability in Trypanosoma cruzi populations from tissues of Colombian chagasic patients. Hum Pathol. 2011;42:1159–1168. doi: 10.1016/j.humpath.2010.11.012. [DOI] [PubMed] [Google Scholar]
- 35.Sanchez B, Monteon V, Reyes PA, Espinoza B. Standardization of micro-enzyme-linked immunosorbent assay (ELISA) and Western blot for detection of Trypanosoma cruzi antibodies using extracts from Mexican strains as antigens. Arch Med Res. 2001;32:382–388. doi: 10.1016/s0188-4409(01)00303-4. [DOI] [PubMed] [Google Scholar]