Abstract
In the Democratic Republic of the Congo (DRC), artesunate-amodiaquine is first-line therapy for falciparum malaria; little is known about the prevalence of molecular markers of parasite drug resistance. Across the DRC, we genotyped 166 parasites in Plasmodium falciparum chloroquine resistance transporter (pfcrt) using polymerase chain reaction (PCR) and sequencing. Of these parasites, 73 (44%) parasites were pure wild-type CVMNK, 55 (31%) parasites were chloroquine-resistant CVIET, 35 (21.1%) parasites were mixed CVMNK and CVIET, and 3 parasites were other genotypes. Ninety-two infections (55.4%) harbored the pfcrt K76T substitution that is highly correlated with chloroquine failure. The amodiaquine-resistant SVMNT haplotype was absent. Geographically, pfcrt haplotypes were not clearly clustered. Chloroquine accounted for 19.4% of antimalarial use, and amodiaquine accounted for 15.3% of antimalarial use; there were no associations between drug use and mutant haplotype prevalence. In the DRC, our molecular survey indicates that resistance to chloroquine is substantial but that resistance to amodiaquine is absent. These contrasting findings highlight the need for molecular surveillance of drug resistance to inform malaria control policies.
Chloroquine (CQ)-resistant Plasmodium falciparum parasites are widespread and undermined prior efforts to control falciparum malaria. Resistance is conferred by polymorphisms in the P. falciparum CQ resistance transporter (PfCRT)1 encoded by the pfcrt gene. Clinically, the K76T substitution elevates the risk of treatment failure sevenfold.2 Although pfcrt was originally associated with CQ susceptibility, a recent chemical genomic screen identified mutations in pfcrt as key mediators of response to a diverse array of drugs,3 suggesting that the biological relevance of pfcrt extends far beyond simply CQ.
Two observations suggest that CQ may be redeployed for malaria control, despite widespread resistance. First, in Malawi, CQ susceptibility returned over time after CQ use was curtailed4 owing to the removal of selective pressure and the reexpansion of CQ-susceptible parasites.5 Additionally, even in the presence of the K76T mutation, the administration of higher doses of CQ can achieve cure rates similar to the rates of artemether-lumefantrine.6 Because of these data, interest persists in possibly redeploying CQ in certain epidemiologic and clinical settings.
Central Africa harbors diverse pfcrt haplotypes that are germane for clinical therapy. The Democratic Republic of the Congo (DRC) adopted artesunate-amodiaquine (ASAQ) as its first-line antimalarial in 2005, and neighboring Burundi, Republic of the Congo, and South Sudan have also adopted ASAQ. In the DRC, ASAQ is highly efficacious: the cure rate of uncomplicated falciparum malaria was > 98% in Katanga province in 2008 and 2009.7 Recent data confirm the appearance in neighboring Tanzania8 and Angola9 of the pfcrt haplotype SVMNT, which may confer resistance to amodiaquine1,10 and herald failures of ASAQ.11 Additionally, the haplotype SVIET has been reported from Kinshasa in 2000, although it is of unclear biological significance.12
To inform current and future guidelines on antimalarial use in Central Africa, we conducted a cross-sectional survey of pfcrt haplotypes in the DRC.
Parasites were obtained from respondents to the 2007 Demographic and Health Survey (DHS) in the DRC.13 Briefly, adults over 15 years old were selected for participation within 300 random population-representative clusters identified across the DRC. Data about household antimalarial use were collected by asking female respondents if children under the age of 5 years in the household with fever during the preceding 2 weeks had received an antimalarial drug; data about drug use by individual respondents who contributed parasite specimens were not collected. Verbal informed consent for the collection of blood spots was obtained from all survey participants in one of the five main languages used in the DRC. The Ethics Committees of Macro International and the School of Public Health of the University of Kinshasa approved the consent procedures, survey administration, and blood sample collection. The Institutional Review Board of the University of North Carolina approved the testing of malaria parasites.
Finger-prick blood samples from the adult survey participants were stored on filter paper as dried blood spots and used to make genomic DNA (gDNA). P. falciparum was detected using real-time polymerase chain reaction (PCR).13 Of 8,838 adult respondents, 2,435 respondents harbored only P. falciparum parasites. Of this subset, 180 P. falciparum parasitemias were randomly selected for pfcrt genotyping.
Samples were genotyped at codons 72–76 by amplifying pfcrt across the codons of interest and Sanger sequencing the product. The 25 μL PCR reaction contained 12.5 μL HotStarTaq Master Mix (Qiagen, Valencia, CA), 4 mM MgCl2, and 800 nM each of primers CRTFwd (GGAAATGGCTCACGTTTAGG) and CRTRev (TGTGAGTTTCGGATGTTACAAAA; adapted from a previous study).14 PCR products were bidirectionally sequenced using ABI BigDye Terminator chemistry, and reads were aligned to National Center for Biotechnology Information reference sequence XM_001348968 using Sequencher (v4.8; Gene Codes, Ann Arbor, MI). All sequences were manually inspected at the loci of interest. We defined mixed alleles as those alleles at which a secondary peak was ≥ 10% of the height of the primary peak.
Of 180 randomly selected parasite isolates, 166 isolates were successfully genotyped across pfcrt codons 72–76. Of these isolates, the wild-type CVMNK haplotype was solely present in 73 (44.0%) isolates, the CQ-resistant haplotype CVIET was solely present in 55 (33.1%) isolates, and 35 (21.1%) isolates harbored parasites with both CVMNK and CVIET haplotypes. Additionally, two isolates bore a CVMNT haplotype, and one isolate had a CVMDK. Therefore, 92 of 166 samples that were successfully genotyped (55.4%) harbored the K76T mutation that most clearly confers CQ resistance.
The SVMNT haplotype reported previously from Angola and Tanzania was absent, and the SVIET that had been reported from Kinshasa was also absent. There were no novel substitutions identified in the sequenced fragment of pfcrt.
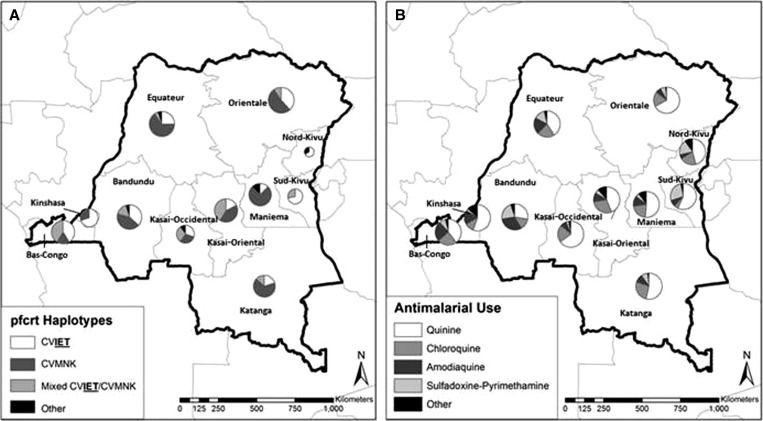
Geographically, the most common wild-type (CVMNK) and mutant (CVIET) haplotypes were widely distributed across the DRC without apparent geographic clustering (Figure 1A). The prevalence of the K76T mutation was not significantly different between 11 DRC provinces.
Figure 1.
Provincial distributions of (A) pfcrt haplotypes and (B) antimalarial drug use for childhood fever.
Of 790 DHS respondents with children under 5 years who received antimalarials in the preceding 2 weeks for fever, the most commonly administered antimalarials were quinine (49.6%), CQ (19.4%), amodiaquine (15.3%), and sulfadoxine-pyrimethamine (9.7%). On a provincial level, there were no significant differences in antimalarial use (Figure 1B).
Overall, we report a higher prevalence of wild-type pfcrt haplotypes than prior studies in the region: 44% of isolates in our study harbored the CVMNK haplotype alone. In three studies in Kinshasa or neighboring Republic of the Congo, wild-type CVMNK haplotype prevalence was 4–12%,15–17 and only 3% of isolates collected in Luanda, Angola in 2007 harbored wild-type pfcrt.9 We note that, consistent with these studies, prevalence of wild-type parasites was lower than the national average in Kinshasa and Bas-Congo provinces (Figure 1A).
What might these data mean for CQ redeployment? Currently, ASAQ is effective therapy for malaria in the DRC, but the recent reports of reduced efficacy of artemisinin therapies for malaria in Asia highlight the need to continually evaluate the efficacy of alternative drugs. The correlation between prevailing K76T mutation frequency and the efficacy of CQ is unknown. In Malawi, 25 mg/kg CQ were highly effective in treating uncomplicated childhood malaria in a setting where the prevalence of the K76T mutation was < 0.2%.18 More surprisingly, in a Guinea-Bissau study in which 31% of parasites harbored K76T, 50 mg/kg CQ were 94% effective as treatment of uncomplicated malaria.6 Given these data, it seems plausible that a threshold of prevailing K76T frequency may define locations where it is likely to be effective. Areas with moderate but heterogeneous mutation prevalence, like the DRC, can provide settings in which to test CQ efficacy and its correlation with molecular markers of resistance. Nonetheless, redeploying CQ in an area with parasites bearing K76T mutations will promote the spread of resistant parasites and ultimately, reduce drug efficacy. For this reason, clinical and molecular monitoring of drug resistance will be critical if CQ is deployed in any limited setting.
Persistent CQ use for childhood fever may be sustaining transmission of K76T-bearing parasites in the DRC. In Malawi, the decrease in CQ use facilitated a return of CQ-susceptible parasites, but in a meta-analysis of population-level data from several other African countries, continued CQ use was associated with the persistence of CQ resistance.19 Although parasites bearing mutant pfcrt haplotypes are believed to suffer an overall fitness disadvantage, it may be counterbalanced in the presence of continued drug pressure by increased infectivity to mosquitos.20 The K76T mutation may be further sustained by the poor quality of CQ formulations in the DRC,21 which may simultaneously fail to clear parasites and promote gametocytogenesis. Until 2002, when failure rates reached 45%,22 CQ was the first-line antimalarial for uncomplicated malaria; our study was conducted in 2007, and its administration for 20% of treated childhood fevers may reflect residual practice patterns, which have since abated.
Notably, no parasites in our study harbored the SVMNT haplotype. This haplotype has been reported in Angola and Tanzania, and it has been associated with resistance to amodiaquine in a clinical study in Afghanistan10 and in vitro experimentation.1 Our study was conducted only 2 years after the adoption of ASAQ as first-line antimalarial in the DRC, and respondents endorsed minimal use for childhood antimalarial treatment of either amodiaquine (4.9%) or artesunate (0.6%). Our data suggest that ASAQ may be a durable antimalarial, although ongoing surveillance is necessary.
Our study has several limitations. We sampled only 166 isolates from a large country. Nevertheless, they were randomly sampled from a nationally representative survey, and we believe that they reflect DRC parasite populations in a less-biased fashion than more local studies. Our genotyping method may have missed minority variant subpopulations, although we set a liberal cutoff for secondary peaks in chromatograms, all of which were scored manually. We have no clinical efficacy data with which to correlate our molecular data, and future studies are needed to understand the impact of the pfcrt prevalence. Finally, the DHS did not collect data on the recent use of antimalarials by the participants themselves. The pfcrt K76T mutation decreases susceptibility to both CQ and amodiaquine; if participants had recently received these antimalarials in the period before the survey, they would be expected to inflate the reported mutant allele frequencies above their true values.
Across the DRC, parasites bearing pfcrt haplotypes conferring CQ resistance remain prevalent, perhaps owing to ongoing CQ use for childhood fever. A pfcrt haplotype associated with amodiaquine resistance was absent, suggesting that first-line antimalarial ASAQ may be a durable therapy. Ongoing population-level molecular surveillance may assist strategies to redeploy CQ in select African settings.
ACKNOWLEDGMENTS
The authors thank Dr. Augustin Okenge (Programme National de Lutte Contre le Syndrome D’Immunodeficience Acquise et Infection Sexuellement Transmissible, Kinshasa, Democratic Republic of the Congo), Dr. Jeremie Mwonga (Laboratoire National de Reference SIDA et IST, Kinshasa, Democratic Republic of the Congo), and Ann Way, Mohamed Ayad, and Martin Vaessen (all of ICF International, Calverton, MD) for help in obtaining access to the dried blood spots. Ultimately, we are indebted to the Demographic and Health Survey respondents for their participation.
Footnotes
Financial support: This work was supported by a Summer Undergraduate Research Fellowship from the Office for Undergraduate Research at the University of North Carolina at Chapel Hill (to A.L.A.), a Gillings Innovation Laboratory Award from the University of North Carolina Gillings School of Global Public Health (to S.R.M.), and National Institute of Allergy and Infectious Diseases Award 1R56AI097909 (to S.R.M.).
Authors' addresses: Alejandro L. Antonia, Mark Janko, Michael Emch, and Steven R. Meshnick, University of North Carolina, Chapel Hill, NC, E-mails: aantonia01@gmail.com, janko@live.unc.edu, emch@unc.edu, and meshnick@unc.edu. Steve M. Taylor, Division of Infectious Diseases and International Health, Duke University Medical Center, Durham, NC, E-mail: steve.taylor@duke.edu. Antoinette K. Tshefu, Ecole de Sante Publique, Faculte de Medicine, University of Kinshasa, Kinshasa, Democratic Republic of the Congo, E-mail: antotshe@yahoo.com.
References
- 1. Sidhu AB, Verdier-Pinard D, Fidock DA. 2002. Chloroquine resistance in Plasmodium falciparum malaria parasites conferred by pfcrt mutations Science 298 210–213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Picot S, Olliaro P, de Monbrison F, Bienvenu AL, Price RN, Ringwald P. A systematic review and meta-analysis of evidence for correlation between molecular markers of parasite resistance and treatment outcome in falciparum malaria. Malar J. 2009;8:89. doi: 10.1186/1475-2875-8-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yuan J, Cheng KC, Johnson RL, Huang R, Pattaradilokrat S, Liu A, Guha R, Fidock DA, Inglese J, Wellems TE, Austin CP, Su XZ. Chemical genomic profiling for antimalarial therapies, response signatures, and molecular targets. Science. 2011;333:724–729. doi: 10.1126/science.1205216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Laufer MK, Thesing PC, Eddington ND, Masonga R, Dzinjalamala FK, Takala SL, Taylor TE, Plowe CV. Return of chloroquine antimalarial efficacy in Malawi. N Engl J Med. 2006;355:1959–1966. doi: 10.1056/NEJMoa062032. [DOI] [PubMed] [Google Scholar]
- 5.Laufer MK, Takala-Harrison S, Dzinjalamala FK, Stine OC, Taylor TE, Plowe CV. Return of chloroquine-susceptible falciparum malaria in Malawi was a reexpansion of diverse susceptible parasites. J Infect Dis. 2010;202:801–808. doi: 10.1086/655659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ursing J, Kofoed PE, Rodrigues A, Blessborn D, Thoft-Nielsen R, Bjorkman A, Rombo L. Similar efficacy and tolerability of double-dose chloroquine and artemether-lumefantrine for treatment of Plasmodium falciparum infection in Guinea-Bissau: a randomized trial. J Infect Dis. 2011;203:109–116. doi: 10.1093/infdis/jiq001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Espie E, Lima A, Atua B, Dhorda M, Flevaud L, Sompwe EM, Palma Urrutia PP, Guerin PJ. Efficacy of fixed-dose combination artesunate-amodiaquine versus artemether-lumefantrine for uncomplicated childhood Plasmodium falciparum malaria in Democratic Republic of Congo: a randomized non-inferiority trial. Malar J. 2012;11:174. doi: 10.1186/1475-2875-11-174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alifrangis M, Dalgaard MB, Lusingu JP, Vestergaard LS, Staalsoe T, Jensen AT, Enevold A, Ronn AM, Khalil IF, Warhurst DC, Lemnge MM, Theander TG, Bygbjerg IC. Occurrence of the Southeast Asian/South American SVMNT haplotype of the chloroquine-resistance transporter gene in Plasmodium falciparum in Tanzania. J Infect Dis. 2006;193:1738–1741. doi: 10.1086/504269. [DOI] [PubMed] [Google Scholar]
- 9.Gama BE, Pereira-Carvalho GA, Lutucuta Kosi FJ, Almeida de Oliveira NK, Fortes F, Rosenthal PJ, Daniel-Ribeiro CT, de Fatima Ferreira-da-Cruz M. Plasmodium falciparum isolates from Angola show the StctVMNT haplotype in the pfcrt gene. Malar J. 2010;9:174. doi: 10.1186/1475-2875-9-174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Beshir K, Sutherland CJ, Merinopoulos I, Durrani N, Leslie T, Rowland M, Hallett RL. Amodiaquine resistance in Plasmodium falciparum malaria in Afghanistan is associated with the pfcrt SVMNT allele at codons 72 to 76. Antimicrob Agents Chemother. 2010;54:3714–3716. doi: 10.1128/AAC.00358-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sa JM, Twu O. Protecting the malaria drug arsenal: halting the rise and spread of amodiaquine resistance by monitoring the PfCRT SVMNT type. Malar J. 2010;9:374. doi: 10.1186/1475-2875-9-374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Severini C, Menegon M, Sannella AR, Paglia MG, Narciso P, Matteelli A, Gulletta M, Caramello P, Canta F, Xayavong MV, Moura IN, Pieniazek NJ, Taramelli D, Majori G. Prevalence of pfcrt point mutations and level of chloroquine resistance in Plasmodium falciparum isolates from Africa. Infect Genet Evol. 2006;6:262–268. doi: 10.1016/j.meegid.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 13.Taylor SM, Messina JP, Hand CC, Juliano JJ, Muwonga J, Tshefu AK, Atua B, Emch M, Meshnick SR. Molecular malaria epidemiology: mapping and burden estimates for the Democratic Republic of the Congo, 2007. PLoS One. 2011;6:e16420. doi: 10.1371/journal.pone.0016420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Juliano JJ, Kwiek JJ, Cappell K, Mwapasa V, Meshnick SR. Minority-variant pfcrt K76T mutations and chloroquine resistance, Malawi. Emerg Infect Dis. 2007;13:872–877. doi: 10.3201/eid1306.061182. [DOI] [PubMed] [Google Scholar]
- 15.Mobula L, Lilley B, Tshefu AK, Rosenthal PJ. Resistance-mediating polymorphisms in Plasmodium falciparum infections in Kinshasa, Democratic Republic of the Congo. Am J Trop Med Hyg. 2009;80:555–558. [PubMed] [Google Scholar]
- 16.Koukouikila-Koussounda F, Malonga V, Mayengue PI, Ndounga M, Vouvoungui CJ, Ntoumi F. Genetic polymorphism of merozoite surface protein 2 and prevalence of K76T pfcrt mutation in Plasmodium falciparum field isolates from Congolese children with asymptomatic infections. Malar J. 2012;11:105. doi: 10.1186/1475-2875-11-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tsumori Y, Ndounga M, Sunahara T, Hayashida N, Inoue M, Nakazawa S, Casimiro P, Isozumi R, Uemura H, Tanabe K, Kaneko O, Culleton R. Plasmodium falciparum: differential selection of drug resistance alleles in contiguous urban and peri-urban areas of Brazzaville, Republic of Congo. PLoS One. 2011;6:e23430. doi: 10.1371/journal.pone.0023430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Laufer MK, Thesing PC, Dzinjalamala FK, Nyirenda OM, Masonga R, Laurens MB, Stokes-Riner A, Taylor TE, Plowe CV. A longitudinal trial comparing chloroquine as monotherapy or in combination with artesunate, azithromycin or atovaquone-proguanil to treat malaria. PLoS One. 2012;7:e42284. doi: 10.1371/journal.pone.0042284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Frosch AE, Venkatesan M, Laufer MK. Patterns of chloroquine use and resistance in sub-Saharan Africa: a systematic review of household survey and molecular data. Malar J. 2011;10:116. doi: 10.1186/1475-2875-10-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ecker A, Lakshmanan V, Sinnis P, Coppens I, Fidock DA. Evidence that mutant PfCRT facilitates the transmission to mosquitoes of chloroquine-treated Plasmodium gametocytes. J Infect Dis. 2011;203:228–236. doi: 10.1093/infdis/jiq036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Atemnkeng MA, Chimanuka B, Plaizier-Vercammen J. Quality evaluation of chloroquine, quinine, sulfadoxine-pyrimethamine and proguanil formulations sold on the market in East Congo DR. J Clin Pharm Ther. 2007;32:123–132. doi: 10.1111/j.1365-2710.2007.00797.x. [DOI] [PubMed] [Google Scholar]
- 22.Kazadi WM, Vong S, Makina BN, Mantshumba JC, Kabuya W, Kebela BI, Ngimbi NP. Assessing the efficacy of chloroquine and sulfadoxine-pyrimethamine for treatment of uncomplicated Plasmodium falciparum malaria in the Democratic Republic of Congo. Trop Med Int Health. 2003;8:868–875. doi: 10.1046/j.1365-3156.2003.01098.x. [DOI] [PubMed] [Google Scholar]