Abstract
Malaria chemoprophylaxis is used as a preventive measure in military personnel deployed to malaria-endemic countries. However, limited information is available on compliance with chemoprophylaxis among trauma patients during hospitalization and after discharge. Therefore, we assessed antimalarial primary chemoprophylaxis and presumptive antirelapse therapy (primaquine) compliance among wounded United States military personnel after medical evacuation from Afghanistan (June 2009–August 2011) to Landstuhl Regional Medical Center in Landstuhl, Germany, and then to three U.S. military hospitals. Among admissions at Landstuhl Regional Medical Center, 74% of 2,540 patients were prescribed primary chemoprophylaxis and < 1% were prescribed primaquine. After transfer of 1,331 patients to U.S. hospitals, 93% received primary chemoprophylaxis and 33% received primaquine. Of 751 trauma patients with available post-admission data, 42% received primary chemoprophylaxis for four weeks, 33% received primaquine for 14 days, and 17% received both. These antimalarial chemoprophylaxis prescription rates suggest that improved protocols to continue malaria chemoprophylaxis in accordance with force protection guidelines are needed.
During the past decade, United States military personnel have been deployed to Afghanistan, where malaria remains a serious health threat. Approximately 77% of the Afghan population resides in areas at risk of disease transmission, which results in an estimated annual average of 77,000 cases per year.1 United States military personnel must rely on preventive measures (e.g., chemoprophylaxis and permethrin-treated uniforms) to mitigate the risk of infection. Despite these efforts, approximately 20–90 cases of malaria per year were diagnosed among U.S. military personnel serving in Afghanistan during 2003–2012.2,3
Antimalarial chemoprophylaxis (doxycycline or mefloquine), per guidelines published by the U.S. Department of Defense (DoD) during 2009–2011, should be initiated before entering Afghanistan, continued during deployment, and for 28 days after returning to the United States. In addition, primaquine is recommended for 14 days after deployment for presumptive antirelapse therapy, except for persons with glucose-6-phosphate dehydrogenase (G6PD) deficiency.4,5 In general, chemoprophylaxis compliance by service members during deployment has been self-reportedly low (38–61% for primary chemoprophylaxis, 41% for primaquine, and 31–56% for primary chemoprophylaxis and primaquine).6–9 Poor compliance has been attributed to gastrointestinal side effects, forgetfulness, and misperception of malaria hazard.8 Moreover, service members may receive suboptimal chemoprophylaxis regimens (i.e., shorter than recommended durations or lack of primaquine) as recently reported among 36% of cases.10
Few studies are available on antimalarial chemoprophylaxis and primaquine prescribed to trauma patients after admission to medical facilities.11,12 The objective of our analysis was to evaluate antimalarial chemoprophylaxis compliance, as prescribed by clinicians, among wounded U.S. military personnel evacuated from Afghanistan after arrival at Landstuhl Regional Medical Center (LRMC) in Germany and subsequent U.S. military treatment facilities (MTFs).
Primary chemoprophylaxis and primaquine prescription data were collected at LRMC and three U.S. MTFs as part of a prospective study on infectious complications after deployment-related injuries (DoD–Department of Veterans Affairs, Trauma Infectious Disease Outcomes Study [TIDOS]).13 Participating U.S. MTFs were the National Naval Medical Center, the Walter Reed Army Medical Center, and the Brooke Army Medical Center. Demographic, trauma, and clinical inpatient data from service members with deployment-related injuries were collected through the TIDOS infectious disease module of the DoD Trauma Registry.13,14 Data on antimalarial prescription and duration were obtained from cohort records for patients who enrolled in TIDOS and consented to follow-up. This study was approved by the Infectious Disease Institutional Review Board of Uniformed Services University of the Health Sciences.
Clinical characteristics and duration of prescribed chemoprophylaxis for wounded military personnel were compared among four hospitalized groups: 1) those who received recommended durations of primary chemoprophylaxis and primaquine; 2) those who received primary chemoprophylaxis for the recommended four weeks, but primaquine for less than two weeks; 3) those who received primary chemoprophylaxis for less than four weeks; and 4) those who never received any chemoprophylaxis during hospitalization. Chi-square and Fisher's exact tests were used to test differences between categorical variables. The non-parametric Kruskal-Wallis test was used to compare the distribution of medians between the groups. Statistical analysis was conducted using SAS version 9.3 (SAS Institute, Cary, NC) and StatXact version 9 (Cytel Inc., Cambridge, MA). Significance was defined as P < 0.05.
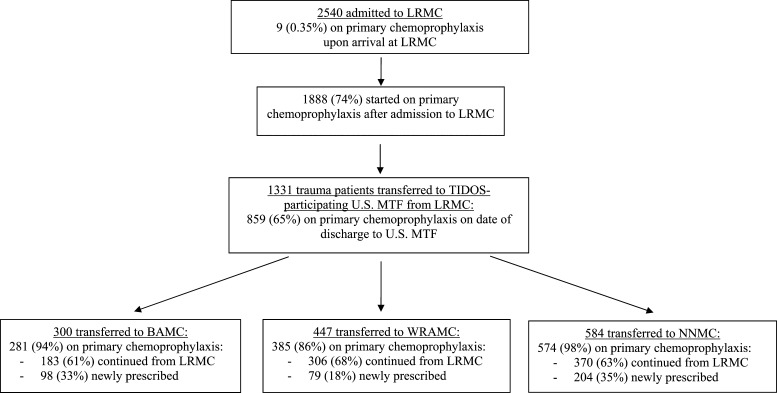
During June 1, 2009–August 31, 2011, 2,540 military personnel were admitted to LRMC with wounds sustained in Afghanistan and < 1% received antimalarial chemoprophylaxis from clinicians in-theater after injury (Figure 1). After admission to LRMC, 74% were prescribed antimalarial chemoprophylaxis. After care at LRMC (median hospitalization = 2 days, interquartile range [IQR] = 1–3 days), 1,331 patients transferred to TIDOS-participating U.S. MTFs (65% receiving antimalarial chemoprophylaxis at time of discharge) and 93% received primary chemoprophylaxis after admission to U.S. facilities. Primaquine was initiated among < 1% of patients at LRMC and an additional 33% (433) at the U.S. MTFs.
Figure 1.
Antimalarial primary chemoprophylaxis flow diagram for United States Military trauma patients from Afghanistan admitted during June 1, 2009–August 31, 2011. LRMC = Landstuhl Regional Medical Center; TIDOS = Trauma Infectious Disease Outcomes Study; MTF = military treatment facility; BAMC = Brooke Army Medical Center; WRAMC = Walter Reed Army Medical Center; NNMC = National Naval Medical Center.
Overall, 751 patients enrolled in the longitudinal TIDOS cohort (Table 1), of whom 42% received either doxycycline or mefloquine for at least four weeks, 53% for less than four weeks, and 5% did not receive any antimalarial during hospitalization at either LRMC or the U.S. MTFs. In addition, 32% (244 patients) received primaquine for at least 14 days, 10% (76 patients) were prescribed it for less than the recommended duration, and 57% (431 patients) did not receive primaquine. Only 17% of TIDOS enrollees received primary chemoprophylaxis and primaquine.
Table 1.
Characteristics of TIDOS-enrolled trauma patients related to antimalarial chemoprophylaxis duration (June 1, 2009–August 31, 2011)*
| Characteristic | Total population (n = 751) | Received 4 weeks of AMC† plus 2 weeks of primaquine (n = 127) | Received 4 weeks of AMC† but not 2 weeks of primaquine (n = 190) | Received less than 4 weeks of AMC† (n = 399) | Never received AMC† (n = 35) | P‡ | P§ |
|---|---|---|---|---|---|---|---|
| Age, median (IQR) | 24.4 (22.0–28.5) | 23.6 (21.9–25.9) | 23.6 (21.6–27.9) | 25.1 (22.3–29.5) | 25.6 (22.1–28.4) | 0.79 | < 0.01 |
| Males, no. (%) | 741 (98.7) | 127 (100) | 189 (99.5) | 391 (98.0) | 34 (97.1) | 1.00 | 0.05 |
| Time from injury to receipt of first antimalarial drug, median days (IQR) | 3 (2–4) | 2 (2–3) | 3 (2–4) | 3 (2–4) | NA | 0.56 | 0.50 |
| LRMC ISS, median (IQR) | 12 (6–18) | 17 (12–24) | 10 (6–17) | 11 (6–18) | 8 (5–10) | < 0.01 | < 0.01 |
| Admitted to LRMC ICU, no. (%) | 364 (48.5) | 96 (75.6) | 90 (47.4) | 174 (43.6) | 4 (11.4) | < 0.01 | < 0.01 |
| LRMC length of ICU stay, median days (IQR) | 2 (1–3) | 2 (1–3) | 2 (1–3) | 2 (1–2) | 1 (1–14) | 0.80 | 0.03 |
| Admitted to U.S. MTF ICU, no. (%) | 275 (36.6) | 86 (67.7) | 70 (36.8) | 116 (29.1) | 3 (8.6) | < 0.01 | < 0.01 |
| MTF length of ICU stay,¶ median days (IQR) | 4 (2–6) | 4 (3–7) | 3 (2–5) | 3 (2–6) | 2 (2–7) | 0.08 | 0.81 |
| First U.S. MTF admission, no. (%) | < 0.01# | < 0.01** | |||||
| BAMC | 197 (26.2) | 0 | 30 (15.8) | 154 (38.6) | 13 (37.1) | ||
| WRAMC | 300 (40.0) | 68 (53.5) | 83 (43.7) | 131 (32.8) | 18 (51.4) | ||
| NNMC | 254 (33.8) | 59 (46.5) | 77 (40.5) | 114 (28.6) | 4 (11.4) | ||
| Total hospitalization at LRMC and U.S. MTFs, median days (IQR) | 23 (13–41) | 45 (30–65) | 19 (11–38) | 19 (12–34) | 13 (9–25) | < 0.01 | < 0.01 |
| Receipt of minocycline at LRMC and U.S. MTFs, no. (%) | 19 (2.5) | 5 (3.9) | 0 | 14 (3.5) | 0 | 0.01 | 0.24 |
| Bacterial infections at LRMC and U.S. MTFs, no. (%)†† | < 0.01 | < 0.01 | |||||
| 0 | 499 (66.4) | 43 (33.9) | 137 (72.1) | 291 (72.9) | 28 (80.0) | ||
| 1 | 107 (14.3) | 29 (22.8) | 19 (10) | 53 (13.3) | 6 (17.1) | ||
| 2 | 62 (8.3) | 24 (18.9) | 15 (7.9) | 23 (5.8) | 0 | ||
| 3 | 35 (4.7) | 15 (11.8) | 8 (4.2) | 12 (3.0) | 0 | ||
| ≥ 4 | 48 (6.4) | 16 (12.6) | 11 (5.8) | 20 (5.0) | 1 (2.9) |
TIDOS = Trauma Infectious Disease Outcomes Study; AMC = antimalarial primary chemoprophylaxis; IQR = interquartile range; NA = not applicable; LRMC = Landstuhl Regional Medical Center; ISS = injury severity score; ICU = intensive care unit; MTF = military treatment facility; BAMC = Brooke Army Military Center; WRAMC = Walter Reed Army Medical Center; NNMC = National Naval Medical Center.
Doxycycline or mefloquine at any dose at either LRMC or the U.S. MTFs.
Compares persons who received at least four weeks of primary chemoprophylaxis plus two weeks of primaquine (n = 127) with those who received a full duration of primary chemoprophylaxis but less than two weeks of primaquine (n = 190).
Compares persons who received at least four weeks of primary chemoprophylaxis (combination of first two groups; n = 317) with those who either received primary chemoprophylaxis for less than four weeks or those who did not receive any antimalarial drugs (combination of last two groups; n = 434).
ICU length of stay data excludes NNMC because the information was not available in the Department of Defense Trauma Registry.
Compares all MTFs. Specific facility comparisons are BAMC and WRAMC (P < 0.01); BAMC and NNMC (P < 0.01); and WRAMC and NNMC (P = 0.78).
Compares all MTFs. Specific facility comparisons are BAMC and WRAMC (P < 0.01); BAMC and NNMC (P < 0.01); and WRAMC and NNMC (P = 0.45).
Bacterial infections were classified on the basis of clinical findings, laboratory and other test results, and standardized definitions. Examples of infections include skin and soft-tissue infections, osteomyelitis, and sepsis.
When the two groups that received four weeks of primary chemoprophylaxis were compared on the basis of primaquine duration (two weeks versus less than two weeks; Table 1), patients who received recommended durations of antimalarial drugs had greater injury severity, more intensive care unit admissions, longer duration of total hospitalization, and a higher number of bacterial infections. There were similar significant differences when patients who received four weeks of primary chemoprophylaxis were compared with those who received it for either less than the recommended duration or not at all. Notably, 43% of patients who received four weeks of chemoprophylaxis had at least one bacterial infection whereas only 26% of patients who either did not receive recommended duration or any antimalarial chemoprophylaxis had a bacterial infection.
Most military personnel wounded in Afghanistan during June 2009–August 2011 received primary chemoprophylaxis (74% at LRMC and 93% at U.S. MTFs), but not as many were prescribed primaquine (< 1% at LRMC and 33% at U.S. MTFs) by clinicians. However, both types of antimalarial drugs were largely not prescribed for recommended durations (primary chemoprophylaxis: median = 13 days, IQR = 4–21 days; primaquine: median = 11 days, IQR = 8–13 days). At the present time, few data are available on receipt of antimalarial chemoprophylaxis among combat casualties during hospitalization. Among wounded British military personnel evacuated from Afghanistan, prescription of appropriate antimalarial chemoprophylaxis after MTF admission has been reported to be low; compliance ranged from 1.64% (5 of 305 patients) to 50% (4 of 8 patients) in various analyses.11,12 Although our analysis reported greater adherence to antimalarial chemoprophylaxis guidelines than the British studies, compliance was still poor, particularly for primaquine and possibly the result of a low perception of malaria risk by the clinicians.
Data reported herein suggest that severely injured patients were more likely to receive antimalarial chemoprophylaxis (Table 1). The greater proportion of infectious complications among patients with severe injuries may have resulted in them being followed by infectious disease specialists; thus, increasing the likelihood of receiving chemoprophylaxis. Moreover, this patient group had longer hospitalizations, which provided greater opportunity for clinicians to prescribe primary chemoprophylaxis for the recommended duration and initiate primaquine for two weeks.
Although a major strength of the analysis is use of prospective data obtained from multiple MTFs, there are study limitations to be considered. Although G6PD status is determined for all service members before deployment, this information was not captured in our study. The prevalence of G6PD deficiency has been estimated at approximately 2.5% among U.S. Army personnel and may account for a small percentage of those service members in which primaquine would be contraindicated.15 Data on duration of antimalarial chemoprophylaxis was only captured for patients who transferred to TIDOS-participating U.S. MTFs and consented to the cohort study, and this population consists of more severely injured patients.13 Thus, it is uncertain if these data are applicable to injured personnel at all MTFs. There also appears to be facility management differences, but we were unable to establish a cause. Finally, we were unable to determine if malaria developed in patients who did not receive antimalarial chemoprophylaxis after discharge because TIDOS does not capture this information.
Malaria remains a health concern for deployers to malaria-endemic areas. In 2002, 38 U.S. Army Rangers were infected with Plasmodium vivax malaria (52.4 cases per 1,000 persons), and were diagnosed a median of 233 days after return from deployment to eastern Afghanistan.7 In 2012, three cases of P. vivax malaria were diagnosed among members of an Army unit who had returned from deployment to Afghanistan approximately seven months earlier.10 The extended duration after return from a malaria-endemic area to diagnosis suggests that improved compliance and/or appropriate prescriptions of primaquine may have prevented these cases. As long as military personnel are deployed to malaria-endemic regions, preventive measures, such as chemoprophylaxis, are critical to lessen the likelihood of contracting this disease. However, potential hazards of large-scale antimalarial drug use among military deployers must also be considered. In recent years, antimalarial chemoprophylaxis recommendations have been adjusted because of neuropsychiatric contraindications with mefloquine, resulting in fewer prescriptions of the antimalarial agent.16 Furthermore, in addition to other adverse effects (e.g., gastrointestinal upset), widespread use of antimalarial drugs may lead to increased antimalarial or bacterial drug resistance. Recent contemporary analyses demonstrated increased tetracycline resistance among colonizing Staphylococcus aureus isolates and enteric flora collected from U.S. military personnel deployed to Afghanistan and Thailand who were receiving long-term doxycycline.17,18 The complexity of these factors may merit further analysis of current policies, as well as increased involvement of infectious disease specialists in the management of antimalarial chemoprophylaxis regimens.
The results of our analysis suggest that more awareness of antimalarial chemoprophylaxis recommendations may be necessary among clinicians to improve compliance for combat casualties. Additional analyses to validate our results and determine reasons for the lower rates of chemoprophylaxis regimens are warranted.
ACKNOWLEDGMENTS
We thank the Infectious Disease Clinical Research Program TIDOS study team of clinical coordinators, microbiology technicians, data managers, clinical site managers, and administrative support personnel for their tireless hours to ensure the success of this project.
Disclaimer: The views expressed are those of the authors do not necessarily reflect the official views of the Uniformed Services University of the Health Sciences, the National Institute of Health or the Department of Health and Human Services, the Department of Defense, or the Departments of the Army, Navy, or Air Force. Mention of trade names, commercial products, or organization does not imply endorsement by the U.S. Government.
Footnotes
Financial support: The study (IDCRP-024) was supported by the Infectious Disease Clinical Research Program, a Department of Defense program executed through the Uniformed Services University of the Health Sciences; the National Institute of Allergy and Infectious Diseases, National Institute of Health, under Inter-Agency Agreement Y1-AI-5072; and the Department of the Navy under the Wounded, Ill, and Injured Program.
Authors' addresses: Elizabeth A. Rini, Landstuhl Regional Medical Center, CMR 402, Box 1838, APO AE 09180-0019, E-mail: elizabeth.a.rini.mil@mail.mil. Amy C. Weintrob, Walter Reed National Military Medical Center, Bethesda, MD, and Infectious Disease Clinical Research Program, Uniformed Services University of the Health Sciences, Bethesda, MD, E-mail: amy.c.weintrob.ctr@health.mil. David R. Tribble, Faraz Shaikh, Ping Li, Deepak Aggarwal, and M. Leigh Carson, Infectious Disease Clinical Research Program, Uniformed Services University of the Health Sciences, Bethesda, MD, E-mails: david.tribble@usuhs.edu, fshaikh@idcrp.org, pli@idcrp.org, daggarwal@idcrp.org, and lcarson@idcrp.org. Bradley A. Lloyd and Clinton K. Murray, San Antonio Military Medical Center, Fort Sam Houston, TX, E-mails: bradley.lloyd@us.af.mil and clinton.k.murray.mil@mail.mil. Tyler E. Warkentien, Walter Reed National Military Medical Center, Bethesda, MD, E-mail: tyler.e.warkentien@health.mil.
References
- 1.World Health Organization . World Malaria Report 2012. 2012. http://www.who.int/malaria/publications/world_malaria_report_2012/en/ Available at. Accessed June 6, 2013. [Google Scholar]
- 2.Armed Forces Health Surveillance Center Update: malaria, U.S. Armed Forces, 2012. MSMR. 2013;20:2–5. [Google Scholar]
- 3.Armed Forces Health Surveillance Center Update: malaria, U.S. Armed Forces, 2010. MSMR. 2011;18:2–6. [Google Scholar]
- 4.Department of Defense . USCENTCOM 051122Z March 2010. MOD Ten to USCENTCOM Individual Protection and Individual-Unit Deployment Policy. 2010. [Superseded by MOD Eleven and no longer publically available] [Google Scholar]
- 5.Department of the Army . Department of the Army Personnel Policy Guidance for Overseas Contingency Operations. 2013. http://www.armyg1.army.mil/MilitaryPersonnel/PPG/PPG_17-May-2013.pdf Available at. Accessed June 6, 2013. [Google Scholar]
- 6.Newton JA, Jr, Schnepf GA, Wallace MR, Lobel HO, Kennedy CA, Oldfield EC ., III Malaria in US Marines returning from Somalia. JAMA. 1994;272:397–399. [PubMed] [Google Scholar]
- 7.Kotwal RS, Wenzel RB, Sterling RA, Porter WD, Jordan NN, Petruccelli BP. An outbreak of malaria in US Army Rangers returning from Afghanistan. JAMA. 2005;293:212–216. doi: 10.1001/jama.293.2.212. [DOI] [PubMed] [Google Scholar]
- 8.Brisson M, Brisson P. Compliance with antimalaria chemoprophylaxis in a combat zone. Am J Trop Med Hyg. 2012;86:587–590. doi: 10.4269/ajtmh.2012.11-0511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Whitman TJ, Coyne PE, Magill AJ, Blazes DL, Green MD, Milhous WK, Burgess TH, Freilich D, Tasker SA, Azar RG, Endy TP, Clagett CD, Deye GA, Shanks GD, Martin GJ. An outbreak of Plasmodium falciparum malaria in U.S. Marines deployed to Liberia. Am J Trop Med Hyg. 2010;83:258–265. doi: 10.4269/ajtmh.2010.09-0774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shaha DP, Pacha LA, Garges EC, Scoville SL, Mancuso JD. Confirmed malaria cases among active component U.S. Army personnel, January–September 2012. MSMR. 2013;20:6–9. [PubMed] [Google Scholar]
- 11.Keene DD, Tong JL, Roughton S, Fadden SJ. Anti-malarial chemoprophylaxis following evacuation from Afghanistan. J R Army Med Corps. 2012;158:38–40. doi: 10.1136/jramc-158-01-09. [DOI] [PubMed] [Google Scholar]
- 12.Penn-Barwell J, Pengelly S. Audit of prescription of anti-malarial prophylaxis to patients admitted to Royal Centre for Defence Medicine (RCDM) following evacuation from Afghanistan. J R Nav Med Serv. 2008;94:112–114. [PubMed] [Google Scholar]
- 13.Tribble DR, Conger NG, Fraser S, Gleeson TD, Wilkins K, Antonille T, Weintrob A, Ganesan A, Gaskins LJ, Li P, Grandits G, Landrum ML, Hospenthal DR, Millar EV, Blackbourne LH, Dunne JR, Craft D, Mende K, Wortmann GW, Herlihy R, McDonald J, Murray CK. Infection-associated clinical outcomes in hospitalized medical evacuees after traumatic injury: trauma infectious disease outcome study. J Trauma. 2011;71:S33–S42. doi: 10.1097/TA.0b013e318221162e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eastridge BJ, Jenkins D, Flaherty S, Schiller H, Holcomb JB. Trauma system development in a theater of war: experiences from Operation Iraqi Freedom and Operation Enduring Freedom. J Trauma. 2006;61:1366–1372. doi: 10.1097/01.ta.0000245894.78941.90. discussion 1372–1373. [DOI] [PubMed] [Google Scholar]
- 15.Chinevere TD, Murray CK, Grant E, Jr, Johnson GA, Duelm F, Hospenthal DR. Prevalence of glucose-6-phosphate dehydrogenase deficiency in U.S. Army personnel. Mil Med. 2006;171:905–907. doi: 10.7205/milmed.171.9.905. [DOI] [PubMed] [Google Scholar]
- 16.Nevin RL. Mefloquine prescriptions in the presence of contraindications: prevalence among US military personnel deployed to Afghanistan, 2007. Pharmacoepidemiol Drug Saf. 2010;19:206–210. doi: 10.1002/pds.1879. [DOI] [PubMed] [Google Scholar]
- 17.Vento TJ, Calvano TP, Cole DW, Mende K, Rini EA, Tully CC, Landrum ML, Zera W, Guymon CH, Yu X, Beckius ML, Cheatle KA, Murray CK. Staphylococcus aureus colonization of health military service members in the United States and Afghanistan. BMC Infect Dis. 2013;13:325. doi: 10.1186/1471-2334-13-325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Arthur JD, Echeverria P, Shanks GD, Karwacki J, Bodhidatta L, Brown JE. A comparative study of gastrointestinal infections in United States soldiers receiving doxycycline or mefloquine for malaria prophylaxis. Am J Trop Med Hyg. 1990;43:608–613. doi: 10.4269/ajtmh.1990.43.608. [DOI] [PubMed] [Google Scholar]