Abstract
Patients with acute uncomplicated Plasmodium falciparum malaria have no evident neurologic disorder, vital organ dysfunction, or other severe manifestations of infection. Nonetheless, parasitized erythrocytes cytoadhere to the endothelium throughout their microvasculature, especially within the brain. We aimed to determine if 3 Tesla magnetic resonance imaging studies could detect evidence of cerebral abnormalities in these patients. Within 24 hours of admission, initial magnetic resonance imaging examinations found a lesion with restricted water diffusion in the mid-portion of the splenium of the corpus callosum of 4 (40%) of 10 male patients. The four patients who had a splenial lesion initially had evidence of more severe hemolysis and thrombocytopenia than the six patients who had no apparent abnormality. Repeat studies four weeks later found no residua of the lesions and resolution of the hematologic differences. These observations provide evidence for acute cerebral injury in the absence of severe or cerebral malaria.
Introduction
Worldwide, the most important parasitic disease infecting the central nervous system is Plasmodium falciparum malaria.1 Of the five protozoan species of the genus Plasmodium that infect humans, P. falciparum causes almost all severe disease and death. P. falciparum is also the only species that modifies the erythrocyte surface so that infected erythrocytes are sequestered within the microvasculature by binding to the vascular endothelium.2 Cytoadherance of erythrocytes in vital organs throughout the body, especially in the brain, is believed to occur in all cases of falciparum malaria.3–5 Cerebral malaria is the most lethal complication of falciparum infection, presenting as a diffuse symmetric encephalopathy with alterations in the level of consciousness, ranging from drowsiness to deep coma, at times precipitated by seizures.6 In cerebral malaria, microvascular obstruction by sequestered parasitized erythrocytes leading to axonal damage has been proposed as a principal pathway responsible for coma and neurologic dysfunction, possibly in concert with a variety of immunopathologic mechanisms.1,7–10 Sequestration and cerebral damage have been documented at autopsy in patients who died of cerebral or other forms of severe malaria.3,11–13
In children in Malawi with cerebral malaria, magnetic resonance imaging (MRI) studies at 0.35 Tesla have identified distinctive findings in cortical, deep gray, and white matter structures.14,15 Acute uncomplicated falciparum malaria is an illness with P. falciparum asexual parasitemia and symptoms similar to those of a minor systemic viral infection, including headache, fever, chills, malaise, abdominal discomfort, and muscle and joint aches, but with no apparent neurologic disorder, vital organ dysfunction, or other severe clinical or laboratory manifestations of infection.16 Sequestration of parasitized erythrocytes within the microcirculation of the brain potentially develops in acute uncomplicated falciparum malaria10 but neuroimaging observations have been lacking.17 We aimed to determine if high-field (3.0 Tesla) MRI studies could detect evidence of cerebral abnormalities in adult patients with acute uncomplicated falciparum malaria in Thailand.
Materials and Methods
Study participants.
This study was a single-site prospective examination of adult patients with acute uncomplicated falciparum malaria admitted to a hospital in Thailand specializing in the care of patients with malaria. Acute uncomplicated falciparum malaria was defined as a febrile symptomatic illness with asexual P. falciparum parasitemia in the absence of any of the clinical features or laboratory findings meeting the World Health Organization criteria for severe malaria.18,19 This study was approved by the Institutional Review Boards of the institutions involved. A detailed verbal and written explanation of the research project was provided and each participant gave fully informed, signed consent to participate in the study. Patients were excluded from the study if there was a history of previous malarial infection, underlying disorders, seizures, splenectomy, drug or alcohol abuse, an age < 18 years or > 65 years, or if they were women who were or could be pregnant. To protect vulnerable populations, patients with a history of treatment for mental illness, imprisonment, or institutionalization were also excluded.
Study procedures.
At admission, a history was obtained and a physical examination, including a standard neurologic evaluation, was performed, and the level of consciousness of each patient was assessed by using the Glasgow coma scale.18 After clinical evaluation and examination of thick and thin blood smears to establish the diagnosis, blood samples were obtained for hematologic and biochemical studies at baseline and periodically thereafter during the four-week hospitalization period. Hematologic studies were performed by using an Advia 120 Hematology Analyzer (Bayer HealthCare, Diagnostics Division, Tarrytown, NY).
All patients received antimalarial treatment with artemesinin combination therapy as part of clinical studies examining regimens for treatment of falciparum malaria and remained in the hospital for four weeks to assess clinical outcome, safety, and tolerance and to evaluate the cure rate at 28-day follow-up. Patients found to be co-infected by asexual forms of P. vivax were treated with the hospital's standard regimen for vivax malaria: chloroquine (30 mg base/kg given over 3 days) and primaquine (15 mg once a day for 14 days).
Parasite counts were determined by microscopy and counting infected erythrocytes per 1,000 erythrocytes in thin blood films, or calculated from the parasite count per 200 leukocytes in thick blood films. Parasite clearance time was defined as the time from the start of treatment until the patient's first negative blood film, with the blood film then remaining negative for 24 hours. Fever clearance time was defined as the time from the start of treatment until the oral temperature decreased below 37.5°C and then remained at or below this level for 48 hours.
MRI techniques and analysis.
Within 24 hours of admission, MRI studies were performed by using a 3.0-Tesla scanner (Philips Achieva; Philips Healthcare Best, The Netherlands) with a phased-array multi-channel head coil and standard MRI pulse sequences to provide anatomic, clinical, and metabolic data. In addition to a standard neuroradiologic protocol, the studies included a T2-weighted fluid-attenuated inversion recovery (T2FLAIR) sequence (TR = 11000 ms, TE = 100 ms, TI = 2800 ms, FOV = 240 × 192 mm, acquisition matrix = 256 × 164, and recon matrix = 512 × 512); and a diffusion-weighted imaging echo-planar imaging (DWI-EPI) sequence (TR = 3000 ms, TE = 88 ms, b = [0,1000] s/mm2, FOV = 240 × 240 mm, acquisition matrix = 112 × 90, and recon matrix = 256 × 256). Using the diffusion-weighted images, maps of the apparent diffusion coefficient (ADC) were generated. The MRI studies were repeated near the end of the four-week hospitalization. All MRI studies were independently reviewed by two clinical radiologists for structural lesions.
Statistical analysis.
Groups of patients were compared by using the unpaired Student's t tests for continuous variables with a Gaussian distribution, the Mann-Whitney test for nonparametric tests of continuous variables without a Gaussian distribution, and Fisher's exact test for proportions. Because the distributions of initial parasite counts were skewed, parasite counts were log-transformed before comparing the means with Student's t test. The results were then retransformed into antilogarithms to recover the original units and were expressed as geometric means with the upper and lower 95% confidence intervals.
Results
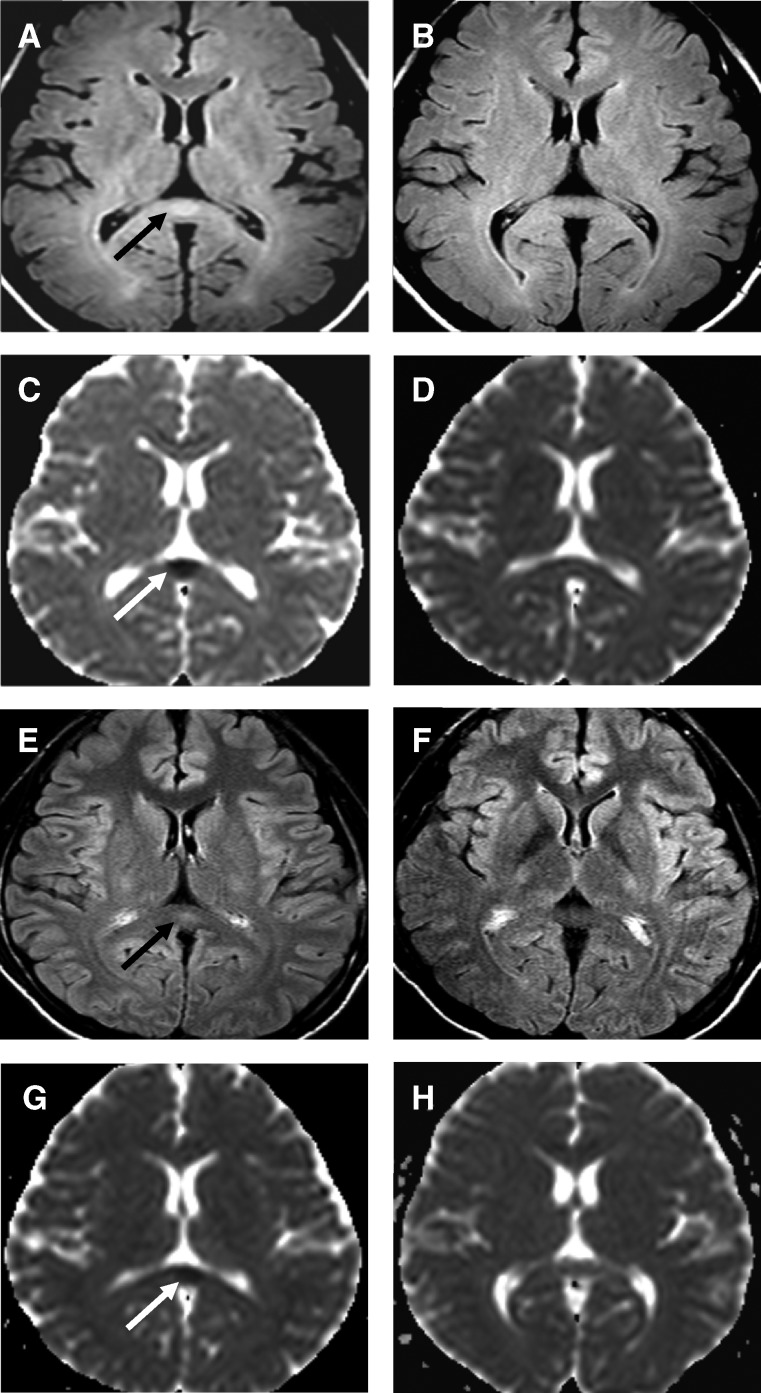
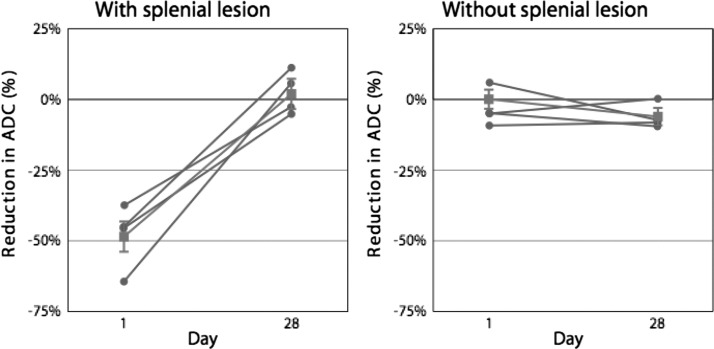
We describe the first 10 consecutive patients with acute uncomplicated malaria who were examined with a 3.0-Tesla MR scanner. Their clinical characteristics are summarized in Table 1 . All patients were men 18–45 years of age. At admission, nine patients had only P. falciparum parasitemia, and one patient had a mixed infection with P. vivax. All patients were conscious and had a Glasgow coma score of 14–15. No patient had a history of coma or brain injury. No neurologic abnormalities were identified by standard examination. In 4 of the 10 patients (40%), the initial magnetic resonance study showed a hyperintense, symmetrical oval lesion in the midline of the splenium of the corpus callosum on images derived from T2FLAIR sequences (Figure 1 ). Using maps of the ADC of water derived from the diffusion-weighted echo-planar imaging studies, we found that measurements of the corresponding regions of interest within the mid portion of the splenium of the corpus callosum showed a decrease in the ADC for each patient in whom a lesion was seen on the T2FLAIR examination. In this group of four patients, comparisons of the ADC estimates with those derived from comparable areas in the remaining six patients indicated a significant reduction in diffusion (Figure 2 ) (P < 0.0001). Visual inspection of the ADC maps found no other focal abnormalities. Follow-up examinations at the end of the 28-day hospitalization found resolution of the splenial lesions on T2FLAIR images in the four patients initially affected. In addition, measurements of corresponding areas of the ADC maps now found no significant difference between the four patients initially affected and the remaining patients (Figure 2) (P > 0.11).
Table 1.
Clinical characteristics of patients*
| Variable | Age (years) | Days of fever before admission | Parasite count on admission (no./μL) | Parasite count at 24 hours (no./μL) | Parasite clearance time (hours) | Fever clearance time (hours) | Splenial lesion on initial T2FLAIR |
|---|---|---|---|---|---|---|---|
| 22 | 3 | 588,000 | 36,800 | 63 | 94 | + | |
| 30 | 6 | 9,200† | 896 | 58 | 48 | + | |
| 34 | 3 | 50,700 | 6,720 | 49 | 18 | + | |
| 23 | 3 | 708,000 | 104,000 | 94 | 80 | + | |
| Mean | 27 | 3‡ | 118,000§ | 12,300§ | 66 | 64 | |
| SEM | 3 | 1–6 | 4,300–3,240,000 | 450–338,000 | 10 | 6–114 | |
| 24 | 30 | 15,300 | 48 | 31 | 20 | − | |
| 45 | 3 | 112,000 | 17,800 | 79 | 154 | − | |
| 26 | 5 | 38,600 | 95 | 48 | 16 | − | |
| 18 | 5 | 78,800 | 120 | 44 | 16 | − | |
| 39 | 3 | 3,580 | 54 | 81 | 12 | − | |
| 18 | 3 | 104,000 | 9,920 | 56 | 42 | − | |
| Mean | 28 | 4‡ | 35,300§ | 485§ | 57 | 18‡ | |
| SEM | 5 | −3 to 20 | 8,580–146,000 | 30–7,840 | 8 | −15–101 | |
| P | 0.85 | 0.36 | 0.29 | 0.08 | 0.48 | 0.26 | |
T2FLAIR = T2-weighted fluid-attenuated inversion recovery. Ranges are 95% confidence intervals.
128 Plasmodium vivax.
Median.
Geometric mean.
Figure 1.
Magnetic resonance imaging studies of two patients with acute malaria who had no neurologic symptoms or signs. A–D, A 30 year-old man with uncomplicated malaria and a mixed infection (Plasmodium falciparum: 9,200 parasites/μL; P. vivax: 128 parasites/μL). E–H, A 22 year-old man with hyperparasitemia (P. falciparum: 588,000 parasites/μL). T2-weighted fluid-attenuated inversion recovery sequences (A and E) obtained shortly after admission show hyperintense, symmetrical oval lesions in the midline of the splenium of the corpus callosum (black arrows). Repeat examinations four weeks later (B and F), show resolution of the lesions. Diffusion-weighted imaging echo-planar imaging studies (C and G) shortly after admission showed relative decreases in the apparent diffusion coefficient within the lesions (white arrows) that had also resolved on the repeat studies (D and H) four weeks later.
Figure 2.
Change in apparent diffusion coefficient (ADC) (%) from day 1 (the first day after admission) to day 28 for four patients with a splenial lesion (left panel) and six patients without a splenial lesion (right panel). Gray circles and lines show the individual values; gray squares and lines show medians for each group of patients. Upper and lower 95% confidence intervals for the median are shown by the vertical gray lines.
Quantitative assessment of the apparent diffusion coefficients (ADC) found an approximately 50% overall reduction in tissue water diffusion rates in the lesion on the initial MR study with recovery to normal four weeks later (Figure 2A). Further analysis of the ADC data showed that diffusion anisotropy was preserved in the lesion. The diffusion rate was approximately four times greater in the transverse direction (left–right, X), parallel to the crossing axons, than in the anterior-posterior direction (front–back, Y) or in the superior-inferior direction (head–toe, Z) (Figure 2B). In the acute phase MRI, diffusion appears to be equally proportionally restricted by approximately 50% in the X, Y, and Z axes but the magnitude of restriction is greatest by approximately four times in the X direction, parallel to the long axis of the crossing axons.
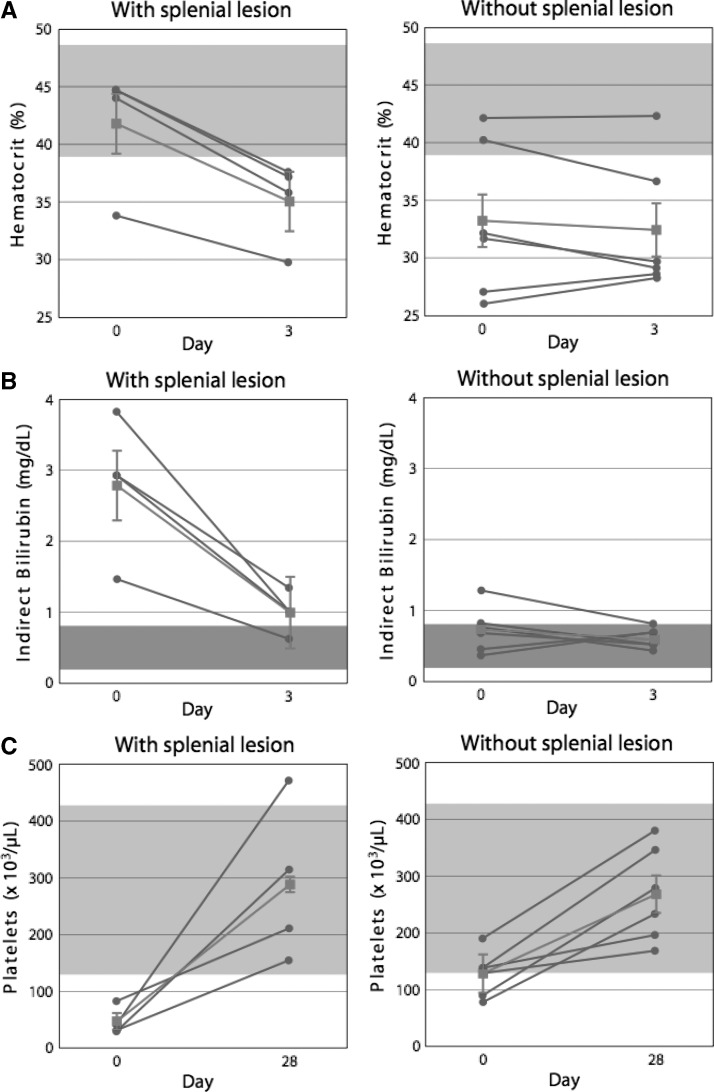
At admission, the four patients with the splenial lesions compared with the six patients with no apparent abnormality initially had a higher median hematocrit (Figure 3A) (P < 0.04), a higher mean serum indirect bilirubin level (Figure 3B) (P < 0.03) and a lower mean platelet count (Figure 3A) (P < 0.01), as well as a greater decrease in hematocrit over the first three hospital days (Figure 3A) (P < 0.003).
Figure 3.
A, Change in hematocrit (%) from day 0 (admission) to day 3 for 4 patients with a splenial lesion (left panel) and six patients without a splenial lesion (right panel). Gray circles and lines show individual values; gray squares and lines show medians for each group of patients. Upper and lower 95% confidence intervals for the median are shown by the vertical gray lines. B, Change in indirect bilirubin concentration (mg/dL) from day 0 (admission) to day 3 for 4 patients with a splenial lesion (left panel) and 6 patients without a splenial lesion (right panel). Gray circles and lines show individual values; gray squares and lines shows medians for each group of patients. Upper and lower 95% confidence intervals for the median are shown by the vertical gray lines. C, Change in platelet count (×103/μL) from day 0 (admission) to day 28 for four patients with a splenial lesion (left panel) and six patients without a splenial lesion (right panel). Gray circles and lines show individual values; gray squares and lines show medians for each group of patients. Upper and lower 95% confidence intervals for the median are shown by the vertical gray lines. Shaded areas in the figure indicate the laboratory reference range for each measurement.
No significant differences were found between the four patients with and the six patients without the splenial lesions with respect to mean age, days of fever before admission, parasite count on admission, parasite count after 24 hours of hospitalization (near the time of the initial MRI examination), parasite clearance time or fever clearance time (Table 1). Mean ± SEM serum creatinine did not differ significantly between the groups with and without the splenial lesion either on admission (0.90 ± 0.04 versus 0.78 ± 0.08 U/L; P = 0.30) or on day 3 of hospitalization (0.73 ± 0.03 versus 0.68 ± 0.06; P = 0.61). Four weeks later, near the time of discharge from the hospital, the groups of patients with and without the splenial lesions did not differ significantly with respect to either median hematocrit (Figure 3A) (P = 0.61) or mean platelet count (Figure 3A) (P = 0.24).
Discussion
Each of these 10 patients had acute uncomplicated falciparum malaria, was fully conscious (Glasgow coma score of 14–15) and had no abnormality detected by standard neurologic examination. In 4 (40%) of the 10 patients, a distinctive symmetrical midline lesion in the central splenium of the corpus callosum was found at admission (Figure 1), which is evidence of a combination of tissue increased water content and diffusion restriction. T2FLAIR images showed that the lesion was hyperintense as a result of prolonged tissue T2 relaxation, an indicator of increased tissue water content. In acute phase diffusion-weighted images, the lesion was also hyperintense, both when compared with more lateral portions of the splenium or to the genu of the corpus callosum and when compared with the same area four weeks later (Figure 1), a sign of transient diffusion restriction. Notably, almost half the children in Malawi with cerebral malaria had increased T2 signal intensity changes in the corpus callosum, usually with diffusion-weighted image abnormalities, and predominantly involving the splenium.15 Lesions in the splenium of the corpus callosum have been reported in three adults with cerebral malaria,20–22 and had resolved in the two patients who were re-examined, 36 days20 and 4 months later.21
Similar changes in the central splenium of the corpus callosum have been described in other, non-malarial disease states,20,23,24 including temporal lobe epilepsy, trauma (shear injury), alcoholism with vitamin deficiency (Marchifava-Bignami), primary demyelination, and encephalitis, but the MRI appearance and time course in our patients most closely resembles high altitude cerebral edema25 and may share common pathophysiologic mechanisms. Observational and experimental studies of high-altitude cerebral edema suggest that the primary etiology is hypoxemia, which leads to tissue oxidative stress, endothelial cell injury, and spreading of endothelial cell junctions with blood–brain barrier opening, resulting in a combination of increased tissue water content from vasogenic interstitial edema and of tissue diffusion restriction.26,27 Hypoxemia in high-altitude cerebral edema may also stimulate a variety of cellular and molecular responses that affect tissue energy metabolism and alter endothelial permeability via vascular endothelial growth factor, oxygen free radicals and upregulation of nitric oxide production.26,27
In falciparum malaria, sequestration of parasitized erythrocytes in the cerebral microvasculature may produce tissue hypoxia by reducing the oxygen carrying capacity of the parasitized erythrocytes and decreasing oxygen delivery by slowing the passage of erythrocytes. Capillary obstruction or occlusion may cause ischemia and reduced delivery of vascular water, oxygen, and glucose. All of these potential consequences of parasitic sequestration could impair cellular energy metabolism and lead to reduced energy-dependent transmembrane water movement and axonal transport.28 The proportional decrease in X-, Y-, and Z-axis ADC measurements in the central splenium of the corpus callosum of our patients suggests a generalized reduction in cellular water motion, including axonal transport. The degree of ADC reduction of approximately 50% seen in our patients is often associated with permanent tissue damage in cerebral ischemia but the observed transient changes seem to have been of insufficient severity to trigger apoptosis and cellular autolysis.
These general considerations do not specifically explain the involvement of the central splenium of the corpus callosum. These focal changes may be related to the vascular anatomy of the splenium where small arterioles penetrate the corpus callosum directly from large arteries, making them more likely to dilate abnormally with increased local blood flow, blood volume, and intra-arterial pressure.29 The splenium is also the only region of the corpus callosum supplied by the posterior circulation, which may be more prone to transient opening of the blood–brain barrier.30 The microscopic anatomy of the dense myelinated axons in relation to the capillary density in the splenium may also play a role in producing relatively high local tissue hypoxia because of increased oxygen diffusion distances (or relative barriers to diffusion such as the myelin sheath or oxygen steal by oligodendrocytes along the oxygen diffusion pathways).31
At admission, the four patients with the splenial lesions had a higher median hematocrit than the six patients without splenial lesions (Figure 3A) (P < 0.04), a difference that would increase blood viscosity, reduce flow, and favor sequestration. In addition, the higher mean indirect bilirubin (Figure 3B) (P < 0.03) initially in the four patients with splenial lesions relative to the remaining six patients is evidence of increased hemolysis at admission. Subsequently, during the first three days of hospitalization, the median hematocrit decreased more rapidly in the group with the splenial abnormality (Figure 3A) (by 7% versus 1%; P < 0.02), despite effective antimalarial treatment. The increased hemolytic activity in the patients with the splenial lesion occurred in conjunction with marked thrombocytopenia: the median platelet count on admission was 48,500 × 103/μL compared with 129,000 × 103/μL in the remaining patients (Figure 3C) (P < 0.02).
Earlier reports from Kenya and studies of patients with acute falciparum malaria in northwestern Thailand have identified platelet-mediated clumping or autoagglutination in P. falciparum isolates.32–34 In Thailand, the agglutination phenotype was found in approximately half of the isolates but, notably, was present in 100% of patients with cerebral malaria.32 Autoagglutination is believed to involve a platelet-mediated ligand-receptor interaction, possibly mediated by CD36 acting as a receptor for the P. falciparum erythrocyte membrane protein 1 on parasitized erythrocytes.33,35–37 In our patients, increased sequestration and destruction of erythrocytes and platelets in platelet-mediated autoagglutinates may have contributed to the severity of the hemolysis and thrombocytopenia, as well as to the microvascular obstruction underlying the lesions in the splenium of the corpus callousum. The power of our study may have been insufficient to detect other differences between those with and without splenial lesions.
The consequences of splenial lesions similar to those shown in Figure 1 would not be evident by standard neurologic examination. The various disconnection syndromes that are produced by damage to the axons passing through the splenium include varieties of alexia, tactile anomia, apraxia, dysgraphia, and other deficits.38–40 Specialized testing is needed to detect these conditions but detailed assessment of neurocognitive functioning is difficult in acutely ill patients with cerebral malaria and is seldom included in clinical evaluation. In an exceptional study, specialized somatosensory examination of 20 children in Ghana with a recent history of cerebral malaria identified tactile discrimination deficits.41 A strong negative correlation (R = −0.72) was found between coma duration and tactile discrimination. The authors concluded on clinical grounds that impaired integrity of axonal tracts in the corpus callosum was likely to be responsible. We plan to include detailed clinical neurocognitive evaluation in future neuroimaging studies of patients with malaria.
The clinical presentation of the neurologic abnormalities associated with falciparum malaria differs between children in Africa and adults in Southeast Asia.42 Although cerebral malaria occurs in both populations, children in Africa living in malaria-endemic areas have a much higher incidence of seizures and persistent neurocognitive impairment in survivors is increasingly recognized.1,6,42–44 In Southeast Asia, cerebral malaria in adults with no antimalarial immunity often occurs in association with multi-system organ failure but many adult survivors of severe malaria seem to make a full neurologic recovery, at least as judged by standard clinical neurologic evaluation.10,45 The means whereby P. falciparum can produce severe but potentially reversible neurologic complications are still uncertain but accumulating evidence supports axonal injury as at least one pathologic mechanism.7,10,28,46
This MRI study of adults with no anti-malarial immunity in Southeast Asia is too small to provide a meaningful estimate of the prevalence of ischemic lesions affecting the brain in patients with acute malaria. In our patients, after effective antimalarial therapy, repeat magnetic resonance studies at the end of the four-week hospitalization found the lesions wholly resolved. We have no grounds for conjecture about the likelihood of resolution of such lesions in the absence of antimalarial therapy or with treatment less effective than prompt administration of the potent antimalarial drugs used in our study. Nonetheless, a large number of persons potentially could be affected. In Southeast Asia, nearly one billion persons are now exposed to malaria and 25% of the world's clinical attacks of malaria occur in this region.47–49 Episodes of uncomplicated falciparum malaria may be an unrecognized source of neurologic disease and disability in affected populations, both in Southeast Asia and globally.
ACKNOWLEDGMENTS
We thank the patients for their participation in this study and the hospital staff for their assistance.
Footnotes
Financial support: This study was supported in part by National Institutes of Health/National Institute of Neurological Disorders and Stroke grant R21 NS055348 and by the U.S. National Institutes of Health (Grant U01 HD061233), Eunice Kennedy Shriver National Institute of Child Health and Human Development, and the Office of Dietary Supplements.
Authors' addresses: Jiraporn Laothamatas, Faculty of Medicine, Department of Radiology, Ramathibodi Hospital, Mahidol University, Bangkok, Thailand, E-mail: laothamatas@yahoo.com. Christina L. Sammet, Department of Medical Imaging, Ann and Robert H. Lurie Children's Hospital of Chicago, Chicago, IL, E-mail: csammet@luriechildrens.org. Xavier Golay, Institute of Neurology, University College London, London WC1N 3BG, UK, E-mail: xgolay@gmail.com. Marc Van Cauteren, Philips Medical Systems Asia Pacific, Tokyo, Japan, E-mail: marc.van.cauteren@philips.com. Varinee Lekprasert, Noppadon Tangpukdee, Srivicha Krudsood, Wattana Leowattana, Polrat Wilairatana, and Sornchai Looareesuwan, Faculty of Tropical Medicine, The Hospital for Tropical Diseases, Mahidol University, Bangkok, Thailand, E-mails: varinee63@aol.com, tmntp@mucc.mahidol.ac.th, tmsks@mucc.mahidol.ac.th, leowattana@yahoo.com, and tmpwl@mucc.mahidol.ac.th. Srirama V. Swaminathan, Philips Healthcare, Andover, MA, E-mail: sri.swaminathan@philips.com. Robert L. DeLaPaz, Department of Radiology, Columbia University, New York, NY, E-mail: rld17@columbia.edu. Truman R. Brown, Department of Radiology and Radiological Sciences, Center for Advanced Imaging Research, Medical University of South Carolina, Charleston, SC, E-mail: brotrr@musc.edu. Gary M. Brittenham, Division of Pediatric Hematology, Oncology and Stem Cell Transplantation, Department of Pediatrics, Columbia University College of Physicians and Surgeons, Childrens' Hospital of New York, CHN 10-08, 3959 Broadway, New York, NY, E-mail: gmb31@columbia.edu.
References
- 1.Idro R, Marsh K, John CC, Newton CR. Cerebral malaria: mechanisms of brain injury and strategies for improved neurocognitive outcome. Pediatr Res. 2010;68:267–274. doi: 10.1203/PDR.0b013e3181eee738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Miller LH, Ackerman HC, Su XZ, Wellems TE. Malaria biology and disease pathogenesis: insights for new treatments. Nat Med. 2013;19:156–167. doi: 10.1038/nm.3073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pongponratn E, Turner GD, Day NP, Phu NH, Simpson JA, Stepniewska K, Mai NT, Viriyavejakul P, Looareesuwan S, Hien TT, Ferguson DJ, White NJ. An ultrastructural study of the brain in fatal Plasmodium falciparum malaria. Am J Trop Med Hyg. 2003;69:345–359. [PubMed] [Google Scholar]
- 4.Cunnington AJ, Bretscher MT, Nogaro SI, Riley EM, Walther M. Comparison of parasite sequestration in uncomplicated and severe childhood Plasmodium falciparum malaria. J Infect. 2013;67:220–230. doi: 10.1016/j.jinf.2013.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rowe JA, Claessens A, Corrigan RA, Arman M. Adhesion of Plasmodium falciparum-infected erythrocytes to human cells: molecular mechanisms and therapeutic implications. Expert Rev Mol Med. 2009;11:e16. doi: 10.1017/S1462399409001082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carter JA, Mung'ala-Odera V, Neville BG, Murira G, Mturi N, Musumba C, Newton CR. Persistent neurocognitive impairments associated with severe falciparum malaria in Kenyan children. J Neurol Neurosurg Psychiatry. 2005;76:476–481. doi: 10.1136/jnnp.2004.043893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Medana IM, Esiri MM. Axonal damage: a key predictor of outcome in human CNS diseases. Brain. 2003;126:515–530. doi: 10.1093/brain/awg061. [DOI] [PubMed] [Google Scholar]
- 8.Medana IM, Lindert RB, Wurster U, Hien TT, Day NP, Phu NH, Mai NT, Chuong LV, Chau TT, Turner GD, Farrar JJ, White NJ. Cerebrospinal fluid levels of markers of brain parenchymal damage in Vietnamese adults with severe malaria. Trans R Soc Trop Med Hyg. 2005;99:610–617. doi: 10.1016/j.trstmh.2004.11.017. [DOI] [PubMed] [Google Scholar]
- 9.Medana IM, Idro R, Newton CR. Axonal and astrocyte injury markers in the cerebrospinal fluid of Kenyan children with severe malaria. J Neurosci. 2007;258:93–98. doi: 10.1016/j.jns.2007.03.005. [DOI] [PubMed] [Google Scholar]
- 10.White NJ, Pukrittayakamee S, Hien TT, Faiz MA, Mokuolu OA, Dondorp AM. Malaria. Lancet. 2013 doi: 10.1016/S0140-6736(13)60024-0. Aug 15 [Epub ahead of print]. doi:10.1016/S0140-6736(13)60024-0. [DOI] [PubMed] [Google Scholar]
- 11.Birbeck GL, Beare N, Lewallen S, Glover SJ, Molyneux ME, Kaplan PW, Taylor TE. Identification of malaria retinopathy improves the specificity of the clinical diagnosis of cerebral malaria: findings from a prospective cohort study. Am J Trop Med Hyg. 2010;82:231. doi: 10.4269/ajtmh.2010.09-0532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dorovini-Zis K, Schmidt K, Huynh H, Fu W, Whitten RO, Milner D, Kamiza S, Molyneux M, Taylor TE. The neuropathology of fatal cerebral malaria in malawian children. Am J Pathol. 2011;178:2146–2158. doi: 10.1016/j.ajpath.2011.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Milner DA, Valim C, Luo R, Playforth KB, Kamiza S, Molyneux ME, Seydel KB, Taylor TE. Supraorbital postmortem brain sampling for definitive quantitative confirmation of cerebral sequestration of Plasmodium falciparum parasites. J Infect Dis. 2012;205:1601–1606. doi: 10.1093/infdis/jis001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kampondeni SD, Potchen MJ, Beare NA, Seydel KB, Glover SJ, Taylor TE, Birbeck GL. MRI findings in a cohort of brain injured survivors of pediatric cerebral malaria. Am J Trop Med Hyg. 2013;88:542–546. doi: 10.4269/ajtmh.12-0538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Potchen MJ, Kampondeni SD, Seydel KB, Birbeck GL, Hammond CA, Bradley WG, DeMarco JK, Glover SJ, Ugorji JO, Latourette MT, Siebert JE, Molyneux ME, Taylor TE. Acute brain MRI findings in 120 Malawian children with cerebral malaria: new insights into an ancient disease. AJNR Am J Neuroradiol. 2012;33:1740–1746. doi: 10.3174/ajnr.A3035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.World Health Organization . Management of Severe Malaria: A Practical Handbook. Geneva: World Health Organization; 2013. [Google Scholar]
- 17.Looareesuwan S, Laothamatas J, Brown TR, Brittenham GM. Cerebral malaria: a new way forward with magnetic resonance imaging (MRI) Am J Trop Med Hyg. 2009;81:545–547. doi: 10.4269/ajtmh.2009.07-0411. [DOI] [PubMed] [Google Scholar]
- 18.World Health Organization Severe falciparum malaria. World Health Organization, Communicable Diseases Cluster. Trans R Soc Trop Med Hyg. 2000;94((Suppl 1)):S1–S90. [PubMed] [Google Scholar]
- 19.World Health Organization . Guidelines for the Treatment of Malaria. Geneva: World Health Organization; 2010. [Google Scholar]
- 20.Hantson P, Hernalsteen D, Cosnard G. Reversible splenial lesion syndrome in cerebral malaria. J Neuroradiol. 2010;37:243–246. doi: 10.1016/j.neurad.2009.12.005. [DOI] [PubMed] [Google Scholar]
- 21.Vyas S, Gupta V, Hondappanavar A, Sakhuja V, Bhardwaj N, Singh P, Khandelwal N. Magnetic resonance imaging of cerebral malaria. J Emerg Med. 2012;42:e117–e119. doi: 10.1016/j.jemermed.2010.05.068. [DOI] [PubMed] [Google Scholar]
- 22.Yadav P, Sharma R, Kumar S, Kumar U. Magnetic resonance features of cerebral malaria. Acta Radiol. 2008;49:566–569. doi: 10.1080/02841850802020476. [DOI] [PubMed] [Google Scholar]
- 23.Polster T, Hoppe M, Ebner A. Transient lesion in the splenium of the corpus callosum: three further cases in epileptic patients and a pathophysiological hypothesis. J Neurol Neurosurg Psychiatry. 2001;70:459–463. doi: 10.1136/jnnp.70.4.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Takanashi J, Imamura A, Fumio H, Terada H. Differences in the time course of splenial and white matter lesions in clinically mild encephalitis/encephalopathy with a reversible splenial lesion (MERS) J Neurol Sci. 2010;292:24–27. doi: 10.1016/j.jns.2010.02.013. [DOI] [PubMed] [Google Scholar]
- 25.Hackett PH, Yarnell PR, Hill R, Reynard K, Heit J, McCormick J. High-altitude cerebral edema evaluated with magnetic resonance imaging: clinical correlation and pathophysiology. JAMA. 1998;280:1920–1925. doi: 10.1001/jama.280.22.1920. [DOI] [PubMed] [Google Scholar]
- 26.Kallenberg K, Bailey DM, Christ S, Mohr A, Roukens R, Menold E, Steiner T, Bartsch P, Knauth M. Magnetic resonance imaging evidence of cytotoxic cerebral edema in acute mountain sickness. J Cereb Blood Flow Metab. 2007;27:1064–1071. doi: 10.1038/sj.jcbfm.9600404. [DOI] [PubMed] [Google Scholar]
- 27.Roach RC, Hackett PH. Frontiers of hypoxia research: acute mountain sickness. J Exp Biol. 2001;204:3161–3170. doi: 10.1242/jeb.204.18.3161. [DOI] [PubMed] [Google Scholar]
- 28.Medana IM, Day NP, Hien TT, Mai NT, Bethell D, Phu NH, Farrar J, Esiri MM, White NJ, Turner GD. Axonal injury in cerebral malaria. Am J Pathol. 2002;160:655–666. doi: 10.1016/S0002-9440(10)64885-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kakou M, Destrieux C, Velut S. Microanatomy of the pericallosal arterial complex. J Neurosurg. 2000;93:667–675. doi: 10.3171/jns.2000.93.4.0667. [DOI] [PubMed] [Google Scholar]
- 30.Bartynski WS, Boardman JF, Zeigler ZR, Shadduck RK, Lister J. Posterior reversible encephalopathy syndrome in infection, sepsis, and shock. AJNR Am J Neuroradiol. 2006;27:2179–2190. [PMC free article] [PubMed] [Google Scholar]
- 31.Klatzo I. Pathophysiological aspects of brain edema. Acta Neuropathol. 1987;72:236–239. doi: 10.1007/BF00691095. [DOI] [PubMed] [Google Scholar]
- 32.Chotivanich K, Sritabal J, Udomsangpetch R, Newton P, Stepniewska KA, Ruangveerayuth R, Looareesuwan S, Roberts DJ, White NJ. Platelet-induced autoagglutination of Plasmodium falciparum-infected red blood cells and disease severity in Thailand. J Infect Dis. 2004;189:1052–1055. doi: 10.1086/381900. [DOI] [PubMed] [Google Scholar]
- 33.Pain A, Ferguson DJ, Kai O, Urban BC, Lowe B, Marsh K, Roberts DJ. Platelet-mediated clumping of Plasmodium falciparum-infected erythrocytes is a common adhesive phenotype and is associated with severe malaria. Proc Natl Acad Sci USA. 2001;98:1805–1810. doi: 10.1073/pnas.98.4.1805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Roberts DJ, Pain A, Kai O, Kortok M, Marsh K. Autoagglutination of malaria-infected red blood cells and malaria severity. Lancet. 2000;355:1427–1428. doi: 10.1016/S0140-6736(00)02143-7. [DOI] [PubMed] [Google Scholar]
- 35.Miao WM, Vasile E, Lane WS, Lawler J. CD36 associates with CD9 and integrins on human blood platelets. Blood. 2001;97:1689–1696. doi: 10.1182/blood.v97.6.1689. [DOI] [PubMed] [Google Scholar]
- 36.Nakamura K, Hasler T, Morehead K, Howard RJ, Aikawa M. Plasmodium falciparum-infected erythrocyte receptor(s) for CD36 and thrombospondin are restricted to knobs on the erythrocyte surface. J Histochem Cytochem. 1992;40:1419–1422. doi: 10.1177/40.9.1380530. [DOI] [PubMed] [Google Scholar]
- 37.Wassmer SC, Lepolard C, Traore B, Pouvelle B, Gysin J, Grau GE. Platelets reorient Plasmodium falciparum-infected erythrocyte cytoadhesion to activated endothelial cells. J Infect Dis. 2004;189:180–189. doi: 10.1086/380761. [DOI] [PubMed] [Google Scholar]
- 38.Fabri M, Del Pesce M, Paggi A, Polonara G, Bartolini M, Salvolini U, Manzoni T. Contribution of posterior corpus callosum to the interhemispheric transfer of tactile information. Brain Res Cogn Brain Res. 2005;24:73–80. doi: 10.1016/j.cogbrainres.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 39.Gazzaniga MS. Principles of human brain organization derived from split-brain studies. Neuron. 1995;14:217–228. doi: 10.1016/0896-6273(95)90280-5. [DOI] [PubMed] [Google Scholar]
- 40.Lee ST, Jung YM, Na DL, Park SH, Kim M. Corpus callosum atrophy in Wernicke's encephalopathy. J Neuroimaging. 2005;15:367–372. doi: 10.1177/1051228405278352. [DOI] [PubMed] [Google Scholar]
- 41.Dugbartey AT, Spellacy FJ, Dugbartey MT. Somatosensory discrimination deficits following pediatric cerebral malaria. Am J Trop Med Hyg. 1998;59:393–396. doi: 10.4269/ajtmh.1998.59.393. [DOI] [PubMed] [Google Scholar]
- 42.Idro R, Jenkins NE, Newton CR. Pathogenesis, clinical features, and neurological outcome of cerebral malaria. Lancet Neurol. 2005;4:827–840. doi: 10.1016/S1474-4422(05)70247-7. [DOI] [PubMed] [Google Scholar]
- 43.Carter JA, Neville BG, White S, Ross AJ, Otieno G, Mturi N, Musumba C, Newton CR. Increased prevalence of epilepsy associated with severe falciparum malaria in children. Epilepsia. 2004;45:978–981. doi: 10.1111/j.0013-9580.2004.65103.x. [DOI] [PubMed] [Google Scholar]
- 44.Carter JA, Lees JA, Gona JK, Murira G, Rimba K, Neville BG, Newton CR. Severe falciparum malaria and acquired childhood language disorder. Dev Med Child Neurol. 2006;48:51–57. doi: 10.1017/S0012162206000107. [DOI] [PubMed] [Google Scholar]
- 45.Warrell DA. Cerebral malaria: clinical features, pathophysiology and treatment. Ann Trop Med Parasitol. 1997;91:875–884. doi: 10.1080/00034989760644. [DOI] [PubMed] [Google Scholar]
- 46.Medana IM, Chaudhri G, Chan-Ling T, Hunt NH. Central nervous system in cerebral malaria: ‘Innocent bystander’ or active participant in the induction of immunopathology? Immunol Cell Biol. 2001;79:101–120. doi: 10.1046/j.1440-1711.2001.00995.x. [DOI] [PubMed] [Google Scholar]
- 47.Hay SI, Guerra CA, Tatem AJ, Noor AM, Snow RW. The global distribution and population at risk of malaria: past, present, and future. Lancet Infect Dis. 2004;4:327–336. doi: 10.1016/S1473-3099(04)01043-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Murray CJ, Rosenfeld LC, Lim SS, Andrews KG, Foreman KJ, Haring D, Fullman N, Naghavi M, Lozano R, Lopez AD. Global malaria mortality between 1980 and 2010: a systematic analysis. Lancet. 2012;379:413–431. doi: 10.1016/S0140-6736(12)60034-8. [DOI] [PubMed] [Google Scholar]
- 49.Snow RW, Guerra CA, Noor AM, Myint HY, Hay SI. The global distribution of clinical episodes of Plasmodium falciparum malaria. Nature. 2005;434:214–217. doi: 10.1038/nature03342. [DOI] [PMC free article] [PubMed] [Google Scholar]