Abstract
Toxoplasma gondii is a ubiquitous parasite that can cause neurologic and ocular disease. We tested sera from 7,072 people ≥ 6 years of age in the 2009–2010 National Health and Nutrition Examination Survey (NHANES) for immunoglobulin G antibodies and compared these results with two previous NHANES studies. The overall T. gondii antibody seroprevalence among persons ≥ 6 years of age in 2009–2010 was 13.2% (95% confidence limit [CL] 11.8%, 14.5%) and age-adjusted seroprevalence was 12.4% (95% CL 11.1%, 13.7%); age-adjusted seroprevalence among women 15–44 years of age was 9.1% (95% CL 7.2%, 11.1%). In U.S. born persons 12–49 years of age, the age-adjusted T. gondii seroprevalence decreased from 14.1% (95% CL 12.7%, 15.5%) in NHANES III (1988–1994) to 9.0% (95% CL 7.6%, 10.5%) in NHANES 1999–2004 to 6.7% (95% CL 5.3%, 8.2%) in NHANES 2009–2010 (P < 0.001 linear trend). Although T. gondii antibody presence is still relatively common, the prevalence in the United States has continued to decline.
Introduction
Toxoplasma gondii is a ubiquitous organism and is one of the most common parasites that infect humans. Each year in the United States, T. gondii is responsible for approximately one million infections,1 thousands of cases of encephalitis and systemic disease in immunosuppressed persons,2 thousands of cases of retinochoroiditis from infection after birth affecting vision,1 and hundreds to thousands of cases of congenital disease in infants that can lead to neurological sequalae or retinochoroiditis.3 In addition, T. gondii infection has been repeatedly found to be associated with mental illnesses, including schizophrenia, self-harm, and bipolar depression, although the evidence is not yet sufficient to imply a causal relationship.4–8
Periodically, the Centers for Disease Control and Prevention (CDC) tests serum samples from the National Health and Nutrition Examination Survey (NHANES) for T. gondii antibodies to estimate the prevalence of T. gondii infection in the United States. Once infected, people generally retain a chronic infection, particularly in the cells of the muscles and brain. This chronic form of the organism (bradyzoites in cysts) is intracellular and cannot be eradicated by currently available medications. Antibodies to T. gondii are thought to persist for life in infected persons. In this work, we report the T. gondii antibody prevalence in the United States from samples collected in the NHANES in 2009–2010. Previous assessments of T. gondii antibody prevalence were done in the NHANES III (1988–1994) and the NHANES 1999–2004.9,10 The T. gondii antibody prevalence reported in this work will be compared with those found in these previous seroprevalence studies.
Materials and Methods
The NHANES is a cross-sectional survey conducted by the National Center for Health Statistics (NCHS), CDC and is based on a stratified, multistage cluster design from which a sample representative of the civilian, non-institutionalized U.S. population is drawn. The NHANES collects information on a variety of health measures and conditions through household interviews, physical examinations, and collection of blood samples in mobile examination centers. Since 1999, data have been collected and released in 2-year cycles. Non-Hispanic blacks, Hispanics, and persons 60 years of age and older were oversampled in NHANES 2009–2010 to ensure adequate sample sizes for these groups. Descriptions of the survey design and sampling methods have been published elsewhere.11 The NHANES surveys were reviewed and approved by the NCHS Research Ethics Review Board and included written consent.12
Surplus serum samples collected in the NHANES 2009–2010 for sampled persons 6 years of age and older were tested for T. gondii antibodies at the CDC's Parasitic Diseases Serology Laboratory in 2013. Primary analyses for NHANES 2009–2010 were conducted on those tested age 6 years and older. For comparisons across all three survey periods (NHANES III [1988–1994], NHANES 1999–2004, and NHANES 2009–2010), serologic data on T. gondii was available only for those 12–49 years of age. Data among women of childbearing age (those 15–44 years of age) were also available and analyzed for all three survey periods.
In NHANES 2009–2010, seroprevalence was calculated for the total population and by age groups (6–11, 12–19, 20–29, 30–39, 40–49, 50–59, 60–69, and 70 + years). Age-adjusted seroprevalence was compared across demographic and socio-economic subgroups that included gender, race, and Hispanic origin based on the respondents' self-assessment and categorized as non-Hispanic white, non-Hispanic black, and Mexican American; subjects who did not self-select into these three groups were classified as “other,” which included all non-Mexican American Hispanics and individuals reporting multiple races, and were only included when all racial/ethnic groups were combined in analyses for the total population, birth place (U.S. versus non-U.S. birth), poverty index (calculated by dividing family income by a poverty threshold specific for family size using the U.S. Department of Health and Human Services' poverty guidelines and categorized as either below poverty [< 1] or at or above poverty [1 or above]),13 and educational level of the head of household (measured as the last year of school completed and self-categorized as having less than a high school education, having completed high school or a general equivalency diploma, or having more than a high school education). Seroprevalence estimates were also calculated among U.S. born persons because this group more accurately represents transmission of T. gondii occurring in the United States. There was not a large enough sample of non-U.S. born persons to evaluate trends in this group. In many countries outside the United States, a relatively high percentage of persons are infected at an early age,14,15 and therefore are already infected when immigrating to the United States. To examine trends in T. gondii seroprevalence over time, age-adjusted estimates from NHANES 2009–2010 among all persons and U.S. born persons 12–49 years of age (the age group in which serum was tested in all three NHANES surveys), and among childbearing age women (15–44 years of age), were compared with estimates from NHANES 1999–2004 and NHANES III (1988–1994). Detailed information on each variable collected can be found in the file documentation.16
Laboratory testing.
Serum samples for NHANES 2009–2010 were tested for T. gondii-specific immunoglobulin G (IgG) using Toxoplasma IgG EIA kits (Bio-Rad, Redmond, WA) according to the manufacturer's instructions (positive test 33 or greater IU/mL). Serum samples for NHANES III and NHANES 1999–2004 were tested with Platelia Toxo-G EIA (Bio-Rad, Hercules, CA). The Platelia Toxo-G EIA was not used for the NHANES 2009–2010 because it was no longer available from the manufacturer. Both the Toxoplasma IgG EIA and the Platelia Toxo-G EIA were evaluated using a battery of 90 sera (23 negative and 67 positive) against the CDC Toxoplasma immunofluorescence assay-IgG test and the Sabin-Feldman dye test (Dr. Jack Remington, Palo Alto, CA) and found to have 100% concordance.
Statistical analysis.
All estimates of seroprevalence were weighted using the NHANES examination weights to represent the total civilian non-institutionalized U.S. household population and to account for oversampling and nonresponse to the household interview and physical examination.17 Standard errors were calculated using SUDAAN software to account for the complex sample design.18 Age adjustment was done using direct standardization with the 2000 census population as the reference population using the following age groups: 6–11, 12–19, 20–29, 30–39, 40–49, 50–59, 60–69, and 70 and above for analyses of those 6 years of age and older; 12–19, 20–29, 30–39, 40–49 for analyses of those age 12–49; and 15–19, 20–29, 30–39, and 40–44 for analyses of those 15–44 years of age. Estimates were considered to be unstable if the relative standard error around the proportion of participants who were seropositive or seronegative was > 30% or if the estimate was based on < 10 seropositive or seronegative persons. Pairwise differences between seroprevalence estimates were evaluated using a t-statistic and test for trends were conducted using a linear orthogonal procedure, both in SUDAAN; P values < 0.05 were considered significant.
Response to T. gondii testing.
Of the 11,357 individuals 6 years of age and above sampled in NHANES 2009–2010, 8,814 (77.6%) were interviewed, 8,591 (97.5% of those interviewed) were examined, and 7,070 (82% of those examined) had sera available for T. gondii antibody testing. The percent of those with sera available for testing varied by age (54% among those age 6–11 years and 83–90% among the remaining age groups), race and Hispanic origin (76% among non-Hispanic blacks, 85% and 84% among non-Hispanic whites, and Mexican Americans, respectively), was lower among those born in the United States (81% versus 87% among non-U.S. born), and varied among those with more education (82% among those who completed high school or more and 84% among those with less than a high school education).
Results
The overall prevalence of T. gondii antibodies among persons 6 years of age and older was 13.2% (95% CL 11.8%, 14.5%) and age-adjusted prevalence was 12.4% (95% CL 11.1%, 13.7%; Table 1). Overall seroprevalence increased with age (P < 0.001, linear test for trend), was higher among males (P < 0.05), was higher among the non-U.S. born than U.S. born persons (P < 0.001), higher among those living below the poverty level compared with those living at or above the poverty level (P < 0.01), and higher among those with less than a high school education compared with either those with only a high school education (P < 0.001) and those with greater than a high school education (P < 0.001) (Table 1). Among U.S. born persons, the same differences among subgroups within these socio-demographic factors remained except the difference between males and females no longer reached statistical significance. However, differences by race and Hispanic origin varied between the total population and those U.S. born. In the total population, non-Hispanic whites had a lower T. gondii seroprevalence compared with Mexican Americans (P < 0.01). In contrast, among those U.S. born, both non-Hispanic whites and non-Hispanic blacks had higher seroprevalence than Mexican Americans (P < 0.001 and P < 0.01, respectively) (Table 1).
Table 1.
Toxoplasma gondii antibody prevalence among persons 6 or more years of age by age groups and age-standardized* by gender, race/ethnicity, poverty index, education, and place of birth, NHANES 2009–2010 (overall and U.S. born)
| Category† | Overall | U.S. born | ||||
|---|---|---|---|---|---|---|
| n | Prevalence | 95% CL‡ | n | Prevalence | 95% CL | |
| Total unadjusted | 7,070 | 13.2 | 11.8, 14.5 | 5,401 | 10.3 | 9.0, 11.5 |
| Total age adjusted | 7,070 | 12.4 | 11.1, 13.7 | 5,401 | 9.6 | 8.5, 10.8 |
| Age group (years) | ||||||
| 6–11 | 664 | 1.5§¶ | 0.2, 2.8 | 618 | 1.5§¶ | 0.1, 2.9 |
| 12–19 | 1,082 | 4.1 | 2.1, 6.0 | 936 | 3.2 | 1.1, 5.3 |
| 20–29 | 893 | 9.3 | 6.8, 11.8 | 661 | 5.4 | 3.2, 7.6 |
| 30–39 | 878 | 10.5 | 8.7, 12.3 | 623 | 6.4 | 4.6, 8.2 |
| 40–49 | 957 | 14.8 | 11.7, 17.9 | 632 | 10.9 | 8.0, 13.8 |
| 50–59 | 818 | 15.0 | 12.2, 17.8 | 578 | 11.6 | 8.5, 14.8 |
| 60–69 | 854 | 17.4 | 14.6, 20.1 | 593 | 15.4 | 12.5, 18.2 |
| 70+ | 924 | 29.9 | 24.8, 34.9 | 760 | 27.6 | 22.9, 32.3 |
| Gender | ||||||
| Male | 3,508 | 13.8∥ | 12.2, 15.5 | 2,679 | 10.7 | 9.0, 12.4 |
| Female | 3,562 | 11.1 | 9.2, 13.1 | 2,722 | 8.7 | 7.0, 10.3 |
| Race/ethnicity | ||||||
| White, not Hispanic (ref)** | 3,173 | 10.2 | 8.8, 11.6 | 3,007 | 9.7 | 8.3, 11.2 |
| Black, not Hispanic | 1,228 | 14.9 | 10.9, 18.9 | 1,085 | 10.5 | 8.0, 13.0 |
| Mexican American | 1,524 | 15.8∥ | 11.8, 19.8 | 805 | 5.7∥ | 3.8, 7.6 |
| Other | 1,145 | 20.2 | 15.5, 24.9 | 504 | 10.8 | 6.2, 15.5 |
| Poverty index | ||||||
| Below | 1,637 | 20.3∥ | 15.4, 25.1 | 1,164 | 17.6∥ | 11.8, 23.4 |
| At or above | 4,836 | 10.9 | 9.7, 12.0 | 3,899 | 8.7 | 7.6, 9.8 |
| Education | ||||||
| < High school (ref) | 2,086 | 19.3 | 16.4, 22.3 | 1,246 | 13.4 | 10.0, 16.8 |
| High school | 1,592 | 12.2∥ | 10.3, 14.0 | 1,361 | 10.4∥ | 8.9, 11.9 |
| > High school | 3,315 | 10.3∥ | 9.1, 11.5 | 2,727 | 8.4∥ | 7.0, 9.8 |
| Place of birth | ||||||
| Non-United States | 1,664 | 25.1∥ | 22.2, 27.9 | NA†† | NA | NA |
| United States | 5,401 | 9.6 | 8.5, 10.8 | 5,401 | 9.6 | 8.5, 10.8 |
All categories except age groups are age-standardized to the 2000 U.S. Census population using the following age groups: 6–11, 12–19, 20–29, 30–39, 40–49, 50–59, 60–69, and age 70 years and above.
Columns for categories may not add to totals because of item non-response.
CL = confidence limits.
P < 0.001 for linear test for trend among age groups.
Estimate for 6–11 year age group considered unstable because its relative standard error is > 40%.
P < 0.05 for comparison of subgroup to reference group (ref) for each factor.
ref = reference group.
NA = not applicable.
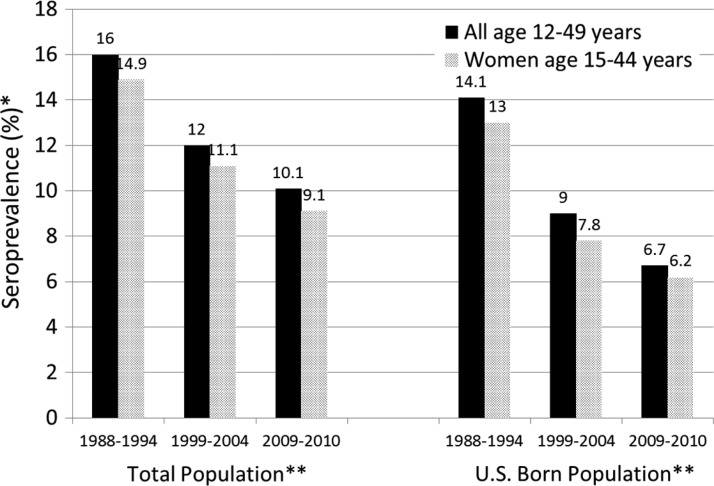
To examine trends in T. gondii seroprevalence across the three NHANES surveys, we restricted the analysis to those 12–49 years of age (see Methods). Among all persons 12–49 years of age, the age-adjusted prevalence values decreased from 16.0% (95% CL 14.5%, 17.5%) in NHANES III to 12.0% (95% CL 10.8%, 13.2%) in NHANES 1999–2004 to 10.1% (95% CL 8.7%, 11.5%) in NHANES 2009–2010 (P < 0.001 linear trend; P < 0.05 for all pairwise comparisons) (Figure 1). Among U.S. born persons 12–49 years of age, the age-adjusted prevalence values decreased from 14.1% (95% CL 12.7%, 15.5%) in NHANES III to 9.0% (95% CL 7.6%, 10.5%) in NHANES 1999–2004 to 6.7% (95% CL 5.3%, 8.2%) in NHANES 2009–2010 (P < 0.001 linear trend) (Figure 1).
Figure 1.
Toxoplasma gondii age-standardized antibody seroprevalence among the total U.S. population and among those U.S. born, NHANES III (1988–1994), NHANES 1999–2004, and NHANES 2009–2010. *Age-adjusted to 2000 Census population using the following age groups: 12–19, 20–29, 30–39, and 40–49 for those 12–49 years of age, and 15–19, 20–29, 30–39, and 40–44 for those 15–44 years of age. **P < 0.001 linear trend for all series.
Age-adjusted seroprevalence among all women 15–44 years of age from NHANES 2009–2010 was 9.1% (95% CL 7.2%, 11.1%) (Figure 1); in this 15–44 age group seroprevalence was 6.2% (95% CL 4.4%, 8.0%) for U.S. born women and 21.7% (95% CL 16.8%, 26.7%) for non-U.S. born women. Seroprevalence decreased among U.S. born women 15–44 years of age from 13.0% (95% CL 11.0%, 15.0%) in NHANES III to 7.8% (95% CL 6.1%, 9.4%) in NHANES 1999–2004, to 6.2% (95% CL 4.4%, 8.0%) in NHANES 2009–2010 (P < 0.001, linear trend) (Figure 1).
Discussion
In NHANES 2009–2010 we found an overall age-adjusted T. gondii seroprevalence of 12.4% among persons 6 years of age or older and a decreasing seroprevalence over the past 20 years among persons 12–49 years of age when comparing the NHANES 2009–2010 to the two previous NHANES T. gondii seroprevalence studies. Among women of childbearing age (15–44 years of age) the overall seroprevalence was 9.1%, indicating that over 90% of women in the United States are susceptible to initial T. gondii infection during pregnancy, and therefore susceptible to congenital transmission of the organism to their offspring because congenital transmission usually occurs with an initial infection during pregnancy. It is important for pregnant women from the United States to practice toxoplasmosis-related soil, water, and food precautions at home and when traveling.19,20
The decrease in T. gondii seroprevalence over the past 20 years may be the result of education of physicians and the public by CDC, public health agencies, and human and animal health practitioners leading to improvement in soil/cat feces-related hygiene, keeping cats indoors where they do not eat prey and become infected with T. gondii, or changes in food storage and preparation. There has been a slight reduction in the estimate of the number of cats in the United States since 2007,21 however, this change is too recent to greatly affect trends in the T. gondii seroprevalence in the U.S. population 12–49 years of age over the past 20 years. Recent findings by Dr. Hill and colleagues using a sporozoite antigen test suggest that the oocyst form of T. gondii, which is associated with soil and cat feces exposure, may be responsible for a majority of human infections in the United States.22,23 If this is currently the case, one possibility is that some of the reduction in human exposure to T. gondii in the past 20 years was caused by a reduction of T. gondii in meats such as pork, which has been documented,24 or less exposure from meat in general because of changes in preparation or freezing practices. An increase in frozen pre-prepared meals and freezing of meat helps reduce exposure to infectious T. gondii cysts because cysts are inactivated by adequate freezing.25 In addition, use of enhancement salt solutions in meat can reduce the viability of T. gondii.26
As has been noted in previous NHANES studies, seroprevalence increased with age and was higher in those who live below the poverty level and had less education. One interesting finding is that although Mexican Americans had a higher seroprevalence overall than non-Hispanic Whites or non-Hispanic Blacks, among U.S. born persons Mexican Americans had a lower T. gondii seroprevalence than either non-Hispanic Whites or non-Hispanic Blacks. The reason for this finding is not known. It may be that U.S. born Mexican Americans cook meat more thoroughly, have a lower exposure to cats in their environment, live in environments where oocysts do not survive as well in soil (for example, dry climates), or have hygiene practices that make them less likely to be exposed to T. gondii. Foreign born Mexican Americans have a higher T. gondii seroprevalence,15 which generally reflects the T. gondii exposure risk of their region of origin.
The strengths of the NHANES include the collection of a representative sample of the U.S. population and the collection of data through highly standardized methods. In addition, there are a number of limitations to our study. At the time of NHANES 2009–2010 testing, the Platelia Toxo-G EIA test used for the NHANES III (1988–1994) and the NHANES 1999–2004 was no longer available from the manufacturer. However, the Toxoplasma IgG EIA used for NHANES 2009–2010 is from the same manufacturer and had 100% agreement when tested against the same serum panel that was used for evaluation of the Platelia Toxo-G EIA test; therefore, this is only a potential limitation. In addition, because the sample was designed to provide national estimates, it was not possible to calculate statistically valid estimates for states, regions, or localities for the 2-year NHANES 2009–2010 survey.
The NHANES has made it possible to examine the seroprevalence of T. gondii over time and estimate the seroprevalence of T. gondii infection in demographic groups in the United States. From these data it is clear that most women of childbearing age in the United States are not infected with T. gondii and therefore susceptible to T. gondii infection and passing the organism congenitally. Precautions to prevent infection are important for this group, especially when traveling to areas of the world with higher rates of infection such as much of Latin America. The potential to cause more severe illness is now being associated with specific serotypes of T. gondii27 and as test development progresses there may be an opportunity in the future to evaluate the prevalence of various T. gondii serotypes in the United States in a representative sample of the population.
Footnotes
Authors' addresses: Jeffrey L. Jones, Hilda Rivera, Courtney Price, and Patricia P. Wilkins, Parasitic Diseases Branch, Division of Parasitic Diseases and Malaria, Center for Global Health, Centers for Disease Control and Prevention, Atlanta, GA, E-mails: jlj1@cdc.gov, igi2@cdc.gov, www4@cdc.gov, and pma1@cdc.gov. Deanna Kruszon-Moran, Division of Health and Nutrition Examination Statistics, National Center for Health Statistics, Centers for Disease Control and Prevention, Hyattsville, MD, E-mail: ddko@cdc.gov.
References
- 1.Jones JL, Holland GN. Annual burden of ocular toxoplasmosis in the U.S. Am J Trop Med Hyg. 2010;82:464–465. doi: 10.4269/ajtmh.2010.09-0664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jones JL, Roberts JM. Toxoplasmosis hospitalizations in the United States, 2008, and trends, 1993–2008. Clin Infect Dis. 2012;54:e58–e61. doi: 10.1093/cid/cir990. [DOI] [PubMed] [Google Scholar]
- 3.Lopez A, Dietz V, Wilson M, Navin TR, Jones JL. Preventing congenital toxoplasmosis. MMWR Recomm Rep. 2000;49:37–75. [PubMed] [Google Scholar]
- 4.Torrey EF, Bartko JJ, Lun ZR, Yolken RH. Antibodies to Toxoplasma gondii in patients with schizophrenia: a meta-analysis. Schizophr Bull. 2007;33:729–736. doi: 10.1093/schbul/sbl050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Torrey EF, Bartko JB, Yolken RH. Toxoplasma gondii and other risk factors for schizophrenia: an update. Schizophr Bull. 2012;38:642–647. doi: 10.1093/schbul/sbs043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pearce BD, Kruszon-Moran D, Jones JL. The relationship between Toxoplasma gondii infection and mood disorders in the third national health and nutrition survey. Biol Psychiatry. 2012;72:290–295. doi: 10.1016/j.biopsych.2012.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Arling TA, Yolken RH, Lapidus M, Langenberg P, Dickerson FB, Zimmerman SA, Balis T, Cabassa JA, Scrandis DA, Tonelli LH, Postolache TT. Toxoplasma gondii antibody titers and history of suicide attempts in patients with recurrent mood disorders. J Nerv Ment Dis. 2009;197:905–908. doi: 10.1097/NMD.0b013e3181c29a23. [DOI] [PubMed] [Google Scholar]
- 8.Pedersen MG, Mortensen PB, Norgaard-Pedersen B, Postolache TT. Toxoplasma gondii infection and self-directed violence in mothers. Arch Gen Psych. 2012;69:1123–1130. doi: 10.1001/archgenpsychiatry.2012.668. [DOI] [PubMed] [Google Scholar]
- 9.Jones JL, Kruzon-Moran D, Wilson M, McQuillan G, Navin T, McAuley JB. Toxoplasma gondii in the United States, seroprevalence and risk factors. Am J Epidemiol. 2001;154:357–365. doi: 10.1093/aje/154.4.357. [DOI] [PubMed] [Google Scholar]
- 10.Jones JL, Kruszon-Moran D, Sanders-Lewis K, Wilson M. Toxoplasma gondii infection in the United States, 1999–2004, decline from the prior decade. Am J Trop Med Hyg. 2007;77:405–410. [PubMed] [Google Scholar]
- 11.Curtin LR, Mohadjer LK, Dohrmann SM, Kruszan-Moran D, Mirel LB, Carroll MD, Hirsch R, Burt VL, Johnson CL. National Health and Nutrition Examination Survey: Sample design, 2007–2010. Vital Health Stat. 2013;2((160)) http://www.cdc.gov/nchs/data/series/sr_02/sr02_160.pdf National Center for Health Statistics. Accessed January 2, 2014. [PubMed] [Google Scholar]
- 12.Zipf G, Chiappa M, Porter KS, Ostchega Y, Lewis BG, Dostal J. National Health and Nutrition Examination Survey: Plan and operations, 1999–2010. Vital Health Stat. 2013;1((56)) http://www.cdc.gov/nchs/data/series/sr_01/sr01_056.pdf National Center for Health Statistics. Available at. Accessed January 2, 2014. [PubMed] [Google Scholar]
- 13.U.S. Department of Health and Human Services . Poverty Guidelines, Research, and Measurement. U.S. Department of Health and Human Services website; 2013. http://aspe.hhs.gov/POVERTY/index.shtml Available at. Accessed January 2, 2014. [Google Scholar]
- 14.Pappas G, Roussos N, Falagas ME. Toxoplasmosis snapshots: global status of Toxoplasma gondii seroprevalence and implications for pregnancy and congenital toxoplasmosis. Int J Parasitol. 2009;39:1385–1394. doi: 10.1016/j.ijpara.2009.04.003. [DOI] [PubMed] [Google Scholar]
- 15.Caballero-Ortega H, Uribe-Salas FJ, Conde-Glez CJ, Cedillo-Pelaez C, Vargas-Villavicencio JA, Luna-Pastén H, Cañedo-Solares I, Ortiz-Alegría LB, Correa D. Seroprevalence and national distribution of human toxoplasmosis in Mexico: analysis of the 2000 and 2006 National Health Surveys. Trans R Soc Trop Med Hyg. 2012;106:653–659. doi: 10.1016/j.trstmh.2012.08.004. [DOI] [PubMed] [Google Scholar]
- 16.Centers for Disease Control and Prevention . NHANES File Documentation. 2013. http://www.cdc.gov/nchs/nhanes/about_nhanes.htm Available at. Accessed January 2, 2014. [Google Scholar]
- 17.Johnson CL, Paulose-Ram R, Ogden CL, Carroll MD, Kruszan-Moran D, Dohrmann SM, Curtin LR. National Health and Nutrition Examination Survey: Analytic guidelines, 1999–2010. Vital Health Stat. 2013;2((161)) http://www.cdc.gov/nchs/data/series/sr_02/sr02_161.pdf National Center for Health Statistics. Accessed January 2, 2014. [PubMed] [Google Scholar]
- 18.Research Triangle Institute . Research Triangle Park, NC: Research Triangle Institute; 2012. SUDAAN user's manual release 10.1. [Google Scholar]
- 19.Jones JL, Dubey JP. Foodborne toxoplasmosis. Clin Infect Dis. 2012;55:845–851. doi: 10.1093/cid/cis508. [DOI] [PubMed] [Google Scholar]
- 20.Centers for Disease Control and Prevention . Parasites – Toxoplasmosis. 2013. http://www.cdc.gov/parasites/toxoplasmosis/ Available at. Accessed January 2, 2014. [Google Scholar]
- 21.U.S. Pet Ownership & Demographics Sourcebook American Veterinary Medical Association. 2012. https://www.avma.org/KB/Resources/Statistics/Pages/Market-research-statistics-US-pet-ownership.aspx Available at. Accessed January 2, 2014.
- 22.Hill DE, Coss C, Dubey JP, Wroblewski K, Sautter M, Hosten T, Muñoz-Zanzi C, Mui E, Withers S, Boyer K, Hermes G, Coyne J, Jagdis F, Burnett A, McLeod P, Morton H, Robinson D, McLeod R. Identification of a sporozoite-specific antigen from Toxoplasma gondii. J Parasitol. 2011;97:328–337. doi: 10.1645/GE-2782.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Boyer K, Hill D, Mui E, Wroblewski K, Karrison T, Dubey JP, Sautter M, Noble AG, Withers S, Swisher C, Heydemann P, Hosten T, Babiarz J, Lee D, Meier P, McLeod R. Unrecognized ingestion of Toxoplasma gondii oocysts leads to congenital toxoplasmosis and causes epidemics in North America. Clin Infect Dis. 2011;53:1081–1089. doi: 10.1093/cid/cir667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hill DE, Dubey JP. Toxoplasma gondii prevalence in farm animals in the United States. Int J Parasitol. 2013;43:107–113. doi: 10.1016/j.ijpara.2012.09.012. [DOI] [PubMed] [Google Scholar]
- 25.Kotula AW, Dubey JP, Sharar AK, Andrews CD, Shen SK, Lindsay DS. Effect of freezing on infectivity of Toxoplasma gondii tissue cysts in pork. J Food Prot. 1991;54:687–690. doi: 10.4315/0362-028X-54.9.687. [DOI] [PubMed] [Google Scholar]
- 26.Hill DE, Sreekumar C, Gamble HR, Dubey JP. Effect of commonly used enhancement solutions on the viability of Toxoplasma gondii tissue cysts in pork loin. J Food Prot. 2004;67:2230–2233. doi: 10.4315/0362-028x-67.10.2230. [DOI] [PubMed] [Google Scholar]
- 27.McLeod R, Boyer KM, Lee D, Mui E, Wroblewski K, Karrison T, Noble AG, Withers S, Swisher CN, Heydemann PT, Sautter M, Babiarz J, Rabiah P, Meier P, Grigg ME. Prematurity and severity are associated with Toxoplasma gondii alleles (NCCCTS, 1981–2009) Clin Infect Dis. 2012;54:1595–1605. doi: 10.1093/cid/cis258. [DOI] [PMC free article] [PubMed] [Google Scholar]