Abstract
Several but not all MRI studies have reported volume reductions in the hippocampus and dorsolateral prefrontal cortex (DLPFC) in patients with schizophrenia. Given the high prevalence of smoking among schizophrenia patients and the fact that smoking has also been associated with alterations in brain morphology, this study evaluated whether a proportion of the known gray matter reductions in key brain regions may be attributed to smoking rather than to schizophrenia alone.
We examined structural MRI data of 112 schizophrenia patients (53 smokers and 59 non-smokers) and 77 healthy non-smoker controls collected by the MCIC study of schizophrenia. An automated atlas based probabilistic method was used to generate volumetric measures of the hippocampus and DLPFC. The two patient groups were matched with respect to demographic and clinical variables.
Smoker schizophrenia patients showed significantly lower hippocampal and DLPFC volumes than non-smoker schizophrenia patients. Gray matter volume reductions associated with smoking status ranged between 2.2% and 2.8%. Furthermore, we found significant volume differences between smoker patients and healthy controls in the hippocampus and DLPFC, but not between non-smoker patients and healthy controls.
Our data suggest that a proportion of the volume reduction seen in the hippocampus and DLPFC in schizophrenia is associated with smoking rather than with the diagnosis of schizophrenia. These results may have important implications for brain imaging studies comparing schizophrenia patients and other groups with a lower smoking prevalence.
Keywords: schizophrenia, smoking, MRI, brain volume, hippocampus, DLPFC
Introduction
Among the variety of brain regions which have been implicated in schizophrenia, the hippocampus and the lateral prefrontal cortex, in particular the dorsolateral prefrontal cortex (DLPFC), have shown some of the most consistent, replicated abnormalities (Heckers 2001; Crespo-Facorro et al. 2007). The hippocampus is part of the medial temporal lobe memory system and is responsible for the consolidation of short-term into long-term memory (Squire and Zola-Morgan 1991). Abnormalities in the hippocampus are thought to play an important role in memory dysfunction in schizophrenia (Weiss and Heckers 2001; Saykin et al. 1991; Saykin et al. 1994; Beatty et al. 1993). Furthermore, changes in the hippocampal formation have been linked to the sensory gating deficits in schizophrenia, leading to a diminished capacity to filter out unimportant features of the environment and to misperception (Adler et al. 1998).
The DLPFC is involved in cognitive control, working memory and in the integration of sensory and mnemonic information (Crespo-Facorro et al. 2007; Zilles et al. 2009; Potkin et al. 2009b; Barbey et al 2012). It has also been implicated in the regulation of mental flexibility specific to the capacity of using context and organized information for information retrieval (Maher et al. 1995). Most importantly, DLPFC dysfunction is associated with the genetic risk for schizophrenia (Becker et al. 2008; Potkin et al. 2009a).
Several magnetic resonance imaging (MRI) studies have demonstrated significant reductions in gray matter (GM) density and volumes in patients with schizophrenia (SCZ), relative to healthy controls (HC) (for review see Wright et al. 2000; Shenton et al. 2001; Haijma et al. 2012 and Shepherd et al. 2012). The two regions most consistently reported to show GM reductions are the hippocampus and prefrontal cortex (Seidman et al. 2003; Adriano et al. 2012; Shepherd et al. 2012). Overall, hippocampal volumes are reduced approximately 4% in each hemisphere in SCZ patients compared to healthy subjects, and slightly smaller reductions have been found in medication-naïve, first episode patients and in individuals at high risk for schizophrenia (Heckers 2001; Nelson et al. 1998; Watson et al. 2012; Adriano et al. 2012; Shepherd et al. 2012). In the DLPFC, studies have shown GM volume reductions of approximately 9-11% (Gur et al. 2000; Lopez-Garcia et al. 2006; Kikinis et al. 2010). Volumetric abnormalities of the DLPFC have been related to impairments in executive functions such as cognitive control and working memory (Crespo-Facorro et al. 2007). In addition to GM volume, reduced cortical thickness has been found in schizophrenia, including thinning in frontal and temporal regions (Voineskos et al. 2013; Ehrlich et al. 2011; Takayanagi et al. 2011; Goldman et al. 2009; Narr et al. 2005). Cortical thickness is assumed to reflect the arrangement and density of neurons in the cortex; the decrease of regional GM volumes in schizophrenia is likely caused by a combination of changes of the GM surface area and cortical thinning (Parent and Carpenter, 1995).
Although GM density, volume or thickness reductions in schizophrenia have been reported in a large number of studies, many structural MRI studies have not confirmed these findings (i.e. Niemann et al. 2000; Sanfilipo et al. 2000; Honea et al. 2005; Shenton et al. 2001; Adriano et al. 2012); surprisingly, evidence for morphometric abnormalities in the hippocampus and prefrontal cortex in schizophrenia is only moderately consistent. It remains unclear whether the observed structural abnormalities are closely tied to the pathophysiology of schizophrenia or whether they are due to confounding variables, such as those associated with schizophrenia, but not a direct consequence of the illness.
One important confounding variable may be smoking behaviour, since the prevalence of smoking is approximately 75%, three- to four-fold higher in patients with schizophrenia compared to the general population (de Leon and Diaz 2005; Ziedonis et al. 2008). Studies in healthy controls have found associations between cigarette smoking and a variety of adverse central nervous system effects, such as global brain atrophy, and structural abnormalities in prefrontal regions as well as reduced GM volumes in the anterior cingulate, occipital and temporal cortices including the parahippocampal structures and hippocampal substructures (Brody et al. 2004; Gallinat et al. 2006; Durazzo et al. 2013; 2010). Moreover, smoking has been related to cerebrovascular changes such as increased cerebral blood flow velocity and reduced vasomotor reactivity (Boyajian and Otis 2000), to biochemical abnormalities such as reduced N-acetylaspartate concentration in hippocampus (Gallinat et al. 2007) and to alteration of DNA methylation of specific genes (Ehrlich et al. 2012).
Despite the high prevalence of smoking in SCZ patients and the well-known adverse effects of smoking on brain structure in healthy subjects, diagnosis-related differences in smoking status have as yet rarely been taken into consideration (Tregellas et al. 2007; Van Haren et al. 2010). In this study, we aimed to determine the percentage of GM volume reduction of the hippocampus and the DLPFC in patients with schizophrenia that can be attributed to smoking rather than to the diagnosis. Based on prior findings in healthy subjects, we hypothesized that patients with schizophrenia who smoke will show greater volume reduction in the hippocampus and DLPFC compared to non-smoker patients. We also performed secondary data-driven analyses to test for similar effects of smoking in alternative brain regions using both a region-of-interest volumetric and a surface-wide cortical thickness approach. Lastly, we examined the effect of additional confounding variables such as premorbid cognitive functioning and antipsychotic medication.
Methods and materials
Participants
The Mind Clinical Imaging Consortium (MCIC) study of schizophrenia (Ehrlich et al. 2010) obtained baseline structural MRI scans on a total of 328 subjects from four participating sites: Massachusetts General Hospital in Boston (MGH) and the Universities of Iowa (UI), Minnesota (UMN) and New Mexico (UNM). All subjects provided written informed consent prior to study enrollment. The human subjects research committees at each of the four sites approved the study protocol. The patient group (SCZ) consists of subjects with a DSM-IV diagnosis of schizophrenia established using structured clinical interviews and review of case files by trained clinicians. Healthy controls (HC) were included if they had no history of a medical or Axis I psychiatric diagnosis. All participants were required to be at least 18 years of age and no older than 60 and to be fluent in English. Participants were excluded if they had a history of neurologic disease, psychiatric disease other than schizophrenia, history of a head injury with loss of consciousness, history of substance abuse or dependence within the past month, severe or disabling medical conditions, contraindication to MR scanning, or a premorbid cognitive achievement score less than 70 (based on the reading subtest from the WRAT-IIIRT (Wilkinson 1993). The final sample with complete and high-quality structural MRI, demographic, clinical and smoking data comprised 112 SCZ (53 smokers and 59 non-smokers) and 77 non-smoker HC.
Instruments
All study participants underwent an extensive clinical diagnostic assessment that included either the Structured Clinical Interview for DSM-IV (SCID) (First et al. 2002) or the Comprehensive Assessment of Symptoms and History (CASH) (Andreasen et al. 1992). To further characterize our sample, the severity of positive and negative symptoms was assessed using the Scale for the Assessment of Positive Symptoms (SAPS), the Scale for the Assessment of Negative Symptoms (SANS) (Andreasen 1984; 1983) and the Calgary Depression Scale for Schizophrenia (Addington et al. 1993). Extrapyramidal symptoms were assessed using the Barnes Akathisia Scale (Barnes 2003) and the Abnormal Involuntary Movement Scale (1988). Premorbid cognitive achievement was estimated by the Reading Subtest of the Wide Range Achievement Test (WRAT-IIIRT) (Wilkinson 1993); parental socioeconomic status (SES) was determined using the Hollingshead index (Hollingshead 1965) and handedness was determined using the Annett Scale of Hand Preference (Annett 1970).
Life-time exposure to smoking was recorded for all participants using site-dependent sources (MGH and UMN: Clinical records and MRI debriefing form, UI: CASH, UNM: smoking-related variables were collected for a site-specific study (Leyba et al. 2008). The total sample included only four HC who smoke, thus these participants were excluded.
Antipsychotic history was collected as part of the psychiatric assessment using the PSYCH instrument (Andreasen 1987), and cumulative antipsychotic exposure (cumulative dose years) was calculated using the chlorpromazine (CPZ) conversion factors of Woods (2003). For a more detailed account of the calculations please refer to Supplementary Material (SM) 1.1.
Structural Image Acquisition
Cross-site MRI acquisition calibration and reliability were established following guidelines developed by the Biomedical Informatics Research Network (BIRN) test bed for morphometry (Jovicich et al. 2009; 2006). The MRI acquisition protocol for high-resolution coronal T1-weighted images was matched across the four different sites: Siemens 3T Trio (UMN): TR/TE/flip angle=2.53s/3.81ms/7°, Siemens 1.5T Sonata (UI): TR/TE/flip angle=20ms/6ms/20°, Siemens 1.5 T Avanto (MGH and UNM): TR/TE/flip angle 12ms/4.76ms/20°; FOV=16×16cm, 128 contiguous slices (imaging matrix=256×256, in plane resolution=0.625×0.625mm, slice thickness=1.5mm), NEX=3. Acquisition time for this gradient echo sequence was approximately 20 min.
Structural Image Data Processing
Structural MRI data from three consecutively-acquired, T1-weighted 3D gradient-echo images were registered, motion corrected, realigned, averaged and analyzed in an automated manner with the atlas-based FreeSurfer software suite (http://surfer.nmr.mgh.harvard.edu, Version 4.0.1). This process included volumetric segmentation and cortical surface reconstruction. Volumes of the amygdala, hippocampus, superior temporal gyrus and frontal lobe regions are a standard output of the FreeSurfer segmentation (Fischl et al. 2002) and parcellation (Desikan et al. 2006) procedures. These procedures rely upon variations in voxel signal intensities, probabilistic atlas location and local spatial relationships between the structures (Fischl et al. 2002). Total intracranial volume (ICV) was estimated with the FreeSurfer standard procedure using the determinant of the transform matrix to align the image with the atlas (Buckner et al. 2004). DLPFC volumes were derived from FreeSurfer cortical parcellations (see also SM 1.2.) as detailed by (Rajkowska and Goldman-Rakic 1995). The cortical surface reconstruction was performed for each hemisphere and cortical thickness was calculated at each vertex on the tessellated surface as the closest distance from the gray/white boundary to the gray/cerebrospinal fluid boundary (Fischl and Dale 2000) (see also SM 1.3).
Segmentation and surface reconstruction quality was assured by manual inspection of all raw MRI volumes, segmented volumes in three planes and pial as well as inflated volumes. In addition, histograms and boxplots were checked for outliers, which can indicate poor segmentation or reconstruction quality. Five participants' MRI data failed the quality assurance and were then recovered with minor manual intervention.
Entire cortex vertex-wise analyses of cortical thickness were performed with FreeSurfer. Briefly, spherical cortical thickness data from all subjects were mapped to an average subject using surface-based registration methods (http://surfer.nmr.mgh.harvard.edu/fswiki/FsAverage) (Fischl et al. 1999). This procedure provides accurate matching of morphologically homologous cortical locations across subjects on the basis of each individual's anatomy while minimizing metric distortion. Cortical thickness maps were smoothed using a Gaussian kernel with a full-width-at-half-maximum of 10mm. Finally, statistical maps were generated by computing general linear models (GLM) of the effects of each predictor variable on cortical thickness at each vertex. Because of known confounding effects and in line with similar studies, we included age, gender and field strength of acquisition site into the models as control variables (Kuperberg et al. 2003; Narr et al. 2005; Goldman et al. 2009; Schultz et al. 2010). All cortical thickness results were corrected for multiple comparisons using a Monte-Carlo simulation (uncorrected results are not reported). This procedure includes the following steps: (1) The initial vertex-wise threshold (VWT) was set to p=0.05 to form spatially contiguous areas of association (referred to as “cluster”). (2) The likelihood that a finding (cluster) of this size and magnitude (difference in thickness as specified by the VWT) would appear by chance, i.e. when using repeated random sampling, was tested using Monte-Carlo simulation with 10,000 repeats. This results in a cluster-wise probability (CWP), which is reported as p-values throughout the results section.
Statistical analyses
Chi-square statistic and analysis of variance (ANOVA) were used to compare demographic and clinical variables between the groups (smoker SCZ patients, non-smoker SCZ patients and HC). Analysis of covariance (ANCOVA) models were employed to compare our main GM structures (right and left hippocampus and right and left DLPFC) across the three groups covarying for age, gender, ICV and scanner field strength. To address the latter variable, we also re-analyzed our data without participants from the acquisition site with a 3T MRI scanner. Post-hoc pairwise tests, Bonferroni-corrected for multiple comparisons, were performed for measures with significant overall group differences. Secondary analyses were conducted for additional regions of interest (superior temporal gyrus, amygdala and thalamus) using the same model strategy. We also tested for possible associations between smoking status and surface-wide cortical thickness. In further analyses (see SM 2.5), we explored the effect of WRAT-IIIRT score as an additional possibly confounding variable. We included this variable as a covariate, using the same ANCOVA models.
The percentage of GM reduction in our main ROIs in SCZ patients that can be attributed to smoking was estimated as an R2 difference between models with and without smoking as binary variable. All statistical analyses were carried out using SPSS Statistics 19.
Results
Sample characteristics
Smoker SCZ patients, non-smoker SCZ patients and HC did not differ in gender, race, parental SES, handedness or ICV. Statistically significant differences were found for age (F(2, 187)=7.19; p=0.001) and WRAT-IIIRT score (F(2, 183)=9.24; p<0.001), with HC being slightly younger and attaining a somewhat higher WRAT-IIIRT score (Table 1). We also found no differences in demographic variables or ICV when stratifying the sample according to the acquisition site-specific scanner field strength.
Table 1. Demographic results for patients with schizophrenia and healthy controls.
| N | Gender | Race | Age | WRAT-IIIRT score | Parental SES | Handedness | ICV | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||||||||
| female | Caucasian | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | ||||||
| N | % | N | % | ||||||||||||||
| Field strength | 1.5 T | Smoker SCZ | 48 | 15 | 31.3 | 41 | 85.4 | 34.88 | 11.18 | 46.21a | 6.91 | 2.82 | 1.01 | 1.19 | 3.23 | 1561549.3 | 223084.4 |
| Non-smoker SCZ | 48 | 10 | 20.4 | 41 | 83.7 | 33.51 | 11.12 | 48.49 | 6.28 | 2.94 | 1.16 | 0.85 | 2.69 | 1678102.0 | 143423.7 | ||
| HC | 68 | 25 | 36.8 | 63 | 93.6 | 28.22 | 10.11 | 50.31a | 5.11 | 2.65 | 0.71 | 0.56 | 1.66 | 1712667.4 | 197535.7 | ||
|
| |||||||||||||||||
| Field strength | 3 T | SmokerSCZ | 5 | 1 | 20.0 | 2 | 40.0 | 42.60 | 7.32 | 40.40a | 6.43 | 3.75 | 0.96 | 1.20 | 1.79 | 1699887.8 | 209254.3 |
| Non-smoker SCZ | 11 | 5 | 45.5 | 9 | 81.8 | 35.64b | 11.34 | 48.27 | 6.45 | 2.36 | 0.67 | 2.09 | 3.45 | 1695741.3 | 218201.1 | ||
| HC | 9 | 5 | 55.6 | 9 | 100.0 | 32.44b | 13.55 | 49.89a | 3.44 | 2.44 | 0.73 | 0.33 | 0.71 | 1733051.3 | 270110.4 | ||
|
| |||||||||||||||||
| Total | SmokerSCZ | 53 | 16 | 30.2 | 43 | 81.1 | 35.60a | 11.06 | 45.66a,c | 7.04 | 2.90 | 1.03 | 1.19 | 3.11 | 1660735.4 | 220257.1 | |
| Non-smokerSCZ | 59 | 15 | 25.0 | 50 | 83.3 | 33.90b | 11.09 | 48.50c | 6.25 | 2.83 | 1.10 | 1.09 | 2.86 | 1690155.6 | 196528.2 | ||
| HC | 77 | 30 | 39.0 | 72 | 93.5 | 28.71a,b | 10.55 | 50.26a | 4.93 | 2.62 | 0.71 | 0.53 | 1.58 | 1723521.2 | 237643.7 | ||
Means and standard deviations are given. HC = healthy controls; SCZ = patients with schizophrenia. Cognitive achievement was measured by the Reading Subtest of the Wide Range Achievement Test (WRAT-IIIRT), parental socioeconomic status (SES) was classified according to Hollingshead and handedness was determined using the Annett Scale of Hand Preference.
significantly different between HC and smoker SCZ patients.
significantly different between HC and non-smoker SCZ patients.
significantly different between smoker SCZ patients and non-smoker SCZ patients. P-values for all group comparisons were calculated on the basis of Scheffé post-hoc test (p<0.05) following a significant F-test (p<0.05).
There was no significant difference between the two patient groups regarding clinical variables such as duration of illness, positive, negative or disorganized symptoms, cumulative antipsychotic drug use or history of alcohol abuse or dependence (Table 2). The two patient groups also did not significantly differ in comorbid depressive symptoms or in extrapyramidal symptoms (Table 2). Non-smoker SCZ patients had a slightly higher WRAT-IIIRT score in comparison to smoker SCZ patients (t(df=107)=2.188; p=0.031).
Table 2. Sample characteristics: Clinical variables for patients with schizophrenia.
| Length of Illness |
SAPS | SANS | Disorganized | Cumulative Dose Year |
Calgary Tot | BA Tot | AIMS Tot | h/o C2 abuse |
h/o C2 dependence |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||||||||||
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | N | N | |||
| Field strength | 1,5T | Smoker SCZ | 12.92 | 10.71 | 5.38 | 2.76 | 8.63 | 4.13 | 2.25 | 1.92 | 49.02 | 63.28 | 3.94 | 4.56 | 0.46 | 0.80 | 0.38 | 0.79 | 9 | 5 |
| Non-smoker SCZ | 10.28 | 10.46 | 4.31 | 2.58 | 8.76 | 4.33 | 1.82 | 2.01 | 29.45 | 48.45 | 2.48 | 2.44 | 0.41 | 0.76 | 0.27 | 0.64 | 5 | 3 | ||
|
| ||||||||||||||||||||
| 3T | Smoker SCZ | 14.20 | 8.93 | 4.60 | 2.79 | 8.40 | 3.85 | 1.80 | 3.03 | 109.26 | 162.50 | 0.50 | 0.58 | 0.40 | 0.89 | 2.20 | 2.28 | 0 | 0 | |
| Non-smoker SCZ | 12.50 | 10.34 | 4.91 | 2.17 | 7.18 | 3.16 | 2.09 | 2.51 | 49.53 | 117.53 | 3,25 | 3.78 | 0.73 | 0.65 | 2.36 | 3.14 | 0 | 0 | ||
|
| ||||||||||||||||||||
| Total | Smoker SCZ | 13.05 | 10.47 | 5.30 | 2.74 | 8.60 | 4.07 | 5.52 | 6.28 | 54.70 | 77.24 | 3.29 | 4.31 | 0.45 | 0.80 | 0.45 | 0.87 | 9 | 5 | |
| Non-smoker SCZ | 10.69 | 10.39 | 4.42 | 2.51 | 8.47 | 4.16 | 4.98 | 5.85 | 33.20 | 65.93 | 2.60 | 2.61 | 0.47 | 0.75 | 0.33 | 0.73 | 5 | 3 | ||
Means and standard deviations are given. SCZ = patients with schizophrenia. SAPS, Scale for the Assessment of Positive Symptoms; SANS, Scale for the Assessment of Negative Symptoms; Calgary Tot, Calgary Depression Scale for Schizophrenia total value; BA Tot, Barnes Akathisia Scale total value; AIMS Tot, Abnormal Involuntary Movement Scale total value; cumulative antipsychotic drug exposure is stated in dose years (1 dose year = 100 chlorpromazine equivalents per day for one year). No significant group differences on the basis of t-tests (p>0.05); h/o C2 abuse, history of alcohol abuse; h/o C2 dependence, history of alcohol dependence. No significant association between group membership and alcohol abuse or dependence in the past (abuse: χ2(1)=2.25, p=0.134; dependence: χ2(1)=1.15, p=0.284).
Hippocampus and DLPFC volumes
Mean volumes in mm3 and standard deviation for each region in each group are reported in Table 3. A one-way ANCOVA across the three groups and controlling for, age, gender, ICV and scanner field strength revealed significant overall group differences in the following main region of interest volumes: right hippocampus, left hippocampus, right DLPF and left DLPFC (for F-and p-values see Table 3). Boxplots are shown in SM 2.1, SM Fig.1.
Table 3. Summary of gray matter volume differences.
| Brain region | ANCOVA F/p | Smoker SCZ | Non-smoker SCZ | HC | Total adjusted R2 | |
|---|---|---|---|---|---|---|
| Main analysis | Right hippocampus | 5.02/0.008 | 4256.9 (529.5) a* | 4514.9 (580.2) b | 4663.0 (490.9) c** | 0.448 |
| Left hippocampus | 3.82/0.024 | 4003.5 (465.8) a | 4160.3 (530.1) b | 4325.9 (440.0) c* | 0.423 | |
| Right DLPFC | 5.95/0.003 | 19569.3 (3656.6) a* | 21183.5 (3622.4) b | 22555.6 (2720.7) c** | 0.568 | |
| Left DLPFC | 5.56/0.005 | 19856.1 (3110.0) a* | 21394.5 (3285.2) b | 22560.1 (2799.4) c** | 0.555 | |
|
| ||||||
| Secondary analysis | Right STG | 2.86/0.060 | 11647.7 (1492.4) a | 12237.6 (1961.0) b | 12924.6 (1559.5) c | 0.449 |
| Left STG | 1.62/0.200 | 12500.0 (1936.8) a | 12900.2 (2106.1) b | 13789.4 (1690.7) c | 0.490 | |
| Right Amygdala | 7.43/0.001 | 1701.2 (193.5) a** | 1853.7 (263.3) b | 1858.0 (232.3) c* | 0.496 | |
| Left Amygdala | 1.53/0.219 | 1701.4 (238.6) a | 1795.2 (284.7) b | 1800.1 (229.6) c | 0.423 | |
| Right Thalamus | 2.62/0.075 | 7127.3 (949.5) a | 7529.0 (1064.8) b | 7611.4 (979.7) c | 0.598 | |
| Left Thalamus | 3.21/0.043 | 7120.8 (851.4) a | 7561.9 (1157.3) b | 7670.4 (986.8) c | 0.568 | |
Means and standard deviations are given in mm3. HC = healthy controls; SCZ = patients with schizophrenia. Statistics are based on ANCOVA models (covarying for age, gender, ICV and site-specific scanner field strength) and if appropriate Bonferroni-corrected post-hoc comparisons between smoker SCZ patients, non-smoker SCZ patients and HC are displayed.
comparison between smoker SCZ and non-smoker SCZ patients;
comparison between non-smoker SCZ patients and HC;
comparison between smoker SCZ patients and HC.
p<0.05;
p<0.01.
Pairwise Bonferroni-corrected post-hoc tests revealed a significant volume reduction of the right hippocampus in smoker SCZ patients compared to HC (p=0.009) and in smoker SCZ patients compared to non-smoker SCZ patients (p=0.042) but no significant difference between non-smoker SCZ patients and HC (p=1.000). For the left hippocampus, smoker SCZ patients had reduced volumes when compared to HC (p=0.019) but we observed no significant differences when comparing non-smoker SCZ patients and HC (p=0.499) as well as when comparing the two patient groups (p=0.429).
We found a significant volume reduction in the DLPFC between smoker SCZ patients when compared to HC (right p=0.003; left p=0.006) but no significant differences between non-smoker SCZ patients and HC (right and left p=1.000). The volume of the right and left DLPFC was significantly lower in smoker SCZ patients compared to non-smoker SCZ patients (p=0.036 for right DLPFC and p=0.027 for left DLPFC). When comparing all SCZ patients (smoker and non-smoker) with HC, the patients have significantly smaller hippocampal and DLPFC volumes bilaterally (all p-values < 0.005) (see also SM 2.2, SM Table 1). Effect sizes are shown in SM 2.3, SM Table 2. Models including only participants measured at sites with a 1.5T MRI scanner showed identical results (see also SM 2.4, SM Table 3).
Additional Analyses
Using the same statistical model and covariates as described above we found significant group differences in the right amygdala and left thalamus; smoker SCZ patients showed significant smaller right amygdala volumes in comparison to HC (p=0.015; p-values refer to pairwise Bonferroni-corrected post-hoc tests) and non-smoker SCZ patients (p=0.001). There was no significant difference between non-smoker SCZ patients and HC in right amygdala volumes (p=1.000). For the left thalamus we found a statistical trend when comparing the two patient groups (p=0.051), but no significant effects for the other group comparisons. The effect of group reached trend-level significance for the right thalamus and right superior temporal gyrus (smoker SCZ patients<HC, p=0.055). All other F-tests were not significant. All mean volumes, standard deviations and F-values are shown in Table 3.
Given the group differences in WRAT-IIIRT score, we ran additional ANCOVA models including this variable as a covariate. All of our findings reported above remained significant. For a more detailed account of these results see SM 2.5, SM Table 4.
Exploratory Analyses - Cortical thickness
Comparison of smoker SCZ patients with non-smoker SCZ patients showed significantly decreased cortical thickness in smokers in the right pericalcarine area of the primary visual cortex (Figure 1). No regions of increased cortical thickness were determined in smoker SCZ patients in comparison to non-smoker SCZ patients.
Fig. 1. Statistical map of cortical thickness.
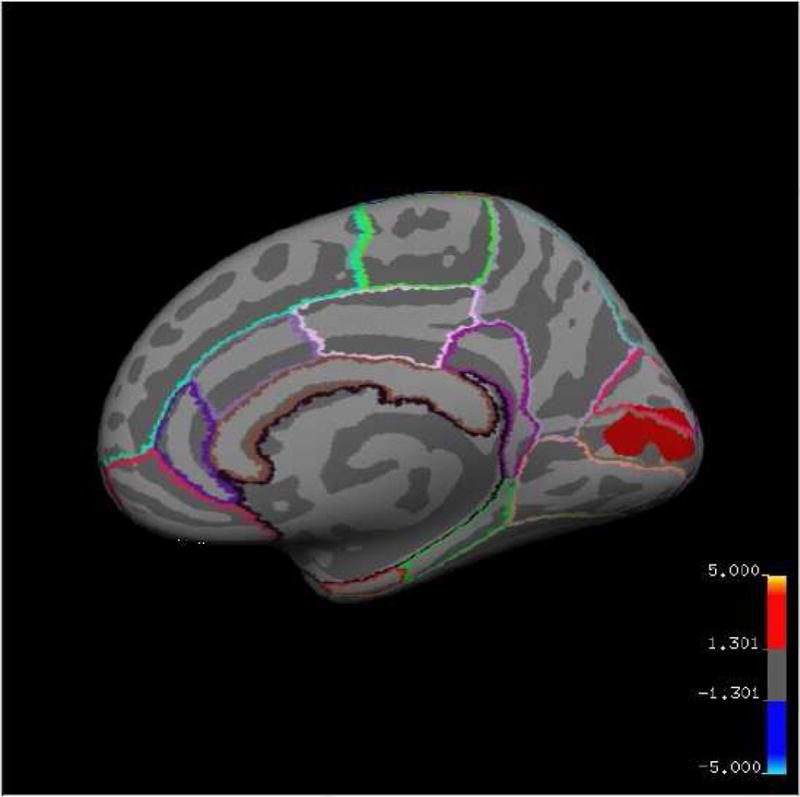
displaying a region of reduced thickness in smoker SCZ patients compared to non-smoker SCZ patients. Cluster-wise probability (CWP) values (corrected for multiple comparisons) are represented according to the color code. The statistical map is shown on the inflated surface of the standard average subject, allowing visualization of data across the entire cortical surface without interference from cortical folding.
Discussion
Using a well-validated automated segmentation procedure (Fischl et al. 2002) to measure regional brain volumes in HC and SCZ, we evaluated the role smoking plays in GM reductions in SCZ. We hypothesized that differences in smoking behaviour in SCZ might explain the variability of GM volume reductions reported within the literature.
Our findings confirm results from previous studies, which have found smaller hippocampus and DLPFC volumes in SCZ compared to HC (Honea et al. 2005; Wright et al. 2000; Heckers and Konradi 2010; Adriano et al. 2012). However, the results of our study indicate significant volume differences between SCZ patients who smoke and HC in the considered brain areas, but non-significant volume differences between non-smoker SCZ patients and HC. Compared to non-smoker SCZ patients, smoker SCZ patients showed significantly lower volumes of the right hippocampus, right and left DLPFC and the right amygdala. It is important to consider, that the two patient groups did not differ in their disease severity and other symptom-related measurements. Even after covarying for premorbid cognitive achievement these results remained the same, i.e. non-smoker SCZ patients do not show the commonly expected volume reductions in the hippocampus and DLPFC. In fact, we found a 2.8% volume reduction in the right hippocampus and a 2.2% and 2.7% reduction in the right and left DLPFC, respectively, in smoker SCZ patients compared to non-smoker SCZ patients.
The smoker SCZ patients did not have lower left hippocampal volumes than the non-smoker patients. However, the estimated mean values show a trend for smaller volumes in the left hippocampus in smoker compared to non-smoker SCZ patients. Our exploratory analyses found an additional significant difference between smoker SCZ patients, non-smoker SCZ patients and HC in the right amygdala, an area that has also been implicated in schizophrenia (Davidson and Heinrichs 2003; Honea et al. 2005; Shenton et al. 2001; Ehrlich et al. 2010; Adriano et al. 2012). The results of our study are in line with previous investigations in HC suggesting that smoking may impact GM (Tregellas et al. 2007; Brody et al. 2004; Liao et al. 2010; Durazzo et al. 2013). In particular, our results parallel those of Gallinat et al. (2006) who reported reduced GM volumes in prefrontal and medial frontal regions and the medial temporal lobe in HC.
To date, only two previous studies have investigated the relationship between smoking and GM volume in SCZ. Tregellas et al. (2007) reported smaller medial frontal gyrus and DLPFC volumes in smoker SCZ patients in comparison to HC. However, they also reported that smoker SCZ patients had larger lateral prefrontal GM volumes relative to non-smoker SCZ patients. This result is inconsistent with results of studies of smoking of GM in HC, which have only found decreased GM volumes in smokers, e.g. in dorsolateral and ventrolateral prefrontal regions (Brody et al. 2004; Gallinat et al. 2006). These discrepancies may possibly be explained by the small sample size in the aforementioned study or methodological differences, e.g. our use of absolute GM volumes in contrast to the voxel-based morphometry approach of Tregalles et al.
A second study found that heavy smoking, defined as consumption of over 25 cigarettes per day, contributes to GM volume loss in SCZ patients (Van Haren et al. 2010). However, in less heavy smokers, there was no difference in cerebral GM volume between smoker and non-smoker SCZ patients. Furthermore, smoking was not associated with brain volume changes in HC.
Our study is the first to investigate the effect of cigarette smoking on GM thickness in SCZ. Our finding of significantly decreased cortical thickness in the right pericalcarine area of the primary visual cortex in smoker SCZ patients compared to non-smoker SCZ patients is consistent with prior evidence for reduced thickness of the medial orbitofrontal cortex in HC who smoke compared to never-smokers (Kühn et al. 2010) and suggests that smoking has widespread effects on the brain.
Cigarette smoking has also been shown to have effects on brain function and white matter integrity. Friedman et al. (2008) examined the effect of smoking history and a diagnosis of schizophrenia on brain function; fMRI data were collected in SCZ patients and HC while they performed a simple visual activation task. They found a statistically significant effect of smoking on median percent signal change in the calcarine cortex, cuneus and occipital lobe. Heavy smokers had a 22% larger signal change, whereas the effects of diagnosis and the diagnosis-by-smoking status interaction were not significant. However, the number of activated voxels in smoker participants was significantly reduced relative to non-smokers. The combination of increased activity and decreased activated volume in the occipital lobe in smokers could be related to an increase in smoking-related cortical pruning (and thus reduced cortical thickness). On the contrary, Leyba et al. (2008) were unable to verify an effect of smoking history on fMRI activity in a simple sensorimotor task in SCZ. In a diffusion tensor imaging study using MCIC participants, Cullen et al. (2012) found lower fractional anisotropy in the frontal lobe in smoker compared to non-smoker SCZ patients. The results from this study are consistent with our hypothesis that smoking adversely affects overall brain structure.
Longitudinal studies are needed to assess to what extent structural brain changes in SCZ can be explained by smoking and/or an interaction between smoking and illness mechanisms. Interestingly, Durazzo et al. (2013) found a greater volume loss with increasing age in healthy smokers. In our study, a group x age interaction term was not a significant predictor of hippocampal or DLPFC volumes (for more details see SM 2.6, SM Table 5). One possible interpretation of our results is that volume differences in the hippocampus and DLPFC in SCZ may be more related to adverse effects of cigarette smoke or to an interaction between smoking and a primary pathological process that affects neurons of the hippocampus and other brain regions. However, the association between smoking in SCZ and more pronounced hippocampal and DLPFC volume reductions could be related to other factors associated with smoking, such as poorer food intake or self-care, i.e. SCZ patients who smoke are somehow different in some lifestyle characteristics than those who do not. Also, the possibility that SCZ patients who smoke carry genetic variants that increase the susceptibility for schizophrenia and addictive behaviour cannot be excluded. Furthermore, smoking behavior could be related to the attempt to diminish side effects of antipsychotic medications by counter-acting dopamine receptor blockade, or to an attempt to ameliorate negative or cognitive symptoms (Winterer 2010). Due to the cross-sectional design of our study, we were not able to measure causal relationships. Thus, it is conceivable that SCZ patients with more pronounced structural brain changes are more prone to smoking.
Our work has to be seen in the light of the following additional limitations. First, the present study involves acquisition sites that used MR scanners with different field strengths (3T vs. 1.5T scanner). We followed the best practice guidelines for multi-site MR acquisition by the Biomedical Informatics Research Network (http://www.nbirn.net/). Given that statistically controlling for site differences and excluding all participants from the sites with a 3T scanner did not alter our results, we believe that it is unlikely that site differences influenced our findings. This is in line with previous multi-site studies which show consistent group differences across the sites after accounting for potential scanner-related effects in the analyses (Stonnington et al. 2008; Segall et al. 2009). A second potential limitation is related to the unknown influence of antipsychotic medication. Due to the fact that there is no control group with similar exposure to antipsychotic medication, it is difficult to distinguish between potential medication effects and the disease effect on brain volume measures. Further, the more pronounced brain atrophy in smoking patients may also be a result of an interaction between smoking and antipsychotic medication, insofar as smoking can increase drug metabolism via pharmacokinetic and pharmacodynamic mechanisms (Lucas & Martin 2013). Given that in this study smoker and non-smoker SCZ patients did not differ in cumulative antipsychotic medication exposure, it seems unlikely that an effect of medication alone could explain the differences in brain volumes found here between the two groups. Third, the lack of a smoker control group limits our ability to make inferences about overall effects of smoking on the brain. A further major limitation of our study is the lack of quantitative measures of lifetime smoking exposure. However, a relatively high stability of smoking status in SCZ patients is supported by a recent meta-analysis (De Leon and Diaz, 2005: smoking cessation rates for SCZ patients are 50-90% lower than in the general population).
In conclusion, our data suggest that volumetric reductions previously reported in SCZ may be partially attributable to smoking. These results may have important implications for structural brain imaging studies of patients with SCZ, whose smoking rate is three- to four-fold higher than in the general population. Therefore, we recommend an assessment of smoking status, e.g. using the Fagerstroem Test for Nicotine Dependence (FNTD; Heatherton et al. 1991) in future MRI studies in samples including psychiatric patients. Using this data the amount of variance explained by cigarette smoking can be estimated and taken into account in statistical models.
Supplementary Material
Acknowledgments
The authors wish to express their gratitude to the many individuals who contributed to the MCIC study of schizophrenia.
Role of funding Source: This work was supported by the National Institutes of Health (NIH/NCRR P41RR14075), Department of Energy (DE-FG02-99ER62764), Mind Research Network, Morphometry BIRN (1U24, RR021382A), Function BIRN (U24RR021992-01, NIH.NCRR MO1 RR025758-01, NIMH 1RC1MH089257 to VDC), the Deutsche Forschungsgemeinschaft (research fellowship to SE) and the NARSAD Young Investigator Grant (SE).
Footnotes
Conflict of Interest: Veit Roessner has received lecture fees from Eli Lilly, Janssen-Cilag, Medice, Novartis, and was member of advisory boards of Eli Lilly, Novartis. All other authors declare no biomedical financial interests or other potential conflict of interests.
Author Contribution: Author Stefan Ehrlich designed the study, wrote the protocol and supervised the statistical analysis and the writing of the manuscript. Author Claudia E. Schneider conducted the statistical analyses, managed the literature searches and wrote the first draft of the manuscript. Randy L. Gollub, Tonya White and Daphne J. Holt were responsible for the study design, the supervision of the subject assessment and the data collection. Stuart R. Wallace was involved in the collection of the data and the interpretation of our results. Johanna Hass helped with the statistical analysis and the interpretation of the data. Daniel Geisler carried out the cortical thickness analyses and helped with the interpretation of this data, Vince D. Calhoun and Veit Roessner helped with the design of this research project and the analysis of the results. All authors contributed to and have approved the final manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abnormal Involuntary Movement Scale (AIMS) Psychopharmacol Bull. 1988;24(4):781–3. [PubMed] [Google Scholar]
- Addington D, Addington J, Maticka-Tyndale E. Assessing depression in schizophrenia: the Calgary Depression Scale. Br J Psychiatry Suppl. 1993 Dec;(22):39–44. [PubMed] [Google Scholar]
- Adler LE, Olincy A, Waldo M, Harris JG, Griffith J, Stevens K. Schizophrenia, sensory gating, and nicotinic receptors. Schizophr Bull. 1998;24(2):189–202. doi: 10.1093/oxfordjournals.schbul.a033320. [DOI] [PubMed] [Google Scholar]
- Adriano F, Caltagirone C, Spalletta G. Hippocampal volume reduction in first-episode and chronic schizophrenia: a review and meta-analysis. Neuroscientist. 2012 Apr;18(2):180–200. doi: 10.1177/1073858410395147. [DOI] [PubMed] [Google Scholar]
- Andreasen NC. Scale of Assessment of Negative Symptoms (SANS) Iowa city: University of Iowa; 1983. [Google Scholar]
- Andreasen NC. Scale of the Assessment of Positive Symptoms (SAPS) Iowa city: University of Iowa; 1984. [Google Scholar]
- Andreasen NC. Psychiatric Symptoms You Currently Have-Baseline (PSYCH-BASE) Iowa city: University of Iowa; 1987. [Google Scholar]
- Andreasen NC, Flaum M, Arndt S. The Comprehensive Assessment of Symptoms and History (CASH). An instrument for assessing diagnosis and psychopathology. Arch Gen Psychiatry. 1992 Aug;49(8):615–23. doi: 10.1001/archpsyc.1992.01820080023004. [DOI] [PubMed] [Google Scholar]
- Annett M. A classification of hand preference by association analysis. Br J Psychol. 1970 Aug;61(3):303–21. doi: 10.1111/j.2044-8295.1970.tb01248.x. [DOI] [PubMed] [Google Scholar]
- Barbey AK, Koenigs M, Grafman J. Dorsolateral prefrontal contributions to human working memory. Cortex; a journal devoted to the study of the nervous system and behavior. 2012 Sep; doi: 10.1016/j.cortex.2012.05.022. http://www.ncbi.nlm.nih.gov/pubmed/22789779. [DOI] [PMC free article] [PubMed]
- Barnes TRE. The Barnes Akathisia Rating Scale--revisited. J Psychopharmacol (Oxford) 2003 Dec;17(4):365–70. doi: 10.1177/0269881103174013. [DOI] [PubMed] [Google Scholar]
- Beatty WW, Jocic Z, Monson N, Staton RD. Memory and frontal lobe dysfunction in schizophrenia and schizoaffective disorder. J Nerv Ment Dis. 1993 Jul;181(7):448–53. doi: 10.1097/00005053-199307000-00008. [DOI] [PubMed] [Google Scholar]
- Becker TM, Kerns JG, Macdonald AW, 3rd, Carter CS. Prefrontal dysfunction in first-degree relatives of schizophrenia patients during a Stroop task. Neuropsychopharmacology. 2008 Oct;33(11):2619–25. doi: 10.1038/sj.npp.1301673. [DOI] [PubMed] [Google Scholar]
- Boyajian RA, Otis SM. Acute effects of smoking on human cerebral blood flow: a transcranial Doppler ultrasonography study. J Neuroimaging. 2000 Oct;10(4):204–8. doi: 10.1111/jon2000104204. [DOI] [PubMed] [Google Scholar]
- Brody AL, Mandelkern MA, Jarvik ME, Lee GS, Smith EC, Huang JC. Differences between smokers and nonsmokers in regional gray matter volumes and densities. Biol Psychiatry. 2004 Jan;55(1):77–84. doi: 10.1016/s0006-3223(03)00610-3. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Head D, Parker J, Fotenos AF, Marcus D, Morris JC. A unified approach for morphometric and functional data analysis in young, old, and demented adults using automated atlas-based head size normalization: reliability and validation against manual measurement of total intracranial volume. Neuroimage. 2004 Oct;23(2):724–38. doi: 10.1016/j.neuroimage.2004.06.018. [DOI] [PubMed] [Google Scholar]
- Crespo-Facorro B, Barbadillo L, Pelayo-Terán JM, Rodríguez-Sánchez JM. Neuropsychological functioning and brain structure in schizophrenia. Int Rev Psychiatry. 2007 Aug;19(4):325–36. doi: 10.1080/09540260701486647. [DOI] [PubMed] [Google Scholar]
- Cullen KR, Wallace S, Magnotta VA, Bockholt J, Ehrlich S, Gollub RL, et al. Cigarette smoking and white matter microstructure in schizophrenia. Psychiatry Res. 2012 Feb;201(2):152–8. doi: 10.1016/j.pscychresns.2011.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson LL, Heinrichs RW. Quantification of frontal and temporal lobe brain-imaging findings in schizophrenia: a meta-analysis. Psychiatry Res. 2003 Feb;122(2):69–87. doi: 10.1016/s0925-4927(02)00118-x. [DOI] [PubMed] [Google Scholar]
- Desikan RS, Ségonne F, Fischl B, Quinn BT, Dickerson BC, Blacker D, et al. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage. 2006 Jul;31(3):968–80. doi: 10.1016/j.neuroimage.2006.01.021. [DOI] [PubMed] [Google Scholar]
- Durazzo TC, Meyerhoff DJ, Nixon SJ. Interactive Effects of Chronic Cigarette Smoking and Age on Hippocampal Volumes. Drug and Alcohol Dependence. 2013 Dec;133(2):704–711. doi: 10.1016/j.drugalcdep.2013.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durazzo TC, Meyerhoff DJ, Nixon SJ. Chronic cigarette smoking: implications for neurocognition and brain neurobiology. Int J Environ Res Public Health. 2010 Oct;7(10):3760–91. doi: 10.3390/ijerph7103760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrlich S, Walton E, Roffman JL, Weiss D, Puls I, Doehler N, et al. Smoking, But Not Malnutrition, Influences Promoter-Specific DNA Methylation of the Proopiomelanocortin Gene in Patients With and Without Anorexia Nervosa. Can J Psychiatry. 2012 Mar;57(3):168–76. doi: 10.1177/070674371205700306. [DOI] [PubMed] [Google Scholar]
- Ehrlich S, Brauns S, Yendiki A, Ho BC, Calhoun V, Schulz SC, et al. Associations of Cortical Thickness and Cognition in Patients With Schizophrenia and Healthy Controls. Schizophr Bull. 2011 Mar; doi: 10.1093/schbul/sbr018. http://www.ncbi.nlm.nih.gov/pubmed/21436318. [DOI] [PMC free article] [PubMed]
- Ehrlich S, Morrow EM, Roffman JL, Wallace SR, Naylor M, Bockholt HJ, et al. The COMT Val108/158Met polymorphism and medial temporal lobe volumetry in patients with schizophrenia and healthy adults. Neuroimage. 2010 Nov;53(3):992–1000. doi: 10.1016/j.neuroimage.2009.12.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B, Dale AM. Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proc Natl Acad Sci U S A. 2000 Sep;97(20):11050–5. doi: 10.1073/pnas.200033797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B, Salat DH, Busa E, Albert M, Dieterich M, Haselgrove C, et al. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron. 2002 Jan;33(3):341–55. doi: 10.1016/s0896-6273(02)00569-x. [DOI] [PubMed] [Google Scholar]
- Fischl B, Sereno MI, Tootell RB, Dale AM. High-resolution intersubject averaging and a coordinate system for the cortical surface. Hum Brain Mapp. 1999;8(4):272–84. doi: 10.1002/(SICI)1097-0193(1999)8:4<272::AID-HBM10>3.0.CO;2-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman L, Turner JA, Stern H, Mathalon DH, Trondsen LC, Potkin SG. Chronic smoking and the BOLD response to a visual activation task and a breath hold task in patients with schizophrenia and healthy controls. Neuroimage. 2008 Apr;40(3):1181–94. doi: 10.1016/j.neuroimage.2007.12.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallinat J, Lang UE, Jacobsen LK, Bajbouj M, Kalus P, von Haebler D, et al. Abnormal hippocampal neurochemistry in smokers: evidence from proton magnetic resonance spectroscopy at 3 T. J Clin Psychopharmacol. 2007 Feb;27(1):80–4. doi: 10.1097/JCP.0b013e31802dffde. [DOI] [PubMed] [Google Scholar]
- Gallinat J, Meisenzahl E, Jacobsen LK, Kalus P, Bierbrauer J, Kienast T, et al. Smoking and structural brain deficits: a volumetric MR investigation. Eur J Neurosci. 2006 Sep;24(6):1744–50. doi: 10.1111/j.1460-9568.2006.05050.x. [DOI] [PubMed] [Google Scholar]
- Goldman AL, Pezawas L, Mattay VS, Fischl B, Verchinski BA, Chen Q, et al. Widespread reductions of cortical thickness in schizophrenia and spectrum disorders and evidence of heritability. Arch Gen Psychiatry. 2009 May;66(5):467–77. doi: 10.1001/archgenpsychiatry.2009.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gur RE, Cowell PE, Latshaw A, Turetsky BI, Grossman RI, Arnold SE, et al. Reduced dorsal and orbital prefrontal gray matter volumes in schizophrenia. Arch Gen Psychiatry. 2000 Aug;57(8):761–8. doi: 10.1001/archpsyc.57.8.761. [DOI] [PubMed] [Google Scholar]
- Haijma SV, Van Haren N, Cahn W, Koolschijn PCMP, Hulshoff Pol HE, Kahn RS. Brain Volumes in Schizophrenia: A Meta-Analysis in Over 18000 Subjects. Schizophr Bull. 2012 Oct; doi: 10.1093/schbul/sbs118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Haren NEM, Koolschijn PCMP, Cahn W, Schnack HG, Hulshoff Pol HE, Kahn RS. Cigarette smoking and progressive brain volume loss in schizophrenia. Eur Neuropsychopharmacol. 2010 Jul;20(7):454–8. doi: 10.1016/j.euroneuro.2010.02.009. [DOI] [PubMed] [Google Scholar]
- Heatherton TF, Kozlowski LT, Frecker RC, Fagerström KO. The Fagerström Test for Nicotine Dependence: A Revision of the Fagerström Tolerance Questionnaire. British Journal of Addiction. 1991 Sep;86(9):1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- Heckers S. Neuroimaging studies of the hippocampus in schizophrenia. Hippocampus. 2001;11(5):520–8. doi: 10.1002/hipo.1068. [DOI] [PubMed] [Google Scholar]
- Heckers S, Konradi C. Hippocampal pathology in schizophrenia. Curr Top Behav Neurosci. 2010;4:529–53. doi: 10.1007/7854_2010_43. [DOI] [PubMed] [Google Scholar]
- Hollingshead A. Two Factor Index of Social Position. Yale University; New Haven: 1965. [Google Scholar]
- Honea R, Crow TJ, Passingham D, Mackay CE. Regional deficits in brain volume in schizophrenia: a meta-analysis of voxel-based morphometry studies. Am J Psychiatry. 2005 Dec;162(12):2233–45. doi: 10.1176/appi.ajp.162.12.2233. [DOI] [PubMed] [Google Scholar]
- Jovicich J, Czanner S, Greve D, Haley E, van der Kouwe A, Gollub R, et al. Reliability in multi-site structural MRI studies: effects of gradient non-linearity correction on phantom and human data. Neuroimage. 2006 Apr;30(2):436–43. doi: 10.1016/j.neuroimage.2005.09.046. [DOI] [PubMed] [Google Scholar]
- Jovicich J, Czanner S, Han X, Salat D, van der Kouwe A, Quinn B, et al. MRI-derived measurements of human subcortical, ventricular and intracranial brain volumes: Reliability effects of scan sessions, acquisition sequences, data analyses, scanner upgrade, scanner vendors and field strengths. Neuroimage. 2009 May;46(1):177–92. doi: 10.1016/j.neuroimage.2009.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikinis Z, Fallon JH, Niznikiewicz M, Nestor P, Davidson C, Bobrow L, et al. Gray matter volume reduction in rostral middle frontal gyrus in patients with chronic schizophrenia. Schizophr Res. 2010 Nov;123(2-3):153–9. doi: 10.1016/j.schres.2010.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kühn S, Schubert F, Gallinat J. Reduced thickness of medial orbitofrontal cortex in smokers. Biol Psychiatry. 2010 Dec;68(11):1061–5. doi: 10.1016/j.biopsych.2010.08.004. [DOI] [PubMed] [Google Scholar]
- Kuperberg GR, Broome MR, McGuire PK, David AS, Eddy M, Ozawa F, et al. Regionally localized thinning of the cerebral cortex in schizophrenia. Arch Gen Psychiatry. 2003 Sep;60(9):878–88. doi: 10.1001/archpsyc.60.9.878. [DOI] [PubMed] [Google Scholar]
- De Leon J, Diaz FJ. A meta-analysis of worldwide studies demonstrates an association between schizophrenia and tobacco smoking behaviors. Schizophr Res. 2005 Jul;76(2-3):135–57. doi: 10.1016/j.schres.2005.02.010. [DOI] [PubMed] [Google Scholar]
- Leyba L, Mayer AR, Gollub RL, Andreasen NC, Clark VP. Smoking status as a potential confound in the BOLD response of patients with schizophrenia. Schizophr Res. 2008 Sep;104(1-3):79–84. doi: 10.1016/j.schres.2008.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao Y, Tang J, Liu T, Chen X, Hao W. Differences between smokers and non-smokers in regional gray matter volumes: a voxel-based morphometry study. Addict Biol. 2010 Aug; doi: 10.1111/j.1369-1600.2010.00250.x. http://www.ncbi.nlm.nih.gov/pubmed/20731627. [DOI] [PubMed]
- Lopez-Garcia P, Aizenstein HJ, Snitz BE, Walter RP, Carter CS. Automated ROI-based brain parcellation analysis of frontal and temporal brain volumes in schizophrenia. Psychiatry Res. 2006 Oct;147(2-3):153–61. doi: 10.1016/j.pscychresns.2006.04.007. [DOI] [PubMed] [Google Scholar]
- Lucas C, Martin J. Smoking and Drug Interactions. 2013 Nov; http://www.australianprescriber.com/magazine/36/3/102/4.
- Maher BA, Manschreck TC, Woods BT, Yurgelun-Todd DA, Tsuang MT. Frontal brain volume and context effects in short-term recall in schizophrenia. Biol Psychiatry. 1995 Feb;37(3):144–50. doi: 10.1016/0006-3223(94)00203-F. [DOI] [PubMed] [Google Scholar]
- Narr KL, Toga AW, Szeszko P, Thompson PM, Woods RP, Robinson D, et al. Cortical thinning in cingulate and occipital cortices in first episode schizophrenia. Biol Psychiatry. 2005 Jul;58(1):32–40. doi: 10.1016/j.biopsych.2005.03.043. [DOI] [PubMed] [Google Scholar]
- Nelson MD, Saykin AJ, Flashman LA, Riordan HJ. Hippocampal volume reduction in schizophrenia as assessed by magnetic resonance imaging: a meta-analytic study. Arch Gen Psychiatry. 1998 May;55(5):433–40. doi: 10.1001/archpsyc.55.5.433. [DOI] [PubMed] [Google Scholar]
- Niemann K, Hammers A, Coenen VA, Thron A, Klosterkötter J. Evidence of a smaller left hippocampus and left temporal horn in both patients with first episode schizophrenia and normal control subjects. Psychiatry Res. 2000 Aug;99(2):93–110. doi: 10.1016/s0925-4927(00)00059-7. [DOI] [PubMed] [Google Scholar]
- Parent A, Carpenter M. Human neuroanatomy. Baltimore: MD: Williams & Wilkins; 1995. [Google Scholar]
- Potkin S, Turner J, Fallon J, Lakatos A, Keator D, Guffanti G, et al. Gene discovery through imaging genetics: identification of two novel genes associated with schizophrenia. Mol Psychiatry. 2009a Apr;14(4):416–28. doi: 10.1038/mp.2008.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potkin SG, Turner JA, Brown GG, McCarthy G, Greve DN, Glover GH, et al. Working memory and DLPFC inefficiency in schizophrenia: the FBIRN study. Schizophr Bull. 2009b Jan;35(1):19–31. doi: 10.1093/schbul/sbn162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajkowska G, Goldman-Rakic PS. Cytoarchitectonic definition of prefrontal areas in the normal human cortex: II. Variability in locations of areas 9 and 46 and relationship to the Talairach Coordinate System. Cereb Cortex. 1995 Aug;5(4):323–37. doi: 10.1093/cercor/5.4.323. [DOI] [PubMed] [Google Scholar]
- Sanfilipo M, Lafargue T, Rusinek H, Arena L, Loneragan C, Lautin A, et al. Volumetric measure of the frontal and temporal lobe regions in schizophrenia: relationship to negative symptoms. Arch Gen Psychiatry. 2000 May;57(5):471–80. doi: 10.1001/archpsyc.57.5.471. [DOI] [PubMed] [Google Scholar]
- Saykin AJ, Gur RC, Gur RE, Mozley PD, Mozley LH, Resnick SM, et al. Neuropsychological function in schizophrenia. Selective impairment in memory and learning. Arch Gen Psychiatry. 1991 Jul;48(7):618–24. doi: 10.1001/archpsyc.1991.01810310036007. [DOI] [PubMed] [Google Scholar]
- Saykin AJ, Shtasel DL, Gur RE, Kester DB, Mozley LH, Stafiniak P, et al. Neuropsychological deficits in neuroleptic naive patients with first-episode schizophrenia. Arch Gen Psychiatry. 1994 Feb;51(2):124–31. doi: 10.1001/archpsyc.1994.03950020048005. [DOI] [PubMed] [Google Scholar]
- Schultz CC, Koch K, Wagner G, Roebel M, Nenadic I, Schachtzabel C, et al. Complex pattern of cortical thinning in schizophrenia: results from an automated surface based analysis of cortical thickness. Psychiatry Res. 2010 May;182(2):134–40. doi: 10.1016/j.pscychresns.2010.01.008. [DOI] [PubMed] [Google Scholar]
- Segall JM, Turner JA, van Erp TGM, White T, Bockholt HJ, Gollub RL, et al. Voxel-based morphometric multisite collaborative study on schizophrenia. Schizophr Bull. 2009 Jan;35(1):82–95. doi: 10.1093/schbul/sbn150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidman LJ, Pantelis C, Keshavan MS, Faraone SV, Goldstein JM, Horton NJ, et al. A review and new report of medial temporal lobe dysfunction as a vulnerability indicator for schizophrenia: a magnetic resonance imaging morphometric family study of the parahippocampal gyrus. Schizophr Bull. 2003;29(4):803–30. doi: 10.1093/oxfordjournals.schbul.a007048. [DOI] [PubMed] [Google Scholar]
- Shenton ME, Dickey CC, Frumin M, McCarley RW. A review of MRI findings in schizophrenia. Schizophr Res. 2001 Apr;49(1-2):1–52. doi: 10.1016/s0920-9964(01)00163-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepherd AM, Laurens KR, Matheson SL, Carr VJ, Green MJ. Systematic meta-review and quality assessment of the structural brain alterations in schizophrenia. Neurosci Biobehav Rev. 2012 Apr;36(4):1342–56. doi: 10.1016/j.neubiorev.2011.12.015. [DOI] [PubMed] [Google Scholar]
- Squire LR, Zola-Morgan S. The medial temporal lobe memory system. Science. 1991 Sep;253(5026):1380–6. doi: 10.1126/science.1896849. [DOI] [PubMed] [Google Scholar]
- Stonnington CM, Tan G, Klöppel S, Chu C, Draganski B, Jack CR, Jr, et al. Interpreting scan data acquired from multiple scanners: a study with Alzheimer's disease. Neuroimage. 2008 Feb;39(3):1180–5. doi: 10.1016/j.neuroimage.2007.09.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takayanagi Y, Takahashi T, Orikabe L, Mozue Y, Kawasaki Y, Nakamura K, et al. Classification of first-episode schizophrenia patients and healthy subjects by automated MRI measures of regional brain volume and cortical thickness. PLoS ONE. 2011;6(6):e21047. doi: 10.1371/journal.pone.0021047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tregellas JR, Shatti S, Tanabe JL, Martin LF, Gibson L, Wylie K, et al. Gray matter volume differences and the effects of smoking on gray matter in schizophrenia. Schizophr Res. 2007 Dec;97(1-3):242–9. doi: 10.1016/j.schres.2007.08.019. [DOI] [PubMed] [Google Scholar]
- Voineskos AN, Foussias G, Lerch J, Felsky D, Remington G, Rajji TK, et al. Neuroimaging evidence for the deficit subtype of schizophrenia. JAMA Psychiatry. 2013 May;70(5):472–80. doi: 10.1001/jamapsychiatry.2013.786. [DOI] [PubMed] [Google Scholar]
- Watson DR, Bai F, Barrett SL, Turkington A, Rushe TM, Mulholland CC, et al. Structural changes in the hippocampus and amygdala at first episode of psychosis. Brain Imaging Behav. 2012 Mar;6(1):49–60. doi: 10.1007/s11682-011-9141-4. [DOI] [PubMed] [Google Scholar]
- Weiss AP, Heckers S. Neuroimaging of declarative memory in schizophrenia. Scand J Psychol. 2001 Jul;42(3):239–50. doi: 10.1111/1467-9450.00234. [DOI] [PubMed] [Google Scholar]
- Wilkinson D. Wide Range Achievement Test. 3rd. Wide Range, Inc.; Wilmington: 1993. [Google Scholar]
- Winterer G. Why do patients with schizophrenia smoke? Curr Opin Psychiatry. 2010 Mar;23(2):112–9. doi: 10.1097/YCO.0b013e3283366643. [DOI] [PubMed] [Google Scholar]
- Woods SW. Chlorpromazine equivalent doses for the newer atypical antipsychotics. J Clin Psychiatry. 2003 Jun;64(6):663–7. doi: 10.4088/jcp.v64n0607. [DOI] [PubMed] [Google Scholar]
- Wright IC, Rabe-Hesketh S, Woodruff PW, David AS, Murray RM, Bullmore ET. Meta-analysis of regional brain volumes in schizophrenia. Am J Psychiatry. 2000 Jan;157(1):16–25. doi: 10.1176/ajp.157.1.16. [DOI] [PubMed] [Google Scholar]
- Ziedonis D, Hitsman B, Beckham JC, Zvolensky M, Adler LE, Audrain-McGovern J, et al. Tobacco use and cessation in psychiatric disorders: National Institute of Mental Health report. Nicotine Tob Res. 2008 Dec;10(12):1691–715. doi: 10.1080/14622200802443569. [DOI] [PubMed] [Google Scholar]
- Zilles D, Burke S, Schneider-Axmann T, Falkai P, Gruber O. Diagnosis-specific effect of familial loading on verbal working memory in schizophrenia. Eur Arch Psychiatry Clin Neurosci. 2009 Sep;259(6):309–15. doi: 10.1007/s00406-009-0001-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


