Abstract
dal-PLAQUE is a placebo-controlled multicenter study designed to assess the effect of dalcetrapib on imaging measures of plaque inflammation and plaque burden. dal-PLAQUE is a multimodality imaging study in the context of the large dal-HEART Program. Decreased high-density lipoprotein cholesterol is linked to increased risk of coronary heart disease (CHD). Dalcetrapib, a compound that increases high-density lipoprotein cholesterol by modulating cholesteryl ester transfer protein, is being studied to assess if it can reduce the progression of atherosclerotic disease and thereby decrease cardiovascular morbidity and mortality. Patients with CHD or CHD-risk equivalents were randomized to receive 600 mg dalcetrapib or placebo daily for 24 months, in addition to conventional lipid-lowering medication and other medications for cardiovascular risk factors. The primary outcomes are the effect of dalcetrapib on 18F-fluorodeoxyglucose positron emission tomography target-to-background ratio after 6 months and magnetic resonance imaging (MRI) plaque burden (wall area, wall thickness, total vessel area, and wall area/total vessel area ratio) after 12 months. Secondary objectives include positron emission tomography target-to-background ratio at 3 months and MRI plaque burden at 6 and 24 months; plaque composition at 6, 12, and 24 months; and aortic compliance at 6 months. A tertiary objective is to examine the dynamic contrast-enhanced MRI parameters of plaque neovascularization. In total, 189 subjects entered screening, and 130 were randomized. dal-PLAQUE will provide important information on the effects of dalcetrapib on markers of inflammation and atherosclerotic plaque burden and, thereby, on the safety of cholesteryl ester transfer protein modulation with dalcetrapib. Results are expected in 2011.
Despite advances in the diagnosis and management of coronary artery disease, acute coronary events continue to occur in many patients. Numerous studies have suggested that high-density lipoprotein (HDL) may exert several potentially important antiatherosclerotic and endothelial-protective effects.1 In particular, the promotion of reverse cholesterol transport, that is, cholesterol efflux from lipid-loaded macrophages in atherosclerotic lesions and the subsequent cholesterol transport back to the liver, has been proposed as an antiatherogenic effect of HDL.2,3 The development of drugs that raise HDL-cholesterol (HDL-C) and produce a functional HDL particle may potentially reduce the progression of atherosclerosis and decrease morbidity and mortality when used alongside established therapies. One strategy for raising HDL-C is to inhibit or modulate the cholesteryl ester transfer protein (CETP). Decreased plasma levels of CETP are associated with increased levels of HDL-C and, in turn, decreased risk of coronary artery disease.4,5
Torcetrapib, the first CETP inhibitor extensively evaluated in humans, was associated with increased blood pressure (BP) in several clinical trials, but this appears to be a compound-specific, off-target effect and not a result of CETP inhibition in general.6,7 Dalcetrapib has been shown to modulate CETP to reduce its activity and thereby increase HDL-C levels in a dose-dependent manner.8 To date, the tolerability of dalcetrapib has been reassuring, with no evidence of clinically relevant increases in BP, electrolytes, or mineralocorticoids at therapeutic doses.9,10
dal-PLAQUE is being conducted to assess the efficacy and safety of dalcetrapib on plaque inflammation as evaluated by 18F-fluorodeoxyglucose positron emission tomography (18F-FDG-PET) and atherosclerotic plaque progression as determined by magnetic resonance imaging (MRI) plaque burden. It is becoming increasingly evident that the lesions responsible for acute coronary events may not be critically obstructive nor associated with inducible ischemia. Computed tomography (CT) and MRI can be used to evaluate several morphologic features of plaque vulnerability11-17 (eg, large-volume necrotic cores, positive outward remodeling, thin fibrous caps).18,19 Increasingly, the role of inflammation has been considered key in the progression of atheromas.20-22 It has also been suggested that 18F-FDG-PET/CT could be used to measure inflammation within atherosclerotic plaque and potentially to track its change with appropriate therapies.23-29 Similarly, high-resolution MRI offers accurate and reproducible measures of atherosclerotic plaque burden and composition.11,30-33
As the key objective, dal-PLAQUE will attempt to refute —at a meaningful pilot scale—a hypothesis proposed following torcetrapib's failure that, as a class, agents acting on CETP that increase HDL-C are proinflammatory or proatherogenic and may, thereby, increase cardiovascular mortality and morbidity. The combined use of 2 imaging modalities—18F-FDG-PET/CT and MRI—showing no progression, or regression, of inflammatory burden or atherosclerotic plaque size despite CETP inhibition would help to disprove this hypothesis and support the safety of dalcetrapib. To transition dalcetrapib from an early-stage clinical trial to clinical practice, in addition to excluding harmful effects, its near-term utility needs to be demonstrated. The use of intermediary surrogate imaging end points may provide an alternative strategy to evaluate the efficacy and safety of a drug and may therefore be a useful tool in a proof of concept study.
Methods
Design
dal-PLAQUE (ClinicalTrials.gov identifier NCT00655473; accessed June 10, 2010) is a phase IIB, double-blind, randomized, placebo-controlled study comparing the efficacy of dalcetrapib 600 mg/day or matching placebo, in addition to current “standard of care,” on the progression or regression of atherosclerosis using MRI and 18F-FDG-PET/CT in patients with existing coronary heart disease (CHD) or CHD-risk equivalents.
This study is an investigator-initiated protocol with the final trial design approved in collaboration with the study sponsor (F. Hoffmann–La Roche Ltd). The first draft of the manuscript was prepared by the first author, with edits and revisions provided by all coauthors. Editorial assistance was provided by Prime Healthcare during the preparation of this report and funded by F. Hoffmann–La Roche Ltd.
This study is being conducted at 11 centers in Canada and the United States, in compliance with the principles of the Declaration of Helsinki and according to Good Clinical Practice guidelines. The protocol has been reviewed and approved by the institutional review board of each center. All participants have provided written informed consent.
Patients meeting the entry criteria enter a prerandomization phase of up to 8 weeks, to adjust lipid-lowering therapy. Eligible patients are randomized according to a computer-generated global randomization code and assigned in a 1:1 double-blind fashion, stratified by center, to receive either dalcetrapib 600 mg/day or matching placebo tablets for 2 years, followed by a 2-week safety follow-up. Patients undergo 18F-FDG-PET/CT at screening; those with an average maximal arterial wall (target) to background (blood) ratio (TBR; a measure of 18F-FDG uptake) ≥1.6 are eligible for randomization. This visit will also be used as the PET/CT baseline. The baseline MRI is performed within 2 weeks of the screening PET/CT, after which patients undergo randomization. Follow-up PET/CT will take place at 3 and 6 months and MRI at 6, 12, and 24 months after randomization (Figure 1).
Figure 1.
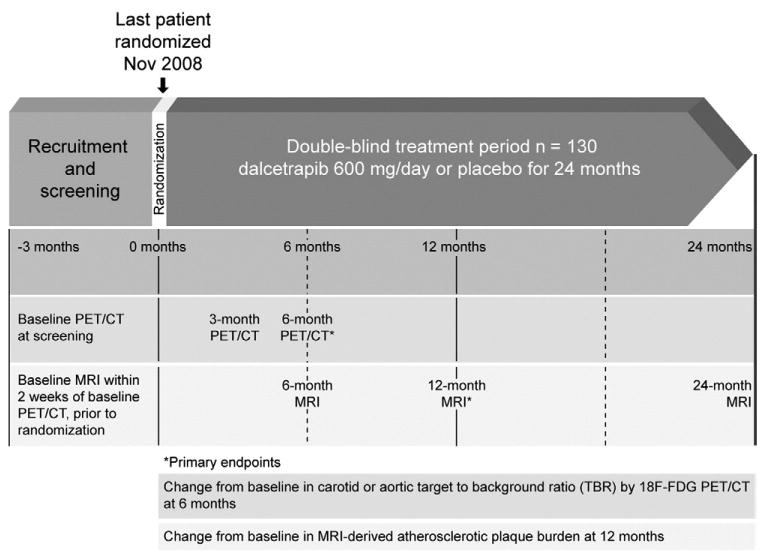
dal-PLAQUE study design and timeline of imaging assessments (see text for details).
Inclusion and exclusion criteria
The target population is patients with CHD or other CHD risk factors (CHD equivalents). The main inclusion criteria at screening are male and female patients aged 18 to 75 years; a diagnosis of CHD or CHD-risk equivalents based on the National Cholesterol Education Program Adult Treatment Panel III, for example, diabetes or >20% 10-year risk of CHD events by Framingham risk score; and triglycerides level ≤400 mg/dL (≤4.5 mmol/L). Additional inclusion criteria at randomization are carotid or aortic plaque inflammation TBR ≥1.6, determined by 18F-FDG uptake on PET/CT. Patients should be clinically stable and on appropriate treatment with statin and/or other low-density lipoprotein cholesterol (LDL-C) lowering drugs to a stable LDL-C level of <100 mg/dL (<2.6 mmol/L), unless taking maximum tolerated doses of therapy based on their medical condition or intolerant to statins.
Female patients are excluded if they are pregnant, breastfeeding, or of childbearing potential. Other exclusion criteria are concomitant HDL-C raising therapy (niacin, fibrates, bile acid sequestrants, rimonabant, CETP therapy, or other), uncontrolled BP, uncontrolled diabetes (hemoglobin A1c >10%), recent (<3 months) clinically significant coronary or cerebral vascular event, patients with familial hypercholesterolemia, glomerular filtration rate <30 mL/min (Cockcroft-Gault formula), liver disease, history of malignancies, and standard contraindications for MRI and PET/CT.
Study end points
Results of PET/CT and MRI are prespecified coprimary imaging end points for this study. The primary PET/CT end point is the change in arterial wall 18F-FDG uptake within an index vessel (right carotid, or left carotid, or ascending thoracic aorta) after 6 months, reported as the TBR (Figure 1). The primary MRI end point is the change in atherosclerotic plaque burden, measured by 4 indices: wall area, wall thickness, total vessel area, and wall area/total vessel area ratio, based on the average of the right and left carotids after 12 months.34,35
Secondary objectives include the following: change from baseline in TBR from the index vessel (right and left carotid or ascending thoracic aorta) based on the standardized 18F-FDG uptake measured with PET after 3 months and change from baseline in MRI-derived atherosclerotic plaque burden indices on the right carotid, left carotid, and descending abdominal aorta after 6 and 24 months. Other objectives include the following: change from baseline in MRI-derived aortic compliance after 6 months; plaque composition and area under signal intensity versus time curves obtained from dynamic contrast-enhanced (DCE) MRI after injection of contrast agent19 after 6, 12, and 24 months; change from baseline in the standardized uptake values with PET/CT; and change from baseline in inflammatory markers (high-sensitivity C-reactive protein, interleukin-6, soluble platelet-selectin, soluble E-selectin, soluble intercellular adhesion molecule) after 3, 12, and 24 months.
Prespecified cardiovascular end points are monitored as a component of the dal-HEART Program: CHD death, major coronary events (nonfatal myocardial infarction, hospitalization for acute coronary syndrome, resuscitated cardiac arrest), and fatal or nonfatal stroke of presumed atherothrombotic origin. End points will be adjudicated by an independent clinical end point committee.
Statistical analyses plan
For all primary and secondary objectives, a linear model will be assumed for the expected value of the variable and will include treatment and center as fixed effects along with a covariate term for the centered baseline value of the variable. To check for consistency of the treatment differences across centers, the results will also be analyzed with a model including the treatment-by-center interaction term.
Hypothesis testing and statistical analyses plan
For the primary and secondary efficacy end points of change from baseline in 18F-FDG uptake and plaque size/burden, the null and alternative hypotheses to be tested at the 5% 1-sided significance level are as follows:
where μD and μP are the mean reductions from baseline for the dalcetrapib and placebo arms, respectively. In addition, point estimates and confidence intervals will be determined for each of the summary statistics associated with the tests of hypotheses. For this exploratory study, no adjustments for multiplicity will be carried out.
Because of the sparseness of the literature reporting similar studies at the time the study was designed, the sample size was not based on power calculations for specified treatment effects. To ensure at least 100 evaluable patients, we defined a nominal sample size of 120 patients to be randomized equally into the 2 treatment groups. Trials of this size are able to detect treatment differences of 0.45 to 0.5 standard deviations in the efficacy variable with at least 80% power at the 5% 1-sided significance level. At the time the study was planned, differences of this magnitude had been observed for both primary end points in 2 studies.25,36 Because of a greater than expected number of patients consenting to be entered into the study, the final sample size achieved was 130.
Analyses will be carried out according to intent to treat and will include all patients randomized. No interim analysis that might result in the early termination of the trial is planned. However, as part of the compilation of evidence to support continuation of a phase III clinical end point trial (www.clinicaltrials.gov identifier NCT00658515), an early assessment of safety and efficacy outcomes will be made. This will occur once the change from baseline in TBR after 3 and 6 months of treatment is available for all patients. Results of analyses will be presented by treatment group, that is, dalcetrapib versus placebo, to the data safety and monitoring board and, upon the discretion of the data safety and monitoring board, to the sponsor. No individual patient data will be presented to the sponsor. An independent statistician will perform the analyses.
18F-fluorodeoxyglucose positron emission tomography/computed tomography
18F-fluorodeoxyglucose positron emission tomography/computed tomography imaging of the carotid arteries and ascending thoracic aorta is performed at screening and at 3 and 6 months. Thirty minutes before 18F-FDG intravenous injection, glucose is measured; if the fasting value is >200 mg/dL, the scan is rescheduled.
After 2 hours of 18F-FDG circulation, patients are imaged, head first, for localization and attenuation correction. A 2-dimensional (2D), chest PET scan using 2-bed positions to cover the aortic arch (upper limit) to the diaphragm (including inferior myocardial wall) and a 3-dimensional PET/CT image of the neck follow.
Images are analyzed at the core laboratory by an experienced reader. The TBR is calculated from the ratio of the standardized uptake values of the artery compared with background venous activity for the index vessel (to be followed throughout the study). The analysis will be performed using a “whole region of interest (ROI) approach.”37,38 Sample PET/CT images are shown in Figure 2.
Figure 2.
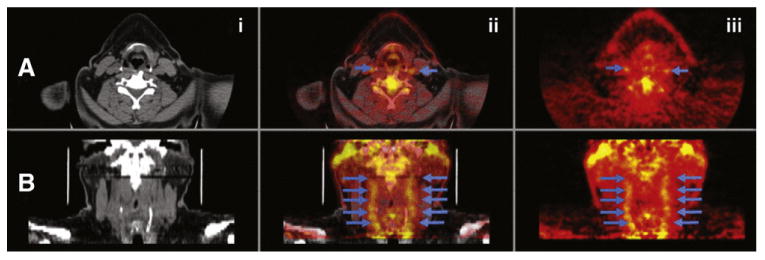
Sample 18F-FDG-PET/CT images of carotids. Transaxial (A) and coronal orientations (B). CT (i), fused PET/CT images (ii), and PET (iii). Blue arrows indicate inflammation in both carotids.
Magnetic resonance imaging
Magnetic resonance imaging of the carotid arteries and descending abdominal aorta is performed at baseline and at 6, 12, and 24 months. Abdominal aorta scout images are obtained for localization of the aorta, followed by 2D dark-blood turbo spin echo (16 slices).39,40 An image of 1 slice of the abdominal aorta is obtained subsequently for calculating aortic compliance.
Carotid bifurcations are localized using phase contrast images, followed by acquisition of 2D dark-blood turbo-spin-echo images of the common carotid arteries. Lumen contours are obtained via a time-of-flight bright magnetic resonance angiography sequence. Finally, DCE images are obtained on 1 slice after administration of 0.2 mmol/kg of gadolinium-based contrast agent.20
At the core laboratory, magnetic resonance images are analyzed at a dedicated workstation by an experienced observer for the presence or absence of plaque components.31,32 The vessel wall boundaries and outer and inner walls of the common carotid and descending abdominal aorta are manually traced to derive vessel dimensions and morphologies. To facilitate comparisons across patients, a normalized parameter (mean wall area divided by mean total vessel area) will be used.35 Sample MRIs are shown in Figure 3.
Figure 3.
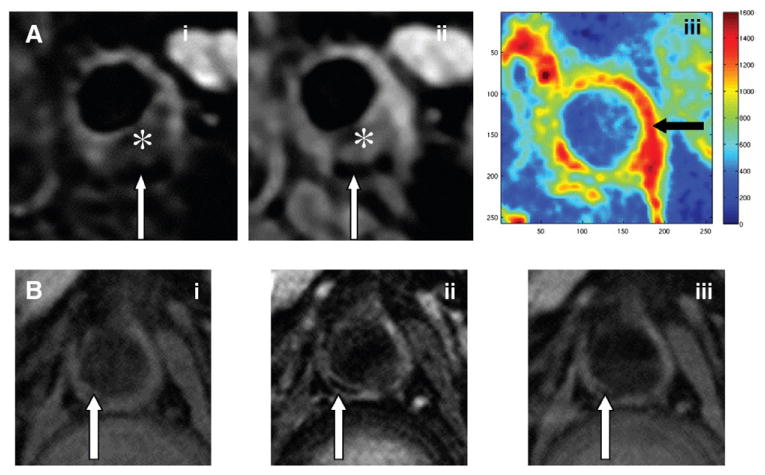
Sample MRIs. A, Cross-sectional carotid images: T2 weighted (i), proton density (PD) weighted (ii), and DCE–area under the curve (iii) 7 minutes. B, Cross-sectional abdominal aorta images: T1 weighted (i), T2 weighted (ii), and PD weighted (iii). * indicates lipid-rich necrotic core; white arrows indicate calcification, and black arrow indicates increased vessel wall neovascularization.
Details of the imaging procedures/analyses are provided in the online Supplementary Appendix.
Results
Enrolment into dal-PLAQUE was initiated in March 2008. A total of 130 patients were randomized, the last in November 2008. Baseline characteristics are typical of the population being studied (Table I).
Table I. Baseline characteristics of the patients.
| Characteristic* | All randomized patients (n = 130) |
|---|---|
| Mean age (y) | 63.6 |
| Male sex, n (%) | 106 (82) |
| Body mass index (kg/m2) | 29.7 ± 5.53 |
| Medical history, n (%) | |
| Type II diabetes | 39 (30) |
| Hypertension | 95 (73) |
| CHD | 111 (85) |
| Abdominal aortic aneurysm | 5 (4) |
| >20% Framingham risk | Yes: 39 (30), no: 57 (44), Unknown: 33 (26) |
| Symptomatic carotid disease | 10 (8) |
| Peripheral arterial disease | 16 (12) |
| Smoking, n (%) | |
| Never | 53 (41) |
| Former | 60 (46) |
| Current | 17 (13) |
| Statin use,† n (%) | 113 (87) |
| Cholesterol (mg/dL) | |
| Total | 145.83 ± 27.39 |
| LDL-C | 74.18 ± 20.98 |
| HDL-C | 44.40 ± 13.52 |
| Triglycerides‡ (mg/dL) | 124.5 (88.0-165.0) |
| CRP (mg/L) | 2.98 ± 5.25 |
CRP indicates C-reactive protein.
All reported as mean ± SD, unless otherwise stated.
Patients with at least 1 treatment.
Median (interquartile range).
Discussion
dal-PLAQUE is a randomized, double-blind, placebo-controlled, multicenter trial designed to assess the effects of dalcetrapib, complementary to statins or other LDL-C– lowering therapy, on vessel wall inflammation and atherosclerotic plaque burden in patients with CHD or CHD-risk equivalents. The primary end points of this study are the 6-month change in plaque inflammation as measured by 18F-FDG-PET TBR and the 12-month change in MRI-derived atherosclerotic plaque burden as measured by 4 indices: wall area, wall thickness, total vessel area, and wall area/total vessel area ratio.
Investigating whether CETP modulation by dalcetrapib proves beneficial in reducing vessel wall inflammation and atherosclerotic plaque progression is imperative for understanding if dalcetrapib is likely to assist in preventing cardiovascular disease. This rationale must take into account the results of trials in which the CETP inhibitor torcetrapib showed no beneficial effect on atherosclerosis progression.41-43 In ILLUMINATE, torcetrapib was associated with an increased risk of cardiovascular events and death,42 potentially linked to the off-target toxicity of torcetrapib (ie, BP increase, electrolyte changes, increase of circulating aldosterone levels) mitigating the benefits of increasing HDL-C. In clinical studies, dalcetrapib alone or in combination with a statin increased HDL-C but did not have a clinically relevant effect on BP.9 An analysis of safety data at 4, 12, and 48 weeks from several phase II trials showed the safety of dalcetrapib both alone and in combination with statins.9,44
It was also postulated that HDL produced from inhibition of CETP by torcetrapib could be proinflammatory or proatherogenic, and the imaging modalities selected for dal-PLAQUE, PET/CT and MRI, will be used to test this hypothesis in dalcetrapib-treated patients. The combined use of these imaging modalities will provide a rigorous assessment of dalcetrapib's safety in this regard and will further add to the overall assessment of safety that is a key aspect of the dal-HEART Program.
In comparing plaque 18F-FDG uptake to anatomical features of corresponding carotid endarterectomy specimens, Tawakol et al27 found no correlation between 18F-FDG uptake and plaque area and thickness. Rudd et al25 demonstrated 18F-FDG uptake in macrophage-rich areas of plaques, predominantly at the lipid-rich necrotic core/fibrous cap border, but little/no uptake in other plaque areas. It seems that subjects with cardiovascular disease, or risk factors for it, have higher levels of 18F-FDG uptake on vascular PET scans.29,45,46 Recent studies linked 18F-FDG uptake with levels of gene expression of inflammatory markers47 and circulating levels of several inflammatory biomarkers,48 showing that PET/CT imaging can help quantify vascular inflammation and provide incremental, independent information beyond atherosclerosis burden measured by MRI.
Noninvasive imaging of atherosclerotic disease using MRI has been well validated histologically.12 It can track disease progression and regression and can quantitatively and qualitatively evaluate parameters associated with in vivo plaque morphology and composition.15,17,18,34 Currently, the presence of intraplaque hemorrhage, lipid-rich necrotic core, or increased wall volume are indicators of lesion vulnerability. Newer MRI methods capable of imaging other important aspects of carotid atherosclerotic disease in vivo including inflammation, neovascularization, such as DCE-MRI20,49,50 and mechanical forces (aortic wall compliance),51,52 may aid in advancing our understanding of the pathophysiology of this multifactorial disease. Previous MRI studies have shown increased plaque burden with the presence of prior major cardiovascular events, establishing that MRI can be used as a surrogate marker for atherosclerotic disease burden.35 Recently, clinical trials have investigated the direct effect of drugs on atheroma using 3-dimensional MRI.53 In light of the ongoing debate surrounding CETP inhibition, these imaging modalities can serve as important tools to shed light, at the level of the vasculature, on the safety of agents acting on CETP.
dal-PLAQUE is the first multicenter drug efficacy and safety study that uses noninvasive multimodality imaging as the primary efficacy end points and includes advanced imaging techniques (ie, MRI-based measures of aortic wall compliance and DCE-MRI of neovascularization) as secondary or tertiary end points. dal-PLAQUE is therefore expected to provide unique data regarding the use of advanced imaging as a surrogate marker for the extent of cardiovascular disease. In dal-PLAQUE 2, atherosclerotic disease progression will be assessed by intravascular ultrasound and carotid B-mode ultrasound, and in dal-VESSEL54 (both part of the dal-HEART Program), brachial artery flow–mediated vasodilatation is quantified by ultrasound. In concert, these trials will greatly increase our understanding of the efficacy, safety, and tolerability of dalcetrapib, whereas dal-OUTCOMES,55 which will randomize more than 15 000 stable CHD patients after recent acute coronary syndrome to 600 mg dalcetrapib or matching placebo beginning 4 to 12 weeks after an index event, will serve to more fully define the clinical utility of dalcetrapib toward reducing cardiovascular morbidity and mortality.
Conclusions
dal-PLAQUE will help to determine the efficacy and safety profile of dalcetrapib by assessing its effects on reducing atherosclerotic plaque inflammation and burden on top of regular lipid-altering treatment in patients with CHD or CHD-risk equivalents. The multimodality approach used in dal-PLAQUE might provide supplementary information regarding the efficacy of dalcetrapib by directly visualizing plaque inflammation and burden after treatment while the results of the dal-OUTCOMES trial are pending.
Supplementary Material
Acknowledgments
This study was supported by Hoffmann–La Roche Inc. Executive Committee: Zahi A. Fayad (Chair), Michael E. Farkouh, David Kallend (nonvoting member), James H.F. Rudd, Ahmed Tawakol, and Mark Woodward (statistics).
Footnotes
Disclosures: This study was funded by F. Hoffmann–La Roche Ltd. Editorial assistance was provided by Prime Healthcare during the preparation of this report and funded by F. Hoffmann–La Roche Ltd. Z.A.F. discloses that he has received research grants from Roche, GlaxoSmithKline, Merck, VBL Therapeutics, Novartis, Bristol-Myers Squibb, and Via Pharmaceuticals and honoraria from Roche. M.E.F., J.H.F.R., A.T., and M.W. disclose that they have received honoraria from Roche. J.H.F.R. is part-supported by the National Institute for Health Research Cambridge Biomedical Research Centre. T.B. and J.P. disclose that they are employees of Hoffmann–La Roche Inc, and D.K. is an employee of F. Hoffmann–La Roche Ltd. V.M., S.B., and V.F. indicate that they have nothing to disclose.
References
- 1.Gordon DJ, Probstfield JL, Garrison RJ, et al. High-density lipoprotein cholesterol and cardiovascular-disease— 4 prospective American studies. Circulation. 1989;79:8–15. doi: 10.1161/01.cir.79.1.8. [DOI] [PubMed] [Google Scholar]
- 2.Quintao ECR, Cazita PM. Lipid transfer proteins: past, present and perspectives. Atherosclerosis. 2010;209:1–9. doi: 10.1016/j.atherosclerosis.2009.08.002. [DOI] [PubMed] [Google Scholar]
- 3.Tomás M, Latorre G, Senti M, et al. The antioxidant function of high density lipoproteins: a new paradigm in atherosclerosis. Rev Esp Cardiol. 2004;57:557–69. [PubMed] [Google Scholar]
- 4.Klerkx AHEM, El Harchaoui K, van der Steeg WA, et al. Cholesteryl ester transfer protein (CETP) inhibition beyond raising high-density lipoprotein cholesterol levels—pathways by which modulation of CETP activity may alter atherogenesis. Arterioscler Thromb Vasc Biol. 2006;26:706–15. doi: 10.1161/01.ATV.0000205595.19612.c9. [DOI] [PubMed] [Google Scholar]
- 5.Thompson A, Di Angelantonio E, Sarwar N, et al. Association of cholesteryl ester transfer protein genotypes with CETP mass and activity, lipid levels, and coronary risk. JAMA. 2008;299:2777–88. doi: 10.1001/jama.299.23.2777. [DOI] [PubMed] [Google Scholar]
- 6.Forrest MJ, Bloomfield D, Briscoe RJ, et al. Torcetrapib-induced blood pressure elevation is independent of CETP inhibition and is accompanied by increased circulating levels of aldosterone. Br J Pharmacol. 2008;154:1465–73. doi: 10.1038/bjp.2008.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nissen SE, Tardif J, Nicholls SJ, et al. Effect of torcetrapib on the progression of coronary atherosclerosis. N Engl J Med. 2007;356:1304–16. doi: 10.1056/NEJMoa070635. [DOI] [PubMed] [Google Scholar]
- 8.Niesor EJ, Magg C, Ogawa N, et al. Modulating cholesterol ester transfer protein activity maintains efficient pre–β-HDL formation and increases reverse cholesterol transport. J Lipid Res. 2010;51:3443–54. doi: 10.1194/jlr.M008706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stein EA, Stroes ESG, Steiner G, et al. Safety and tolerability of dalcetrapib. Am J Cardiol. 2009;104:82–91. doi: 10.1016/j.amjcard.2009.02.061. [DOI] [PubMed] [Google Scholar]
- 10.Stroes ESG, Kastelein JJP, Benardeau A, et al. Dalcetrapib: no off-target toxicity on blood pressure or on genes related to the renin-angiotensin-aldosterone system in rats. Br J Pharmacol. 2009;158:1763–70. doi: 10.1111/j.1476-5381.2009.00460.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Saam T, Schoenberg SO, Hatsukami TS, et al. High-resolution magnetic resonance imaging of carotid atherosclerotic plaque. Rofo. 2008;180:100–11. doi: 10.1055/s-2007-963666. [DOI] [PubMed] [Google Scholar]
- 12.Saam T, Ferguson MS, Yarnykh VL, et al. Quantitative evaluation of carotid plaque composition by in vivo MRI. Arterioscler Thromb Vasc Biol. 2005;25:234–9. doi: 10.1161/01.ATV.0000149867.61851.31. [DOI] [PubMed] [Google Scholar]
- 13.Yuan C, Kerwin WS, Ferguson MS, et al. Contrast-enhanced high resolution MRI for atherosclerotic carotid artery tissue characterization. J Magn Reson Imaging. 2002;15:62–7. doi: 10.1002/jmri.10030. [DOI] [PubMed] [Google Scholar]
- 14.Saam T, Hatsukami TS, Takaya N, et al. The vulnerable, or high-risk, atherosclerotic plaque: noninvasive MR imaging for characterization and assessment. Radiology. 2007;244:64–77. doi: 10.1148/radiol.2441051769. [DOI] [PubMed] [Google Scholar]
- 15.Yuan C, Kerwin WS, Yarnykh VL, et al. MRI of atherosclerosis in clinical trials. NMR Biomed. 2006;19:636–54. doi: 10.1002/nbm.1065. [DOI] [PubMed] [Google Scholar]
- 16.Chu BC, Hatsukami TS, Polissar NL, et al. Determination of carotid artery atherosclerotic lesion type and distribution in hypercholesterolemic patients with moderate carotid stenosis using noninvasive magnetic resonance imaging. Stroke. 2004;35:2444–8. doi: 10.1161/01.STR.0000144686.57135.98. [DOI] [PubMed] [Google Scholar]
- 17.Saam T, Cai JM, Cai YQ, et al. Carotid plaque composition differs between ethno-racial groups. An MRI pilot study comparing mainland Chinese and American Caucasion patient. Arterioscler Thromb Vasc Biol. 2005;25:611–6. doi: 10.1161/01.ATV.0000155965.54679.79. [DOI] [PubMed] [Google Scholar]
- 18.Cai JM, Hatsukami TS, Ferguson MS, et al. In vivo quantitative measurement of intact fibrous cap and lipid-rich necrotic core size in atherosclerotic carotid plaque—comparison of high-resolution, contrast-enhanced magnetic resonance imaging and histology. Circulation. 2005;112:3437–44. doi: 10.1161/CIRCULATIONAHA.104.528174. [DOI] [PubMed] [Google Scholar]
- 19.Mitsumori LM, Hatsukami TS, Ferguson MS, et al. In vivo accuracy of multisequence MR imaging for identifying unstable fibrous caps in advanced human carotid plaques. J Magn Reson Imaging. 2003;17:410–20. doi: 10.1002/jmri.10264. [DOI] [PubMed] [Google Scholar]
- 20.Calcagno C, Cornily JC, Hyafil F, et al. Detection of neovessels in atherosclerotic plaques of rabbits using dynamic contrast enhanced MRI and 18F-FDG PET. Arterioscler Thromb Vasc Biol. 2008;28:1311–7. doi: 10.1161/ATVBAHA.108.166173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Purushothaman KR, Sanz J, Zias E, et al. Neovascularization in human atherosclerosis. Curr Mol Med. 2006;6:457–77. doi: 10.2174/156652406778018635. [DOI] [PubMed] [Google Scholar]
- 22.Libby P. Inflammation in atherosclerosis. Nature. 2002;420:868–74. doi: 10.1038/nature01323. [DOI] [PubMed] [Google Scholar]
- 23.Davies JR, Rudd JHF, Fryer TD, et al. Identification of culprit lesions after transient ischemic attack by combined F-18 fluorodeoxyglucose positron-emission tomography and high-resolution magnetic resonance imaging. Stroke. 2005;36:2642–7. doi: 10.1161/01.STR.0000190896.67743.b1. [DOI] [PubMed] [Google Scholar]
- 24.Tawakol A, Migrino RQ, Hoffmann U, et al. Noninvasive in vivo measurement of vascular inflammation with F-18 fluorodeoxyglucose positron emission tomography. J Nucl Cardiol. 2005;12:294–301. doi: 10.1016/j.nuclcard.2005.03.002. [DOI] [PubMed] [Google Scholar]
- 25.Rudd JHF, Warburton EA, Fryer TD, et al. Imaging atherosclerotic plaque inflammation with [F-18]-fluorodeoxyglucose positron emission tomography. Circulation. 2002;105:2708–11. doi: 10.1161/01.cir.0000020548.60110.76. [DOI] [PubMed] [Google Scholar]
- 26.Tahara N, Imaizumi T, Virmani R, et al. Clinical feasibility of molecular imaging of plaque inflammation in atherosclerosis. J Nucl Med. 2009;50:331–4. doi: 10.2967/jnumed.108.060376. [DOI] [PubMed] [Google Scholar]
- 27.Tawakol A, Migrino RQ, Bashian GG, et al. In vivo F-18-fluorodeoxyglucose positron emission tomography imaging provides a noninvasive measure of carotid plaque inflammation in patients. J Am Coll Cardiol. 2006;48:1818–24. doi: 10.1016/j.jacc.2006.05.076. [DOI] [PubMed] [Google Scholar]
- 28.Ogawa M, Ishino S, Mukai T, et al. F-18-FDG accumulation in atherosclerotic plaques: immunohistochemical and PET imaging study. J Nucl Med. 2004;45:1245–50. [PubMed] [Google Scholar]
- 29.Tahara N, Kai H, Yamagishi S, et al. Vascular inflammation evaluated by [F-18]-fluorodeoxyglucose positron emission tomography is associated with the metabolic syndrome. J Am Coll Cardiol. 2007;49:1533–9. doi: 10.1016/j.jacc.2006.11.046. [DOI] [PubMed] [Google Scholar]
- 30.Silvera SS, el Aidi H, Rudd JHF, et al. Multimodality imaging of atherosclerotic plaque activity and composition using FDG-PET/CT and MRI in carotid and femoral arteries. Atherosclerosis. 2009;207:139–43. doi: 10.1016/j.atherosclerosis.2009.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Anderson RW, Stomberg C, Hahm CW, et al. Automated classification of atherosclerotic plaque from magnetic resonance images using predictive models. Biosystems. 2007;90:456–66. doi: 10.1016/j.biosystems.2006.11.005. [DOI] [PubMed] [Google Scholar]
- 32.Itskovich VV, Samber DD, Mani V, et al. Quantification of human atherosclerotic plaques using spatially enhanced cluster analysis of multicontrast-weighted magnetic resonance images. Magn Reson Med. 2004;52:515–23. doi: 10.1002/mrm.20154. [DOI] [PubMed] [Google Scholar]
- 33.Mani V, Aguiar SH, Itskovich VV, et al. Carotid black blood MRI burden of atherosclerotic disease assessment correlates with ultrasound intima-media thickness. J Cardiovasc Magn Reson. 2006;8:529–34. doi: 10.1080/10976640600675245. [DOI] [PubMed] [Google Scholar]
- 34.El Aidi H, Mani V, Weinshelbaum KB, et al. Cross-sectional, prospective study of MRI reproducibility in the assessment of plaque burden of the carotid arteries and aorta. Nat Clin Pract Cardiovasc Med. 2009;6:219–28. doi: 10.1038/ncpcardio1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mani V, Muntner P, Gidding SS, et al. Cardiovascular magnetic resonance parameters of atherosclerotic plaque burden improve discrimination of prior major adverse cardiovascular events. J Cardiovasc Magn Reson. 2009;11:10. doi: 10.1186/1532-429X-11-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tahara N, Kai H, Ishibashi M, et al. Simvastatin attenuates plaque inflammation—evaluation by fluorodeoxyglucose positron emission tomography. J Am Coll Cardiol. 2006;48:1825–31. doi: 10.1016/j.jacc.2006.03.069. [DOI] [PubMed] [Google Scholar]
- 37.Rudd JHF, Myers KS, Bansilal S, et al. Atherosclerosis inflammation imaging with F-18-FDG PET: carotid, iliac, and femoral uptake reproducibility, quantification methods, and recommendations. J Nucl Med. 2008;49:871–8. doi: 10.2967/jnumed.107.050294. [DOI] [PubMed] [Google Scholar]
- 38.Rudd JHF, Myers KS, Bansilal S, et al. 18Fluorodeoxyglucose positron emission tomography imaging of atherosclerotic plaque inflammation is highly reproducible—implications for atherosclerosis therapy trials. J Am Coll Cardiol. 2007;50:892–6. doi: 10.1016/j.jacc.2007.05.024. [DOI] [PubMed] [Google Scholar]
- 39.Mani V, Itskovich VV, Szimtenings M, et al. Rapid extended coverage simultaneous multisection black-blood vessel wall MR imaging. Radiology. 2004;232:281–8. doi: 10.1148/radiol.2321031022. [DOI] [PubMed] [Google Scholar]
- 40.Mani V, Itskovich VV, Aguiar SH, et al. Comparison of gated and nongated fast multislice black-blood carotid imaging using rapid extended coverage and inflow/outflow saturation techniques. J Magn Reson Imaging. 2005;22:628–33. doi: 10.1002/jmri.20428. [DOI] [PubMed] [Google Scholar]
- 41.Nicholls SJ, Tuzcu EM, Brennan DM, et al. Cholesteryl ester transfer protein inhibition, high-density lipoprotein raising, and progression of coronary atherosclerosis: insights from ILLUSTRATE (Investigation of Lipid Level Management Using Coronary Ultrasound to Assess Reduction of Atherosclerosis by CETP Inhibition and HDL Elevation) Circulation. 2008;118:2506–14. doi: 10.1161/CIRCULATIONAHA.108.790733. [DOI] [PubMed] [Google Scholar]
- 42.Barter PJ, Caulfield M, Eriksson M, et al. Effects of torcetrapib in patients at high risk for coronary events. N Engl J Med. 2007;357:2109–22. doi: 10.1056/NEJMoa0706628. [DOI] [PubMed] [Google Scholar]
- 43.Bots ML, Visseren FL, Evans GW, et al. Torcetrapib and carotid intima-media thickness in mixed dyslipidaemia (RADIANCE 2 study): a randomised, double-blind trial. Lancet. 2007;370:153–60. doi: 10.1016/S0140-6736(07)61088-5. [DOI] [PubMed] [Google Scholar]
- 44.Stein EA, Roth EM, Rhyne JM, et al. Safety and tolerability of dalcetrapib (RO4607381/JTT-705): results from a 48-week trial. Eur Heart J. 2010;31:480–8. doi: 10.1093/eurheartj/ehp601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rudd JHF, Myers KS, Bansilal S, et al. Relationships among regional arterial inflammation, calcification, risk factors, and biomarkers: a prospective fluorodeoxyglucose positron-emission tomography/computed tomography imaging study. Circ Cardiovasc Imaging. 2009;2:107–15. doi: 10.1161/CIRCIMAGING.108.811752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kim TN, Kim S, Yang SJ, et al. Vascular inflammation in patients with impaired glucose tolerance and type 2 diabetes: analysis with 18F-fluorodeoxyglucose positron emission tomography. Circ Cardiovasc Imaging. 2010;3:142–8. doi: 10.1161/CIRCIMAGING.109.888909. [DOI] [PubMed] [Google Scholar]
- 47.Pedersen SF, Graebe M, Fisker Hag AM, et al. Gene expression and 18FDG uptake in atherosclerotic carotid plaques. Nucl Med Commun. 2010;31:423–9. doi: 10.1097/MNM.0b013e32833767e0. [DOI] [PubMed] [Google Scholar]
- 48.Wu YW, Kao HL, Chen MF, et al. Characterization of plaques using 18F-FDG PET/CT in patients with carotid atherosclerosis and correlation with matrix metalloproteinase-1. J Nucl Med. 2007;48:227–33. [PubMed] [Google Scholar]
- 49.Dong L, Kerwin WS, Chen HJ, et al. Effect of intensive lipid therapy on atherosclerotic plaque inflammation: evaluation using dynamic contrast-enhanced magnetic resonance imaging (DCE-MRI) in carotid disease. Circulation. 2009;120:S342–3. [Google Scholar]
- 50.Kerwin WS, Oikawa M, Yuan C, et al. MR imaging of adventitial vasa vasorum in carotid atherosclerosis. Magn Reson Med. 2008;59:507–14. doi: 10.1002/mrm.21532. [DOI] [PubMed] [Google Scholar]
- 51.Herrington DM, Brown V, Mosca L, et al. Relationship between arterial stiffness and subclinical aortic atherosclerosis. Circulation. 2004;110:432–7. doi: 10.1161/01.CIR.0000136582.33493.CC. [DOI] [PubMed] [Google Scholar]
- 52.Metafratzi ZM, Efremidis SC, Skopelitou AS, et al. The clinical significance of aortic compliance and its assessment with magnetic resonance imaging. J Cardiovasc Magn Reson. 2002;4:481–91. doi: 10.1081/jcmr-120016386. [DOI] [PubMed] [Google Scholar]
- 53.Varghese A, Yee MS, Chan CF, et al. Effect of rosiglitazone on progression of atherosclerosis: insights using 3D carotid cardiovascular magnetic resonance. J Cardiovasc Magn Reson. 2009;11:24. doi: 10.1186/1532-429X-11-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kastelein JJP, Duivenvoorden R, Deanfield J, et al. Rationale and design of dal-VESSEL: a study to assess the safety and efficacy of dalcetrapib on endothelial function using brachial artery flow-mediated vasodilatation. Curr Med Res Opin. 2011;27:141–50. doi: 10.1185/03007995.2010.536207. [DOI] [PubMed] [Google Scholar]
- 55.Schwartz GG, Olsson AG, Ballantyne CM, et al. Rationale and design of the dal-OUTCOMES trial: efficacy and safety of dalcetrapib in patients with recent acute coronary syndrome. Am Heart J. 2009;158:896–901. doi: 10.1016/j.ahj.2009.09.017. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


