 A new modified paullone ligand bearing a TEMPO free-radical unit (HL2) and its copper(ii) complex have been prepared. The compounds demonstrate high cytotoxicity in vitro and strongly inhibit cell-free hR2 RNR activity.
A new modified paullone ligand bearing a TEMPO free-radical unit (HL2) and its copper(ii) complex have been prepared. The compounds demonstrate high cytotoxicity in vitro and strongly inhibit cell-free hR2 RNR activity.
Abstract
A new paullone–TEMPO conjugate and its copper(ii) complex inhibit RNR activity and show high antiproliferative activity in human cancer cell lines.
Platinum-based drugs are widely used in the clinic against cancer. They exert their effect mainly by DNA binding leading finally to apoptosis. This mode of action is highly unselective, and the clinical application of these drugs is accompanied by severe side effects.1 Therefore, the development of metal-based drugs with targets other than DNA is an extremely important task. Over the last ten years we have reported metal complexes, organoruthenium(ii) and organoosmium(ii) compounds with a series of modified paullone ligands as potential Cdk inhibitors and showed that they possess high antiproliferative activity in vitro.2 However, a correlation between the Cdk inhibitory activity and cytotoxicity has not been established. Quite recently we have prepared highly antiproliferative ruthenium(ii)- and osmium(ii)-arene-based paullones bearing a TEMPO free-radical unit.3 The antitumour activity of nitroxyl radicals is well-documented in the literature.4 They show cytotoxicity in tumour cell lines5,6 and activity in animal tumour models.6,7 Investigations of conjugates, in which nitroxyl radical-containing units are covalently bound to antitumour compounds have attracted growing attention of researchers.8 Nitroxyl radicals possess unique antioxidant properties due to their ability to interact with free radicals.4 On the other hand, 4-hydroxy-2,2,6,6-tetramethyl-piperidine-1-oxyl (Tempol) has been reported to promote oxidative stress in leukemia cells.5 This prompted us to investigate the anti-/pro-oxidative properties of paullone–TEMPO conjugates, in particular their interaction with the tyrosyl radical [Y˙] in the R2 subunit of human ribonucleotide reductase enzyme (hR2 RNR) as well as their influence on cellular levels of reactive oxygen species (ROS). We show that a new type of modified paullones bearing a tridentate binding site and a free-radical TEMPO unit and its copper(ii) complex are highly cytotoxic and are able to destroy the [Y˙] in hR2 RNR. The RNR enzyme catalyzes the reduction of four ribonucleotides to their corresponding deoxyribonucleotides, providing precursors required for both synthesis and repair of DNA.9
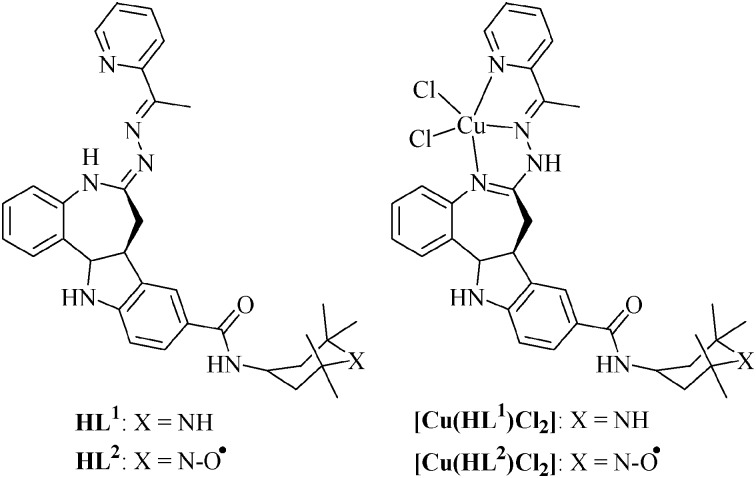
The synthesis of HL1 and HL2 was performed in three steps, starting from 9-carboxy-7,12-dihydroindolo[3,2-d][1]benzazepin-6(5H)-one, as shown in Scheme S1, ESI.† First the lactam group was thionated as reported previously,3 and then thiolactam A was allowed to react with 2-acetylpyridine hydrazone with formation of species B, containing a tridentate binding site to accommodate metal ions. 2,2,6,6-Tetramethylpiperidine or TEMPO radical units were attached onto the paullone backbone through a carboxylic group by formation of an amide bond in a DMF solution in the presence of triethylamine, 1-hydroxybenzotriazole and 1-ethyl-3-(3-dimethylaminopropyl)-carbodiimide hydrochloride (EDCI). Final purification of ligands HL1 and HL2 was performed by HPLC.
Using the reaction of HL1 and HL2 with CuCl2 in methanol or water–THF, copper(ii) complexes [Cu(HL1)Cl2]·3H2O·0.5MeOH (1) and [Cu(HL2)Cl2]·1.8H2O (2) were prepared in 85 and 87% yields, respectively (Fig. 1). Both the ligands and complexes (1 and 2) were characterized using elemental analysis, IR, EPR spectroscopy and ESI mass spectrometry. The presence of five-coordinate copper(ii) in 1 and 2 was assumed from previous X-ray diffraction analysis of related complexes with a bromo substituent in position 9 of the paullone backbone.2d Re-crystallization of 2 from THF is accompanied by deprotonation of the ligand and isolation of the complex [Cu(L2)Cl]·0.5THF, which has been studied by X-ray crystallography (vide infra).‡ The formation of ligands and complexes 1 and 2 is corroborated by ESI mass spectrometry. The peaks with m/z 645 and 660 were attributed to [M–Cl]+ ions, while those with m/z 609 and 624 are due to [M–Cl–HCl]+ ions. The presence of a TEMPO radical in HL2 and 2 was confirmed by EPR spectra of their 10–4 M solutions in methanol or in 1 : 1 v/v MeOH–DMF. A typical triplet as reported previously3 with a tumbling effect pattern was observed (Fig. S1, ESI†). Interaction between TEMPO radical (S = 1/2) and the paramagnetic copper(ii) ion (S = 1/2) has not been observed. Note that the intramolecular separation between these two paramagnetic centres is about 14.25 Å.
Fig. 1. Structures of ligands and their copper(ii) complexes.
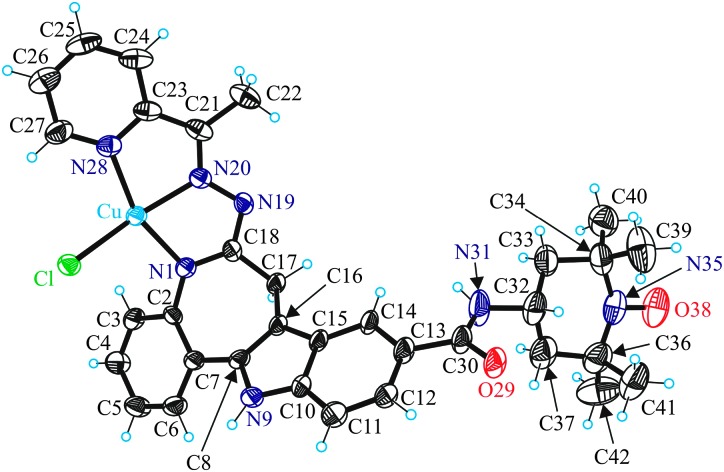
The copper(ii) ion in [Cu(L2)Cl] has a square-pyramidal coordination environment (τ = 0.04)10 with a tridentate monodeprotonated ligand (L2)– bound to copper(ii) via the azepine ring nitrogen atom N1, the hydrazine group nitrogen atom N20 and the pyridine nitrogen atom N28, as well as a chlorido ligand in the basal plane and an amide oxygen of a neighbouring metal complex in the apical position (Fig. 2 and Fig. S2, ESI†).
Fig. 2. ORTEP view of a molecule of [Cu(L2)Cl] with atom labeling, showing thermal ellipsoids at 50% probability level. Selected bond distances (Å) and bond angles (deg): Cu–N1 1.978(5), Cu–N20 1.951(4), Cu–N28 2.028(5), Cu–Cl 2.2529(15), Cu–O29i 2.271(4), N35–O38 1.285(7), N1–Cu–N20 79.19(19), N20–Cu–N28 79.64(19). Symmetry code i: x, y, z + 1.
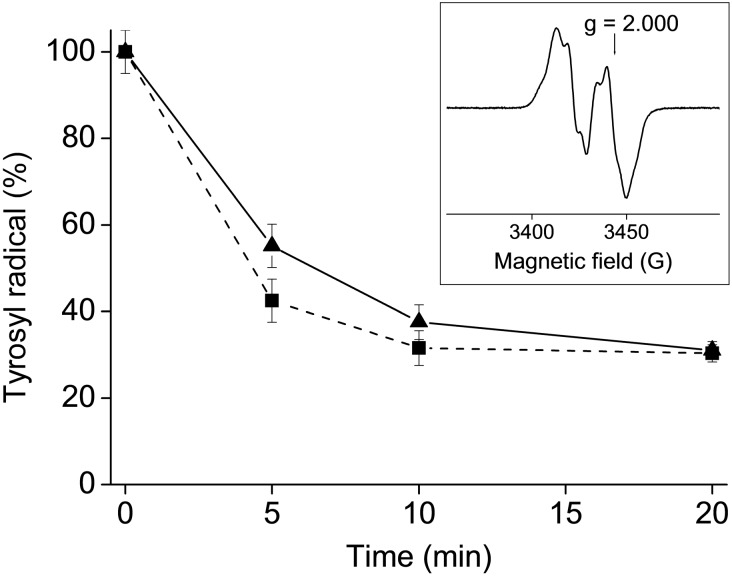
The sensitivity of the R2 specific [Y˙] in hRNR to HL2 and 2 was tested. A highly purified hR2 RNR protein (20 μM R2 monomer) in Tris buffer, pH 7.60/100 mM KCl/5% glycerol was incubated with 20 μM of the corresponding compound at 298 K. The samples were analysed by EPR spectroscopy at 20 K. The results obtained are shown in Fig. 3.
Fig. 3. Tyrosyl radical [Y˙] destruction in human R2 RNR protein by HL2 (triangles) and 2 (squares). Samples containing 20 μM human R2 protein and 20 μM compound (1% (w/w) DMSO–H2O) in Tris buffer, pH 7.60/100 mM KCl/5% glycerol, were incubated for indicated times and quickly frozen in cold isopentane. The natural decay of tyrosyl radical in the R2 protein was subtracted for each point. Inset: X-band EPR spectrum of the tyrosyl radical in human R2 RNR protein at 20 K. Experimental conditions: frequency 9.63 GHz, microwave power 3.2 mW, modulation amplitude 0.5 mT.
Both the ligand HL2 and copper(ii) complex 2 show marked hR2 RNR inhibitory activity destroying more than 60% of [Y˙] after 20 min incubation. Addition of 2 mM dithiothreitol (DTT) to hR2 and 2 leads to complete tyrosyl radical destruction after 30 s incubation, while in the case of HL2 the remaining radical content after 30 s is 12%.
All compounds show high antiproliferative activity in vitro with IC50 values in the nanomolar range (Table 1 and Fig. S3, ESI†). CH1 ovarian cancer cells are the most sensitive to all four compounds, whereas SW480 colon cancer cells or SK-Mel 28 melanoma cells are the least sensitive to compounds containing or lacking the radical unit, respectively. On average, the presence of a TEMPO radical instead of 2,2,6,6-tetramethylpiperidine results in increased cytotoxicity, but the actual effect depends very much on the cell line, varying from a maximum of 23 and 14 times increased potency of ligand and copper(ii) complex, respectively, in SK-Mel-28 melanoma cells to an even slightly reverse effect in SW480 colon cancer cells. Complexation with copper(ii) has little or no effect on the cytotoxicity in the presence or absence of the radical unit, respectively.
Table 1. Cytotoxicity of paullone ligands HL1 and HL2, and copper(ii) complexes 1 and 2 in six human tumour cell lines.
| IC50,
a
96 h |
||||
| HL1 | 1 | HL2 | 2 | |
| A549 | 0.22 ± 0.02 | 0.19 ± 0.04 | 0.063 ± 0.009 | 0.093 ± 0.009 |
| CH1 | 0.081 ± 0.002 | 0.056 ± 0.003 | 0.02 ± 0.003 | 0.037 ± 0.007 |
| SW480 | 0.17 ± 0.03 | 0.15 ± 0.02 | 0.27 ± 0.05 | 0.39 ± 0.05 |
| N87 | 0.22 ± 0.04 | 0.18 ± 0.02 | 0.027 ± 0.009 | 0.056 ± 0.013 |
| SK-Mel 28 | 0.70 ± 0.03 | 0.64 ± 0.07 | 0.031 ± 0.005 | 0.047 ± 0.011 |
| T47D | 0.13 ± 0.01 | 0.12 ± 0.02 | 0.13 ± 0.03 | 0.22 ± 0.05 |
a50% inhibitory concentrations (means ± standard deviations from at least three independent experiments), as obtained by the MTT assay using exposure times of 96 h.
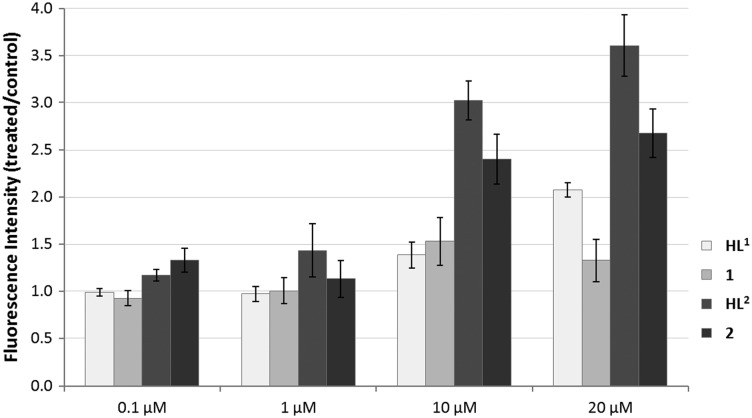
Generation of intracellular ROS by the compounds was determined by using the DCFH-DA assay in HL-60 leukemia cells. In general the compounds with a TEMPO radical moiety show a stronger induction of ROS than the compounds without the radical moiety (Fig. 4). Treatment with 20 μM of 2 or HL2 results in a 2.7-fold or 3.5-fold enhancement of ROS levels, respectively, whereas HL1 increases ROS levels by only two times and 1 shows negligible activity.
Fig. 4. Generation of intracellular ROS induced by treatment with compounds in the DCFH-DA assay. H2O2 (500 μM; 10 min incubation) was used as a positive control.
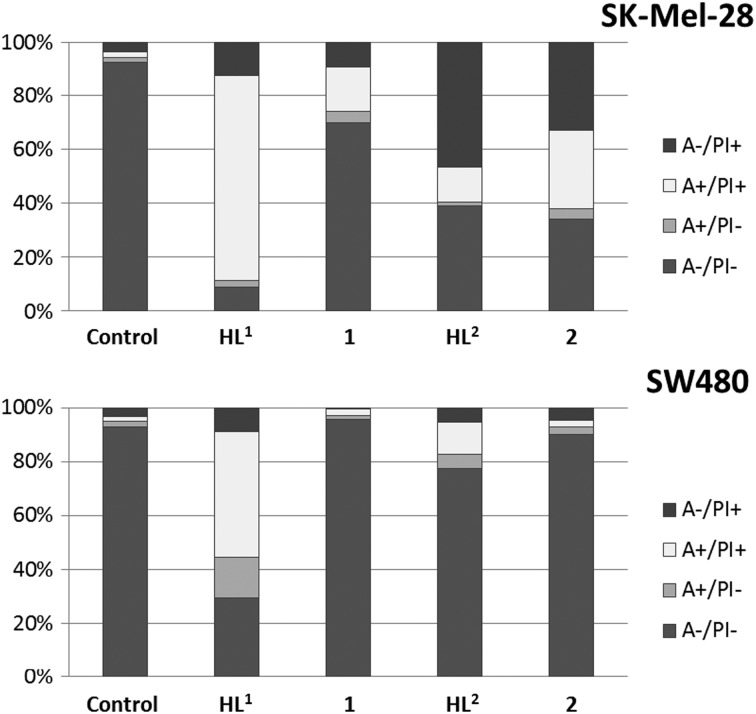
To determine apoptosis induction, SW480 and SK-Mel-28 cells were treated with different concentrations of the compounds for 24 h. Afterwards, the cells were stained with Annexin V-FITC and propidium iodide, and 5000 cells were measured by flow cytometry. HL1 at 20 μM concentration shows a remarkable induction of apoptosis of up to 61% in SW480 and 79% in SK-Mel-28 cells. In contrast, the corresponding copper(ii) complex shows no pronounced apoptosis induction in SW480, but up to 20% apoptosis in SK-Mel-28 cells. The ligand with a TEMPO-radical moiety (HL2) induces less apoptosis in both cell lines, whereas enhanced necrosis was observed in SK-Mel-28 cells. The corresponding complex 2 induces apoptosis and necrosis in SK-Mel-28, but shows no activity in SW480 cells (Fig. 5 and Fig. S4, ESI†). A comparison with results from the MTT assay suggests that especially the copper(ii) complexes exert merely antiproliferative effects in SW480 cells, virtually without killing cells.
Fig. 5. Induction of apoptosis (annexin-positive and double positive cells) and necrosis (PI-only positive cells) after treatment with 20 μM of compounds for 24 h in SW480 and SK-Mel-28 cells.
In conclusion, these compounds are extraordinarily cytotoxic in human cancer cell lines, with structural modifications (presence/absence of the radical moiety, complexation with copper(ii)) exerting comparatively moderate effects on cytotoxic potency. On average, the presence of the TEMPO radical is advantageous but not in all cell lines. Whereas cell-free experiments suggest inhibition of ribonucleotide reductase activity by the anti-oxidative properties of the radical unit as a possible mechanism of action, enhanced generation of reactive oxygen species was observed in leukemia cells. Whether this is a direct or an indirect effect remains unclear. Similar divergent findings regarding anti-/pro-oxidative properties have been reported for 4-hydroxy-2,2,6,6-tetramethylpiperidine-1-oxyl (Tempol) by different authors (compare ref. 4 and 5). Nevertheless, the major contribution to biological activity has to be attributed to the modified paullone structure of the compounds at the original lactam unit with creation of potential tridentate binding site.
We thank Alexander Roller for collection of X-ray diffraction data. Financial support from Austrian Science Fund (FWF), project number P20897, is acknowledged.
Footnotes
†Electronic supplementary information (ESI) available: Materials and methods, synthetic procedures, EPR spectra, details of crystal structure of [Cu(L2)Cl], concentration effect curves of the tested compounds in six human cancer cell lines, quantification of apoptosis induction in SK-Mel-28 and SW480 cells. CCDC 952588. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c3cc45743e
‡Crystal data for [Cu(L2)Cl]·0.5THF: C35H39ClCuN7O2.5 (M r = 696.72 g mol–1), monoclinic, a = 12.7891(7) Å, b = 28.6933(15) Å, c = 11.9318(6) Å, β = 104.934(3)°, V = 4230.6(4) Å3, T = 100(2) K, space group P21/c, Z = 4, 64 065 coll. refl., 7391 indep. refl. (R int = 0.1051), GoF = 1.031, R 1 = 0.0829, wR(F 2) = 0.2386.
References
- (a) Hartinger C. G., Metzler-Nolte N., Dyson P. J. Organometallics. 2012;31:5677. [Google Scholar]; (b) Casini A., Hartinger C. G., Nazarov A. A., Dyson P. J. Top. Organomet. Chem. 2010;32:57. [Google Scholar]; (c) Therrien B. Top. Curr. Chem. 2012;319:35. doi: 10.1007/128_2011_272. [DOI] [PubMed] [Google Scholar]; (d) Sava G., Jaouen G., Hillard E. A., Bergamo A. Dalton Trans. 2012;41:8226. doi: 10.1039/c2dt30075c. [DOI] [PubMed] [Google Scholar]; (e) Gianni G., Bergamo A., Dyson P. J. Dalton Trans. 2011;40:9069. doi: 10.1039/c1dt10522a. [DOI] [PubMed] [Google Scholar]; (f) Gasser G., Ott I., Metzler-Nolte N. J. Med. Chem. 2011;54:3. doi: 10.1021/jm100020w. [DOI] [PMC free article] [PubMed] [Google Scholar]; (g) Noffke A. L., Habtemariam A., Pizarro A. M., Sadler P. J. Chem. Commun. 2012;48:5219. doi: 10.1039/c2cc30678f. [DOI] [PubMed] [Google Scholar]; (h) Bruijnincx P. C. A., Sadler P. J. Curr. Opin. Chem. Biol. 2008;12:197. doi: 10.1016/j.cbpa.2007.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (a) Dobrov A., Arion V. B., Kandler N., Ginzinger W., Jakupec M. A., Rufinska A., Graf von Keyserlingk N., Galanski M., Kowol C., Keppler B. K. Inorg. Chem. 2006;45:1945. doi: 10.1021/ic0511120. [DOI] [PubMed] [Google Scholar]; (b) Schmid W. F., John R. O., Mühlgassner G., Heffeter P., Jakupec M. A., Galanski M., Berger W., Arion V. B., Keppler B. K. J. Med. Chem. 2007;50:6343. doi: 10.1021/jm701042w. [DOI] [PubMed] [Google Scholar]; (c) Schmid W. F., Zorbas-Seifried S., John R. O., Arion V. B., Jakupec M. A., Roller A., Galanski M., Chiorescu I., Zorbas H., Keppler B. K. Inorg. Chem. 2007;46:3645. doi: 10.1021/ic070098j. [DOI] [PubMed] [Google Scholar]; (d) Primik M. F., Mühlgassner G., Jakupec M. A., Zava O., Dyson P. J., Arion V. B., Keppler B. K. Inorg. Chem. 2010;49:302. doi: 10.1021/ic902042a. [DOI] [PubMed] [Google Scholar]; (e) Filak L. K., Mühlgassner G., Jakupec M. A., Heffeter P., Berger W., Arion V. B., Keppler B. K. JBIC, J. Biol. Inorg. Chem. 2010;15:903. doi: 10.1007/s00775-010-0653-y. [DOI] [PMC free article] [PubMed] [Google Scholar]; (f) Mühlgassner G., Bartel C., Schmid W. F., Jakupec M. A., Arion V. B., Keppler B. K. J. Inorg. Biochem. 2012;116:180. doi: 10.1016/j.jinorgbio.2012.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arion V. B., Dobrov A., Göschl S., Jakupec M. A., Keppler B. K., Rapta P. Chem. Commun. 2012;48:8559. doi: 10.1039/c2cc33786j. [DOI] [PubMed] [Google Scholar]
- Soule B. P., Hyodo F., Matsumoto K., Simone N. L., Cook J. A., Krishna M. C., Mitchell J. B. Free Radicals Biol. Med. 2007;42:1632. doi: 10.1016/j.freeradbiomed.2007.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gariboldi M. B., Rimoldi V., Supino R., Favini E., Monti E. Free Radicals Biol. Med. 2000;29:633. doi: 10.1016/s0891-5849(00)00347-6. [DOI] [PubMed] [Google Scholar]
- Suy S., Mitchell J. B., Samuni A., Mueller S., Kasid U. Cancer. 2005;103:1302. doi: 10.1002/cncr.20898. [DOI] [PubMed] [Google Scholar]
- Konovalova N. P., Bogdanov G. N., Miller V. B., Rozantsev E. G., Neiman M. B., Emanuel N. M. Dokl. Akad. Nauk SSSR. 1964;157:707. [PubMed] [Google Scholar]
- Sen' V. D., Terent'ev A. A., Konovalova N. P. Russ. Chem. Bull., Int. Ed. 2011;60:7. [Google Scholar]
- Reichard P. Science. 1993;260:1773–1777. doi: 10.1126/science.8511586. [DOI] [PubMed] [Google Scholar]
- Addison A. W., Rao T. N., Reedijk J., Van Rijn J., Verschoor C. G. J. Chem. Soc., Dalton Trans. 1984:1349. [Google Scholar]