Abstract
The objective of the current study was to determine the classification accuracy of serum S100B and apolipoprotein (apoA-I) for mild traumatic brain injury (mTBI) and abnormal initial head computed tomography (CT) scan, and to identify ethnic, racial, age, and sex variation in classification accuracy. We performed a prospective, multi-centered study of 787 patients with mTBI who presented to the emergency department within 6 h of injury and 467 controls who presented to the outpatient laboratory for routine blood work. Serum was analyzed for S100B and apoA-I. The outcomes were disease status (mTBI or control) and initial head CT scan. At cutoff values defined by 90% of controls, the specificity for mTBI using S100B (0.899 [95% confidence interval (CI): 0.78–0.92]) was similar to that using apoA-I (0.902 [0.87–0.93]), and the sensitivity using S100B (0.252 [0.22–0.28]) was similar to that using apoA-I (0.249 [0.22–0.28]). The area under the receiver operating characteristic curve (AUC) for the combination of S100B and apoA-I (0.738, 95% CI: 0.71, 0.77), however, was significantly higher than the AUC for S100B alone (0.709, 95% CI: 0.68, 0.74, p=0.001) and higher than the AUC for apoA-I alone (0.645, 95% CI: 0.61, 0.68, p<0.0001). The AUC for prediction of abnormal initial head CT scan using S100B was 0.694 (95%CI: 0.62, 0.77) and not significant for apoA-I. At a S100B cutoff of <0.060 μg/L, the sensitivity for abnormal head CT was 98%, and 22.9% of CT scans could have been avoided. There was significant age and race-related variation in the accuracy of S100B for the diagnosis of mTBI. The combined use of serum S100B and apoA-I maximizes classification accuracy for mTBI, but only S100B is needed to classify abnormal head CT scan. Because of significant subgroup variation in classification accuracy, age and race need to be considered when using S100B to classify subjects for mTBI.
Key words: biomarkers, CT scanning, head trauma, human studies, traumatic brain injury
Introduction
More than 1.7 million Americans incur a traumatic brain injury (TBI) annually.1 Most are classified as mild, a potentially misleading term. Mild TBI (mTBI) is associated with significant morbidity, including long-term physical and behavioral symptoms, cognitive dysfunction, and an increased risk for development of neurodegenerative disease.2
The mTBI diagnosis is based on witnessed or self-reported symptoms at the time of injury, including a brief loss of consciousness, brief period of amnesia, or transiently altered mental state.3,4 Unfortunately, self-report of symptoms may be unobtainable (e.g., pre-verbal children, demented elders, polytrauma patients) or unreliable (e.g., intoxicated patients).5,6 Further, some patients may intentionally misreport injury events to avoid the immediate consequences of a mTBI diagnosis, such as being removed from a sporting contest or combat unit.7 The subjective nature of this diagnosis complicates efforts to identify patients at risk for adverse sequelae and to develop effective treatments. A practical and objective diagnostic is clearly needed.
Brain-related proteins that enter the peripheral circulation after mTBI can act as objective diagnostic aids. Serum S100B, an astrocyte-related protein, is already used as a pre-head CT screen in 17 European and Asian countries [personal written communication, Prof. Dr. Peter Biberthaler]. Its diagnostic ability for mTBI is unknown; however, it has excellent sensitivity for abnormal head CT scan when measured within 3 h of injury.8–10 S100B's poor specificity may result from its presence in adipocytes and melanocytes (resulting in higher baseline levels among non-Caucasians), its release from extracranial injuries such as fractures, and its inability to cross an intact blood–brain barrier (BBB).11–13 An ideal diagnostic marker would not depend on BBB permeability to accurately diagnose mTBI. Apolipoprotein A1 (apoA-I) does not have these limitations and has potential to serve as a diagnostic aid.
ApoA-I is a 28 kDa apolipoprotein primarily synthesized in the liver and small intestine.14 It is the major protein component of high-density lipoprotein particles and plays an important role in reverse cholesterol transport. ApoA-I is a negative marker of inflammation, decreasing more than 25% during sepsis and burns.15 ApoA-I is thought to transport lipids and interact with the BBB in a fashion analogous to apoE in the brain.16 We recently identified apoA-I as a potential mTBI biomarker through a proteomic screen of sera collected from mTBI subjects.17 As a peripheral protein, apoA-I has the potential to counter S100B's weakness in specificity. The combined use of apoA-I and S100B thus may be more accurate for disease classification and prognostication than either marker individually.
This study's primary objective was to determine the classification accuracy of serum S100B and apoA-I, individually and in combination, for mTBI and abnormal initial head CT scan. The secondary objective was to describe age, sex, racial, and ethnic variation in classification accuracy.
Methods
We performed a prospective study of serum S100B and apoA-I after mTBI at six emergency departments (EDs) between 2008 and 2010. These sites represented two pre-existing research groups: the Academic Health Center Consortium and the Emergency Research Network of the Empire State (University of Rochester Medical Center, Erie County Medical Center, State University of New York Upstate Medical Center, Albany Medical Center, Guthrie Health and Medical Center, and Bassett Hospital). The University of Rochester Medical Center served as the coordinating center for all study activities. The Institutional Review Boards for each center approved this study and the process of informed consent.
Inclusion and exclusion criteria
Patients ≥1 year of age were eligible for inclusion as mTBI subjects if they met the study definition of mTBI, arrived at the ED, and were able to have blood drawn within 6 h of injury, and underwent head CT scanning as part of their clinical care. The mTBI study definition was adapted from the Centers for Disease Control and Prevention's definition3 and consists of a blow to the head or rapid acceleration/deceleration resulting in at least one of the following: a loss of consciousness (LOC) ≤30 minutes, post-traumatic amnesia ≤24 h, neuropsychological abnormality (any transient period of confusion, disorientation, or impaired consciousness; in children ≤2 years old: irritability, lethargy, or vomiting post-injury), or neurological abnormality (seizure acutely after injury, hemiplegia, or diplopia). To meet the mTBI definition, the subject must have a Glasgow Coma Scale (GCS) score of 13 or greater within 30 min of the injury.
Subjects participated after providing written informed consent (>17 years), written informed assent with written parental/guardian permission (13–17 years), verbal assent with written parental/guardian permission (7–12 years), or written parental/guardian permission (1–6 years).
Participating subjects were interviewed in the ED for information regarding their injury, demographics, and medical history. Subjects were asked by interviewers to classify their ethnicity as Hispanic/Latino or not Hispanic/Latino, and to classify their race as African-American, Caucasian, Native American/Alaskan Native, Asian, or Native Hawaiian/Pacific Islander. The emergency provider was interviewed, and the medical record was reviewed to determine associated injuries and GCS score. Providers and interviewers were blinded to S100B and apoA-I results.
Patients presenting to the University of Rochester Medical Center for routine blood work were eligible to participate as control subjects if they were ≥1 year of age. They were ineligible if they had a history of brain tumor, melanoma, or Alzheimer disease; a history of concussion,18,19 bone fracture, or stroke within the previous month; or underwent surgery within the previous month. Interviewers were blinded to S100B and apoA-I results.
Blood draw and sample handling
Blood for S100B and apoA-I was drawn from participating control subjects and mTBI subjects within 6 h of the time of injury. Four milliliters of whole blood was drawn into a serum separator tube and immediately placed on ice. Within 60 min, the blood was centrifuged at 3000 rpms for 10 min; the serum was aliquoted into 500 μL tubes frozen at –80°C.
S100B and apoA-I assays
Serum S100B concentrations were determined by a fully automatic electrochemoluminometric immunoassay (Elecsys S100®; Roche Diagnostics, Penzberg, Germany) with a detection limit of 0.005–39 μg/L. The analyte was sandwiched between two monoclonal antibodies directed against the beta-chain of the S100 dimer. Then, strepavidin-coated microparticles were added and the immunocomplex binds to the solid phase. In the measurement cell, unbound components were removed and a defined voltage used to initiate the electrochemiluminescent reaction. The resultant light emission was then measured using a photomultiplier. S100B assays were performed between November and December 2010.
Serum apoA-I concentrations were measured in duplicate by enzyme-linked immunosorbent assay (ELISA) (Mabtech; Cincinnati, OH). This assay uses ELISA strip plates pre-coated with a capture monoclonal antibody, to which serum samples are added. Captured apoA-I was detected by adding a biotinylated monoclonal antibody followed by streptavidin-horseradish peroxidase. The addition of the enzyme substrate TMB resulted in a colored substrate product with an intensity that was directly proportional to the concentration of apoA-I in the sample. The concentration of the ApoA-I in the sample was determined by comparison with a serial dilution of purified apoA-I standard analyzed in parallel.
Normal and potentially impaired patients were tested together and randomly intermixed across plates. Calibrators were run with every assay plate, and all calibrators were run in duplicate. The apoA-I value range of the calibration curve was 0.1 ng/mL–100 ng/mL. A four parameter curve was used to generate the standard curve from the calibrator data using software in the plate reader. R2 values ranged from 0.994–1.000 for all assay runs. Result standard deviations between duplicates were routinely calculated for all samples by the reader software. ApoA-I assays were performed between June and November 2011.
Head CT scans
At each study site, head CT scans were interpreted by board-certified radiologists who were blinded to the laboratory results. The final reading entered into the radiology image database at each institution was used to determine the presence or absence of intracranial abnormalities. Traumatic CT abnormalities were defined as subdural hematomas (SDH), epidural hematomas, subarachnoid hemorrhage (SAH), edema, skull fracture, and cerebral contusions.
Analysis
mTBI subjects and controls were compared using chi-square, Fisher exact test, and Student t test. A Mann-Whitney test was used to compare S100B because of its highly skewed distribution. Receiver operating characteristic (ROC) analyses were performed to evaluate classification accuracy. When considering each marker individually, standard ROC procedures were followed.20 For the combination of the two markers, however, a sequential strategy was used. See online supplementary material 1 at ftp.liebertpub.com.
To determine which marker was more accurate for mTBI classification, AUCs as well as the sensitivity (proportion of subjects with mTBI correctly classified as injured, i.e., true positives) and specificity (proportion of controls correctly classified as uninjured, i.e., true negatives) at several relevant cutoffs were compared. These cutoffs included the value (1) defined by 90% of control subjects, (2) reported in previous studies, (3) maximizing sensitivity (≥98%), and (4) maximizing specificity (≥98%), (5) corresponding to 90% sensitivity, and (6) corresponding to 90% specificity. The 90% threshold was considered because it is the minimum level of certainty at which clinicians feel comfortable ruling in or ruling out a disease.21 Identical procedures were used to determine which marker was more accurate for classifying mTBI subjects with an abnormal head CT. AUCs were compared using the methods described by DeLong and associates22; sensitivities and specificities were compared using chi-square. AUCs within each subgroup category were compared using the method described by Hanley and colleagues.23,24 For subgroups with significant differences, the mean (apoA-I) or median (S100B) marker level in controls was compared, as was the relative difference in marker levels between controls and mTBI. Relative difference calculation is described in online supplementary material 2, at ftp.liebertpub.com.
Subjects with missing data points were included if variables related to inclusion and exclusion criteria were present. A p value of≤0.05 was considered statistically significant. For sample size determination, see online supplement 3, at ftp.liebertpub.com.
Results
We enrolled 787 subjects with mTBI and 467 control subjects (Table 1). See Supplementary Figure 1 at ftp.liebertpub.com. The distributions of S100B and apoA-I levels in subjects with mTBI and controls subjects are shown in Figure 1. Compared with controls, median S100B levels were significantly higher in subjects with mTBI subjects (0.149 μg/L vs. 0.071 g/L, p<0.0001), while mean apoA-I levels were significantly lower (0.834±0.25 mg/mL vs. 0.950±0.26 mg/mL, p<0.0001).
Table 1.
Subject Characteristics
| Characteristic | mTBI (787) | Controls (467) |
|---|---|---|
| Age, mean (SD) | 38.2 (19.5) | 39.2 (20.2) |
| Female, n (%)a | 287 (36.5) | 309 (66.2) |
| Race | ||
| African American, n (%)a | 115 (14.6) | 122 (26.1) |
| Asian, n (%) | 7 (0.9) | 8 (1.7) |
| Caucasian, n (%)a | 642 (81.6) | 322 (69.0) |
| Native American/Alaska Native, n (%) | 4 (0.5) | 4 (0.86) |
| Native Hawaiian/Pacific Islander, n (%) | 1 (0.1) | 0 |
| Refused/missing, n (%) | 18 (2.2) | 11 (2.4) |
| Ethnicity | ||
| Hispanic/Latino, n (%)a | 35 (4.4) | 44 (9.4) |
| Not Hispanic/Latino, n (%) | 726 (92.2) | 422 (90.4) |
| Refused/missing, n (%)a | 26 (3.3) | 1 (0.2) |
| Mechanism of injury | ||
| Assault, n (%) | 43 (5.1) | |
| Bicycling, n (%) | 37 (4.7) | |
| Fall <3 feet/5 stairs, n (%) | 85 (10.8) | |
| Fall >3 feet/5 stairs, n (%) | 133 (16.9) | |
| Motorcycle crash, n (%) | 44 (5.6) | |
| Motor vehicle crash, ejection, n (%) | 17 (2.2) | |
| Motor vehicle crash, no ejection, n (%) | 261 (33.2) | |
| Pedestrian struck, n (%) | 35 (4.4) | |
| Sport, n (%) | 47 (6.0) | |
| Other, n (%) | 79 (10.0) | |
| Missing, n (%) | 7 (1.3) | |
| GCS on arrival | ||
| 15, n (%) | 705 (89.2) | |
| 14, n (%) | 51 (6.5) | |
| 13, n (%) | 10 (1.3) | |
| “13–15”, n (%) | 21 (2.5) | |
| Initial Head CT Scan | ||
| Normal, n (%) | 737 (93.6) | |
| Abnormal, n (%)b | 45 (5.7) | |
| Missing, n (%) | 5 (0.64) | |
| Extracranial Injuries | ||
| None, n (%) | 412 (52.4) | |
| Fracture, n (%) | 176 (22.4) | |
| Laceration requiring stitches, n (%) | 96 (12.2) | |
| Internal organ injury, n (%) | 15(1.9) | |
| Spinal cord injury, n (%) | 15 (1.9) | |
| Blast/burn/electrocution | 3 (0.38) | |
| Don't know/missing, n (%) | 70 (8.9) | |
| Disposition | ||
| Discharged from ED, n (%) | 541 (68.7) | |
| Discharged after 23 hours observation, n (%) | 13 (1.7) | |
| Discharged from inpatient unit, n (%) | 223 (28.3) | |
| Left ED before evaluation complete, n (%) | 2 (0.25) | |
| Died in ED, n (%) | 0 | |
| Died in inpatient unit, n (%) | 1 (0.13) | |
| Missing, n (%) | 7 (0.89) | |
Proportions in mTBI and controls is significantly different (p<0.05).
Cerebral contusion (n=5), subarachnoid hemorrhage (8), subdural hematoma (8), linear skull fracture (5), epidural hematoma (3), intraventricular hemorrhage (3), pneumocephalus (2), edema (1), and depressed skull fracture (1). Some subjects had more than one abnormality; none needed neurosurgical intervention.
GCS, Glasgow Coma Scale; ED, emergency department.
FIG. 1.
S100B and apoA-I levels in subjects with mild traumatic brain injury (TBI) and control subjects. Distribution of S100B (light grey bars) and apoA-I (dark grey bars) concentrations among controls (top) and subjects with mild TBI (bottom). Ninety percent of controls (vertical dashed lines) had a S100B concentration<0.290 μg/L, while 90% had an apoA-I concentration >0.675 mg/dL. Mean/median S100B concentrations were 0.141/0.071 μg/L among controls and 0.286/0.149 μg/L among subjects with mild TBI. Mean/median apoA-1 concentrations were 0.950/0.920 mg/mL among controls and 0.834/0.804 mg/mL among subjects with mild TBI.
Classification accuracy for mTBI
The ability of S100B and apoA-I to separate subjects with mTBI from control subjects— that is, to diagnose mTBI—is represented by the ROC curves in Figure 2 (left panel). The AUC for S100B (0.709 [95% CI: 0.68, 0.74]) was significantly higher than the AUC for apoA-I (0.645 [95% CI: 0.61, 0.68]) (p=0.004). Because S100B had a higher AUC than apoA-I, it was considered first in the combined analysis. The upper and lower S100B cutoffs maximizing the overall AUC of both markers were identified as 0.828 μg/L and 0.086 μg/L, respectively. The AUC for the combined use of S100B and apoA-I (0.738 [95%CI: 0.71, 0.77]) was significantly higher than the AUC for S100B alone (p=0.001) and higher than the AUC for apoA-I alone (p<0.0001).
FIG. 2.
Receiver operating characteristics of S100B and apoA-I for mild traumatic brain injury (TBI) and abnormal initial head CT scan. Left: The area under the curve (AUC) for mild TBI classification among all subjects (n=1254) was 0.709 [95% CI: 0.68, 0.74] for S100B (solid line), 0.645 [95% CI: 0.61, 0.68] for apoA-1 (dashed line), and 0.738 [95%CI: 0.71, 0.77] for both together (dotted line). Right: The AUC for classification of abnormal initial head CT scan among subjects with mild TBI (n=787) was 0.694 [95%CI: 0.62, 0.77] for S100B (solid line), 0.502 [95% CI: 0.41, 0.59] for apoA-1 (dashed line), and 0.712 [95%CI: 0.64, 0.78] for both (dotted line).
The sensitivity and specificity of the markers alone and combined at several relevant cutoffs are shown in Table 2. At control-defined cutoffs, both S100B and apoA-I had similar sensitivity and specificity. However, at cutoffs defining a high level of sensitivity (90% and ≥98%), however, S100B and the combination of S100B and apoA-I had higher specificity than apoA-I alone, while at cutoffs defining a high specificity (90% and ≥98%), the combination of S100B and apoA-I had the highest sensitivity.
Table 2.
Sensitivity and Specificity of S100B and apoA-I at Relevant Cutoffs
| Cutoffa | Rationale | Sensitivity (95% CI) | Specificity (95% CI) | |
|---|---|---|---|---|
| mTBI vs. control | ||||
| S100B | >0.290 μg/L | Control-defined | 0.252 (.22–.28) | 0.899 (.78–.92) |
| >0.028 μg/L | ≥98% sensitivity | 0.991 (.98–.996) | 0.028 (.01–.05) | |
| >1.355 μg/L | ≥98% specificity | 0.037 (.025–.053) | 0.991 (.98–.997) | |
| >0.054 μg/L | 90% sensitivity | 0.898 (.87–.92) | 0.336 (.29–.38) | |
| >0.294 μg/L | 90% specificity | 0.249 (.22–.28) | 0.901 (.87–.93) | |
| ApoA-I | <0.675 mg/mL | Control-defined | 0.249 (.22–.28) | 0.902 (.87–.93) |
| <1.597 mg/mL | ≥98% sensitivity | 0.991 (.98–.997) | 0.016 (.007–.03) | |
| <0.372 mg/mL | ≥98% specificity | 0.017 (.009–.031) | 0.991 (.98–.998) | |
| <1.108 mg/mL | 90% sensitivity | 0.913 (.89–.93) | 0.167 (.13–.21) | |
| <0.677 mg/mL | 90% specificity | 0.253 (.22–.29) | 0.898 (.87–.92) | |
| Both | S100B >0.028 μg/L | ≥98% sensitivity | 0.991 (.98–.996) | 0.028 (.01–.05) |
| S100B ≥0.828 μg/L or S100B 0.086–0.828 μg/L and ApoA-I <0.470 mg/mL | ≥98% specificity | 0.102 (.08–.12) | 0.981(.97–.99) | |
| S100B >0.054 μg/L | 90% sensitivity | 0.898 (.87–.92) | 0.336 (.29–.38) | |
| S100B >0.828 or S100B 0.086–0.828 μg/L and ApoA-I <0.797 mg/mL | 90% specificity | 0.390 (.36–.42) | 0.901 (.87–.93) | |
| CT+ vs. CT- | ||||
| S100B | >0.290 μg/L | Control-defined | 0.511 (.35–.66) | 0.763 (.73–.79) |
| >0.060 μg/L | ≥98% sensitivity | 1.00 (.92–1.0) | 0.123 (.10–.15) | |
| >2.391 μg/L | ≥98% specificity | 0.044 (.005–.15) | 0.991 (.98–.996) | |
| >0.097 μg/L | 90% sensitivity | 0.889 (.76–.96) | 0.317 (.28–.35) | |
| >0.521 μg/L | 90% specificity | 0.244 (.13–40) | 0.902 (.88–.92) | |
| >0.10 μg/L | From previous studyb | 0.867 (.73–.95) | 0.358 32–.39) | |
Cutoffs defining mTBI or abnormal initial head CT scan.
From previous, mostly European studies.9,10,23–25,27,28
CI, confidence interval; mTBI, mild traumatic brain injury.
Using 90% sensitivity and 90% specificity to rule out and rule in mTBI, respectively, the proportion of subjects correctly classified as either having or not having mTBI using S100B was 38.1%, using apoA-I was 30.4%, and using both was 45.2%. The proportion of subjects correctly classified using both markers together was significantly higher than the proportion classified using S100B alone (p<0.0001) and apoA1 alone (p<0.0001).
Classification accuracy for abnormal initial head CT scan
Subjects wit mTBI with an abnormal initial head CT had significantly higher median S100B levels compared with subjects with mTBI with a normal head CT (0.292 μg/L vs. 0.144 μg/L, p<0.0001). There was no significant difference in mean apoA-I levels between subjects with mTBI with (0.82±0.25 mg/mL) and without (0.83±0.25 mg/mL) head CT abnormalities (p=0.92). The ability of S100B and apoA-I to separate subjects with mTBI with intracranial injury on head CT tfrom sibjects with mTBI with normal scans—that is, to predict abnormal initial head CT—is represented by the ROC curves in Figure 2 (right panel). The AUC for prediction of abnormal initial head CT was significantly higher for S100B (0.694 [95%CI: 0.62, 0.77]) than for apoA-I (0.502 [95% CI: 0.41, 0.59]) (p=0.001) but no different than S100B and apoA-I combined (0.71 [95%CI: 0.64, 0.78]) (p=0.49). Because the 95% CI for apoA-I AUC included 0.50, and the addition of apoA-I to S100B did not significantly increase the AUC of S100B alone, apoA-I was not analyzed further.
The sensitivity and specificity of S100B at several relevant cutoffs are shown in Table 2. Using the 90% sensitivity cutoff (0.097 μg/L), 30.5% of head CT scans could have been avoided, but six subjects with mTBI with abnormal head CT scans would have been missed. Their CT abnormalities included contusion, intraventricular hemorrhage, SDH, SAH, and edema. (None needed neurosurgical intervention). Two of these six subjects had blood drawn to injury times greater than 3 h (3.4 and 4.9 h). Limiting the analysis to those with S100B measured within 3 h of injury did not improve the ability of S100B to predict head CT scan (AUC 0.60 [95%CI: 0.46, 0.74]). If the more conservative ≥98% sensitivity level (<0.060 μg/L) is used to decide who should get a head CT, 22.9% of CT scans could have been avoided, and only one subject with abnormal head CT would have been missed (small cerebral contusion, 0.06 μg/L).
Using the 90% sensitivity and 90% specificity level, the proportion of subjects with mTBI correctly classified as either having or not having an abnormal initial head CT scan was 40.8%.
Subgroup variation in classification accuracy
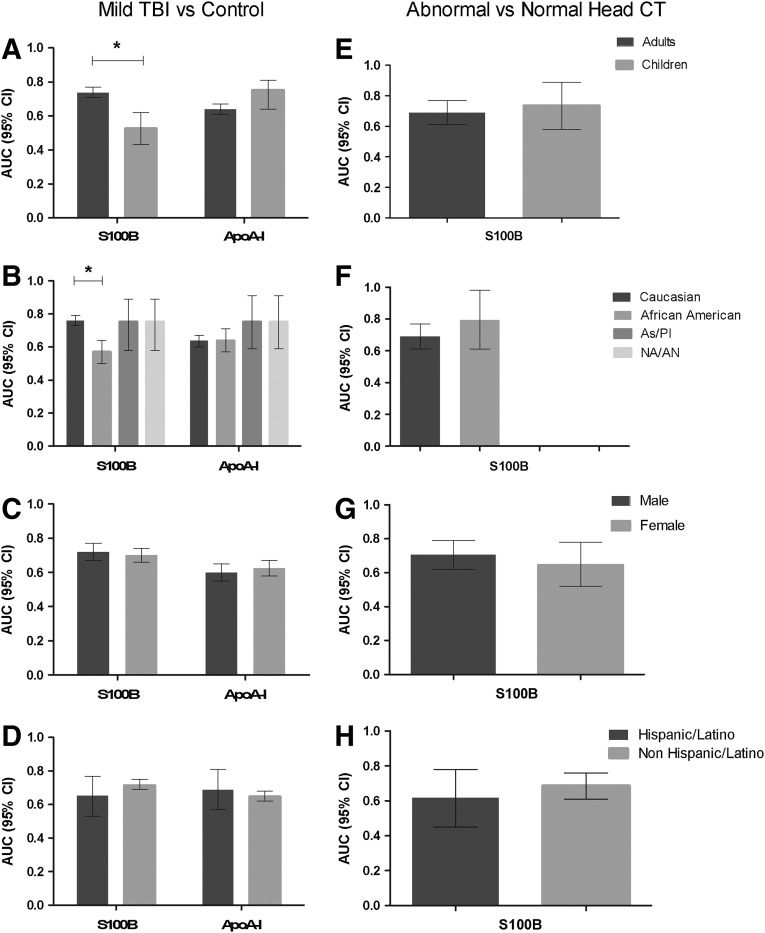
Variation in AUCs for mTBI classification among subject subgroups is shown in Figure 3 (panels A–D). The AUC for S100B was significantly higher in adults compared with children (p<0.0001). Although children controls had higher median S100B values than adult controls (0.128 μg/L vs. 0.065 μg/L), the relative increase in median S100B levels among subjects with mTBI compared with controls was much higher for adults (129% vs. 16%). The AUC for S100B was also significantly higher in Caucasians compared with African Americans (p<0.0001). While African American controls had higher median S100B values than Caucasian controls (0.118 μg/L vs. 0.065 μg/L), the relative increase in median S100B levels among subjects with mTBI compared with controls was much higher for Caucasians (138% vs. 13%). Variation in AUC for abnormal head CT classification using S100B is shown in Figure 3 (panels E–H). There was no significant difference between AUCs in any subgroup. Marker cutoff values defining 90% sensitivity and specificity for mild TBI classification and abnormal head CT among subgroups are shown in Supplementary Tables 1 and 2, respectively, at ftp.liebertpub.com.
FIG. 3.
Subgroup variation in classification accuracy of S100B and apoA-I. Classification accuracy of S100B and apoA-I for mild traumatic brain injury (TBI) (A–D) and of S100B for abnormal head CT (E–H) among age, race, sex, and ethnic subgroups. (*p<0.0001) Area under the curves (AUCs) could not be calculated in two race subgroups (F) because of lack of subjects with abnormal head CT. CI, confidence interval; As/PI, Asian, Pacific Islander; NA/AN, Native American/Alaskan Native.
Discussion
This study is notable for several “firsts.” It is the first large, multicentered brain marker study in North America, the first brain marker study to combine the clinical utility of two markers, the first to determine marker accuracy in ethnic and racial subgroups, and the first to use the peripheral protein, apoA-I.
There are three major findings in the current study. The first is the observation that combining S100B with apoA-I increased classification accuracy for mTBI over either marker alone. Using AUC, the classification accuracy of S100B was higher than apoA-I, but accuracy was highest using the combination of the two markers (although both would still be considered in the fair range, 0.7–0.8). The same was true using the proportion of subjects correctly classified as a measure of classification accuracy. The addition of apoA-I to S100B appeared to reduce S100B's false negative rate. At controlled-defined cutoffs, both S100B and apoA-I had low sensitivity and high specificity for mTBI classification. The combination of apoA-I and S100B increased the sensitivity at cutoffs defining high levels of specificity. For example, at a fixed specificity of 90%, the sensitivity for mTBI using either marker alone was 24.9–25.3%, but using the combination, the sensitivity rose to 39%. When the specificity was fixed even higher at ≥98%, the sensitivity increased from 1.7–3.7% using the markers individually to 10.2% using them together.
The improvement in true positive rate by adding apoA-I to S100B may be because it is not affected by extracranial injuries. ApoA-1 levels were not significantly different among those with lacerations, fractures, or internal organ injuries, compared with those with isolated mTBI. Median S100B levels, on the other hand, were significantly higher among those with bone fractures (0.265ug/L vs. 0.116 μg/L, p=0.001), a finding that has been reported by others.12 Also, as a peripheral protein, apoA-I concentration changes after mTBI likely do not depend on changes in BBB permeability.
The second important finding in our study was that serum S100B was best at predicting abnormal initial head CT scan after mTBI. Neither apoA-I alone nor in combination with S100B improved S100B's ability to predict CT findings. Using the same single, control-defined cutoff to diagnose mTBI (0.290 μg/L), S100B had a low sensitivity (51%) and specificity (76%) for abnormal initial head CT scan. Previous studies identified maximum sensitivity for abnormal head CT at an S100B level of 0.10–0.12 μg/L.9,10,25–29 In our cohort, the 98% sensitivity level was not reached until the S100B level was below 0.060 μg/L. At a cutoff of 0.10 μg/L, the sensitivity for initial abnormal head CT scan in our cohort was 86% (95% CI 73–95%). The lower sensitivity in our cohort may have resulted from the inclusion of patient subgroups where baseline S100B levels were higher (i.e., children and African Americans) than the cohorts used in these previous, mostly European, studies.
Despite the relatively lower performance of S100B in our cohort, initial head CT results were accurately predicted in more than 40% of subjects with mTBI. In addition, a significant proportion of head CT scans could have been avoided using S100B as a pre-head CT screen. This proportion varied from 22.9% to 30.5% depending on the cutoff used.
A third and final important finding of this study was the observation that the classification accuracy of S100B for mTBI varied by race and age. This variation appeared to be related to relative increases in S100B levels in subjects with mTBI compared with controls. S100B was significantly more accurate in Caucasians and adults (where the relative S100B increase was more than 125%) than in African Americans and children (where the relative S100B increase was less than 20%). Reasons for the low relative S100B increase with mTBI were not specifically explored in this study, but should be the focus of future research. We could identify no significant subgroup variation in classification accuracy of apoA-I.
There are several limitations to our study. Head CT scan readings from the six centers were interpreted by certified radiologists at each center. While this could produce variation in the classification of initial head CT scans as normal or abnormal, this reflects real-world practice. Prolonged storage of serum samples (1–3 years in our study) can potentially confound results if protein degradation occurs. If stored at −80C, however, as our samples were, apoA-I is stable in ethylenediaminetetracetic acid plasma samples for up to 13 years [personal written communication, Dr. Sarah Clark, University of Oxford], and there are no reports of S100B concentration rising with storage at −80C. Finally, the control and mTBI groups were not demographically balanced. Because healthy females have higher apoA-I levels than males, the higher apoA-I levels observed in controls may have been because of this gender imbalance rather than to mTBI-related differences in apoA-I levels. Because mean apoA-I levels in controls were higher for both males (.882±.28 vs .799±.25, p=0.0005) and females (.984±.25 vs .886±.25 p<0.0001), however, this is unlikely to be the case.
Conclusion
The combined use of serum S100B and apoA-I values maximizes classification accuracy for mTBI as well as the proportion of subjects that can be classified as either having or not having mTBI. S100B alone predicts an abnormal head CT scan; at a cutoff defining 98% sensitivity, 22.9% of head CT scans could have been avoided. Because of significant subgroup variation in classification accuracy, age and race need to be considered when estimating the sensitivity and specificity of S100B for mTBI. This may be best accomplished by development of a multivariable predictive model that estimates the risk of mTBI and abnormal head CT scan by incorporating marker values with age and race.
Supplementary Material
Acknowledgments
Authors' Contributions: Conception and design: Bazarian, Blyth, Khan, Mookerjee; acquisition of data: Blyth, Mookerjee, Moscati, Secreti, Leinhart, Kiechle, Triner, Ellis, Wojcik, Grant; analysis and interpretation of data: Bazarian, Blyth, He, Jones, Moynihan; drafting of the manuscript: Bazarian, Blyth; critical revision of the manuscript for important intellectual content: Blyth, Ellis, Grant, He, Jones, Kiechle, Khan, Leinhart, Mookerjee, Moscati, Secreti, Triner, Wojcik; statistical analysis: Grant, He, Jones, Moynihan; obtaining funding: Bazarian; administrative, technical or material support: Blyth, Kiechle, Khan, Mookerjee, Leinhart, Moscati, Triner, Wojcik; supervision: Bazarian, Blyth, Ellis, Mookerjee, Moscati, Secreti.
Funding/Support: This study was supported by funds from the New York State Department of Health, the Academic Health Center Consortium, and the Emergency Research Network of the Empire State (ERNIES). ERNIES is supported by the Pilot Research Collaborative Program of the Foundation for Healthy Living, the University of Rochester's Clinical and Translational Science Award, the Upstate New York Translational Research Network (UNYTRN), and the Upstate New York Consortium for Healthcare Research and Quality (UNYCHRQ).
Other contributions: The authors gratefully acknowledge the assistance Liana Dypka, Nancy Robak, RN, MPH, Donna Tyburczy, RN, BSN, Kamie Hoey, RN, and Coleen Vesely, RN, who coordinated enrollment activities; Akshata Nayak, Ming Ji, and Emily Tuttle who processed serum samples and performed apoA-I assays; Susanne Greub, PhD, and Dr. Marion Niessner who performed S100B assays; and Prof. Dr. Peter Biberthaler, Glenn Currier, MD, and Janet Williams, MD who assisted with study design. This study would not have been possible without the support and encouragement of New York State Senator Joseph Robach (R) and of the late Bruce Holme, PhD, who was Director of the New York State Center of Excellence in Life Sciences Director.
Data access and responsibility: Jeffrey J. Bazarian and Hua He had full access to all the data in the study and take full responsibility for the integrity of the data and the accuracy of the data analysis.
Author Disclosure Statement
Drs. Bazarian, Blyth have a Patent Pending, “Method of Diagnosing Mild Traumatic Brain Injury.” Dr. Bazarian is a consultant for Banyan Biomarkers and Roche Diagnostics. For all other authors, No competing financial interests exist.
References
- 1.Faul M. Xu L. Wald M.M. Coronado V.G. Atlanta, GA: Centers for Disease Control and Prevention, National Center for Injury Prevention and Control; [Jun 8;2010 ]. Traumatic Brain Injury in the United States: Emergency Department Visits, Hospitalizations and Deaths 2002–2006. [Google Scholar]
- 2.Bazarian J.J. Cernak I. Noble-Haeusslein L. Potolicchio S. Temkin N. Long-term neurologic outcomes after traumatic brain injury. J. Head Trauma Rehabil. 2009;24:439–451. doi: 10.1097/HTR.0b013e3181c15600. [DOI] [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention. National Center for Injury Prevention and Control. Report to Congress. Mild Traumatic Brain Injury in the United States: Steps to Prevent a Serious Public Health Problem. Centers for Disease Control and Prevention. 2003. http://www.cdc.gov/ncipc/pub-res/mtbi/mtbireport.pdf. [Aug 1;2012 ]. http://www.cdc.gov/ncipc/pub-res/mtbi/mtbireport.pdf
- 4.American Congress of Rehabilitation Medicine. Definition of mild traumatic brain injury. J. Head Trauma Rehabil. 1993;8:86–87. [Google Scholar]
- 5.Powell J.M. Ferraro J.V. Dikmen S.S. Temkin N.R. Bell K.R. Accuracy of mild traumatic brain injury diagnosis. Arch. Phys. Med. Rehabil. 2008;89:1550–1555. doi: 10.1016/j.apmr.2007.12.035. [DOI] [PubMed] [Google Scholar]
- 6.Rutland-Brown W. Langlois J.A. Bazarian J.J. Warden D. Improving identification of traumatic brain injury after nonmilitary bomb blasts. J. Head Trauma Rehabil. 2008;23:84–91. doi: 10.1097/01.HTR.0000314527.78134.70. [DOI] [PubMed] [Google Scholar]
- 7.Tanielian T. Jaycox L. The RAND Center for Military Health Policy Research; Washington, D.C.: 2008. Invisible Wounds of War: Psychological and Cognitive Injuries, Their Consequences, and Services to Assist Recovery. [Google Scholar]
- 8.Unden J. Romner B. Can low serum levels of S100B predict normal CT findings after minor head injury in adults? An evidence-based review and meta-analysis. J. Head Trauma Rehabil. 2010;25:228–240. doi: 10.1097/HTR.0b013e3181e57e22. [DOI] [PubMed] [Google Scholar]
- 9.Biberthaler P. Linsenmeier U. Pfeifer K.J. Kroetz M. Mussack T. Kanz K.G. Hoecherl E.F. Jonas F. Marzi I. Leucht P. Jochum M. Mutschler W. Serum S-100B concentration provides additional information for the indication of computed tomography in patients after minor head injury: a prospective multicenter study. Shock. 2006;25:446–453. doi: 10.1097/01.shk.0000209534.61058.35. [DOI] [PubMed] [Google Scholar]
- 10.Zongo D. Ribereau-Gayon R. Masson F. Laborey M. Contrand B. Salmi L.R. Mantaudon D. Beaudeux J.L. Meurin A. Dousset V. Loiseau H. Lagarde E. S100-B protein as a screening tool for the early assessment of minor head injury. Ann. Emerg. Med. 2012;59:209–218. doi: 10.1016/j.annemergmed.2011.07.027. [DOI] [PubMed] [Google Scholar]
- 11.Ben Abdesselam O. Vally J. Adem C. Foglietti M.J. Beaudeux J.L. Reference values for serum S-100B protein depend on the race of individuals. Clin. Chem. 2003;49:836–837. doi: 10.1373/49.5.836. [DOI] [PubMed] [Google Scholar]
- 12.Anderson R.E. Hansson L.O. Nilsson O. Dijlai-Merzoug R. Settergren G. High serum S100B levels for trauma patients without head injuries. Neurosurgery. 2001;48:1255–1260. doi: 10.1097/00006123-200106000-00012. [DOI] [PubMed] [Google Scholar]
- 13.Blyth B.J. Farhavar A. Gee C. Hawthorn B. He H. Nayak A. Stöcklein V. Bazarian J.J. Validation of serum markers for blood-brain barrier disruption in traumatic brain injury. J. Neurotrauma. 2009;26:1497–1507. doi: 10.1089/neu.2008.0738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rader D.J. Molecular regulation of HDL metabolism and function: implications for novel therapies. J. Clin. Invest. 2006;116:3090–3100. doi: 10.1172/JCI30163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Van Lenten B.J. Reddy S.T. Navab M. Fogelman A.M. Understanding changes in high density lipoproteins during the acute phase response. Arterioscler. Thromb. Vasc. Biol. 2006;26:1687–1688. doi: 10.1161/01.ATV.0000232522.47018.a6. [DOI] [PubMed] [Google Scholar]
- 16.Yu C. Youmans K.L. LaDu M.J. Proposed mechanism for lipoprotein remodelling in the brain. Biochim. Biophys. Acta. 2010;1801:819–823. doi: 10.1016/j.bbalip.2010.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Blyth B.J. Nayak A. Sparks J.D. Khan J. Apolipoprotein A1 is increased in the serum of mild traumatic brain injury patients. J. Neurotrauma. 2010;26:25. [Google Scholar]
- 18.Towend W. Dibble C. Abid K. Vail A. Sherwood R. Lecky F. Rapid elimination of protein S-100B from serum after minor head trauma. J. Neurotrauma. 2006;23:149–155. doi: 10.1089/neu.2006.23.149. [DOI] [PubMed] [Google Scholar]
- 19.Tape C. Kisilevsky R. Apolipoprotein A-I and apolipoprotein SAA half-lives during acute inflammation and amyloidogenesis. Biochim. Biophys. Acta. 1990;11043:295–300. doi: 10.1016/0005-2760(90)90030-2. [DOI] [PubMed] [Google Scholar]
- 20.Tang W. He H. Tu X.M. Applied Categorical and Count Data Analysis. CRC Press, Taylor & Francis Group; Boca Raton, FL: 2012. [Google Scholar]
- 21.Allman R.M. Steinberg E.P. Keruly J.C. Dans P.E. Physician tolerance for uncertainty. Use of liver-spleen scans to detect metastases. JAMA. 1985;254:246–248. doi: 10.1001/jama.254.2.246. [DOI] [PubMed] [Google Scholar]
- 22.DeLong E.R. DeLong D.M. Clarke-Pearson D.L. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometric. 1988;44:837–845. [PubMed] [Google Scholar]
- 23.Hanley J.A. McNeil B.J. A method of comparing the areas under receiver operating characteristic curves derived from the same cases. Radiology. 1983;148:839–843. doi: 10.1148/radiology.148.3.6878708. [DOI] [PubMed] [Google Scholar]
- 24.Hanley J.A. McNeil B.J. The meaning and use of the area under a receiver operating characteristic (ROC) curve. Radiology. 1982;143:29–36. doi: 10.1148/radiology.143.1.7063747. [DOI] [PubMed] [Google Scholar]
- 25.Bouvier D. Oddoze C. Ben Haim D. Moustafa F. Legrand A. Alazia M. Jehle E. Schmidt J. Sapin V. [Interest of S100B protein blood level determination for the management of patients with minor head trauma] (Fre) Ann. Biol. Clin. (Paris) 2009;67:425–431. doi: 10.1684/abc.2009.0347. [DOI] [PubMed] [Google Scholar]
- 26.Morochovic R. Racz O. Kitka M. Pingorova S. Cibur P. Tomkova D. Lenartova R. Serum S100B protein in early management of patients after mild traumatic brain injury. Eur. J. Neurol. 2009;16:1112–1117. doi: 10.1111/j.1468-1331.2009.02653.x. [DOI] [PubMed] [Google Scholar]
- 27.Müller K. Townend W. Biasca N. Unden J. Waterloo K. Romner B. Ingebrigtsen T. S100B serum level predicts computed tomography findings after minor head injury. J. Trauma. 2007;62:1452–1456. doi: 10.1097/TA.0b013e318047bfaa. [DOI] [PubMed] [Google Scholar]
- 28.Poli-de-Figueiredo LF. Biberthaler P. Simao Filho C. Hauser C. Mutschler W. Jochum M. Measurement of S-100B for risk classification of victims sustaining minor head injury—first pilot study in Brazil. Clinics (Sao Paulo) 2006;61:41–46. doi: 10.1590/s1807-59322006000100008. [DOI] [PubMed] [Google Scholar]
- 29.Nygren De Boussard C. Fredman P. Lundin A. Andersson K. Edman G. Borg J. S100 in mild traumatic brain injury. Brain Inj. 2004;18:671–683. doi: 10.1080/02699050310001646215. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.