Abstract
The platinum derivative cis-diamminedichloroplatinum(II), best known as cisplatin, is currently employed for the clinical management of patients affected by testicular, ovarian, head and neck, colorectal, bladder and lung cancers. For a long time, the antineoplastic effects of cisplatin have been fully ascribed to its ability to generate unrepairable DNA lesions, hence inducing either a permanent proliferative arrest known as cellular senescence or the mitochondrial pathway of apoptosis. Accumulating evidence now suggests that the cytostatic and cytotoxic activity of cisplatin involves both a nuclear and a cytoplasmic component. Despite the unresolved issues regarding its mechanism of action, the administration of cisplatin is generally associated with high rates of clinical responses. However, in the vast majority of cases, malignant cells exposed to cisplatin activate a multipronged adaptive response that renders them less susceptible to the antiproliferative and cytotoxic effects of the drug, and eventually resume proliferation. Thus, a large fraction of cisplatin-treated patients is destined to experience therapeutic failure and tumor recurrence. Throughout the last four decades great efforts have been devoted to the characterization of the molecular mechanisms whereby neoplastic cells progressively lose their sensitivity to cisplatin. The advent of high-content and high-throughput screening technologies has accelerated the discovery of cell-intrinsic and cell-extrinsic pathways that may be targeted to prevent or reverse cisplatin resistance in cancer patients. Still, the multifactorial and redundant nature of this phenomenon poses a significant barrier against the identification of effective chemosensitization strategies. Here, we discuss recent systems biology studies aimed at deconvoluting the complex circuitries that underpin cisplatin resistance, and how their findings might drive the development of rational approaches to tackle this clinically relevant problem.
Keywords: BCL-2, carboplatin, CTR1, DNA damage response, oxaliplatin, p53
Facts
A large fraction of human malignancies rapidly becomes (or intrinsically is) insensitive to the cytostatic/cytotoxic effects of cisplatin.
Cisplatin resistance is generally multifactorial, that is, it relies on the activation of multiple, non-redundant molecular or cellular circuitries.
Throughout the past two decades, a number of systems biology studies has been performed to obtain additional insights into cisplatin resistance.
The results of such an intense wave of investigation may promote the development of strategies to circumvent this clinically relevant hurdle.
Open Questions
The precise molecular mechanisms whereby cisplatin exerts antineoplastic effects have not yet been elucidated.
In particular, it remains unclear to which extent the cytoplasmic and nuclear events elicited by cisplatin contribute to its cytostatic/cytotoxic activity.
Moreover, the possibility that cisplatin resistance may originate (at least in part) from cell-extrinsic (stromal or immune system-related) mechanisms has not yet been investigated in detail.
First described by the Italian chemist Michele Peyrone as early as in 1845, cis-diamminedichloroplatinum(II) (CDDP, best known as cisplatin or cisplatinum), is the founding member of a class of antineoplastic agents born in the second half of the 19th century around the peculiar atomic configuration of platinum.1, 2 More than 120 years had indeed to pass from Peyrone's observations for the American chemist Barnett Rosenberg to characterize the robust antiproliferative effects of CDDP, first in Escherichia coli cultures, then in solid and hematopoietic tumor xenografts.3, 4 Rosenberg's findings initiated an intense wave of preclinical and clinical investigation aimed at elucidating not only the molecular mechanisms that underpin the cytostatic/cytotoxic effects of CDDP, but also its safety and therapeutic profile.5, 6, 7 Such an experimental effort culminated in 1978 with the approval of CDDP by the US Food and Drug Administration (FDA) for use in testicular and bladder cancer patients.7 Since then, CDDP has been licensed worldwide for the treatment of multiple other solid neoplasms, including head and neck, lung, colorectal and ovarian cancers.5, 8, 9 Thus, during the last 35 years, several millions of cancer patients have received CDDP-based antineoplastic regimens, either as part of consolidated therapeutic procedures or in the context of clinical studies. Unfortunately, CDDP-based chemo(radio)therapy was often destined to fail.
As a matter of fact, CDDP is highly efficient only against testicular germ cell cancer, leading to a durable complete remission in >80% of the patients.10, 11 Conversely, the clinical responses elicited by CDDP-based chemo(radio)therapeutic regimens in patients affected by other solid tumors (e.g., ovarian carcinoma) are temporary and vanish as malignant cells become chemoresistant. Moreover, a significant fraction of lung, prostate and colorectal cancer patients bears neoplastic lesions that are intrinsically resistant to the cytostatic/cytotoxic activity of CDDP.12, 13, 14 Thus, although the use of CDDP (which is generally administered i.v. as a short-term infusion in physiological saline) has been associated with mild-to-moderate nephrotoxic, neurotoxic, cardiotoxic and ototoxic side effects,7, 15 chemoresistance (be it intrinsic or acquired) constitutes the most prominent obstacle against the use of this drug. The biological reasons underlying the exquisite sensitivity of testicular germ cell cancers to CDDP have not been completely elucidated. Indeed, although defects in several DNA repair pathways (see below) have been associated with improved disease outcome in CDDP-treated testicular germ cell cancer patients,16, 17, 18, 19 additional hitherto unidentified factors are likely to influence this phenomenon.
In the early 1980s, investigators and clinicians began to refocus their attention on the development of CDDP derivatives that would elicit robust therapeutic responses accompanied by clinically manageable side effects. Such an effort led to the discovery of two additional platinum derivatives that are nowadays approved by FDA for use in cancer patients: cis-diammine (cyclobutane-1,1-dicarboxylate-O,O′) platinum(II) (carboplatin) and [(1R,2R)-cyclohexane-1,2-diamine](ethanedioato-O,O')platinum(II) (oxaliplatin).20, 21 The former has been licensed in 1989 for the treatment of ovarian cancer,20 whereas the latter has entered clinical practice in 2002, as part of a 5-fluorouracil (5-FU)- and folinic acid-containing cocktail (the so-called FOLFOX regimen) that is routinely employed as a neo-adjuvant or adjuvant intervention in colorectal cancer patients.21 Carboplatin and CDDP exert antineoplastic effects via very similar, if not undistinguishable, mechanisms, reflecting the fact that the active forms of these drugs are identical. Still, carboplatin appears to be less nephro- and neurotoxic than CDDP, perhaps reflecting its reduced biological potency.22 Conversely, oxaliplatin and CDDP constitute two chemically distinct entities and as such exhibit distinct pharmacological and immunological properties.23, 24, 25 Nonetheless, a significant proportion of CDDP-insensitive tumors is resistant not only to carboplatin but also to oxaliplatin as well as to a large array of antineoplastic agents with a completely unrelated mechanism of action.26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36 These observations, which reflect the multilevel and multifactorial nature of CDDP resistance (see below), have fostered the development of additional platinum derivatives, including amminedichloro(2-methylpyridine)platinum (picoplatin) and (OC-6-43)-bis(acetato)amminedichloro(cyclohexylamine)platinum (satraplatin). As it stands, however, neither picoplatin- nor satraplatin-containing regimens provide consistent advantages over CDDP-, oxaliplatin- and carboplatin-based chemotherapy.37, 38, 39, 40 Thus, the interest in developing novel platinum derivatives as well as clinically applicable strategies to increase the sensitivity of human neoplasms to CDDP remains high.
In this review, we summarize the mechanisms whereby CDDP exerts antineoplastic effects as well as the major determinants of CDDP resistance, laying special emphasis on recent systems biology studies aimed at avoiding or overcoming this crucial clinical issue.
Mode of Action
CDDP is chemically inert until one or both of its cis chloro groups are replaced by water molecules.41, 42 Such an ‘aquation' occurs spontaneously in the cytoplasm, presumably due to the relatively low concentration of chloride ions (∼2–10 mM, as compared with ∼100 mM in the extracellular space).43, 44 Mono- and bi-aquated forms of CDDP are highly electrophilic and hence prone to form covalent bonds with methionine as well as with a large panel of cysteine-containing peptides and polypeptides, including reduced glutathione (GSH) and metallothioneins.45 The interaction between aquated CDDP and endogenous nucleophiles has dual consequences. On one hand, it depletes the cytosol of reducing equivalents, hence promoting the establishment of oxidative stress, which may have direct cytotoxic effects or provoke DNA damage (see below).46, 47 On the other hand, it results in the inactivation of (at least a fraction of) chemically reactive CDDP, hence functioning as a cytoprotective buffer.12, 48
Upon aquation, CDDP also binds with high affinity to mitochondrial and nuclear DNA, in particular to nucleophilic N7 sites on purines, thus favoring the generation of heterotypic protein–DNA complexes as well as homotypic inter- and intra-strand DNA adducts.49, 50, 51 If limited in amount, the DNA lesions provoked by CDDP can be recognized and safely removed by several repair systems that normally operate in the context of a temporary cell cycle arrest.52, 53, 54, 55, 56 Conversely, when the CDDP-induced DNA damage is irreparable, either such a cell cycle arrest becomes permanent (an oncosuppressive response known as cellular senescence)57, 58 or cells become committed to die, most often via mitochondrial apoptosis.59, 60, 61, 62, 63, 64 Both these processes can be initiated by the sequential activation of ATM- and RAD3-related protein (ATR, a sensor of DNA damage) and checkpoint kinase 1 (CHEK1, its major downstream effector), eventually resulting in the stabilizing phosphorylation of the oncosuppressor protein p53.65, 66, 67, 68, 69 A precise description of the molecular mechanisms whereby an excess of DNA lesions promotes widespread mitochondrial outer membrane permeabilization (MOMP), and hence cell death, exceeds the scope of this review and can be found in other publications.65, 66, 67, 70, 71
Importantly, it is now clear that the cytostatic/cytotoxic effects of CDDP do not represent a mere consequence of its genotoxic activity, but originate from both nuclear and cytoplasmic signaling pathways.48, 72 In line with this notion, (i) only ∼1% of intracellular CDDP forms covalent bonds with nuclear DNA73 and (ii) CDDP (as well as oxaliplatin) exerts prominent cytotoxic effects in enucleated cells (cytoplasts).51, 74, 75, 76, 77, 78 The molecular mechanisms that underlie the cytotoxic potential of cytoplasmic CDDP are poorly understood, yet may involve: (i) the accumulation of reactive oxygen species (ROS) and nitric oxide (NO), which not only exacerbate CDDP genotoxicity but also exert direct cytotoxic effects by favoring the opening of the so-called permeability transition pore complex (PTPC);70, 79, 80 (ii) the transduction of a MOMP-stimulatory signal via the pro-apoptotic BCL-2 family member BAK1, the PTPC component voltage-dependent anion channel 1 (VDAC1) and the BAK1 homolog BAX;81, 82 (iii) the activation of a cytoplasmic pool of p53 that is capable of promoting MOMP via various mechanisms83, 84, 85, 86 and (iv) in specific cellular models (see below), the establishment of an endoplasmic reticulum (ER) stress response.77, 87
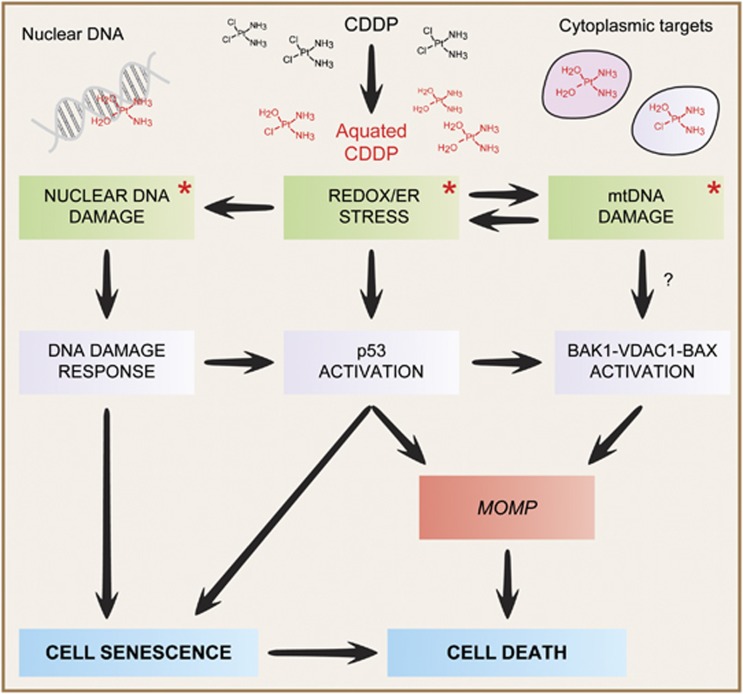
The relative contribution of all these nuclear and cytoplasmic effects to the cytostatic/cytotoxic activity of CDDP remains to be deciphered and may exhibit a consistent degree of context dependency (Figure 1).
Figure 1.
Mode of action of cisplatin. As a result of the reduced cytoplasmic concentration of chloride ions, intracellular cisplatin (CDDP) is rapidly ‘aquated', hence acquiring a pronounced electrophilic reactivity. Aquated CDDP binds with high affinity to nuclear DNA, in particular to nucleophilic N7 sites on purines, thereby promoting the activation of the DNA damage response. In addition, CDDP can physically interact with several cytoplasmic nucleophiles, including mitochondrial DNA (mtDNA) as well as multiple mitochondrial and extramitochondrial proteins, hence (i) favoring the establishment of oxidative and reticular stress; (ii) eliciting a signal transduction cascade that involves the pro-apoptotic BCL-2 family members BAK1 and BAX, as well as voltage-dependent anion channel 1 (VDAC1) and (iii) activating the cytoplasmic pool of p53. The relative contribution of these nuclear and cytoplasmic modules to the cytostatic/cytotoxic activity of CDDP remains to be precisely elucidated and may exhibit an elevated degree of context dependency. Asterisks tag the primary consequences of CDDP reactivity. ER, endoplasmic reticulum; MOMP, mitochondrial outer membrane permeabilization
Mechanisms of Resistance
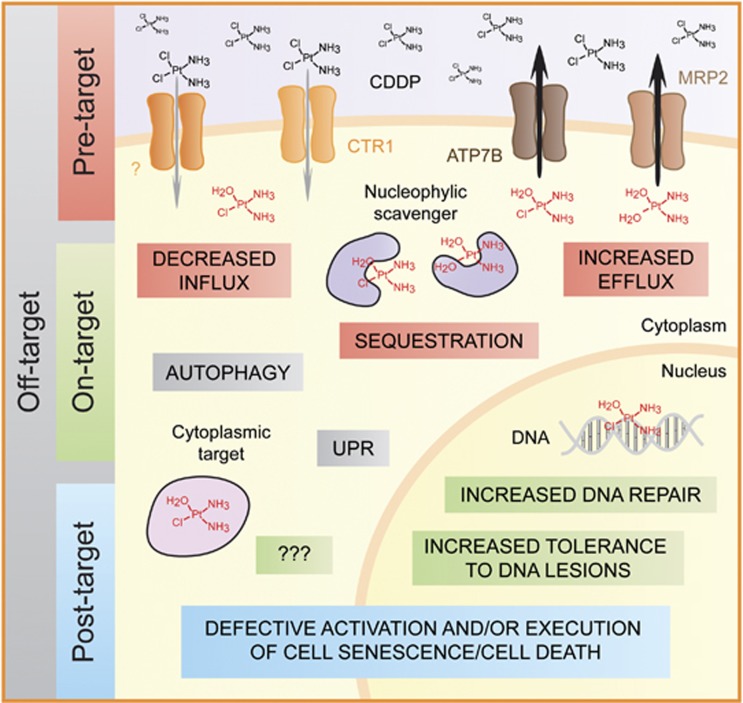
We have recently discussed the molecular mechanisms that reduce the sensitivity of malignant cells to CDDP, and classified them based on functional and hierarchical parameters. In particular, we proposed that CDDP resistance can surge from alterations (i) in processes that precede the binding of CDDP to its actual targets, including DNA and cytoplasmic structures (pre-target resistance), (ii) directly related to the molecular damage provoked by CDDP (on-target resistance), (iii) in the lethal signaling pathways triggered by such molecular lesions (post-target resistance) and (iv) influencing molecular circuitries that are not (or at least have not yet been) intimately associated with CDDP-elicited signals (off-target resistance).48
Pre-target resistance
CDDP resistance can develop along with alterations that reduce the amount of aquated CDDP forms in the cytoplasm. Although for a long time CDDP has been believed to get access to the cytoplasmic compartment by passively diffusing across the plasma membrane,42, 88 it is now clear that the majority of CDDP is actively moved in and out of the cell by copper transporters. In particular, copper transporter 1 (CTR1) has turned out to mediate a significant fraction of CDDP intake,89, 90, 91, 92, 93 whereas ATPase, Cu2+ transporting, β polypeptide (ATP7B), a Cu2+-extruding P-type ATPase involved in the pathogenesis of Wilson's disease,94 plays a significant role in CDDP export.90, 95 In line with this notion, alterations in the expression level, subcellular localization and/or functionality of CTR1 and ATP7B have been associated with CDDP resistance, both in preclinical models and in cancer patients.90, 91, 93, 96, 97, 98, 99, 100, 101, 102 Promising (though very preliminary) results from a pilot clinical trial investigating the safety and chemosensitizing potential of trientine (a copper chelator) plus platinum-based chemotherapy in advanced cancer patients (NCT01178112) have recently been disseminated.102, 103, 104
Other plasma membrane transporters have been suggested to contribute to the extrusion of CDDP (hence promoting CDDP resistance in preclinical as well as clinical settings), notably ATP-binding cassette, subfamily C (CFTR/MRP), member 2 (ABCC2), best known as multidrug resistance-associated protein 2 (MRP2)105, 106, 107, 108, 109 and ATPase, class VI, type 11B (ATP11B).110 Moreover, CDDP-resistant cancer cells may exhibit increased levels of GSH, the enzyme that catalyzes GSH synthesis (i.e., γ-glutamylcysteine synthetase), the enzyme that conjugates CDDP with GSH (i.e., glutathione S-transferase), or metallothioneins.111, 112, 113, 114 Although none of these alterations has been shown to be clinically relevant, all of them may contribute to pre-target resistance by incrementing the capacity of the cytoplasmic CDDP buffer.
On-target resistance
The sensitivity of cancer cells to the cytostatic/cytotoxic effects of CDDP is limited in the presence of a proficient DNA repair apparatus. In particular, the nucleotide excision repair (NER) system is believed to resolve the majority of DNA lesions provoked by CDDP,53, 54 although components of the mismatch repair (MMR) machinery have also been implicated in this process (at least in its detection, as opposed to resolution, phase).55 In line with this notion, an increased NER proficiency has been associated with CDDP resistance in vitro, in murine models as well as in cohorts of cancer patients.115, 116, 117, 118, 119, 120, 121, 122, 123 Convenient biomarkers for assessing NER proficiency in human tumors are still missing, as the actual predictive value of excision repair cross-complementing rodent repair deficiency, complementation group 1 (ERCC1) expression levels (first proposed in 2006),118 has recently been reconsidered.124 The MMR system is believed to detect DNA adducts caused by CDDP, engage in their repair and ultimately fail, thus transmitting a pro-apoptotic signal.125 Accordingly, genes encoding MMR components such as mutS homolog 2 (MSH2) and mutL homolog 1 (MLH1) are frequently mutated or downregulated in the context-acquired CDDP resistance.126, 127, 128, 129 Moreover, non-small cell lung carcinoma (NSCLC) lesions expressing high MSH2 levels exhibit an improved prognosis upon surgical resection (in the absence of adjuvant chemotherapy).130 Thus, high expression levels of MMR components may exert a positive influence both on the propensity of chemotherapy-naive tumors to relapse (perhaps because MMR limits the accumulation of additional genetic alterations) and on the probability that neoplasms exposed to CDDP become chemoresistant. MLH1 mutations are also associated with increased rates of translesion synthesis (TLS), the process whereby DNA is replicated (by a peculiar class of polymerases) in spite of unrepaired lesions.131 In line with this notion, defects in TLS polymerases including polymerase (DNA-directed), η (POLH) and REV3-like, polymerase (DNA directed), ζ, catalytic subunit (REV3L) have been linked to increased CDDP sensitivity in vitro.132, 133, 134, 135 However, compelling clinical evidence in support of the translational relevance of these findings is elusive.
CDDP-elicited DNA adducts can engender double-strand breaks (DSBs), which are normally repaired along with DNA synthesis (or shortly after) via homologous recombination (HR).136 Accordingly, HR-deficient neoplasms, such as those bearing loss-of-function mutations in the genes encoding breast cancer 1, early onset (BRCA1) or breast cancer 2, early onset (BRCA2),137, 138 are generally more susceptible to the genotoxic effects of CDDP than HR-proficient cancers of the same type.139, 140, 141 Moreover, the appearance of compensatory mutations in BRCA1 and BRCA2 that restore the functionality of HR has been show to favor CDDP resistance in breast carcinoma cells.142 Mutations of this type not only have been associated with the resistance of ovarian carcinoma to platinum-based chemotherapy142, 143 but also appear to influence the sensitivity of pancreatic and ovarian neoplasms to inhibitors of poly(ADP-ribose) polymerase 1 (PARP1), another important regulator of DNA repair.144 Of note, while the innate resistance of pancreatic carcinoma cells to CDDP and PARP1 inhibitors resolves simultaneously in response to HR-restoring BRCA2 mutations,143 we have recently reported that NSCLC cells cultured for prolonged periods in the presence of subtoxic amounts of CDDP often (but not always) manifest a constitutive hyperactivation of PARP1, hence becoming susceptible to pharmacological or genetic PARP inhibition.145 In line with these notions, the concomitant administration of CDDP and PARP inhibitors appears to elicit at least additive antineoplastic effects, in vitro and in vivo.146, 147, 148
Although the identity of the cytoplasmic components that account for the extranuclear toxicity of CDDP has only recently begun to emerge, these molecules as well as the enzymatic systems that regulate their preservation/turnover may also be involved in the development of on-target CDDP resistance.72 So far, only a few extranuclear CDDP-binding partners have been identified, including near-to-ubiquitous (e.g., valosin-containing protein), as well as mostly cytosolic (e.g., myosin IIa, HSP90), ribosomal (e.g., ribosomal protein L5), reticular (e.g., calreticulin) and mitochondrial components (e.g., mitochondrial DNA, VDAC1).51, 149 Remarkably, human NSCLC cells developing CDDP resistance as they are exposed for long periods to sublethal doses of the drug appear to be positively selected for specific mitochondrial DNA mutations, resulting in partial defects of the respiratory chain.150, 151 Two mechanisms that may account for this effect are the compensatory stimulation of mitochondrial biogenesis by retrograde mitochondrio-nuclear signaling and the activation of a peroxisome proliferator-activated receptor γ, coactivator 1β (PPARGC1B)-dependent (but mitochondrion-independent) signal transduction cascade.150, 152 Moreover, both rho0 cells and cells depleted of VDAC1 by RNA interference are less susceptible to CDDP cytotoxicity than their wild-type counterparts.51, 81 In line with this notion, the transfection-enforced overexpression of VDAC1 reportedly sensitizes carcinoma cells to cell death induction by CDDP.82 However, elevated expression levels of VDAC1 may exert broad chemosensitization effects.153 Thus, whether these observations constitute examples of bona fide on-target resistance or rather reflect the critical role of mitochondria (and in particular of the PTPC) in CDDP-elicited cell death, hence constituting examples of post-target resistance, remains an open conundrum. Along similar lines, although physical interactions between CDDP and cyclin-dependent kinase 2 (CDK2) have not yet been documented, CDK2 has been shown to contribute to extranuclear CDDP toxicity (by inducing ER stress, at least in some cellular models), and CDK2-deficient cells exhibit some extent of CDDP resistance.77, 87
Post-target resistance
In line with the notion that regulated cell death (be it apoptotic or necrotic) is under the control of a plethora of checkpoints and safeguard mechanisms, post-target CDDP resistance may develop along with a wide panel of alterations in the systems that detect the molecular damage caused by CDDP and convert it into a lethal signal, as well as in the machinery that de facto executes cell death.12, 48, 154 Malignant cells are (i) intrinsically more resistant to adverse microenvironmental and intracellular cues than their non-transformed counterparts155, 156 and (ii) highly prone to acquire additional genetic and epigenetic alterations.157, 158 Thus, (at least some degree of) post-target resistance can be documented in most, if not all, CDDP-exposed tumors. Importantly, post-target CDDP resistance is rarely (if ever) specific, rather extending to other DNA-damaging agents (including radiation),27, 33, 159 as well as to cytotoxic stimuli that engage a wide panel of primary targets (e.g., death receptor agonists).160
As a general rule, intracellular stress conditions (such as those elicited by CDDP) promote the rapid activation of an integrated adaptive response aimed at the re-establishment of cellular homeostasis.161 This is generally accompanied by the emission of robust anti-apoptotic cues and only when homeostasis cannot be restored (for instance, when stress conditions are excessive in intensity or duration) lethal signals are transmitted, de facto representing a mechanism for the preservation of organismal homeostasis.162 In the case of CDDP, these signals consist in (i) the switch of the DNA damage response from a cytoprotective to a cytotoxic mode, followed by the (often, but not always, p53-dependent) activation of BAX and BAK1163 and (ii) the accumulation of ROS and consequent PTPC opening.81, 164 Both these processes eventually promote MOMP, in turn resulting in the functional and physical breakdown of mitochondria followed by the activation of caspase-dependent and independent mechanisms of cell death.70, 165 Thus, post-target CDDP resistance has been associated not only with genetic and epigenetic alterations that impair p53 signaling (in vitro, in murine tumor models as well as in several distinct clinical scenarios)11, 166, 167, 168, 169, 170 but also with defects in several other pro-apoptotic signal transducers, including mitogen-activated protein kinase 14 (MAPK14, best known as p38MAPK) and c-Jun N-terminal kinase 1 (JNK1).171, 172 Along similar lines, post-target CDDP resistance appears to be significantly influenced by the expression levels and functional status of BCL-2 family members and caspases, a class of cysteine proteases that has a major role in the execution of apoptotic cell death.81, 173, 174 This said, only a few of these factors have been formally correlated with CDDP resistance in clinical studies, including various BCL-2-like proteins such as BCL-2 itself, BCL-XL and MCL-1,175, 176, 177, 178 survivin, a caspase inhibitor that is often upregulated in response to CDDP,179, 180, 181, 182 as well as other members of the baculoviral IAP repeat-containing (BIRC) protein family.183 In line with this notion, small molecules that antagonize the anti-apoptotic effects of BCL-2-like proteins (e.g., ABT-263) as well as IAP inhibitors (e.g., YM155, LY2181308, LBW242) have been intensively investigated in clinical trials, either as standalone anticancer interventions or in combination with various chemotherapeutics including CDDP.184, 185, 186, 187, 188 In spite of an acceptable safety profile and promising antineoplastic activity, the clinical development of all these agents appears to stand at an impasse, at least as judged by the low rate of ongoing versus completed, terminated or withdrawn trials (source www.clinicaltrials.gov).
Off-target resistance
The susceptibility of cancer cells to CDDP can also be limited by off-target mechanisms, that is, molecular circuitries that deliver compensatory survival signals even though they are not directly activated by CDDP.48 For instance, the overexpression of v-erb-b2 avian erythroblastic leukemia viral oncogene homolog 2 (ERBB2), which is common in breast and ovarian carcinomas,189, 190 has been suggested to promote CDDP resistance not only by delivering robust pro-survival signals via the v-akt murine thymoma viral oncogene homolog 1 (AKT1) signaling axis, but also by finely regulating the transitory cell cycle arrest that is required for the repair of CDDP-induced DNA lesions.191, 192 Along similar lines, dual-specificity Y-phosphorylation-regulated kinase 1B (DYRK1B, also known as MIRK) appears to sustain CDDP resistance as it favors the expression of various antioxidant enzymes.193 By augmenting the intracellular pool of antioxidants, DYRK1B may actually reduce the susceptibility of cancer cells to CDDP via on-target, post-target as well as off-target mechanisms.48 Recently, a poorly characterized transmembrane protein (TMEM205) has been shown to favor CDDP resistance via a molecular cascade that involves the small RAS-like GTPase RAB8A.194, 195 Whether this constitutes an off-target mechanism of resistance or rather reflects the inhibition of caspase-9 by AKT1-transduced signals196 (hence representing a post-target effect) remains to be clarified. Finally, several relatively unspecific adaptive responses to stress have been implicated in CDDP resistance. Such general responses include macroautophagy and the so-called heat-shock response, that is, the adaptation of cells to increased temperatures and other conditions that promote protein unfolding.161 Thus, various components of the autophagic machinery and several chaperones of the heat-shock protein (HSP) family reportedly impair the cytostatic/cytotoxic response of cultured cancer cells to CDDP.149, 197, 198, 199, 200, 201 Moreover, the expression levels of HSP27 may constitute a predictive biomarkers of clinical responses to CDDP in esophageal squamous cell carcinoma patients.202
In conclusion, malignant cells can lose their sensitivity to CDDP owing to a wide panel of molecular and functional alterations, many of which are a priori connected to oncogenesis and tumor progression. CDDP resistance is often multifactorial, as it relies on the simultaneous activation of multiple, non-redundant molecular circuitries (Figure 2).48, 203 This obviously poses a major hurdle against the development of therapeutically useful chemosensitization strategies. As summarized below, several systems biology studies have recently been undertaken to address this clinically relevant issue.
Figure 2.
Molecular mechanisms of cisplatin resistance. Malignant cells can lose their sensitivity to the cytostatic/cytotoxic activity of cisplatin (CDDP) as a result of a wide panel of genetic or epigenetic defects. These alterations can (i) affect processes that precede the actual binding of CDDP to its targets (pre-target resistance); (ii) potentiate the ability of cells to repair the molecular damage caused by CDDP (on-target resistance); (iii) impair the transmission of signals that normally relay such a CDDP-induced damage to cell senescence or apoptosis (post-target resistance) or (iv) stimulate the delivery of pro-survival signals that antagonize CDDP cytotoxicity although they are normally not elicited by this drug (off-target resistance). Of note, CDDP resistance is generally multifactorial, that is, it relies on the activation of several, non-overlapping mechanisms that concur to limit the cytostatic/cytotoxic effects of CDDP at multiple levels. At least in part, this explains why efficient strategies to increase the sensitivity of human neoplasms to CDDP are still lacking in spite of a prolonged and intense wave of investigation. ATP7B, ATPase, Cu2+ transporting, β polypeptide; CTR1, copper transporter 1; MRP2, multidrug resistance-associated protein 2; UPR, unfolded protein response. CDDP aquation is depicted in red
Systems Biology and Cisplatin Resistance
Throughout the last decade, a huge number of high-content and/or high-throughput studies have been initiated to tackle – by means of an empirical approach – the complex and clinically relevant problem of CDDP resistance. For illustrative purposes, these studies can be classified into three large groups: (i) genomic, transcriptomic, methylomic and proteomic approaches, investigating the correlation between CDDP resistance and gene expression/regulation or the activation of specific signal transduction pathways (in preclinical models or patient material); (ii) large-scale silencing approaches and functional screenings, determining whether the genetic or pharmacological inhibition of specific proteins alters CDDP sensitivity (in preclinical models only) and (iii) multiplex genotyping studies, assessing in a high-throughput manner whether particular (combinations of) single-nucleotide polymorphisms (SNPs) are associated with increased or reduced CDDP sensitivity (in clinical settings). Each of these approaches obviously brings about specific advantages and disadvantages, which we will not systematically discuss here (for further details please consult a recent study by He et al.204). Still, it is important to note that, with a few exceptions (including some genotyping studies), the majority of these strategies have relied on relatively reductionistic models (e.g., cultured cancer cells) or readouts (e.g., mRNA levels in whole-tumor lysates). Considering tumors as relatively homogeneous entities exclusively composed of malignant cells and attributing CDDP resistance only to cancer cell-intrinsic mechanisms (hence neglecting the prominent structural and functional impact of stromal and immune cells) appears indeed as an excessive oversimplification (see below), and may in part explain why clinically applicable strategies for restoring CDDP sensitivity are still missing. Nonetheless, these studies have provided us with ever more precise insights into the molecular mechanisms that account for CDDP resistance, at least at the cell-intrinsic level. Here, we provide an overview of recent systems biology studies (for the most part published in the last 3 years) that have significantly added to our understanding of CDDP resistance (Supplementary Table 1).
Genomic, methylomic, transcriptomic and proteomic approaches
For nearly one decade, it has been possible – and cost-efficient – to employ microarrays for quantifying (i) variations in gene copy number, by comparative genomic hybridization (CGH), (ii) the abundance of mRNAs and (iii) the expression levels of regulatory RNA species (including microRNAs and long non-coding RNAs) in both human and murine samples. Thus, several research groups worldwide, including us, have adopted one of these approaches to get further insights into CDDP resistance. Nonetheless, the potential of these methods is intrinsically limited by the frequent lack of correlation between copy number or mRNA/microRNA expression levels and phenotype, calling for robust rounds of functional validation.205 Recently, along with the evolution of mass spectrometry and the development of high-throughput techniques for the study of post-translational protein modifications (including, but not limited to, phosphorylation and acetylation),206, 207, 208, 209 attention has been refocused to proteomic/interactomic approaches as well as to the identification of novel signal transduction cascades that underpin CDDP resistance. In addition, great enthusiasm has been generated by the possibility to sequence the whole genome of neoplastic lesions, as first achieved for acute myeloid leukemia and quickly thereafter for various other malignancies.210, 211, 212, 213, 214 Further increasing the potential of this approach, Murtaza et al.215 have just demonstrated that advanced solid tumors can be sequenced starting from DNA molecules released by malignant cells into the plasma, de facto constituting a non-invasive liquid biopsy. Again, although these approaches can undoubtedly improve our understanding of CDDP resistance, caution should be taken while interpreting sequencing data in the absence of functional validation.
Several factors have recently been revealed or confirmed to contribute to CDDP resistance as a result of genomic, methylomic, transcriptomic and proteomic studies (Supplementary Table 1). Using CGH arrays, Cui et al.216 identified an interleukin-7 (IL-7)-dependent autocrine loop specifically activated in glioma cells that have lost their sensitivity to CDDP. By means of an integrated approach involving CGH arrays and transcriptomic studies, Jiffar et al.218 demonstrated that head and neck squamous cell carcinomas (HNSCCs) can become resistant to CDDP along with the loss of KiSS-1 metastasis-suppressor (KISS1), a G protein-coupled receptor ligand that also mediates CDDP-independent oncosuppressive functions.217, 218 By comparing the methylome of CDDP-sensitive and CDDP-resistant isogenic ovarian cancer cells, Yu et al.219 found that the latter exhibit not only a global decrease in the methylation of CpG islands but also specific alterations including the hypomethylation (which is generally associated with increased gene expression) of protein tyrosine kinase 6 (PTK6), coding for a non-receptor tyrosine kinase with prominent pro-survival functions; protein kinase Cɛ (PRKCE), encoding a Ca2+- and diacylglycerol-activated serine/threonine kinase, and BCL-2-like 1 (BCL2L1), coding for BCL-XL.219 By means of a systems biology approach comprising a methylomic and a transcriptomic component, Zeller et al.220 identified a set of nine genes that are hypermethylated (resulting in decreased transcription) both in several, independent CDDP-resistant derivatives of an ovarian carcinoma cell line (i.e., A2780 cells), and in clinical samples from ovarian carcinoma patients relapsing upon platinum-based chemotherapy. Besides MLH1, which is often mutated or downregulated in the context of CDDP resistance,126, 127, 128, 129 these genes include Rho GDP dissociation inhibitor β beta (ARHGDIB), armadillo repeat containing, X-linked 2 (ARMCX2), collagen, type I, α1 (COL1A1), filamin Aα (FLNA) filamin Cγ (FLNC), mesoderm-specific transcript (MEST), neurotensin (NTS) and proteasome (prosome, macropain) subunit, β type, 9 (PSMB9), pointing to the existence of hitherto unexplored mechanisms whereby malignant cells can lose their sensitivity to CDDP.220 Ivanova et al.221 applied a very similar experimental strategy to study gastric cancer, identifying bone morphogenetic protein 4 (BMP4) as an epigenetically regulated factor that underpins CDDP (but not oxaliplatin) resistance and exerts oncogenic functions (at least in this setting) by promoting the epithelial-to-mesenchymal transition (EMT). It remains unclear whether the oncogenic activity of BMP4, similar to that of IL-7, relies on autocrine signaling circuitries.
The profound implication of the DNA repair machinery and the BCL-2 protein family in CDDP resistance has been confirmed by a large number of recent transcriptomic studies.118, 124, 222, 223, 224, 225, 226 However, none of these factors has yet been validated as a reliable clinical predictor of CDDP sensitivity.124 Alongside, the abundance of transcripts coding for several proteins that have no obvious links with CDDP-elicited signaling pathway has been correlated with clinical CDDP resistance. These factors include AKT1, eukaryotic translation initiation factor 4B (EIF4B) and ribosomal protein S6 (RPS6), defining a metabolic signature linked to the activity of mammalian target of rapamycin (mTOR);227 cytochrome P450, family 4, subfamily B, polypeptide 1 (CYP4B1), choline/ethanolamine phosphotransferase 1 (CEPT1) and charged multivesicular body protein 4A (CHMP4A),228 as well as several proteins implicated in the EMT.229 However, whether the EMT triggers a specific pathway of CDDP resistance or these observations rather reflect the high number of genetic and epigenetic alterations that characterize advanced neoplastic lesions (underpinning their aggressiveness and chemoresistance) remains to be determined. Furthermore, by analyzing HNSCC biopsies with whole-genome microarrays and quantitative reverse transcriptase PCR cards, Tomkiewicz et al.230 delineated a 10-gene signature that predicts the response of HNSCC patients to CDDP/5-FU-based induction chemotherapy. As it stands, most of these genes have not been previously implicated in CDDP resistance, although some of them code for factors that have a role in the cytoprotective response to redox stress, such as DnaJ (Hsp40) homolog, subfamily A, member 1 (DNAJA1) and thioredoxin domain containing 9 (TXNDC9).230
By comparing the transcriptomic profile of NSCLC A549 cells exposed to cytotoxic doses of CDDP, cadmium dichloride (CdCl2, an environmental pollutant that triggers oxidative stress-dependent apoptosis) and C2-ceramide (a plasma membrane permeant ceramide with mitochondriotoxic effects), we have recently demonstrated that CDDP elicits signaling cascades (encompassing both pro-survival and pro-death pathways) that are for the most part ‘private', that is, they manifest limited overlap with the molecular mechanisms set in motion by other inducers of mitochondrial apoptosis.231 Along similar lines, by studying the microRNAome of A549 cells succumbing to CDDP, CdCl2 or C2-ceramide, we identified miR-181a and miR-630 as functional components of the molecular mechanisms that underpin CDDP toxicity and the general cytoprotective response of A549 cells to stress, respectively.232 On a similar note, several research groups have recently investigated the microRNAomic profile of CDDP-sensitive versus CDDP-resistant cancer cell lines of various origin, including glioblastoma,233 tongue squamous cell carcinoma (TSCC),234 NSCLC,235, 236, 237, 238 hepatocellular carcinoma (HCC)239 and ovarian carcinoma.240, 241 Several of these studies identified the downregulation of microRNAs that inhibit the expression of anti-apoptotic BCL-2-like proteins (e.g., miR-135 and miR-497) as a prominent mechanism of CDDP resistance.235, 236 In addition, a decrease in the sensitivity of cancer cells to the cytostatic/cytotoxic effects of CDDP has been associated with the upregulation of microRNAs that target the major oncosuppressive factor phosphatase and tensin homolog (PTEN), such as miR-130a and miR-93,240, 241 and ERCC1, like miR-138,237 as well as with the downregulation of microRNAs that normally limit the expression of cell cycle-associated proteins such as let-7b.233 Similarly, CDDP resistance in cultured A549 cells has been correlated with the downregulation of a long non-coding RNA (AK126698) that inhibits WNT/β-catenin signaling.238
At odds with our observations, elevated expression levels of miR-181a have been associated with a decreased (rather than increased) sensitivity of cultured HCC cells to multiple antineoplastic agents, including CDDP.239 Such an apparent discrepancy may originate from the experimental systems employed (i.e., acute response to CDDP in NSCLC cells versus stably acquired resistance in HCC cells) or reflect a context-dependent role of miR-181a. This said, elevated expression levels of miR-181 species (in the context of a 58-component microRNA signature) have been shown to predict the response of gastric carcinoma patients to CDDP/5-FU-based chemotherapy.242 The precise elucidation of the molecular targets of miR-181 may provide important insights this issue. In another clinical study, the upregulation of miR-135b and miR-145 in response to trimodal therapy (concurrent CDDP, irinotecan and irradiation, followed by surgery) has been associated with poor disease-free survival (DFS) among esophageal carcinoma patients,243 but the molecular mechanisms underpinning these observations have not been further investigated. Interestingly, microRNAomic studies also implicate the EMT in CDDP resistance. In particular, miR-200b and miR-15b (which are downregulated in CDDP-resistant TSCC cells) turned out to actively inhibit the EMT (hence exerting oncosuppressive effects) by targeting the polycomb ring finger oncogenic factor BMI1.234 In line with this notion, reduced intratumoral levels of miR-200b or miR-15b have been associated with clinical CDDP resistance and poor survival in a cohort of TSCC patients.234 Of note, the expression of several microRNAs and proteins that contribute to CDDP resistance is under the control of epigenetic mechanisms, including DNA methylation and histone acetylation.244, 245, 246, 247 This raises the intriguing possibility that pharmacological inhibitors of DNA methyltransferases and histone deacetylases, some of which are currently approved by international regulatory agencies for use in cancer patients, may limit CDDP resistance at least in part by modulating the abundance of specific microRNAs.247, 248, 249, 250, 251, 252 Robust experimental evidence in support of this hypothesis is lacking.
Various aspects of CDDP resistance have been uncovered by proteomic approaches. By combining quantitative proteomics with interaction network analyses, Chavez et al.253 demonstrated that the levels of no less than 374 proteins are altered in CDDP-resistant (as compared with wild-type, CDDP-sensitive) cervical carcinoma HeLa cells, including various components of energy-producing metabolic pathways as well as proteins involved in DNA repair and other stress response mechanisms. Zeng et al.254 found CDDP-insensitive NSCLC A549 cells to differ from their CDDP-susceptible counterparts in the expression levels of 9 proteins, including parkinson protein 7 (PARK7, best known as DJ-1), a transcription-modulatory and antioxidant factor implicated in the development of familiar Parkinson disease. These findings had an important clinical correlate, as elevated amounts of DJ-1 turned out to predict poor disease outcome in a cohort of 67 locally advanced NSCLC patients.254 Along similar lines, Roesch et al.255 demonstrated that melanoma cells exposed for long periods to suboptimal CDDP concentrations not only express increased levels of the histone demethylase Jumonji, AT-rich interactive domain 1 (JARID1) but also contain increased amounts of several enzymes implicated in mitochondrial respiration. Importantly, inhibition of the mitochondrial respiratory chain appears to prevent the accumulation of these slowly proliferating CDDP-insensitive cells, delineating a strategy for the selective elimination of malignant cell clones that initially resist CDDP-based chemotherapy.255 Accordingly, the response of esophageal adenocarcinoma patients to neo-adjuvant CDDP-based chemotherapy correlates with mitochondrial defects provoked by the loss of specific cytochrome c oxidase (COX) subunits.256 On a slightly different note, Vasko et al.257 investigated the acute proteomic alterations caused by therapeutic concentrations of CDDP in renal cell carcinoma cells. In this context, CDDP-treated cells were found to express increased levels of multiple cytoskeletal proteins, molecular chaperones of the HSP family and glycolytic enzymes.257 Taken together, these observations suggest that cancer cells acutely exposed to CDDP might temporarily rely on glycolysis as a source of energy (presumably reflecting the mitochondriotoxic effects of cytoplasmic CDDP), whereas the acquisition of stable CDDP resistance might require a robust respiratory activity.255, 256, 257 At least in part, this is at odds with our recent findings, indicating that CDDP resistance can be mediated by the activation of a PPARGC1B-dependent signaling pathway that is elicited by respiratory chain defects but does not depend on mitochondrial biogenesis.150 Thus, the precise contribution of glycolysis and mitochondrial respiration to the development of CDDP resistance remains to be clarified.
As an alternative to conventional whole-cell approaches, proteomic studies to elucidate CDDP resistance have been performed on enriched subcellular compartments (e.g., nuclei, mitochondria),258, 259, 260 purified components known to participate in CDDP-elicited signaling pathways (e.g., immobilized CDDP, Y-box binding protein 1),149, 261 as well as on serum samples from cancer patients.262, 263 The latter approach allowed for the identification of α1-antitripsin (a circulating protease inhibitor with anti-inflammatory functions) and S100A9 (a Ca2+-binding protein that exerts immunomodulatory effects) as possible biomarkers of clinical CDDP resistance.262, 263 Moreover, CDDP resistance has been associated with immunopeptidomic alterations, that is, changes in the composition and abundance of peptides presented on the cell surface in complex with MHC molecules.260 This is particularly important in view of the facts that (i) several anticancer regimens operate by eliciting or boosting anticancer immunity, some of them by altering the immunopeptidome of malignant cells264, 265 and that (ii) CDDP is generally considered as a non-immunogenic anticancer agent.24, 25 Thus, it is tempting to speculate that CDDP resistance might involve specific changes in the immunopeptidome of neoplastic cells as well as in the tumor microenvironment that prevent the activation of anticancer immune responses. Formal evidence in support of this hypothesis is missing.
Large-scale silencing approaches and functional screenings
In the mid 2000s, key technological advances have allowed researchers to tackle CDDP resistance with large-scale functional/empirical approaches, as opposed to high-throughput observational studies followed by low-throughput functional validation (such as the genomic, methylomic, transcriptomic and proteomic approaches described above). These developments include (but perhaps are not limited to): (i) the availability of the first genome-wide siRNA sets, at least theoretically permitting the downregulation of each mRNA encoded by the human or murine genome;266, 267 (ii) the explosion of combinatorial chemistry, allowing for the generation of huge libraries of compounds for investigational purposes268, 269 and (iii) the rapid diffusion of fully robotized platforms compatible with the sterile handling of 96- and 384-well plates.270, 271 Thus, along with the establishment of reproducible and cost-efficient workflows, large-scale silencing approaches and ample chemical screenings have been undertaken by several groups worldwide, providing profound insights into the phenomenon of CDDP resistance.231, 272, 273, 274, 275, 276, 277 It should be noted that these functional/empirical approaches are not exempted from validation. Rather, primary hits must be confirmed upon (i) the exclusion of putative off-target effects and the establishment of a robust cause–effect relationship between the downregulation of a specific protein and CDDP resistance (possibly coupled to the elucidation of the underlying mechanisms) or (ii) the precise identification of the molecular target(s) through which a given chemical alters the sensitivity of cancer cells to CDDP (and again the clarification of mechanistic aspects of this phenomenon). Similar to the genomic, transcriptomic, methylomic and proteomic approaches described above, these functional/empiric approaches rely on homotypic experimental settings, mainly cultured cancer cells, de facto overlooking the notion that CDDP resistance (at least in some settings) might originate from alterations of the tumor microenvironment or systemic defects (see below). Approximately in the same period, investigators have attempted to elucidate the mechanisms that limit the sensitivity of malignant cells to CDDP by taking advantage of well-established, genetically tractable models that would be compatible with ample knockout strategies, such as Saccharomyces cerevisiae, Schizosaccharomyces pombe and Dictyostelium discoideum.89, 231, 278, 279, 280, 281 Although these genetic approaches are cost-efficient and straightforward, hits must be subjected to multiple, intense rounds of cross-species functional validation.
We have been working intensively along these lines to obtain clinically relevant insights into CDDP resistance. As a result of a genome-wide siRNA screening approach, we identified the metabolism of vitamin B6 as a central regulator of the response of cancer cells to a wide array of nutritional, physical and chemical stress conditions, including the administration of CDDP.272, 282 In particular, we found that the cytotoxic response of a large panel of cancer cells to stress is significantly exacerbated in the presence of a cell permeant B6 vitamer, pyridoxine. Such an effect was only observed when cells expressed pyridoxal kinase (PDXK), the enzyme that generates the bioactive form of vitamin B6 (pyridoxal-5-phosphate). In line with this notion, the downregulation of PDXK conferred a significant degree of protection from stress to cancer cells, while that of pyridoxal phosphatase (PDXP), the enzyme that degrades bioactive vitamin B6, caused a robust increase in the cytotoxicity of several stimuli.272 Importantly, NSCLC patients (from two distinct cohorts, one from Europe and one from North America) bearing lesions that expressed high levels of PDXK had an improved disease-free and overall survival (OS) than individuals affected by tumors with low PDXK levels, irrespective of therapy.272 Of note, the prognostic value of the vitamin B6 metabolism among NSCLC patients was clear when PDXK levels were quantified by immunohistochemistry (IHC) in malignant cells, but neither when the adjacent, non-malignant lung tissue was analyzed (implying that the effect of vitamin B6 on disease progression originates in the tumor) nor when the amount of the PDXK-coding mRNA was assessed in tumor biopsies (perhaps suggesting that PDXK is subjected to a relevant degree of post-transcriptional regulation).272 Although the precise molecular mechanisms whereby vitamin B6 sensitizes malignant cells to adverse conditions remain unclear, we found that the administration of pyridoxine (i) facilitates the establishment of oxidative stress (presumably explaining the influence of vitamin B6 on tumor progression)272 and (ii) promotes the intracellular accumulation of CDDP (at least in part accounting for the ability of vitamin B6 to promote the cytotoxicity of CDDP, in vitro and in vivo).273 Both these effects were strictly dependent on the presence of PDXK, implying that they both are mediated by pyridoxal-5-phosphate.272, 273, 282 Of note, the same study led us to identify 84 additional factors that influence the response of NSCLC to CDDP, including lipase, hepatic (LIPC).283 Similar to those of PDXK, the intratumoral levels of LIPC turned out to positively correlate with disease outcome (independent of several other prognostic factors, including PDXK expression) in two distinct cohorts of NSCLC patients.283 Moreover, in one of these relatively small cohorts, LIPC expression levels tended to have a predictive (as opposed to merely prognostic) value.283 Thus, although validation in large patient cohorts is still pending, LIPC expression levels may constitute a biomarker that allows for the identification of patients who obtain a true clinical benefit from the administration of platinum-based chemotherapy. This said, the molecular mechanisms through which LIPC influences tumor progression (and perhaps response to chemotherapy) remain obscure.
A few other groups have undertaken global silencing approaches to discover novel factors involved in CDDP resistance. For instance, Ho et al.277 found that the GTPase RAS homolog family member J (RHOJ) and p21 protein (CDC42/RAC)-activated kinase 1 (PAK1) render melanoma cells resistant to DNA-damaging agents (including dacarbazine and CDDP) by uncoupling ATR from its downstream effectors, resulting in the expression of the survival gene SRY (sex determining region Y)-box 10 (SOX10). By focusing this strategy on lipid and protein kinases, Guerreiro et al.284 identified a set of six factors involved in the decreased sensitivity of medulloblastoma cells to CDDP, including ATR, membrane protein, palmitoylated 2 (MPP2), phosphatidylinositol 4-kinase, catalytic, α (PI4KA), phosphatidylinositol-4,5-bisphosphate 3-kinase, catalytic subunit γ (PIK3CG), STE20-related kinase adaptor α (STRADA, a pseudokinase also known as LYK5) and WNK lysine-deficient protein kinase 4 (WNK4). A kinome-wide siRNA screen allowed Salm et al.276 to identify fibroblast growth factor receptor 2 (FGFR2) as an important determinant of CDDP resistance in neuroblastoma. FGFR2 turned out to decrease the sensitivity of neuroblastoma cells to CDDP via an autocrine loop promoting the expression of BCL-2 and BCL-XL. Moreover, the expression levels of FGFR2 were shown to have a clinical relevance, as they correlated with the amplification of v-myc avian myelocytomatosis viral oncogene neuroblastoma-derived homolog (MYCN) and advanced disease stage.276 Of note, silencing approaches can also be implemented by means of microRNA mimics. By screening a panel of these reagents, Pouliot et al.285 demonstrated that several microRNAs of the miR-15 family, including miR-15a, miR-15b, miR-16, miR-16-1* and miR-424*, alter the sensitivity of epidermoid carcinoma cells to CDDP as they regulate the expression of cell cycle-associated kinases.
Large chemical libraries have been screened for the presence of compounds that may increase the susceptibility of malignant cells to the cytostatic/cytotoxic effects of CDDP. Using this approach, Chen et al.275 identified pimozide and GW7647, two distinct inhibitors of the deubiquitinase complex assembled by ubiquitin specific peptidase 1 (USP1) and WD repeat domain 48 (WDR48, also known as UAF1), as putative chemosensitizers in NSCLC cells. As the USP1/UAF1 complex is involved in TLS, pimozide and GW7647 may operate by limiting the ability of cancer cells to bypass CDDP-induced DNA lesions. By screening a validated library of kinase inhibitors, Wong et al.286 demonstrated that the inhibition of several signal transducers including AKT1, mTOR, NF-κB, transforming growth factor β receptor 1 (TGFBR1), phosphoinositide-3-kinase (PI3K) and various MAPKs sensitizes basal-like breast carcinoma cells to the toxic effects of CDDP. Specifically, the mTOR inhibitor rapamycin was shown to synergize with CDDP in the killing of basal-like MDA-MB-468, MDA-MB-231 and HCC1937 cells, but not luminal-like T47D or MCF-7 cells, owing to the induction of the p53-related protein p73.286 Jacquemont et al.274 tested >16 000 compounds for their ability to inhibit the formation of DNA damage foci triggered by irradiation or CDDP, identifying a dozen molecules that sensitize ovarian cancer cells to the cytotoxic effects of the latter. These molecules include several inhibitors of the Fanconi anemia (FA) DNA repair pathway (which coordinates HR and TLS for the repair of interstrand crosslinks) and indeed were confirmed to exacerbate the cytostatic/cytotoxic potential of CDDP in FA-proficient but not in FA-deficient ovarian cancer cells. Finally, Garnett et al.287 recently undertook a large screen to identify genes associated with the cellular responses to most anticancer agents available to date. To this aim, 130 drugs currently approved by regulatory agencies for use in cancer patients or under clinical development were tested on several hundred cancer cell lines, representing much of the tissue-type and genetic diversity of human tumors. This systematic screening approach generated several unexpected findings, including the extremely high sensitivity to both PARP inhibitors and CDDP of Ewing's sarcoma cells harboring a translocation that involves EWS RNA-binding protein 1 (EWSR1) and Fli-1 proto-oncogene, ETS transcription factor (FLI1).287
Functional screenings recently performed in genetically tractable organisms have also unveiled various aspects of CDDP resistance. For instance, by screening the clonogenic potential of a collection of S. cerevisiae mutants exposed to an overtly toxic amount of CDDP, we demonstrated that the molecular cascades elicited by CDDP, CdCl2 and C2-ceramide in yeast exhibit very limited overlaps.231 Indeed, none of the S. cerevisiae mutants tested was protected from the cytostatic/cytotoxic potential of all these compounds. Of note, Δpor2 cells, lacking one isoform of the yeast ortholog of human VDAC1, turned out to be specifically protected against CDDP cytotoxicity, indicating that the molecular mechanisms that underlie CDDP resistance are phylogenetically conserved.231 Upon the development of a high-resolution high-throughput screening (HTS) platform, Sletta et al.281 tested the response of >1400 S. cerevisiae deletion mutants to three distinct DNA-damaging agents, namely, methyl methanesulphonate, 5-FU and CDDP, identifying MAPK7 (also known as ERK5) as an evolutionarily ancient regulator of the DNA damage response that also operates in human cells. Using 2359 non-essential haploid deletion mutants of the fission yeast S. pombe, Gatti et al.280 identified no less than 13 distinct gene products involved in the ubiquitin-proteasome system whose absence increases (3 proteins) or decreases (10 proteins) CDDP sensitivity. Although these authors did not validate their findings in mammalian systems, we and others confirmed the implication of the proteasome in the response of human cells to CDDP.220, 272
Multiplex genotyping studies
During the last decade, dozens of studies have been carried out to determine whether SNPs affecting one or more genetic loci would influence the natural course of multiple neoplasms (leading to the identification of prognostic biomarkers) or the propensity of neoplastic lesions to respond to specific therapeutic regimens, including CDDP-based chemotherapy (resulting in the characterization of predictive biomarkers).288, 289, 290, 291 This approach has obvious clinical implications because it may lead to the identification of patient subgroups that will benefit from treatment, and hence allow for the implementation of personalized medicine.292 Moreover, the identification of genetic backgrounds that influence the antineoplastic activity of CDDP would have one major advantage as compared with most strategies for the elucidation of resistance discussed above: it would intrinsically consider all possible cancer cell-extrinsic factors that may impact on this phenomenon, irrespective of whether they have previously been described or not. However, until recently, genotyping studies could only focus on SNPs affecting one (or a few) protein(s) at the same time, owing to technical limitations. Thus, near-to-invariably, these approaches would study SNPs known or expected to influence cellular or organismal processes linked to CDDP sensitivity, such as CDDP extrusion (e.g., SNPs affecting ABCC2), DNA damage-elicited signal transduction (e.g., SNPs affecting ERCC1), apoptosis (e.g., SNPs affecting caspases) or immune responses (e.g., SNPs affecting cytokines or their receptors).293, 294, 295, 296, 297, 298 This limitation has nowadays been circumvented by the introduction of technical platforms that allow for the simultaneous determination of up to 384 different SNPs in one single assay.299, 300, 301
A few genotyping studies of this type have already been performed to obtain insights into the organismal aspects of CDDP resistance. Tan et al.302 reported that two SNPs affecting the expression levels of death-associated protein kinase 3 (DAPK3) and methyltransferase like 6 (METTL6) influenced disease outcome in 222 small cell lung carcinoma and 961 NSCLC patients treated with platinum-based therapy. Accordingly, the knockdown of DAPK3 and METTL6 was shown to significantly decrease CDDP sensitivity in lung carcinoma cells.302 By adopting a similar approach, Wheeler et al.303 tested the association between 2 million SNPs and the response of lymphoblastoid cell lines (LCLs) derived from 83 individuals of African American descent to cytarabine, 5′-deoxyfluorouridine, carboplatin and CDDP. This study led to the identification of 41 genes that putatively influence the response of African Americans to CDDP-based chemotherapy. Notably, the set of SNPs associated with the sensitivity of LCLs to CDDP exhibited some degree of overlap with that linked to carboplatin susceptibility, reinforcing the notion that platinum derivatives activate similar, but not identical, signal transduction cascades.303 More recently, the same authors expanded their analysis to a total of 608 LCLs and >3 million SNPs, reporting a robust association between CDDP susceptibility and a SNP upstream of keratin 16 pseudogene 2 (KRT16P2) as well as a link between the sensitivity of LCLs to CDDP and SNPs affecting BCL2, ERCC2, ERCC6, glutathione S-transferase μ1 (GSTM1) and glutathione S-transferase θ1 (GSTT1).304 Li et al.305 analyzed the association between 894 SNPs affecting 70 genes involved in glutathione metabolism, DNA repair, cell cycle and epidermal growth factor receptor (EGFR) signaling and disease outcome among 1076 NSCLC patients treated with various platinum-based chemotherapeutic regimens. Globally, SNPs affecting glutathione synthetase (GSS) and mitogen-activated protein kinase kinase kinase 1 (MAP3K1) were linked to poor OS. Moreover, SNPs affecting MAP3K1 turned out to negatively influence the OS of NSCLC patients treated with platinum derivatives plus a taxane, whereas SNPs in the v-raf-1 murine leukemia viral oncogene homolog 1 (RAF1) had an opposite effect on the OS of this patient subgroup. Along similar lines, SNPs involving RAF1 (as well as glutathione peroxidase 5, GPX5) were associated with improved OS among patients receiving platinum derivatives plus gemcitabine. Conversely, SNPs affecting GPX7 and neuroblastoma RAS viral (v-ras) oncogene homolog (NRAS) had a negative influence on OS in these individuals.305 Wu et al.306 studied the influence of 307 260 distinct SNPs on the survival of three distinct cohorts of (327, 315 and 420) advanced-stage NSCLC patients who received platinum-based chemotherapy with or without irradiation, identifying a relatively reproducible (though not absolute) association between SNPs in chemokine-like receptor 1 (CMKLR1) and sortilin-related VPS10 domain containing receptor 2 (SORCS2) and poor OS. Along similar lines, Hu et al.307 performed a fast-track genotyping study on three distinct cohorts of (307, 228 and 340) NSCLC patients receiving first-line platinum-based chemotherapy, finding an association between three SNPs (rs7629386 at 3p22.1, rs969088 at 5p14.1 and rs3850370 at 14q24.3) and poor OS as well as a link between two SNPs (rs41997 at 7q31.31 and rs12000445 at 9p21.3) and improved OS. These studies and others of a similar type to come may provide significant insights into the organismal aspect of CDDP resistance.
Concluding Remarks
Throughout the last 4 decades, several millions of cancer patients have been treated with CDDP-based chemo(radio)therapeutic regimens. Exception made for individuals bearing testicular germ cell tumors, most of these patients only obtained temporary clinical benefits, which were destined to vanish along with the progressive transition of malignant cells from a chemosensitive to a chemoresistant state. Thus, even though CDDP provokes moderate-to-severe adverse effects in a fraction of patients, the most prominent hurdle related to the use of CDDP in the clinical practice is posed by the elevated incidence of chemoresistance, be it innate or acquired. An intense wave of preclinical and clinical investigation aimed at developing strategies to restore the sensitivity of human neoplasms to the cytostatic/cytotoxic effects of CDDP has been initiated worldwide in the late 1970s. Reflecting the huge clinical and socioeconomic impact of CDDP resistance, such an investigational effort has not yet come to an end. Indeed, although several strategies have been devised to circumvent CDDP resistance in cancer patients, including (but not limited to) the use of CDDP in combination with targeted anticancer agents such as erlotinib (a pharmacological inhibitor of the tyrosine kinase activity of EGFR), trastuzumab (an EGFR-specific antibody) and bevacizumab (a vascular endothelial growth factor-blocking antibody), a majority of these combinatorial regimens failed to improve the therapeutic profile of CDDP in randomized clinical trials.308, 309, 310
Past
Before the advent of high-content, high-throughput technologies, the elucidation of the molecular mechanisms underpinning the cytostatic/cytotoxic effects of CDDP has long been under the limelight. It was indeed tempting to accept the relatively straightforward assumptions that (i) CDDP would exert antineoplastic effects by binding one (or a few) molecular target(s), hence activating relatively simple signaling pathways, and (ii) that CDDP resistance would originate from the activation of mutually exclusive mechanisms. Starting with the early 1990s, the attention of molecular oncologists focused on the cellular factors that are responsible for the detection and repair of CDDP-elicited DNA lesions, and on the mechanisms whereby these systems transduce a lethal signal when damage is irreparable (on-target and post-target resistance). The increasingly more precise characterization of plasma membrane transporters that actively extrude various xenobiotics, including several members of the ATP-binding cassette (ABC) protein family,311 led to the hypothesis that CDDP resistance would mainly involve pre-target mechanisms. Such a hypothesis, however, was soon disconfirmed by findings from many groups indicating that the inhibition of ABC transporters does not suffice to reinstate CDDP sensitivity. In the early 2000s, the role of copper transporters in the uptake and extrusion of CDDP became clear, yet the experimental modulation of these systems also failed to completely abolish the insensitivity of some cancer cells to CDDP. Thus, the redundant and multifactorial nature of CDDP resistance has been established even before the advent of high-content, high-throughput technologies.
Present
The automation of functional screenings and the introduction of high-content experimental platforms have literally revolutionized the study of several biological phenomena, including CDDP resistance. The first high-content, high-throughput technologies (such as mRNA and CGH microarrays and mass spectrometry) have been available for more than a decade, but the true explosion of this area has occurred in the late 2000s, along with the commercialization of platforms such as automated fluorescence microscopes and the introduction of innovative protocols like deep sequencing. Several attempts have recently been made to harness these technologies for empirically resolving the clinical hurdle posed by CDDP resistance, with very promising results. In addition, increasingly more detailed insights into the complexity of the signal transduction pathways triggered by CDDP in cancer and normal cells have been obtained. Nonetheless, no chemosensitization strategies have yet been introduced into the clinical practice (though a few of them are currently being investigated in clinical settings).
Future
Accumulating evidence indicates that, contrarily to long-standing beliefs, the limited sensitivity of human neoplasms to CDDP may reflect not only cell-intrinsic but also cell-extrinsic alterations.24, 25, 312, 313 On one hand, CDDP appears to be much less effective than other chemotherapeutics, including oxaliplatin (in spite of their chemical resemblance), at inducing the immunogenic demise of cancer cells.314, 315 On the other hand, it has recently been shown that CDDP resistance is significantly influenced by non-transformed mesenchymal stem cells and other components of the tumor stroma, including proteins of the extracellular matrix and immunosuppressive cell populations.312, 313, 316 Until now, such a cell-extrinsic dimension of CDDP resistance was largely underestimated. We surmise that the application of high-content, high-throughput technologies to heterotypic experimental settings that involve not only malignant cells but also various other components of the tumor microenvironment (including endothelial, mesenchymal and immune cells) is the key for the development of efficient strategies to restore the sensitivity of multiple human neoplasms to CDDP. Along similar lines, we believe that the untargeted metabolomic profiling of CDDP-sensitive versus CDDP-resistant tumor models, an approach that only recently has begun to be applied to the study of chemoresistance,313, 317, 318 may yield important insights into this considerable clinical impediment.
Acknowledgments
The authors are supported by the Ligue contre le Cancer (équipe labellisée); Agence National de la Recherche (ANR); Association pour la recherche sur le cancer (ARC); Associazione Italiana per la Ricerca sul Cancro (AIRC); Cancéropôle Ile-de-France; AXA Chair for Longevity Research; Institut National du Cancer (INCa); Fondation Bettencourt-Schueller; Fondation de France; Fondation pour la Recherche Médicale (FRM); the European Commission (ArtForce); the European Research Council (ERC); the LabEx Immuno-Oncology; the LabEx LERMIT; the SIRIC Stratified Oncology Cell DNA Repair and Tumor Immune Elimination (SOCRATE); the SIRIC Cancer Research and Personalized Medicine (CARPEM) and the Paris Alliance of Cancer Research Institutes (PACRI).
Glossary
- 5-FU
5-fluorouracil
- ABCC2
ATP-binding cassette, subfamily C (CFTR/MRP), member 2
- AKT1
v-akt murine thymoma viral oncogene homolog 1
- ATP7B
ATPase, Cu2+ transporting, β polypeptide
- BMP4
bone morphogenetic protein 4
- BRCA1
breast cancer 1, early onset
- BRCA2
breast cancer 2, early onset
- CDDP
cis-diamminedichloroplatinum(II)
- CDK2
cyclin-dependent kinase 2
- CTR1
copper transporter 1
- DAPK3
death-associated protein kinase 3
- DFS
disease-free survival
- DSB
double-strand break
- DYRK1B
dual-specificity Y-phosphorylation regulated kinase 1B
- EGFR
epidermal growth factor receptor
- EMT
epithelial-to-mesenchymal transition
- ER
endoplasmic reticulum
- ERCC1
excision repair cross-complementing rodent repair deficiency, complementation group 1
- FA
Fanconi anemia
- FDA
Food and Drug Administration
- FGFR2
fibroblast growth factor receptor 2
- GSH
reduced glutathione
- GST
glutathione S-transferase
- HCC
hepatocellular carcinoma
- HNSCC
head and neck squamous cell carcinoma
- HR
homologous recombination
- HSP
heat-shock protein
- HTS
high-throughput screening
- IL-7
interleukin-7
- LCL
lymphoblastoid cell line
- LIPC
lipase, hepatic
- MAPK
mitogen-activated protein kinase
- MAP3K1
mitogen-activated protein kinase kinase kinase 1
- METTL6
methyltransferase like 6
- MLH1
mutL homolog 1
- MMR
mismatch repair
- MOMP
mitochondrial outer membrane permeabilization
- MSH2
mutS homolog 2
- mTOR
mammalian target of rapamycin
- NER
nucleotide excision repair
- NSCLC
non-small cell lung carcinoma
- OS
overall survival
- PARP1
poly(ADP-ribose) polymerase 1
- PDXK
pyridoxal kinase
- PPARGC1B
peroxisome proliferator-activated receptor γ, coactivator 1β
- PTPC
permeability transition pore complex
- RAF1
v-raf-1 murine leukemia viral oncogene homolog 1
- ROS
reactive oxygen species
- SNP
single-nucleotide polymorphism
- TLS
translesion synthesis
- TSCC
tongue squamous cell carcinoma
- USP1
ubiquitin specific peptidase 1
- VDAC1
voltage-dependent anion channel 1
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies this paper on Cell Death and Disease website (http://www.nature.com/cddis)
Edited by B Zhivotovsky
Supplementary Material
References
- Peyrone M. Ueber die Einwirkung des Ammoniaks auf Platinchlorür. Ann Chemie Pharm. 1844;51:1–29. [Google Scholar]
- Burchenal JH, Kalaher K, Dew K, Lokys L. Rationale for development of platinum analogs. Cancer Treat Rep. 1979;63:1493–1498. [PubMed] [Google Scholar]
- Rosenberg B, Vancamp L, Krigas T. Inhibition of cell division in Escherichia coli by electrolysis products from a platinum electrode. Nature. 1965;205:698–699. doi: 10.1038/205698a0. [DOI] [PubMed] [Google Scholar]
- Rosenberg B, VanCamp L, Trosko JE, Mansour VH. Platinum compounds: a new class of potent antitumour agents. Nature. 1969;222:385–386. doi: 10.1038/222385a0. [DOI] [PubMed] [Google Scholar]
- Lebwohl D, Canetta R. Clinical development of platinum complexes in cancer therapy: an historical perspective and an update. Eur J Cancer. 1998;34:1522–1534. doi: 10.1016/s0959-8049(98)00224-x. [DOI] [PubMed] [Google Scholar]
- Kelland LR, Sharp SY, O'Neill CF, Raynaud FI, Beale PJ, Judson IR. Mini-review: discovery and development of platinum complexes designed to circumvent cisplatin resistance. J Inorg Biochem. 1999;77:111–115. [PubMed] [Google Scholar]
- Kelland L. The resurgence of platinum-based cancer chemotherapy. Nat Rev Cancer. 2007;7:573–584. doi: 10.1038/nrc2167. [DOI] [PubMed] [Google Scholar]
- Prestayko AW, D'Aoust JC, Issell BF, Crooke ST. Cisplatin (cis-diamminedichloroplatinum II) Cancer Treat Rev. 1979;6:17–39. doi: 10.1016/s0305-7372(79)80057-2. [DOI] [PubMed] [Google Scholar]
- Galanski M. Recent developments in the field of anticancer platinum complexes. Recent Pat Anticancer Drug Discov. 2006;1:285–295. doi: 10.2174/157489206777442287. [DOI] [PubMed] [Google Scholar]
- Winter C, Albers P. Testicular germ cell tumors: pathogenesis, diagnosis and treatment. Nat Rev Endocrinol. 2011;7:43–53. doi: 10.1038/nrendo.2010.196. [DOI] [PubMed] [Google Scholar]
- Feldman DR, Bosl GJ, Sheinfeld J, Motzer RJ. Medical treatment of advanced testicular cancer. JAMA. 2008;299:672–684. doi: 10.1001/jama.299.6.672. [DOI] [PubMed] [Google Scholar]
- Koberle B, Tomicic MT, Usanova S, Kaina B. Cisplatin resistance: preclinical findings and clinical implications. Biochim Biophys Acta. 2010;1806:172–182. doi: 10.1016/j.bbcan.2010.07.004. [DOI] [PubMed] [Google Scholar]
- Ozols RF. Ovarian cancer: new clinical approaches. Cancer Treat Rev. 1991;18 (Suppl A:77–83. doi: 10.1016/0305-7372(91)90027-w. [DOI] [PubMed] [Google Scholar]
- Giaccone G.Clinical perspectives on platinum resistance Drugs 200059(Suppl 49–17.discussion 37-18. [DOI] [PubMed] [Google Scholar]
- Cvitkovic E, Spaulding J, Bethune V, Martin J, Whitmore WF. Improvement of cis-dichlorodiammineplatinum (NSC 119875): therapeutic index in an animal model. Cancer. 1977;39:1357–1361. doi: 10.1002/1097-0142(197704)39:4<1357::aid-cncr2820390402>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- Mendoza J, Martinez J, Hernandez C, Perez-Montiel D, Castro C, Fabian-Morales E, et al. Association between ERCC1 and XPA expression and polymorphisms and the response to cisplatin in testicular germ cell tumours. Br J Cancer. 2013;109:68–75. doi: 10.1038/bjc.2013.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Usanova S, Piee-Staffa A, Sied U, Thomale J, Schneider A, Kaina B, et al. Cisplatin sensitivity of testis tumour cells is due to deficiency in interstrand-crosslink repair and low ERCC1-XPF expression. Mol Cancer. 2010;9:248. doi: 10.1186/1476-4598-9-248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welsh C, Day R, McGurk C, Masters JR, Wood RD, Koberle B. Reduced levels of XPA, ERCC1 and XPF DNA repair proteins in testis tumor cell lines. Int J Cancer. 2004;110:352–361. doi: 10.1002/ijc.20134. [DOI] [PubMed] [Google Scholar]
- Cavallo F, Graziani G, Antinozzi C, Feldman DR, Houldsworth J, Bosl GJ, et al. Reduced proficiency in homologous recombination underlies the high sensitivity of embryonal carcinoma testicular germ cell tumors to Cisplatin and poly (adp-ribose) polymerase inhibition. PLoS One. 2012;7:e51563. doi: 10.1371/journal.pone.0051563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tattersall MN. Ovarian cancer chemotherapy: carboplatin as standard. Lancet. 2002;360:500–501. doi: 10.1016/s0140-6736(02)09757-x. [DOI] [PubMed] [Google Scholar]
- Mandala M, Ferretti G, Barni S.Oxaliplatin in colon cancer N Engl J Med 20043511691–1692.(author reply 1691–1692). [DOI] [PubMed] [Google Scholar]
- Harrap KR. Preclinical studies identifying carboplatin as a viable cisplatin alternative. Cancer Treat Rev. 1985;12 (Suppl A:21–33. doi: 10.1016/0305-7372(85)90015-5. [DOI] [PubMed] [Google Scholar]
- Kidani Y, Inagaki K, Iigo M, Hoshi A, Kuretani K. Antitumor activity of 1,2-diaminocyclohexane–platinum complexes against sarcoma-180 ascites form. J Med Chem. 1978;21:1315–1318. doi: 10.1021/jm00210a029. [DOI] [PubMed] [Google Scholar]
- Martins I, Kepp O, Schlemmer F, Adjemian S, Tailler M, Shen S, et al. Restoration of the immunogenicity of cisplatin-induced cancer cell death by endoplasmic reticulum stress. Oncogene. 2011;30:1147–1158. doi: 10.1038/onc.2010.500. [DOI] [PubMed] [Google Scholar]
- Tesniere A, Schlemmer F, Boige V, Kepp O, Martins I, Ghiringhelli F, et al. Immunogenic death of colon cancer cells treated with oxaliplatin. Oncogene. 2010;29:482–491. doi: 10.1038/onc.2009.356. [DOI] [PubMed] [Google Scholar]
- Gore ME, Fryatt I, Wiltshaw E, Dawson T, Robinson BA, Calvert AH. Cisplatin/carboplatin cross-resistance in ovarian cancer. Br J Cancer. 1989;60:767–769. doi: 10.1038/bjc.1989.356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamaguchi K, Godwin AK, Yakushiji M, O'Dwyer PJ, Ozols RF, Hamilton TC. Cross-resistance to diverse drugs is associated with primary cisplatin resistance in ovarian cancer cell lines. Cancer Res. 1993;53:5225–5232. [PubMed] [Google Scholar]
- Negoro K, Yamano Y, Nakashima D, Saito K, Nakatani K, Shiiba M, et al. Cross-resistance of platinum derivatives in H-1R, a cisplatin-resistant cell line. Oncol Rep. 2009;21:443–449. [PubMed] [Google Scholar]
- Johnson SW, Laub PB, Beesley JS, Ozols RF, Hamilton TC. Increased platinum-DNA damage tolerance is associated with cisplatin resistance and cross-resistance to various chemotherapeutic agents in unrelated human ovarian cancer cell lines. Cancer Res. 1997;57:850–856. [PubMed] [Google Scholar]
- Shen DW, Akiyama S, Schoenlein P, Pastan I, Gottesman MM. Characterisation of high-level cisplatin-resistant cell lines established from a human hepatoma cell line and human KB adenocarcinoma cells: cross-resistance and protein changes. Br J Cancer. 1995;71:676–683. doi: 10.1038/bjc.1995.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naredi P, Heath DD, Enns RE, Howell SB. Cross-resistance between cisplatin and antimony in a human ovarian carcinoma cell line. Cancer Res. 1994;54:6464–6468. [PubMed] [Google Scholar]
- Jones M, Siracky J, Kelland LR, Harrap KR. Acquisition of platinum drug resistance and platinum cross resistance patterns in a panel of human ovarian carcinoma xenografts. Br J Cancer. 1993;67:24–29. doi: 10.1038/bjc.1993.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill BT, Shellard SA, Hosking LK, Dempke WC, Fichtinger-Schepman AM, Tone T, et al. Characterization of a cisplatin-resistant human ovarian carcinoma cell line expressing cross-resistance to 5-fluorouracil but collateral sensitivity to methotrexate. Cancer Res. 1992;52:3110–3118. [PubMed] [Google Scholar]
- Nishio K, Sugimoto Y, Nakagawa K, Niimi S, Fujiwara Y, Bungo M, et al. Cross-resistance to tumour promoters in human cancer cell lines resistant to adriamycin or cisplatin. Br J Cancer. 1990;62:415–419. doi: 10.1038/bjc.1990.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz JL, Rotmensch J, Beckett MA, Jaffe DR, Toohill M, Giovanazzi SM, et al. X-ray and cis-diamminedichloroplatinum(II) cross-resistance in human tumor cell lines. Cancer Res. 1988;48:5133–5135. [PubMed] [Google Scholar]
- Shen D, Pastan I, Gottesman MM. Cross-resistance to methotrexate and metals in human cisplatin-resistant cell lines results from a pleiotropic defect in accumulation of these compounds associated with reduced plasma membrane binding proteins. Cancer Res. 1998;58:268–275. [PubMed] [Google Scholar]
- Choy H. Satraplatin: an orally available platinum analog for the treatment of cancer. Expert Rev Anticancer Ther. 2006;6:973–982. doi: 10.1586/14737140.6.7.973. [DOI] [PubMed] [Google Scholar]
- Eckardt JR, Bentsion DL, Lipatov ON, Polyakov IS, Mackintosh FR, Karlin DA, et al. Phase II study of picoplatin as second-line therapy for patients with small-cell lung cancer. J Clin Oncol. 2009;27:2046–2051. doi: 10.1200/JCO.2008.19.3235. [DOI] [PubMed] [Google Scholar]
- Monneret C. Platinum anticancer drugs. From serendipity to rational design. Ann Pharm Fr. 2011;69:286–295. doi: 10.1016/j.pharma.2011.10.001. [DOI] [PubMed] [Google Scholar]
- Sternberg CN, Petrylak DP, Sartor O, Witjes JA, Demkow T, Ferrero JM, et al. Multinational, double-blind, phase III study of prednisone and either satraplatin or placebo in patients with castrate-refractory prostate cancer progressing after prior chemotherapy: the SPARC trial. J Clin Oncol. 2009;27:5431–5438. doi: 10.1200/JCO.2008.20.1228. [DOI] [PubMed] [Google Scholar]
- el-Khateeb M, Appleton TG, Gahan LR, Charles BG, Berners-Price SJ, Bolton AM. Reactions of cisplatin hydrolytes with methionine, cysteine, and plasma ultrafiltrate studied by a combination of HPLC and NMR techniques. J Inorg Biochem. 1999;77:13–21. doi: 10.1016/s0162-0134(99)00146-4. [DOI] [PubMed] [Google Scholar]
- Kelland LR.Preclinical perspectives on platinum resistance Drugs 200059(Suppl 41–8.(discussion 37–38). [DOI] [PubMed] [Google Scholar]
- Eastman A. Cross-linking of glutathione to DNA by cancer chemotherapeutic platinum coordination complexes. Chem Biol Interact. 1987;61:241–248. doi: 10.1016/0009-2797(87)90004-4. [DOI] [PubMed] [Google Scholar]
- Michalke B. Platinum speciation used for elucidating activation or inhibition of Pt-containing anti-cancer drugs. J Trace Elem Med Biol. 2010;24:69–77. doi: 10.1016/j.jtemb.2010.01.006. [DOI] [PubMed] [Google Scholar]
- Timerbaev AR, Hartinger CG, Aleksenko SS, Keppler BK. Interactions of antitumor metallodrugs with serum proteins: advances in characterization using modern analytical methodology. Chem Rev. 2006;106:2224–2248. doi: 10.1021/cr040704h. [DOI] [PubMed] [Google Scholar]
- Jordan P, Carmo-Fonseca M. Molecular mechanisms involved in cisplatin cytotoxicity. Cell Mol Life Sci. 2000;57:1229–1235. doi: 10.1007/PL00000762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slater AF, Nobel CS, Maellaro E, Bustamante J, Kimland M, Orrenius S. Nitrone spin traps and a nitroxide antioxidant inhibit a common pathway of thymocyte apoptosis. Biochem J. 1995;306 (Pt 3:771–778. doi: 10.1042/bj3060771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galluzzi L, Senovilla L, Vitale I, Michels J, Martins I, Kepp O, et al. Molecular mechanisms of cisplatin resistance. Oncogene. 2012;31:1869–1883. doi: 10.1038/onc.2011.384. [DOI] [PubMed] [Google Scholar]
- Murata T, Hibasami H, Maekawa S, Tagawa T, Nakashima K. Preferential binding of cisplatin to mitochondrial DNA and suppression of ATP generation in human malignant melanoma cells. Biochem Int. 1990;20:949–955. [PubMed] [Google Scholar]
- Eastman A. The formation, isolation and characterization of DNA adducts produced by anticancer platinum complexes. Pharmacol Ther. 1987;34:155–166. doi: 10.1016/0163-7258(87)90009-x. [DOI] [PubMed] [Google Scholar]
- Yang Z, Schumaker LM, Egorin MJ, Zuhowski EG, Guo Z, Cullen KJ. Cisplatin preferentially binds mitochondrial DNA and voltage-dependent anion channel protein in the mitochondrial membrane of head and neck squamous cell carcinoma: possible role in apoptosis. Clin Cancer Res. 2006;12:5817–5825. doi: 10.1158/1078-0432.CCR-06-1037. [DOI] [PubMed] [Google Scholar]
- Bellon SF, Coleman JH, Lippard SJ. DNA unwinding produced by site-specific intrastrand cross-links of the antitumor drug cis-diamminedichloroplatinum(II) Biochemistry. 1991;30:8026–8035. doi: 10.1021/bi00246a021. [DOI] [PubMed] [Google Scholar]
- Chaney SG, Sancar A. DNA repair: enzymatic mechanisms and relevance to drug response. J Natl Cancer Inst. 1996;88:1346–1360. doi: 10.1093/jnci/88.19.1346. [DOI] [PubMed] [Google Scholar]
- Furuta T, Ueda T, Aune G, Sarasin A, Kraemer KH, Pommier Y. Transcription-coupled nucleotide excision repair as a determinant of cisplatin sensitivity of human cells. Cancer Res. 2002;62:4899–4902. [PubMed] [Google Scholar]
- Kunkel TA, Erie DA. DNA mismatch repair. Annu Rev Biochem. 2005;74:681–710. doi: 10.1146/annurev.biochem.74.082803.133243. [DOI] [PubMed] [Google Scholar]
- Kazak L, Reyes A, Holt IJ. Minimizing the damage: repair pathways keep mitochondrial DNA intact. Nat Rev Mol Cell Biol. 2012;13:659–671. doi: 10.1038/nrm3439. [DOI] [PubMed] [Google Scholar]
- Campisi J. Aging, cellular senescence, and cancer. Annu Rev Physiol. 2013;75:685–705. doi: 10.1146/annurev-physiol-030212-183653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Otin C, Blasco MA, Partridge L, Serrano M, Kroemer G. The hallmarks of aging. Cell. 2013;153:1194–1217. doi: 10.1016/j.cell.2013.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitale I, Galluzzi L, Castedo M, Kroemer G. Mitotic catastrophe: a mechanism for avoiding genomic instability. Nat Rev Mol Cell Biol. 2011;12:385–392. doi: 10.1038/nrm3115. [DOI] [PubMed] [Google Scholar]
- Galluzzi L, Vitale I, Abrams JM, Alnemri ES, Baehrecke EH, Blagosklonny MV, et al. Molecular definitions of cell death subroutines: recommendations of the Nomenclature Committee on Cell Death 2012. Cell Death Differ. 2012;19:107–120. doi: 10.1038/cdd.2011.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galluzzi L, Kepp O, Kroemer G. Mitochondria: master regulators of danger signalling. Nat Rev Mol Cell Biol. 2012;13:780–788. doi: 10.1038/nrm3479. [DOI] [PubMed] [Google Scholar]
- Deniaud A, Sharaf el dein O, Maillier E, Poncet D, Kroemer G, Lemaire C, et al. Endoplasmic reticulum stress induces calcium-dependent permeability transition, mitochondrial outer membrane permeabilization and apoptosis. Oncogene. 2008;27:285–299. doi: 10.1038/sj.onc.1210638. [DOI] [PubMed] [Google Scholar]
- Tait SW, Green DR. Mitochondria and cell death: outer membrane permeabilization and beyond. Nat Rev Mol Cell Biol. 2010;11:621–632. doi: 10.1038/nrm2952. [DOI] [PubMed] [Google Scholar]
- Vandenabeele P, Galluzzi L, Vanden Berghe T, Kroemer G. Molecular mechanisms of necroptosis: an ordered cellular explosion. Nat Rev Mol Cell Biol. 2010;11:700–714. doi: 10.1038/nrm2970. [DOI] [PubMed] [Google Scholar]
- Cimprich KA, Cortez D. ATR: an essential regulator of genome integrity. Nat Rev Mol Cell Biol. 2008;9:616–627. doi: 10.1038/nrm2450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vousden KH, Lane DP. p53 in health and disease. Nat Rev Mol Cell Biol. 2007;8:275–283. doi: 10.1038/nrm2147. [DOI] [PubMed] [Google Scholar]
- Sperka T, Wang J, Rudolph KL. DNA damage checkpoints in stem cells, ageing and cancer. Nat Rev Mol Cell Biol. 2012;13:579–590. doi: 10.1038/nrm3420. [DOI] [PubMed] [Google Scholar]
- Huntoon CJ, Flatten KS, Wahner Hendrickson AE, Huehls AM, Sutor SL, Kaufmann SH, et al. ATR inhibition broadly sensitizes ovarian cancer cells to chemotherapy independent of BRCA status. Cancer Res. 2013;73:3683–3691. doi: 10.1158/0008-5472.CAN-13-0110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitale I, Galluzzi L, Vivet S, Nanty L, Dessen P, Senovilla L, et al. Inhibition of Chk1 kills tetraploid tumor cells through a p53-dependent pathway. PLoS One. 2007;2:e1337. doi: 10.1371/journal.pone.0001337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroemer G, Galluzzi L, Brenner C. Mitochondrial membrane permeabilization in cell death. Physiol Rev. 2007;87:99–163. doi: 10.1152/physrev.00013.2006. [DOI] [PubMed] [Google Scholar]
- Galluzzi L, Blomgren K, Kroemer G. Mitochondrial membrane permeabilization in neuronal injury. Nat Rev Neurosci. 2009;10:481–494. doi: 10.1038/nrn2665. [DOI] [PubMed] [Google Scholar]
- Sancho-Martinez SM, Prieto-Garcia L, Prieto M, Lopez-Novoa JM, Lopez-Hernandez FJ. Subcellular targets of cisplatin cytotoxicity: an integrated view. Pharmacol Ther. 2012;136:35–55. doi: 10.1016/j.pharmthera.2012.07.003. [DOI] [PubMed] [Google Scholar]
- Gonzalez VM, Fuertes MA, Alonso C, Perez JM. Is cisplatin-induced cell death always produced by apoptosis. Mol Pharmacol. 2001;59:657–663. doi: 10.1124/mol.59.4.657. [DOI] [PubMed] [Google Scholar]
- Mandic A, Hansson J, Linder S, Shoshan MC. Cisplatin induces endoplasmic reticulum stress and nucleus-independent apoptotic signaling. J Biol Chem. 2003;278:9100–9106. doi: 10.1074/jbc.M210284200. [DOI] [PubMed] [Google Scholar]
- Berndtsson M, Hagg M, Panaretakis T, Havelka AM, Shoshan MC, Linder S. Acute apoptosis by cisplatin requires induction of reactive oxygen species but is not associated with damage to nuclear DNA. Int J Cancer. 2007;120:175–180. doi: 10.1002/ijc.22132. [DOI] [PubMed] [Google Scholar]
- Obeid M, Tesniere A, Ghiringhelli F, Fimia GM, Apetoh L, Perfettini JL, et al. Calreticulin exposure dictates the immunogenicity of cancer cell death. Nat Med. 2007;13:54–61. doi: 10.1038/nm1523. [DOI] [PubMed] [Google Scholar]
- Yu F, Megyesi J, Price PM. Cytoplasmic initiation of cisplatin cytotoxicity. Am J Physiol Renal Physiol. 2008;295:F44–F52. doi: 10.1152/ajprenal.00593.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gourdier I, Crabbe L, Andreau K, Pau B, Kroemer G. Oxaliplatin-induced mitochondrial apoptotic response of colon carcinoma cells does not require nuclear DNA. Oncogene. 2004;23:7449–7457. doi: 10.1038/sj.onc.1208047. [DOI] [PubMed] [Google Scholar]
- Brenner C, Grimm S. The permeability transition pore complex in cancer cell death. Oncogene. 2006;25:4744–4756. doi: 10.1038/sj.onc.1209609. [DOI] [PubMed] [Google Scholar]
- Godoy LC, Anderson CT, Chowdhury R, Trudel LJ, Wogan GN. Endogenously produced nitric oxide mitigates sensitivity of melanoma cells to cisplatin. Proc Natl Acad Sci USA. 2012;109:20373–20378. doi: 10.1073/pnas.1218938109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tajeddine N, Galluzzi L, Kepp O, Hangen E, Morselli E, Senovilla L, et al. Hierarchical involvement of Bak, VDAC1 and Bax in cisplatin-induced cell death. Oncogene. 2008;27:4221–4232. doi: 10.1038/onc.2008.63. [DOI] [PubMed] [Google Scholar]
- Sharaf el dein O, Gallerne C, Brenner C, Lemaire C. Increased expression of VDAC1 sensitizes carcinoma cells to apoptosis induced by DNA cross-linking agents. Biochem Pharmacol. 2012;83:1172–1182. doi: 10.1016/j.bcp.2012.01.017. [DOI] [PubMed] [Google Scholar]
- Vaseva AV, Marchenko ND, Ji K, Tsirka SE, Holzmann S, Moll UM. p53 opens the mitochondrial permeability transition pore to trigger necrosis. Cell. 2012;149:1536–1548. doi: 10.1016/j.cell.2012.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenbussche F, Vaseva I, Vissenberg K, Van Der Straeten D. Ethylene in vegetative development: a tale with a riddle. New Phytol. 2012;194:895–909. doi: 10.1111/j.1469-8137.2012.04100.x. [DOI] [PubMed] [Google Scholar]
- Mihara M, Erster S, Zaika A, Petrenko O, Chittenden T, Pancoska P, et al. p53 has a direct apoptogenic role at the mitochondria. Mol Cell. 2003;11:577–590. doi: 10.1016/s1097-2765(03)00050-9. [DOI] [PubMed] [Google Scholar]
- Chipuk JE, Kuwana T, Bouchier-Hayes L, Droin NM, Newmeyer DD, Schuler M, et al. Direct activation of Bax by p53 mediates mitochondrial membrane permeabilization and apoptosis. Science. 2004;303:1010–1014. doi: 10.1126/science.1092734. [DOI] [PubMed] [Google Scholar]
- Hodeify R, Megyesi J, Tarcsafalvi A, Safirstein RL, Price PM. Protection of cisplatin cytotoxicity by an inactive cyclin-dependent kinase. Am J Physiol Renal Physiol. 2010;299:F112–F120. doi: 10.1152/ajprenal.00151.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida M, Khokhar AR, Siddik ZH. Biochemical pharmacology of homologous alicyclic mixed amine platinum(II) complexes in sensitive and resistant tumor cell lines. Cancer Res. 1994;54:3468–3473. [PubMed] [Google Scholar]
- Ishida S, Lee J, Thiele DJ, Herskowitz I. Uptake of the anticancer drug cisplatin mediated by the copper transporter Ctr1 in yeast and mammals. Proc Natl Acad Sci USA. 2002;99:14298–14302. doi: 10.1073/pnas.162491399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katano K, Kondo A, Safaei R, Holzer A, Samimi G, Mishima M, et al. Acquisition of resistance to cisplatin is accompanied by changes in the cellular pharmacology of copper. Cancer Res. 2002;62:6559–6565. [PubMed] [Google Scholar]
- Holzer AK, Manorek GH, Howell SB. Contribution of the major copper influx transporter CTR1 to the cellular accumulation of cisplatin, carboplatin, and oxaliplatin. Mol Pharmacol. 2006;70:1390–1394. doi: 10.1124/mol.106.022624. [DOI] [PubMed] [Google Scholar]
- More SS, Akil O, Ianculescu AG, Geier EG, Lustig LR, Giacomini KM. Role of the copper transporter, CTR1, in platinum-induced ototoxicity. J Neurosci. 2010;30:9500–9509. doi: 10.1523/JNEUROSCI.1544-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishida S, McCormick F, Smith-McCune K, Hanahan D. Enhancing tumor-specific uptake of the anticancer drug cisplatin with a copper chelator. Cancer Cell. 2010;17:574–583. doi: 10.1016/j.ccr.2010.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zischka H, Lichtmannegger J, Schmitt S, Jagemann N, Schulz S, Wartini D, et al. Liver mitochondrial membrane crosslinking and destruction in a rat model of Wilson disease. J Clin Invest. 2011;121:1508–1518. doi: 10.1172/JCI45401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komatsu M, Sumizawa T, Mutoh M, Chen ZS, Terada K, Furukawa T, et al. Copper-transporting P-type adenosine triphosphatase (ATP7B) is associated with cisplatin resistance. Cancer Res. 2000;60:1312–1316. [PubMed] [Google Scholar]
- Safaei R, Holzer AK, Katano K, Samimi G, Howell SB. The role of copper transporters in the development of resistance to Pt drugs. J Inorg Biochem. 2004;98:1607–1613. doi: 10.1016/j.jinorgbio.2004.05.006. [DOI] [PubMed] [Google Scholar]
- Aida T, Takebayashi Y, Shimizu T, Okamura C, Higasimoto M, Kanzaki A, et al. Expression of copper-transporting P-type adenosine triphosphatase (ATP7B) as a prognostic factor in human endometrial carcinoma. Gynecol Oncol. 2005;97:41–45. doi: 10.1016/j.ygyno.2004.12.042. [DOI] [PubMed] [Google Scholar]
- Nakayama K, Kanzaki A, Ogawa K, Miyazaki K, Neamati N, Takebayashi Y. Copper-transporting P-type adenosine triphosphatase (ATP7B) as a cisplatin based chemoresistance marker in ovarian carcinoma: comparative analysis with expression of MDR1, MRP1, MRP2, LRP and BCRP. Int J Cancer. 2002;101:488–495. doi: 10.1002/ijc.10608. [DOI] [PubMed] [Google Scholar]
- Nakayama K, Kanzaki A, Terada K, Mutoh M, Ogawa K, Sugiyama T, et al. Prognostic value of the Cu-transporting ATPase in ovarian carcinoma patients receiving cisplatin-based chemotherapy. Clin Cancer Res. 2004;10:2804–2811. doi: 10.1158/1078-0432.ccr-03-0454. [DOI] [PubMed] [Google Scholar]
- Kalayda GV, Wagner CH, Buss I, Reedijk J, Jaehde U. Altered localisation of the copper efflux transporters ATP7A and ATP7B associated with cisplatin resistance in human ovarian carcinoma cells. BMC Cancer. 2008;8:175. doi: 10.1186/1471-2407-8-175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen HH, Yan JJ, Chen WC, Kuo MT, Lai YH, Lai WW, et al. Predictive and prognostic value of human copper transporter 1 (hCtr1) in patients with stage III non-small-cell lung cancer receiving first-line platinum-based doublet chemotherapy. Lung Cancer. 2012;75:228–234. doi: 10.1016/j.lungcan.2011.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo MT, Fu S, Savaraj N, Chen HH. Role of the human high-affinity copper transporter in copper homeostasis regulation and cisplatin sensitivity in cancer chemotherapy. Cancer Res. 2012;72:4616–4621. doi: 10.1158/0008-5472.CAN-12-0888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu S, Naing A, Fu C, Kuo MT, Kurzrock R. Overcoming platinum resistance through the use of a copper-lowering agent. Mol Cancer Ther. 2012;11:1221–1225. doi: 10.1158/1535-7163.MCT-11-0864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang ZD, Long Y, Tsai WB, Fu S, Kurzrock R, Gagea-Iurascu M, et al. Mechanistic basis for overcoming platinum resistance using copper chelating agents. Mol Cancer Ther. 2012;11:2483–2494. doi: 10.1158/1535-7163.MCT-12-0580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui Y, Konig J, Buchholz JK, Spring H, Leier I, Keppler D. Drug resistance and ATP-dependent conjugate transport mediated by the apical multidrug resistance protein, MRP2, permanently expressed in human and canine cells. Mol Pharmacol. 1999;55:929–937. [PubMed] [Google Scholar]
- Koike K, Kawabe T, Tanaka T, Toh S, Uchiumi T, Wada M, et al. A canalicular multispecific organic anion transporter (cMOAT) antisense cDNA enhances drug sensitivity in human hepatic cancer cells. Cancer Res. 1997;57:5475–5479. [PubMed] [Google Scholar]
- Liedert B, Materna V, Schadendorf D, Thomale J, Lage H. Overexpression of cMOAT (MRP2/ABCC2) is associated with decreased formation of platinum-DNA adducts and decreased G2-arrest in melanoma cells resistant to cisplatin. J Invest Dermatol. 2003;121:172–176. doi: 10.1046/j.1523-1747.2003.12313.x. [DOI] [PubMed] [Google Scholar]
- Korita PV, Wakai T, Shirai Y, Matsuda Y, Sakata J, Takamura M, et al. Multidrug resistance-associated protein 2 determines the efficacy of cisplatin in patients with hepatocellular carcinoma. Oncol Rep. 2010;23:965–972. doi: 10.3892/or_00000721. [DOI] [PubMed] [Google Scholar]
- Yamasaki M, Makino T, Masuzawa T, Kurokawa Y, Miyata H, Takiguchi S, et al. Role of multidrug resistance protein 2 (MRP2) in chemoresistance and clinical outcome in oesophageal squamous cell carcinoma. Br J Cancer. 2011;104:707–713. doi: 10.1038/sj.bjc.6606071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno-Smith M, Halder JB, Meltzer PS, Gonda TA, Mangala LS, Rupaimoole R, et al. ATP11B mediates platinum resistance in ovarian cancer. J Clin Invest. 2013;123:2119–2130. doi: 10.1172/JCI65425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis AD, Hayes JD, Wolf CR. Glutathione and glutathione-dependent enzymes in ovarian adenocarcinoma cell lines derived from a patient before and after the onset of drug resistance: intrinsic differences and cell cycle effects. Carcinogenesis. 1988;9:1283–1287. doi: 10.1093/carcin/9.7.1283. [DOI] [PubMed] [Google Scholar]
- Chen HH, Kuo MT.Role of glutathione in the regulation of cisplatin resistance in cancer chemotherapy Met Based Drugs 20102010Article ID: 430939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley SL, Basu A, Teicher BA, Hacker MP, Hamer DH, Lazo JS. Overexpression of metallothionein confers resistance to anticancer drugs. Science. 1988;241:1813–1815. doi: 10.1126/science.3175622. [DOI] [PubMed] [Google Scholar]
- Kasahara K, Fujiwara Y, Nishio K, Ohmori T, Sugimoto Y, Komiya K, et al. Metallothionein content correlates with the sensitivity of human small cell lung cancer cell lines to cisplatin. Cancer Res. 1991;51:3237–3242. [PubMed] [Google Scholar]
- Dabholkar M, Bostick-Bruton F, Weber C, Bohr VA, Egwuagu C, Reed E. ERCC1 and ERCC2 expression in malignant tissues from ovarian cancer patients. J Natl Cancer Inst. 1992;84:1512–1517. doi: 10.1093/jnci/84.19.1512. [DOI] [PubMed] [Google Scholar]
- Metzger R, Leichman CG, Danenberg KD, Danenberg PV, Lenz HJ, Hayashi K, et al. ERCC1 mRNA levels complement thymidylate synthase mRNA levels in predicting response and survival for gastric cancer patients receiving combination cisplatin and fluorouracil chemotherapy. J Clin Oncol. 1998;16:309–316. doi: 10.1200/JCO.1998.16.1.309. [DOI] [PubMed] [Google Scholar]
- Shirota Y, Stoehlmacher J, Brabender J, Xiong YP, Uetake H, Danenberg KD, et al. ERCC1 and thymidylate synthase mRNA levels predict survival for colorectal cancer patients receiving combination oxaliplatin and fluorouracil chemotherapy. J Clin Oncol. 2001;19:4298–4304. doi: 10.1200/JCO.2001.19.23.4298. [DOI] [PubMed] [Google Scholar]
- Olaussen KA, Dunant A, Fouret P, Brambilla E, Andre F, Haddad V, et al. DNA repair by ERCC1 in non-small-cell lung cancer and cisplatin-based adjuvant chemotherapy. N Engl J Med. 2006;355:983–991. doi: 10.1056/NEJMoa060570. [DOI] [PubMed] [Google Scholar]
- Handra-Luca A, Hernandez J, Mountzios G, Taranchon E, Lacau-St-Guily J, Soria JC, et al. Excision repair cross complementation group 1 immunohistochemical expression predicts objective response and cancer-specific survival in patients treated by Cisplatin-based induction chemotherapy for locally advanced head and neck squamous cell carcinoma. Clin Cancer Res. 2007;13:3855–3859. doi: 10.1158/1078-0432.CCR-07-0252. [DOI] [PubMed] [Google Scholar]
- Bellmunt J, Paz-Ares L, Cuello M, Cecere FL, Albiol S, Guillem V, et al. Gene expression of ERCC1 as a novel prognostic marker in advanced bladder cancer patients receiving cisplatin-based chemotherapy. Ann Oncol. 2007;18:522–528. doi: 10.1093/annonc/mdl435. [DOI] [PubMed] [Google Scholar]
- Kim MK, Cho KJ, Kwon GY, Park SI, Kim YH, Kim JH, et al. Patients with ERCC1-negative locally advanced esophageal cancers may benefit from preoperative chemoradiotherapy. Clin Cancer Res. 2008;14:4225–4231. doi: 10.1158/1078-0432.CCR-07-4848. [DOI] [PubMed] [Google Scholar]
- Jun HJ, Ahn MJ, Kim HS, Yi SY, Han J, Lee SK, et al. ERCC1 expression as a predictive marker of squamous cell carcinoma of the head and neck treated with cisplatin-based concurrent chemoradiation. Br J Cancer. 2008;99:167–172. doi: 10.1038/sj.bjc.6604464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olaussen KA. A new step ahead for the consideration of ERCC1 as a candidate biomarker to select NSCLC patients for the treatment of cetuximab in combination with cisplatin. Cancer Biol Ther. 2009;8:1922–1923. doi: 10.4161/cbt.8.20.9785. [DOI] [PubMed] [Google Scholar]
- Friboulet L, Olaussen KA, Pignon JP, Shepherd FA, Tsao MS, Graziano S, et al. ERCC1 isoform expression and DNA repair in non-small-cell lung cancer. N Engl J Med. 2013;368:1101–1110. doi: 10.1056/NEJMoa1214271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaisman A, Varchenko M, Umar A, Kunkel TA, Risinger JI, Barrett JC, et al. The role of hMLH1, hMSH3, and hMSH6 defects in cisplatin and oxaliplatin resistance: correlation with replicative bypass of platinum-DNA adducts. Cancer Res. 1998;58:3579–3585. [PubMed] [Google Scholar]
- Aebi S, Kurdi-Haidar B, Gordon R, Cenni B, Zheng H, Fink D, et al. Loss of DNA mismatch repair in acquired resistance to cisplatin. Cancer Res. 1996;56:3087–3090. [PubMed] [Google Scholar]
- Drummond JT, Anthoney A, Brown R, Modrich P. Cisplatin and adriamycin resistance are associated with MutLalpha and mismatch repair deficiency in an ovarian tumor cell line. J Biol Chem. 1996;271:19645–19648. doi: 10.1074/jbc.271.33.19645. [DOI] [PubMed] [Google Scholar]
- Fink D, Aebi S, Howell SB. The role of DNA mismatch repair in drug resistance. Clin Cancer Res. 1998;4:1–6. [PubMed] [Google Scholar]
- Brown R, Hirst GL, Gallagher WM, McIlwrath AJ, Margison GP, van der Zee AG, et al. hMLH1 expression and cellular responses of ovarian tumour cells to treatment with cytotoxic anticancer agents. Oncogene. 1997;15:45–52. doi: 10.1038/sj.onc.1201167. [DOI] [PubMed] [Google Scholar]
- Kamal NS, Soria JC, Mendiboure J, Planchard D, Olaussen KA, Rousseau V, et al. MutS homologue 2 and the long-term benefit of adjuvant chemotherapy in lung cancer. Clin Cancer Res. 2010;16:1206–1215. doi: 10.1158/1078-0432.CCR-09-2204. [DOI] [PubMed] [Google Scholar]
- Bassett E, Vaisman A, Tropea KA, McCall CM, Masutani C, Hanaoka F, et al. Frameshifts and deletions during in vitro translesion synthesis past Pt-DNA adducts by DNA polymerases beta and eta. DNA Repair (Amst) 2002;1:1003–1016. doi: 10.1016/s1568-7864(02)00150-7. [DOI] [PubMed] [Google Scholar]
- Zhao Y, Biertumpfel C, Gregory MT, Hua YJ, Hanaoka F, Yang W. Structural basis of human DNA polymerase eta-mediated chemoresistance to cisplatin. Proc Natl Acad Sci USA. 2012;109:7269–7274. doi: 10.1073/pnas.1202681109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittschieben JP, Reshmi SC, Gollin SM, Wood RD. Loss of DNA polymerase zeta causes chromosomal instability in mammalian cells. Cancer Res. 2006;66:134–142. doi: 10.1158/0008-5472.CAN-05-2982. [DOI] [PubMed] [Google Scholar]
- Roos WP, Tsaalbi-Shtylik A, Tsaryk R, Guvercin F, de Wind N, Kaina B. The translesion polymerase Rev3L in the tolerance of alkylating anticancer drugs. Mol Pharmacol. 2009;76:927–934. doi: 10.1124/mol.109.058131. [DOI] [PubMed] [Google Scholar]
- Wang H, Zhang SY, Wang S, Lu J, Wu W, Weng L, et al. REV3L confers chemoresistance to cisplatin in human gliomas: the potential of its RNAi for synergistic therapy. Neuro Oncol. 2009;11:790–802. doi: 10.1215/15228517-2009-015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith J, Tho LM, Xu N, Gillespie DA. The ATM-Chk2 and ATR-Chk1 pathways in DNA damage signaling and cancer. Adv Cancer Res. 2010;108:73–112. doi: 10.1016/B978-0-12-380888-2.00003-0. [DOI] [PubMed] [Google Scholar]
- Narod SA, Foulkes WD. BRCA1 and BRCA2: 1994 and beyond. Nat Rev Cancer. 2004;4:665–676. doi: 10.1038/nrc1431. [DOI] [PubMed] [Google Scholar]
- Venkitaraman AR. Cancer susceptibility and the functions of BRCA1 and BRCA2. Cell. 2002;108:171–182. doi: 10.1016/s0092-8674(02)00615-3. [DOI] [PubMed] [Google Scholar]
- Farmer H, McCabe N, Lord CJ, Tutt AN, Johnson DA, Richardson TB, et al. Targeting the DNA repair defect in BRCA mutant cells as a therapeutic strategy. Nature. 2005;434:917–921. doi: 10.1038/nature03445. [DOI] [PubMed] [Google Scholar]
- Bryant HE, Schultz N, Thomas HD, Parker KM, Flower D, Lopez E, et al. Specific killing of BRCA2-deficient tumours with inhibitors of poly(ADP-ribose) polymerase. Nature. 2005;434:913–917. doi: 10.1038/nature03443. [DOI] [PubMed] [Google Scholar]
- Ratnam K, Low JA. Current development of clinical inhibitors of poly(ADP-ribose) polymerase in oncology. Clin Cancer Res. 2007;13:1383–1388. doi: 10.1158/1078-0432.CCR-06-2260. [DOI] [PubMed] [Google Scholar]
- Sakai W, Swisher EM, Karlan BY, Agarwal MK, Higgins J, Friedman C, et al. Secondary mutations as a mechanism of cisplatin resistance in BRCA2-mutated cancers. Nature. 2008;451:1116–1120. doi: 10.1038/nature06633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards SL, Brough R, Lord CJ, Natrajan R, Vatcheva R, Levine DA, et al. Resistance to therapy caused by intragenic deletion in BRCA2. Nature. 2008;451:1111–1115. doi: 10.1038/nature06548. [DOI] [PubMed] [Google Scholar]
- Gibson BA, Kraus WL. New insights into the molecular and cellular functions of poly(ADP-ribose) and PARPs. Nat Rev Mol Cell Biol. 2012;13:411–424. doi: 10.1038/nrm3376. [DOI] [PubMed] [Google Scholar]
- Michels J, Vitale I, Galluzzi L, Adam J, Olaussen KA, Kepp O, et al. Cisplatin resistance associated with PARP hyperactivation. Cancer Res. 2013;73:2271–2280. doi: 10.1158/0008-5472.CAN-12-3000. [DOI] [PubMed] [Google Scholar]
- Michels J, Vitale I, Senovilla L, Enot DP, Garcia P, Lissa D, et al. Synergistic interaction between cisplatin and PARP inhibitors in non-small cell lung cancer. Cell Cycle. 2013;12:877–883. doi: 10.4161/cc.24034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donawho CK, Luo Y, Luo Y, Penning TD, Bauch JL, Bouska JJ, et al. ABT-888, an orally active poly(ADP-ribose) polymerase inhibitor that potentiates DNA-damaging agents in preclinical tumor models. Clin Cancer Res. 2007;13:2728–2737. doi: 10.1158/1078-0432.CCR-06-3039. [DOI] [PubMed] [Google Scholar]
- Evers B, Drost R, Schut E, de Bruin M, van der Burg E, Derksen PW, et al. Selective inhibition of BRCA2-deficient mammary tumor cell growth by AZD2281 and cisplatin. Clin Cancer Res. 2008;14:3916–3925. doi: 10.1158/1078-0432.CCR-07-4953. [DOI] [PubMed] [Google Scholar]
- Karasawa T, Sibrian-Vazquez M, Strongin RM, Steyger PS. Identification of cisplatin-binding proteins using agarose conjugates of platinum compounds. PLoS One. 2013;8:e66220. doi: 10.1371/journal.pone.0066220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao Z, Jones AW, Fassone E, Sweeney MG, Lebiedzinska M, Suski JM, et al. PGC-1beta mediates adaptive chemoresistance associated with mitochondrial DNA mutations. Oncogene. 2013;32:2592–2600. doi: 10.1038/onc.2012.259. [DOI] [PubMed] [Google Scholar]
- Mizutani S, Miyato Y, Shidara Y, Asoh S, Tokunaga A, Tajiri T, et al. Mutations in the mitochondrial genome confer resistance of cancer cells to anticancer drugs. Cancer Sci. 2009;100:1680–1687. doi: 10.1111/j.1349-7006.2009.01238.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones AW, Yao Z, Vicencio JM, Karkucinska-Wieckowska A, Szabadkai G. PGC-1 family coactivators and cell fate: roles in cancer, neurodegeneration, cardiovascular disease and retrograde mitochondria-nucleus signalling. Mitochondrion. 2012;12:86–99. doi: 10.1016/j.mito.2011.09.009. [DOI] [PubMed] [Google Scholar]
- Abu-Hamad S, Sivan S, Shoshan-Barmatz V. The expression level of the voltage-dependent anion channel controls life and death of the cell. Proc Natl Acad Sci USA. 2006;103:5787–5792. doi: 10.1073/pnas.0600103103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siddik ZH. Cisplatin: mode of cytotoxic action and molecular basis of resistance. Oncogene. 2003;22:7265–7279. doi: 10.1038/sj.onc.1206933. [DOI] [PubMed] [Google Scholar]
- Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- Negrini S, Gorgoulis VG, Halazonetis TD. Genomic instability–an evolving hallmark of cancer. Nat Rev Mol Cell Biol. 2010;11:220–228. doi: 10.1038/nrm2858. [DOI] [PubMed] [Google Scholar]
- Vitale I, Galluzzi L, Senovilla L, Criollo A, Jemaa M, Castedo M, et al. Illicit survival of cancer cells during polyploidization and depolyploidization. Cell Death Differ. 2011;18:1403–1413. doi: 10.1038/cdd.2010.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao CC, Huang SL, Huang HM, Lin-Chao S. Cross-resistance to UV radiation of a cisplatin-resistant human cell line: overexpression of cellular factors that recognize UV-modified DNA. Mol Cell Biol. 1991;11:2075–2080. doi: 10.1128/mcb.11.4.2075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okouoyo S, Herzer K, Ucur E, Mattern J, Krammer PH, Debatin KM, et al. Rescue of death receptor and mitochondrial apoptosis signaling in resistant human NSCLC in vivo. Int J Cancer. 2004;108:580–587. doi: 10.1002/ijc.11585. [DOI] [PubMed] [Google Scholar]
- Kroemer G, Marino G, Levine B. Autophagy and the integrated stress response. Mol Cell. 2010;40:280–293. doi: 10.1016/j.molcel.2010.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vicencio JM, Galluzzi L, Tajeddine N, Ortiz C, Criollo A, Tasdemir E, et al. Senescence, apoptosis or autophagy? When a damaged cell must decide its path–a mini-review. Gerontology. 2008;54:92–99. doi: 10.1159/000129697. [DOI] [PubMed] [Google Scholar]
- Mandic A, Viktorsson K, Varsanyi M, Hansson J, Linder S, Shoshan M. BAK, BAX and p53 proteins in the apoptotic response to cisplatin. Nat Genet. 2001;27:86. [Google Scholar]
- Kim JS, Lee JH, Jeong WW, Choi DH, Cha HJ, Kim do H, et al. Reactive oxygen species-dependent EndoG release mediates cisplatin-induced caspase-independent apoptosis in human head and neck squamous carcinoma cells. Int J Cancer. 2008;122:672–680. doi: 10.1002/ijc.23158. [DOI] [PubMed] [Google Scholar]
- Galluzzi L, Kepp O, Trojel-Hansen C, Kroemer G. Mitochondrial control of cellular life, stress, and death. Circ Res. 2012;111:1198–1207. doi: 10.1161/CIRCRESAHA.112.268946. [DOI] [PubMed] [Google Scholar]
- Branch P, Masson M, Aquilina G, Bignami M, Karran P. Spontaneous development of drug resistance: mismatch repair and p53 defects in resistance to cisplatin in human tumor cells. Oncogene. 2000;19:3138–3145. doi: 10.1038/sj.onc.1203668. [DOI] [PubMed] [Google Scholar]
- O'Connor PM, Jackman J, Bae I, Myers TG, Fan S, Mutoh M, et al. Characterization of the p53 tumor suppressor pathway in cell lines of the National Cancer Institute anticancer drug screen and correlations with the growth-inhibitory potency of 123 anticancer agents. Cancer Res. 1997;57:4285–4300. [PubMed] [Google Scholar]
- Hengstler JG, Pilch H, Schmidt M, Dahlenburg H, Sagemuller J, Schiffer I, et al. Metallothionein expression in ovarian cancer in relation to histopathological parameters and molecular markers of prognosis. Int J Cancer. 2001;95:121–127. doi: 10.1002/1097-0215(20010320)95:2<121::aid-ijc1021>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- Gadducci A, Cosio S, Muraca S, Genazzani AR. Molecular mechanisms of apoptosis and chemosensitivity to platinum and paclitaxel in ovarian cancer: biological data and clinical implications. Eur J Gynaecol Oncol. 2002;23:390–396. [PubMed] [Google Scholar]
- Goloudina AR, Tanoue K, Hammann A, Fourmaux E, Le Guezennec X, Bulavin DV, et al. Wip1 promotes RUNX2-dependent apoptosis in p53-negative tumors and protects normal tissues during treatment with anticancer agents. Proc Natl Acad Sci USA. 2012;109:E68–E75. doi: 10.1073/pnas.1107017108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brozovic A, Fritz G, Christmann M, Zisowsky J, Jaehde U, Osmak M, et al. Long-term activation of SAPK/JNK, p38 kinase and fas-L expression by cisplatin is attenuated in human carcinoma cells that acquired drug resistance. Int J Cancer. 2004;112:974–985. doi: 10.1002/ijc.20522. [DOI] [PubMed] [Google Scholar]
- Mansouri A, Ridgway LD, Korapati AL, Zhang Q, Tian L, Wang Y, et al. Sustained activation of JNK/p38 MAPK pathways in response to cisplatin leads to Fas ligand induction and cell death in ovarian carcinoma cells. J Biol Chem. 2003;278:19245–19256. doi: 10.1074/jbc.M208134200. [DOI] [PubMed] [Google Scholar]
- Sakamoto M, Kondo A, Kawasaki K, Goto T, Sakamoto H, Miyake K, et al. Analysis of gene expression profiles associated with cisplatin resistance in human ovarian cancer cell lines and tissues using cDNA microarray. Hum Cell. 2001;14:305–315. [PubMed] [Google Scholar]
- de La Motte Rouge T, Galluzzi L, Olaussen KA, Zermati Y, Tasdemir E, Robert T, et al. A novel epidermal growth factor receptor inhibitor promotes apoptosis in non-small cell lung cancer cells resistant to erlotinib. Cancer Res. 2007;67:6253–6262. doi: 10.1158/0008-5472.CAN-07-0538. [DOI] [PubMed] [Google Scholar]
- Han JY, Hong EK, Choi BG, Park JN, Kim KW, Kang JH, et al. Death receptor 5 and Bcl-2 protein expression as predictors of tumor response to gemcitabine and cisplatin in patients with advanced non-small-cell lung cancer. Med Oncol. 2003;20:355–362. doi: 10.1385/MO:20:4:355. [DOI] [PubMed] [Google Scholar]
- Michaud WA, Nichols AC, Mroz EA, Faquin WC, Clark JR, Begum S, et al. Bcl-2 blocks cisplatin-induced apoptosis and predicts poor outcome following chemoradiation treatment in advanced oropharyngeal squamous cell carcinoma. Clin Cancer Res. 2009;15:1645–1654. doi: 10.1158/1078-0432.CCR-08-2581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams J, Lucas PC, Griffith KA, Choi M, Fogoros S, Hu YY, et al. Expression of Bcl-xL in ovarian carcinoma is associated with chemoresistance and recurrent disease. Gynecol Oncol. 2005;96:287–295. doi: 10.1016/j.ygyno.2004.10.026. [DOI] [PubMed] [Google Scholar]
- Erovic BM, Pelzmann M, Grasl M, Pammer J, Kornek G, Brannath W, et al. Mcl-1, vascular endothelial growth factor-R2, and 14-3-3sigma expression might predict primary response against radiotherapy and chemotherapy in patients with locally advanced squamous cell carcinomas of the head and neck. Clin Cancer Res. 2005;11:8632–8636. doi: 10.1158/1078-0432.CCR-05-1170. [DOI] [PubMed] [Google Scholar]
- Nakamura M, Tsuji N, Asanuma K, Kobayashi D, Yagihashi A, Hirata K, et al. Survivin as a predictor of cis-diamminedichloroplatinum sensitivity in gastric cancer patients. Cancer Sci. 2004;95:44–51. doi: 10.1111/j.1349-7006.2004.tb03169.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karczmarek-Borowska B, Filip A, Wojcierowski J, Smolen A, Pilecka I, Jablonka A. Survivin antiapoptotic gene expression as a prognostic factor in non-small cell lung cancer: in situ hybridization study. Folia Histochem Cytobiol. 2005;43:237–242. [PubMed] [Google Scholar]
- Kato J, Kuwabara Y, Mitani M, Shinoda N, Sato A, Toyama T, et al. Expression of survivin in esophageal cancer: correlation with the prognosis and response to chemotherapy. Int J Cancer. 2001;95:92–95. doi: 10.1002/1097-0215(20010320)95:2<92::aid-ijc1016>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- Ikeguchi M, Kaibara N. Changes in survivin messenger RNA level during cisplatin treatment in gastric cancer. Int J Mol Med. 2001;8:661–666. doi: 10.3892/ijmm.8.6.661. [DOI] [PubMed] [Google Scholar]
- Keating J, Tsoli M, Hallahan AR, Ingram WJ, Haber M, Ziegler DS. Targeting the inhibitor of apoptosis proteins as a novel therapeutic strategy in medulloblastoma. Mol Cancer Ther. 2012;11:2654–2663. doi: 10.1158/1535-7163.MCT-12-0352. [DOI] [PubMed] [Google Scholar]
- Wilson WH, O'Connor OA, Czuczman MS, LaCasce AS, Gerecitano JF, Leonard JP, et al. Navitoclax, a targeted high-affinity inhibitor of BCL-2, in lymphoid malignancies: a phase 1 dose-escalation study of safety, pharmacokinetics, pharmacodynamics, and antitumour activity. Lancet Oncol. 2010;11:1149–1159. doi: 10.1016/S1470-2045(10)70261-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudin CM, Hann CL, Garon EB, Ribeiro de Oliveira M, Bonomi PD, Camidge DR, et al. Phase II study of single-agent navitoclax (ABT-263) and biomarker correlates in patients with relapsed small cell lung cancer. Clin Cancer Res. 2012;18:3163–3169. doi: 10.1158/1078-0432.CCR-11-3090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giaccone G, Zatloukal P, Roubec J, Floor K, Musil J, Kuta M, et al. Multicenter phase II trial of YM155, a small-molecule suppressor of survivin, in patients with advanced, refractory, non-small-cell lung cancer. J Clin Oncol. 2009;27:4481–4486. doi: 10.1200/JCO.2008.21.1862. [DOI] [PubMed] [Google Scholar]
- Tolcher AW, Mita A, Lewis LD, Garrett CR, Till E, Daud AI, et al. Phase I and pharmacokinetic study of YM155, a small-molecule inhibitor of survivin. J Clin Oncol. 2008;26:5198–5203. doi: 10.1200/JCO.2008.17.2064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanioka M, Nokihara H, Yamamoto N, Yamada Y, Yamada K, Goto Y, et al. Phase I study of LY2181308, an antisense oligonucleotide against survivin, in patients with advanced solid tumors. Cancer Chemother Pharmacol. 2011;68:505–511. doi: 10.1007/s00280-010-1506-7. [DOI] [PubMed] [Google Scholar]
- Slamon DJ, Godolphin W, Jones LA, Holt JA, Wong SG, Keith DE, et al. Studies of the HER-2/neu proto-oncogene in human breast and ovarian cancer. Science. 1989;244:707–712. doi: 10.1126/science.2470152. [DOI] [PubMed] [Google Scholar]
- Hengstler JG, Lange J, Kett A, Dornhofer N, Meinert R, Arand M, et al. Contribution of c-erbB-2 and topoisomerase IIalpha to chemoresistance in ovarian cancer. Cancer Res. 1999;59:3206–3214. [PubMed] [Google Scholar]
- Fijolek J, Wiatr E, Rowinska-Zakrzewska E, Giedronowicz D, Langfort R, Chabowski M, et al. p53 and HER2/neu expression in relation to chemotherapy response in patients with non-small cell lung cancer. Int J Biol Markers. 2006;21:81–87. doi: 10.1177/172460080602100203. [DOI] [PubMed] [Google Scholar]
- Zhou BP, Liao Y, Xia W, Spohn B, Lee MH, Hung MC. Cytoplasmic localization of p21Cip1/WAF1 by Akt-induced phosphorylation in HER-2/neu-overexpressing cells. Nat Cell Biol. 2001;3:245–252. doi: 10.1038/35060032. [DOI] [PubMed] [Google Scholar]
- Deng X, Ewton DZ, Friedman E. Mirk/Dyrk1B maintains the viability of quiescent pancreatic cancer cells by reducing levels of reactive oxygen species. Cancer Res. 2009;69:3317–3324. doi: 10.1158/0008-5472.CAN-08-2903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen DW, Gottesman MM. RAB8 enhances TMEM205-mediated cisplatin resistance. Pharm Res. 2012;29:643–650. doi: 10.1007/s11095-011-0562-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen DW, Ma J, Okabe M, Zhang G, Xia D, Gottesman MM. Elevated expression of TMEM205, a hypothetical membrane protein, is associated with cisplatin resistance. J Cell Physiol. 2010;225:822–828. doi: 10.1002/jcp.22287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messina S, Leonetti C, De Gregorio G, Affatigato V, Ragona G, Frati L, et al. Ras inhibition amplifies cisplatin sensitivity of human glioblastoma. Biochem Biophys Res Commun. 2004;320:493–500. doi: 10.1016/j.bbrc.2004.06.003. [DOI] [PubMed] [Google Scholar]
- Yu H, Su J, Xu Y, Kang J, Li H, Zhang L, et al. p62/SQSTM1 involved in cisplatin resistance in human ovarian cancer cells by clearing ubiquitinated proteins. Eur J Cancer. 2011;47:1585–1594. doi: 10.1016/j.ejca.2011.01.019. [DOI] [PubMed] [Google Scholar]
- Ren JH, He WS, Nong L, Zhu QY, Hu K, Zhang RG, et al. Acquired cisplatin resistance in human lung adenocarcinoma cells is associated with enhanced autophagy. Cancer Biother Radiopharm. 2010;25:75–80. doi: 10.1089/cbr.2009.0701. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Shen X. Heat shock protein 27 protects L929 cells from cisplatin-induced apoptosis by enhancing Akt activation and abating suppression of thioredoxin reductase activity. Clin Cancer Res. 2007;13:2855–2864. doi: 10.1158/1078-0432.CCR-06-2090. [DOI] [PubMed] [Google Scholar]
- Yamamoto K, Okamoto A, Isonishi S, Ochiai K, Ohtake Y. Heat shock protein 27 was up-regulated in cisplatin resistant human ovarian tumor cell line and associated with the cisplatin resistance. Cancer Lett. 2001;168:173–181. doi: 10.1016/s0304-3835(01)00532-8. [DOI] [PubMed] [Google Scholar]
- Ren A, Yan G, You B, Sun J. Down-regulation of mammalian sterile 20-like kinase 1 by heat shock protein 70 mediates cisplatin resistance in prostate cancer cells. Cancer Res. 2008;68:2266–2274. doi: 10.1158/0008-5472.CAN-07-6248. [DOI] [PubMed] [Google Scholar]
- Miyazaki T, Kato H, Faried A, Sohda M, Nakajima M, Fukai Y, et al. Predictors of response to chemo-radiotherapy and radiotherapy for esophageal squamous cell carcinoma. Anticancer Res. 2005;25:2749–2755. [PubMed] [Google Scholar]
- Shen DW, Pouliot LM, Hall MD, Gottesman MM. Cisplatin resistance: a cellular self-defense mechanism resulting from multiple epigenetic and genetic changes. Pharmacol Rev. 2012;64:706–721. doi: 10.1124/pr.111.005637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He JC, Chuang PY, Ma'ayan A, Iyengar R. Systems biology of kidney diseases. Kidney Int. 2012;81:22–39. doi: 10.1038/ki.2011.314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee NH, Saeed AI. Microarrays: an overview. Methods Mol Biol. 2007;353:265–300. doi: 10.1385/1-59745-229-7:265. [DOI] [PubMed] [Google Scholar]
- Nilsson T, Mann M, Aebersold R, Yates JR, 3rd, Bairoch A, Bergeron JJ. Mass spectrometry in high-throughput proteomics: ready for the big time. Nat Methods. 2010;7:681–685. doi: 10.1038/nmeth0910-681. [DOI] [PubMed] [Google Scholar]
- Monetti M, Nagaraj N, Sharma K, Mann M. Large-scale phosphosite quantification in tissues by a spike-in SILAC method. Nat Methods. 2011;8:655–658. doi: 10.1038/nmeth.1647. [DOI] [PubMed] [Google Scholar]
- Johnson SA, Hunter T. Kinomics: methods for deciphering the kinome. Nat Methods. 2005;2:17–25. doi: 10.1038/nmeth731. [DOI] [PubMed] [Google Scholar]
- Mertins P, Qiao JW, Patel J, Udeshi ND, Clauser KR, Mani DR, et al. Integrated proteomic analysis of post-translational modifications by serial enrichment. Nat Methods. 2013;10:634–637. doi: 10.1038/nmeth.2518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger MF, Lawrence MS, Demichelis F, Drier Y, Cibulskis K, Sivachenko AY, et al. The genomic complexity of primary human prostate cancer. Nature. 2011;470:214–220. doi: 10.1038/nature09744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ley TJ, Mardis ER, Ding L, Fulton B, McLellan MD, Chen K, et al. DNA sequencing of a cytogenetically normal acute myeloid leukaemia genome. Nature. 2008;456:66–72. doi: 10.1038/nature07485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pleasance ED, Cheetham RK, Stephens PJ, McBride DJ, Humphray SJ, Greenman CD, et al. A comprehensive catalogue of somatic mutations from a human cancer genome. Nature. 2010;463:191–196. doi: 10.1038/nature08658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pleasance ED, Stephens PJ, O'Meara S, McBride DJ, Meynert A, Jones D, et al. A small-cell lung cancer genome with complex signatures of tobacco exposure. Nature. 2010;463:184–190. doi: 10.1038/nature08629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah SP, Morin RD, Khattra J, Prentice L, Pugh T, Burleigh A, et al. Mutational evolution in a lobular breast tumour profiled at single nucleotide resolution. Nature. 2009;461:809–813. doi: 10.1038/nature08489. [DOI] [PubMed] [Google Scholar]
- Murtaza M, Dawson SJ, Tsui DW, Gale D, Forshew T, Piskorz AM, et al. Non-invasive analysis of acquired resistance to cancer therapy by sequencing of plasma DNA. Nature. 2013;497:108–112. doi: 10.1038/nature12065. [DOI] [PubMed] [Google Scholar]
- Cui L, Fu J, Pang JC, Qiu ZK, Liu XM, Chen FR, et al. Overexpression of IL-7 enhances cisplatin resistance in glioma. Cancer Biol Ther. 2012;13:496–503. doi: 10.4161/cbt.19592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JH, Miele ME, Hicks DJ, Phillips KK, Trent JM, Weissman BE, et al. KiSS-1, a novel human malignant melanoma metastasis-suppressor gene. J Natl Cancer Inst. 1996;88:1731–1737. doi: 10.1093/jnci/88.23.1731. [DOI] [PubMed] [Google Scholar]
- Jiffar T, Yilmaz T, Lee J, Hanna E, El-Naggar A, Yu D, et al. KiSS1 mediates platinum sensitivity and metastasis suppression in head and neck squamous cell carcinoma. Oncogene. 2011;30:3163–3173. doi: 10.1038/onc.2011.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu W, Jin C, Lou X, Han X, Li L, He Y, et al. Global analysis of DNA methylation by Methyl-Capture sequencing reveals epigenetic control of cisplatin resistance in ovarian cancer cell. PLoS One. 2011;6:e29450. doi: 10.1371/journal.pone.0029450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeller C, Dai W, Steele NL, Siddiq A, Walley AJ, Wilhelm-Benartzi CS, et al. Candidate DNA methylation drivers of acquired cisplatin resistance in ovarian cancer identified by methylome and expression profiling. Oncogene. 2012;31:4567–4576. doi: 10.1038/onc.2011.611. [DOI] [PubMed] [Google Scholar]
- Ivanova T, Zouridis H, Wu Y, Cheng LL, Tan IB, Gopalakrishnan V, et al. Integrated epigenomics identifies BMP4 as a modulator of cisplatin sensitivity in gastric cancer. Gut. 2013;62:22–33. doi: 10.1136/gutjnl-2011-301113. [DOI] [PubMed] [Google Scholar]
- Chon HS, Marchion DC, Xiong Y, Chen N, Bicaku E, Stickles XB, et al. The BCL2 antagonist of cell death pathway influences endometrial cancer cell sensitivity to cisplatin. Gynecol Oncol. 2012;124:119–124. doi: 10.1016/j.ygyno.2011.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu HM, Wang TC. Mechanism of cisplatin resistance in human urothelial carcinoma cells. Food Chem Toxicol. 2012;50:1226–1237. doi: 10.1016/j.fct.2012.01.040. [DOI] [PubMed] [Google Scholar]
- Lai TC, Chow KC, Fang HY, Cho HC, Chen CY, Lin TY, et al. Expression of xeroderma pigmentosum complementation group C protein predicts cisplatin resistance in lung adenocarcinoma patients. Oncol Rep. 2011;25:1243–1251. doi: 10.3892/or.2011.1184. [DOI] [PubMed] [Google Scholar]
- Koch M, Krieger ML, Stolting D, Brenner N, Beier M, Jaehde U, et al. Overcoming chemotherapy resistance of ovarian cancer cells by liposomal cisplatin: molecular mechanisms unveiled by gene expression profiling. Biochem Pharmacol. 2013;85:1077–1090. doi: 10.1016/j.bcp.2013.01.028. [DOI] [PubMed] [Google Scholar]
- Marchion DC, Cottrill HM, Xiong Y, Chen N, Bicaku E, Fulp WJ, et al. BAD phosphorylation determines ovarian cancer chemosensitivity and patient survival. Clin Cancer Res. 2011;17:6356–6366. doi: 10.1158/1078-0432.CCR-11-0735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HK, Choi IJ, Kim CG, Kim HS, Oshima A, Michalowski A, et al. A gene expression signature of acquired chemoresistance to cisplatin and fluorouracil combination chemotherapy in gastric cancer patients. PLoS One. 2011;6:e16694. doi: 10.1371/journal.pone.0016694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barlin JN, Jelinic P, Olvera N, Bogomolniy F, Bisogna M, Dao F, et al. Validated gene targets associated with curatively treated advanced serous ovarian carcinoma. Gynecol Oncol. 2013;128:512–517. doi: 10.1016/j.ygyno.2012.11.018. [DOI] [PubMed] [Google Scholar]
- Marchini S, Fruscio R, Clivio L, Beltrame L, Porcu L, Nerini IF, et al. Resistance to platinum-based chemotherapy is associated with epithelial to mesenchymal transition in epithelial ovarian cancer. Eur J Cancer. 2013;49:520–530. doi: 10.1016/j.ejca.2012.06.026. [DOI] [PubMed] [Google Scholar]
- Tomkiewicz C, Hans S, Mucchielli MH, Agier N, Delacroix H, Marisa L, et al. A head and neck cancer tumor response-specific gene signature for cisplatin, 5-fluorouracil induction chemotherapy fails with added taxanes. PLoS One. 2012;7:e47170. doi: 10.1371/journal.pone.0047170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galluzzi L, Vitale I, Senovilla L, Eisenberg T, Carmona-Gutierrez D, Vacchelli E, et al. Independent transcriptional reprogramming and apoptosis induction by cisplatin. Cell Cycle. 2012;11:3472–3480. doi: 10.4161/cc.21789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galluzzi L, Morselli E, Vitale I, Kepp O, Senovilla L, Criollo A, et al. miR-181a and miR-630 regulate cisplatin-induced cancer cell death. Cancer Res. 2010;70:1793–1803. doi: 10.1158/0008-5472.CAN-09-3112. [DOI] [PubMed] [Google Scholar]
- Guo Y, Yan K, Fang J, Qu Q, Zhou M, Chen F. Let-7b expression determines response to chemotherapy through the regulation of cyclin D1 in glioblastoma. J Exp Clin Cancer Res. 2013;32:41. doi: 10.1186/1756-9966-32-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun L, Yao Y, Liu B, Lin Z, Lin L, Yang M, et al. MiR-200b and miR-15b regulate chemotherapy-induced epithelial-mesenchymal transition in human tongue cancer cells by targeting BMI1. Oncogene. 2012;31:432–445. doi: 10.1038/onc.2011.263. [DOI] [PubMed] [Google Scholar]
- Zhou L, Qiu T, Xu J, Wang T, Wang J, Zhou X, et al. miR-135a/b modulate cisplatin resistance of human lung cancer cell line by targeting MCL1. Pathol Oncol Res. 2013;19:677–683. doi: 10.1007/s12253-013-9630-4. [DOI] [PubMed] [Google Scholar]
- Zhu W, Zhu D, Lu S, Wang T, Wang J, Jiang B, et al. miR-497 modulates multidrug resistance of human cancer cell lines by targeting BCL2. Med Oncol. 2012;29:384–391. doi: 10.1007/s12032-010-9797-4. [DOI] [PubMed] [Google Scholar]
- Wang Q, Zhong M, Liu W, Li J, Huang J, Zheng L. Alterations of microRNAs in cisplatin-resistant human non-small cell lung cancer cells (A549/DDP) Exp Lung Res. 2011;37:427–434. doi: 10.3109/01902148.2011.584263. [DOI] [PubMed] [Google Scholar]
- Yang Y, Li H, Hou S, Hu B, Liu J, Wang J. The noncoding RNA expression profile and the effect of lncRNA AK126698 on cisplatin resistance in non-small-cell lung cancer cells. PLoS One. 2013;8:e65309. doi: 10.1371/journal.pone.0065309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuo L, Liu J, Wang B, Gao M, Huang A. Differential miRNA expression profiles in hepatocellular carcinoma cells and drug-resistant sublines. Oncol Rep. 2013;29:555–562. doi: 10.3892/or.2012.2155. [DOI] [PubMed] [Google Scholar]
- Yang L, Li N, Wang H, Jia X, Wang X, Luo J. Altered microRNA expression in cisplatin-resistant ovarian cancer cells and upregulation of miR-130a associated with MDR1/P-glycoprotein-mediated drug resistance. Oncol Rep. 2012;28:592–600. doi: 10.3892/or.2012.1823. [DOI] [PubMed] [Google Scholar]
- Fu X, Tian J, Zhang L, Chen Y, Hao Q. Involvement of microRNA-93, a new regulator of PTEN/Akt signaling pathway, in regulation of chemotherapeutic drug cisplatin chemosensitivity in ovarian cancer cells. FEBS Lett. 2012;586:1279–1286. doi: 10.1016/j.febslet.2012.03.006. [DOI] [PubMed] [Google Scholar]
- Kim CH, Kim HK, Rettig RL, Kim J, Lee ET, Aprelikova O, et al. miRNA signature associated with outcome of gastric cancer patients following chemotherapy. BMC Med Genomics. 2011;4:79. doi: 10.1186/1755-8794-4-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko MA, Zehong G, Virtanen C, Guindi M, Waddell TK, Keshavjee S, et al. MicroRNA expression profiling of esophageal cancer before and after induction chemoradiotherapy. Ann Thorac Surg. 2012;94:1094–1102. doi: 10.1016/j.athoracsur.2012.04.145. [DOI] [PubMed] [Google Scholar]
- Scott GK, Mattie MD, Berger CE, Benz SC, Benz CC. Rapid alteration of microRNA levels by histone deacetylase inhibition. Cancer Res. 2006;66:1277–1281. doi: 10.1158/0008-5472.CAN-05-3632. [DOI] [PubMed] [Google Scholar]
- Zhu J, Jiang Z, Gao F, Hu X, Zhou L, Chen J, et al. A systematic analysis on DNA methylation and the expression of both mRNA and microRNA in bladder cancer. PLoS One. 2011;6:e28223. doi: 10.1371/journal.pone.0028223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bangert A, Hacker S, Cristofanon S, Debatin KM, Fulda S. Chemosensitization of glioblastoma cells by the histone deacetylase inhibitor MS275. Anticancer Drugs. 2011;22:494–499. doi: 10.1097/CAD.0b013e32834631e0. [DOI] [PubMed] [Google Scholar]
- Rikiishi H, Shinohara F, Sato T, Sato Y, Suzuki M, Echigo S. Chemosensitization of oral squamous cell carcinoma cells to cisplatin by histone deacetylase inhibitor, suberoylanilide hydroxamic acid. Int J Oncol. 2007;30:1181–1188. [PubMed] [Google Scholar]
- Wang F, Liu M, Li X, Tang H. MiR-214 reduces cell survival and enhances cisplatin-induced cytotoxicity via down-regulation of Bcl2l2 in cervical cancer cells. FEBS Lett. 2013;587:488–495. doi: 10.1016/j.febslet.2013.01.016. [DOI] [PubMed] [Google Scholar]
- Eriksson I, Joosten M, Roberg K, Ollinger K. The histone deacetylase inhibitor trichostatin A reduces lysosomal pH and enhances cisplatin-induced apoptosis. Exp Cell Res. 2013;319:12–20. doi: 10.1016/j.yexcr.2012.10.004. [DOI] [PubMed] [Google Scholar]
- Ozaki K, Kishikawa F, Tanaka M, Sakamoto T, Tanimura S, Kohno M. Histone deacetylase inhibitors enhance the chemosensitivity of tumor cells with cross-resistance to a wide range of DNA-damaging drugs. Cancer Sci. 2008;99:376–384. doi: 10.1111/j.1349-7006.2007.00669.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steele N, Finn P, Brown R, Plumb JA. Combined inhibition of DNA methylation and histone acetylation enhances gene re-expression and drug sensitivity in vivo. Br J Cancer. 2009;100:758–763. doi: 10.1038/sj.bjc.6604932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu YY, Mirkin BL, Dwivedi RS. Inhibition of DNA methyltransferase reverses cisplatin induced drug resistance in murine neuroblastoma cells. Cancer Detect Prev. 2005;29:456–463. doi: 10.1016/j.cdp.2005.05.004. [DOI] [PubMed] [Google Scholar]
- Chavez JD, Hoopmann MR, Weisbrod CR, Takara K, Bruce JE. Quantitative proteomic and interaction network analysis of cisplatin resistance in HeLa cells. PLoS One. 2011;6:e19892. doi: 10.1371/journal.pone.0019892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng HZ, Qu YQ, Zhang WJ, Xiu B, Deng AM, Liang AB. Proteomic analysis identified DJ-1 as a cisplatin resistant marker in non-small cell lung cancer. Int J Mol Sci. 2011;12:3489–3499. doi: 10.3390/ijms12063489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roesch A, Vultur A, Bogeski I, Wang H, Zimmermann KM, Speicher D, et al. Overcoming intrinsic multidrug resistance in melanoma by blocking the mitochondrial respiratory chain of slow-cycling JARID1B(high) cells. Cancer Cell. 2013;23:811–825. doi: 10.1016/j.ccr.2013.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aichler M, Elsner M, Ludyga N, Feuchtinger A, Zangen V, Maier SK, et al. Clinical response to chemotherapy in oesophageal adenocarcinoma patients is linked to defects in mitochondria. J Pathol. 2013;230:410–419. doi: 10.1002/path.4199. [DOI] [PubMed] [Google Scholar]
- Vasko R, Mueller GA, von Jaschke AK, Asif AR, Dihazi H. Impact of cisplatin administration on protein expression levels in renal cell carcinoma: a proteomic analysis. Eur J Pharmacol. 2011;670:50–57. doi: 10.1016/j.ejphar.2011.08.030. [DOI] [PubMed] [Google Scholar]
- Chappell NP, Teng PN, Hood BL, Wang G, Darcy KM, Hamilton CA, et al. Mitochondrial proteomic analysis of cisplatin resistance in ovarian cancer. J Proteome Res. 2012;11:4605–4614. doi: 10.1021/pr300403d. [DOI] [PubMed] [Google Scholar]
- Zhang G, Sun L, Lu X, Chen Z, Duerksen-Hughes PJ, Hu H, et al. Cisplatin treatment leads to changes in nuclear protein and microRNA expression. Mutat Res. 2012;746:66–77. doi: 10.1016/j.mrgentox.2012.03.004. [DOI] [PubMed] [Google Scholar]
- Shetty V, Nickens Z, Testa J, Hafner J, Sinnathamby G, Philip R. Quantitative immunoproteomics analysis reveals novel MHC class I presented peptides in cisplatin-resistant ovarian cancer cells. J Proteomics. 2012;75:3270–3290. doi: 10.1016/j.jprot.2012.03.044. [DOI] [PubMed] [Google Scholar]
- Garand C, Guay D, Sereduk C, Chow D, Tsofack SP, Langlois M, et al. An integrative approach to identify YB-1-interacting proteins required for cisplatin resistance in MCF7 and MDA-MB-231 breast cancer cells. Cancer Sci. 2011;102:1410–1417. doi: 10.1111/j.1349-7006.2011.01948.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang W, Ding X, Li B, Fan M, Zhou T, Sun H, et al. Serum biomarkers analyzed by LC-MS/MS as predictors for short outcome of non-small cell lung cancer patients treated with chemoradiotherapy. Neoplasma. 2013;60:11–18. doi: 10.4149/neo_2013_002. [DOI] [PubMed] [Google Scholar]
- Han M, Dai J, Zhang Y, Lin Q, Jiang M, Xu X, et al. Support vector machines coupled with proteomics approaches for detecting biomarkers predicting chemotherapy resistance in small cell lung cancer. Oncol Rep. 2012;28:2233–2238. doi: 10.3892/or.2012.2037. [DOI] [PubMed] [Google Scholar]
- Zitvogel L, Galluzzi L, Smyth MJ, Kroemer G. Mechanism of action of conventional and targeted anticancer therapies: reinstating immunosurveillance. Immunity. 2013;39:74–88. doi: 10.1016/j.immuni.2013.06.014. [DOI] [PubMed] [Google Scholar]
- Galluzzi L, Senovilla L, Zitvogel L, Kroemer G. The secret ally: immunostimulation by anticancer drugs. Nat Rev Drug Discov. 2012;11:215–233. doi: 10.1038/nrd3626. [DOI] [PubMed] [Google Scholar]
- Kittler R, Putz G, Pelletier L, Poser I, Heninger AK, Drechsel D, et al. An endoribonuclease-prepared siRNA screen in human cells identifies genes essential for cell division. Nature. 2004;432:1036–1040. doi: 10.1038/nature03159. [DOI] [PubMed] [Google Scholar]
- Sachse C, Echeverri CJ. Oncology studies using siRNA libraries: the dawn of RNAi-based genomics. Oncogene. 2004;23:8384–8391. doi: 10.1038/sj.onc.1208072. [DOI] [PubMed] [Google Scholar]
- Schimmer AD, Welsh K, Pinilla C, Wang Z, Krajewska M, Bonneau MJ, et al. Small-molecule antagonists of apoptosis suppressor XIAP exhibit broad antitumor activity. Cancer Cell. 2004;5:25–35. doi: 10.1016/s1535-6108(03)00332-5. [DOI] [PubMed] [Google Scholar]
- Newman MJ, Rodarte JC, Benbatoul KD, Romano SJ, Zhang C, Krane S, et al. Discovery and characterization of OC144-093, a novel inhibitor of P-glycoprotein-mediated multidrug resistance. Cancer Res. 2000;60:2964–2972. [PubMed] [Google Scholar]
- Alcala S, Klee M, Fernandez J, Fleischer A, Pimentel-Muinos FX. A high-throughput screening for mammalian cell death effectors identifies the mitochondrial phosphate carrier as a regulator of cytochrome c release. Oncogene. 2008;27:44–54. doi: 10.1038/sj.onc.1210600. [DOI] [PubMed] [Google Scholar]
- Sun C, Newbatt Y, Douglas L, Workman P, Aherne W, Linardopoulos S. High-throughput screening assay for identification of small molecule inhibitors of Aurora2/STK15 kinase. J Biomol Screen. 2004;9:391–397. doi: 10.1177/1087057104264071. [DOI] [PubMed] [Google Scholar]
- Galluzzi L, Vitale I, Senovilla L, Olaussen KA, Pinna G, Eisenberg T, et al. Prognostic impact of vitamin B6 metabolism in lung cancer. Cell Rep. 2012;2:257–269. doi: 10.1016/j.celrep.2012.06.017. [DOI] [PubMed] [Google Scholar]
- Galluzzi L, Marsili S, Vitale I, Senovilla L, Michels J, Garcia P, et al. Vitamin B6 metabolism influences the intracellular accumulation of cisplatin. Cell Cycle. 2013;12:417–421. doi: 10.4161/cc.23275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacquemont C, Simon JA, D'Andrea AD, Taniguchi T. Non-specific chemical inhibition of the Fanconi anemia pathway sensitizes cancer cells to cisplatin. Mol Cancer. 2012;11:26. doi: 10.1186/1476-4598-11-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Dexheimer TS, Ai Y, Liang Q, Villamil MA, Inglese J, et al. Selective and cell-active inhibitors of the USP1/ UAF1 deubiquitinase complex reverse cisplatin resistance in non-small cell lung cancer cells. Chem Biol. 2011;18:1390–1400. doi: 10.1016/j.chembiol.2011.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salm F, Cwiek P, Ghosal A, Lucia Buccarello A, Largey F, Wotzkow C, et al. RNA interference screening identifies a novel role for autocrine fibroblast growth factor signaling in neuroblastoma chemoresistance. Oncogene. 2013;32:3944–3953. doi: 10.1038/onc.2012.416. [DOI] [PubMed] [Google Scholar]
- Ho H, Aruri J, Kapadia R, Mehr H, White MA, Ganesan AK. RhoJ regulates melanoma chemoresistance by suppressing pathways that sense DNA damage. Cancer Res. 2012;72:5516–5528. doi: 10.1158/0008-5472.CAN-12-0775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li G, Alexander H, Schneider N, Alexander S. Molecular basis for resistance to the anticancer drug cisplatin in Dictyostelium. Microbiology. 2000;146 (Pt 9:2219–2227. doi: 10.1099/00221287-146-9-2219. [DOI] [PubMed] [Google Scholar]
- Liao C, Hu B, Arno MJ, Panaretou B. Genomic screening in vivo reveals the role played by vacuolar H+ ATPase and cytosolic acidification in sensitivity to DNA-damaging agents such as cisplatin. Mol Pharmacol. 2007;71:416–425. doi: 10.1124/mol.106.030494. [DOI] [PubMed] [Google Scholar]
- Gatti L, Hoe KL, Hayles J, Righetti SC, Carenini N, Bo LD, et al. Ubiquitin-proteasome genes as targets for modulation of cisplatin sensitivity in fission yeast. BMC Genomics. 2011;12:44. doi: 10.1186/1471-2164-12-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sletta H, Klinkenberg G, Winnberg A, Kvitvang HF, Nilsen MB, Krokan HE, et al. A new high resolution screening method for study of phenotype stress responses of Saccharomyces cerevisae mutants. J Microbiol Methods. 2011;87:363–367. doi: 10.1016/j.mimet.2011.10.003. [DOI] [PubMed] [Google Scholar]
- Galluzzi L, Vacchelli E, Michels J, Garcia P, Kepp O, Senovilla L, et al. Effects of vitamin B6 metabolism on oncogenesis, tumor progression and therapeutic responses. Oncogene. 2013;32:4995–5004. doi: 10.1038/onc.2012.623. [DOI] [PubMed] [Google Scholar]
- Galluzzi L, Goubar A, Olaussen KA, Vitale I, Senovilla L, Michels J, et al. Prognostic value of LIPC in non-small cell lung carcinoma. Cell Cycle. 2013;12:647–654. doi: 10.4161/cc.23517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerreiro AS, Fattet S, Kulesza DW, Atamer A, Elsing AN, Shalaby T, et al. A sensitized RNA interference screen identifies a novel role for the PI3K p110gamma isoform in medulloblastoma cell proliferation and chemoresistance. Mol Cancer Res. 2011;9:925–935. doi: 10.1158/1541-7786.MCR-10-0200. [DOI] [PubMed] [Google Scholar]
- Pouliot LM, Chen YC, Bai J, Guha R, Martin SE, Gottesman MM, et al. Cisplatin sensitivity mediated by WEE1 and CHK1 is mediated by miR-155 and the miR-15 family. Cancer Res. 2012;72:5945–5955. doi: 10.1158/0008-5472.CAN-12-1400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong SW, Tiong KH, Kong WY, Yue YC, Chua CH, Lim JY, et al. Rapamycin synergizes cisplatin sensitivity in basal-like breast cancer cells through up-regulation of p73. Breast Cancer Res Treat. 2011;128:301–313. doi: 10.1007/s10549-010-1055-0. [DOI] [PubMed] [Google Scholar]
- Garnett MJ, Edelman EJ, Heidorn SJ, Greenman CD, Dastur A, Lau KW, et al. Systematic identification of genomic markers of drug sensitivity in cancer cells. Nature. 2012;483:570–575. doi: 10.1038/nature11005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krajinovic M, Costea I, Chiasson S. Polymorphism of the thymidylate synthase gene and outcome of acute lymphoblastic leukaemia. Lancet. 2002;359:1033–1034. doi: 10.1016/S0140-6736(02)08065-0. [DOI] [PubMed] [Google Scholar]
- Shahbazi M, Pravica V, Nasreen N, Fakhoury H, Fryer AA, Strange RC, et al. Association between functional polymorphism in EGF gene and malignant melanoma. Lancet. 2002;359:397–401. doi: 10.1016/S0140-6736(02)07600-6. [DOI] [PubMed] [Google Scholar]
- Hildebrandt MA, Lippman SM, Etzel CJ, Kim E, Lee JJ, Khuri FR, et al. Genetic variants in the PI3K/PTEN/AKT/mTOR pathway predict head and neck cancer patient second primary tumor/recurrence risk and response to retinoid chemoprevention. Clin Cancer Res. 2012;18:3705–3713. doi: 10.1158/1078-0432.CCR-11-3271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichikawa W, Takahashi T, Suto K, Sasaki Y, Hirayama R. Orotate phosphoribosyltransferase gene polymorphism predicts toxicity in patients treated with bolus 5-fluorouracil regimen. Clin Cancer Res. 2006;12:3928–3934. doi: 10.1158/1078-0432.CCR-05-2665. [DOI] [PubMed] [Google Scholar]
- Hood L, Heath JR, Phelps ME, Lin B. Systems biology and new technologies enable predictive and preventative medicine. Science. 2004;306:640–643. doi: 10.1126/science.1104635. [DOI] [PubMed] [Google Scholar]
- Han B, Gao G, Wu W, Gao Z, Zhao X, Li L, et al. Association of ABCC2 polymorphisms with platinum-based chemotherapy response and severe toxicity in non-small cell lung cancer patients. Lung Cancer. 2011;72:238–243. doi: 10.1016/j.lungcan.2010.09.001. [DOI] [PubMed] [Google Scholar]
- Cheng J, Ha M, Wang Y, Sun J, Chen J, Wang Y, et al. A C118T polymorphism of ERCC1 and response to cisplatin chemotherapy in patients with late-stage non-small cell lung cancer. J Cancer Res Clin Oncol. 2012;138:231–238. doi: 10.1007/s00432-011-1090-1. [DOI] [PubMed] [Google Scholar]
- Yu D, Shi J, Sun T, Du X, Liu L, Zhang X, et al. Pharmacogenetic role of ERCC1 genetic variants in treatment response of platinum-based chemotherapy among advanced non-small cell lung cancer patients. Tumour Biol. 2012;33:877–884. doi: 10.1007/s13277-011-0314-y. [DOI] [PubMed] [Google Scholar]
- Qian J, Gu S, Wu Q, Zhao X, Wu W, Gao Z, et al. Association of CASP7 polymorphisms and survival of patients with non-small cell lung cancer with platinum-based chemotherapy treatment. Chest. 2012;142:680–689. doi: 10.1378/chest.11-2522. [DOI] [PubMed] [Google Scholar]
- Qian J, Qu HQ, Yang L, Yin M, Wang Q, Gu S, et al. Association between CASP8 and CASP10 polymorphisms and toxicity outcomes with platinum-based chemotherapy in Chinese patients with non-small cell lung cancer. Oncologist. 2012;17:1551–1561. doi: 10.1634/theoncologist.2011-0419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu D, O'Day SJ, Yang D, Boasberg P, Milford R, Kristedja T, et al. Impact of gene polymorphisms on clinical outcome for stage IV melanoma patients treated with biochemotherapy: an exploratory study. Clin Cancer Res. 2005;11:1237–1246. [PubMed] [Google Scholar]
- Lin CH, Yeakley JM, McDaniel TK, Shen R. Medium- to high-throughput SNP genotyping using VeraCode microbeads. Methods Mol Biol. 2009;496:129–142. doi: 10.1007/978-1-59745-553-4_10. [DOI] [PubMed] [Google Scholar]
- Paynter RA, Skibola DR, Skibola CF, Buffler PA, Wiemels JL, Smith MT. Accuracy of multiplexed Illumina platform-based single-nucleotide polymorphism genotyping compared between genomic and whole genome amplified DNA collected from multiple sources. Cancer Epidemiol Biomarkers Prev. 2006;15:2533–2536. doi: 10.1158/1055-9965.EPI-06-0219. [DOI] [PubMed] [Google Scholar]
- Vacchelli E, Galluzzi L, Rousseau V, Rigoni A, Tesniere A, Delahaye N, et al. Loss-of-function alleles of P2RX7 and TLR4 fail to affect the response to chemotherapy in non-small cell lung cancer. Oncoimmunology. 2012;1:271–278. doi: 10.4161/onci.18684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan XL, Moyer AM, Fridley BL, Schaid DJ, Niu N, Batzler AJ, et al. Genetic variation predicting cisplatin cytotoxicity associated with overall survival in lung cancer patients receiving platinum-based chemotherapy. Clin Cancer Res. 2011;17:5801–5811. doi: 10.1158/1078-0432.CCR-11-1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler HE, Gorsic LK, Welsh M, Stark AL, Gamazon ER, Cox NJ, et al. Genome-wide local ancestry approach identifies genes and variants associated with chemotherapeutic susceptibility in African Americans. PLoS One. 2011;6:e21920. doi: 10.1371/journal.pone.0021920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler HE, Gamazon ER, Stark AL, O'Donnell PH, Gorsic LK, Huang RS, et al. Genome-wide meta-analysis identifies variants associated with platinating agent susceptibility across populations. Pharmacogenomics J. 2013;13:35–43. doi: 10.1038/tpj.2011.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Sun Z, Cunningham JM, Aubry MC, Wampfler JA, Croghan GA, et al. Genetic variations in multiple drug action pathways and survival in advanced stage non-small cell lung cancer treated with chemotherapy. Clin Cancer Res. 2011;17:3830–3840. doi: 10.1158/1078-0432.CCR-10-2877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X, Ye Y, Rosell R, Amos CI, Stewart DJ, Hildebrandt MA, et al. Genome-wide association study of survival in non-small cell lung cancer patients receiving platinum-based chemotherapy. J Natl Cancer Inst. 2011;103:817–825. doi: 10.1093/jnci/djr075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu L, Wu C, Zhao X, Heist R, Su L, Zhao Y, et al. Genome-wide association study of prognosis in advanced non-small cell lung cancer patients receiving platinum-based chemotherapy. Clin Cancer Res. 2012;18:5507–5514. doi: 10.1158/1078-0432.CCR-12-1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatzemeier U, Pluzanska A, Szczesna A, Kaukel E, Roubec J, De Rosa F, et al. Phase III study of erlotinib in combination with cisplatin and gemcitabine in advanced non-small-cell lung cancer: the Tarceva Lung Cancer Investigation Trial. J Clin Oncol. 2007;25:1545–1552. doi: 10.1200/JCO.2005.05.1474. [DOI] [PubMed] [Google Scholar]
- Gatzemeier U, Groth G, Butts C, Van Zandwijk N, Shepherd F, Ardizzoni A, et al. Randomized phase II trial of gemcitabine-cisplatin with or without trastuzumab in HER2-positive non-small-cell lung cancer. Ann Oncol. 2004;15:19–27. doi: 10.1093/annonc/mdh031. [DOI] [PubMed] [Google Scholar]
- Kindler HL, Karrison TG, Gandara DR, Lu C, Krug LM, Stevenson JP, et al. Multicenter, double-blind, placebo-controlled, randomized phase II trial of gemcitabine/cisplatin plus bevacizumab or placebo in patients with malignant mesothelioma. J Clin Oncol. 2012;30:2509–2515. doi: 10.1200/JCO.2011.41.5869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher JI, Haber M, Henderson MJ, Norris MD. ABC transporters in cancer: more than just drug efflux pumps. Nat Rev Cancer. 2010;10:147–156. doi: 10.1038/nrc2789. [DOI] [PubMed] [Google Scholar]
- Andjilani M, Droz JP, Benahmed M, Tabone E. Alpha6 integrin subunit mediates laminin enhancement of cisplatin-induced apoptosis in testicular tumor germ cells. Int J Cancer. 2005;117:68–81. doi: 10.1002/ijc.21144. [DOI] [PubMed] [Google Scholar]
- Roodhart JM, Daenen LG, Stigter EC, Prins HJ, Gerrits J, Houthuijzen JM, et al. Mesenchymal stem cells induce resistance to chemotherapy through the release of platinum-induced fatty acids. Cancer Cell. 2011;20:370–383. doi: 10.1016/j.ccr.2011.08.010. [DOI] [PubMed] [Google Scholar]
- Kepp O, Menger L, Vacchelli E, Locher C, Adjemian S, Yamazaki T, et al. Crosstalk between ER stress and immunogenic cell death. Cytokine Growth Factor Rev. 2013;24:311–318. doi: 10.1016/j.cytogfr.2013.05.001. [DOI] [PubMed] [Google Scholar]
- Kroemer G, Galluzzi L, Kepp O, Zitvogel L. Immunogenic cell death in cancer therapy. Annu Rev Immunol. 2013;31:51–72. doi: 10.1146/annurev-immunol-032712-100008. [DOI] [PubMed] [Google Scholar]
- Nakasone ES, Askautrud HA, Kees T, Park JH, Plaks V, Ewald AJ, et al. Imaging tumor-stroma interactions during chemotherapy reveals contributions of the microenvironment to resistance. Cancer Cell. 2012;21:488–503. doi: 10.1016/j.ccr.2012.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasada S, Miyata Y, Tsutani Y, Tsuyama N, Masujima T, Hihara J, et al. Metabolomic analysis of dynamic response and drug resistance of gastric cancer cells to 5-fluorouracil. Oncol Rep. 2013;29:925–931. doi: 10.3892/or.2012.2182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Yan SK, Dai WX, Liu XR, Zhang WD, Wang JJ. A metabonomic approach to chemosensitivity prediction of cisplatin plus 5-fluorouracil in a human xenograft model of gastric cancer. Int J Cancer. 2010;127:2841–2850. doi: 10.1002/ijc.25294. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.