Abstract
Bioassay-guided fractionation of a commercial sample of African mango (Irvingia gabonensis) that was later shown to be contaminated with goji berry (Lycium sp.) led to the isolation of a new pyrrole alkaloid, methyl 2-[2-formyl-5-(hydroxymethyl)-1H-pyrrol-1-yl]propanoate, 1, along with seven known compounds, 2–8. The structures of the isolated compounds were established by analysis of their spectroscopic data. The new compound 1g showed hydroxyl radical-scavenging activity with an ED50 value of 16.7 μM, whereas 4-[formyl-5-(methoxymethyl)-1H-pyrrol-1-yl]butanoic acid (2) was active in both the hydroxyl radical-scavenging (ED50 11.9 μM) and quinone reductase-induction [CD (concentration required to double QR activity) 2.4 μM)] assays used. The isolated compounds were shown to be absent in a taxonomically authenticated African mango sample but present in three separate authentic samples of goji berry (Lycium barbarum) using LC-MS and 1H NMR fingerprinting analysis, including one sample that previously showed inhibitory activity in vivo in a rat esophageal cancer model induced with N-nitrosomethylbenzylamine. Additionally, microscopic features characteristic of goji berry were observed in the commercial African mango sample.
Keywords: Lycium barbarum, goji berry, Solanaceae, pyrrole alkaloids, hydroxyl radical-scavenging activity, quinone reductase-inducing activity, Irvingia gabonensis, African mango, LC-MS, 1H NMR fingerprinting, microscopic comparison
Introduction
Irvingia gabonensis (Aubry-Lecomte ex O’Rorke) Baill. (Irvingiaceae) has become known as African mango and is unrelated botanically to the common, or Indian, mango [Mangifera indica (Anacardiaceae)].1 The fruits of African mango are edible, and their use in traditional medicine has been documented for the treatment of gastrointestinal or hepatic disorders, diarrhea, infections, and as a purgative.2,3 Recently, African mango seeds have become available on the U.S. market as a dietary supplement and have shown high in vitro antioxidant capacity,4 significant effects on body weight loss,5,6 blood lipid decreases,5 and a lowering of plasma glucose7 in experimental animal or human subject studies. Phytochemical investigations of the stem bark of I. gabonensis have led to the isolation of antioxidant and hepatoprotective triterpenes and phenols.2 Nevertheless, very limited studies on the chemical constituents of the seeds have been reported. In 2011, Atawodi and co-workers8 published the first report on the phenolic compound profile of I. gabonensis seeds, in which methyl gallate, ellagic acid, and other ellagic acid derivatives were detected by LC-UV-MS in the methanol extract of the seeds, which also demonstrated strong in vitro antioxidant activity. A recent study of dietary supplements wholly or partially containing African mango seed extracts using UHPLC-HRMS found evidence of contamination, adulteration, and/or mislabeling among multiple commercial samples when compared to authentic samples.9 It is noteworthy that no alkaloids have been previously isolated or detected spectroscopically from African mango, according to the accessible and surveyed literature.
Scavenging reactive oxygen species by antioxidants and enhancing carcinogen detoxification via induction of phase II enzymes such as quinone reductase (QR) are two important cancer chemopreventive strategies.10,11 In a continuing search for natural inhibitors of carcinogenesis, the chloroform-soluble extract of a commercially sourced African mango seed extract showed in vitro hydroxyl radical-scavenging and QR-inducing activities, and thus was selected for further study. Bioactivity-guided fractionation of this sample, using both hydroxyl radical-scavenging and QR-inducing assays, led to the isolation of a new pyrrole alkaloid, 1, along with seven known compounds, 2–8. Compounds 1–4 represent structural analogues of substituted pyrrole alkaloids reported recently from Lycium chinense Mill. (Solanaceae).12,13
The fruits of L. chinense and of the closely related species Lycium barbarum L. are commonly known as goji berry or wolfberry and are used almost interchangeably.14,15 Goji has a long history of usage in Asian countries as a culinary ingredient, traditional tonic medicine, and functional food for its perceived benefits in antiaging, as well as enhancements in vision and liver and kidney functions.16,17 In recent years, goji has become increasingly popular in Europe and North America as a “superfruit” and botanical dietary supplement. The in vivo inhibitory activity of powdered L. barbarum (wolfberry) fruits when evaluated in an N-nitrosomethylbenzylamine-induced esophageal cancer model was reported recently in rats.18 Other recent studies have indicated that goji berry extracts and L. barbarum polysaccharides possess a range of biological effects, including antiaging, antioxidant, antitumor, immunomodulatory, and cytoprotective activities.17,19−21 The published chemical constituents of goji have been thoroughly reviewed, with the occurrence of pyrrole alkaloids well established.16
Due to the unexpected presence of the pyrrole alkaloids isolated, 1–4, and their structural similarity to constituents found in goji berries,12,13 as well as the recent report of contamination in commercial African mango samples,9 the identity of the source material investigated came into question. The present study includes the bioactivity-guided isolation, identification, and biological evaluation of compounds 1–8 in addition to comparison of the commercial product with authentic African mango seeds and L. barbarum samples by chemotaxonomy using LC-MS and 1H NMR fingerprinting as well as microscopic analysis.
Materials and Methods
Instrumentation for Compound Isolation and Characterization
Optical rotations were measured using a PerkinElmer 343 automatic polarimeter (PerkinElmer, Waltham, MA, USA). UV spectra were collected on a Hitachi U-2910 spectrophotometer (Hitachi, Tokyo, Japan). IR spectra were obtained with a Nicolet 6700 FT-IR spectrometer (Thermo Scientific, Waltham, MA, USA). NMR spectroscopic data were recorded at room temperature on a Bruker Avance DRX-400 MHz spectrometer (Bruker, Billerica, MA, USA) using standard Bruker pulse sequences. High-resolution electrospray ionization mass spectra (HRESIMS) were obtained on a Micromass Q-Tof II (Micromass, Wythenshawe, UK) mass spectrometer operated in the positive-ion mode, with sodium iodide being used for mass calibration. Column chromatography was performed with Sephadex LH-20 (Supelco, Bellefonte, PA, USA) and 65 × 250 or 230 × 400 mesh silica gel (Sorbent Technologies, Atlanta, GA, USA). Analytical thin-layer chromatography (TLC) was conducted on precoated 250 μm thickness Partisil Si gel 60F254 glass plates. A 150 mm × 19 mm i.d., 5 μm, XBridge PrepC18 column with a 10 mm × 19 mm i.d. guard column of the same material (Waters, Milford, MA, USA) was used for semipreparative HPLC, along with a Hitachi system composed of an L-2130 prep pump, an L-2200 autosampler, and an L-2450 diode array detector (Hitachi, Tokyo, Japan).
Chemicals
All solvents used for chromatographic separations were purchased from Fisher Scientific (Fair Lawn, NJ, USA). 2′,7′-Dichlorodihydrofluorescein diacetate (H2DCF-DA), esterase, ferrous sulfate (FeSO4), hydrogen peroxide (H2O2), quercetin, dimethyl sulfoxide (DMSO), digitonin, EDTA, Trizma base, Tween 20, flavin adenine dinucleotide phosphate (FAD), glucose-6-phosphate (G-6-P), nicotinamide adenine dinucleotide phosphate (NADP), glucose-6-phosphate dehydrogenase (G-6-P-D), menadione, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT), bovine serum albumin (BSA), l-sulforaphane, and deuterated NMR solvents were purchased from Sigma-Aldrich (St. Louis, MO, USA). Cell culture media and supplements were obtained from Life Technologies, Inc. (Grand Island, NY, USA). Gallic acid, methyl gallate, and ellagic acid that served as standards for HPLC-PDA analysis were authentic samples isolated in previous studies and stored in a compound library at The Ohio State University.
Plant Material
The commercial sample labeled as powdered seeds of African mango (I. gabonensis), used for isolation, was obtained by Nature’s Sunshine Products, Inc., from a manufacturer in the People’s Republic of China. A representative sample (code OSUADK-CCP0024) was deposited in the Division of Medicinal Chemistry and Pharmacognosy, College of Pharmacy, The Ohio State University. An authentic sample of African mango seed powder, collected in Nigeria and imported by Nutralliance (Yorba Linda, CA, USA), was purchased by Nature’s Sunshine Products, Inc. (code OSUADK-CCP0025; same repository). Three samples of authentic goji berries were obtained, two from the Ningxia province of People’s Republic of China, by Nature’s Sunshine, Inc., and another directly from a drug store in Chengdu, Sichuan, People’s Republic of China (codes OSUADK-CCP0011, OSUADK-CCP0015, and OSUADK-CCP0026, respectively; same repository). The seeds of the last-mentioned product were separated manually from the fruit pulp. These five samples (OSUADK-CCP0024, OSUADK-CCP0026, OSUADK-CCP0015, OSUADK-CCP0011, and OSUADK-CCP0025) are referred to as products A–E, respectively, henceforth in this paper.
Extraction and Isolation
The commercial sample labeled as the powdered seeds of African mango (product A, 5 kg) was extracted by maceration with MeOH (3 × 10 L) at room temperature, for 3 days each. After filtration and evaporation of the solvent under reduced pressure, the combined crude methanol extract (ca. 1000 g) was suspended in H2O (1 L) and then partitioned in turn with hexanes (3 × 1 L), CHCl3 (3 × 1 L), EtOAc (3 × 1 L), and n-BuOH (3 × 1 L) to afford dried hexanes (40.1 g), CHCl3 (5.0 g), EtOAc (6.5 g), n-BuOH (166.0 g), and H2O-soluble (ca. 800 g) extracts. The CHCl3-soluble extract showed activities in both hydroxyl radical-scavenging (ED50 6.3 μg/mL) and QR induction (CD 12.4 μg/mL) assays and hence was selected for further fractionation. The CHCl3-soluble extract was subjected initially to chromatography over a silica gel column, eluted with CHCl3/MeOH (50:1 to pure MeOH, stepwise), to afford 12 fractions (fractions F01–F12). Fractions F01–F03 showed activity in either the hydroxyl radical-scavenging assay or the QR induction assay and were chosen for further purification.
Fraction F01 (ca. 890 mg) was chromatographed over an open C18 reversed-phase column, eluted with MeOH/H2O (5:95 to pure MeOH, stepwise) to afford eight subfractions. Subfraction F0102 (ca. 400 mg, eluted by MeOH/H2O 20:80) was subjected to passage over a Sephadex LH-20 column with MeOH/H2O (20:80 to pure MeOH, stepwise) as the eluting solvent system to yield compound 8 (eluted by MeOH/H2O 50:50; 3.0 mg) and a further subfraction F010211 (ca. 65 mg). Subfraction F010211 was separated by HPLC using a 150 mm × 19 mm i.d., 5 μm, XBridge PrepC18 column with a 10 mm × 19 mm i.d. guard column of the same material (Waters) and with CH3CN/H2O by isocratic elution (3:97; 8 mL/min) to afford compounds 5 (tR 68.6 min; 5.7 mg) and 6 (tR 55.0 min; 0.8 mg) and a further subfraction, F01021103 (ca. 40 mg, eluted with pure CH3CN). F01021103 was purified further by HPLC on the same column with CH3CN/H2O (0.025% NH4OH) isocratic elution (3:97; 8 mL/min) to obtain compound 2 (tR 12.3 min; 4.5 mg).
Fractions F02 (ca. 180 mg) and F03 (ca. 120 mg) were subjected separately to Sephadex LH-20 column chromatography, eluted by MeOH/H2O (50:50, 75:25, and pure MeOH) to remove pigments and afforded several subfractions. Subfraction F0203 (ca. 100 mg) was purified further by HPLC on the same XBridge PrepC18 column as above, by a gradient elution (A, CH3CN; B, H2O, 3–15% A over 70 min, 15–100% A from 70 to 85 min; 8 mL/min), to render the purification of compounds 1 (tR 49.5 min; 4.8 mg), 3 (tR 46.7 min; 1.3 mg), and 7 (tR 16.5 min; 20.0 mg). Subfraction F0303 (ca. 80 mg) was also purified by HPLC on the same XBridge PrepC18 column, eluted by a CH3CN/H2O gradient (A, CH3CN; B, H2O, 3–15% A over 70 min, 15–100% A from 70 to 85 min; 8 mL/min), to yield compound 4 (tR 22.7 min; 6.8 mg).
Methyl 2-[2-formyl-5-(hydroxymethyl)-1H-pyrrol-1-yl]propanoate, 1, was isolated (ca. 0.0001% of dry weight) as a colorless resin: [α]20D +28.0 (c 0.1, MeOH); UV (MeOH) λmax (log ε) 291 (4.09) nm; IR (film) νmax 3424, 3120, 2946, 2850, 2803, 2727, 1749, 1655 cm–1; 1H NMR (400 MHz, CD3OD) δ 9.31 (1H, s, CHO), 7.07 (1H, d, J = 4.0 Hz, H-3), 6.28 (1H, d, J = 4.0 Hz, H-4), 5.43 (1H, q, J = 7.0 Hz, H-1′), 4.63 (2H, ABq, Δν = 8.1 Hz, J = 13.9 Hz, H-6), 3.67 (3H, s, OCH3), 1.67 (3H, d, J = 7.0 Hz, CH3); 13C NMR (100 MHz, CD3OD) δ 180.5 (CHO), 172.5 (COO), 144.6 (C-5), 133.4 (C-2), 127.4 (C-3), 111.4 (C-4), 56.6 (C-6), 55.8 (C-1′), 52.8 (OCH3), 18.0 (CH3); HRESIMS m/z 234.0733 [M + Na]+ (calcd for C10H13NO4Na, 234.0742).
Evaluation of Hydroxyl Radical-Scavenging Activity
Hydroxyl radical-scavenging activity was tested according to the method previously reported.22,23
Evaluation of Quinone Reductase-Inducing Activity
The in vitro QR-inducing activity of the extracts, fractions, and pure isolates was assayed according to a previously published method.24,25
Microscopic Examination Procedure
The clean samples of products B–D were pulverized and passed through a no. 4 sieve to obtain fine granules. Products A and E were obtained as powders and thus passed through the sieve directly. The powdered materials of products A–E were each placed on a slide and stirred with a fine-pointed needle to distribute the powders evenly, followed by the application of 2 drops of clearing agent, Visikol (Phytosys LLC, New Brunswick, NJ, USA), and gentle heat until air bubbles moved to the edge of the slide. Two drops of diluted glycerin and a coverslip were added to the slide, followed by immediate observation under a Nikon Eclipse TE300 optical microscope (Nikon, Tokyo, Japan), along with an A01F881036 camera module (Roper Scientific, Trenton, NJ, USA) for digital imaging of the microscopic structures observed.
Sample Preparation and HPLC-PDA, LC-IT-MS, and 1H NMR Fingerprinting Analysis
Each sample (3 g) was extracted with 10 mL of methanol by sonication at room temperature for 30 min. The methanol extracts were dried in vacuo (40 °C), followed by another ultrasonic extraction with 10 mL of chloroform at room temperature for 30 min, to obtain a chloroform-soluble extract of each sample, which was then dried in vacuo at 40 °C and stored at −20 °C before analysis. All analyses were completed within 24 h of extraction.
The HPLC-PDA analysis was performed using a 150 mm × 4.6 mm i.d., 5 μm, XBridge C18 analytical column (Waters), along with a Hitachi system composed of an L-2130 pump and an L-2450 diode array detector (Hitachi). The mobile phase consisted of 0.05% trifluoroacetic acid in water (A) and acetonitrile (B) using a gradient program of 3–20% B from 0 to 60 min and 20–100% B from 60 to 70 min. The mobile phase flow rate and the injection volume were 1 mL/min and 10 μL, respectively. The dried chloroform-soluble extract of each sample was dissolved in 1 mL of HPLC grade methanol, and filtered through a Fisher Scientific 13 mm syringe filter (0.2 μm) prior to injection. After comparison of the chromatograms of the chloroform-soluble extract solution recorded at wavelengths within 200–550 nm, it was found that 254 nm could best represent the profile of the analytes.
The LC-IT-MS analysis employed the same separation conditions and Xbridge C18 analytical column as used for the HPLC-PDA analysis mentioned above, on a Waters Alliance 2690 separation module. The injection volume was 10 μL. The mobile phase flow rate was maintained at 1 mL/min and was split approximately 50:1 postcolumn using a microsplitter valve (Upchurch Scientific, Oak Harbor, WA, USA) for the introduction to the ESI source. The electrospray ionization ion trap mass spectrometry (ESI-IT-MS) was performed on a Bruker dual funnel amaZon ETD ion trap mass spectrometer (Bremen, Germany) equipped with an orthogonal electrospray source operated in positive-ion mode. Sodium iodide was used for mass calibration for a calibration range of m/z 100–1000. Optimal ESI conditions were as follows: capillary voltage, 4500 V; source temperature, 250 °C; ESI drying gas (nitrogen), 4.0 L/min; and nebulizer, 10 psi. The ion trap was set to UltraScan mode with a target mass of m/z 500 pass ions from m/z 100 to 1000.
The 1H NMR spectroscopic fingerprinting was conducted using the previously described chloroform-soluble extracts of products A–E. The samples were dissolved in 600 μL of CDCl3, and spectra were measured at 300 K using a Bruker Avance-III HD400 spectrometer.
Results and Discussion
Isolation, Structure Elucidation, and Biological Evaluation of the Constituents
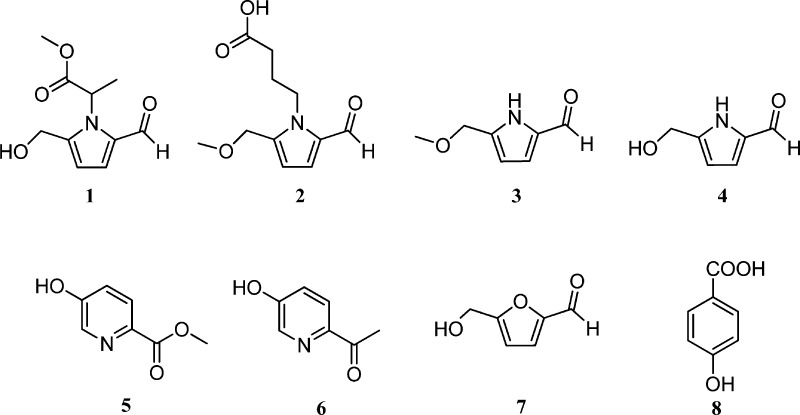
The CHCl3-soluble extract of product A (a commercial sample labeled as powdered seeds of African mango) was found to be the most potent among the hexanes-, CHCl3-, EtOAc-, n-BuOH-, and H2O-soluble extracts in the in vitro hydroxyl radical-scavenging (ED50, concentration scavenging hydroxyl radical by 50%, 6.3 μg/mL) and QR induction (CD, concentration required to double QR activity, 12.4 μg/mL) assays. Therefore, it was selected for fractionation. Fractions F01–F03 of the CHCl3-soluble extract showed hydroxyl radical-scavenging activity, with ED50 values of 3.3, 5.6, and 6.8 μg/mL, respectively. In addition, fractions F01 and F02 exhibited QR-inducing activity, with CD values of 6.9 and 10.2 μg/mL, respectively. Accordingly, fractions F01–F03 were used for further detailed purification. In this way, bioactivity-guided fractionation of product A led to the isolation of a new pyrrole alkaloid, 1, and seven known compounds, 2–8, as shown in Figure 1.
Figure 1.
Structures of compounds isolated from a goji berry-contaminated commercial sample labeled African mango (Irvingia gabonensis).
The new compound, 1, was isolated as a colorless resin. The molecular formula was determined as C10H13NO4 on the basis of the sodiated molecular ion peak in the HRESIMS. The IR spectrum showed absorptions of hydroxy (3424 cm–1), ester carbonyl (1749 cm–1), and conjugated aldehyde (2803, 2727, and 1655 cm–1) groups. The UV spectrum of 1 exhibited a maximum absorption at 291 nm, which is characteristic of pyrrole 2-carbaldehyde.13 The proton signal at δH 9.31 and the carbon signal at δC 180.5 correlated in the HSQC spectrum and indicated the presence of an aldehyde group. Two proton signals at δH 4.63 (2H, ABq, Δν = 8.1 Hz, J = 13.9 Hz, H-6) resulted from a magnetically inequivalent oxygenated methylene group with geminal coupling. The HMBC correlation (Figure 2) of the methoxy group (δH 3.67, 3H, s, OCH3) to the carbonyl at δC 172.5 revealed that this methoxy group forms an ester with this carbonyl. The quartet of H-1′ (δH 5.43, J = 7.0 Hz) and doublet of the methyl group (δH 1.67, J = 7.0 Hz), as well as their correlation in the COSY spectrum, clearly indicated the connection of the methyl group (δC 18.1) to C-1′ (δC 55.8). Furthermore, HMBC correlation of this methyl group (δH 1.67) to the ester carbonyl C-2′ (δC 172.5) suggested the connection of C-1′ (δC 55.8) to C-2′. The chemical shifts and coupling constants of the two protons at δH 7.07 (1H, d, J = 4.0 Hz, H-3) and δH 6.28 (1H, d, J = 4.0 Hz, H-4) implied the presence of a pyrrole ring. Moreover, the substitution pattern of the pyrrole ring should be 2,5- because a 2,3- or a 2,4-disubstituted pyrrole would have coupling constants of 1.3–2.9 or 2.3–3.2 Hz,13 respectively. Also, the two carbons of the pyrrole ring at the lower field region (δC 144.6 and 133.4) were not present in the 13C DEPT 135 spectrum, suggesting that C-2 and C-5 of this ring system are substituted (quaternary carbons). This was confirmed by the HMBC correlation of H-6 (δH 4.63) to C-4 (δC 111.4) and C-5 (δC 144.6), H-4 (δH 6.28) to C-6 (δC 56.6), H-3 (δH 7.07) to the aldehyde carbon (δC 180.5), and the aldehyde proton (δH 9.31) to C-2 (δC 133.4). In addition, the connection between C-1′ and N-1 was clearly indicated by the HMBC correlation of H-1′ (δH 5.43) to C-2 (δC 133.4). Therefore, the structure of 1 was established unambiguously as methyl 2-[2-formyl-5-(hydroxymethyl)-1H-pyrrol-1-yl]propanoate.
Figure 2.
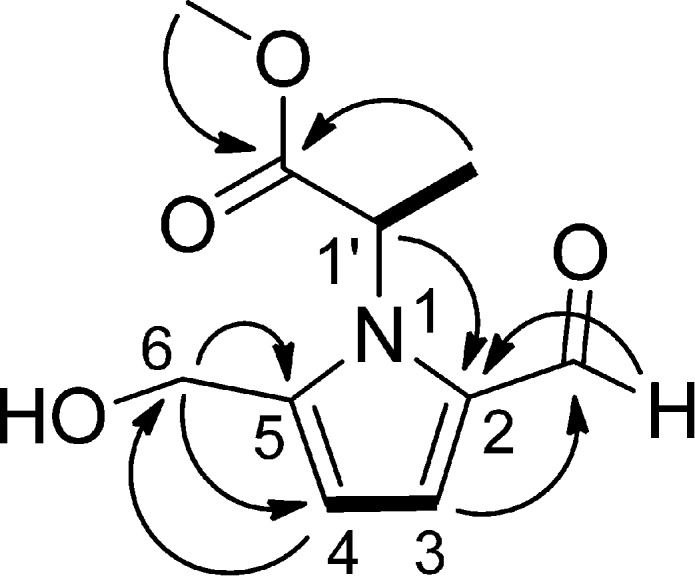
1H–1H COSY (bold lines) and key HMBC (arrows) correlations of the new compound 1.
On the basis of the spectroscopic data measurements and the comparison of the data obtained with published values, the structures of the known compounds 2–8 were identified as 4-[formyl-5-(methoxymethyl)-1H-pyrrol-1-yl]butanoic acid, 2,13 5-(methoxymethyl)-1H-pyrrole-2-carbaldehyde, 3,26 5-(hydroxymethyl)-1H-pyrrole-2-carbaldehyde, 4,27,28 methyl-5-hydroxy-2-pyridinecarboxylate, 5,29 5-hydroxy-2-pyridyl methyl ketone, 6,30 5-hydroxymethyl-2-furancarbaldehyde, 7,31 and 4-hydroxybenzoic acid, 8.32
In the present study, all of the isolates, 1–8, were evaluated in vitro using the hydroxyl radical-scavenging and QR induction assays. The new compound, 1, and the known pyrrole alkaloid, 2, showed activities in both assays, as shown in Table 1. When the QR-inducing activities were compared among the structurally related pyrrole alkaloids, 1–4, it was observed that 2, containing a butanoic acid side chain at position N-1, exhibited the greatest potency (CD 2.4 μM); 1, with a methyl propanoate substitution attached to N-1, showed much less potency (CD 43.1 μM), whereas 3 and 4, each bearing no substituent to N-1, displayed no discernible activity (both CD >100 μM), suggesting that the length of the side chain at N-1 may significantly affect the QR-inducing activity of this type of compound.
Table 1. Hydroxyl Radical-Scavenging and Quinone Reductase-Inducing Activities of the Isolated Active Compounds 1, 2, and 6.
| hydroxyl radical scavenging | QR induction |
|||
|---|---|---|---|---|
| compounda | ED50b (μM) | CDc (μM) | IC50d (μM) | CIe |
| 1 | 16.7 | 43.1 | >100 | >2.3 |
| 2 | 11.9 | 2.4 | >100 | >42.6 |
| 6 | >20 | 48.5 | >100 | >2.1 |
| quercetinf | 1.3 | |||
| l-sulforaphaneg | 0.77 | 16.7 | 21.7 | |
Compounds 3–5, 7, and 8 did not show hydroxyl radical-scavenging or QR-inducing activity at the concentrations tested.
ED50, concentration scavenging hydroxyl radical by 50%. Compounds with ED50 values of <20 μM are considered active.
CD, concentration required to double QR activity. Compounds with CD values of <20, 20–100, and >100 μM are considered significantly active, weakly active, and inactive, respectively.
IC50, concentration inhibiting cell growth by 50%.
CI, chemoprevention index (= IC50/CD).
Positive control for hydroxyl radical-scavenging assay.
Positive control for QR induction assay.
Pyrrole alkaloid 2 was initially isolated from goji berry (L. chinense).13 Compounds 1, 3, and 4 are analogues of 2 and are also pyrrole alkaloids. In contrast, no alkaloid has been previously isolated or detected spectroscopically from African mango. In addition, both goji berry and African mango reportedly can be used as antiobesity medicinal plants.33 Due to the unexpected presence of these pyrrole alkaloids and their structural similarity to and occurrence as constituents found in goji berries,12,13 as well as the public concern and a recent report about frequent contamination in commercial African mango samples,9 a comprehensive comparison of the commercial sample labeled as African mango seeds (product A) with authentic goji berry samples (products B–D) and African mango seeds (product E) was conducted via microscopic analysis as well as chemotaxonomy using LC-MS and 1H NMR fingerprinting, as shown in the following sections.
Microscopic Examination
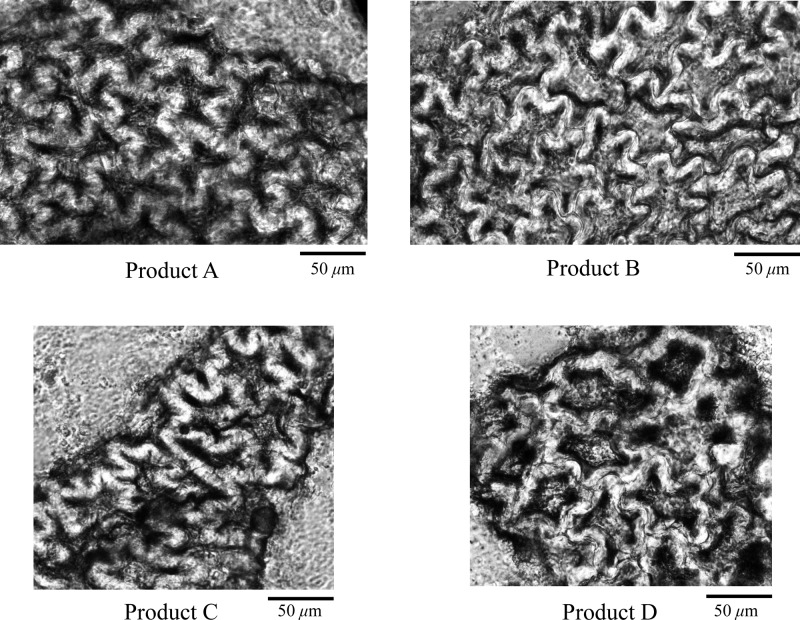
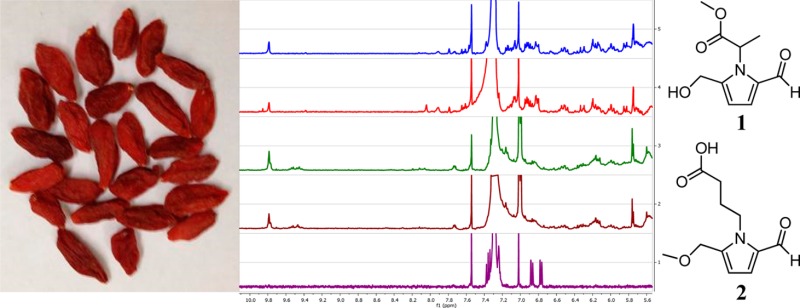
In the powders of products A–D, irregular polygonal testa sclereids (stone cells) were observed with thickened and curved walls as well as distinct striations in surface view (Figure 3), characteristic of the seeds of goji berry.14 This microscopic feature was not found in the authentic sample of African mango seed powder (product E).
Figure 3.
Microscopic structures of testa sclereids (stone cells) observed in products A–D.
HPLC-PDA and LC-IT-MS Analysis
Products A–D shared very similar HPLC chromatograms, which differed significantly from that of product E (Figure 4). Compound 3 had been all consumed for bioassay and thus could not be used as a standard. However, compounds 1, 2, and 4–8 isolated from product A were present in the authentic goji berry samples (products B–D), as identified by comparison of the retention times, UV profiles, and mass spectra of the peaks with standard compounds that had been identified by NMR (Table 2). In contrast, the HPLC chromatogram of the authentic sample of African mango seeds (product E) was shown to be very different, containing gallic acid, methyl gallate, ellagic acid, and their derivatives as major constituents, as identified by the same analytical methods mentioned above. These substances have been previously detected in African mango.8,9
Figure 4.
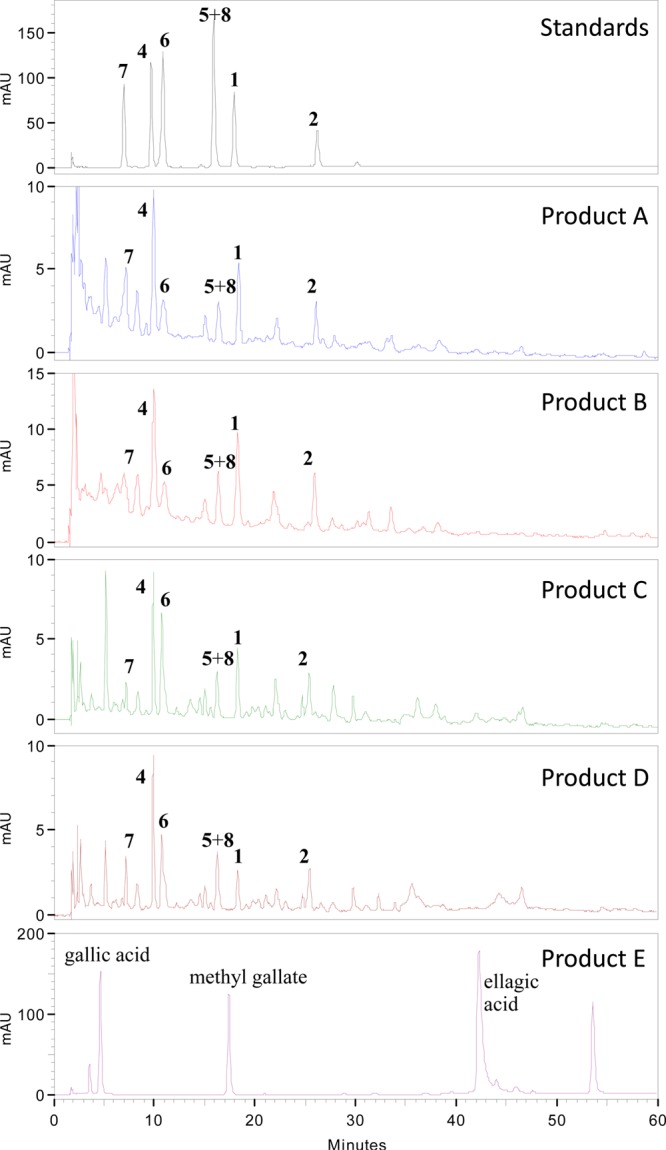
HPLC chromatograms (254 nm) of products A–E and standard compounds isolated from product A.
Table 2. LC-MS Data of Constituents Identified in a Representative Authentic Goji Berry Sample (Product C).
| tR (min) | UV λmax (nm) | molecular formula | [M + H]+ | [M + Na]+ | identification |
|---|---|---|---|---|---|
| 7.0 | 230, 284 | C6H6O3 | 127.1 | 149.1 | compound 7 |
| 9.7 | 254, 299 | C6H7NO2 | 126.1 | 148.1 | compound 4 |
| 10.9 | 217, 261, 294 | C7H7NO2 | 138.1 | 160.0 | compound 6 |
| 15.9 | 254, 285 | C7H6O3, C7H7NO3 | 139.1, 154.1 | 161.1, 176.0 | compounds 5 and 8 |
| 18.0 | 258, 296 | C10H13NO4 | 212.1 | 234.1 | compound 1 |
| 26.2 | 258, 296 | C11H15NO4 | 226.1 | 248.1 | compound 2 |
1H NMR Fingerprinting
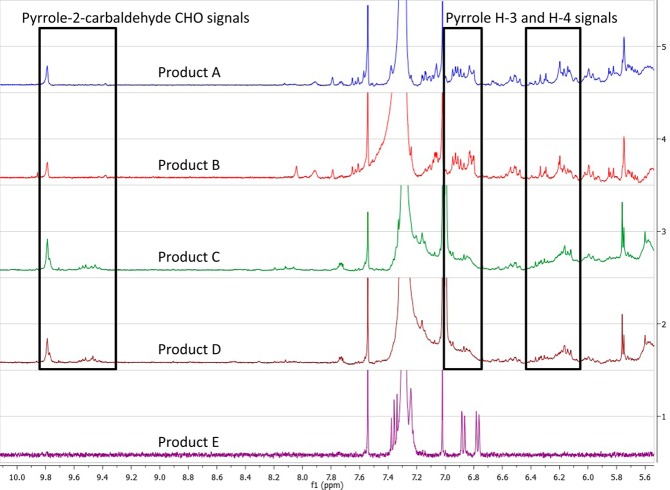
Visual inspection of the 1H NMR spectra revealed that all samples tested contained triacylglycerols as major constituents. By comparison, however, products A–D each contained pyrrole alkaloids as minor constituents with characteristic signals at δH 6.0–6.4 ppm that were not observed in product E. The presence of signals in the region of δH 9.4–9.8 ppm can be attributed to the aldehyde functionality of the isolated pyrrole-2-carbaldehyde compounds and further distinguished the botanical identity of product A from product E. Additionally, the signals that were detected only in product E may be ascribed to the previously reported constituents of African mango, namely, methyl gallate, ellagic acid, or their derivatives.8,9 The region of the NMR spectra pertinent to these comparisons is shown in Figure 5. The relative cleanness of the proton NMR spectrum of the authentic African mango seed extract suggests that 1H NMR fingerprinting may be a useful tool for the future authentication and/or quality control of such samples.
Figure 5.
1H NMR fingerprinting (δH 5.5–10.1 ppm) of products A–E (CDCl3, 400 MHz).
Accordingly, chemotaxonomic examination by LC-IT-MS and 1H NMR fingerprinting using authentic samples of goji berries and African mango suggested that there was evidence of contamination, adulteration, and/or mislabeling of the commercial African mango sample evaluated (product A), in agreement with a concerning trend previously reported.9 It is worth noting that goji berry from the same batch as product C was previously reported to exhibit in vivo inhibitory activity in a rat N-nitrosomethylbenzylamine (NMBA)-induced esophageal cancer model, and the detection of the in vitro active compounds 1, 2, and 6 in product C suggests that these compounds may possess potential in vivo cancer chemopreventive activity and hence merit further investigation.
Acknowledgments
We thank John Fowble and Dr. Craig McElroy (College of Pharmacy, The Ohio State University) for facilitating the acquisition of NMR data and Nanette Kleinholz from the Mass Spectrometry and Proteomics Facility at The Ohio State University for the acquisition of LC-MS data.
Supporting Information Available
1H NMR, 13C NMR, 13C DEPT 135, HSQC, HMBC, 1H–1H COSY, HRESIMS, and UV and IR spectra for compound 1; HPLC-PDA and LC-IT-MS data for identification of the isolated compounds in the authentic goji berry and African mango samples; 1H NMR fingerprinting of the CHCl3-soluble extracts of products A–E, and protocols used to evaluate hydroxyl radical-scavenging and QR-inducing activity. This material is available free of charge via the Internet at http://pubs.acs.org.
C.B.N. acknowledges financial support provided by ongoing predoctoral fellowships from the American Foundation for Pharmaceutical Education (AFPE) and an NIH Chemistry-Biology Interface Training Program (T32 GM008512).
The authors declare no competing financial interest.
Funding Statement
National Institutes of Health, United States
Supplementary Material
References
- Kuete V.; Wabo G. F.; Ngameni B.; Mbaveng A. T.; Metuno R.; Etoa F. X.; Ngadjui B. T.; Beng V. P.; Meyer J. J.; Lall N. Antimicrobial activity of the methanolic extract, fractions and compounds from the stem bark of Irvingia gabonensis (Ixonanthaceae). J. Ethnopharmacol. 2007, 114, 54–60. [DOI] [PubMed] [Google Scholar]
- Donfack J. H.; Fosto G. W.; Ngameni B.; Tsofack F. N.; Tchoukoua A.; Ambassa P.; Abia W.; Tchana A. N.; Giardina S.; Buonocore D.; Finzi P. V.; Vidari G.; Marzatico F.; Ngadjui B. T.; Moundipa P. F. In vitro hepatoprotective and antioxidant activities of the crude extract and isolated compounds from Irvingia gabonensis. Asian J. Tradit. Med. 2010, 5, 79–88. [Google Scholar]
- Okolo C. O.; Johnson P. B.; Abdurahman E. M.; Abdu-Aguye I.; Hussaini I. M. Analgesic effect of Irvingia gabonensis stem bark extract. J. Ethnopharmacol. 1995, 45, 125–129. [DOI] [PubMed] [Google Scholar]
- Agbor G. A.; Oben J. E.; Ngogang J. Y.; Xinxing C.; Vinson J. A. Antioxidant capacity of some herbs/spices from Cameroon: a comparative study of two methods. J. Agric. Food Chem. 2005, 53, 6819–6824. [DOI] [PubMed] [Google Scholar]
- Ngondi J. L.; Oben J. E.; Minka S. R. The effect of Irvingia gabonensis seeds on body weight and blood lipids of obese subjects in Cameroon. Lipids Health Dis. 2005, 4, 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oben J. E.; Ngondi J. L.; Momo C. N.; Agbor G. A.; Sobgui C. S. The use of a Cissus quadrangularis/Irvingia gabonensis combination in the management of weight loss: a double-blind placebo-controlled study. Lipids Health Dis. 2008, 7, 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ngondi J. L.; Djiotsa E. J.; Fossouo Z.; Oben J. Hypoglycaemic effect of the methanol extract of Irvingia gabonensis seeds on streptozotocin diabetic rats. Virol. J. 2006, 3, 74–77.16956415 [Google Scholar]
- Atawodi S. E. Polyphenol content and in vitro antioxidant activity of methanol extract of seeds of Irvingia gabonensis Baill. of Nigerian origin. Elec. J. Environ. Agric. Food Chem. 2011, 10, 2314–2321. [Google Scholar]
- Sun J.; Chen P. Ultra high-performance liquid chromatography with high-resolution mass spectrometry analysis of African mango (Irvingia gabonensis) seeds, extract, and related dietary supplements. J. Agric. Food Chem. 2012, 60, 8703–8709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuendet M.; Oteham C. P.; Moon R. C.; Pezzuto J. M. Quinone reductase induction as a biomarker for cancer chemoprevention. J. Nat. Prod. 2006, 69, 460–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waris G.; Ahsan H. Reactive oxygen species: role in the development of cancer and various chronic conditions. J. Carcinog. 2006, 5, 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youn U. J.; Kil Y.; Nam J.; Lee Y.; Kim J.; Lee D.; Lee J.; Seo E. New pyrrole alkaloids with bulky N-alkyl side chains containing stereogenic centers from Lycium chinense. Helv. Chim. Acta 2013, 96, 1482–1487. [Google Scholar]
- Chin Y. W.; Lim S. W.; Kim S. H.; Shin D. Y.; Suh Y. G.; Kim Y. B.; Kim Y. C.; Kim J. Hepatoprotective pyrrole derivatives of Lycium chinense fruits. Bioorg. Med. Chem. Lett. 2003, 13, 79–81. [DOI] [PubMed] [Google Scholar]
- American Herbal Pharmacopoeia. Lycium chinense Mill., L. barbarum L. In Botanical Pharmacognosy – Microscopic Characterization of Botanical Medicines; Upton R., Graff A., Jolliffe G., Langer R., Williamson E., Eds.; CRC Press: Boca Raton, FL, USA, 2011; pp 468–470. [Google Scholar]
- Sze S. C.; Song J. X.; Wong R. N.; Feng Y. B.; Ng T. B.; Tong Y.; Zhang K. Y. Application of SCAR (sequence characterized amplified region) analysis to authenticate Lycium barbarum (wolfberry) and its adulterants. Biotechnol. Appl. Biochem. 2008, 51, 15–21. [DOI] [PubMed] [Google Scholar]
- Potterat O. Goji (Lycium barbarum and L. chinense): phytochemistry, pharmacology and safety in the perspective of traditional uses and recent popularity. Planta Med. 2010, 76, 7–19. [DOI] [PubMed] [Google Scholar]
- Chang R. C.; So K. F. Use of anti-aging herbal medicine, Lycium barbarum, against aging-associated diseases. What do we know so far?. Cell Mol. Neurobiol. 2008, 28, 643–652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoner G. D.; Wang L. S.; Seguin C.; Rocha C.; Stoner K.; Chiu S.; Kinghorn A. D. Multiple berry types prevent N-nitrosomethylbenzylamine-induced esophageal cancer in rats. Pharm. Res. 2010, 27, 1138–1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo Q.; Cai Y.; Yan J.; Sun M.; Corke H. Hypoglycemic and hypolipidemic effects and antioxidant activity of fruit extracts from Lycium barbarum. Life Sci. 2004, 76, 137–149. [DOI] [PubMed] [Google Scholar]
- Amagase H.; Farnsworth N. R. A review of botanical characteristics, phytochemistry, clinical relevance in efficacy and safety of Lycium barbarum fruit (goji). Food Res. Int. 2011, 44, 1702–1717. [Google Scholar]
- Yu M. S.; Ho Y. S.; So K. F.; Yuen W. H.; Chang R. C. Cytoprotective effects of Lycium barbarum against reducing stress on endoplasmic reticulum. Int. J. Mol. Med. 2006, 17, 1157–1161. [PubMed] [Google Scholar]
- LeBel C. P.; Ischiropoulos H.; Bondy S. C. Evaluation of the probe 2′,7′-dichlorofluorescin as an indicator of reactive oxygen species formation and oxidative stress. Chem. Res. Toxicol. 1992, 5, 227–231. [DOI] [PubMed] [Google Scholar]
- Chin Y. W.; Chai H. B.; Keller W. J.; Kinghorn A. D. Lignans and other constituents of the fruits of Euterpe oleracea (Açaí) with antioxidant and cytoprotective activities. J. Agric. Food Chem. 2008, 56, 7759–7764. [DOI] [PubMed] [Google Scholar]
- Li J.; Pan L.; Deng Y.; Munoz-Acuna U.; Yuan C.; Lai H.; Chai H.; Chagwedera T. E.; Farnsworth N. R.; Carcache de Blanco E. J.; Li C.; Soejarto D. D.; Kinghorn A. D. Sphenostylisins A-K: bioactive modified isoflavonoid constituents of the root bark of Sphenostylis marginata ssp. erecta. J. Org. Chem. 2013, 78, 10166–10177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prochaska H. J.; Santamaria A. B. Direct measurement of NAD(P)H:quinone reductase from cells cultured in microtiter wells: a screening assay for anticarcinogenic enzyme inducers. Anal. Biochem. 1988, 169, 328–336. [DOI] [PubMed] [Google Scholar]
- Don M. J.; Shen C. C.; Lin Y. L.; Syu W. J.; Ding Y. H.; Sun C. M. Nitrogen-containing compounds from Salvia miltiorrhiza. J. Nat. Prod. 2005, 68, 1066–1070. [DOI] [PubMed] [Google Scholar]
- Hiermann A.; Kedwani S.; Schramm H. W.; Seger C. A new pyrrole alkaloid from seeds of Castanea sativa. Fitoterapia 2002, 73, 22–27. [DOI] [PubMed] [Google Scholar]
- Ahmed F. R.; Cheung K. M.; Toube T. P.; Utley J. H. P. Approaches to a cathodic route to polypyrrolylvinylenes. J. Mater. Chem. 1995, 5, 837–844. [Google Scholar]
- Wu B.; Lin W. H.; Gao H. Y.; Kim C. Antibacterial constituents of Senecio cannabifolius (II). Chin. Tradit. Herbal Drugs 2005, 36, 1447–1450. [Google Scholar]
- Olsson K.; Pernemalm P. A.; Theander O. Formation of aromatic compounds from carbohydrates. VII. Reaction of d-glucose and glycine in slightly acidic, aqueous solution. Acta Chem. Scand. B 1978, B32, 249–256. [Google Scholar]
- Pyo M. K.; Jin J. L.; Koo Y. K.; Yun-Choi H. S. Phenolic and furan type compounds isolated from Gastrodia elata and their anti-platelet effects. Arch. Pharm. Res. 2004, 27, 381–385. [DOI] [PubMed] [Google Scholar]
- Rukachaisirikul V.; Khamthong N.; Sukpondma Y.; Phongpaichit S.; Hutadilok-Towatana N.; Graidist P.; Sakayaroj J.; Kirtikara K. Cyclohexene, diketopiperazine, lactone and phenol derivatives from the sea fan-derived fungi Nigrospora sp. PSU-F11 and PSU-F12. Arch. Pharm. Res. 2010, 33, 375–380. [DOI] [PubMed] [Google Scholar]
- Hasani-Ranjbar S.; Jouyandeh Z.; Abdollahi M. A systematic review of anti-obesity medicinal plants – an update. J. Diabetes Metab. Disord. 2013, 12, 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.