Graphical abstract
Keywords: Lungworm, Angiostrongylosis, Canid, Epidemiology, Wolf, Wildlife disease, Pathology, One health
Highlights
-
•
Nematodes found in dead wolves in Rome province, Italy, were confirmed to be Angiostrongylus vasorum.
-
•
Molecular evidence further showed homology with lungworm larvae in sympatric dogs.
-
•
It is not known whether wolves represent ancentral hosts or are subject to more recent spill-over from dogs and foxes.
-
•
The parasite is pathogenic, emerging, and should be borne in mind in wolf conservation programmes in endemic areas.
Abstract
In the past decade, the parasitic nematode Angiostrongylus vasorum has attracted attention for its emergence in previously free areas and for the rise in clinical cases in domestic dogs. Italy is regarded as one of the countries where this potentially life-threatening parasite is spreading, especially due to bridging infections between wildlife and domestic dogs. The present article describes the presence of A. vasorum in wolves from Italy. Nematodes were observed in histological sections of three wolves found dead in Rome province, central Italy. Morphological and molecular identification of the nematodes, by polymerase chain reaction of rDNA ITS-2 and sequencing, confirmed the nematodes to be A. vasorum, with 99% genetic homology with A. vasorum from sympatric dogs. This is the second report of this species in wolves and the first in this host in Italy, and coincides with increasing records of A. vasorum in dogs and foxes in Italy. Implications for the epidemiology of this emerging parasite and for wildlife health are concisely discussed.
1. Introduction
The wolf (Canis lupus) is undergoing a population recovery in Europe, aided by legal protection and changes in human attitudes towards wolves and their conservation (Boitani, 2003). Disease has been frequently acknowledged as a major issue in the conservation of wild carnivores, especially where potential exists for spillover of generalist pathogens from domestic and wild canids (Craft et al., 2009; Alexander et al., 2010), and disease control has been employed to support conservation of wolves in Ethiopea (Randall et al., 2006). However, limited published information exists on the health status or parasite fauna of wolves in Europe. Sarcoptic mange is considered to have potential but unconfirmed impacts on wolf populations in Spain (Oleaga et al., 2011). In Italy, wolves are also expanding from previously small, isolated populations (Lucchini et al., 2004; Galaverni et al., 2013). The emerging eye-worm Thelazia callipaeda has been found in Italian wolves and discussed in the light of reservoirs of zoonotic disease (Otranto et al., 2007), but parasite impacts on Italian wolf health and population viability remain unstudied.
Angiostrongylus vasorum is a nematode parasite of dogs and other canids, occupying the heart and pulmonary blood vessels in its adult form, and using gastropod mollusc intermediate hosts (Morgan et al., 2005). Infection can cause a wide range of disease outcomes in dogs, including mild to severe respiratory disease, as well as bleeding disorders that manifest in various ways (Koch and Willesen, 2009), while in foxes cardio-respiratory pathology has been demonstrated (Morgan et al., 2008; Willesen et al., 2008). Although the parasite has long been recognised, in recent years it has emerged as an increasing clinical concern in dogs in endemic areas, as well as in new areas in which parasite presence was not previously recorded (Jefferies et al., 2010; Traversa et al., 2010; Conboy, 2011). The expansion of current endemic foci of A. vasorum and the establishment of further new endemic foci are supported by recent events described from Italy, with several published clinical cases in dogs and antibody seroprevalence of 0.8% in the general canine population in Tuscany (Guardone et al., 2013). In fact, in this country A. vasorum infection was first reported over 20 years ago in red foxes and for a long time the infection was likely confined to this host with no descriptions in domestic dogs. By 2002 angiostrongylosis was reported with increasing frequency in dogs, in which it can cause severe pathology, and now it is considered endemic throughout the country (Traversa et al., 2013).
This paper reports the discovery of A. vasorum in wolves in the Rome region, the second report in this host species and the first in Italy. This finding is discussed in the context of the current epidemiological situation in Italy with respect to angiostrongylosis, and possible effects on wolf health and conservation.
2. Materials and methods
Between December 2011 and January 2012, three wolves (Canis lupus) found dead in Rome province (Central Italy) were submitted to Istituto Zooprofilattico Sperimentale delle Regioni Lazio e Toscana for post mortem examination to investigate the cause of death. Animals were a juvenile female (W.1), an adult female (W.2) and an adult male (W.3). In order to ascertain if they were pure wolves or possible hybrids with the dog (Boitani, 2003), 18 autosomal microsatellite markers were used to genotype each of the three animals (Lorenzini et al., 2013).
For histological tests, representative samples of lungs, heart, liver, spleen, kidneys and brain, were taken from all three animals and fixed in buffered formalin, embedded in paraffin wax, sectioned at 4 μm and stained with haematoxylin and eosin. Histopathological changes in the lung and the presence of nematode material (see below) prompted further investigation of parasitic infection. Hence, although A. vasorum was previously reported only once from a wolf, in Spain (Segovia et al., 2001), microscopic examination of the larvae (McGarry and Morgan, 2009; Taubert et al., 2009) and anatomical localization of adults, led to the suspicion that this could be the parasite present in the three examined wolves. To confirm this possible unusual host/parasite association, the parasites were subjected to molecular identification. In order to further discern the relationship between the nematodes infecting these wolves and in local dogs naturally infected with A. vasorum, faecal samples from three dogs that had tested positive for A. vasorum L1s on clinical investigation were also subjected to DNA extraction and compared with the material from the wolves.
Molecular analysis was carried out as previously described (Jefferies et al., 2009a), with minor modification. Briefly, DNA was extracted from the lung tissues using DNeasy blood and tissue kit (Qiagen, Germany) following the manufacturer’s instructions. The lysing step was performed overnight instead of over 3 h to ensure complete lysis of the tissues. The manufacturer’s protocol was then followed until DNA was eluted in 200 μl of RNAse free water. DNA was stored at −20 °C until required. The internal transcribed spacer (ITS2) of ribosomal DNA was amplified using primers NC1 (5′-ACGTCTGGTTCAGGGTTGTT-3′) AND NC2 (5′-TTAGTTTCTTTTCCTCCGCT-3′) as described by Gasser et al. (1993). Polymerase chain reactions (PCR) were performed in a final total volume of 25 μl consisting of 2.5 μl of 10× polymerase buffer, 0.5 μl each of the deoxyribonucleotide triphosphate (dNTPs; 10 mmol each), 0.125 μl of Hot star Taq DNA polymerase(5 U/ml), 18.625 μl of dH2O and 2 μl of DNA.
Targets were amplified under the PCR conditions of 95 °C for 15 min followed by 40 cycles of 95 °C for 30 s, 55 °C for 30 s and 72 °C for 1 min; followed by a final extension step of 72 °C for 5 min. The PCR products were visualised with use of ethidium bromide and ultraviolet illumination on 1% agarose gel. Un-purified PCR products were sent for purification and sequencing using the value read tube sequencing service (Eurofins MWG Operon, London). Sequences were compared with those available in the GenBank database by nucleotide sequence homology searches made at the network server of the National Centre for Biotechnology Information (NCBI) using BLAST. Multiple sequence alignments were performed using the program ClustalW2 (EMBL-EBI database).
3. Results and discussion
Genotyping showed no evidence of genetic admixture with dogs for any of the three wolves. At necropsy, all wolves showed traumatic lesions, with multiple bone fractures, in particular of the cranium. Anatomo-pathological examination of cavitary organs of W.1 revealed congestion and presence of small, whitish and firm nodules scattered in lung parenchyma, especially near the edges of pulmonary lobes. W.2 and W.3 showed diffuse lung congestion and rare foci, similar to W.1. No macroscopic lesion was detected in any of the other organs of the three animals. At histological examination, microscopic changes consisted of the presence of parasitic granulomatous foci in lung parenchyma, in all individuals, characterized by peripheral fibrosis and inflammatory infiltrates of lymphocytes, plasma cells, macrophages, granulocytes and a few multinucleated giant cells (Fig. 1). In the centre of granulomata, numerous nematode larvae and eggs were visible. In each host, one or more adult worms were detected in pulmonary arteries (Fig. 2), occasionally associated with obliterative thrombotic endarteritis. These findings were more severe in W.1, where histological examination showed also rare microgranulomata in the heart. The anatomo-pathological and histological pictures in the three animals were overlapping with those usually shown by dogs harbouring A. vasorum (Bourque et al., 2008). No significant microscopic lesions were observed in other organs. Given the small sample size and the destructive nature of sampling for adult nematodes (Morgan et al., 2008), correlation between nematode burden and extent of histopathological change was not attempted.
Fig. 1.
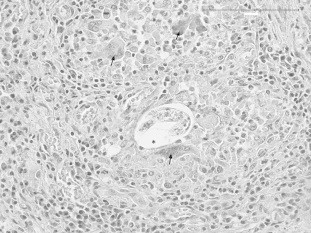
Lung: granuloma containing a parasitic developing larva (asterisk), surrounded by multinucleated giant cells (arrows). Hematoxylin and eosin. Scale bar represents 100 μm.
Fig. 2.
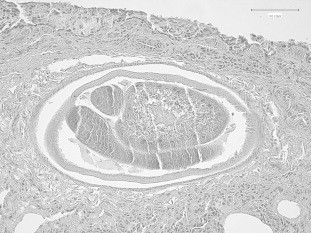
Lung: adult nematode within a pulmonary artery. Hematoxylin and eosin. Scale bar represents 100 μm.
Sequencing of the PCR products confirmed the presence of A. vasorum in the faecal sediments of the infected dogs as well as the lung tissues of the wolves. The ITS-2 sequences of both the L1s from dogs and lung tissues from wolves were 99% identical to A. vasorum (GenBank EU627595.1). Table 1 shows the alignment scores with different sequences available on GenBank.
Table 1.
Alignment scores of ITS-2 rDNA PCR products from the nematode samples recovered from dogs (sequence 1) and wolves (sequence 2), and publicly available sequences from Genbank. Angiostrongylus cantonensis was included as an outlier, and sequence 4 is from a recently described novel metastrongyloid nematode described in badgers in Spain (Gerrikagoitia et al., 2010). Sequence 3 is the closest matching confirmed Angiostrongylus vasorum sequence in Genbank. bp = base pairs.
| Sequence | Identifier | Source | Length (bp) | Percent alignment |
||||
|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | ||||
| 1 | LARVfs_1.0 | Dogs, Italy | 532 | – | 98 | 99 | 89 | 84 |
| 2 | Ltw_1.0 | Wolves, Italy | 490 | – | 99 | 89 | 82 | |
| 3 | gi|194246349|gb|EU627595.1| | A. vasorum, Genbank | 580 | – | 90 | 82 | ||
| 4 | gi|299835292|gb|GU323341.1| | Badgers, Spain | 533 | – | 82 | |||
| 5 | gi|321270990|gb|HQ540548.1 | A. cantonensis | 1547 | – | ||||
A wide range of wild carnivores has been found to be infected by A. vasorum to date, as well as domestic dogs in several geographic areas (Jefferies et al., 2009b, 2010). In Newfoundland, Canada, this nematode is found in red foxes and more recently has been described in coyotes (Bridger et al., 2009). In several European countries A. vasorum is commonly found in wild red foxes (e.g. Gerrikagoitia et al., 2010), in which it causes detectable pathology (Morgan et al., 2008). Only one previous record has been published from wolves (Segovia et al., 2001). This raises the question of whether wolves have acted as ancestral hosts to this parasite, forming part of the sylvatic reservoir from which emerging cases in dogs are presumably infected, or whether infection in wolves results from more recent spill-over from dogs. Molecular analyses using several mitochondrial as well as nuclear DNA markers confirm that A. vasorum genotypes are well mixed between dogs and foxes in Europe (Jefferies et al., 2010). More detailed molecular studies would be needed to better elucidate the evolutionary relationships and transmission dynamics of A. vasorum between wild canids (especially foxes) and dogs in Italy, where infection with the parasite appears to be expanding in dogs (Traversa et al., 2010, 2013). In conclusion, although death of these wolves cannot be ascribed to angiostrongylosis, with the injuries described likely to be fatal, the parasite is known to be highly pathogenic in dogs and to cause significant pathology in foxes, and should be considered as a wildlife health issue in regions in which the parasitosis is endemic and wolves are of conservation concern.
Acknowledgements
We thank Ryan Jefferies for advice on the molecular analyses, Rita Lorenzini for genetic analysis of wolves and Mark Viney for providing laboratory assistance. The authors have no conflicts of interest.
Footnotes
This is an open-access article distributed under the terms of the Creative Commons Attribution-NonCommercial-No Derivative Works License, which permits non-commercial use, distribution, and reproduction in any medium, provided the original author and source are credited.
References
- Alexander K.A., McNutt J.W., Briggs M.B., Standers P.E., Funston P., Hemson G., Keet D., van Vuuren M. Multi-host pathogens and carnivore management in southern Africa. Comp. Immunol. Microbiol. Infect. Dis. 2010;33:249–265. doi: 10.1016/j.cimid.2008.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boitani L. Wolf conservation and recovery. In: Mech L.D., Boitani L., editors. Wolves: Behaviour Ecology and Conservation. University of Chicago Press; Chicago: 2003. pp. 317–340. [Google Scholar]
- Bourque A.C., Conboy G., Miller L.M., Whitney H. Pathological findings in dogs naturally infected with Angiostrongylus vasorum in Newfoundland and Labrador, Canada. J. Vet. Diagn. Invest. 2008;20:11–20. doi: 10.1177/104063870802000103. [DOI] [PubMed] [Google Scholar]
- Bridger K.E., Baggs E.M., Finney-Crawley J. Endoparasites of the coyote (Canis latrans), a recent migrant to insular Newfoundland. J. Wildl. Dis. 2009;45:1221–1226. doi: 10.7589/0090-3558-45.4.1221. [DOI] [PubMed] [Google Scholar]
- Conboy G.A. Canine angiostrongylosis: the French heartworm: an emerging threat in North America. Vet. Parasitol. 2011;176:382–389. doi: 10.1016/j.vetpar.2011.01.025. [DOI] [PubMed] [Google Scholar]
- Craft M.E., Volz E., Packer C., Meyers L.A. Distinguishing epidemic waves from disease spillover in a wildlife population. Proc. R. Soc. London, Sect. B. 2009;276:1777–1785. doi: 10.1098/rspb.2008.1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galaverni M., Caniglia R., Fabbri E., Lapalombella S., Randi E. MHC variability in an isolated wolf population in Italy. J. Hered. 2013;104:601–612. doi: 10.1093/jhered/est045. [DOI] [PubMed] [Google Scholar]
- Gasser R.B., Chilton N.B., Hoste H., Beveridge I. Rapid sequencing of rDNA from single worms and eggs of parasitic helminths. Nucleic Acids Res. 1993;21:2525–2526. doi: 10.1093/nar/21.10.2525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerrikagoitia X., Barral M., Juste R.A. Angiostrongylus species in wild carnivores in the Iberian peninsula. Vet. Parasitol. 2010;174:175–180. doi: 10.1016/j.vetpar.2010.07.015. [DOI] [PubMed] [Google Scholar]
- Guardone L., Schnyder M., Macchioni F., Deplazes P., Magi M. Serological detection of circulating Angiostrongylus vasorum antigen and specific antibodies in dogs from central and northern Italy. Vet. Parasitol. 2013;192:192–198. doi: 10.1016/j.vetpar.2012.10.016. [DOI] [PubMed] [Google Scholar]
- Jefferies R., Morgan E.R., Shaw S.E. A SYBR green real-time PCR assay for the detection of the nematode Angiostrongylus vasorum in definitive and intermediate hosts. Vet. Parasitol. 2009;166:112–118. doi: 10.1016/j.vetpar.2009.07.042. [DOI] [PubMed] [Google Scholar]
- Jefferies R., Shaw S.E., Viney M.E., Morgan E.R. Angiostrongylus vasorum from South America and Europe represent distinct lineages. Parasitology. 2009;136:107–115. doi: 10.1017/S0031182008005258. [DOI] [PubMed] [Google Scholar]
- Jefferies R., Shaw S.E., Willesen J., Viney M.E., Morgan E.R. Elucidating the spread of the emerging canid nematode Angiostrongylus vasorum between Palearctic and Nearctic ecozones. Infect. Genet. Evol. 2010;10:561–568. doi: 10.1016/j.meegid.2010.01.013. [DOI] [PubMed] [Google Scholar]
- Koch J., Willesen J. Canine pulmonary angiostrongylosis: an update. Vet. J. 2009;179:348–359. doi: 10.1016/j.tvjl.2007.11.014. [DOI] [PubMed] [Google Scholar]
- Lorenzini, R., Fanelli, R., Grifoni, G., Scholl, F., Fico, R., 2013. Wolf–dog crossbreeding: “smelling” a hybrid may not be easy. Mamm. Biol. (in press).
- Lucchini V., Galov A., Randi E. Evidence of genetic distinction and long-term population decline in wolves (Canislupus) in the Italian Apennines. Mol. Ecol. 2004;13:523–536. doi: 10.1046/j.1365-294x.2004.02077.x. [DOI] [PubMed] [Google Scholar]
- McGarry J.W., Morgan E.R. Identification of first-stage larvae of metastrongyles from dogs. Vet. Rec. 2009;165:258–261. doi: 10.1136/vr.165.9.258. [DOI] [PubMed] [Google Scholar]
- Morgan E.R., Shaw S.E., Brennan S.F., De Waal T.D., Jones B.R., Mulcahy G. Angiostrongylus vasorum: a real heartbreaker. Trends Parasitol. 2005;21:49–51. doi: 10.1016/j.pt.2004.11.006. [DOI] [PubMed] [Google Scholar]
- Morgan E.R., Tomlinson A., Hunter S., Nichols T., Roberts E., Fox M.T., Taylor M.A. Angiostrongylus vasorum and Eucoleus aerophilus in foxes (Vulpes vulpes) in Great Britain. Vet. Parasitol. 2008;154:48–57. doi: 10.1016/j.vetpar.2008.02.030. [DOI] [PubMed] [Google Scholar]
- Oleaga A., Casais R., Balseiro A., Espi A., Llaneza L., Hartasanchez A., Gortazar C. New techniques for an old disease: sarcoptic mange in the Iberian wolf. Vet. Parasitol. 2011;181:255–266. doi: 10.1016/j.vetpar.2011.04.036. [DOI] [PubMed] [Google Scholar]
- Otranto D., Cantacessi C., Mallia E., Lia R.P. First report of Thelazia callipaeda (Spirurida, Thelaziidae) in wolves in Italy. J. Wildl. Dis. 2007;43:508–511. doi: 10.7589/0090-3558-43.3.508. [DOI] [PubMed] [Google Scholar]
- Randall D.A., Marino J., Haydon D.T., Sillero-Zubiri C., Knobel D.L., Tallents L.A., MacDonald D.W., Laurenson M.K. An integrated disease management strategy for the control of rabies in Ethiopean wolves. Biol. Conserv. 2006;131:151–162. [Google Scholar]
- Segovia J.M., Torres J., Miguel J. Helminths in the wolf, Canis lupus, from north-western Spain. J. Helminthol. 2001;75:183–192. [PubMed] [Google Scholar]
- Taubert A., Pantchev N., Vrhovec M.G., Bauer C., Hermosilla C. Lungworm infections (Angiostrongylus vasorum, Crenosoma vulpis, Aelurostrongylus abstrusus) in dogs and cats in Germany and Denmark in 2003–2007. Vet. Parasitol. 2009;159:175–180. doi: 10.1016/j.vetpar.2008.10.005. [DOI] [PubMed] [Google Scholar]
- Traversa D., Di Cesare A., Conboy G. Canine and feline cardiopulmonary parasitic nematodes in Europe: emerging and underestimated. Parasites Vectors. 2010;3:62. doi: 10.1186/1756-3305-3-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traversa D., Di Cesare A., Meloni S., di Regalbono A.F., Milillo P., Pampurini F., Venco L. Canine angiostrongylosis in Italy: occurrence of Angiostrongylus vasorum in dogs with compatible clinical pictures. Parasitol. Res. 2013;112:2473–2480. doi: 10.1007/s00436-013-3412-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willesen J.L., McEvoy F.J., Webster P., Monrad J., Jensen A.T., Svalatoga E.L., Koch J. Radiological changes in foxes (Vulpes vulpes) experimentally infected with Angiostrongylus vasorum. J. Vet. Int. Med. 2008;22:707. [Google Scholar]


