Summary
Background
Epigenetic studies that utilize peripheral tissues to identify molecular substrates of neuropsychiatric disorders rely on the assumption that disease-relevant, cellular alterations that occur in the brain are mirrored and detectable in peripheral tissues such as blood. We sought to test this assumption by using a mouse model of Cushing’s disease and asking whether epigenetic changes induced by glucocorticoids can be correlated between these tissue types.
Methods
Mice were treated with different doses of glucocorticoids in their drinking water for four weeks to assess gene expression and DNA methylation (DNAm) changes in the stress response gene Fkbp5.
Results
Significant linear relationships were observed between DNAm and four-week mean plasma corticosterone levels for both blood (R2 = 0.68, P = 7.1×10−10) and brain (R2 = 0.33, P = 0.001). Further, degree of methylation change in blood correlated significantly with both methylation (R2 = 0.49, P = 2.7×10−5) and expression (R2 = 0.43, P = 3.5×10−5) changes in hippocampus, with the notable observation that methylation changes occurred at different intronic regions between blood and brain tissues.
Conclusion
Although our findings are limited to several intronic CpGs in a single gene, our results demonstrate that DNA from blood can be used to assess dynamic, glucocorticoid-induced changes occurring in the brain. However, for such correlation analyses to be effective, tissue-specific locations of these epigenetic changes may need to be considered when investigating brain-relevant changes in peripheral tissues.
Keywords: Fkbp5, Epigenetics, DNA methylation, Blood-brain correlation, Biomarker, and Glucocorticoids
1. Introduction
Levels of the glucocorticoid cortisol constitute one of the key determinants of allostasis or allostatic load, and prolonged exposure to its catabolic properties leads to numerous diseases that include diabetes, cardiovascular disease, and obesity (Karlamangla et al., 2002). For the brain, exposure to glucocorticoids is a robust risk factor for neuropsychiatric illnesses and cognitive decline, as numerous lines of evidence implicate exposure to stress or glucocorticoids in the etiology of several psychiatric disorders (McEwen and Gianaros, 2010, Fardet et al., 2012, Theall et al., 2012). In particular, hypercortisolemia or dysregulation of the hypothalamic-pituitary-adrenal-axis (HPA-axis) system is often associated with mood disorders (Gillespie and Nemeroff, 2005), and genetic association studies have linked single nucleotide polymorphisms in “HPA-axis genes” that govern the stress-response with post-traumatic stress disorder (PTSD), suicide, and depression (Binder et al., 2004, Binder et al., 2008, Roy et al., 2010, Roy et al., 2012, Sinclair et al., 2012). Large-scale epidemiological studies have also implicated iatrogenic glucocorticoid administration with psychiatric symptoms in hundreds of thousands of patients prescribed steroids for non-psychiatric diseases (Fardet et al., 2012). In addition, a more direct, causal relationship between glucocorticoid exposure and psychiatric illnesses can be found in Cushing’s disease, where endogenous or iatrogenic elevation in plasma glucocorticoid levels has led to depression in 60–90% of patients (Cohen, 1980, Flitsch et al., 2000). Notably, the psychiatric symptoms are often alleviated with resolution of hypercortisolemia, strongly suggesting involvement of glucocorticoids in depressive symptoms (Starkman et al., 1986, Dorn et al., 1997). In animals, stress-induced deficits in both dopamine signaling and behavior are rescued by the glucocorticoid receptor antagonist mifepristone (RU486), further implicating glucocorticoid action (Niwa et al., 2013). Therefore, the ability to measure glucocorticoid exposure in the brain would serve as a useful gauge for assessing susceptibility to neuropsychiatric illnesses.
Previously, we found that degree of change in DNA methylation (DNAm) at a key regulatory locus within the stress-response gene Fkbp5 robustly reflected the previous 30-day mean plasma glucocorticoid values in the mouse blood (Lee et al., 2011). In fact, methylation changes in blood also correlated with anxiety-like behavior on the elevated plus maze. In addition, these epigenetic changes strongly correlated with functionally relevant physiological changes in glucocorticoid target tissues, such as atrophy of the thymus, spleen, and the adrenal glands. Thus we suggested in our previous study that using such an approach is highly practical since only one small sampling (~20 μL of blood) accurately reflected and obviated the need for multiple daily measurements of plasma glucocorticoid levels when determining glucocorticoid burden (Lee et al., 2011).
In this study, we extend our analysis to the hippocampus, a particularly glucocorticoid-sensitive brain region (Kaouane et al., 2012), to determine whether the correlations between mean glucocorticoid exposure over a four-week treatment period and changes in DNAm and expression levels of the Fkbp5 gene in the blood accurately reflect changes in the brain. In addition to its function as a stress-response gene and a regulator of glucocorticoid signaling (Scammell et al., 2001, Wochnik et al., 2005), FKBP5 has been implicated in several candidate gene association studies of depression, bipolar disorder, and PTSD (Binder et al., 2004, Binder et al., 2008, Willour et al., 2009, Roy et al., 2012). A recent clinical study implicating methylation changes of FKBP5 in blood to childhood trauma exposure (Klengel et al., 2013) further warrants a closer examination of the relationship between blood and brain epigenetic changes in this important gene.
Given the link between glucocorticoid exposure and neuropsychiatric disorders, development of a tool that can assess exposure in the brain through the use of a surrogate, easily accessible tissue would be of great clinical utility. While reported as a case study of a single but an important gene in stress response, our results provide useful insights into the use of surrogate tissues for brain and suggest guidelines for future biomarker discoveries.
2. Materials and methods
2.1. Animals
At five weeks of age, male C57BL/6J mice (N=12 in each group; Jackson Laboratories, Bar Harbor, ME) were given ad libitum access to solutions containing the rodent stress glucocorticoid corticosterone (Sigma-Aldrich, St. Louis, MO; 100 μg/ml with 1% ethanol; “CORT” group) or 1% ethanol (“VEHICLE” group) in place of their normal drinking water, and this treatment continued for four weeks. This group is referred to as the first cohort. A second cohort of animals (N = 8 in each group, 5 dose groups, and 40 total) was treated with five doses of corticosterone (0, 25, 50, 75, or 100 μg/ml with 1% ethanol), once again with ad libitum access to the solution in place of their drinking water, in order to obtain a wider dose-effect relationship. At the end of the treatment period, animals were euthanized, tissues harvested, and their brains frozen on powdered dry ice and subsequently stored at −80°C. All procedures were approved by the Institutional Animal Care and Use Committee at Johns Hopkins University School of Medicine and were performed in accordance with guidelines established in the National Research Council’s Guide for the Care and Use of Laboratory Animals.
2.2. Blood collection
20 μL of whole blood was collected weekly (0900 hr) from each mouse into heparinized glass capillary tubes through a small nick at the tip of the tail. Blood samples were centrifuged at 4°C and plasma was collected and frozen at −80°C for further analysis of total (free and bound) plasma CORT by radioimmunoassay, according to the manufacturer’s instructions (MP Biomedicals, Costa Mesa, CA). All samples were run in duplicates with an intra-assay coefficient of variance (CV) of 3.9% and inter-assay CV of 7.5%. Animals were euthanized at the end of the treatment period, and trunk blood (~250 μl) was collected. Blood samples were incubated with three-volumes of Ack Lysing Buffer (Quality Biological, Gaithersburg, MD) to lyse red blood cells, centrifuged to collect white blood cells, and divided into two fractions for subsequent genomic DNA (gDNA) and (mRNA) messenger RNA analysis.
2.3. Brain dissection
Frozen mouse brains were sectioned using a cryostat, and 400 μm or 500 μm sections were mounted on glass slides. 19-gauge needles (0.686 mm inner diameter and 1.086 mm outer diameter) were used to punch out the hippocampal dentate gyrus (bregma −0.98 through −2.0 mm). Punched tissues were stored at −80°C in either Tissue & Cell Lysis Buffer (Epicentre Biotechnologies, Madison, WI) or Qiazol (Qiagen, Valencia, CA) until processed for gDNA and mRNA analysis, respectively.
2.4. Messenger RNA extraction and gene expression
Messenger RNA from white blood cells and the hippocampal dentate gyrus was obtained using the RNeasy Lipid Tissue Mini kit (Qiagen, Valencia, CA). QuantiTect Reverse Transcription Kit (Qiagen) was used to generate cDNA for quantitative real-time PCR. Calculation of expression values has been previously described (Lee et al., 2011).
2.5. Genomic DNA extraction and bisulfite conversion
gDNA from mouse brain tissues and blood was isolated with the Masterpure DNA Purification Kit, according to manufacturer’s instructions (Epicentre Biotechnologies, Madison, WI). Concentration of gDNA was determined using a NanoDrop 1000 Spectrophotometer (Thermo Scientific, Rockford, IL), and EZ DNA Methylation Gold Kit was used on ~500 ng of gDNA for bisulfite conversion, according to manufacturer’s protocol (Zymo Research, Irvine, CA).
2.6. Bisulfite PCR and pyrosequencing
DNA methylation (DNAm) status was measured by pyrosequencing of the PCR products, which measures methylation variation at >90% precision (Colella et al., 2003). Procedures for PCR of bisulfite-converted DNA for pyrosequencing, including primer sequences, have been described previously (Lee et al., 2010). Percentage of methylation at each CpG (seven total: three CpGs from intron 1 and four CpGs from intron 5) as determined by pyrosequencing was compared between DNA from 0 μg/mL (or VEHICLE) vs. all other CORT doses for both cohorts. Based on the UCSC Genome Browser mouse build mm10, genomic coordinates for the CpGs examined are: intron 1 CpG-1: 28,441,243; CpG-2: 28,441,253; CpG-3: 28,441,288; and intron 5 CpG-1: 28,420,359; CpG-2: 28,420,385; CpG-3: 28,420,541; CpG-4: 28,420,548, which are all located on chromosome 17.
2.7. Statistical Analysis
For the first cohort, comparisons of mean plasma CORT, tissue-specific DNAm and gene expression between CORT- and VEHICLE-treated animals were analyzed by Student’s t-tests. In the second cohort, we used linear regression to assess the relationships of CORT exposure (captured as an ordinal variable for doses of 0, 25, 50, 75 and 100 μg/mL) with mean plasma CORT levels, mean plasma CORT levels with tissue specific DNAm and gene expression, and DNAm of specific CpGs in blood with DNAm of CpGs in the hippocampus. We computed R2 and p-values from the linear regression models. P-values were adjusted by Bonferroni correction for multiple testing by the number of CpGs tested for each tissue. For instance, where all blood CpG DNAm values were compared to all hippocampal CpG DNAm values, corrected p < 0.05 [or uncorrected p-value × (7 blood CpGs × 7 hippocampal CpGs)] was considered study-wide significant. All analyses were performed using R statistical programming (R, 2010).
3. Results
3.1. Glucocorticoid exposure and changes in Fkbp5 expression
Genomic structure of the stress-response gene Fkbp5 (Fig. 1a) includes two promoter CpG islands and two bioinformatically-annotated and functionally-characterized glucocorticoid response elements (GREs) that reside in the first and fifth introns. We first assessed the functional and epigenetic consequences of glucocorticoid exposure on the Fkbp5 locus. A (first) cohort of mice were treated with 100 μg/mL of corticosterone (CORT) in the drinking water for four weeks. To adequately capture glucocorticoid exposure, plasma samples were obtained at weekly intervals. In the treated group, plasma CORT levels (mean ± SEM) became significantly elevated following the first week of treatment and remained elevated throughout the treatment period. In contrast, plasma CORT levels in the vehicle-treated animals remained less than 40 ng/mL during all sampling times (Fig. 1b, left panel; p < 0.01). The mean plasma CORT levels over the four weeks of treatment was 309.7 ± 27.0 ng/mL for treated animals (CORT, N=12) versus 28.2 ± 3.2 ng/mL for vehicle-treated animals (VEHICLE, N=12; p = 2.1×10−7, Fig. 1b, right panel). An additional repeated measures ANOVA test to assess the impact of chronic CORT administration on plasma CORT levels showed that four-week CORT administration had a significant effect on the difference in measured plasma CORT levels between exposed (CORT) and unexposed (VEHICLE) groups (p = 2.0×10−7).
Figure 1.
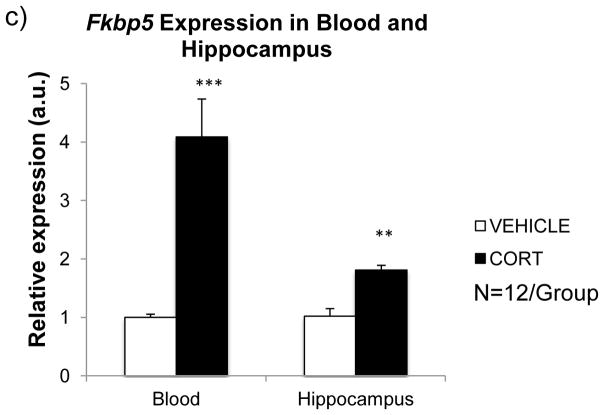
Genomic organization of the mouse Fkbp5 gene and effect of chronic glucocorticoid exposure on its expression and DNA methylation. (a) Mouse Fkbp5 genomic locus (Chr.17: 28,399,095 – 28,517,524, UCSC Genome Browser, mm10) consists of eleven exons (black boxes), four alternate exons (gray boxes), and three CpG islands (thick horizontal lines), two of which are located upstream of the first alternate exon and the third one immediately downstream of the first exon. Two GREs (glucocorticoid response elements) assayed by pyrosequencing for tissue-specific DNA methylation are represented by thin horizontal lines. (b) To assess the impact of chronic CORT exposure on Fkbp5 gene expression and methylation, animals were treated for four weeks and serial, weekly measurements for plasma CORT levels were determined. For each group (VEHICLE vs. CORT), an average was calculated from individual animals at each of the weekly CORT measurements (left panel), and weekly mean values from each group were averaged further to obtain mean plasma CORT level differences between the two groups (right panel). (c) After four weeks of CORT treatment mRNA was extracted from blood and hippocampus. Tissues examined from the CORT-treated group showed elevation of Fkbp5 expression, with blood showing the greater increase. Data are shown as mean ± SEM. ** p ≤ 0.01 and *** p ≤ 0.001: compared to the VEHICLE group.
In order to examine the impact of elevated CORT levels on Fkbp5 gene function, mRNA was extracted from blood lymphocytes and hippocampus at the end of the treatment period and assayed for expression levels. We observed greater than four-fold increase in blood Fkbp5 (p < 0.001) and a more modest ~80% increase in hippocampal Fkbp5 (p < 1.7×10−3) in the CORT vs. VEHICLE animals (Fig. 1c). As Fkbp5 is an immediate-early stress-response gene, our results further confirm robust CORT-induced increase in its expression levels in both tissues examined.
3.2. Glucocorticoid exposure and changes in DNA methylation
We then examined whether increases in expression levels observed in these tissues are accompanied by epigenetic alterations. In order to maximize observed epigenetic changes in the hippocampus, we carried out hole-punch dissection of the granule neuron-rich dentate gyrus. gDNA from white blood cells and the dentate was then bisulfite-treated and subjected to pyrosequencing. Two intronic regions previously characterized as glucocorticoid response elements (GREs) were assayed for changes in DNAm levels (Magee et al., 2006). The GRE in intron 1 includes three CpGs, whereas the GRE of intron 5 includes four CpGs. In blood, CpGs in intron 1 GRE showed significant changes in DNAm in CORT-treated animals, with notable changes in CpG-1 (36.7%, p = 3.7×10−17) and CpG-2 (34.4%, p = 3.1×10−15), and a more modest change in CpG-3 (11.6%, p = 6.6×10−14, Fig. 2a). In contrast, the intron 5 GRE in blood showed relatively small DNAm changes, with the magnitude of DNAm differences less than 6.5% at all four CpGs (Fig. 2b). A small but statistically significant DNAm change at CpG-4 (5.5%, p = 0.003) showed an increase in methylation that was inconsistent with the other CpGs in intron 5 and did not replicate in the second cohort of animals.
Figure 2.
Glucocorticoid-induced DNA methylation changes in blood and hippocampus. Bisulfite-pyrosequencing was performed at three CpG dinucleotides in intron 1 (a) and four CpG dinucleotides in intron 5 (b) of the mouse Fkbp5 gene in white blood cells of mice treated with corticosterone (“CORT”; N=12) and compared to those of vehicle-solution treated controls (“VEHICLE”; N=12). Bisulfite-pyrosequencing was also performed at three CpG dinucleotides in intron 1 (c) and four CpG dinucleotides in intron 5 (d) of the mouse Fkbp5 gene in the hippocampus of mice treated with corticosterone and compared to those of vehicle-solution treated controls. Data are shown as mean ± SEM. * p ≤ 0.05, ** p ≤ 0.01, and *** p ≤ 0.001: compared to the VEHICLE group.
In the hippocampus, we observed small but significant differences in CORT-induced DNAm changes in intron 1 CpG-1 (2.7%, p = 6.0×10−5, Fig. 2c), which we deemed biologically irrelevant due to the small effect size. In contrast, all four CpGs in intron 5 in the dentate underwent significant DNAm changes with CORT treatment. The most remarkable epigenetic changes were observed at CpG-4, where the magnitude of methylation differences was 14.0% (p = 1.8×10−5, Fig. 2d). Pyrosequencing performed on other potential regulatory cis elements, including few CpGs within two of the three CpG islands and 10 CpGs of the promoter, were hypomethylated and did not show appreciable differences in DNAm (data not shown) (Lee et al., 2010).
3.3. Dose-response changes in plasma corticosterone levels, expression, and DNA methylation
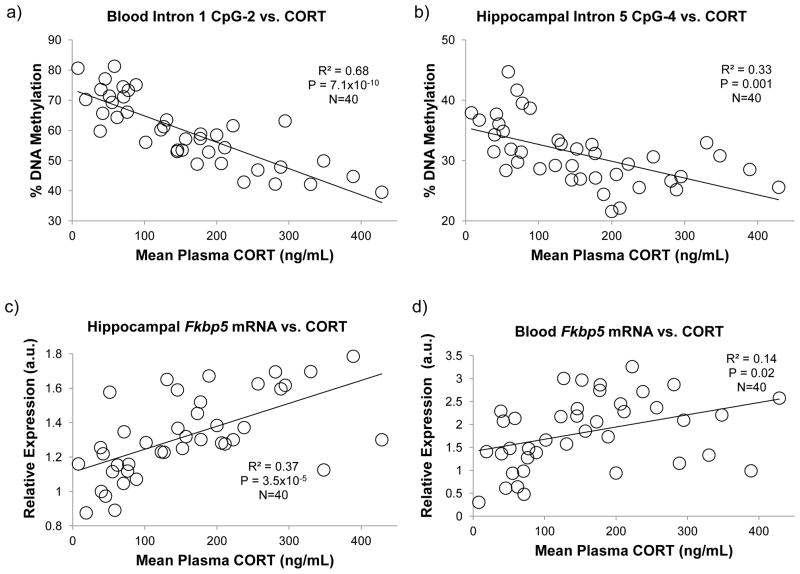
In order to determine whether there were dose-response relationships between CORT exposure and changes in plasma CORT levels, gene expression, and DNAm, we treated another (second) cohort of mice with different doses of CORT (0, 25, 50, 75, and 100 μg/mL). A trend analysis revealed that mean plasma CORT levels were linearly related to increasing doses of CORT administration (R2 = 0.74, p = 1.18×10−12). In turn, there were significant linear relationships between mean plasma CORT levels and epigenetic and expression changes in Fkbp5 in both blood and brain tissue. In general, the most significant correlations between mean plasma CORT levels and DNAm were observed with the intron 1 CpGs in blood whereas they were with the intron 5 CpGs in the hippocampus (Table 1). In particular, a strong correlation was observed with intron 1 CpG-2 in blood (R2 = 0.68, p = 7.1×10−10, Fig. 3a), which replicated our previous work (Lee et al., 2011). On the other hand, the most significant correlation was observed with intron 5 CpG-4 in the hippocampus (R2 = 0.33, p = 0.001, Fig. 3b). There were also significant correlations between mean plasma CORT levels and expression of Fkbp5, but this was more apparent in the hippocampus (R2 = 0.37, p = 3.5×10−5, Figure 3c) compared to blood (R2 = 0.14, p = 0.02, Figure 3d).
Table 1. Relationship between mean plasma CORT levels and DNAm of intronic CpGs.
Correlation between mean plasma CORT levels and DNA methylation in blood and hippocampus. For both tissues, correlation values between DNA methylation versus four-week mean CORT levels for the two intronic regions are shown. R2, or square of the correlation coefficient and P-values are included for each comparison. P-values have been adjusted by Bonferroni correction to reflect the number of CpGs tested, and a “1” was assigned to those comparisons that were p ≥ 1 following Bonferroni correction.
| Tissues | Fkbp5 Intron | CpG Position | Comparison to Mean Plasma CORT | |
|---|---|---|---|---|
| R2 | P-value | |||
| Blood | Intron 1 | CpG-1 | 0.52 | 2.2×10−6 |
| CpG-2 | 0.68 | 7.1×10−10 | ||
| CpG-3 | 0.17 | 0.12 | ||
| Intron 5 | CpG-1 | 0.03 | 1 | |
| CpG-2 | 0.30 | 0.003 | ||
| CpG-3 | 0.07 | 1 | ||
| CpG-4 | 0.08 | 1 | ||
| Hippocampus | Intron 1 | CpG-1 | 0.01 | 1 |
| CpG-2 | 0.04 | 1 | ||
| CpG-3 | 0.07 | 1 | ||
| Intron 5 | CpG-1 | 0.24 | 0.020 | |
| CpG-2 | 0.34 | 0.001 | ||
| CpG-3 | 0.30 | 0.003 | ||
| CpG-4 | 0.33 | 0.001 | ||
Figure 3.
Correlation between mean plasma CORT vs. DNA methylation and gene expression. For all of the forty animals in the five groups (0, 25, 50, 75, or 100 μg/ml of CORT), linear regression analysis was performed between the calculated mean plasma CORT levels versus: (a) DNA methylation of blood intron 1 CpG-2; (b) DNA methylation of hippocampal intron 5 CpG-4; (c) expression of hippocampal Fkbp5; and (d) expression of blood Fkbp5. R2, or square of the correlation coefficient and P-values are included for each comparison. For comparisons involving DNA methylation measurements, P-values have been adjusted by Bonferroni correction to reflect the number of CpGs tested.
3.4. Correlation between blood and brain DNA methylation
Finally, we examined the pairwise associations between DNAm values of all seven intronic CpGs in blood versus the hippocampus in this second cohort of mice (Suppl. Table). Taking the conventional approach of comparing the same CpGs across tissues, no significant correlations were observed (R2 ≤ 0.24 and p ≥ 0.064, Suppl. Table). In contrast, more significant correlations were observed comparing different CpGs across introns. Comparisons between the three intron 1 CpGs in blood and the four intron 5 CpGs in hippocampus are shown in Table 2. The most significant correlation was observed between intron 1 CpG-2 DNAm in blood and intron 5 CpG-4 DNAm in the hippocampus (R2 = 0.49 and p = 2.7×10−5, Fig. 4a). Interestingly, while expression of Fkbp5 in the hippocampus was significantly correlated with intron 5 CpG-4 DNAm in the hippocampus (R2 = 0.29 and p = 0.003, Fig. 4b), it was even more highly correlated with intron 1 CpG-2 DNAm (R2 = 0.43 and p = 3.5×10−5, Fig. 4c). Taken together, these data suggest that glucocorticoid-induced methylation changes observed in blood gDNA can serve as a proxy to both methylation and expression changes in the brain.
Table 2. Cross-tissue comparisons of DNAm between intron 1 CpGs in blood and intron 5 CpGs in brain.
Inter-intronic correlation between blood and hippocampus DNA methylation. R2 and P-values for intron 1 CpGs in blood vs. intron 5 CpGs in hippocampus are shown. P-values have been adjusted by Bonferroni correction to reflect the number of CpGs tested, which totaled 7 CpGs in blood × 7 CpGs in hippocampus, or 49 CpGs.
| Tissues | Blood | |||||||
|---|---|---|---|---|---|---|---|---|
| Fkbp5 Intronic CpGs | Intron 1 | |||||||
| CpG-1 | CpG-2 | CpG-3 | ||||||
| R2 | P-value | R2 | P-value | R2 | P-value | |||
| Hippocampus | Intron 5 | CpG-1 | 0.17 | 0.42 | 0.26 | 0.036 | 0.23 | 0.087 |
| CpG-2 | 0.29 | 0.015 | 0.31 | 0.009 | 0.22 | 0.127 | ||
| CpG-3 | 0.23 | 0.077 | 0.37 | 0.002 | 0.26 | 0.034 | ||
| CpG-4 | 0.33 | 0.005 | 0.49 | 2.7×10−5 | 0.30 | 0.011 | ||
Figure 4.
Correlation between DNA methylation of hippocampal intron 5 CpG-4 versus (a) DNA methylation of blood intron 1 CpG-2 and (b) expression of hippocampal Fkbp5. Relationship between DNA methylation of blood intron 1 CpG-2 versus expression in the hippocampus was also calculated (c). There was a relatively poor correlation between DNAm of intron 1 CpG-2 vs. expression in blood (R2 = 0.12, p = 0.026, graph not shown). All of the forty animals, eight from each of the five treatment groups, are included. R2, or square of the correlation coefficient and P-values are included for each comparison. For comparisons involving DNA methylation measurements, P-values have been adjusted by Bonferroni correction to reflect the number of CpGs tested.
4. Discussion
Many epigenetic studies that employ DNA from peripheral sources such as blood are predicated on the assumption that non-genetic alterations that have occurred in target CNS regions are mirrored in the periphery. This assumption is especially prevalent in epigenetic studies of neuropsychiatric disorders, where essential brain tissues are virtually inaccessible in patients, and investigators must rely on other sources of DNA, such as from blood draws (Mehta et al., 2013) or buccal swabs (Yang et al., 2013). However, there are only a few studies that have tested this assumption by capturing disease-relevant, dynamic changes in the brain and linking them to those occurring in peripheral tissues.
One such study examined the 17 promoter of the glucocorticoid receptor (GR) and reported that the sum of methylation values between whole blood cells and the prefrontal cortex of stressed and unstressed animals show moderate association on a Pearson correlation heat map (Witzmann et al., 2012). Another study has performed a methylomic profile of blood, cortex, and hippocampus and found relatively high degree of concordance of 62.3% in DNAm among the three tissues and similar concordance rates of methylation changes among tissues of mice that were treated with haloperidol (Aberg et al., 2013). Other interesting blood-correlation studies have identified numerous loci that have both similar and tissue-specific epigenetic patterns between blood and brain tissues of “control” individuals (Davies et al., 2012), and loci that maintain strong across-tissue concordance across age (Horvath et al., 2012). High across-tissue concordance rates provide support for using blood DNA as a proxy for that of brain tissues and the motivation to investigate specific genomic loci for underlying mechanisms in greater detail and under different conditions and treatments.
In this study, we sought to establish a correlative relationship between two tissues by measuring dose-dependent DNA methylation changes that occur following exposure to glucocorticoids. Our work on the Fkbp5 gene was initiated out of a search, both in the literature and by pyrosequencing, for candidate loci that could account for the expression changes observed in both blood and brain of CORT-treated mice. Intron 1 CpGs had been characterized as an important binding site for MECP2 (Nuber et al., 2005), was located adjacently to a potential GRE (Magee et al., 2006), and had shown substantial CORT-induced reduction of DNAm in blood (Lee et al., 2010). However, intron 1 CpGs were mostly unaffected in the hippocampus. Pyrosequencing of additional candidate regions revealed significant DNAm changes in a highly conserved GRE in intron 5, prompting us to ask whether any significant relationships could be derived between measurements made in blood and those made in hippocampus for glucocorticoid exposure.
Despite the presence of tissue-specific changes in DNAm, we were able to derive modest, yet significant relationships between blood DNAm versus hippocampal DNAm and expression. As DNAm changes in both tissues were significantly correlated to overall CORT-exposure for each animal, DNAm measurements made from a drop of blood could provide useful information about the impact of CORT exposure in the hippocampus, thereby supplanting requisite serial measurements to properly assess CORT exposure and direct measurements of DNAm in the hippocampus. Since DNAm changes in blood was also significantly correlated with expression changes of Fkbp5 in the hippocampus, it may also be possible to assess sensitivity and resistance of this tissue to glucocorticoids (Scammell et al., 2001). Similar studies with additional “HPA-axis” genes, such as Gr, Fkbp4, and Hsp90, are necessary to provide a more complete picture of glucocorticoid signaling in the brain.
Our findings also raise an intriguing question regarding the nature of the epigenetic architecture at the Fkbp5 locus. We observed very similar DNAm levels in the intronic regions of both blood and hippocampus. In both tissue types, two of the three CpGs of intron 1 are highly methylated at 80% DNAm, whereas intron 5 is relatively hypomethylated, with three of the four CpGs close to 20% DNAm. Why are the DNAm changes intron- and tissue-specific, despite the presence of similar methylation patterns at these two loci in both tissues? It is unclear to us at this time as to the different functional, regulatory modalities that may exist at these intronic regions between these tissues. It is possible that despite similar DNAm patterns, blood and hippocampus may harbor vastly dissimilar histone modification patterns. Histone modifications and chromatin remodeling have been investigated for intron 5 (Paakinaho et al., 2010), and it would be interesting to see whether similar or different histone marks exist in intron 1 for blood and brain tissues. Given this possibility, differential histone patterns, or differential chromatin conformation, may confer differential accessibility of the GR-complex to the intronic regions. Differential histone and GR occupancy may work in conjunction to affect gene expression during acute CORT exposure, and these may then interact with CORT/GR-mediated changes in DNAm to affect the timing and robustness of gene expression during chronic CORT exposure. A rigorous investigation of histone modifications and CORT-mediated occupancy of GR at these two introns is needed to adequately answer this question. While these additional mechanisms have the potential to confound current and future studies that compare across-tissue epigenetic marks, they also provide additional avenues to alter or mitigate the impact of excessive exposure to glucocorticoids.
Our findings highlight several other biological considerations. First, our ability to observe blood-brain correlation of CORT-exposure depended largely on the presence of functional glucocorticoid signaling in both of the tissues examined. It was due to this fact that we were able to obtain dose-dependent DNAm changes at GREs that significantly reflected CORT exposure in both tissue types, and made it possible to correlate those changes between the two tissues. Further, expression changes caused by elevated glucocorticoid levels in the brain provided another parameter that was significantly associated with DNA methylation changes in the blood, and this was also due to active GR-signaling. It is unknown at this time whether loci that are transcriptionally active in only one of the two tissues both undergo similar epigenetic changes.
Second, tissue heterogeneity represented a significant confounding factor in assessing epigenetic changes. This issue was especially relevant in the brain where presence of astrocytes, glia, oligodendrocytes, and microglia vastly outnumber neurons, and any significant epigenetic changes that occur in one population of cells is likely to be diluted by static epigenetic values of others. In the current study, targeting specific sub-regions such as the granule neuron-rich dentate gyrus allowed us to observe significantly greater differences in glucocorticoid-induced DNAm changes that were not observed in a previous study (Lee et al., 2010). Based on our data and work by others, we suggest that fluorescence-activated cell sorting (FACS) of neurons may be a more effective way to enrich for DNAm differences between treated and control groups (Iwamoto et al., 2011). However, since FACS does not discriminate among subregions of the hippocampus such as the CA1, CA3, and dentate gyrus, we opted to hole-punch dissect the dentate. While white blood cell populations are also likely to be affected by tissue heterogeneity, we speculate that the large effect size observed in one or more tissue types has allowed us to derive a strong correlation of blood DNAm to mean CORT levels. Immunophenotyping can accurately identify and isolate subpopulations of white blood cells that undergo glucocorticoid-induced epigenetic changes. Recent epigenetic studies have begun to employ isolation techniques such as retrograde bead labeling coupled with FACS (Niwa et al., 2013) and laser capture microdissection (Hackler et al., 2012) to maximize the observable epigenetic changes. Additionally, bioinformatics approaches have enabled the development and use of novel statistical methods to account for cellular heterogeneity in brain (Guintivano et al., 2013) and blood tissues (Houseman et al., 2012).
Third, contrary to our initial expectations, DNAm changes in the current study did not occur in CpG islands or promoters, despite the fact that mouse Fkbp5 gene possesses three CpG islands, one intronic and two promoter, that could have served as potential sites of glucocorticoid regulation and epigenetic alteration. In fact, emerging evidence in epigenomics demonstrates that much of tissue-specific, disease-relevant epigenetic changes occur within CpG island “shores,” or ~2.5kb regions that flank CpG islands (Irizarry et al., 2009). In our study, we found that glucocorticoid-induced DNAm changes occurred tens of thousands of kilobases away from the Fkbp5 promoter and CpG islands. These targets of epigenetic modification were bioinformatically and/or experimentally characterized intronic GREs that have been demonstrated to bind the methylation-dependent repressor MECP2 or the activated GR complex (Nuber et al., 2005, Magee et al., 2006, Paakinaho et al., 2010). These findings necessitate a careful consideration in the design and implementation of target probes used in genome-wide DNAm microarray or bisulfite sequencing platforms. For instance, additional regions such as DNase- hypersensitive sites that encompass binding of transcription regulators or cis-elements (Boyle et al., 2008), or regulatory regions as demonstrated by chromatin immunoprecipitation (ChIP) with specific transcription factors or histone modications followed by next-generation sequencing (ChIP-Seq) (Rye et al., 2011), may need to be included in platform design to identify regions of active epigenetic alterations.
Our current study has several limitations. First, although observed levels of CORT may be relevant to endogenous or iatrogenic Cushing’s disease, the exposure amount is likely excessive for modeling conditions of chronic stress exposure. Therefore, it is important to verify whether the findings from the present model are also identified in stress models. Second, it is not clear whether our findings can be translated to humans. While the intron 5 GRE is highly conserved in all of the rodent and primate species examined, the intron 1 GRE is limited to mice and rats (UCSC Genome Browser). Therefore, it is not clear whether patients exposed to high levels of glucocorticoids will exhibit blood DNAm changes in a homologous region in intron 1. For example, a recent study in human blood has reported lymphocyte-specific DNAm changes near the intron 2 GRE (Klengel et al., 2013), raising the possibility that cross-species GRE concordance may not be perfect. Lastly, it is possible that the observed tissue-specific epigenetic patterns may only apply to the Fkbp5 gene. Until similar across-tissue DNAm changes are identified in other glucocorticoid-responsive genes, it is imprudent to make any generalizations regarding our observations. Specifically, it is not clear whether other genes that are important for mood disorders and have been demonstrated to be expressed in lymphocytes, such as Crh, Gr, and Bdnf (Baker et al., 2003, Zuccato et al., 2011, Maranville et al., 2013), exhibit similar across-tissue, CpG-to-CpG correlations. A methylomic assay, such as next-generation sequencing of bisulfite-converted target DNA, is warranted to establish whether the observed tissue-specific DNAm of Fkbp5 is the norm or the exception, and to determine what other genes, pathways, and physiological processes are affected by exposure to glucocorticoids.
Despite these limitations, our study lends support to using surrogate tissue for the brain with the caveat that the particulars of epigenetics biology that determine across-tissue correlates be taken into consideration. A next step in this regard is to determine how much of the results from this preclinical model may apply to the human condition.
Supplementary Material
Acknowledgments
Role of funding source
This study was funded by NIH grants UO1 AA020890 (GSW) and HD055030 (KLT), the Kenneth A. Lattman Foundation (GSW), a NARSAD Young Investigator Award (RSL), Margaret Ann Price Investigator Fund (RSL), and the James Wah Mood Disorders Scholar Fund via the Charles T. Bauer Foundation (RSL).
We thank Tim Moran, Ph.D., who kindly provided edits and comments of the manuscript.
Footnotes
Conflict of interest for all authors
None
Financial disclosures for all authors
None
Contributors
RSL designed the study and wrote the manuscript. ERE performed most of the experiments (pyrosequencing and radioimmunoassays) and co-wrote the manuscript. GSW helped with interpretation of data. FS and PZ performed and oversaw the statistical analysis of results. XY performed the realtime PCR and some of the pyrosequencing. KLT provided her expertise in animal husbandry and sample collection. JBP provided valuable clinical insights and edited the manuscript. All authors contributed to and have approved the final manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aberg KA, Xie LY, McClay JL, Nerella S, Vunck S, Snider S, Beardsley PM, van den Oord EJ. Testing two models describing how methylome-wide studies in blood are informative for psychiatric conditions. Epigenomics. 2013;5:367–377. doi: 10.2217/epi.13.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker C, Richards LJ, Dayan CM, Jessop DS. Corticotropin-releasing hormone immunoreactivity in human T and B cells and macrophages: colocalization with arginine vasopressin. J Neuroendocrinol. 2003;15:1070–1074. doi: 10.1046/j.1365-2826.2003.01099.x. [DOI] [PubMed] [Google Scholar]
- Binder EB, Bradley RG, Liu W, Epstein MP, Deveau TC, Mercer KB, Tang Y, Gillespie CF, Heim CM, Nemeroff CB, Schwartz AC, Cubells JF, Ressler KJ. Association of FKBP5 polymorphisms and childhood abuse with risk of posttraumatic stress disorder symptoms in adults. JAMA. 2008;299:1291–1305. doi: 10.1001/jama.299.11.1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder EB, Salyakina D, Lichtner P, Wochnik GM, Ising M, Putz B, Papiol S, Seaman S, Lucae S, Kohli MA, Nickel T, Kunzel HE, Fuchs B, Majer M, Pfennig A, Kern N, Brunner J, Modell S, Baghai T, Deiml T, Zill P, Bondy B, Rupprecht R, Messer T, Kohnlein O, Dabitz H, Bruckl T, Muller N, Pfister H, Lieb R, Mueller JC, Lohmussaar E, Strom TM, Bettecken T, Meitinger T, Uhr M, Rein T, Holsboer F, Muller-Myhsok B. Polymorphisms in FKBP5 are associated with increased recurrence of depressive episodes and rapid response to antidepressant treatment. Nat Genet. 2004;36:1319–1325. doi: 10.1038/ng1479. [DOI] [PubMed] [Google Scholar]
- Boyle AP, Davis S, Shulha HP, Meltzer P, Margulies EH, Weng Z, Furey TS, Crawford GE. High-resolution mapping and characterization of open chromatin across the genome. Cell. 2008;132:311–322. doi: 10.1016/j.cell.2007.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen SI. Cushing’s syndrome: a psychiatric study of 29 patients. Br J Psychiatry. 1980;136:120–124. doi: 10.1192/bjp.136.2.120. [DOI] [PubMed] [Google Scholar]
- Colella S, Shen L, Baggerly KA, Issa JP, Krahe R. Sensitive and quantitative universal Pyrosequencing methylation analysis of CpG sites. Biotechniques. 2003;35:146–150. doi: 10.2144/03351md01. [DOI] [PubMed] [Google Scholar]
- Davies MN, Volta M, Pidsley R, Lunnon K, Dixit A, Lovestone S, Coarfa C, Harris RA, Milosavljevic A, Troakes C, Al-Sarraj S, Dobson R, Schalkwyk LC, Mill J. Functional annotation of the human brain methylome identifies tissue-specific epigenetic variation across brain and blood. Genome Biol. 2012;13:R43. doi: 10.1186/gb-2012-13-6-r43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorn LD, Burgess ES, Friedman TC, Dubbert B, Gold PW, Chrousos GP. The longitudinal course of psychopathology in Cushing’s syndrome after correction of hypercortisolism. J Clin Endocrinol Metab. 1997;82:912–919. doi: 10.1210/jcem.82.3.3834. [DOI] [PubMed] [Google Scholar]
- Fardet L, Petersen I, Nazareth I. Suicidal behavior and severe neuropsychiatric disorders following glucocorticoid therapy in primary care. Am J Psychiatry. 2012;169:491–497. doi: 10.1176/appi.ajp.2011.11071009. [DOI] [PubMed] [Google Scholar]
- Flitsch J, Spitzner S, Ludecke DK. Emotional disorders in patients with different types of pituitary adenomas and factors affecting the diagnostic process. Exp Clin Endocrinol Diabetes. 2000;108:480–485. doi: 10.1055/s-2000-8144. [DOI] [PubMed] [Google Scholar]
- Gillespie CF, Nemeroff CB. Hypercortisolemia and depression. Psychosom Med. 2005;67(Suppl 1):S26–28. doi: 10.1097/01.psy.0000163456.22154.d2. [DOI] [PubMed] [Google Scholar]
- Guintivano J, Aryee MJ, Kaminsky ZA. A cell epigenotype specific model for the correction of brain cellular heterogeneity bias and its application to age, brain region and major depression. Epigenetics. 2013;8:290–302. doi: 10.4161/epi.23924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackler L, Jr, Masuda T, Oliver VF, Merbs SL, Zack DJ. Use of laser capture microdissection for analysis of retinal mRNA/miRNA expression and DNA methylation. Methods Mol Biol. 2012;884:289–304. doi: 10.1007/978-1-61779-848-1_21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horvath S, Zhang Y, Langfelder P, Kahn RS, Boks MP, van Eijk K, van den Berg LH, Ophoff RA. Aging effects on DNA methylation modules in human brain and blood tissue. Genome Biol. 2012;13:R97. doi: 10.1186/gb-2012-13-10-r97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houseman EA, Accomando WP, Koestler DC, Christensen BC, Marsit CJ, Nelson HH, Wiencke JK, Kelsey KT. DNA methylation arrays as surrogate measures of cell mixture distribution. BMC Bioinformatics. 2012;13:86. doi: 10.1186/1471-2105-13-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irizarry RA, Ladd-Acosta C, Wen B, Wu Z, Montano C, Onyango P, Cui H, Gabo K, Rongione M, Webster M, Ji H, Potash JB, Sabunciyan S, Feinberg AP. The human colon cancer methylome shows similar hypo- and hypermethylation at conserved tissue-specific CpG island shores. Nat Genet. 2009;41:178–186. doi: 10.1038/ng.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwamoto K, Bundo M, Ueda J, Oldham MC, Ukai W, Hashimoto E, Saito T, Geschwind DH, Kato T. Neurons show distinctive DNA methylation profile and higher interindividual variations compared with non-neurons. Genome Res. 2011;21:688–696. doi: 10.1101/gr.112755.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaouane N, Porte Y, Vallee M, Brayda-Bruno L, Mons N, Calandreau L, Marighetto A, Piazza PV, Desmedt A. Glucocorticoids can induce PTSD-like memory impairments in mice. Science. 2012;335:1510–1513. doi: 10.1126/science.1207615. [DOI] [PubMed] [Google Scholar]
- Karlamangla AS, Singer BH, McEwen BS, Rowe JW, Seeman TE. Allostatic load as a predictor of functional decline. MacArthur studies of successful aging. J Clin Epidemiol. 2002;55:696–710. doi: 10.1016/s0895-4356(02)00399-2. [DOI] [PubMed] [Google Scholar]
- Klengel T, Mehta D, Anacker C, Rex-Haffner M, Pruessner JC, Pariante CM, Pace TW, Mercer KB, Mayberg HS, Bradley B, Nemeroff CB, Holsboer F, Heim CM, Ressler KJ, Rein T, Binder EB. Allele-specific FKBP5 DNA demethylation mediates gene-childhood trauma interactions. Nat Neurosci. 2013;16:33–41. doi: 10.1038/nn.3275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee RS, Tamashiro KL, Yang X, Purcell RH, Harvey A, Willour VL, Huo Y, Rongione M, Wand GS, Potash JB. Chronic corticosterone exposure increases expression and decreases deoxyribonucleic acid methylation of Fkbp5 in mice. Endocrinology. 2010;151:4332–4343. doi: 10.1210/en.2010-0225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee RS, Tamashiro KL, Yang X, Purcell RH, Huo Y, Rongione M, Potash JB, Wand GS. A measure of glucocorticoid load provided by DNA methylation of Fkbp5 in mice. Psychopharmacology (Berl) 2011;218:303–312. doi: 10.1007/s00213-011-2307-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magee JA, Chang LW, Stormo GD, Milbrandt J. Direct, androgen receptor-mediated regulation of the FKBP5 gene via a distal enhancer element. Endocrinology. 2006;147:590–598. doi: 10.1210/en.2005-1001. [DOI] [PubMed] [Google Scholar]
- Maranville JC, Baxter SS, Witonsky DB, Chase MA, Di Rienzo A. Genetic mapping with multiple levels of phenotypic information reveals determinants of lymphocyte glucocorticoid sensitivity. Am J Hum Genet. 2013;93:735–743. doi: 10.1016/j.ajhg.2013.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS, Gianaros PJ. Central role of the brain in stress and adaptation: links to socioeconomic status, health, and disease. Ann N Y Acad Sci. 2010;1186:190–222. doi: 10.1111/j.1749-6632.2009.05331.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta D, Klengel T, Conneely KN, Smith AK, Altmann A, Pace TW, Rex-Haffner M, Loeschner A, Gonik M, Mercer KB, Bradley B, Muller-Myhsok B, Ressler KJ, Binder EB. Childhood maltreatment is associated with distinct genomic and epigenetic profiles in posttraumatic stress disorder. Proc Natl Acad Sci USA. 2013;110:8302–8307. doi: 10.1073/pnas.1217750110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niwa M, Jaaro-Peled H, Tankou S, Seshadri S, Hikida T, Matsumoto Y, Cascella NG, Kano S, Ozaki N, Nabeshima T, Sawa A. Adolescent stress-induced epigenetic control of dopaminergic neurons via glucocorticoids. Science. 2013;339:335–339. doi: 10.1126/science.1226931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuber UA, Kriaucionis S, Roloff TC, Guy J, Selfridge J, Steinhoff C, Schulz R, Lipkowitz B, Ropers HH, Holmes MC, Bird A. Up-regulation of glucocorticoid-regulated genes in a mouse model of Rett syndrome. Hum Mol Genet. 2005;14:2247–2256. doi: 10.1093/hmg/ddi229. [DOI] [PubMed] [Google Scholar]
- Paakinaho V, Makkonen H, Jaaskelainen T, Palvimo JJ. Glucocorticoid receptor activates poised FKBP51 locus through long-distance interactions. Mol Endocrinol. 2010;24:511–525. doi: 10.1210/me.2009-0443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Development Core Team. R: A language and environment for statistical computing. Viena, Austria: R Foundation for Statistical Computing; 2010. URL: http://www.R-project.org. [Google Scholar]
- Roy A, Gorodetsky E, Yuan Q, Goldman D, Enoch MA. Interaction of FKBP5, a stress-related gene, with childhood trauma increases the risk for attempting suicide. Neuropsychopharmacology. 2010;35:1674–1683. doi: 10.1038/npp.2009.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy A, Hodgkinson CA, Deluca V, Goldman D, Enoch MA. Two HPA axis genes, CRHBP and FKBP5, interact with childhood trauma to increase the risk for suicidal behavior. J Psychiatr Res. 2012;46:72–79. doi: 10.1016/j.jpsychires.2011.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rye M, Saetrom P, Handstad T, Drablos F. Clustered ChIP-Seq-defined transcription factor binding sites and histone modifications map distinct classes of regulatory elements. BMC Biol. 2011;9:80. doi: 10.1186/1741-7007-9-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scammell JG, Denny WB, Valentine DL, Smith DF. Overexpression of the FK506-binding immunophilin FKBP51 is the common cause of glucocorticoid resistance in three New World primates. Gen Comp Endocrinol. 2001;124:152–165. doi: 10.1006/gcen.2001.7696. [DOI] [PubMed] [Google Scholar]
- Sinclair D, Fullerton JM, Webster MJ, Shannon Weickert C. Glucocorticoid receptor 1B and 1C mRNA transcript alterations in schizophrenia and bipolar disorder, and their possible regulation by GR gene variants. PLoS One. 2012;7:e31720. doi: 10.1371/journal.pone.0031720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starkman MN, Schteingart DE, Schork MA. Cushing’s syndrome after treatment: changes in cortisol and ACTH levels, and amelioration of the depressive syndrome. Psychiatry Res. 1986;19:177–188. doi: 10.1016/0165-1781(86)90096-x. [DOI] [PubMed] [Google Scholar]
- Theall KP, Drury SS, Shirtcliff EA. Cumulative neighborhood risk of psychosocial stress and allostatic load in adolescents. Am J Epidemiol. 2012;176(Suppl 7):S164–174. doi: 10.1093/aje/kws185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willour VL, Chen H, Toolan J, Belmonte P, Cutler DJ, Goes FS, Zandi PP, Lee RS, MacKinnon DF, Mondimore FM, Schweizer B, DePaulo JR, Jr, Gershon ES, McMahon FJ, Potash JB. Family-based association of FKBP5 in bipolar disorder. Mol Psychiatry. 2009;14:261–268. doi: 10.1038/sj.mp.4002141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witzmann SR, Turner JD, Meriaux SB, Meijer OC, Muller CP. Epigenetic regulation of the glucocorticoid receptor promoter 1(7) in adult rats. Epigenetics. 2012;7:1290–1301. doi: 10.4161/epi.22363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wochnik GM, Ruegg J, Abel GA, Schmidt U, Holsboer F, Rein T. FK506-binding proteins 51 and 52 differentially regulate dynein interaction and nuclear translocation of the glucocorticoid receptor in mammalian cells. J Biol Chem. 2005;280:4609–4616. doi: 10.1074/jbc.M407498200. [DOI] [PubMed] [Google Scholar]
- Yang BZ, Zhang H, Ge W, Weder N, Douglas-Palumberi H, Perepletchikova F, Gelernter J, Kaufman J. Child abuse and epigenetic mechanisms of disease risk. Am J Prev Med. 2013;44:101–107. doi: 10.1016/j.amepre.2012.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuccato C, Marullo M, Vitali B, Tarditi A, Mariotti C, Valenza M, Lahiri N, Wild EJ, Sassone J, Ciammola A, Bachoud-Levi AC, Tabrizi SJ, Di Donato S, Cattaneo E. Brain-derived neurotrophic factor in patients with Huntington’s disease. PLoS One. 2011;6:e22966. doi: 10.1371/journal.pone.0022966. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.