Abstract
Background: Fluorescence-guided surgery (FGS) can enable successful cancer surgery where bright-light surgery often cannot. There are three important issues for FGS going forward toward the clinic: (a) proper tumor labeling, (b) a simple portable imaging system for the operating room, and (c) patient-like mouse models in which to develop the technology. The present report addresses all three.
Materials and Methods: Patient colon tumors were initially established subcutaneously in nonobese diabetic (NOD)/severe combined immune deficiency (SCID) mice immediately after surgery. The tumors were then harvested from NOD/SCID mice and passed orthotopically in nude mice to make patient-derived orthotopic xenograft (PDOX) models. Eight weeks after orthotopic implantation, a monoclonal anti-carcinoembryonic antigen (CEA) antibody conjugated with AlexaFluor® 488 (Molecular Probes Inc., Eugene, OR) was delivered to the PDOX models as a single intravenous dose 24 hours before laparotomy. A hand-held portable fluorescence imaging device was used.
Results: The primary tumor was clearly visible at laparotomy with the portable fluorescence imaging system. Frozen section microscopy of the resected specimen demonstrated that the anti-CEA antibody selectively labeled cancer cells in the colon cancer PDOX. The tumor was completely resected under fluorescence navigation. Histologic evaluation of the resected specimen demonstrated that cancer cells were not present in the margins, indicating successful tumor resection. The FGS animals remained tumor free for over 6 months.
Conclusions: The results of the present report indicate that FGS using a fluorophore-conjugated anti-CEA antibody and portable imaging system improves efficacy of resection for CEA-positive colorectal cancer. These data provide the basis for clinical trials.
Introduction
The intent of cancer surgery is to remove malignant tissue together with margins of presumably normal tissue to ensure complete removal of abnormal cells.1–3 The surgeon is currently limited by the contrast between the tumor and surrounding tissues. The ability to make tumors glow offers great potential advantages for tumor detection during fluorescence-guided surgery (FGS).2
In a previous study, the green fluorescent protein (GFP)–containing OBP-401 adenovirus,4,5 which contains the replication cassette with the human telomerase reverse transcriptase promoter driving the expression of the viral E1 gene, was used to label an intraperitoneal model of disseminated cancer for FGS.6
In another study, we have shown that all mice with orthotopic primary colon tumors expressing GFP, that had undergone FGS, had complete resection compared with 58% of mice in the bright-light surgery (BLS) group. FGS resulted in decreased recurrence compared with BLS (33% versus 62%) and lengthened disease-free median survival from 9 to >36 weeks. FGS resulted in a cure in 67% of mice (alive without evidence of tumor at >6 months after surgery) compared with only 37% of mice that underwent BLS.7
A more complete resection of orthotopic pancreatic cancer in nude mice was achieved using FGS compared with BLS (98.9% versus 77.1%). The majority of mice undergoing BLS (63.2%) had evidence of gross disease. In contrast, 20% of mice undergoing FGS had complete resection, and an additional 75% had only minimal residual disease (P=0.0001). FGS resulted in significantly longer disease-free survival than BLS (P=0.02; hazard ratio=0.39; 95% confidence interval 0.17, 0.88).8
5-Aminolevulinic acid (ALA) accumulates as fluorescent protoporphyrin IX in malignant glioma tissue. Compared with normal cortex, mean protoporphyrin IX fluorescence in vital tumor was found to be increased >100-fold. FGS resulted in complete resection in 65% of patients in the ALA group compared with 36% in the white-light group (P<0.0001). Progression-free survival was superior in the FGS group compared with BLS patients, with cumulative 6-month progression-free survival rates of 41% and 21%, respectively.9
Patient-derived orthotopic xenograft (PDOX) models developed in mice preserve peritumoral stroma and recapitulate the biological characteristics of the disease of origin.10–12 Making the PDOX model “glow” with fluorescence presents an ideal model to develop FGS.13–15
We have previously reported that a blue light-emitting diode flashlight (LDP LLC, Woodcliff Lake, NJ) (www.maxmax.com/OpticalProducts.htm) with an excitation filter (midpoint wavelength peak of 470 nm) and an emission D470/40 filter (Chroma Technology, Brattleboro, VT) for viewing could be used for noninvasive whole-body imaging of mice with GFP- and red fluorescent protein–expressing tumors growing in or on internal organs.16
There are three important issues going forward toward the clinic for FGS: (a) proper tumor labeling, (b) a simple portable imaging system for the operating room, and (c) patient-like mouse models in which to develop the technology. In the present study, we demonstrate the effectiveness of a fluorophore-conjugated anti-carcinoembryonic antigen (CEA) antibody for FGS of a colon cancer PDOX nude mouse model using a hand-held, portable, highly sensitive imaging system. The results of the present report suggest the technology described here can enable FGS of clinical cancer in the near future.
Materials and Methods
Animals
Nonobese diabetic (NOD)/severe combined immune deficiency (SCID) and athymic (nu/nu) nude mice (AntiCancer, Inc., San Diego, CA), 4–6 weeks of age, were used in this study. Mice were kept in a barrier facility under high-efficiency particulate absorption filtration. Mice were fed with autoclaved laboratory rodent diet. All surgical procedures and imaging were performed with the animals anesthetized by intramuscular injection of 0.02 mL of a solution of 50% ketamine, 38% xylazine, and 12% acepromazine maleate. All animal studies were conducted in accordance with the principles and procedures outlined in the National Institutes of Health Guide for the Care and Use of Laboratory Animals under Public Health Service Assurance Number A3873-1.
Specimen collection
All patients provided informed consent, samples were obtained, and the study was conducted under the approval of the Institutional Review Board of the University of California San Diego Medical Center.
Establishment of PDOX of colon cancer patient tumors
Colon cancer patient tumor tissue was obtained at surgery, cut into 3-mm3 fragments, and transplanted subcutaneously in NOD/SCID mice.17,18 The patient tumors were then harvested from NOD/SCID mice and passed orthotopically in nude mice.12,19–22 A single 3-mm3 tumor fragment was sutured to the mesenteric border of the cecal wall using 8-0 nylon surgical sutures (Ethilon™; Ethicon Inc., Somerville, NJ). On completion, the cecum was returned to the abdomen, and the incision was closed in one layer using 6-0 nylon surgical sutures (Ethilon).12,19–22
Antibody conjugation
Monoclonal antibody specific for CEA was purchased from RayBiotech, Inc. (Norcross, GA). The antibody was labeled with the AlexaFluor® 488 protein labeling kit (Molecular Probes Inc., Eugene, OR) according to the manufacturers instructions and as previously described.23
FGS
Eight weeks after orthotopic implantation of the colon cancer PDOX, a monoclonal anti-CEA antibody conjugated with AlexaFluor® 488 was delivered to tumor-bearing mice as a single intravenous dose. After 24 hours, mice were anesthetized as described above, and their abdomens were sterilized. The cecum was delivered through a midline incision, and the exposed colon tumor was imaged preoperatively with the Olympus OV100 small animal imaging system (Olympus Corp., Tokyo, Japan) under both standard bright-field and fluorescence illumination.
Portable imaging system for FGS
Resection of the primary colon tumor was performed using the portable imaging system equipped with a Dino-Lite digital camera (AM4113T-GFBW Dino-Lite Premier; AnMo Electronics Corp., Hsinchu, Taiwan). Postoperatively, the surgical resection bed was imaged with the Olympus OV100 small animal imaging system24 under both standard bright-field and fluorescence illumination to assess the completeness of surgical resection. The cecal stump was sutured closed in a running fashion with 8-0 nylon surgical sutures.
Tissue histology
Tumor samples were removed with surrounding normal tissues at the time of resection. Fresh tissue samples were fixed in 10% formalin and embedded in paraffin before sectioning and staining. Tissue sections (3 μm thick) were deparaffinized in xylene and rehydrated in an ethanol series. For immunohistochemistry, the sections were then treated for 30 minutes with 0.3% hydrogen peroxide to block endogenous peroxidase activity. The sections were subsequently washed with-phosphate-buffered saline and unmasked in citrate antigen unmasking solution (Mitsubishi Kagaku Iatron, Inc., Tokyo) in a water bath for 40 minutes at 98°C. After incubation with 10% normal goat serum, the sections were incubated with anti-CEA (1:100; RayBiotech, Inc.) at 4°C overnight. The binding of primary antibodies was detected using anti-mouse secondary antibodies and an avidin/biotin/horseradish peroxidase complex (DAKO Cytomation, Kyoto, Japan) for 30 minutes at room temperature. The labeled antigens were visualized with the DAB kit (DAKO Cytomation). The sections were counterstained with hematoxylin and examined using a BH-2 microscope (Olympus) equipped with an INFINITY1 2.0-megapixel CMOS digital camera (Lumenera Corp., Ottawa, ON, Canada). All images were acquired using INFINITY ANALYZE software (Lumenera Corp.) without postacquisition processing.
Frozen sections and 4′,6-diamidino-2-phenylindole staining
The resected specimens were embedded with optimal cutting temperature compound (Tissue-Tek®; Sakura Finetek Europe BV, Zoeterwude, The Netherlands) and preserved in liquid nitrogen. Frozen sections 7–10 μm thick were prepared with a CM1850 cryostat (Leica, Buffalo Grove, IL). The frozen sections were washed with phosphate-buffered saline three times and then incubated with 4′,6-diamidino-2-phenylindole staining solution (diluted 1:48,000; Invitrogen, Life Technologies, Carlsbad, CA) at room temperature for 3 minutes in darkness. Some frozen sections were processed for hematoxylin and eosin staining. Slides were mounted with Fluoromount™ (Sigma, St. Louis, MO). 4′,6-Diamidino-2-phenylindole-stained sections were observed with confocal microscopy (Fluoview FV1000; Olympus). Excitation sources were semiconductor lasers at 405 nm for 4′,6-diamidino-2-phenylindole and 473 nm for AlexaFluor® 488. Fluorescence images were obtained using the 20×/1.0 XLUMPLFLN objective.25
Results
Tumor histology and peritumoral stroma were well preserved in colon-cancer PDOX
Our laboratory pioneered surgical orthotopic implantation mouse models of PDOX in the early 1990s.10–12,26–28 The PDOX models are more patient-like than ectopic subcutaneous models, which do not metastasize.29,30 The colon-cancer PDOX showed tumor gland formation and the presence of peritumoral stroma, thereby recapitulating the original tumor (Fig. 1). The tumor glands were strongly stained by anti-CEA antibody (Fig. 1B).
FIG. 1.
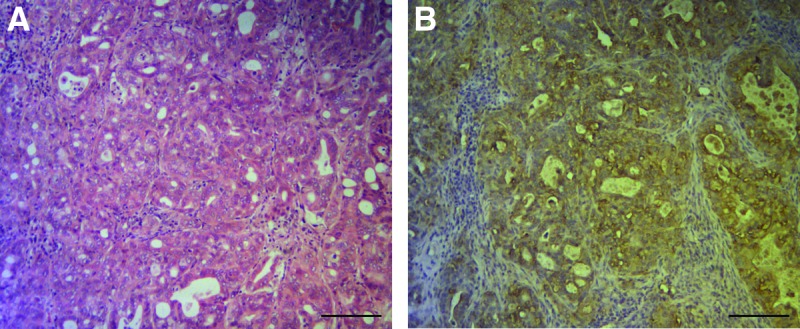
Representative images of the colon cancer patient-derived orthotopic xenograft (PDOX) sections. (A) Hematoxylin and eosin staining of a patient-derived orthotopic xenograft section. The tumor was diagnosed as moderately differentiated adenocarcinoma. (B) Immunohistochemistry for human carcinoembryonic antigen on a sister slide of A. High-intensity brown staining was observed in the cancer cells. Scale bars=250 μm.
Fluorophore-conjugated anti-CEA antibody selectively labeled cancer cells in the colon cancer PDOX
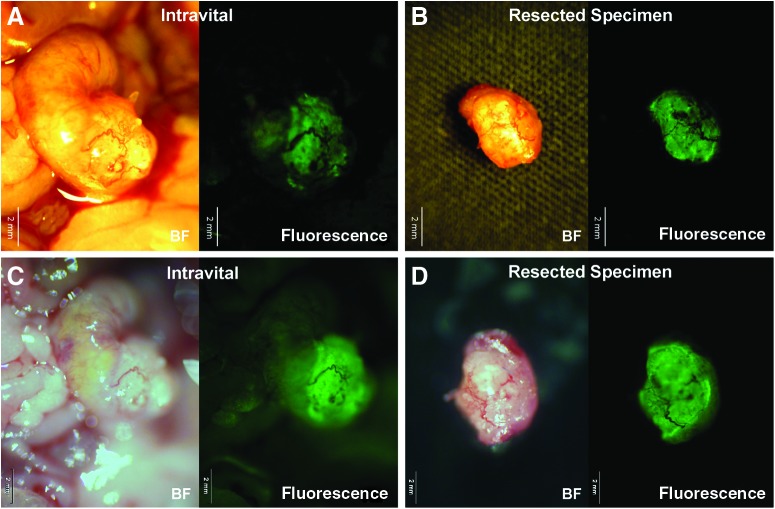
Eight weeks after orthotopic implantation of colon cancer PDOX, a monoclonal anti-CEA antibody conjugated with AlexaFluor® 488 was delivered to tumor-bearing mice as a single intravenous dose. After 24 hours, FGS of the primary colon tumor was performed using the portable imaging system (Fig. 2). Using fluorescence imaging, the primary tumor was clearly visible at laparotomy (Fig. 3A). The tumor margin was sharply visualized because the portable imaging system had very little autofluorescence signal (Fig. 3).
FIG. 2.
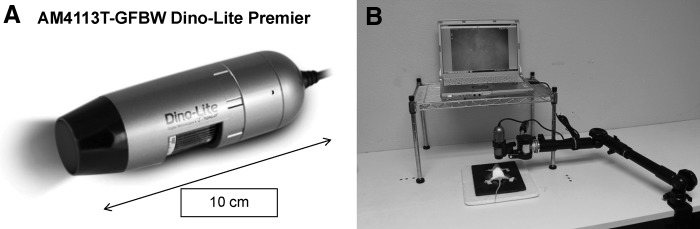
Fluorescence-guided surgery of the primary colon tumor was performed using the portable imaging system with (A) the Dino-Lite digital camera. Eight weeks after orthotopic implantation of a colon cancer patient-derived orthotopic xenograft (PDOX), a monoclonal anti-carcinoembryonic antigen antibody conjugated with AlexaFluor® 488 was delivered to tumor-bearing mice as a single intravenous dose. After 24 hours, fluorescence-guided surgery of the primary colon tumor was performed using the portable imaging system (B).
FIG. 3.
The colon cancer patient-derived orthotopic xenograft (PDOX) was imaged with the portable imaging system (A and B) or the Olympus OV100 small animal imaging system (C and D). During fluorescence-guided surgery, the tumor was clearly visible (A). The portable imaging system had less autofluorescence signal compared with the OV100 system. Scale bars=2 mm. BF, bright-field.
In a previous study, the fluorophore-conjugated anti-CEA antibody was detectable by 30 minutes after injection and reached its peak at 24 hours after systemic antibody delivery. Tumor fluorescence persisted for at least 2 weeks, with minimal in vivo photobleaching after exposure to standard operating room lighting.23 In the present study, frozen-section microscopy of the resected specimen demonstrated that anti-CEA antibody administered as a single intravenous dose 24 hours before laparotomy selectively labeled cancer cells in the colon cancer PDOX (Fig. 4), and tumor fluorescence was present for at least 1 week.
FIG. 4.

Frozen section microscopy of the resected specimen. (A) Hematoxylin and eosin staining of the resected specimen. (B) 4′,6-Diamidino-2-phenylindole staining of the resected specimen. Arrowheads indicate the border of the tumor. Tumor cells were clearly stained by anti-carcinoembryonic antigen antibody administered by intravenous injection 24 hours before laparotomy. Blue, 4′,6-diamidino-2-phenylindole; green, anti-carcinoembryonic antigen antibody conjugated with AlexaFluor® 488. The sections were observed with confocal microscopy (Fluoview FV1000; Olympus). Scale bar=50 μm.
The tumor was completely resected under fluorescence navigation
After FGS, the surgical resection bed was imaged with the Olympus OV100 small animal imaging system under both standard bright-field and fluorescence illumination to assess the completeness of surgical resection (Fig. 5). A complete absence of tumor was detected in the postsurgical bed after FGS (Fig. 5B). Histological evaluation of the margin of the resected specimen demonstrated that cancer cells were not present in the margins (Fig. 6B).
FIG. 5.
Tumor-bearing mice were imaged (A) before and (B) after resection. Lower panels are high magnification (×0.56) images of the upper panels. A complete absence of tumor was detected in the postsurgical bed. The images were taken with the Olympus OV100 Small Animal Imaging System under both standard bright-field (BF) and fluorescence illumination. Scale bars=10 mm in upper panels and 2 mm in lower panels.
FIG. 6.

Histological analysis of the margin of the resected specimen. (A) The box indicates the margin. (B) Hematoxylin and eosin staining of the margin. Cancer cells were not detected in the margin. Scale bars=2 mm in (A) and 250 μm in (B).
We have shown previously that FGS decreased recurrence and lengthened both overall and disease-free survival compared with BLS in orthotopic mouse models of human colon cancer cells expressing a fluorescent protein.7 In the present study, we performed FGS on five nude mouse colon cancer PDOX models. All mice that had undergone FGS had complete resection, and they were all alive without evidence of tumor for over 6 months after surgery.
Discussion
Various methods of fluorescently labeling tumors have been reported.2,6,31–35 Urano et al.34 used fluorescence-guided laparoscopy to visualize and remove tumors illuminated with γ-glu-hydroxymethyl rhodamine green that effectively and selectively labeled invasive human ovarian cancer expressing the probe-activating γ-glutamyltranspeptidase enzyme. Tumors were labeled by topically spraying the tumor-specific probe. However, this method is limited to those tumors that overexpress γ-glutamyltranspeptidase. Van Dam et al.35 conjugated folate to fluorescein isothiocyanate for targeting folate receptor-α, which is often overexpressed in ovarian cancers. The use of folate receptor-α for targeted fluorescence labeling also may be limited to a few tumor types such as ovarian cancer overexpressing folate receptor-α. ALA has been successfully used for glioma FGS.33
The potential for improved detection of a mucosal-based tumor on the inside of the colon versus one that was implanted on the outside surface of the colon needs to be investigated. We have previously developed a mouse model in which human colorectal cancer invades the mucosa of the colon.36 This model will allow future experiments that will optimize labeling of the mucosal surface of the tumor with a fluorophore-conjugated antibody directed against CEA for FGS as well as for fluorescence colonoscopy for improved tumor resection and detection.
In the present report, a monoclonal antibody directed against CEA was conjugated to a green fluorophore and then delivered intravenously into nude mice with a colon cancer PDOX. The use of fluorescent tumor-specific antibodies may have wide applicability, but antibodies require intravenous injection, and labeling takes hours to days.23,31 Furthermore, this method is also limited to only those cancers for which tumor-specific antigens have been characterized. Hence, further investigation is needed to enable all types of tumors to glow selectively for FGS.
Acknowledgments
This study was supported in part by National Cancer Institute grants CA132971 and CA142669 (to M.B. and AntiCancer, Inc.), T32 training grant CA121938 (to C.A.M.), and Grants-in-Aid from the Japanese Ministry of Education, Culture, Sports, Science and Technology for Fundamental Research(C) (number 23592018 to I.E. and number 24592009 to K.T.).
This paper is dedicated to the memory of A.R. Moossa, MD.
Disclosure Statement
Y.Z. is an employee of AntiCancer, Inc. Y.H., F.U., S.M., S.Y., and R.M.H. are associates of AntiCancer, Inc. A.M., C.A.M., S.S., T.M., M.M., T.C., K.T., M.B., and I.E. declare no competing interests exist.
References
- 1.Andreoni B, Chiappa A, Bertani E, et al. Surgical outcomes for colon and rectal cancer over a decade: Results from a consecutive monocentric experience in 902 unselected patients. World J Surg Oncol 2007;5:73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bouvet M, Hoffman RM. Glowing tumors make for better detection and resection. Sci Transl Med 2011;3(110):110fs110. [DOI] [PubMed] [Google Scholar]
- 3.Ruo L, Guillem JG. Surgical management of primary colorectal cancer. Surg Oncol 1998;7:153–163 [DOI] [PubMed] [Google Scholar]
- 4.Fujiwara T, Kagawa S, Kishimoto H, et al. Enhanced antitumor efficacy of telomerase-selective oncolytic adenoviral agent OBP-401 with docetaxel: Preclinical evaluation of chemovirotherapy. Int J Cancer 2006;119:432–440 [DOI] [PubMed] [Google Scholar]
- 5.Kishimoto H, Kojima T, Watanabe Y, et al. In vivo imaging of lymph node metastasis with telomerase-specific replication-selective adenovirus. Nat Med 2006;12:1213–1219 [DOI] [PubMed] [Google Scholar]
- 6.Kishimoto H, Zhao M, Hayashi K, et al. In vivo internal tumor illumination by telomerase-dependent adenoviral GFP for precise surgical navigation. Proc Natl Acad Sci U S A 2009;106:14514–14517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Metildi CA, Kaushal S, Snyder CS, Hoffman RM, Bouvet M. Fluorescence-guided surgery of human colon cancer increases complete resection resulting in cures in an orthotopic nude mouse model. J Surg Res 2013;179:87–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Metildi CA, Kaushal S, Hardamon CR, et al. Fluorescence-guided surgery allows for more complete resection of pancreatic cancer, resulting in longer disease-free survival compared with standard surgery in orthotopic mouse models. J Am Coll Surg 2012;215:126–135; discussion 135–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stepp H, Beck T, Pongratz T, et al. ALA and malignant glioma: Fluorescence-guided resection and photodynamic treatment. J Environ Pathol Toxicol Oncol 2007;26:157–164 [DOI] [PubMed] [Google Scholar]
- 10.Fu X, Guadagni F, Hoffman RM. A metastatic nude-mouse model of human pancreatic cancer constructed orthotopically with histologically intact patient specimens. Proc Natl Acad Sci U S A 1992;89:5645–5649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fu X, Hoffman RM. Human ovarian carcinoma metastatic models constructed in nude mice by orthotopic transplantation of histologically-intact patient specimens. Anticancer Res 1993;13:283–286 [PubMed] [Google Scholar]
- 12.Fu XY, Besterman JM, Monosov A, Hoffman RM. Models of human metastatic colon cancer in nude mice orthotopically constructed by using histologically intact patient specimens. Proc Natl Acad Sci U S A 1991;88:9345–9349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Suetsugu A, Hassanein MK, Reynoso J, et al. The cyan fluorescent protein nude mouse as a host for multicolor-coded imaging models of primary and metastatic tumor microenvironments. Anticancer Res 2012;32:31–38 [PubMed] [Google Scholar]
- 14.Suetsugu A, Katz M, Fleming J, et al. Multi-color palette of fluorescent proteins for imaging the tumor microenvironment of orthotopic tumorgraft mouse models of clinical pancreatic cancer specimens. J Cell Biochem 2012;113:2290–2295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Suetsugu A, Katz M, Fleming J, et al. Non-invasive fluorescent-protein imaging of orthotopic pancreatic-cancer-patient tumorgraft progression in nude mice. Anticancer Res 2012;32:3063–3067 [PubMed] [Google Scholar]
- 16.Yang M, Luiken G, Baranov E, Hoffman RM. Facile whole-body imaging of internal fluorescent tumors in mice with an LED flashlight. Biotechniques 2005;39:170–172 [DOI] [PubMed] [Google Scholar]
- 17.Kim MP, Evans DB, Wang H, Abbruzzese JL, Fleming JB, Gallick GE. Generation of orthotopic and heterotopic human pancreatic cancer xenografts in immunodeficient mice. Nat Protoc 2009;4:1670–1680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim MP, Truty MJ, Choi W, et al. Molecular profiling of direct xenograft tumors established from human pancreatic adenocarcinoma after neoadjuvant therapy. Ann Surg Oncol 2012;19(Suppl 3):S395–403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.An Z, Wang X, Willmott N, et al. Conversion of highly malignant colon cancer from an aggressive to a controlled disease by oral administration of a metalloproteinase inhibitor. Clin Exp Metastasis 1997;15:184–195 [DOI] [PubMed] [Google Scholar]
- 20.Furukawa T, Kubota T, Watanabe M, et al. Immunochemotherapy prevents human colon cancer metastasis after orthotopic onplantation of histologically-intact tumor tissue in nude mice. Anticancer Res 1993;13:287–291 [PubMed] [Google Scholar]
- 21.Hoffman RM. Orthotopic metastatic mouse models for anticancer drug discovery and evaluation: A bridge to the clinic. Invest New Drugs 1999;17:343–359 [DOI] [PubMed] [Google Scholar]
- 22.Wang X, Fu X, Brown PD, Crimmin MJ, Hoffman RM. Matrix metalloproteinase inhibitor BB-94 (batimastat) inhibits human colon tumor growth and spread in a patient-like orthotopic model in nude mice. Cancer Res 1994;54:4726–4728 [PubMed] [Google Scholar]
- 23.Kaushal S, McElroy MK, Luiken GA, et al. Fluorophore-conjugated anti-CEA antibody for the intraoperative imaging of pancreatic and colorectal cancer. J Gastrointest Surg 2008;12:1938–1950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yamauchi K, Yang M, Jiang P, et al. Development of real-time subcellular dynamic multicolor imaging of cancer cell-trafficking in live mice with a variable-magnification whole-mouse imaging system. Cancer Res 2006;66:4208–4214 [DOI] [PubMed] [Google Scholar]
- 25.Uchugonova A, Zhao M, Weinigel M, et al. Multiphoton tomography visualizes collagen fibers in the tumor microenvironment that maintain cancer-cell anchorage and shape. J Cell Biochem 2013;114:99–102 [DOI] [PubMed] [Google Scholar]
- 26.Fu X, Hoffman RM. Human ovarian carcinoma metastatic models constructed in nude mice by orthotopic transplantation of histologically-intact patient specimens. Anticancer Res 1993;13:283–286 [PubMed] [Google Scholar]
- 27.Fu X, Le P, Hoffman RM. A metastatic-orthotopic transplant nude-mouse model of human patient breast cancer. Anticancer Res 1993;13:901–904 [PubMed] [Google Scholar]
- 28.Furukawa T, Kubota T, Watanabe M, et al. Orthotopic transplantation of histologically intact clinical specimens of stomach cancer to nude mice: Correlation of metastatic sites in mouse and individual patient donors. Int J Cancer 1993;53:608–612 [DOI] [PubMed] [Google Scholar]
- 29.Bertotti A, Migliardi G, Galimi F, et al. A molecularly annotated platform of patient-derived xenografts (“xenopatients”) identifies HER2 as an effective therapeutic target in cetuximab-resistant colorectal cancer. Cancer Discov 2011;1:508–523 [DOI] [PubMed] [Google Scholar]
- 30.Talmadge JE, Singh RK, Fidler IJ, Raz A. Murine models to evaluate novel and conventional therapeutic strategies for cancer. Am J Pathol 2007;170:793–804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McElroy M, Kaushal S, Luiken GA, et al. Imaging of primary and metastatic pancreatic cancer using a fluorophore-conjugated anti-CA19-9 antibody for surgical navigation. World J Surg 2008;32:1057–1066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nguyen QT, Olson ES, Aguilera TA, et al. Surgery with molecular fluorescence imaging using activatable cell-penetrating peptides decreases residual cancer and improves survival. Proc Natl Acad Sci U S A 2010;107:4317–4322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stummer W, Pichlmeier U, Meinel T, Wiestler OD, Zanella F, Reulen HJ. Fluorescence-guided surgery with 5-aminolevulinic acid for resection of malignant glioma: A randomised controlled multicentre phase III trial. Lancet Oncol 2006;7:392–401 [DOI] [PubMed] [Google Scholar]
- 34.Urano Y, Sakabe M, Kosaka N, et al. Rapid cancer detection by topically spraying a gamma-glutamyltranspeptidase-activated fluorescent probe. Sci Transl Med 2011;3:110–119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.van Dam GM, Themelis G, Crane LM, et al. Intraoperative tumor-specific fluorescence imaging in ovarian cancer by folate receptor-alpha targeting: First in-human results. Nat Med 2011;17:1315–1319 [DOI] [PubMed] [Google Scholar]
- 36.Sakuma S, Yano T, Masaoka Y, et al. Detection of early colorectal cancer imaged with peanut agglutinin-immobilized fluorescent nanospheres having surface poly(N-vinylacetamide) chains. Eur J Pharm Biopharm 2010;74:451–460 [DOI] [PubMed] [Google Scholar]