Abstract
Herpes simplex virus type 1 (HSV-1) mutants lacking the γ134.5 neurovirulence loci are promising agents for treating malignant glioma. Arming oncolytic HSV-1 to express immunostimulatory genes may potentiate therapeutic efficacy. We have previously demonstrated improved preclinical efficacy, biodistribution, and safety of M002, a γ134.5-deleted HSV-1 engineered to express murine IL-12. Herein, we describe the safety and biodistribution of M032, a γ134.5-deleted HSV-1 virus that expresses human IL-12 after intracerebral administration to nonhuman primates, Aotus nancymae. Cohorts were administered vehicle, 106, or 108 pfu of M032 on day 1 and subjected to detailed clinical observations performed serially over a 92-day trial. Animals were sacrificed on days 3, 31, and 91 for detailed histopathologic assessments of all organs and to isolate and quantify virus in all organs. With the possible exception of one animal euthanized on day 16, neither adverse clinical signs nor sex- or dose-related differences were attributed to M032. Elevated white blood cell and neutrophil counts were observed in virus-injected groups on day 3, but no other significant changes were noted in clinical chemistry or coagulation parameters. Minimal to mild inflammation and fibrosis detected, primarily in meningeal tissues, in M032-injected animals on days 3 and 31 had mostly resolved by day 91. The highest viral DNA levels were detected at the injection site and motor cortex on day 3 but decreased in central nervous system tissues over time. These data demonstrate the requisite safety of intracerebral M032 administration for consideration as a therapeutic for treating malignant brain tumors.
Introduction
Malignant gliomas remain the most therapeutically challenging primary brain tumors. Among these, glioblastoma multiforme (GBM) is the most common and the most lethal. Despite combined treatment approaches with surgical resection, chemotherapy, and radiotherapy, the 5-year survival rate for patients with GBM is less than 10% and the median survival is ∼15 months (Stupp et al., 2009; Preusser et al., 2011). Thus, more effective therapies for these malignancies are needed, and oncolytic viruses have been devised as a therapeutic strategy to address this need (Shah et al., 2003; Parker et al., 2009; Cassady et al., 2010). These strategies utilize intrinsic or engineered viral properties to impart tumor cell-selective infection and/or replication (Parker et al., 2009).
Oncolytic viruses have been developed to treat a broad spectrum of malignancies, but those based on herpes simplex virus type 1 (HSV) are particularly suited for malignant brain tumors. This is primarily because HSV-1 has a natural tropism for neural tissues, but can be rendered safe by deletion of the diploid γ134.5 neurovirulence gene, which abrogates replication in nonmalignant cells (Chou et al., 1990). The γ134.5 gene encodes the neurovirulence factor, ICP34.5 (Chou et al., 1990). In addition to its multiple roles in supporting infection, ICP34.5 also acts to suppress host antiviral responses (He et al., 1997, 1998). Since some of the host antiviral responses inhibited by ICP34.5 are often defective in malignant cells (Chou et al., 1990; Farassati et al., 2001; Smith et al., 2006), they are permissive to γ134.5-deleted oHSV replication (Markert et al., 1993; Chambers et al., 1995). Additional safety can be achieved by inactivating mutations in other viral genes, such as UL2 (Pyles et al., 1997), UL23 (Martuza et al., 1991), and UL39 (Mineta et al., 1995; Kramm et al., 1997) genes.
A number of oncolytic HSV-1 (oHSV) have been evaluated and shown efficacious in murine brain tumor models. Among these, two have been evaluated in clinical trials for patients with primary brain tumor malignancies. The first, G207, lacks both copies of γ134.5 and is further attenuated by lacZ insertion in place of the gene encoding ribonucleotide reductase (UL39) (Markert et al., 2000, 2009). Intratumoral injection of up to 3×109 pfu of G207 was well tolerated in patients with recurrent malignant glioma. No dose-limiting toxicities were observed in the trial (Markert et al., 2009). The second oHSV evaluated, HSV1716, lacks both copies of γ134.5. HSV1716 was administered to patients with malignant glioma at doses up to 1×105 pfu, either by intratumoral injection or into the resected tumor bed, and no clinical evidence of toxicity attributed to the virus was reported (Rampling et al., 2000; Papanastassiou et al., 2002; Harrow et al., 2004).
Attenuating mutations are necessary to ensure safety of oHSV. However, an additional observation of the γ134.5-deleted virus is attenuated replication in malignant cells, as compared with that of parental viruses. Presumed clinical benefit was observed in some G207- and HSV1716-treated patients. Except for a single patient in the initial G207 trial who died from an unrelated stroke, all patients ultimately succumbed to recurrent disease. These results, as well as the aggressive nature of the disease, suggest that the therapeutic potency of attenuated oHSV requires enhancement.
We and others have evaluated oHSV armed with additional gene-based therapeutics to enhance tumor suppression. A range of transgene inserts have been evaluated, including suicide genes (Chase et al., 1998; Aghi et al., 1999; Nakamura et al., 2001; Guffey et al., 2007), or those expressing antiangiogenic (Liu et al., 2006; Goodwin et al., 2012; Yoo et al., 2012), apoptotic (Han et al., 2007; Prabhakar et al., 2010), fusogenic (Nakamori et al., 2004; Simpson et al., 2009; Takaoka et al., 2011), and immunomodulatory proteins (Andreansky et al., 1998; Liu et al., 2003; Parker et al., 2005; Walker et al., 2011). Our group has focused on oHSV that express immunostimulatory cytokines (Andreansky et al., 1998; Parker et al., 2000; Hellums et al., 2005). This strategy combines oHSV-based oncolysis with cytokine-mediated induction of antitumor immunity. In particular, we have evaluated the therapeutic potential of oHSV that express IL-12, a heterodimeric cytokine composed of the IL-12A (p35) and IL-12B (p40) gene products. IL-12 suppresses angiogenesis (Voest et al., 1995; Albini et al., 2009) and mediates antitumor responses by enhancing the effector function of both innate and adaptive immune cells (Trinchieri, 2003). In addition to stimulating stable production of IFN-γ and cytotoxic mediators, IL-12 also acts, directly or synergistically, to enhance T helper type 1 and cytotoxic T lymphocyte responses (Manetti et al., 1994; Koch et al., 1996; Wigginton et al., 2002; Murphy et al., 2003). The IFN-γ upregulates class I and class II MHC molecules and, through induction of CXCL10, also acts to enhance NK and T cell recruitment (Koch et al., 1996; Sgadari et al., 1996; Suzuki et al., 1998). We have previously described a γ134.5-deleted oHSV, designated M002 that expresses the p35 and p40 subunits of murine IL-12 from a bicistronic transcript under control of the murine early growth response-1 (Egr-1) promoter. Compared with the IL-12-deficient parental virus, R3659, M002 administration enhanced survival and tumor growth inhibition in syngeneic murine models of glioblastoma (Parker et al., 2000) and glioma (Hellums et al., 2005). Further, IL-12 activity was demonstrated by increased tumor infiltration of CD4+ and CD8+ T cells (Hellums et al., 2005; Parker et al., 2005). Recently, we reported on the preclinical evaluation of M002 (Markert et al., 2012). Compared with the parental oHSV (R3659) and another attenuated oHSV approved for clinical trial (G207), intracranial administration of M002 resulted in enhanced survival of mice bearing intracranial brain tumors. This study also demonstrated a lack of significant neurologic or systemic toxicity after intracerebral administration of 1.2×108–4.2×108 pfu of M002 into the New World nonhuman primate (NHP) model, A. nancymae.
Despite the preclinical efficacy and safety demonstrated for M002, the potential for an undesirable immune response against murine IL-12 protein existed should this virus be studied in human clinical trials. Therefore, we constructed M032, a γ134.5-deleted oHSV that has an identical configuration to that of M002, but expresses the human IL-12 p35 and p40 subunits. The current study was carried out to determine whether M032 is safe for clinical evaluation in patients with malignant glioma. To this end, NHP were evaluated after intracerebral injection of saline, 1×106 pfu, or 1×108 pfu of M032 per NHP. This report summarizes preclinical data obtained after M032 administration. In the aggregate, no adverse clinical signs were directly attributed to M032 administration, which supports its consideration for entry into phase I evaluation.
Results and Discussion
Clinical trial
M032 is a derivative of HSV-1 strain F. For a detailed description of its derivation see Supplementary Fig. S1 and Materials and Methods (Supplementary Data are available online at www.liebertpub.com/humc). A clinical-grade preparation of M032 (NSC733972) was generated by the NCI RAID (5M01RR000032-420636) core at SAIC Frederick for the IND-directed safety and biodistribution study reported herein, and for future clinical evaluation. Clinical evaluation of M032 will entail a standard phase I dose escalation trial. Patients with recurrent malignant glioma who have failed standard treatment with biopsy or resection and then fractionated radiation and temozolomide therapy will be candidates for the trial. Patient will undergo stereotactic placement of 1–4 catheters, depending on tumor size, location, and surgeon choice, in the operating room after stereotactic biopsy confirms recurrent glioma. On postbiopsy day 1, after confirmation of catheter placement, patients will undergo infusion of virus at an initial total dose of 105 pfu. Patients will be monitored for side effects, including encephalitis and cytokine storm, as well as potential tumor response. Three patients will be enrolled per cohort. If no side effects are reported, dose will be escalated to the next level. If one patient suffers a severe adverse event (SAE) felt to be related to M032, the cohort will be expanded to six at the level. If no further SAEs are noted, dose escalation will occur as above. If another SAE is recorded, the trial will be stopped and the next lower dose level reported as the maximum tolerated dose.
Objectives and study design
The objective of the current study was to evaluate the safety and biodistribution of M032 after intracerebral administration into the Aotus nancymae NHP model. Aotus were selected as an appropriate large animal model for this study because this genus is both permissive to HSV-1 replication (Katzin et al., 1967; Barahona et al., 1976; Meignier et al., 1990; Stewart, 2003) and responsive to hIL-12 (HSV-M032 Toxicology Report, from SAIC-Frederick, Inc., Frederick, MD, 2008). Only 100 pfu of wild-type HSV-1 is required to induce systemic infection and acute hemorrhagic encephalitis (Meignier et al., 1990). Thus, a productive HSV-1 infection in the Aotus is routinely fatal and this makes it an excellent NHP model for evaluating the safety of HSV-based therapeutics. Fifteen male and 15 female 2–7-year-old NHPs were used in the study. Groups were assigned using a computer-generated randomization procedure, with equal numbers of males and females assigned to each group. NHPs were injected on day 1 with one intracerebral dose of vehicle (0.9% saline, cohort 1, n=6), 1×106 pfu (cohort 2, n=12), or 1×108 pfu (cohort 3, n=12) of M032 per NHP (Table 1). Based on average body weights, these doses are equivalent to ∼5×107 and 5×109 pfu in humans, respectively. Thus, the high-dose NHP group utilized a dose-equivalent ∼150 times higher than the highest dose planned for potential dose escalation in the clinical trial.
Table 1.
Nonhuman Primate Groups, Time Points, and Analyses
| Group | Animal no. | M032 dose (pfu/NHP) | Gender | Initial weight (g) | Sacrifice weight (g) | Day of sacrifice |
|---|---|---|---|---|---|---|
| 1 | 930 | Saline | F | 850.6 | 860.5 | 3 |
| 1 | 909 | Saline | M | 812.1 | 762.2 | 3 |
| 1 | 928 | Saline | F | 887.4 | 1049 | 31 |
| 1 | 915 | Saline | M | 952.5 | 942.1 | 31 |
| 1 | 929 | Saline | F | 1091.9 | 1087.4 | 91 |
| 1 | 917 | Saline | M | 1138.5 | 1131.3 | 91 |
| 2 | 921 | 1×106 | F | 846.2 | 801.8 | 3 |
| 2 | 934 | 1×106 | F | 917.7 | 890 | 3 |
| 2 | 912 | 1×106 | M | 1051 | 1024.5 | 3 |
| 2 | 937 | 1×106 | M | 1151 | 1076.5 | 3 |
| 2 | 919 | 1×106 | F | 855.6 | 789.5 | 31 |
| 2 | 923 | 1×106 | F | 1152.4 | 1045.9 | 31 |
| 2 | 910 | 1×106 | M | 1011.4 | 950.8 | 31 |
| 2 | 916 | 1×106 | M | 879.4 | 876.5 | 31 |
| 2 | 922 | 1×106 | F | 826 | 885.7 | 91 |
| 2 | 924 | 1×106 | F | 913.1 | 952.9 | 91 |
| 2 | 906 | 1×106 | M | 1269.2 | 1165.3 | 91 |
| 2 | 914 | 1×106 | M | 1064.3 | 1152.2 | 91 |
| 3 | 920 | 1×108 | F | 888.2 | 821 | 3 |
| 3 | 927 | 1×108 | F | 983.4 | 946.1 | 3 |
| 3 | 903 | 1×108 | M | 795.5 | 761.6 | 3 |
| 3 | 905 | 1×108 | M | 836.1 | 841.7 | 3 |
| 3 | 926 | 1×108 | F | 1047 | 942.3 | 31 |
| 3 | 931 | 1×108 | F | 786.1 | 734.4 | 31 |
| 3 | 911 | 1×108 | M | 1104.5 | 1082.3 | 31 |
| 3 | 918 | 1×108 | M | 1023.2 | 949.3 | 31 |
| 3 | 925 | 1×108 | F | 1002 | 1050.1 | 91 |
| 3 | 933 | 1×108 | F | 849.8 | 913.7 | 91 |
| 3 | 904 | 1×108 | M | 1003.8 | 982.7 | 91 |
| 3 | 907a | 1×108 | M | 945.1 | 774.8 | 16a |
| Observation | Days measured or samples collected |
|---|---|
| Clinical evaluation | 1, 3, 8, 14, 16,a 22, 31, 36, 43, 50, 57, 64, 71, 78, 85, 91 |
| Body weights | −7, 1, 3, 8, 14, 16,a 22, 31, 36, 43, 50, 57, 64, 71, 78, 85, 91 |
| Hematology | −7, 3, 10, 14, 16,a 31, 91 |
| Clinical chemistry/coagulation | −7, 3, 10, 14, 16,a 31, 91 |
| qPCR | 3, 16,a 31, 91 (at sacrifice) |
| Pathology | 3, 16,a 31, 91 (at sacrifice) |
Three groups, consisting of both male and female NHPs, were intracerebrally injected with 0, 1×106, or 1×108 pfu of M032/NHP on day 1. Clinical observations, body weights, and samples for hematology and clinical chemistry were recorded before intracerebral administration and throughout the study at the designated time points. Male and female cohorts within each group were sacrificed on days 3, 31, and 91, and samples for macroscopic and microscopic observations and qPCR-based (group 3 only) assessment of viral biodistribution were obtained at sacrifice.
NHP #907 was found moribund on day 16 and euthanized early.
NHP, nonhuman primate; qPCR, quantitative polymerase chain reaction.
All monkeys were observed twice daily throughout the study for signs of morbidity and mortality and for adverse clinical signs. Cohorts of male and female NHPs within each group were scheduled for euthanasia on days 3, 31, and 91. Clinical observations and body weights, as well as blood and cerebrospinal fluid (CSF) sample collections, were carried out to establish baseline laboratory values before intracerebral injection on day 1. These studies were subsequently repeated at specified time points after injection. Blood samples were utilized for analyses of hematologic and biochemical parameters. Quantitative polymerase chain reaction (PCR)-based detection of the UL27 gene was used as a measurement of HSV-1 DNA content. In addition to blood and CSF, tissue samples were also collected for qPCR and pathology. Tissues collected from the central nervous system (CNS) included the spinal cord (cervical), CSF, the injection site, and the pons/medulla and motor cortex regions of the brain (injection side). Lymphatic tissues collected included the bronchial, mandibular, and mesenteric lymph nodes, as well as the spleen and right tonsil. Peripheral tissue samples were obtained from colon, heart, ileum, and right kidney (see Materials and Methods for detailed assay descriptions).
Summary of data
Body weights
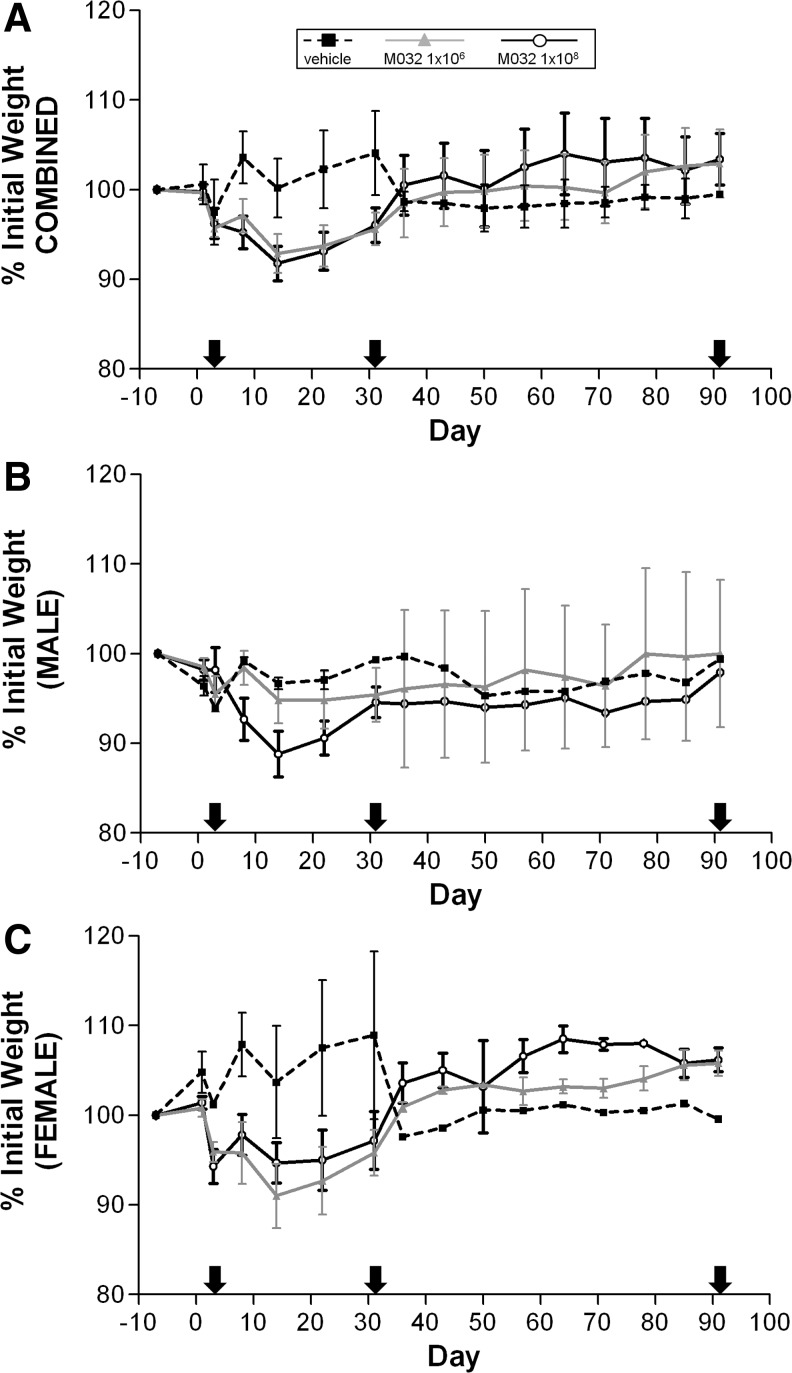
Animals were weighed 7 days before injection, on day 1 (just before injection), day 3 (only animals scheduled for euthanasia), and on days 8, 14, 22, 31, 36, 43, 50, 57, 64, 71, 78, 85, and 91. Values obtained 7 days before administration were used as a baseline to calculate the percent change in body weight over time, which were averaged for all NHPs in each dose group (Fig. 1A). Slight body weight loss (≤5% for most animals) was observed between day 1 and 3 for all NHPs euthanized on day 3. Since these losses were observed in all groups, they were attributed to sedation, dosing, and other procedures on day 1 that altered the animals feeding schedules and activity. Beyond day 3, only minor week-to-week fluctuations in individual body weights were observed for the saline-treated NHPs, but overall, their body weights remained essentially constant or increased slightly between day 1 and the day of euthanasia. Among M032-administered animals euthanized after day 3, minimal (≥5% to <10%) to moderate (≥10%) body weight loss was observed between day 1 and day 22 or 31 for two or more male and female NHPs in the 1×106 or 1×108 pfu/NHP dose groups. However, the incidence or extent of body weight loss did not appear to be related to the dose of M032 used. Included in this number was the male NHP (#907) euthanized because of its moribund condition on day 16. This animal displayed progressive body weight loss between day 1 and day 14, with a total body weight loss during this period of 14%. Between days 31 and 91 the body weight of individual NHPs in each dose group slowly increased over time. Slightly different trends were observed when the body weight data for male (Fig. 1B) and female (Fig. 1C) NHPs were plotted separately. In this regard, saline-treated females exhibited the largest weight gains during the initial 30 days, whereas the weight changes for saline-treated males did not significantly change over the same time period. Although M032 treatment correlated with decreased weights in both male and female NHP over the first 30 days, a dose correlation was only suggestive for male NHP treatment groups; weights of female NHP treated with 1×106 M032 trended lower than that of female NHP treated with 1×108 M032 over the same time period.
FIG. 1.
Combined and sex-specific NHP weight changes after intracerebral injection of M032 and saline. NHPs were weighed 1 week before and just before intracerebral injection of saline or M032 on day 1. The percent change in body weights from preinjection weights was averaged for all NHPs within each group and plotted (A), or plotted separately as male (B) and female (C) NHP groups, over the course of the 91-day study. Arrows indicate days in which cohorts within each group were sacrificed for additional endpoints. Error bars represent mean±SEM. For combined weights: preinjection to day 3; vehicle (n=6), M032 (n=12/dose). Days 4–31; vehicle (n=4), 1E6 M032 (n=8), 1E8 M032 (n=8 or 7; 1 animal euthanized moribund on day 16). Days 32–91; vehicle (n=2), 1E6 M032 (n=4), 1E8 M032 (n=3). NHP, nonhuman primate.
Hematology
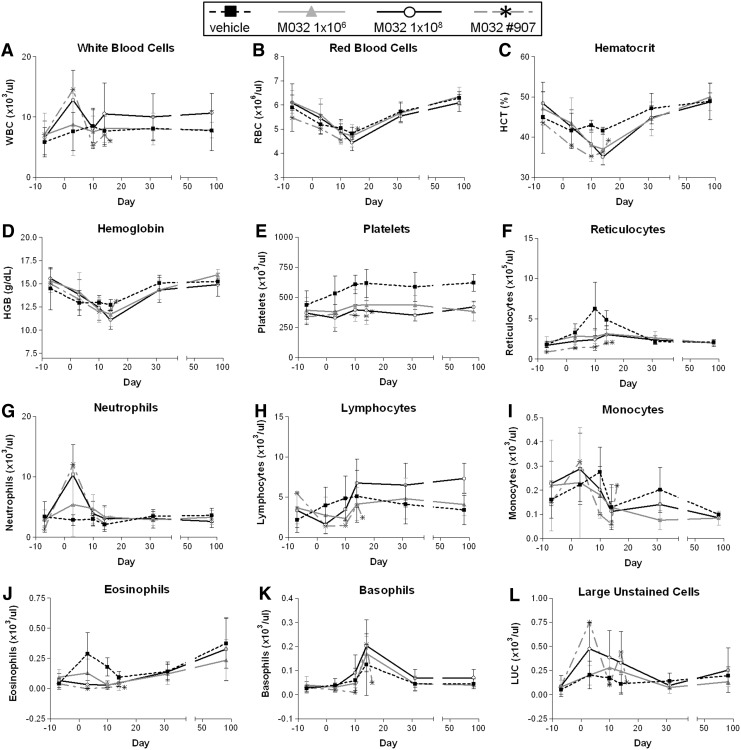
Blood samples for hematologic analyses were collected from each NHP one week before injection on day 1, for baseline values, and on days 3, 10, 14, 31, and 91. Samples were measured for total white blood cells (WBC) (Fig. 2A), red blood cells (Fig. 2B), hematocrit (Fig. 2C), hemoglobin (Fig. 2D), platelets (Fig. 2E), reticulocytes (Fig. 2F), neutrophils (Fig. 2G), lymphocytes (Fig. 2H), monocytes (Fig. 2I), eosinophils (Fig. 2J), basophils (Fig. 2K), and large unstained cells (virus-activated lymphocytes or myeloperoxidase-negative cells; Fig. 2L). Hematologic data were collected on an ADVIA 120 Hematology Analyzer at Southern Research Institute. Values obtained for animals within dose group were averaged at each time point. Values for the male NHP (#907) in the 1×108 pfu/NHP dose group that was euthanized on day 16 are plotted separately for visual comparison to mean values. Noted changes in hematology were based on the percent change from baseline values and on the absolute counts (as noted with each cell type assessed, below).
FIG. 2.
Hematology. Blood samples for hematology were collected from each NHP during the week before intracerebral injection of saline or M032. After injection on day 1, samples were collected on days 3, 10, 14, 31, and 91. Among the measurements carried out (A) white blood cells, (B) red blood cells, (C) hematocrit, (D) hemoglobin, (E) platelets, (F) reticulocytes, (G) neutrophils, (H) lymphocytes, (I) monocytes, (J) eosinophils, (K) basophils, and (L) large unstained (myeloperoxidase negative) cell counts are presented. Blood samples obtained from one male NHP before moribund euthanasia on day 16 are depicted as “X” in each plot. Error bars represent mean±SD. Preadministration to day 3; vehicle (n=6), M032 (n=12/dose). Days 4–31; vehicle (n=4), 1E6 M032 (n=8), 1E8 M032 (n=7 or 8; 1 animal euthanized moribund on day 16). Days 32–91; vehicle (n=2), 1E6 M032 (n=4), 1E8 M032 (n=3).
Compared with baseline data, increased WBC counts (≥100% higher than baseline values of 11.0×103/mm3; range of 102–370% higher) (Fig. 2A), neutrophil counts (≥100% higher than baseline values of 7.0×103/mm3; range of 169–794% higher) (Fig. 2G), and/or decreased lymphocyte counts (≥40% lower than baseline values of 1.5×103/mm3; range of 40–85% lower) (Fig. 2H) were observed for NHPs administered 1×106 pfu/NHP or 1×108 pfu M032/NHP. WBC and neutrophil counts and/or low lymphocyte counts were observed most frequently on day 3. Male NHPs in the 1×106 pfu M032/NHP dose group were observed to have the fewest changes among M032-injected cohorts, with only 1 of 6 male NHPs (#910) with low (0.9×103/mm2) lymphocyte counts. An M032 dose relationship was not observed for female NHPs. High neutrophil counts were observed on both day 10 and 14 in one female (#923) in the 1×106 pfu/NHP (7.0 and 9.4×103/mm2 on day 10 and 14, respectively) and one female (#931) in the 1×108 pfu/NHP (7.0 and 7.8×103/mm2 on day 10 and 14, respectively) dose groups, compared with baseline values for the same NHPs (2.5–4.5×103/mm2), or values from saline-injected controls at the same time points (1.4–3.0×103/mm2). However, the average WBC values observed in all M032-injected animals on day 31 (9±3×103/mm2) and day 91 (9±3×103/mm2) were generally comparable to baseline values (6±3×103/mm2). While inflammation observed in histopathologic studies (described below) may have contributed to the early WBC increases observed in the periphery of M032-injected NHPs, the pathologist assessment (based on simultaneous low lymphocyte counts and sporadic low eosinophil counts) was that the peripheral blood WBC changes were more consistent with an immune response to M032 or a stress response to the surgical procedures, rather than a direct toxic effect of M032. Changes in WBC counts were observed only in NHPs dosed with M032 and not in NHPs given the vehicle control. Since there was no evidence of viral replication, these differences are likely reflective of virus- and/or IL-12-induced tissue inflammation in the brain. However, the use of vehicle as a control for procedure-related responses precluded our ability to determine the extent to which either virus activity or IL-12 expression was involved in the observed immune responses.
Clinical chemistries and coagulation studies
Blood samples were collected during week −1 (for baseline values) and on days 3, 10, 14, 31, and 91 for biochemical analyses. Blood for coagulation studies was obtained during week −1 and on the day the animals were scheduled for euthanasia. Blood samples for NHP #907 were also obtained before moribund euthanasia on day 16.
Clinical chemistry values were averaged for all NHPs within each group (Supplementary Fig. S2). As for the hematologic values, the clinical chemistry data for NHP #907 euthanized on day 16 are plotted separately for comparison to the mean values of all other animals in each group. No clear M032-related clinical chemistry changes were observed for NHPs administered M032 at either 1×106 or 1×108 pfu/NHP. NHP #907 had increased AST (2.7-fold higher than baseline; Supplementary Fig. S2F), increased cholesterol (115% higher than baseline; Supplementary Fig. S2H), and increased triglyceride (108% higher than baseline; Supplementary Fig. S2I) values on the day of euthanasia. However, these changes were consistent with those observed in the control NHPs on the same days (days 3, 20, 24, 31, and/or 91). Compared with baseline values, increased AST and ALT values observed for NHPs (predominantly on day 3) in both the vehicle- and M032-injected groups were attributed to tissue damage from the operative and virus injection procedures on day 1 and/or trauma associated with blood collection.
Relative to baseline values decreased BUN (Supplementary Fig. S2M), creatinine (Supplementary Fig. S2N), K (Supplementary Fig. S2K), and ALP (Supplementary Fig. S2D) values observed for NHPs in the vehicle control and/or M032-injected groups were attributed to variability in body weight and associated differences in food consumption. Decreased total protein (Supplementary Fig. S2Q) and/or albumin (Supplementary Fig. S2O) in vehicle control- and/or M032-injected groups were attributed to protein loss occurring from blood collection. Increased glucose (Supplementary Fig. S2R), cholesterol (Supplementary Fig. S2H), and triglycerides (Supplementary Fig. S2I) and variability in sodium (Supplementary Fig. S2L) and chloride (Supplementary Fig. S2J) values observed for NHPs in the vehicle control- and/or M032-injected groups were attributed to variability in fasting periods before blood collection (overnight fasting for baseline time point; variable fasting for other time points).
Significant increases in cholesterol levels were observed in both M032 treatment groups on days 10 (p<0.01) and 14(p<0.05), compared with saline-treated NHPs. However, there was no significant difference in the levels between the two M032-treated groups. These increases likely reflect variability in fasting periods before blood collection, or acute phase responses to infection, which are associated with alterations in the metabolism of lipids and lipoproteins, including cholesterol (Khovidhunkit et al., 2004; Feingold et al., 2010). Of note, elevated cholesterol levels were previously demonstrated in A. nancymae after intraprostatic injection of an oHSV (Varghese et al., 2001). Other variability observed in clinical chemistry values was too sporadic in occurrence to be clearly M032 related.
No clear changes in coagulation values were observed for NHPs administered M032; however, procedure-related increases in fibrinogen values (≥50% higher than baseline values; range of 51–150% higher) were noted for all 5 male NHPs and 4/5 female NHPs assessed on day 3 (data not shown).
Macroscopic evaluation
Although one male NHP (#907) in the high-dose M032 group was euthanized ahead of schedule, because of morbidity on day 16, all other NHPs in vehicle control and both M032 cohorts were euthanized according to schedule on days 3, 31, and 91. A complete gross necropsy examination was performed on each animal, and a summary of the macroscopic observations for each dose group is listed in Supplementary Table S1. Hemorrhage of the brain meninges was noted in 1 female in the 1×106 pfu/NHP dose group and 1 male and 1 female in the 1×108 pfu/NHP dose groups, but only among animals euthanized on day 3. The carcass and thymus of the moribund animal euthanized on day 16 were noted as “thin,” likely because of generalized meningitis, although this was characterized as mild by histologic criteria. On day 31, the skin of male or female NHPs injected with M032 at 1×106 or 1×108 pfu/NHP was also observed to be gelatinous at the surgical site, but these lesions resolved in the other NHPs by day 91. A number of other macroscopic findings, including discoloration of the kidney, lung, and small intestine, or enlarged heart, spleen, or testis, were sporadically noted among animals on various days of the study, but their incidence and severity did not follow a dose-related pattern and were therefore not considered to be related to M032 administration.
Microscopic evaluations
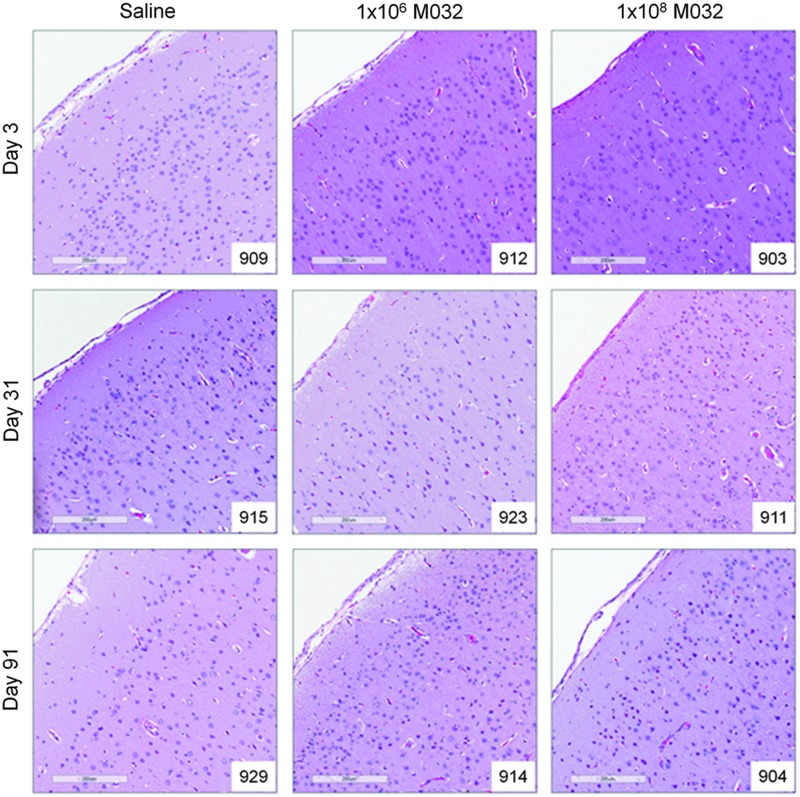
Inflammatory changes associated with the intracerebral administration of M032 (summarized in Supplementary Table S2) were observed in the motor cortex, pons, and medulla, as well as the skin at the surgical site. Microscopic findings in the brain, mainly restricted to minimum-mild levels of inflammation, were greatest on days 3 and 31 in the 1×106 and 1×108 pfu/NHP dose groups, with minimal inflammation observed on day 91. Parasagittal sections of the cerebrum from representative animals in each cohort are depicted in Fig. 3. The inflammation observed consisted of minimum to mild chronic or mixed acute and chronic inflammation of the meninges, chronic (lymphoplasmacytic) inflammation and gliosis of the neuropil, and fibrosis of the meninges. Inflammation in the meninges and neuropil was also observed on the side of the brain contralateral to the injection site, suggesting possible diffusion of M032 and/or IL-12 across the brain after injection.
FIG. 3.
Parasagittal sections of the cerebral cortex of representative animals in each cohort (10× magnification, scale bar=200 μm).
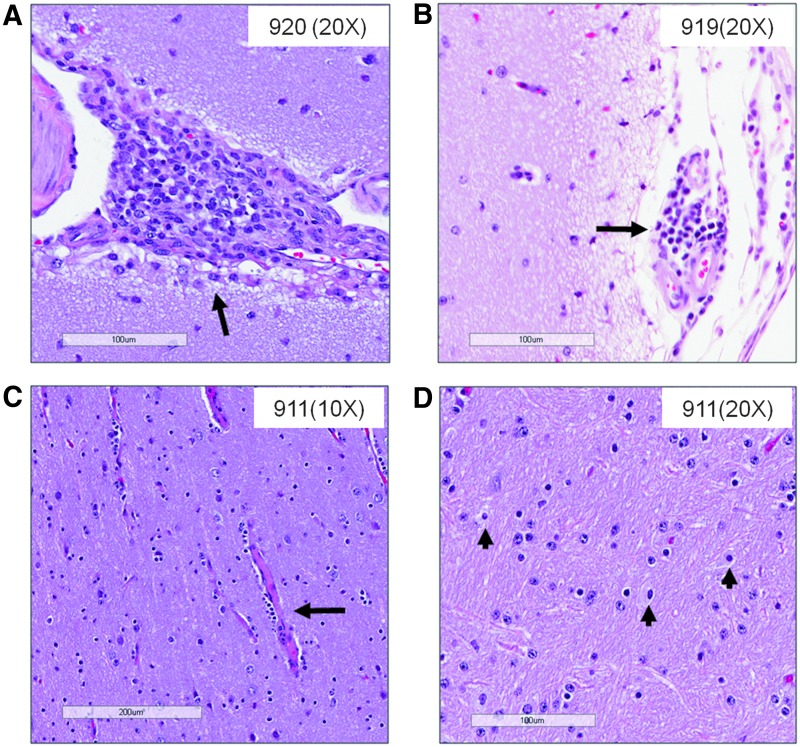
Mild to moderate perivascular inflammation was observed in the motor cortex of a single NHP (#920) treated with 1×108 pfu/NHP M032 on day 3 (Fig. 4A), and in both treatment groups on day 31 (#919 and #911; Fig. 4B and C). Gliosis was also observed in the motor cortex of two female NHPs (#919 and #923) in the 1×106 pfu/NHP M032 dose group and 1 male NHP (#911) in the 1×108 pfu/NHP M032 dose group, but only on day 31 (Fig. 4D). Whereas fibrosis was observed in the meninges of the medulla and pons of approximately half of the M032-injected animals, fibrosis in the motor cortex meninges was observed in both M032- and saline-injected animals. The limited inflammation and fibrosis noted in the brains on day 91 suggests that they resolve with time. In a previous study, perivascular inflammation and gliosis were reported along the needle track after administration of G207 to Aotus (Hunter et al., 1999), but not to the same degree as observed in our study, which is consistent with an inflammatory response mediated by IL-12 expression.
FIG. 4.
Inflammatory changes in M032-treated animals. (A–C) Mild to moderate perivascular inflammation observed in parasagittal cerebrum sections of some NHP treated with (A) 1×108 M032 on day 3, (B) 1×106 M032 on day 31, and (C) 1×108 M032 on day 31. Minimum to mild gliosis was observed on day 31 in some M032-treated NHP. (D) Minimum gliosis of an NHP treated with 1×108 M032 on day 31. Scale bars: 20×, 100 μm (A, B, D); 10×, 200 μm (C).
Inflammation, fibrosis, epidermal hyperplasia, hyperkeratosis, mineralization, and ulceration were also evident in the surgical sites of male and female NHPs in both M032-dose groups, but were only observed for animals sacrificed on day 31. Overall, the microscopic observations between male and female NHPs in each group were similar, but a dose-related response to M032 administration was not detected.
In the NHP euthanized early on day 16 (#907), generalized chronic meningitis was observed in the analyzed sections of the brain (motor cortex, medulla oblongata, and pons) and spinal cord (cervical, thoracic, and lumbar). Gliosis and chronic inflammation of the neuropil of the motor cortex was also observed in NHP #907. Whereas the pathologist's general assessment of this animal reported widespread, but low-level inflammatory changes in the CNS, the inflammation within individual brain sections was described as mild, multifocal inflammation, consistent with meningoencephalitis (Supplementary Fig. S3A–F). No microglial nodules, neuronophagia, or viral inclusions were observed, as would be expected in acute herpes simplex encephalitis in humans (Kennedy et al., 1988) and has been reported in Aotus (Hunter et al., 1999; Todo et al., 2000). Compared with animals at day 3, the reduced viral genome levels and similar biodistribution patterns also suggest a lack of active M032 replication, which, if present, would have been lethal to all of the M032-treated animals. There was also no evidence of an acute neutrophil response, as occurs in a bacterial CNS infection. Instead, the inflammatory infiltrate was chronic in nature, consistent with the histopathology findings of the animals scheduled for sacrifice on day 3 and 31, and consisted primarily of lymphocytes and plasma cells. Thus, the pathology in this NHP may have been M032-related, associated with a secondary infection acquired during surgery, or a manifestation of another concurrent illness. In this regard, A. nancymae are susceptible to a wide variety of illnesses in captivity and it is not uncommon to have to euthanize control NHPs when undertaking such studies (Gozalo et al., 1990; Lowenstine, 2003).
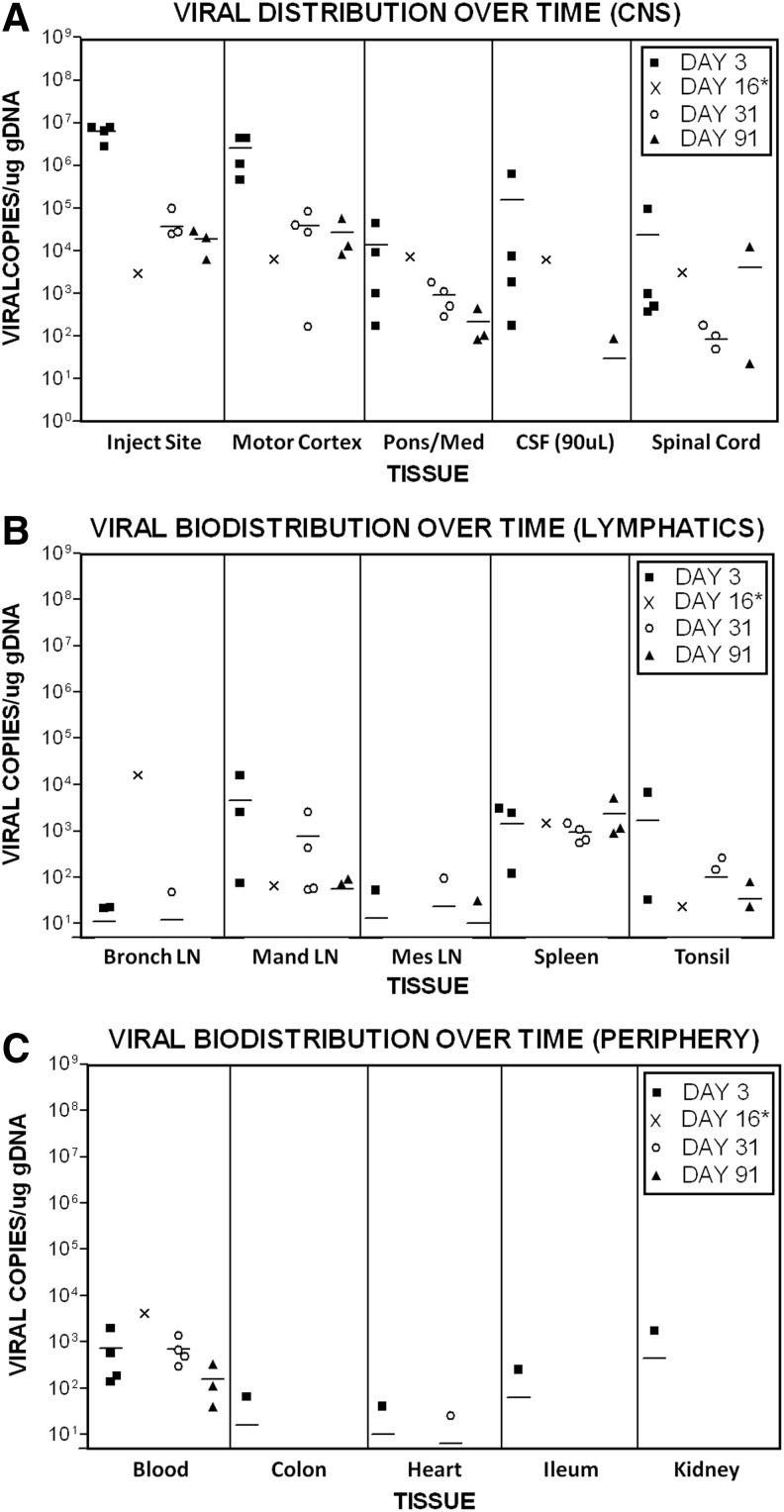
qPCR-based detection of M032 levels and distribution
After intracerebral administration, the biodistribution of M032 was evaluated in group 1 (vehicle) and group 3 (1×108 pfu M032) NHP tissues obtained on days 3, 16, 31, and 91 at necropsy. As shown in Fig. 5, HSV DNA was detectable in multiple tissues obtained from the M032 treated NHP. The highest levels of HSV DNA were observed on day 3 in tissues obtained from the injection site (2.76×106 to 7.93×106 copies/μg gDNA), motor cortex (4.57×105 to 4.40×106 copies/μg gDNA), pons/medulla of the brain (1.74×102 to 4.48×104 copies/μg gDNA), and spinal cord (3.72×102 to 9.42×104 copies/μg gDNA) (Fig. 5A). Viral DNA was also detected in blood and CSF collected from all 4 M032 treated NHPs euthanized on day 3. The relative tissue distribution of HSV DNA was similar among treated animals on each day of scheduled necropsy (day 3, 31, or 91). With the exception of the spleen, the levels of HSV DNA decreased over time. Although reduced on day 91, HSV DNA was still present in the injection site, pons/medulla, motor cortex, spinal cord, CSF, blood, and other tissues, including the mandibular lymph nodes and spleen. These latter findings were indicative of systemic distribution of HSV DNA, presumably as a consequence of initial passage into the CSF with subsequent distribution to tissues.
FIG. 5.
Viral biodistribution over time. The biodistribution of M032 after intracerebral administration was evaluated in NHP groups 1 (vehicle) and 3 (1×108 pfu M032). Plots are organized into (A) central nervous system tissues, including the injection site, motor cortex, pons/medulla oblongata (med), cerebral spinal fluid (CSF), and spinal cord; (B) lymphatic tissues (Bronch LN, bronchial lymph node; Man LN, mandibular lymph node; Mes LN, mesenteric lymph node); and (C) peripheral tissues. PCRs were monitored for contamination and the ability to amplify DNA through the use of both negative and positive control primer and probe sets within each reaction. The quality of samples was monitored by spiking one replicate of each sample with 50 copies of M032 vDNA and through the use of an internal amplification control. Data points represent the mean copy number of two replicates in each tissue per μg of gDNA (or per 90 μl for CSF). Less than 5 copies per μg gDNA represented the limit of detection. Horizontal bars represent the mean. Values obtained from the tissues of male NHP #907 euthanized moribund on day 16* are represented as “X.” PCRs, polymerase chain reactions.
The presence of HSV DNA in the tissues comprising the reticuloendothelial system supported the hypothesis that the hematological changes and histopathological findings induced by M032 were reflective of an immune response to the virus and/or IL-12. Previous reports of oHSV biodistribution after intracerebral administration to Aotus were based on nonquantitative PCR measurements (Todo et al., 2000; Markert et al., 2012), which may lack the sensitivity needed to detect the low levels of HSV DNA we observed in non-CNS tissues. Although the total HSV DNA levels detected in the spleen were orders of magnitude less than levels detected in CNS tissues, there was a trend toward increasing HSV DNA levels with time. This was consistent with the presence of HSV DNA in blood on day 91 and the role of the spleen as the primary “filter” of antigens in blood. In fact, the HSV DNA load in each animal decreased with time, as indicated by decreased copy numbers detected in most tissues.
Conclusions
Despite preclinical studies demonstrating safety and efficacy of M002, the potential exists for the generation of an unfavorable immune response using a murine cytokine in humans and therefore led to the construction of M032, an oHSV expressing human IL-12. The studies described demonstrate the safety and biodistribution data for the humanized M032 virus. Compared with saline-injected controls, all animals injected with M032 showed a characteristic CNS inflammatory change associated with M032 and/or M032-mediated expression of the proinflammatory cytokine, IL-12. There was one untoward event in the 1×108 pfu/NHP dose group; however, the pathologic findings did not show evidence of acute HSV-mediated encephalitis in this animal and did not differ from CNS changes in the other animals administered M032. Clinical evaluation of M032 is anticipated to begin as a phase I dose escalation trial in the near future. These preclinical safety studies have established that Aotus tolerate 1×106 pfu/NHP (equivalent to ∼5×107 pfu in humans) and potentially as high as 1×108 pfu/NHP (equivalent to ∼5×109 pfu in humans). While one NHP (#907, day 16) did become moribund at the highest dose level, no attempt to rescue the animal using antiviral drug (e.g., acyclovir) administration was attempted. Should such toxic symptomatology be evident in humans, both viral replication and further expression of IL-12 could likely be halted by administration of acyclovir, as M032 is intact for the HSV thymidine kinase gene and has demonstrated sensitivity to acyclovir comparable to wild-type HSV, as previously reported (Markert et al., 2012). Further, phase I clinical trial evaluation of M032 proposes an initial dose of 1×105, which is 500 times lower than the equivalent dose established as safe in Aotus. These data support consideration of M032 for entry into phase I evaluation.
Supplementary Material
Acknowledgments
This work was supported by the National Institutes of Health, National Cancer Institute Grants P01 CA71933 (J.M.M., J.C.R., K.A.C., G.Y.G., and R.J.W.) and P20 CA151129 (J.M.M., G.Y.G., K.A.C.), as well as Department of Defense Grant W81XWH-11-1-0498 (J.M.M, G.Y.G., K.A.C.). This research was also supported in part by the RAID program of the Developmental Therapeutics Program in the Division of Cancer Treatment and Diagnosis of the National Cancer Institute. (J.M.M.). We would also like to posthumously acknowledge Dr. Ronna Fulton, from Fulton Veterinary Clinical Pathology Consulting, for her clinical assessment of the NHP study samples.
Author Disclosure Statement
The content of this article does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government.
J.M.M., R.J.W., and G.Y.G. are stockholders, cofounders, and consultants for Catherex, Inc., which has licensed M032 from UAB. UAB, J.M.M., G.Y.G., and R.J.W. hold the intellectual property rights to M032. UAB is the employer of J.M.M., J.C.R., J.J.C., K.A.C., K.H.P., J.M.C., S.L.C., G.Y.G., and R.J.W. No competing financial interests exist for J.O.P., P.E.N., N.W.P., S.D.G., and Dr. Ronna Fulton.
References
- Aghi M., Chou T.C., Suling K., et al. (1999). Multimodal cancer treatment mediated by a replicating oncolytic virus that delivers the oxazaphosphorine/rat cytochrome P450 2B1 and ganciclovir/herpes simplex virus thymidine kinase gene therapies. Cancer Res. 59, 3861–3865 [PubMed] [Google Scholar]
- Albini A., Brigati C., Ventura A., et al. (2009). Angiostatin anti-angiogenesis requires IL-12: the innate immune system as a key target. J. Transl. Med. 7, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreansky S., He B., Van Cott J., et al. (1998). Treatment of intracranial gliomas in immunocompetent mice using herpes simplex viruses that express murine interleukins. Gene Ther. 5, 121–130 [DOI] [PubMed] [Google Scholar]
- Barahona H., Melendez L.V., Hunt R.D., and Daniel M.D. (1976). The owl monkey (Aotus trivirgatus) as an animal model for viral diseases and oncologic studies. Lab. Anim. Sci. 26, 1104–1112 [PubMed] [Google Scholar]
- Cassady K.A., and Parker J.N. (2010). Herpesvirus vectors for therapy of brain tumors. Open. Virol. J 4, 103–108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers R., Gillespie G.Y., Soroceanu L., et al. (1995). Comparison of genetically engineered herpes simplex viruses for the treatment of brain tumors in a scid mouse model of human malignant glioma. Proc. Natl. Acad. Sci. USA 92, 1411–1415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chase M., Chung R.Y., and Chiocca E.A. (1998). An oncolytic viral mutant that delivers the CYP2B1 transgene and augments cyclophosphamide chemotherapy. Nat. Biotechnol. 16, 444–448 [DOI] [PubMed] [Google Scholar]
- Chou J., Kern E.R., Whitley R.J., and Roizman B. (1990). Mapping of herpes simplex virus-1 neurovirulence to gamma 134.5, a gene nonessential for growth in culture. Science 250, 1262–1266 [DOI] [PubMed] [Google Scholar]
- Farassati F., Yang A.D., and Lee P.W. (2001). Oncogenes in Ras signalling pathway dictate host-cell permissiveness to herpes simplex virus 1. Nat. Cell Biol. 3, 745–750 [DOI] [PubMed] [Google Scholar]
- Feingold K.R., and Grunfeld C. (2010). The acute phase response inhibits reverse cholesterol transport. J. Lipid Res. 51, 682–684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodwin J.M., Schmitt A.D., Mcginn C.M., et al. (2012). Angiogenesis inhibition using an oncolytic herpes simplex virus expressing endostatin in a murine lung cancer model. Cancer Invest. 30, 243–250 [DOI] [PubMed] [Google Scholar]
- Gozalo A., and Montoya E. (1990). Mortality causes of owl monkeys (Aotus nancymae and Aotus vociferans) in captivity. J. Med. Primatol. 19, 69–72 [PubMed] [Google Scholar]
- Guffey M.B., Parker J.N., Luckett W.S. Jr., et al. (2007). Engineered herpes simplex virus expressing bacterial cytosine deaminase for experimental therapy of brain tumors. Cancer Gene Ther. 14, 45–56 [DOI] [PubMed] [Google Scholar]
- Han Z.Q., Assenberg M., Liu B.L., et al. (2007). Development of a second-generation oncolytic Herpes simplex virus expressing TNFalpha for cancer therapy. J. Gene Med. 9, 99–106 [DOI] [PubMed] [Google Scholar]
- Harrow S., Papanastassiou V., Harland J., et al. (2004). HSV1716 injection into the brain adjacent to tumour following surgical resection of high-grade glioma: safety data and long-term survival. Gene Ther. 11, 1648–1658 [DOI] [PubMed] [Google Scholar]
- He B., Gross M., and Roizman B. (1997). The gamma(1)34.5 protein of herpes simplex virus 1 complexes with protein phosphatase 1alpha to dephosphorylate the alpha subunit of the eukaryotic translation initiation factor 2 and preclude the shutoff of protein synthesis by double-stranded RNA-activated protein kinase. Proc. Natl. Acad. Sci. USA 94, 843–848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He B., Gross M., and Roizman B. (1998). The gamma134.5 protein of herpes simplex virus 1 has the structural and functional attributes of a protein phosphatase 1 regulatory subunit and is present in a high molecular weight complex with the enzyme in infected cells. J. Biol. Chem. 273, 20737–20743 [DOI] [PubMed] [Google Scholar]
- Hellums E.K., Markert J.M., Parker J.N., et al. (2005). Increased efficacy of an interleukin-12-secreting herpes simplex virus in a syngeneic intracranial murine glioma model. Neuro Oncol. 7, 213–224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter W.D., Martuza R.L., Feigenbaum F., et al. (1999). Attenuated, replication-competent herpes simplex virus type 1 mutant G207: safety evaluation of intracerebral injection in nonhuman primates. J. Virol. 73, 6319–6326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katzin D.S., Connor J.D., Wilson L.A., and Sexton R.S. (1967). Experimental herpes simplex infection in the owl monkey. Proc. Soc. Exp. Biol. Med. 125, 391–398 [DOI] [PubMed] [Google Scholar]
- Kennedy P.G., Adams J.H., Graham D.I., and Clements G.B. (1988). A clinico-pathological study of herpes simplex encephalitis. Neuropathol. Appl. Neurobiol. 14, 395–415 [DOI] [PubMed] [Google Scholar]
- Khovidhunkit W., Kim M.S., Memon R.A., et al. (2004). Effects of infection and inflammation on lipid and lipoprotein metabolism: mechanisms and consequences to the host. J. Lipid Res. 45, 1169–1196 [DOI] [PubMed] [Google Scholar]
- Koch F., Stanzl U., Jennewein P., et al. (1996). High level IL-12 production by murine dendritic cells: upregulation via MHC class II and CD40 molecules and downregulation by IL-4 and IL-10. J. Exp. Med. 184, 741–746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramm C.M., Chase M., Herrlinger U., et al. (1997). Therapeutic efficiency and safety of a second-generation replication-conditional HSV1 vector for brain tumor gene therapy. Hum. Gene Ther. 8, 2057–2068 [DOI] [PubMed] [Google Scholar]
- Liu B.L., Robinson M., Han Z.Q., et al. (2003). ICP34.5 deleted herpes simplex virus with enhanced oncolytic, immune stimulating, and anti-tumour properties. Gene Ther. 10, 292–303 [DOI] [PubMed] [Google Scholar]
- Liu T.C., Zhang T., Fukuhara H., et al. (2006). Oncolytic HSV armed with platelet factor 4, an antiangiogenic agent, shows enhanced efficacy. Mol. Ther. 14, 789–797 [DOI] [PubMed] [Google Scholar]
- Lowenstine L.J. (2003). A primer of primate pathology: lesions and nonlesions. Toxicol. Pathol. 31Suppl, 92–102 [DOI] [PubMed] [Google Scholar]
- Manetti R., Gerosa F., Giudizi M.G., et al. (1994). Interleukin 12 induces stable priming for interferon gamma (IFN-gamma) production during differentiation of human T helper (Th) cells and transient IFN-gamma production in established Th2 cell clones. J. Exp. Med. 179, 1273–1283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markert J.M., Malick A., Coen D.M., and Martuza R.L. (1993). Reduction and elimination of encephalitis in an experimental glioma therapy model with attenuated herpes simplex mutants that retain susceptibility to acyclovir. Neurosurgery 32, 597–603 [DOI] [PubMed] [Google Scholar]
- Markert J.M., Medlock M.D., Rabkin S.D., et al. (2000). Conditionally replicating herpes simplex virus mutant, G207 for the treatment of malignant glioma: results of a phase I trial. Gene Ther. 7, 867–874 [DOI] [PubMed] [Google Scholar]
- Markert J.M., Liechty P.G., Wang W., et al. (2009). Phase Ib trial of mutant herpes simplex virus G207 inoculated pre-and post-tumor resection for recurrent GBM. Mol. Ther. 17, 199–207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markert J.M., Cody J.J., Parker J.N., et al. (2012). Preclinical evaluation of a genetically engineered herpes simplex virus expressing interleukin-12. J. Virol. 86, 5304–5313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martuza R.L., Malick A., Markert J.M., et al. (1991). Experimental therapy of human glioma by means of a genetically engineered virus mutant. Science 252, 854–856 [DOI] [PubMed] [Google Scholar]
- Meignier B., Martin B., Whitley R.J., and Roizman B. (1990). In vivo behavior of genetically engineered herpes simplex viruses R7017 and R7020. II. Studies in immunocompetent and immunosuppressed owl monkeys (Aotus trivirgatus). J. Infect. Dis. 162, 313–321 [DOI] [PubMed] [Google Scholar]
- Mineta T., Rabkin S.D., Yazaki T., et al. (1995). Attenuated multi-mutated herpes simplex virus-1 for the treatment of malignant gliomas. Nat. Med. 1, 938–943 [DOI] [PubMed] [Google Scholar]
- Murphy W.J., Welniak L., Back T., et al. (2003). Synergistic anti-tumor responses after administration of agonistic antibodies to CD40 and IL-2: coordination of dendritic and CD8+ cell responses. J. Immunol. 170, 2727–2733 [DOI] [PubMed] [Google Scholar]
- Nakamori M., Fu X., Pettaway C.A., and Zhang X. (2004). Potent antitumor activity after systemic delivery of a doubly fusogenic oncolytic herpes simplex virus against metastatic prostate cancer. Prostate 60, 53–60 [DOI] [PubMed] [Google Scholar]
- Nakamura H., Mullen J.T., Chandrasekhar S., et al. (2001). Multimodality therapy with a replication-conditional herpes simplex virus 1 mutant that expresses yeast cytosine deaminase for intratumoral conversion of 5-fluorocytosine to 5-fluorouracil. Cancer Res. 61, 5447–5452 [PubMed] [Google Scholar]
- Papanastassiou V., Rampling R., Fraser M., et al. (2002). The potential for efficacy of the modified (ICP 34.5(−)) herpes simplex virus HSV1716 following intratumoural injection into human malignant glioma: a proof of principle study. Gene Ther. 9, 398–406 [DOI] [PubMed] [Google Scholar]
- Parker J.N., Gillespie G.Y., Love C.E., et al. (2000). Engineered herpes simplex virus expressing IL-12 in the treatment of experimental murine brain tumors. Proc. Natl. Acad. Sci. USA 97, 2208–2213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker J.N., Meleth S., Hughes K.B., et al. (2005). Enhanced inhibition of syngeneic murine tumors by combinatorial therapy with genetically engineered HSV-1 expressing CCL2 and IL-12. Cancer Gene Ther. 12, 359–368 [DOI] [PubMed] [Google Scholar]
- Parker J.N., Bauer D.F., Cody J.J., and Markert J.M. (2009). Oncolytic viral therapy of malignant glioma. Neurotherapeutics 6, 558–569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prabhakar S., Brenner G.J., Sung B., et al. (2010). Imaging and therapy of experimental schwannomas using HSV amplicon vector-encoding apoptotic protein under Schwann cell promoter. Cancer Gene Ther. 17, 266–274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preusser M., De Ribaupierre S., Wohrer A., et al. (2011). Current concepts and management of glioblastoma. Ann. Neurol. 70, 9–21 [DOI] [PubMed] [Google Scholar]
- Pyles R.B., Warnick R.E., Chalk C.L., et al. (1997). A novel multiply-mutated HSV-1 strain for the treatment of human brain tumors. Hum. Gene Ther. 8, 533–544 [DOI] [PubMed] [Google Scholar]
- Rampling R., Cruickshank G., Papanastassiou V., et al. (2000). Toxicity evaluation of replication-competent herpes simplex virus (ICP 34.5 null mutant 1716) in patients with recurrent malignant glioma. Gene Ther. 7, 859–866 [DOI] [PubMed] [Google Scholar]
- Sgadari C., Angiolillo A.L., and Tosato G. (1996). Inhibition of angiogenesis by interleukin-12 is mediated by the interferon-inducible protein 10. Blood 87, 3877–3882 [PubMed] [Google Scholar]
- Shah A.C., Benos D., Gillespie G.Y., and Markert J.M. (2003). Oncolytic viruses: clinical applications as vectors for the treatment of malignant gliomas. J. Neurooncol. 65, 203–226 [DOI] [PubMed] [Google Scholar]
- Simpson G.R., and Coffin R.S. (2009). Construction and characterization of an oncolytic HSV vector containing a fusogenic glycoprotein and prodrug activation for enhanced local tumor control. Methods Mol. Biol. 542, 551–564 [DOI] [PubMed] [Google Scholar]
- Smith K.D., Mezhir J.J., Bickenbach K., et al. (2006). Activated MEK suppresses activation of PKR and enables efficient replication and in vivo oncolysis by Deltagamma(1)34.5 mutants of herpes simplex virus 1. J. Virol. 80, 1110–1120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart V.A. (2003). Plasmodium vivax under the microscope: the Aotus model. Trends Parasitol. 19, 589–594 [DOI] [PubMed] [Google Scholar]
- Stupp R., Hegi M.E., Mason W.P., et al. (2009). Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 10, 459–466 [DOI] [PubMed] [Google Scholar]
- Suzuki S., Umezu Y., Saijo Y., et al. (1998). Exogenous recombinant human IL-12 augments MHC class I antigen expression on human cancer cells in vitro. Tohoku J. Exp. Med. 185, 223–226 [DOI] [PubMed] [Google Scholar]
- Takaoka H., Takahashi G., Ogawa F., et al. (2011). A novel fusogenic herpes simplex virus for oncolytic virotherapy of squamous cell carcinoma. Virol. J. 8, 294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todo T., Feigenbaum F., Rabkin S.D., et al. (2000). Viral shedding and biodistribution of G207, a multimutated, conditionally replicating herpes simplex virus type 1, after intracerebral inoculation in Aotus. Mol. Ther. 2, 588–595 [DOI] [PubMed] [Google Scholar]
- Trinchieri G. (2003). Interleukin-12 and the regulation of innate resistance and adaptive immunity. Nat. Rev. Immunol. 3, 133–146 [DOI] [PubMed] [Google Scholar]
- Varghese S., Newsome J.T., Rabkin S.D., et al. (2001). Preclinical safety evaluation of G207, a replication-competent herpes simplex virus type 1, inoculated intraprostatically in mice and nonhuman primates. Hum. Gene Ther. 12, 999–1010 [DOI] [PubMed] [Google Scholar]
- Voest E.E., Kenyon B.M., O'Reilly M.S., et al. (1995). Inhibition of angiogenesis in vivo by interleukin 12. J. Natl. Cancer Inst. 87, 581–586 [DOI] [PubMed] [Google Scholar]
- Walker J.D., Sehgal I., and Kousoulas K.G. (2011). Oncolytic herpes simplex virus 1 encoding 15-prostaglandin dehydrogenase mitigates immune suppression and reduces ectopic primary and metastatic breast cancer in mice. J. Virol. 85, 7363–7371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wigginton J.M., Lee J.K., Wiltrout T.A., et al. (2002). Synergistic engagement of an ineffective endogenous anti-tumor immune response and induction of IFN-gamma and Fas-ligand-dependent tumor eradication by combined administration of IL-18 and IL-2. J. Immunol. 169, 4467–4474 [DOI] [PubMed] [Google Scholar]
- Yoo J.Y., Haseley A., Bratasz A., et al. (2012). Antitumor efficacy of 34.5ENVE: a transcriptionally retargeted and “Vstat120”-expressing oncolytic virus. Mol. Ther. 20, 287–297 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.