Abstract
Glycogen storage disease type Ia (GSD-Ia) is the inherited deficiency of glucose-6-phosphatase (G6Pase), primarily found in liver and kidney, which causes life-threatening hypoglycemia. Dogs with GSD-Ia were treated with double-stranded adeno-associated virus (AAV) vectors encoding human G6Pase. Administration of an AAV9 pseudotyped (AAV2/9) vector to seven consecutive GSD-Ia neonates prevented hypoglycemia during fasting for up to 8 hr; however, efficacy eventually waned between 2 and 30 months of age, and readministration of a new pseudotype was eventually required to maintain control of hypoglycemia. Three of these dogs succumbed to acute hypoglycemia between 7 and 9 weeks of age; however, this demise could have been prevented by earlier readministration an AAV vector, as demonstrated by successful prevention of mortality of three dogs treated earlier in life. Over the course of this study, six out of nine dogs survived after readministration of an AAV vector. Of these, each dog required readministration on average every 9 months. However, two were not retreated until >34 months of age, while one with preexisting antibodies was re-treated three times in 10 months. Glycogen content was normalized in the liver following vector administration, and G6Pase activity was increased in the liver of vector-treated dogs in comparison with GSD-Ia dogs that received only with dietary treatment. G6Pase activity reached approximately 40% of normal in two female dogs following AAV2/9 vector administration. Elevated aspartate transaminase in absence of inflammation indicated that hepatocellular turnover in the liver might drive the loss of vector genomes. Survival was prolonged for up to 60 months in dogs treated by readministration, and all dogs treated by readministration continue to thrive despite the demonstrated risk for recurrent hypoglycemia and mortality from waning efficacy of the AAV2/9 vector. These preclinical data support the further translation of AAV vector–mediated gene therapy in GSD-Ia.
Demaster and colleagues report preclinical results of treating dogs with glycogen storage disease type Ia (GSD-Ia) with double-stranded adeno-associated viral vector type 9 (AAV2/9) encoding human glucose-6-phosphatase (G6Pase). Vector treatment was able to prevent hypoglycemia and led to normalized liver glycogen levels and increased G6Pase activity, but carefully timed readministration with a different pseudotype was required to prevent the therapeutic effect from waning. Survival was prolonged for up to 60 months in dogs treated by readministration, with all dogs continuing to thrive despite the demonstrated risk for recurrent hypoglycemia and mortality from waning vector efficacy.
Introduction
Glycogen storage disease type Ia (GSD-Ia) has great potential for early, effective treatment following detection by newborn screening. Like many other inherited disorders, it can be readily detected by newborn screening for several common mutations (Chen, 2001). The importance of early diagnosis and treatment of GSD-Ia is emphasized by the severe complications of untreated GSD-Ia, which include life-threatening hypoglycemia, severe lactic acidosis, growth failure, renal Fanconi syndrome, and pancreatitis (Chen, 2001). Early complications of GSD-Ia respond favorably to dietary therapy to prevent hypoglycemia, as well as renal tubular dysfunction and growth failure (Chen et al., 1984, 1993). However, long-term complications of GSD-Ia, including growth retardation, proteinuria occasionally progressing to renal failure, osteopenia, formation of hepatic adenomas and hepatocellular carcinoma, and rarely pancreatitis or pulmonary hypertension, frequently fail to respond to dietary therapy (Koeberl et al., 2009). Current management of GSD-Ia requires constant vigilance to avoid acute metabolic decompensation accompanied by life-threatening hypoglycemia and lactic acidosis (Chen et al., 1993; Wolfsdorf and Crigler, 1999).
The initial characterization of dogs with GSD-Ia revealed that their clinical abnormalities most closely resemble severe neonatal onset GSD-Ia in humans (Brix et al., 1995; Kishnani et al., 2001). The canine model more closely mimics GSD-Ia in humans than does the murine model with respect to out-bred genetics, larger size, and the presence of lactic acidosis, and therefore the canine model could be preferable for preclinical development of gene therapy in GSD-Ia (Kishnani et al., 2001). Administration of an adeno-associated virus serotype 2 (AAV2) vector encoding canine glucose-6-phosphatase (G6Pase) corrected hypercholesterolemia and prolonged survival in comparison with untreated GSD-Ia dogs; however, biochemical correction was partial and survival was abbreviated (Beaty et al., 2002).
Previously an AAV2 vector pseudotyped as AAV8 (AAV2/8) achieved efficacy only transiently in a puppy with GSD-Ia prior to the onset of recurrent hypoglycemia before 2 months of age (Weinstein et al., 2010). Weinstein et al. (2010) administered an AAV2/8 vector containing the ubiquitously active CBA (cytomegalovirus enhancer/chicken β-actin) regulatory cassette to drive G6Pase expression, and waning transgene expression prompted re-treating the puppy with an AAV2/1 vector (Weinstein et al., 2010). The AAV2/8 vector containing the CBA regulatory cassette was associated with cytotoxic T cell responses and rapid clearance in the liver of young G6pase−/− mice (Yiu et al., 2010), and that immune response might explain the loss of efficacy from that particular AAV2/8 vector in a puppy with GSD-Ia.
Readministering an AAV vector to prevent recurrent hypoglycemia in GSD-Ia might be limited by the presence of anticapsid antibodies that decrease the efficacy of a second vector. Varying the AAV serotype used for pseudotyping the AAV vector has been proposed as a strategy for readministration of a therapeutic AAV vector (Halbert et al., 1997, 2000). This strategy is feasible, because antisera generated against one AAV vector pseudotype failed to neutralize other pseudotypes (Gao et al., 2002). Moreover, AAV2-pretreated dogs with hemophilia were successfully re-treated with an AAV2/8 vector, indicating a lack of cross-reacting neutralizing antibodies in another dog model (Wang et al., 2005).
In contrast with the experience in canine GSD-Ia, we demonstrated long-term efficacy with a double-stranded AAV2/8 vector containing the human G6Pase minimal promoter to drive expression of the human G6Pase cDNA, AAV-G6Pase, which prolonged survival for >11 months in three consecutive dogs with GSD-Ia (Koeberl et al., 2008). More recently, we demonstrated efficacy with the AAV2/2-, AAV2/7-, AAV2/8-, and AAV2/9-G6Pase vectors in G6pase−/− mice with GSD-Ia treated at 2 weeks of age, with the AAV2/9-G6Pase vector achieving the highest degree of hepatorenal correction (Luo et al., 2011). G6pase−/− mice responded quickly to administration of the double-stranded AAV-G6Pase vector at 2 weeks of age, as demonstrated by their survivability in comparison to the untreated mice that uniformly died by 3 weeks of age (Koeberl et al., 2008). AAV2/9-G6Pase prevented hypoglycemia during fasting for >8 hr at 6 months of age in G6pase−/− mice (Luo et al., 2011). Both AAV2/7 and AAV2/8-G6Pase were highly efficacious in infant G6pase−/− mice with GSD-Ia, based upon prolonged survival and correction of hypoglycemia (Luo et al., 2011).
The efficacy of AAV-G6Pase was further evaluated in dogs with GSD-Ia, because the gene therapy in a large animal model might predict the response of human patients more accurately than would a mouse model. We have currently evaluated the long-term efficacy from AAV-G6Pase pseudotyped as either AAV2/8 or AAV2/9 in dogs with GSD-Ia. The primary endpoint was prevention of hypoglycemic during fasting, and secondary endpoints included long-term survival and the biochemical correction of liver and kidney variables. Seven consecutive puppies with GSD-Ia were treated as neonates with the AAV2/9 vector, and symptoms recurred 6 weeks to 13 months later. Subsequently, the onset of symptoms was managed by readministration of a new pseudotype of AAV vector in a group of three puppies with GSD-Ia at the time of symptoms in any puppy. After the first 3 months of life readministration of a new pseudotype was performed infrequently. Two dogs with GSD-Ia, previously treated as neonates with the AAV2/8 vector (Koeberl et al., 2008), were followed for >2.5 years before efficacy waned and vector readministration was needed. The primary endpoints were prevention of hypoglycemia during fasting and biochemical correction of glycogen storage in the liver, both of which demonstrated efficacy following AAV vector–mediated gene therapy.
Materials and Methods
Breeding and neonatal care of puppies, vector administration, and monitoring
Genetic analysis, vector administration to affected puppies via jugular venipuncture at 2 or 3 days of age, and subsequent feeding and monitored were performed as described by Crane et al. (2011). Following weaning, dogs were fed and monitored three times daily. Blood samples for routine serum chemistry panels and plasma lactate analysis were collected from affected dogs via the jugular vein with minimal restraint. Samples were analyzed by the clinical laboratory at Department of Laboratory Animal Resources or with a Kodak Biolyzer benchtop blood chemistry analyzer (Rochester, NY). The established canine reference range for glucose was 60–120 mg/dL (per the Clinical Pathology Laboratory at North Carolina State University College of Veterinary Medicine). All procedures were done in accordance with Duke University Animal Care and Use Committee-approved guidelines.
Survival analysis was performed using the Wilcoxon-Breslow-Gehan test of equality with Stata 10 (StataCorp, College Station, TX). All viral vector stocks were handled according to the Biohazard Safety Level 2 guidelines published by the National Institutes of Health.
Clinical synopses
The clinical histories for dogs that developed recurrent symptoms of GSD-Ia are detailed as follows.
Puppy F developed acute lethargy, diarrhea, and clinical signs of dehydration between 8 and 9 weeks of age. Blood chemistry panel was normal including serum creatinine. Puppy F also had hypoglycemia (blood glucose 40 mg/dL). Stool evaluation for parasites or bacterial infection was negative. The physical exam and vital signs were normal. Glucose therapy consisting of twice daily subcutaneous 10% glucose injections and frequent syringe feeding was initiated because of a decrease in appetite. Puppy F spontaneously recovered and fed normally for several days; however, he developed lethargy again 6 days later and glucose therapy was resumed. Tachypnea was the only abnormal physical finding in the evening preceding acute demise at 9 weeks of age. Postmortem was performed, and the histopathology of kidneys revealed multiple tubules with acute necrosis of the tubular epithelium (acute tubular necrosis). There was mild to moderate cytoplasmic vacuolation of the proximal convoluted and adjacent tubules and mild cytoplasmic vacuolation of hepatocytes in liver, which were consistent with glycogen accumulation.
Puppy Ro expired at 7 weeks of age on the morning after repeat vector administration, 1 day following demise of Puppy F. Puppy Ro developed shivering and lethargy at 6 weeks of age, and despite refusing food its blood glucose was 100 mg/dL. Blood chemistry panel including blood glucose and creatinine was normal except for elevated blood urea nitrogen (70 mg/dL, normal 7–25 mg/dL). Physical examinations revealed low body temperature and tachypnea; radiographic examination was within normal limits. Glucose therapy was initiated as described for Puppy F, and the puppy was housed in an incubator to maintain body temperature. Blood urea nitrogen value normalized after 24 hr; however, preprandial blood glucose dropped to 43 mg/dL, and therefore AAV2/8-G6Pase vector was administered (1×1013 vector particles [vp]/kg). Over the next several hours Puppy Ro began vocalizing and pacing, vomited once, and passed soft stools. Treatment was initiated including ampicillin, ondansetron, metoclopramide, vitamin B complex, and intermittent intravenous fluids. A parvovirus enzyme-linked immunosorbent assay (ELISA) test was negative. Demise occurred approximately 18 hr following vector administration. Postmortem was performed, and the histopathology of kidneys revealed advanced acute tubular necrosis. Additionally, there was mild to moderate cytoplasmic vacuolation of the proximal convoluted and adjacent tubules in kidney and mild cytoplasmic vacuolation of hepatocytes in liver, which were consistent with glycogen accumulation.
Puppy Du developed poor appetite and tachypnea at 9 weeks of age. Blood glucose was low before feeding (61 mg/dL) without demonstrating hypoglycemia (<60 mg/dL). Puppy Du ate adequately at 10:00 p.m.; however, it was lethargic and hypothermic the following morning. Within 20 min following injection of intravenous glucose the puppy ate and reacted normally. Blood glucose was elevated (165 mg/dL), serum sodium decreased (130 mmol/L, normal 138–160 mmol/L), and blood urea nitrogen elevated (46 mg/dL, normal 7–25 mg/dL). Within 10 min acute cardiac arrest occurred and resuscitation was unsuccessful. Necropsy revealed no cause of death.
Dog T was treated with AAV2/7-G6Pase vector at 4 weeks of age to prevent onset of symptoms, when AAV2/7-G6Pase was also administered to other dogs in the treatment group (H and De). Fasting at 7 weeks old was stopped at 6 hr due to low blood glucose (45 mg/dL). Dog T was treated with AAV2/8-G6Pase vector at 8 weeks of age in coordination with the treatment group. Fasting at 10 weeks of age was stopped after 6 hr due to low blood glucose (41 mg/dL). Over the next several weeks, Dog T displayed ataxia associated with moderate hypoglycemia prior to the morning feeding on four occasions (blood glucose 31–47 mg/dL), which responded promptly to feeding. At 18 weeks of age Dog T developed ataxia and lethargy in association with hypoglycemia (blood glucose 28 mg/dL), which was treated with glucose therapy as described above. AAV2/1-G6Pase was administered the next day, and acute symptoms resolved. However, intermittent moderate hypoglycemia reoccurred on approximately a weekly basis from 22 to 45 weeks of age prior to the morning feeding (blood glucose 26–42 mg/dL). Dog T was subsequently transferred to another research protocol for treatment with a different vector.
Liver biopsy and histology
Liver biopsies were performed at 6 months, 1 year, and 3 years of age, unless otherwise indicated. Biopsies were performed earlier than scheduled when a puppy developed symptoms and was to receive a new AAV vector to treat hypoglycemia. Dogs were anesthetized with isoflurane and a 100-mg liver sample was taken via laparotomy. Part of the liver sample was frozen on dry ice and stored at −70° C, while the remainder was fixed in 10% buffered neutral formalin. Formalin-fixed liver samples were embedded in paraffin, sectioned at 3 to 6 μm, and stained with hematoxylin and eosin. Microscopic sections were examined by light microscopy by a board-certified veterinary pathologist.
Analysis of transduction in liver and kidney
G6Pase enzyme and glycogen content analyses were performed by using the flash frozen liver sample taken during liver biopsy as described (Brix et al., 1995). Glycogen content was measured by complete digestion of polysaccharide using amyloglucosidase. The structure of the polysaccharide was inferred by using phosphorylase-free of debranching enzyme to measure the yield of glucose-1-phosphate. Comparisons of two groups were assessed by a homoscedastic Student t-test. A p-value of <0.05 was considered to be statistically significant.
ELISA detection of plasma anti-AAV antibodies
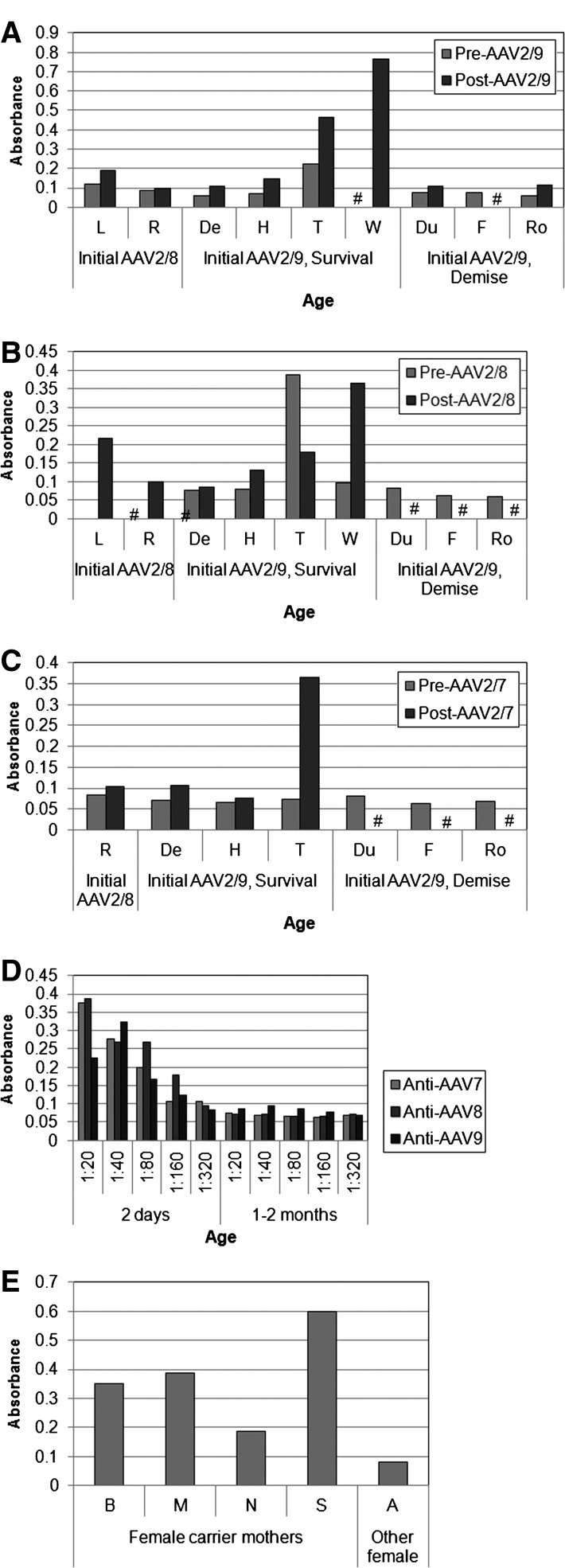
Wells were coated with 2×109 AAV vector particles per well at 4°C overnight for detection of anti-AAV9 antibody. After washing with phosphate-buffered saline (PBS) containing 0.05% Tween 20, a 1:20 dilution of the plasma were added to the wells, and incubated for 1 hr at room temperature. The wells were washed with 0.05% Tween 20+PBS, incubated with a 1:2500 dilution of alkaline phosphatase–conjugated sheep anti-mouse IgG1 at room temperature for 1 hr, and washed, and alkaline phosphatase substrate (p-nitrophenyl phosphate) was added. The absorbance at 411 nm was measured with a Tecan SpectraFluor (MTX Lab Systems, Vienna, VA ) microplate reader. Each sample was analyzed in duplicate at each dilution. The titer of antibody was determined as the highest dilution at which the value for absorbance exceeded 0.2.
Results
Neonatal administration of AAV2/9-G6Pase demonstrated early efficacy
AAV2/9-G6Pase (Luo et al., 2011) was initially administered to seven GSD-Ia puppies at 2–3 days of age to evaluate its efficacy with regard to prevention of hypoglycemia during prolonged fasting (2×1013 vp/kg for the first puppy, and subsequently 4×1013 vp/kg; Table 1). Puppies with GSD-Ia develop hypoglycemia without fasting, and typically do not survive for greater than 2 months when maintained by dietary therapy consisting of frequent feedings (Koeberl et al., 2008). Administration of the AAV2/9 vector prevented hypoglycemia (glucose <60 mg/dL) during periodic fasting tests for >6 hr in the initial puppy that was treated for the entire first year of life (Fig. 1A). The only residual effect of G6Pase deficiency in the first 2 months of age was small size relative to unaffected littermates (not shown).
Table 1.
Vector Administration and Readministration to Dogs with Glycogen Storage Disease Type Ia
| 2nd Vector | 3rd Vector | 4th Vector | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dog (sex, follow-up) | Initial Neonatal vector | Pseudotype | Age (months) | Quantity | Reasona | Pseudotype | Age | Quantity | Reasona | Pseudotype | Age | Quantity | Reasona |
| W (female, 30 months) | AAV2/9, 2×1013 vp/kg | AAV2/8 | 15 | 1×1012 vp/kg | A, E, P | ||||||||
| Du (male, 3 months) | AAV2/9, 4×1013 vp/kg | — | |||||||||||
| F (male, 2 months) | AAV2/9, 4×1013 vp/kg | — | |||||||||||
| Ro (male, 2 months) | AAV2/9, 4×1013 vp/kg | AAV2/8 | 2 | 1×1013 vp/kg | A, E | ||||||||
| De (male, 18 months) | AAV2/9, 4×1013 vp/kg | AAV2/7 | 2 | 2×1012 vp/kg | H, S | AAV2/8 | 4 months | 1×1013 vp/kg | C | ||||
| H (female, 18 months) | AAV2/9, 4×1013 vp/kg | AAV2/7 | 3 | 2×1012 vp/kg | H | AAV2/8 | 5 months | 1×1013 vp/kg | H | ||||
| T (male, 10 months) | AAV2/9, 4×1013 vp/kg | AAV2/7 | 1 month | 1×1013 vp/kg | C | AAV2/8 | 2 months | 1×1013 vp/kg | C | AAV2/1 | 4.5 months | 2×1012 vp/kg | H |
| R (male, 58 months) | AAV2/8, 1×1013 vp/kg | AAV2/9 | 34 | 3×1012 vp/kg | A,E | AAV2/7 | 43 months | 1×1013 vp/kg | E, I | ||||
| L (female, 60 months) | AAV2/8, 1×1013 vp/kg | AAV2/9 | 36 | 3×1012 vp/kg | H | ||||||||
C, synchronize with cohort; E, emesis; H, hypoglycemia; I, anorexia; P, pancreatitis; S, seizures.
FIG. 1.
Prevention of hypoglycemia following neonatal AAV2/9-G6Pase administration. Blood glucose was determined every 2 hr following fasting of duration indicated. (A) Dog W was treated with AAV2/9-G6Pase at 2 days of age (2×1013 vp/kg), and subsequently fasted for 8 hr at the indicated ages. (B) Puppies Du, F, and Ro were treated with AAV2/9-G6Pase at 2 days of age (4×1013 vp/kg), and subsequently fasted for 8 hr at 2–3 weeks of age. These three puppies did not survive past 3 months of age. (C) Puppies De, H, and T were treated with AAV2/9-G6Pase at 2 days of age (4×1013 vp/kg), and subsequently fasted for 8 hr at 2–3 weeks of age. These three puppies were re-treated with AAV2/7- and AAV2/8-G6Pase as a group to prevent mortality (Table 1). Glucose curves for normal puppies (n=10) and GSD-Ia puppies treated with dietary therapy alone (n=4) are shown for comparison. AAV, adeno-associated virus; AAV2, adeno-associated virus serotype 2; G6Pase, glucose-6-phosphatase; GSD-Ia, glycogen storage disease type Ia; vp, vector particles. Color images available online at www.liebertonline.com/hum
Subsequently, the second to fourth puppies were treated with a higher dose of AAV2/9-G6Pase to better prevent hypoglycemia during fasting (4×1013 vp/kg); however, after 2 months of age each of these puppies succumbed to complications of GSD-Ia, which is associated with very high mortality in the first 2 months of life (Koeberl et al., 2008). Glucose administration did not prevent demise following a brief period of poor feeding and inactivity (Table 2; see Clinical synopses for details). The onset of symptoms was unexpected, due to the demonstration of normal blood glucose during prolonged fasting for these vector-treated puppies (Fig. 1B). Prevention of hypoglycemia confirmed efficacy from AAV2/9-G6Pase administration, because GSD-Ia puppies that were not vector-treated developed hypoglycemia following only 2 hr of fasting (blood glucose 11±15 mg/dL). Symptoms preceding demise included lethargy, and two of the three puppies had one episode of moderate hypoglycemia documented between 24 and 48 hr prior to demise (31–37 mg/dL). Frequent hand-feeding and intermittent subcutaneous dextrose failed to resolve symptoms; the latter being effective in mice with GSD-Ia (Sun et al., 2002; Koeberl et al., 2006).
Table 2.
Features Associated with Acute Demise
| Dog | Initial vector, subsequent vector | Age of demise (weeks) | Histological abnormalities |
|---|---|---|---|
| F | AAV2/9 | 9 | Acute tubular necrosis, mild vacuolation of hepatocytes and proximal renal tubules |
| Du | AAV2/9 | 9 | Vacuolation of hepatocytes and proximal renal tubules |
| Ro | AAV2/9, followed by AAV2/8 at 7 weeks | 7 | Acute tubular necrosis, mild vacuolation of hepatocytes and proximal renal tubules |
Readministration of AAV-G6Pase of alternative pseudotype restores long-term efficacy
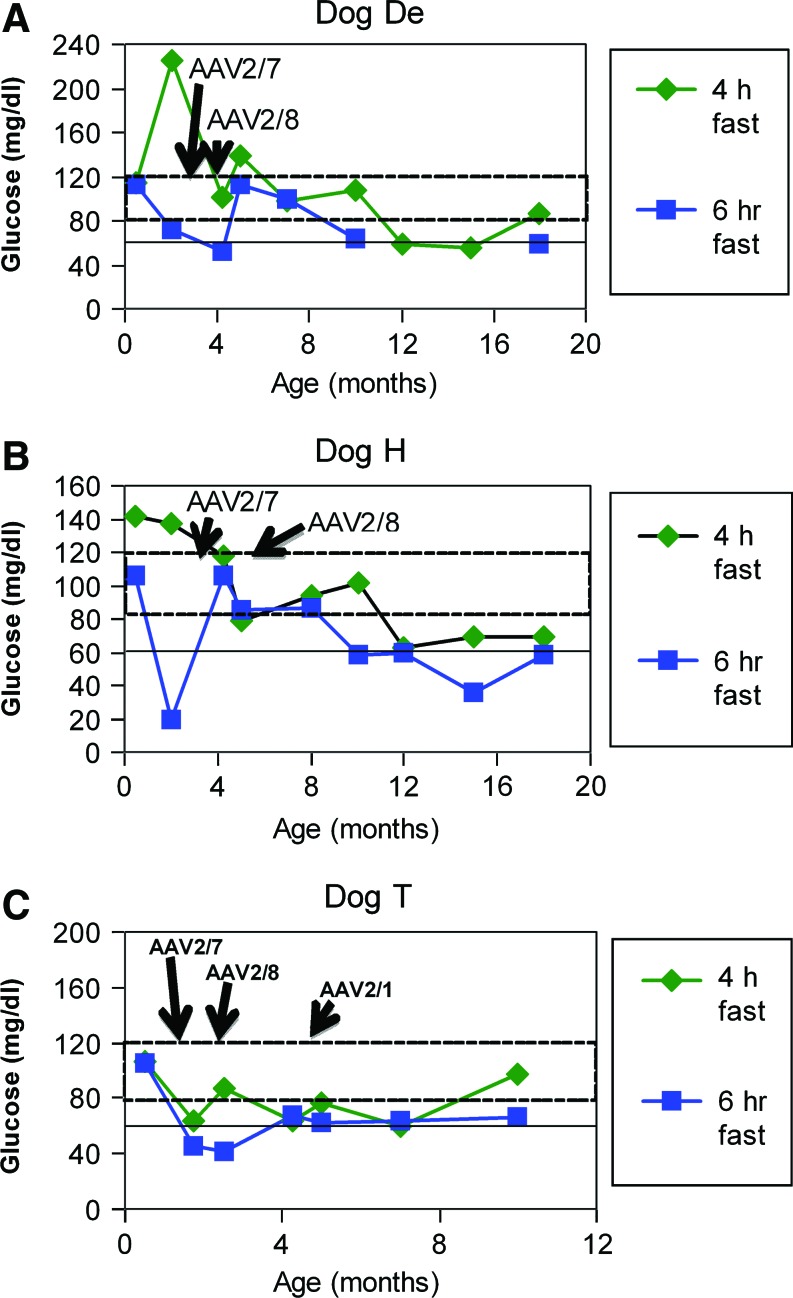
The ability of AAV vectors of an alternative pseudotype to restore efficacy has been established in hemophilia (Wang et al., 2005) and more recently in GSD-Ia (Weinstein et al., 2010). Three additional puppies were treated by readministration of AAV-G6Pase pseudotyped as AAV2/7 and AAV2/8, following neonatal AAV2/9 administration (Table 1, Dogs De, H, and T). The next three puppies demonstrated an initial efficacious response to AAV2/9-G6Pase because hypoglycemia during timed fasting was prevented at 1 month of age by the initial AAV2/9 vector strategy for >6 hr (Fig. 1C). Fasting for 6 hr at age 1 month earlier revealed slightly higher blood glucose for the initial three deceased puppies treated with the higher dose of AAV2/9-G6Pase (Fig. 1B), in comparison with the next three puppies (Fig. 1C); therefore, lower blood glucose during fasting of the latter group supported the need for additional vector treatment (141±16 mg/dL versus 108±4 mg/dL, respectively; p=0.02). Subsequently AAV2/7-G6Pase (and later AAV2/8-G6Pase) was administered to all three puppies in the readministration group, when one of these puppies developed symptoms. At the time of initial symptoms each puppy was supported by frequent feeding until the time of vector readministration (Fig. 2A–2C). This practice resulted in vector readministration at earlier ages to the youngest puppy (Table 1, Dog T). Dog T subsequently developed anorexia and hypoglycemia, prompting the administration of an AAV2/1 at 4.5 months of age. Subsequently Dog T's blood glucose was normal for >4 hr of fasting at 10 months of age (Fig. 2C).
FIG. 2.
Sustained efficacy following readministration of AAV-G6Pase. Puppies De, H, and T were treated with AAV2/9-G6Pase at 2 days of age (4×1013 vp/kg), and subsequently re-treated with AAV2/7- and AAV2/8-G6Pase as a group to prevent mortality. Blood glucose following 4 and 6 hr of fasting at the indicated ages. Fasting was performed 2 weeks following vector administration, and every 2–3 months subsequently. (A) Dog De. (B) Dog H. (C) Dog T. Dog T had recurrent hypoglycemia and anorexia at 5 months of age that precipitated re-treatment with AAV2/1-G6Pase (Table 1). Color images available online at www.liebertonline.com/hum
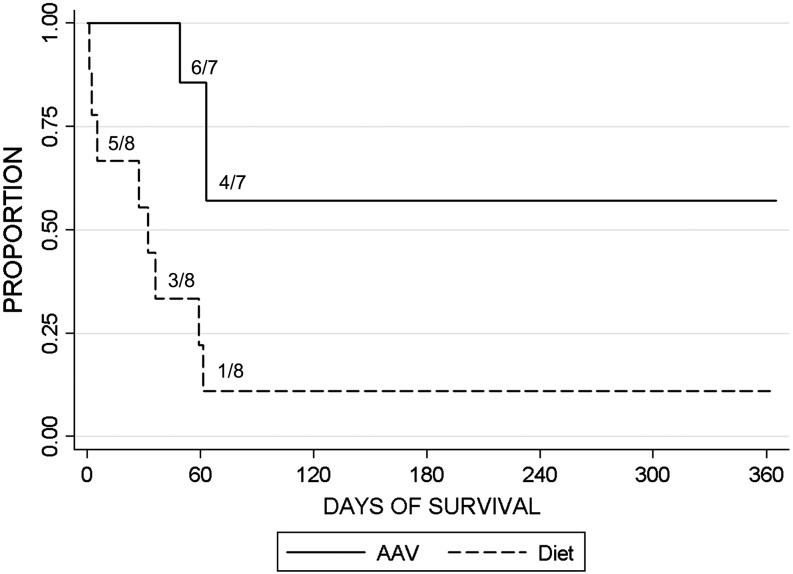
The administration of AAV2/9-G6Pase prolonged survival to greater than 2 months of age in six of seven puppies (Fig. 3), which was markedly improved in comparison with the history for dietary therapy alone that was associated with 88% mortality in the first 2 months of life (Koeberl et al., 2008). As already detailed, readministration of AAV-G6Pase pseudotyped with a new serotype prolonged survival to >1 year of age; moreover, the overall median survival in the neonatal AAV2/9 group was 365 days; in contrast, the median survival in the diet-treated group was 32 days (p=0.007).
FIG. 3.
Survival following initial AAV2/9 vector administration. Survival following neonatal administration of AAV2/9-G6Pase. Proportion surviving by age shown (n=7), in comparison with puppies historically treated with dietary therapy alone (n=8; Koeberl et al., 2009). The fraction surviving is indicated for major steps in each survival curve, for example 1/8 diet-treated puppies at 2 months of age.
Long-term efficacy was sustained by infrequent AAV vector readministration in adult dogs with GSD-Ia
Two of three dogs with GSD-Ia that were originally treated with AAV2/8-G6Pase as neonates (Koeberl et al., 2008) were further evaluated by prolonged fasting at later ages (Fig. 4A). Initially these dogs were fasted for only 2 hr and maintained normal blood glucose levels (Koeberl et al., 2008). When prolonged fasting for 6 hr was performed at 7 months of age hypoglycemia (glucose <60 mg/dL) occurred in both dogs. Later, when these two dogs were fasted for 6 hr at 14 months (Fig. 4A) and 16 months of age (not shown), both maintained normal blood glucose. The prolonged maintenance of normoglycemia at a later age was attributed to the decreased glucose requirements of adults with GSD-Ia, in comparison with younger patients (Chen, 2001). The third dog treated initially treated with AAV2/8-G6Pase developed intermittent hypoglycemia, despite the prevention of hypoglycemia during 2 hr of fasting, and met humane endpoints requiring euthanasia (not shown).
FIG. 4.
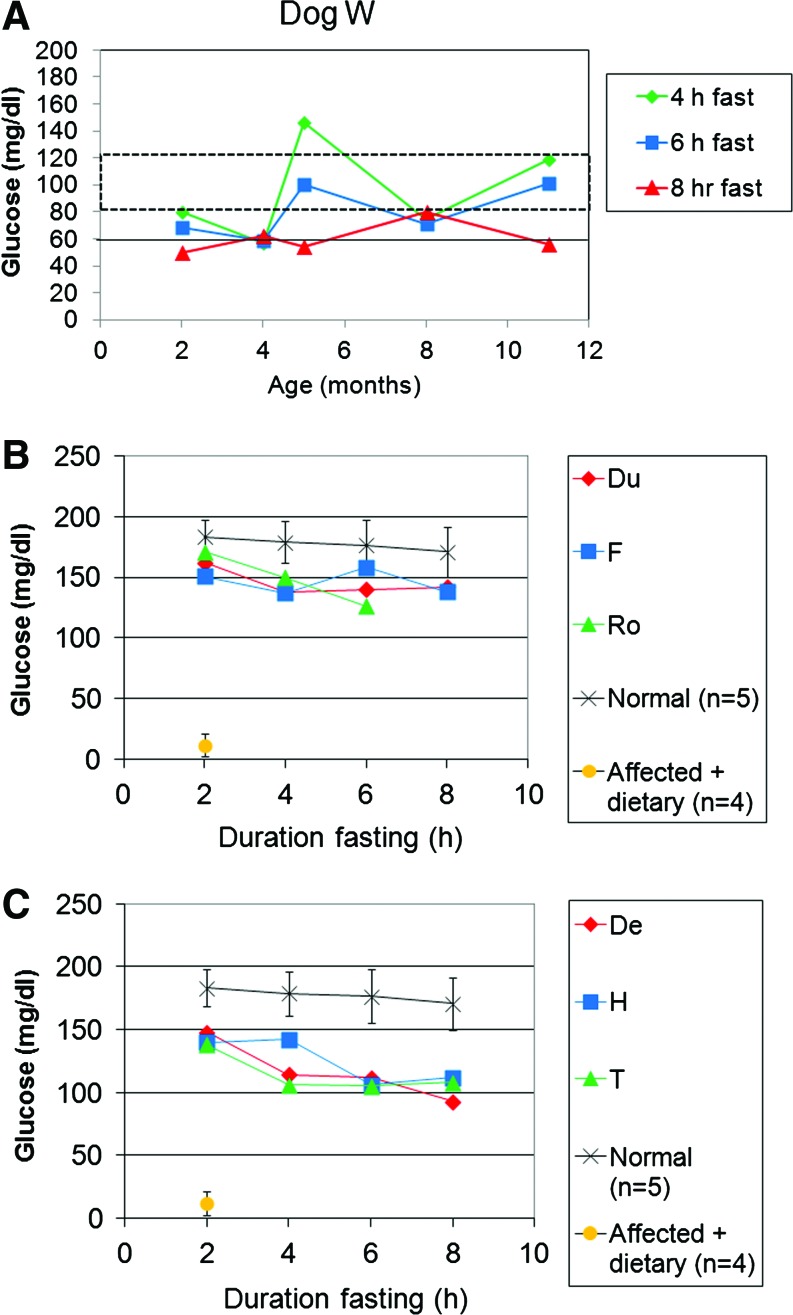
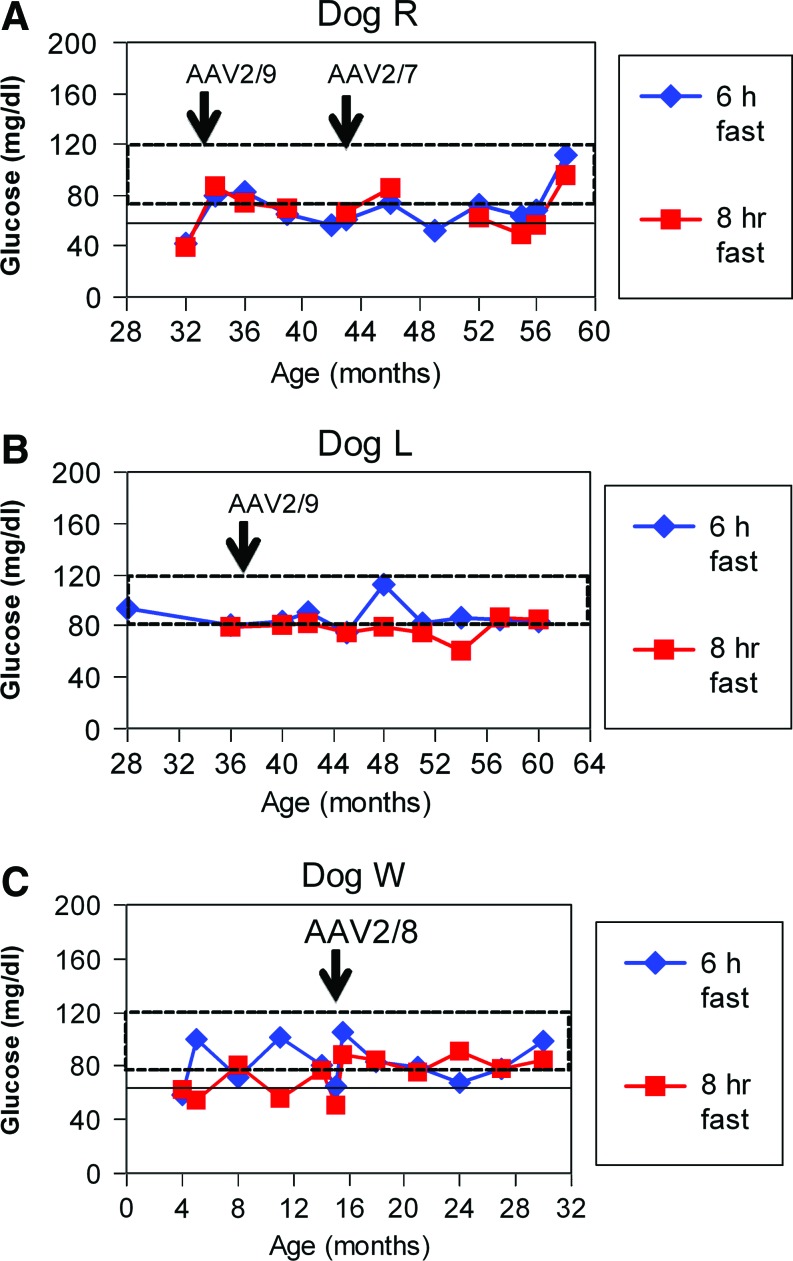
Evaluation of efficacy in adult dogs following AAV-G6Pase administration. Blood glucose during prolonged fasting for the indicated duration in older dogs (Table 1). (A) Fasting for 6 and 8 hr for Dog R at older ages. Dog R was treated by readministration of AAV-G6Pase in response to recurrent symptoms. (B) Fasting for 6 and 8 hr for Dog L at older ages. Dog L was treated by readministration of AAV-G6Pase to prevent recurrence of symptoms. (C) Fasting for 6 and 8 hr for Dog W at older ages. Dog W was treated by readministration of AAV-G6Pase to in response to life-threatening hypoglycemia, anorexia, and pancreatitis (Table 1). Color images available online at www.liebertonline.com/hum
Monitoring blood glucose during fasting tests demonstrated the prevention of hypoglycemia for almost 3 yr following AAV2/8-G6Pase administration in two dogs (Fig. 4B, 4C); however, hypoglycemia with or without accompanying symptoms during fasting occurred eventually and prompted administration of a new pseudotype, AAV2/9-G6Pase, to these dogs (Table 1, Dogs L and R). Dog R developed anorexia and vomiting at 34 months of age, which resolved within 1 day following administration of AAV2/9-G6Pase. Subsequently Dog R's blood glucose was maintained at higher levels during fasting tests (Fig. 4A). Dog R developed recurrent anorexia and hypoglycemia at 43 months of age, which resolved following administration of AAV2/7-G6Pase. Dog L was treated with AAV2/9-G6Pase at 36 months of age to prevent the recurrence of symptoms (Fig. 4B).
The first AAV2/9 vector–treated puppy, Dog W, had normal glucose during serial fasting tests for 1 year following neonatal AAV2/9 vector administration (2×1013 vp/kg). Dog W later developed hypoglycemia during fasting by 12 months of age (Fig. 4C), which progressed to anorexia accompanied by hypoglycemia and lethargy that prompted administration of AAV2/8-G6Pase at 15 months of age (Table 1). Dog W was diagnosed with pancreatitis (emesis, highly elevated serum amylase), a severe complication of GSD-Ia that is typically lethal (Kikuchi et al., 1991). Despite the observation that she was unable to feed for >24 hr and therefore unlikely to respond to dietary therapy, anorexia resolved along with associated symptoms within 1 day following AAV2/8 vector administration.
The frequency of vector readministration was every 9.2 months following neonatal treatment (Table 1). AAV-G6Pase of any serotype was readministered to males every 6.6 months, whereas females were re-treated only every 15 months. Excluding Dog T, readministration was required only every 13 months.
Anti-capsid antibodies of maternal origin were present prior to vector administration in Dog T
The presence of anti-capsid antibodies was evaluated as a potential explanation for the diminished efficacy from AAV-G6Pase in Dog T and others treated with neonatal AAV2/9 administration. Anti-AAV9 was quantified prior to and following AAV2/9 vector administration (Fig. 5A). When GSD-Ia puppies were treated with an AAV2/9 vector at 2–3 days of age, four of seven puppies failed to form detectable anti-AAV9 IgG at a low titer of 1:20 (Fig. 5A). Only one puppy, T, had anti-AAV9 antibodies (>0.2 absorbance at 1:20 dilution) prior to treatment with the AAV2/9 vector (Fig. 5A). Two GSD-Ia dogs treated with AAV2/8 at birth had low anti-AAV2/9 2 years later (<1:20), prior to AAV2/9-G6Pase administration (Fig. 5A, L, and R).
FIG. 5.
Anti-AAV antibody formation prior to and following AAV-G6Pase administration. Plasma was retrospectively analyzed from samples drawn during fasting for dogs with GSD-Ia or drawn following birth of litters from carrier females. Samples for dogs treated with AAV-G6Pase were obtained 2 months following vector administration, with the exception of Dog Du, which was obtained at 0.75 months. Dogs were treated initially with AAV2/8 or AAV2/9 as indicated. The elevation of absorbance >0.2 indicated detectable anti-capsid IgG antibodies. Absorbance for titer of 1:20 shown, except where otherwise indicated. Samples that were unavailable are indicated (#). (A) Anti-AAV9 in vector-treated dogs. (B) Anti-AAV8 in vector-treated dogs. (C) Anti-AAV7 in vector treated dogs. (D) Titering of anti-capsid IgG for Dog T at 2 days of age, and at 1 month (anti-AAV7) to 2 months (anti-AAV8 and anti-AAV9) of age. (E) Anti-AAV9 in carrier females. Dog A was not exposed to puppies treated with AAV2/9-G6Pase, unlike the other carrier females.
The puppy with pre-existing antibodies to AAV9, Puppy T, also had detectable anti-AAV8 antibodies prior to AAV2/8 vector administration (Fig. 5B), although T and all other puppies lacked anti-AAV7 antibodies prior to AAV2/7 vector administration (Fig. 5C). The possibility that puppy T had acquired anti-AAV antibodies as a neonate from cross-placental passage of maternal antibodies was investigated by titering anti-AAV IgG at 2 days and at 1–2 months of age. Antibodies against AAV7–9 were detected at 2 days of age, but antibody titers waned by 1 month (anti-AAV7) to 2 months (anti-AAV8) of age. Therefore, anti-AAV7 and anti-AAV8 were undetectable prior to retreatment with AAV2/7 and AAV2/8 vectors, respectively (Fig. 5D). The likely source of Dog T's anti-AAV antibodies was transplacental passage of maternal antibodies, because the carrier female mothers had detectable anti-AAV9 following delivery of GSD-Ia puppies (Fig. 5E); however, a carrier female that was never exposed to the vector did not form antibodies against AAV2/9 (Fig. 5E, Dog A).
Residual hepatorenal involvement persisted despite biochemical correction
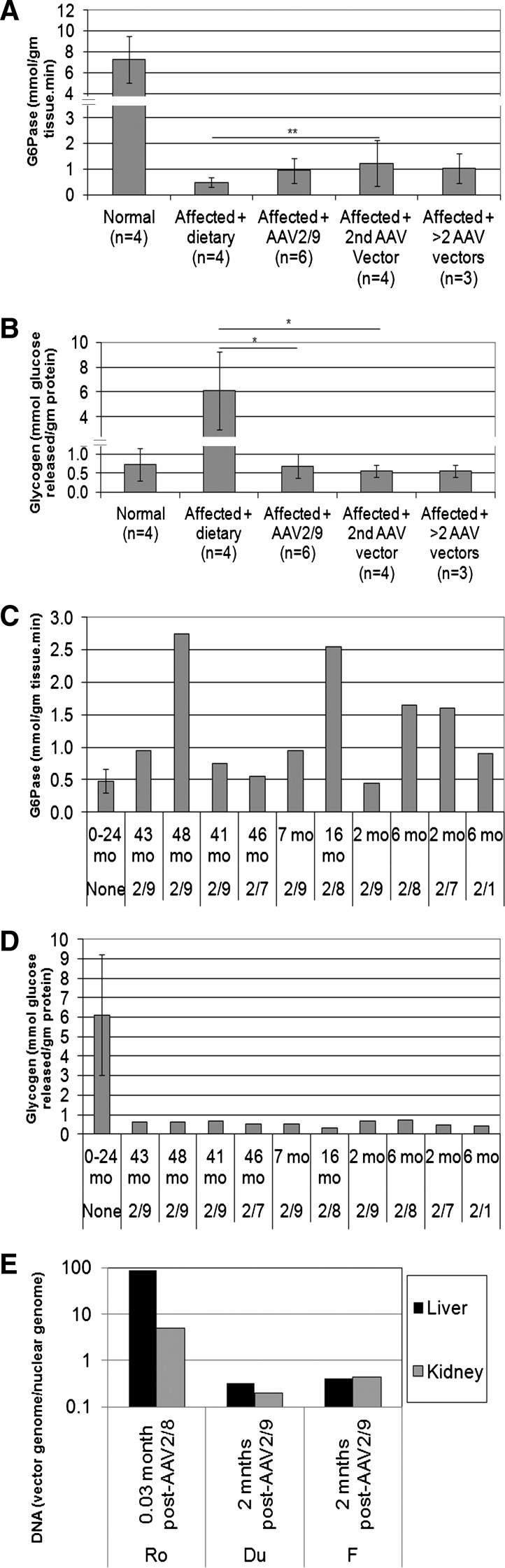
The efficacy from AAV-G6Pase vector administration can be demonstrated by the increased G6Pase activity and reduced glycogen content in the GSD-Ia liver. AAV2/9-G6Pase administration to neonatal dogs with GSD-Ia resulted in 13% of normal G6Pase activity (Fig. 6A; 0.94±0.5 μmol/g tissue/min following AAV2/9-G6Pase; normal range 7.3±2.2 μmol/g tissue/min). Administration of a second AAV vector of a new pseudotype, following neonatal AAV2/8- or AAV2/9-G6Pase administration, increased the mean G6Pase activity to 17% of normal (Fig. 6A). The associated prevention of hypoglycemia during fasting supported the estimate that >10% of normal G6Pase activity is sufficient to prevent hypoglycemia (Keller et al., 1998). The increase in G6Pase activity following vector treatment did not reach statistical significance (Fig. 6A); however, the significant reduction of glycogen content reflected efficient transduction of liver following initial administration of the AAV2/9-G6Pase vector (Fig. 6B). Degradation of G6Pase during handling of the liver biopsies could not be excluded as a cause for low G6Pase activity. Liver biopsies performed following vector readministration revealed sustained normalization of glycogen content, which was significantly reduced in comparison with dietary treatment alone (Fig. 6B). Glycogen content was normalized in the kidney following AAV2/9-G6Pase administration from postmortem samples, in comparison with kidney following dietary treatment alone (0.6±0.8 versus 9.2 mmol glucose released/g protein, not shown).
FIG. 6.
Biochemical correction and vector genome quantification for liver following initial AAV-G6Pase administration and following readministration. G6Pase activity and glycogen content in liver following AAV vector administration or dietary therapy alone for dogs with GSD-Ia (Affected+dietary therapy), and for normal dogs. Mean±standard deviation shown. (A) Liver G6Pase analysis 2 months following AAV2/9-G6Pase administration (Affected+AAV2/9), following a second AAV vector administration (Affected+2nd AAV vector), and following administration of >2 vectors. (B) Liver glycogen content analysis for samples from (A). (C) G6Pase analysis for serial liver biopsies from individual dogs with GSD-Ia following the indicated number of vector treatments (see Table 1 for additional details). GSD-Ia dogs treated with dietary therapy (Aff.). (D) Liver glycogen content for samples from (C). (E) Vector quantification for postmortem tissue samples. Limit of detection was 0.1.
G6Pase activity in liver was stable or increased for individual dogs that were biopsied more than once (Fig. 6C). All but one dog had G6Pase activity increased above the range for untreated affected dog liver on the final biopsy. The G6Pase activity for each of two female dogs, L and W, was increased to approximately 40% of the normal level in the final biopsy. Consistent with G6Pase expression in the liver, glycogen content was markedly reduced in all liver biopsies following administration of any of the AAV pseudotyped vectors (Fig. 6D). Glycogen content remained low >12 months following vector administration for Dog L.
Vector genomes were rapidly reduced in the weeks following vector administration as demonstrated by vector genome quantification. Vector genomes were markedly higher for both liver and kidney of Dog Ro, which received AAV2/8-G6Pase approximately 18 hr earlier in an attempt to treat acute hypoglycemia, in comparison with Dogs F and Du, which received AAV2/9-G6Pase only as neonates (Fig. 6E).
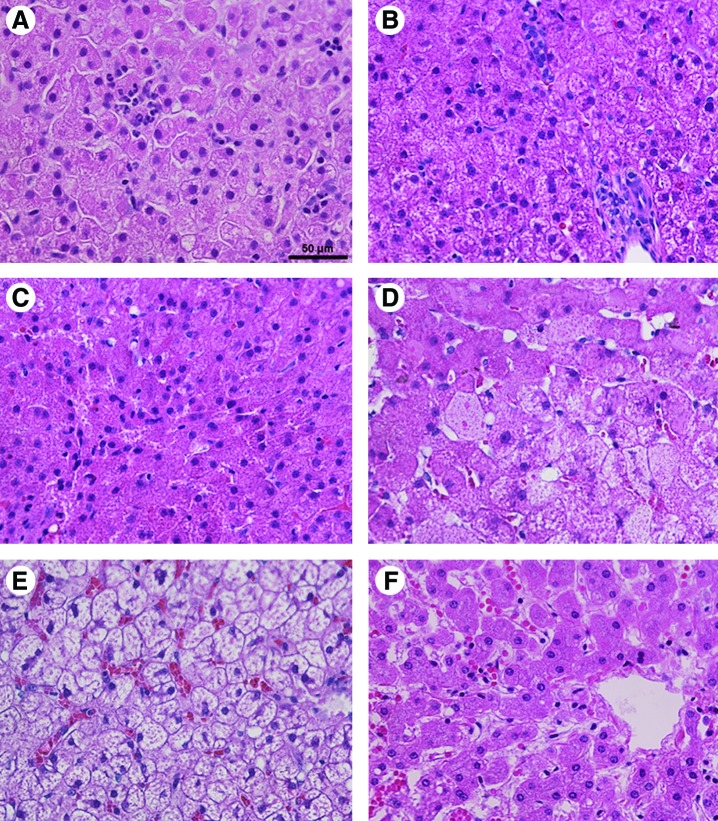
Reduced glycogen content was confirmed by histological examination of liver, which revealed decreased hepatocyte vacuolization following AAV vector administration (Fig. 7A–D), in comparison with the non–vector-treated GSD-Ia liver (Fig. 7E). Slightly less vacuolization was present at ages 6 weeks following initial AAV2/9 vector administration, in comparison with older GSD dogs at 6 months of age (Fig. 7B) and 16 months of age (Fig. 7C). The appearance of liver for Dog R at 41 months of age (Fig. 7D) was intermediate between that for untreated GSD-Ia liver (Fig. 7E) and that for normal liver (Fig. 7F). Lymphocytic infiltrates were not detected by histological examination of liver biopsies at 6 weeks (Fig. 7A) to 5 months (Fig. 7D) following vector administration, although the presence of infiltrates at earlier timepoints could not be excluded.
FIG. 7.
Histology of GSD-Ia following AAV-G6Pase administration. Liver biopsies obtained at the indicated ages. Hematoxylin and eosin staining shown. Original magnification, ×400, and scale bar indicates 50 μm. (A) Dog Ro at 6 weeks of age, following AAV2/9 vector administration at 2 days of age. (B) Dog T at 6 months of age, 6 weeks following AAV2/1 vector administration. (C) Dog W at 16 months of age, 1 month following AAV2/8 vector administration. (D) Dog R at 46 months of age, 3 months following AAV2/7 vector administration. (E) GSD-Ia dog following dietary therapy alone at 2 years of age. (F) Normal dog liver. Color images available online at www.liebertonline.com/hum
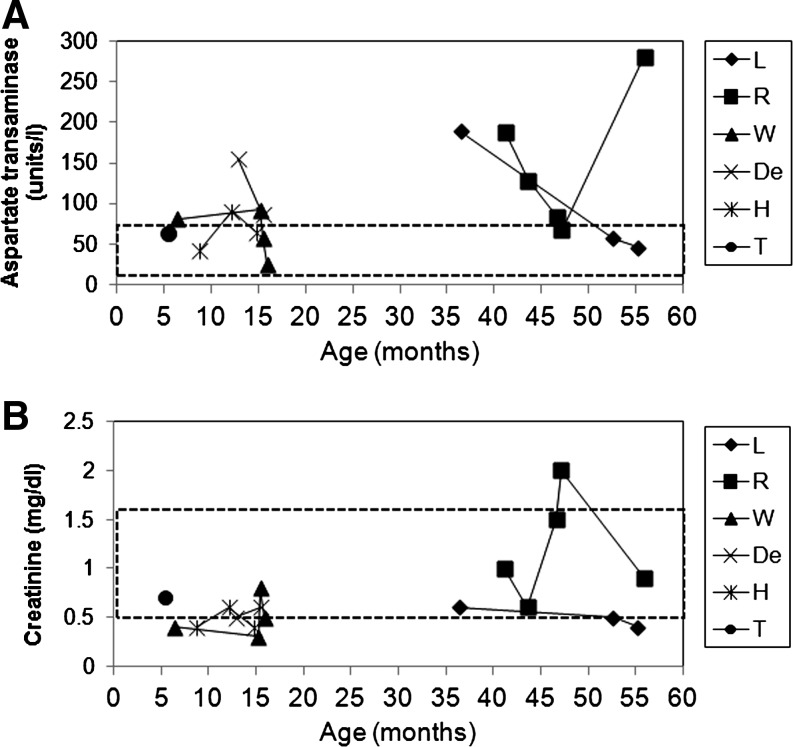
T-cell responses against transduced hepatocytes have been associated with elevated liver transaminases, reflecting liver damage, in a clinical trial of a liver-targeted AAV2 vector (Manno et al., 2006). The onset of elevated transaminases at 4 weeks correlated with the appearance of T-cell responses that eliminated transgene expression in that clinical trial. Transaminases were monitored during episodes of recurrent hypoglycemia in dogs with GSD-Ia, and aspartate aminotransferase (AST) was intermittently elevated (Fig. 8A). However, AST was not elevated in Dog T at 5.5 months of age, which was 4 weeks following AAV2/1 vector administration. The slightly elevated tranaminase levels observed in other dogs were consistent with the mild liver toxicity observed in dogs with GSD-Ia (Crane et al., 2011). Renal failure is associated with elevated serum creatinine in dogs with GSD-Ia (Crane et al., 2011). Serum creatinine was elevated only at one instance in Dog R, and it normalized after fluid and dietary therapy were administered (Fig. 8B).
FIG. 8.
Monitoring for liver damage or renal failure following AAV-G6Pase administration. (A) Serum aspartate aminotransferase was quantified during episodes of recurrent hypoglycemia in dogs with GSD-Ia at the indicated ages. (B) Serum creatinine. Normal ranges indicated (dashed boxes).
Discussion
The efficacy of AAV-G6Pase has now been demonstrated in dogs with GSD-Ia both by prolonged survival and the prevention of hypoglycemia during fasting (Koeberl et al., 2008; Weinstein et al., 2010). The survival of these dogs with GSD-Ia for 12 to 60 months (and counting) demonstrated the efficacy because early mortality usually cannot be prevented by any other treatment than efficacious gene therapy in this disorder (Koeberl et al., 2008; Weinstein et al., 2010). Furthermore, both AAV2/8 and AAV2/9 vectors prevented hypoglycemia for >8 hr of fasting, although the AAV2/8 vector described previously (Koeberl et al., 2008) was effective for many months longer than the current AAV2/9 vector. AAV vector administration was associated with normalization of glycogen content in the liver (Fig. 6), demonstrating biochemical correction. Both AAV2/8- and AAV2/9-G6Pase prolonged survival in dogs with GSD-Ia, in comparison with dietary therapy alone. The initial three dogs studied were treated with an AAV vector of a new serotype that prevented recurrent hypoglycemia from 1 to 2.5 years following neonatal AAV2/8 (Dogs L and R) or AAV2/9 (Dog W) vector administration; thereafter, puppies treated with neonatal administration of the AAV2/9 vector developed symptoms from hypoglycemia at approximately 2 months of age and three affected puppies succumbed to acute complications of GSD-Ia (Dogs F, Du, and Ro). Subsequently, three consecutive puppies were sustained by readministration of AAV-G6Pase early in life, and the benefit of readministering an AAV vector of a new pseudotype was emphasized by the recurrence and subsequent resolution of hypoglycemia. The reversal of symptoms following AAV vector readministration occurred quickly, within 1 to 2 days, consistent with the rapid onset of biochemical correction observed within 72 hr of AAV2/8-G6Pase administration observed in mice with GSD-Ia (Koeberl et al., 2008). Changing pseudotypes has restored the efficacy of a readministered therapeutic vector in dogs with a genetic disease (Wang et al., 2005), endorsing the strategy employed here to treat dogs with GSD-Ia following reoccurrence of hypoglycemia.
Early mortality associated with hypoglycemia in patients with GSD-Ia can be managed by intensive nutritional therapy; therefore, long-term complications that might be prevented by transduction of the liver and kidney include growth retardation, proteinuria occasionally progressing to renal failure, osteopenia, formation of hepatic adenomas, and hepatocellular carcinoma (Koeberl et al., 2009). Hepatorenal correction was demonstrated with AAV2/9-G6Pase in G6pase−/− mice (Luo et al., 2011) and 40%–50% of normal G6Pase activity was sufficient to reduced liver glycogen content by 90% and to arrest the progression of renal involvement. Biochemical correction has now been achieved in the liver of GSD-Ia dogs with multiple pseudotypes of AAV-G6Pase as demonstrated by the marked reduction of glycogen storage in the liver. Nonetheless, demonstrating the prevention of long-term complications in animal models remains challenging due to the limited time and numbers available. Thus, the most relevant endpoint in preclinical experiments involving gene therapy in GSD-Ia is the prevention of hypoglycemia.
Dietary glucose requirements for patients with GSD-Ia decline in adulthood (Chen, 2001). Indeed, fasting of dogs originally treated with AAV2/8-G6Pase demonstrated greater resistance to hypoglycemia after 1 year of age without re-treatment in the current study. The biochemical correction of the liver and prevention of renal failure with AAV-G6Pase in dogs with GSD-Ia supports further development of gene therapy for GSD-Ia, especially because long-term complications cannot be prevented with dietary therapy in human patients (Chen, 2001).
One of the limitations associated with gene therapy is a waning effect of transgene expression over time after initial vector injection, which has been encountered previously with an AAV2/8 vector in canine GSD-Ia by another group (Weinstein et al., 2010). The loss of transgene expression explains the early demise of three of seven puppies with GSD-Ia following neonatal treatment with AAV2/9-G6Pase. Survival was subsequently maintained by adopting an alternative strategy, which consisted of reflexive administration of a new AAV-G6Pase pseudotype following onset of hypoglycemia.
The loss of transgene expression seems to be accelerated in young GSD-Ia animals following administration of AAV vectors, even in comparison with the rapid loss of AAV vectors previously demonstrated in young normal animals (Herzog et al., 2011; Wang et al., 2011a). When an AAV2/8 vector was administered to infant rhesus monkeys, transduction and vector genomes in the liver decreased approximately 10-fold in the liver with 1 month (Wang et al., 2011a). In GSD-Ia dog liver samples we estimate that vector genomes rapidly declined by approximately two orders of magnitude to greater than one copy per cell in the liver of GSD-Ia puppies treated with AAV2/9-G6Pase within 2 months, even in absence of cellular or humoral immune responses (Fig. 6E). In G6pase−/− mice, AAV2/8 vector genomes decreased 10-fold between 3 weeks and 6 months in the liver , and even at 3 weeks of age the vector copy number was lower than expected at only three copies per nucleus (Koeberl et al., 2006). We recently demonstrated the gradual loss of vector genomes from the liver of mice with GSD-Ia between 6 and 12 months following administration of AAV2/9-G6Pase (Luo et al., 2011), thereby confirming ongoing loss of episomal vector genomes in the liver of adult animals with GSD-Ia. The presence of elevated AST in absence of inflammation indicated that hepatocyte death related to glycogen accumulation might drive the loss of vector genomes in the GSD-Ia dog liver (Fig. 8). However, the normal concentration of AST in Dog T's serum and the lack of lymphocytic infiltrates in its liver did not support ongoing liver inflammation as the cause for the low efficacy following later AAV2/1 vector administration. While we have not demonstrated liver inflammation in vector-treated dogs with GSD-Ia, elevated AST in association with recurrent hypoglycemia indicated that ongoing liver damage related to glycogen storage might underlie the loss of efficacy from AAV vectors in GSD-Ia. Humans with GSD-Ia develop mildly elevated transaminase levels in the setting of ongoing hypoglycemia (Chen, 2001), supporting the possibility that elevated transaminase levels reflected a lack of complete biochemical correction in these dogs with GSD-Ia. Additionally, it is possible that increased apoptosis, which was detected in the G6pase−/− mouse liver, drives the loss of vector genomes due to accelerated liver cell death in GSD-Ia (Sun et al. 2009).
The correction of G6Pase deficiency in liver alone following liver transplantation has been insufficient to prevent the progression of renal complications in human patients with GSD-Ia (Matern et al., 1999). Renal transduction was evident following AAV2/9-G6Pase administration, as demonstrated by the normalization of glycogen content in the kidney. Renal involvement has been described previously in canine GSD-Ia in the form of focal segmental glomerulosclerosis (Kishnani et al., 2001) and acute tubular necrosis (Weinstein et al., 2010). The latter abnormality was associated with early demise in two puppies, despite the reversal of glycogen storage in the kidney. Acute tubular necrosis was most likely a secondary abnormality associated with mortality, because none of the dogs developed renal failure.
Anti-capsid antibody formation has been implicated in prior studies as a predictor of low transduction in the liver. Transduction of liver with an AAV2/8 vector was markedly reduced in rhesus macaques with pre-existing anti-AAV8 antibodies, in comparison to seronegative macaques (Wang et al., 2011b). Similarly, transduction of liver by an AAV vector of a particular pseudotype was impaired following immunization with another AAV vector of the same pseudotype (Gao et al., 2002). It is possible that low titer anti-capsid antibodies interfered with the efficacy from the AAV2/9 vector in puppies with GSD-Ia, although anti-AAV2/9 was detected at a titer of >1:20 only in Dog T prior to AAV2/9 vector administration. Unexpectedly, carrier females had formed anti-AAV9 vectors, either from immunization, exposure to wild-type AAV, or from exposure to AAV2/9 vector–treated puppies (Fig. 5). Maternal antibodies against AAV9 were identified among the carrier females following exposure to AAV2/9 vector–treated puppies, although anti-AAV9 antibodies were absent from a naïve carrier female never exposed to a vector-treated puppy (Fig. 5E). Maternal antibodies are transferred transplacentally and persist in the blood of human infants for several months (Leuridan and Van Damme, 2007; Holmlund et al., 2011). Passively acquired maternal antibodies might be expected to interfere with liver transduction in puppies with GSD-Ia by AAV vectors. Dog T developed recurrent hypoglycemia despite repeat administration of the AAV vector, and it had high-level maternally acquired anti-AAV9 antibodies that might have interfered with efficacy for the initial AAV2/9 vector treatment.
Rescue administration of AAV-G6Pase reversed clinical symptoms of GSD-Ia and sustained long-term survival in six of nine dogs with GSD-Ia. The success of AAV-G6Pase at prolonging survival in dogs with GSD-Ia to the greatest extent to date, up to 60 months, justifies additional preclinical experiments in the anticipation that gene therapy will be translated to clinical trials. The acute risks of hypoglycemia in GSD-Ia justify the development of new therapy such as gene therapy. The favorable safety profile of AAV vectors (Snyder and Francis, 2005) and current degree of efficacy with regard to prevention of hypoglycemia indicate that AAV vector-mediated gene therapy might have an acceptable risk/benefit ratio in GSD-Ia and should be considered for further preclinical development.
Acknowledgments
This project was supported by the Children's Fund for GSD Research, Children's Miracle Network, and the Association for Glycogen Storage Disease. We wish to acknowledge inspiration and support from Dr. Emory and Mrs. Mary Chapman and their son Christopher, and from Dr. and Mrs. John Kelly. DDK, FS, XY, and AB were supported by the Children's Fund for GSD Research. We deeply appreciate the dedication shown by Duke DLAR staff, when caring for puppies with GSD-Ia. We appreciate the contributions of Hiruni Amarasekara and Michael Maranzano (extremely dedicated student laboratory assistants), Sean Prater (medical student), and Dr. Suhrad Banugaria (postdoctoral fellow). We wish to thank Ms. Songtao Li for excellent technical support. A debt of gratitude is extended to members of the NCSU GSD Puppy Team, CVM-LAR staff, and CVM-CPL staff, all of whom played a vital role in the success and survival of the GSD colony.
Author Disclosure Statement
Dr. Koeberl has a consulting agreement with Glygenix, a company developing gene therapy for GSD-Ia.
References
- Beaty R.M. Jackson M. Peterson D., et al. Delivery of glucose-6-phosphatase in a canine model for glycogen storage disease, type Ia, with adeno-associated virus (AAV) vectors. Gene Ther. 2002;9:1015–1022. doi: 10.1038/sj.gt.3301728. [DOI] [PubMed] [Google Scholar]
- Brix A.E. Howerth E.W. McConkie-Rosell A., et al. Glycogen storage disease type Ia in two littermate Maltese puppies. Vet. Pathol. 1995;32:460–465. doi: 10.1177/030098589503200502. [DOI] [PubMed] [Google Scholar]
- Chen Y.T. Glycogen storage diseases. In: Scriver C.R., editor; Beaudet A.L., editor; Sly W.S., editor; Valle D., editor. The Metabolic and Molecular Bases of Inherited Disease. McGraw-Hill; New York: 2001. pp. 1521–1551. [Google Scholar]
- Chen Y.T. Cornblath M. Sidbury J.B. Cornstarch therapy in type I glycogen-storage disease. N. Engl. J. Med. 1984;310:171–175. doi: 10.1056/NEJM198401193100306. [DOI] [PubMed] [Google Scholar]
- Chen Y.T. Bazzarre C.H. Lee M.M., et al. Type I glycogen storage disease: nine years of management with cornstarch. Eur. J. Pediatr. 1993;152(Suppl. 1):S56–S59. doi: 10.1007/BF02072090. [DOI] [PubMed] [Google Scholar]
- Crane B. Luo X. Demaster A., et al. Rescue administration of a helper-dependent adenovirus vector with long-term efficacy in dogs with glycogen storage disease type Ia. Gene. Ther. 2011 doi: 10.1038/gt.2011.86. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Gao G.P. Alvira M.R. Wang L., et al. Novel adeno-associated viruses from rhesus monkeys as vectors for human gene therapy. Proc. Natl. Acad. Sci. U. S. A. 2002;99:11854–11859. doi: 10.1073/pnas.182412299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halbert C.L. Standaert T.A. Aitken M.L., et al. Transduction by adeno-associated virus vectors in the rabbit airway: efficiency, persistence, and readministration. Virology. 1997;71:5932–5941. doi: 10.1128/jvi.71.8.5932-5941.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halbert C.L. Rutledge E.A. Allen J.M., et al. Repeat transduction in the mouse lung by using adeno-associated virus vectors with different serotypes. Virology. 2000;74:1524–1532. doi: 10.1128/jvi.74.3.1524-1532.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herzog R.W. Davidoff A.M. Markusic D.M. Nathwani A.C. AAV vector biology in primates: finding the missing link? Mol. Ther. 2011;19:1923–1924. doi: 10.1038/mt.2011.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmlund E. Nohynek H. Quiambao B., et al. Mother-infant vaccination with pneumococcal polysaccharide vaccine: persistence of maternal antibodies and responses of infants to vaccination. Vaccine. 2011 doi: 10.1016/j.vaccine.2011.04.068. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Keller K.M. Schutz M. Podskarbi T., et al. A new mutation of the glucose-6-phosphatase gene in a 4-year-old girl with oligosymptomatic glycogen storage disease type 1a. J. Pediatr. 1998;132:360–361. doi: 10.1016/s0022-3476(98)70463-9. [DOI] [PubMed] [Google Scholar]
- Kikuchi M. Hasegawa K. Handa I., et al. Chronic pancreatitis in a child with glycogen storage disease type 1. Eur. J. Pediatr. 1991;150:852–853. doi: 10.1007/BF01955007. [DOI] [PubMed] [Google Scholar]
- Kishnani P.S. Faulkner E. Vancamp S., et al. Canine model and genomic structural organization of glycogen storage disease type Ia (GSD Ia) Vet. Pathol. 2001;38:83–91. doi: 10.1354/vp.38-1-83. [DOI] [PubMed] [Google Scholar]
- Koeberl D.D. Sun B.D. Damodaran T.V., et al. Early, sustained efficacy of adeno-associated virus vector-mediated gene therapy in glycogen storage disease type Ia. Gene Ther. 2006;13:1281–1289. doi: 10.1038/sj.gt.3302774. [DOI] [PubMed] [Google Scholar]
- Koeberl D.D. Pinto C. Sun B., et al. AAV vector-mediated reversal of hypoglycemia in canine and murine glycogen storage disease type Ia. Mol. Ther. 2008;16:665–672. doi: 10.1038/mt.2008.15. [DOI] [PubMed] [Google Scholar]
- Koeberl D.D. Kishnani P.S. Bali D. Chen Y.T. Emerging therapies for glycogen storage disease type I. Trends Endocrinol. Metab. 2009;20:252–258. doi: 10.1016/j.tem.2009.02.003. [DOI] [PubMed] [Google Scholar]
- Leuridan E. Van Damme P. Passive transmission and persistence of naturally acquired or vaccine-induced maternal antibodies against measles in newborns. Vaccine. 2007;25:6296–6304. doi: 10.1016/j.vaccine.2007.06.020. [DOI] [PubMed] [Google Scholar]
- Luo X. Hall G. Li S., et al. Hepatorenal correction in murine glycogen storage disease type I with a double-stranded adeno-associated virus vector. Mol. Ther. 2011 doi: 10.1038/mt.2011.126. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manno C.S. Pierce G.F. Arruda V.R., et al. Successful transduction of liver in hemophilia by AAV-factor IX and limitations imposed by the host immune response. Nat. Med. 2006;12:342–347. doi: 10.1038/nm1358. [DOI] [PubMed] [Google Scholar]
- Matern D. Starzl T.E. Arnaout W., et al. Liver transplantation for glycogen storage disease types I, I.I.I, and IV. Eur. J. Pediatr. 1999;158(Suppl. 2):S43–S48. doi: 10.1007/pl00014320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder R.O. Francis J. Adeno-associated viral vectors for clinical gene transfer studies. Curr. Gene Ther. 2005;5:311–321. doi: 10.2174/1566523054065066. [DOI] [PubMed] [Google Scholar]
- Sun B. Li S. Yang L., et al. Activation of glycolysis and apoptosis in glycogen storage disease type Ia. Mol. Genet. Metab. 2009;97:267–271. doi: 10.1016/j.ymgme.2009.04.003. [DOI] [PubMed] [Google Scholar]
- Sun M.S. Pan C.J. Shieh J.J., et al. Sustained hepatic and renal glucose-6-phosphatase expression corrects glycogen storage disease type Ia in mice. Hum. Mol. Genet. 2002;11:2155–2164. doi: 10.1093/hmg/11.18.2155. [DOI] [PubMed] [Google Scholar]
- Wang L. Calcedo R. Nichols T.C., et al. Sustained correction of disease in naive and AAV2-pretreated hemophilia B dogs: AAV2/8-mediated, liver-directed gene therapy. Blood. 2005;105:3079–3086. doi: 10.1182/blood-2004-10-3867. [DOI] [PubMed] [Google Scholar]
- Wang L. Bell P. Lin J., et al. AAV8-mediated hepatic gene transfer in infant rhesus monkeys (Macaca mulatta) Mol. Ther. 2011a;19:2012–2020. doi: 10.1038/mt.2011.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L. Calcedo R. Bell P., et al. Impact of pre-existing immunity on gene transfer to nonhuman primate liver with adeno-associated virus 8 vectors. Hum. Gene Ther. 2011b doi: 10.1089/hum.2011.031. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstein D.A. Correia C.E. Conlon T., et al. Adeno-associated virus-mediated correction of a canine model of glycogen storage disease type Ia. Hum Gene. Ther. 2010;21:903–910. doi: 10.1089/hum.2009.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfsdorf J.I. Crigler J.F., Jr. Effect of continuous glucose therapy begun in infancy on the long-term clinical course of patients with type I glycogen storage disease. J. Pediatr. Gastroenterol. Nutr. 1999;29:136–143. doi: 10.1097/00005176-199908000-00008. [DOI] [PubMed] [Google Scholar]
- Yiu W.H. Lee Y.M. Peng W.T., et al. Complete normalization of hepatic G6pc deficiency in murine glycogen storage disease type Ia using gene therapy. Mol. Ther. 2010;18:1076–1084. doi: 10.1038/mt.2010.64. [DOI] [PMC free article] [PubMed] [Google Scholar]