Abstract
Objective
Nevirapine is an important component of highly active antiretroviral therapy used in the treatment of human immunodeficiency virus infection. There is considerable variation in the pharmacokinetics of nevirapine and this variation can impact the efficacy and toxicity of nevirapine. While some of this variation can be attributed to environmental factors, the degree to which heritability influences nevirapine pharmacokinetics is unknown. This study aims to estimate how much variation in nevirapine pharmacokinetics is due to genetic factors and to investigate the contribution of selected polymorphisms to this variability.
Methods
Two doses of immediate-release nevirapine were administered to European (n=11) and African American (n=6) subjects recruited from the Research in Access to Care in the Homeless (REACH) cohort. A repeated drug administration (RDA) method was then used to determine the relative genetic contribution (rGC) to variability in nevirapine AUC0–6h. Nevirapine plasma levels were quantified using LC-MS/MS. Patients were also genotyped for selected polymorphisms in candidate genes that may influence nevirapine pharmacokinetics.
Results
A significant rGC for nevirapine AUC0–6h was found in Europeans (p = 0.02) and African Americans (p = 0.01). A trend towards higher nevirapine AUC0–6h for the CYP2B6 516TT (rs3745274; Q172H) genotype was observed in European Americans (p = 0.19).
Conclusions
This study demonstrates that there is a significant genetic component to variability in nevirapine pharmacokinetics. While genetic variants such as CYP2B6 polymorphisms attributed to some of this variation, these data suggest that there may be additional genetic factors that influence nevirapine pharmacokinetics.
Keywords: HIV, Nevirapine, Pharmacogenetics, Pharmacokinetics, CYP2B6
Introduction
The importance of understanding the role of genetics in variation in pharmacokinetics and pharmacodynamics has been recognized since the 1950s [1–3]. Twin studies have historically been used to determine the heritability of genetic diseases and traits; these studies have also been used to determine the heritability of pharmacodynamic and pharmacokinetic parameters [4]. While twin studies are a useful technique to determine genetic contributions to pharmacokinetic variation, it can be impractical to use twins in pharmacogenetic studies due to difficulty in recruitment and the need to expose them to drugs. A statistical technique that was specifically developed to address this issue is the repeated drug administration (RDA) method, which uses repeated administrations of a drug to the same individuals to compare the within-subject and between-subject variation in pharmacokinetic parameters [5]. This comparison can be used to quantify the relative genetic contribution to variations in pharmacokinetic parameters of a drug. While the RDA method is useful in determining whether pharmacokinetic or pharmacodynamic parameters of a drug have strong genetic components, it may vary with the route of administration, dose of drug or patient population [5]. Additionally, while one pharmacokinetic parameter for a given drug may have a strong relative genetic component, other parameters may be more highly influenced by environmental factors than by genetic factors [6]. Repeated drug administration has successfully been employed to characterize the genetic contribution to variability in pharmacokinetic parameters of several drugs, including erythromycin, midazolam and metformin [7,8]. However, the genetic contribution to pharmacokinetic parameter variability for many drugs is still unknown.
Nevirapine is a non-nucleoside reverse transcriptase inhibitor widely used as a component of antiretroviral therapy in the treatment of human immunodeficiency virus (HIV) [9]. Nevirapine exhibits considerable variability in its pharmacokinetic properties; however, only part of this variability can be explained by environmental factors and concomitant conditions [10]. Variation in nevirapine pharmacokinetics can lead to reduced efficacy, increased viral resistance and increased toxicities [11]. Nevirapine is metabolized to its primary metabolite 3-hydroxynevirapine by CYP2B6 [12]. The CYP2B6 516G>T and CYP2B6 983T>C variant alleles have a significant effect on nevirapine plasma levels and the CYP2B6 516T allele has also been associated with increased recovery of CD4+ T-cell populations in pediatric patients following initiation of nevirapine-containing antiretroviral therapy [13–15]. Additionally, ABCB1 3435C>T has been associated with protection against nevirapine-induced hepatotoxicity and increased nevirapine concentrations in cerebral spinal fluid [16,17]. Despite evidence that nevirapine pharmacokinetics are influenced by specific polymorphisms, there has not been a study conducted to quantify the relative genetic contribution to variability in nevirapine pharmacokinetics.
This study uses the repeated drug administration method to quantify the relative genetic contribution to variability in nevirapine pharmacokinetics. A significant relative genetic contribution to variation in nevirapine exposure was shown in two ethnic populations. The contribution of CYP2B6 516G>T and ABCB1 3435C>T to variability in nevirapine pharmacokinetics was also investigated.
Materials and Methods
Study Design and Subjects
Subjects were recruited from the Research in Access to Care in the Homeless (REACH) cohort as previously described [18]. Study participants are marginally housed HIV positive individuals living in San Francisco. Nineteen patients were recruited to participate in a pharmacokinetic study where subjects receiving 200 mg nevirapine twice daily consented to pharmacokinetic blood sampling. All subjects were on therapy at least four months and were concomitantly receiving two nucleoside reverse transcriptase inhibitors. Subjects were presumed to have reached steady state concentrations. Blood samples were drawn at 0, 1, 2, 3 and 6 hr post-dose. The time between the two measured doses varied from 13 days to 173 days. European American (n=11) and African American (n=6) patients were included in this study. Ethnicity was self reported and verified through genotyping of 112 ancestry informative markers and analysis using the STRUCTURE program [19–21]. The study was approved by the University of California Institutional Review Board and all subjects provided written informed consent prior to participation.
Nevirapine Quantification
Plasma was prepared from blood samples by centrifugation and stored at −80°C until analysis. Nevirapine was extracted using Oasis HLB SPE columns (Waters Corp., Milford, MA) and plasma concentrations were quantified by LC/MS/MS analysis as described by Mistri et. al [22]. Briefly, each 0.5 mL plasma aliquot was heated for 1.5 hrs at 56°C to inactivate HIV-1 virus and then spiked with 25 µl of 20 µM metaxolone (Toronto Research Chemicals, Toronto, Ontario) in methanol, which served as an internal standard. SPE columns were equilibrated with 1 mL methanol followed by 1 mL distilled water. Samples were then loaded on the column and washed with 1 mL of 2 mM ammonium acetate followed by 1 mL of water. Samples were eluted in 1 mL mobile phase (80:20 acetonitrile:water, 0.1% acetic acid) and a 5 µl aliquot was injected onto a 5 µm Hypersil BDS C18 column, 50 × 4.6 µm (Thermo Fisher Scientific, Waltham, MA). The flow rate into the API4000 mass spectrometer (AbSciex, Framingham, MA) was 0.2 mL/min and nevirapine retention time was 1.7 min. The parent ion (267.2 m/z, amu) and product ion (226.2 m/z, amu) were monitored at Q1 and Q3, respectively. Nevirapine standard curves were linear from 50–5000 ng/mL (r2 > 0.9). Assay accuracy was between 100.3% and 112.9% relative standard deviation. Assay precision ranged from 8.2 – 18.5% CV.
Genotyping
Genomic DNA was extracted from whole blood samples. Genotyping of polymorphisms of interest (CYP2B6 516G>T and ABCB1 3435C>T) was accomplished using the ABI Prism 7900HT Sequence Detection System (Applied Biosystems, Foster City, CA). TaqMan assays were used to genotype CYP2B6 516G>T (rs3745274, Assay ID: C___7817765_60) and ABCB1 3435C>T (rs1045642, Assay ID: C___7586657_20). Genotypes were called with ABI Sequence Detection System software (version 2.1; Applied Biosystems, Foster City, CA).
Calculation of Pharmacokinetic Parameters
Due to the long half-life of nevirapine (45 hrs), only AUC0–6h was calculated [23]. AUC0–6h was calculated for each dose administration using the trapezoidal rule.
Calculation of Relative Genetic Component
The genetic contribution to the variability in nevirapine AUC0–6h was assessed with a modified ANOVA formula for estimating the relative genetic component or rGC and 95% confidence intervals proposed by Kalow et.al. [24]:
rGC = (SDb2−SDw2)/SDb2
which can be rearranged as
rGC= 1−(1/F) where F = SDb2/SDw2
Upper and Lower Confidence intervals can be calculated as follows:
Lower 95% confidence interval = Fobserved/F0.025,b.d.f,w.d.f
Upper 95% confidence interval = Fobserved*F0.025,b.d.f,w.d.f
where rGC represents the estimated relative genetic component, SDb2 is the between subjects variation, SDw2 is the within subject variation, b.d.f. is the between subjects degrees of freedom, w.d.f. is the within subject degrees of freedom and F0.025 is the tabulated F statistic at the 2.5% significance level at the appropriate degrees of freedom. Due to well characterized differences in allele frequency and linkage disequilibrium patterns, European Americans (n=11 and African Americans (n=6) were analyzed separately in this study.
Statistical Methods
Statistical significance for genetic contribution to AUC0–6h variability was calculated using an F-test, α=0.05, to determine if the inter- and intra-individual variation was significantly different. One-way ANOVA, α=0.05, was used to determine significance for the effect of genetic polymorphisms on AUC0–6h values. All other calculations of p-values were obtained using two-sided t-tests or one-way ANOVA as appropriate [25]. Calculations were performed using R and Microsoft Excel [26]. All figures were produced in Prism Version 5.01 (GraphPad Software Inc., San Diego, CA).
Results
Ethnicity does not play a role in nevirapine AUC0–6h variability
Since there are well characterized differences in the genetic structure and linkage disequilibrium patterns in various ethnic populations, a statistical analysis to examine any overall differences in nevirapine AUC0–6h between African and European Americans was conducted. A total of 17 subjects were included in this study, 11 European Americans and six African Americans (Table 1). Median ages and concomitant medications were similar in the two ethnic groups, while the African American group had a higher proportion of females than the European American group.
Table 1.
Patient demographics and relative genetic contribution (rGC) to nevirapine AUC0–6h
| European Americans |
African Americans |
||
|---|---|---|---|
| Sample Size | n | 11 | 6 |
| Gender | Male (%) | 4 (36) | 1 (17) |
| Female (%) | 7 (64) | 5 (83) | |
| Age (years) | Median | 45 | 49 |
| Range | 29 – 57 | 33 – 74 | |
| Nevirapine AUC0–6h (mg/L*hr)1 | SDw2 | 2.39 | 5.34 |
| SDb2 | 24.9 | 54.7 | |
| Estimated Relative Genetic Component | rGC2 (95% CI) | 0.904 (0.64 – 0.97) | 0.902 (0.42 – 0.98) |
| F | 10.4 | 10.2 | |
| p | 0.02 | 0.01 |
SDw2 is within individual variation and SDb2 is within subjects variation.
Estimated relative genetic component
Analysis of nevirapine plasma concentrations indicated very little intrasubject variability in concentrations during the six hours following drug administration, consistent with the long terminal half-life of this drug (see Figures 1 and 2, Supplemental Digital Content Figure 1). In contrast, there is considerable variation in nevirapine concentrations between individuals; three individuals in the African American and two in the European American groups never reach plasma concentrations above the minimum effective concentration (MEC) for nevirapine of 3000 µg/L [27]. Average AUC0–6h did not differ between the two visits, although there was significant interpatient variability in these values (Table 1). For example, the mean AUC0–6h was 22.5 mg nevirapine/L*hr (SEM = 3.81 mg nevirapine/L*hr) and 18.3 mg nevirapine/L*hr (SEM = 2.69 mg nevirapine/L*hr) for European and African Americans, respectively. There was not a significant difference in AUC0–6h between the two populations (t-test, p = 0.45).
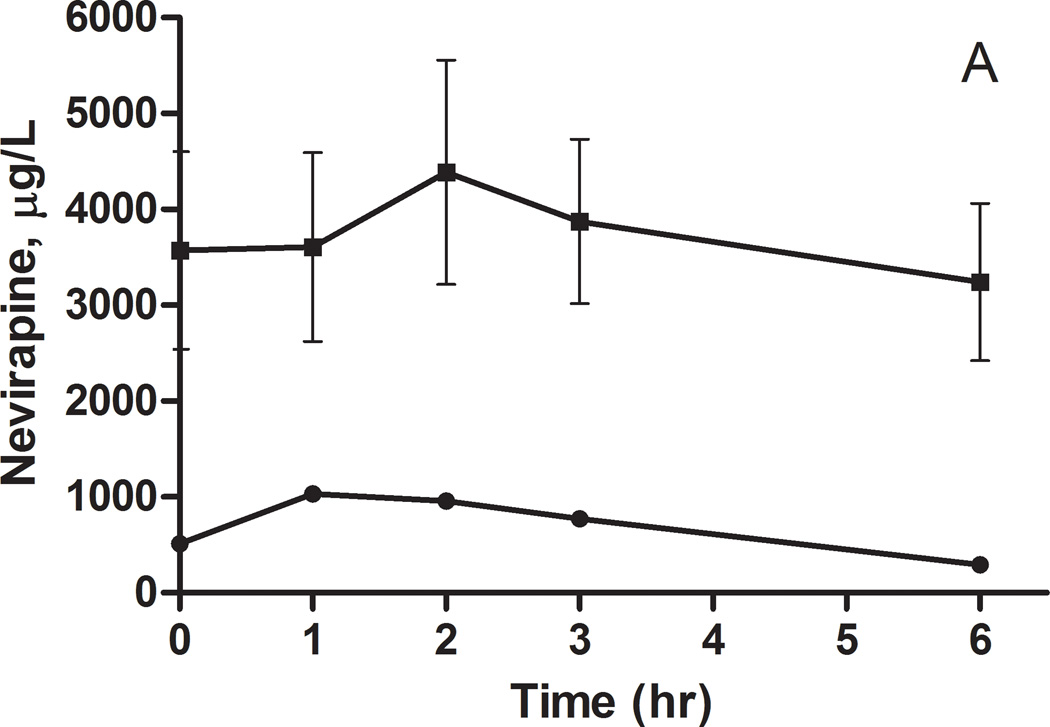
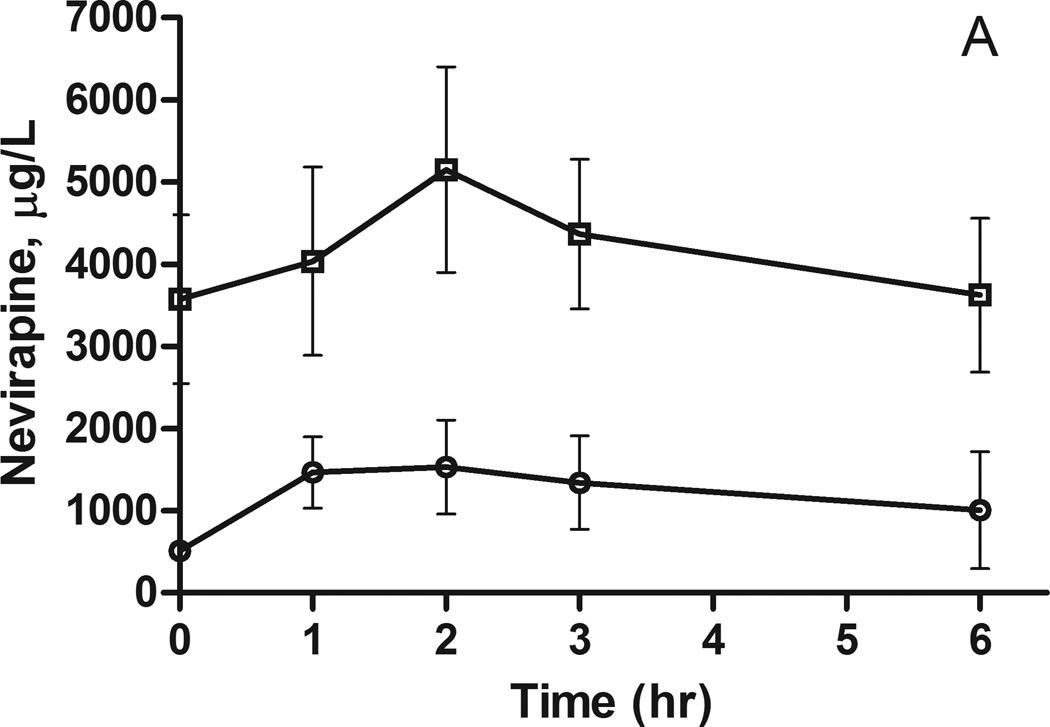
Figure 1.
Nevirapine plasma concentrations following a 200 mg dose to A) African Americans and B) European Americans. The concentrations (mean ± SEM) are stratified by CYP2B6 516G>T genotype: circles GG, squares GT and triangles TT.
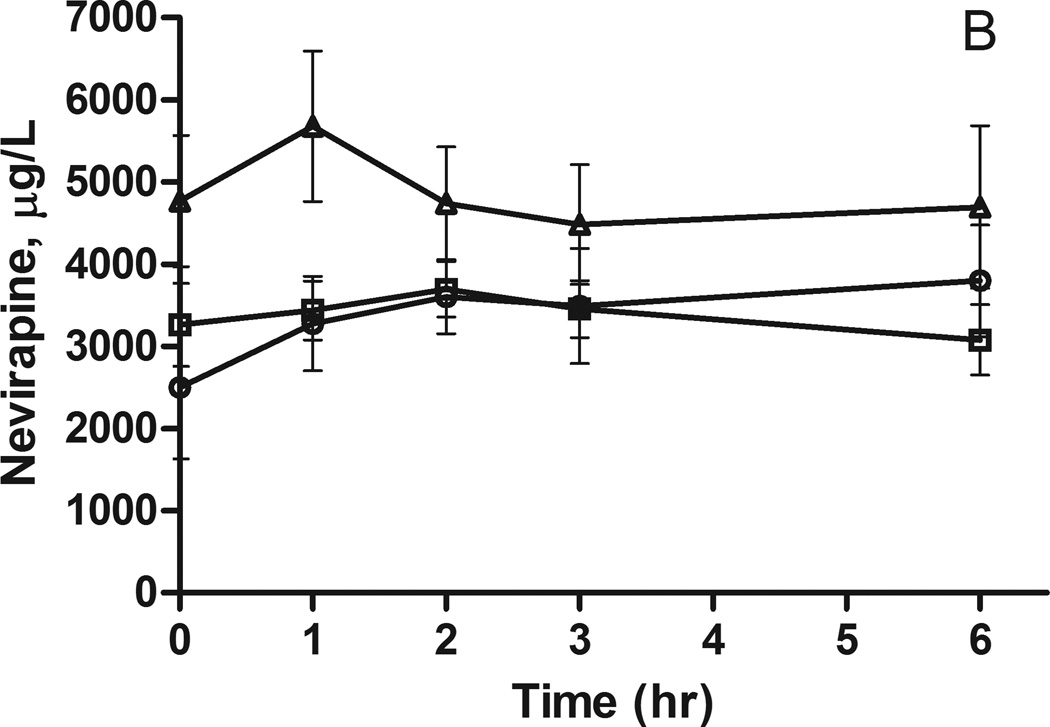
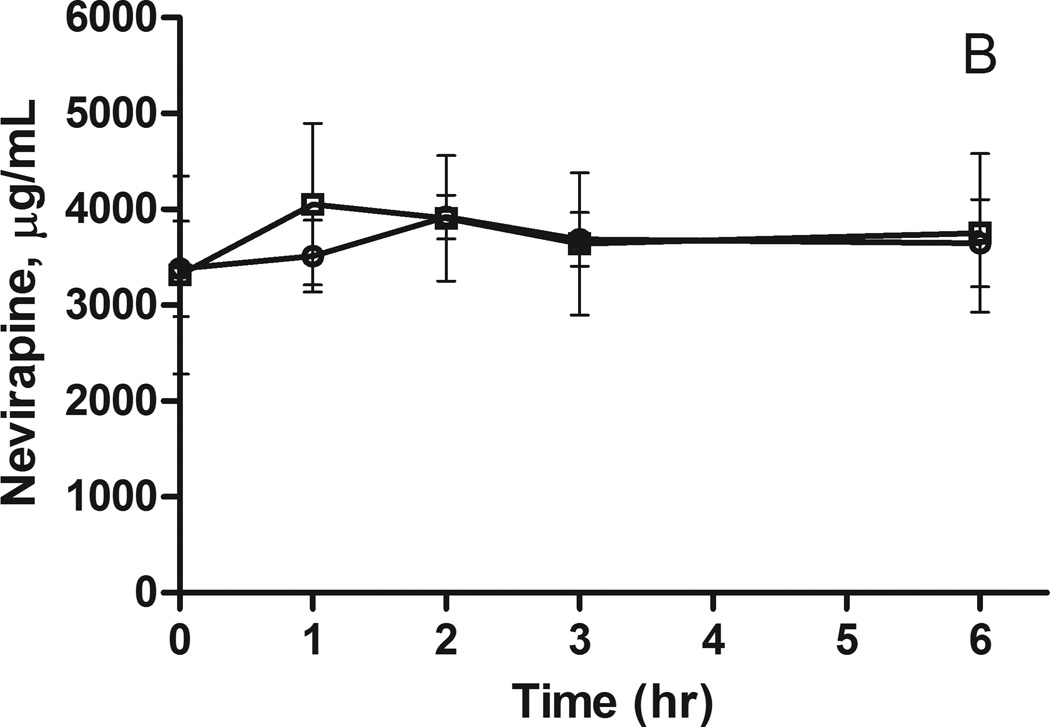
Figure 2.
Nevirapine plasma concentrations following a 200 mg dose to A) African Americans and B) European Americans. The concentrations (mean ± SEM) are stratified by ABCB1 3435C>T genotype: circles CC and squares CT.
Age and sex do not play a role in the variability of nevirapine AUC0–6h
To insure further analyses were not confounded by demographic factors, the effect of age and sex on nevirapine AUC0–6h was examined by linear regression and t-tests, respectively. Age had no effect on nevirapine AUC0–6h with an r2 of 0.04. Males tended to have slightly lower AUC0–6h (16.2 mg nevirapine/L*hr, SEM = 37.0 mg nevirapine/L*hr) than females (23.0 mg nevirapine/L*hr, SEM=24.2 mg nevirapine/L*hr), however this difference was not statistically significant (p=0.14).
There is a significant genetic contribution to variation in nevirapine AUC0–6h
The relative genetic contribution to nevirapine pharmacokinetics was calculated using the RDA method described previously [5,7]. The between subject (SDb2) variation in AUC0–6h was about 10-fold greater than the within subject variation (SDw2) in both ethnic groups (Table 2). The rGC calculated for the European Americans and African Americans was 0.902 (95% confidence intervals 0.64–0.97) and 0.904 (95% confidence intervals 0.42–0.98), respectively. F-tests indicate there is a significant genetic contribution to the variability in AUC0–6h in both Europeans (p = 0.02) and African Americans (p = 0.01).
Table 2.
The effect of ethnicity and genotype on nevirapine exposure
| Ethnicity | n | Nevirapine AUC0–6h (mg/L*h)1 | p | |
|---|---|---|---|---|
| African American | 6 | 18.3 ± 3.81 | 0.45 | |
| European American | 12 | 22.5 ± 2.69 | ||
| CYP2B6 516G>T | African Americans | |||
| GG | 1 | 4.23 | ND | |
| GT | 5 | 21.2 ± 5.63 | ||
| TT | 0 | - | ||
| European Americans | ||||
| GG | 4 | 20.5 ± 3.16 | 0.19 | |
| GT | 5 | 20.3 ± 2.82 | ||
| TT | 3 | 28.8 ± 3.64 | ||
| ABCB1 3435C>T | African Americans | |||
| CC | 2 | 7.31 ± 7.97 | 0.17 | |
| CT | 4 | 23.8 ± 12.8 | ||
| European Americans | ||||
| CC | 6 | 22.0 ± 3.03 | 0.96 | |
| CT | 5 | 22.2 ± 3.32 | ||
Mean ± SEM
CYP2B6 516G>T may influence nevirapine AUC0–6h
Considering the evidence for a significant genetic contribution to the variability in nevirapine exposure, polymorphisms in candidate genes implicated in the metabolism and transport of nevirapine were tested for association with nevirapine pharmacokinetics. In African Americans, there is a trend for increased plasma nevirapine levels in individuals carrying the CYP2B6 516G>T allele or the ABCB1 3435C>T allele (Figures 1A and 2A); however, the sample sizes are too small for formal statistical analysis (Table 2). A similar trend was observed for the CYP2B6 516G>T allele in European Americans, but these differences did not reach statistical significance (Figure 1B and Table 2). There was no indication of an association between the ABCB1 3435C>T polymorphism and nevirapine pharmacokinetics in European Americans (Figure 2B and Table 2).
Discussion
While there have been many candidate gene studies on nevirapine pharmacokinetics, this is the first study to determine the overall relative genetic influence on nevirapine pharmacokinetics. A significant relative genetic contribution to the variability in nevirapine pharmacokinetics was demonstrated in European and African Americans. This supports previous findings that have implicated polymorphisms in drug metabolism and transport genes in nevirapine pharmacokinetic variability and toxicity [14–16]. A trend consistent with previous studies of elevated plasma concentrations in subjects homozygous for the CYP2B6 516G>T allele was also observed [13,15].
Variability in nevirapine pharmacokinetics and toxicity has been observed since its approval for the treatment of HIV. Many candidate gene studies have confirmed that a portion of pharmacokinetic variability is due to polymorphisms in CYP2B6 [14,28,29]. However, the variation in pharmacokinetics due to genetic versus environmental factors has never been examined. The current study demonstrates that there is a significant genetic component to nevirapine pharmacokinetics in African and European Americans. While the population examined here is small, one advantage of the RDA method is the ability to use small populations to estimate relative genetic components of drugs [6]. In our European population, we have a reasonable number of subjects to estimate a 95% lower confidence limit of ~0.65 for an rGC of 0.9 [6]. This suggests that interindividual variation in nevirapine drug levels could be reduced through knowledge of a patient’s genetic background. The importance of this is reflected in the observation that several patients did not reach the minimum effective concentration of nevirapine, which could lead to decreased efficacy against HIV and increased viral resistance to nevirapine. The RDA method has been successfully employed to identify drugs whose renal clearance has a strong genetic component and could also be used to identify antiretroviral drugs that are good candidates for pharmacogenomics research [8].
To further investigate the influence of genetics on nevirapine pharmacokinetics, two candidate polymorphisms were selected for study. A trend was observed towards elevated AUC0–6h of nevirapine in both European and African Americans homozygous for the CYP2B6 516G>T polymorphism. This polymorphism has been associated with a slight decrease in hepatic protein expression and function, therefore increases in AUC0–6 are expected [30]. While the results in European Americans did not reach statistical significance, the analysis was limited by a small sample size and may have been confounded by unidentified environmental factors. The trend observed is consistent with other published work, which supports the need for a larger study population [14,15,31]. No association of ABCB1 3435C>T with nevirapine exposure was observed in our study. The effect of this polymorphism on nevirapine pharmacokinetics remains controversial, with many studies not showing an effect on nevirapine plasma pharmacokinetics [13–15,29,32]. AUC0–6h may not be the most appropriate pharmacokinetic parameter to observe the effects of these polymorphisms; however, due to the long half-life of nevirapine, it was not possible to calculate other pharmacokinetic parameters such as half-life or oral clearance.
The current study demonstrates that there is a significant relative genetic component to nevirapine pharmacokinetics. While CYP2B6 polymorphisms have been attributed to some of this variation [14,15,31], this study suggests that there may be additional genetic factors that influence nevirapine pharmacokinetics. These data support additional research to discover novel genetic factors influencing nevirapine variability. Furthermore, the RDA method could also be used to study endpoints of antiretroviral drugs other than pharmacokinetic parameters, including pharmacodynamic and metabolomic endpoints [33]. Additional knowledge of genetic factors that affect nevirapine pharmacokinetics may help increase the efficacy of nevirapine in the treatment of HIV and lead to less viral resistance.
Supplementary Material
Acknowledgments
Funding: This work was funded by NIH grants GM61390 and MH54907. Janine Micheli was supported by NIH T32 GM007175.
Footnotes
Conflicts of interest: None declared
References
- 1.Motulsky AG. Drug Reactions, Enzymes and Biochemical Genetics. Council on Drugs. 1957;165:835–837. doi: 10.1001/jama.1957.72980250010016. [DOI] [PubMed] [Google Scholar]
- 2.Carson PE, Flanagan CL, Ickes CE, Alving AS, Relationships S. Enzymatic Deficiency in Primaquine-Sensitive Erythrocytes. Science. 1956;124:484–485. doi: 10.1126/science.124.3220.484-a. [DOI] [PubMed] [Google Scholar]
- 3.Kalow W. GENETIC FACTORS IN RELATION TO DRUGS. Annual Review of Pharmacology. 1964;5:9–26. doi: 10.1146/annurev.pa.05.040165.000301. [DOI] [PubMed] [Google Scholar]
- 4.Wesell ES. Pharmacogenetic Perspectives Gained From Twin and Family Studies. Pharmac Ther. 1989;41:535–552. doi: 10.1016/0163-7258(89)90130-7. [DOI] [PubMed] [Google Scholar]
- 5.Kalow W, Endrenyi L, Tang B. Repeat administration of drugs as a means to assess the genetic component in pharmacological variability. Pharmacology. 1999;58:281–284. doi: 10.1159/000028292. [DOI] [PubMed] [Google Scholar]
- 6.Ozdemir V, Kalow W, Tothfalusi L, Bertilsson L, Endrenyi L, Graham JE. Multigenic Control of Drug Response and Regulatory Decision-Making in Pharmacogenomics: The Need for an Upper-Bound Estimate of Genetic Contributions. Current Pharmacogenomics. 2005;3:53–71. [Google Scholar]
- 7.Ozdemir V, Kalow W, Tang BK, Paterson aD, Walker SE, Endrenyi L, et al. Evaluation of the genetic component of variability in CYP3A4 activity: a repeated drug administration method. Pharmacogenetics. 2000;10:373–388. doi: 10.1097/00008571-200007000-00001. [DOI] [PubMed] [Google Scholar]
- 8.Leabman MK, Giacomini KM. Estimating the contribution of genes and environment to variation in renal drug clearance. Pharmacogenetics. 2003;13:581–584. doi: 10.1097/00008571-200309000-00007. [DOI] [PubMed] [Google Scholar]
- 9.Department of Health and Human Services. Guidelines for the use of antiretroviral agents in HIV-infected adults and adolescents. 2009:1–161. [Google Scholar]
- 10.Gandhi M, Benet LZ, Bacchetti P, Kalinowski A, Anastos K, Wolfe AR, et al. Nonnucleoside reverse transcriptase inhibitor pharmacokinetics in a large unselected cohort of HIV-infected women. Journal of Acquired Immune Deficiency Syndromes (1999) 2009;50:482–491. doi: 10.1097/qai.0b013e31819c3376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cooper CL, Van Heeswijk RPG. Once-daily nevirapine dosing: a pharmacokinetics, efficacy and safety review. HIV Medicine. 2007;8:1–7. doi: 10.1111/j.1468-1293.2007.00426.x. [DOI] [PubMed] [Google Scholar]
- 12.Riska P, Lamson M, Macgregor T, Sabo J, Hattox S, Pav J, et al. DISPOSITION AND BIOTRANSFORMATION OF THE ANTIRETROVIRAL DRUG NEVIRAPINE IN HUMANS ABSTRACT. Pharmacology. 1999;27 [PubMed] [Google Scholar]
- 13.Mahungu T, Smith C, Turner F, Egan D, Youle M, Johnson M, et al. Cytochrome P450 2B6 516G-->T is associated with plasma concentrations of nevirapine at both 200 mg twice daily and 400 mg once daily in an ethnically diverse population. HIV Medicine. 2009;10:310–317. doi: 10.1111/j.1468-1293.2008.00689.x. [DOI] [PubMed] [Google Scholar]
- 14.Penzak SR, Kabuye G, Mugyenyi P, Mbamanya F, Natarajan V, Alfaro RM, et al. Cytochrome P450 2B6 (CYP2B6) G516T influences nevirapine plasma concentrations in HIV-infected patients in Uganda. HIV Medicine. 2007;8:86–91. doi: 10.1111/j.1468-1293.2007.00432.x. [DOI] [PubMed] [Google Scholar]
- 15.Haas DWW, Gebretsadik T, Mayo G, Menon UNN, Acosta EPP, Shintani A, et al. Associations between CYP2B6 polymorphisms and pharmacokinetics after a single dose of nevirapine or efavirenz in African americans. The Journal of Infectious Diseases. 2009;199:872–880. doi: 10.1086/597125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ciccacci C, Borgiani P, Ceffa S, Sirianni E, Marazzi MC, Altan AMD, et al. Nevirapine-induced hepatotoxicity and pharmacogenetics: a retrospective study in a population from Mozambique. Pharmacogenomics. 2007;11:23–31. doi: 10.2217/pgs.09.142. [DOI] [PubMed] [Google Scholar]
- 17.Varatharajan L, Thomas Sa. The transport of anti-HIV drugs across blood-CNS interfaces: summary of current knowledge and recommendations for further research. Antiviral Research. 2009;82:A99–A109. doi: 10.1016/j.antiviral.2008.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moss AR, Hahn Ja, Perry S, Charlebois ED, Guzman D, Clark Ra, et al. Adherence to highly active antiretroviral therapy in the homeless population in San Francisco: a prospective study. Clinical Infectious Diseases: an Official Publication of the Infectious Diseases Society of America. 2004;39:1190–1198. doi: 10.1086/424008. [DOI] [PubMed] [Google Scholar]
- 19.Tsai H-J, Choudhry S, Naqvi M, Rodriguez-Cintron W, Burchard EG, Ziv E. Comparison of three methods to estimate genetic ancestry and control for stratification in genetic association studies among admixed populations. Human Genetics. 2005;118:424–433. doi: 10.1007/s00439-005-0067-z. [DOI] [PubMed] [Google Scholar]
- 20.Pritchard JK, Stephens M, Donnelly P. Inference of population structure using multilocus genotype data. Genetics. 2000;155:945–959. doi: 10.1093/genetics/155.2.945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Falush D, Stephens M, Pritchard JK. Inference of population structure using multilocus genotype data: linked loci and correlated allele frequencies. Genetics. 2003;164:1567–1587. doi: 10.1093/genetics/164.4.1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mistri HN, Jangid AG, Pudage A, Gomes N, Sanyal M, Shrivastav P. High throughput LC-MS/MS method for simultaneous quantification of lamivudine, stavudine and nevirapine in human plasma. Journal of Chromatography B, Analytical Technologies in the Biomedical and Life Sciences. 2007;853:320–332. doi: 10.1016/j.jchromb.2007.03.047. [DOI] [PubMed] [Google Scholar]
- 23.Pharmacists A, Guide M. Viramune ® (nevirapine) Tablets Viramune ® (nevirapine) Oral Suspension Rx only. Nursing. 1:4–28. n.d. [Google Scholar]
- 24.Kalow W, Tang BK, Endrenyi L. Hypothesis: Comparisons of inter- and intra-individual variations can substitute for twin studies in drug research. Pharmacogenetics. 1998;8:283–289. doi: 10.1097/00008571-199808000-00001. [DOI] [PubMed] [Google Scholar]
- 25.Venables WN, Ripley BD. Modern Applied Statistics with S-Plus. Springer-Verlag; 1994. [Google Scholar]
- 26.R Core Team. R Foundation for Statistical Computing. R: A Language and Environment for Statistical Computing. 2012 [Google Scholar]
- 27.Vries-sluijs TEMS De, Dieleman JP, Arts D, Huitema ADR, Beijnen JH, Schutten M, et al. Concentrations Predict Virological Failure in an Unselected HIV-1-Infected Population. Clinical Pharmacokinetics. 2003;42:599–605. doi: 10.2165/00003088-200342060-00009. [DOI] [PubMed] [Google Scholar]
- 28.Ramachandran G, Ramesh K, Hemanth Kumar AK, Jagan I, Vasantha M, Padmapriyadarsini C, et al. Association of high T allele frequency of CYP2B6 G516T polymorphism among ethnic south Indian HIV-infected patients with elevated plasma efavirenz and nevirapine. The Journal of Antimicrobial Chemotherapy. 2009;63:841–843. doi: 10.1093/jac/dkp033. [DOI] [PubMed] [Google Scholar]
- 29.Saitoh A, Sarles E, Capparelli E, Aweeka F, Kovacs A, Burchett SK, et al. CYP2B6 genetic variants are associated with nevirapine pharmacokinetics and clinical response in HIV-1-infected children. AIDS (London, England) 2007;21:2191–2199. doi: 10.1097/QAD.0b013e3282ef9695. [DOI] [PubMed] [Google Scholar]
- 30.Lang T, Klein K, Fischer J, Nüssler AK, Neuhaus P, Hofmann U, et al. Extensive genetic polymorphism in the human CYP2B6 gene with impact on expression and function in human liver. Pharmacogenetics. 2001;11:399–415. doi: 10.1097/00008571-200107000-00004. [DOI] [PubMed] [Google Scholar]
- 31.Heil SG, Ende ME Van Der, Schenk PW, Heiden I Van Der, Lindemans J, Burger D, et al. Associations Between ABCB1, CYP2A6, CYP2B6, CYP2D6, and CYP3A5 Alleles in Relation to Efavirenz and Nevirapine Pharmacokinetics in HIV-Infected Individuals. Therapeutic Drug Monitoring. 2012;34:153–159. doi: 10.1097/FTD.0b013e31824868f3. [DOI] [PubMed] [Google Scholar]
- 32.Chen J, Sun J, Ma Q, Yao Y, Wang Z, Zhang L, et al. CYP2B6 polymorphism and nonnucleoside reverse transcriptase inhibitor plasma concentrations in Chinese HIV-infected patients. Therapeutic Drug Monitoring. 2010;32:573–578. doi: 10.1097/FTD.0b013e3181ea953c. [DOI] [PubMed] [Google Scholar]
- 33.Ghannoum Ma, Mukherjee PK, Jurevic RJ, Retuerto M, Brown RE, Sikaroodi M, et al. Metabolomics reveals differential levels of oral metabolites in HIV-infected patients: toward novel diagnostic targets. Omics: a Journal of Integrative Biology. 2013;17:5–15. doi: 10.1089/omi.2011.0035. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.