Abstract
Background
A computed tomography (CT) scan is often the only study needed prior to surgery for resectable solid pancreas masses. However, many patients are evaluated with multiple studies and interventions that may be unnecessary.
Methods
We conducted a retrospective review of patients who presented to the Johns Hopkins Multidisciplinary Pancreas Cancer Clinic with a clearly resectable solid pancreas mass, >1 cm in size over a 2-year period (6/2007–6/2009) and underwent resection. Pancreas specialists reviewed patient records and identified an index CT with a solid pancreas mass deemed to be resectable for curative intent. Data were collected on all studies and interventions between the index CT and the surgery.
Results
A total of 101 patients had an index CT. Following the index CT and before surgery, 78 patients had at least one CT, 19 had magnetic resonance imaging, 9 had a positron emission tomography scan, and 66 underwent pancreatic biopsy. Patients underwent a mean of three studies with a mean added cost of $3,371 per patient. Preoperative tests and interventions were associated with a longer time to definitive surgical intervention.
Conclusion
Wide variation exists for evaluation of newly discovered resectable solid pancreas masses, which is associated with delays to surgical intervention and added costs.
Keywords: Pancreatic cancer, Cost, Unnecessary, Test, Variation
Introduction
In order to achieve high-quality medical care, new technologies should be adopted based on their clinical utility to providers and the risk-benefit ratio to patients.1–4 However, many new technologies are incorporated into routine patient care before they are formally evaluated and many tests may be ordered by providers who are unaware of current best practices among specialists.1–4 Ultimately, the merits of a test or intervention should depend on how it can change management decisions and affect patient outcome.
Pancreas cancer has one of the fastest doubling times of any type of cancer in medicine.5 As such, timely care and referral for definitive surgical resection when indicated is important. While provider preferences to evaluate a newly discovered pancreatic abnormality vary, there is a general consensus regarding the optimal work-up and management of a solid tumor that does not have any suggestion of local invasion or distant metastasis. Current evidence6,7 and consensus statements8 recommend that solid tumors that are surgically resectable should undergo surgical exploration with no need for further testing. At Johns Hopkins, we do not routinely order an MRI for a lesion meeting the above criteria. We used the clinical scenario of this highly standardized presentation to study the variation in work-up among patients with solid pancreas tumors and associated delays to surgical intervention. Elucidation of the variation in practice patterns can help guide efforts to better standardize best practices when these practices are consensus-led and clinically feasible.
Methods
Data Base
Patients undergoing pancreatic resection at the Johns Hopkins Hospital were identified in the Johns Hopkins Pancreas Database, a repository of clinical information on all patients who have presented to our institution and are seen through the Multidisciplinary Pancreas Cancer Clinic. This data is collected prospectively and maintained for research purposes in accordance with HIPPA regulations and with approval from the Institutional Review Board at Johns Hopkins Hospital. For this study, patient information was available from December 2006 to September 2010.
Patient Selection
We retrospectively identified patients who underwent surgical resection of the pancreas for a solid pancreas mass greater than or equal to 1 cm where the intent of surgery was curative. Patients with masses less than 1 cm were excluded to ensure that those patients that were selected had definitively resectable lesions without further imaging. The size of the mass was determined by preoperative computed tomography (CT) scan. We also excluded patients with liver metastasis, peritoneal implants, pancreas cysts, renal insufficiency, previous palliative surgery or those treated with neoadjuvant therapy. Additionally, we excluded patients where imaging indicated abutment or encasement of the portal vein, hepatic artery, superior mesenteric vein, superior mesenteric artery, the celiac trunk, or any other vessel involvement that would deem the mass to be borderline resectable or unresectable. Our rationale in choosing these criteria was to identify patients with whom it would be expected to determine their eligibility for surgical intervention without need for studies and interventions beyond a CT scan.
A multidisciplinary pancreas team including oncologists, radiologists and surgeons reviewed the medical records of each patient meeting the above inclusion/exclusion criteria. For each patient, the team used all available records to identify an “index CT” defined as the first CT imaging that clearly identified the patient as meeting criteria for surgical resection without further evaluation. The index CT was either a CT angiogram or a pancreas protocol CT.
Data Extraction and Statistical Analysis
Patient records were reviewed to identify studies and interventions that occurred after the index CT but before surgery. Specifically, we extracted data on the following studies: CT scan, magnetic resonance imaging (MRI), positron emission tomography scan (PET), abdominal ultrasound (US), esophagogastroduodenoscopy, endoscopic ultrasound with and without biopsy, endoscopic retrograde cholangiopancreatography (ERCP) with and without biopsy, percutaneous tube cholangiogram and biopsy, and percutaneous biopsy.
The date of index CT was recorded and subtracted from the date of the operative note to determine days between diagnosis and surgical intervention. The costs of the studies that we report are the Medicare facility fees. This was used as it was the most consistent way to report the study costs.
Descriptive statistics were used to describe the frequency and distribution of studies per patient work-up. A t test analysis was performed to determine an association between the number of tests and the days between the diagnosis and surgery and the days between diagnosis and presentation at the multi-disciplinary clinic. Charge to costs ratios from the Johns Hopkins Hospital were used to estimate the total added costs of these studies including studies performed at other institutions. Significance for all tests was set at a p value less than 0.05. All p values are two-tailed. All the analyses were performed using JMP® 9.0.0 software (SAS Institute Inc., Cary, NC).
Results
A total of 101 patients with a solid resectable mass of the pancreas greater than or equal to 1 cm who underwent resection for curative intent met the inclusion criteria of this study. Demographic and clinical data are shown in Table 1. Fifty-two percent were women and the age range was 31–89 years with a mean age of 63.6 years. Seventy-four percent of patients underwent a pancreaticoduodenectomy and 22 % underwent a distal pancreatectomy. Adenocarcinoma was the most common tumor type.
Table 1.
Patient characteristics
| Total patients | 101 |
| Age (mean, range) | 63.6 (31–89) |
| Sex | n (%) |
| Female | 53 (52) |
| Male | 48 (48) |
| Surgery | n (%) |
| Pancreaticoduodenectomy | 74 (73) |
| Distal pancreatectomy | 22 (22) |
| Total pancreatectomy | 4 (4) |
| Unresectable | 1 (1) |
| Tumor type/pathology | n (%) |
| Adenocarcinoma | 81 (80) |
| Neuroendocrine | 4 (4) |
| Cholangiocarcinoma | 3 (3) |
| Pancreatitis | 3 (3) |
| IPMN | 3 (3) |
| Serous cystadenoma | 2 (2) |
| Hemangioma | 1 (1) |
| Pseudopapillary | 1 (1) |
| Carcinoid | 1 (1) |
| Pancreaticoblastoma | 1 (1) |
| Intrapancreatic | 1 (1) |
| Heterotropic splenic tissue | |
| Tumor size (cm) | n (%) |
| 1.0–1.99 | 19 (19) |
| 2.0–2.99 | 25 (25) |
| 3.0–3.99 | 35 (34) |
| 4.0–4.99 | 11 (11) |
| 5.0–5.99 | 6 (6) |
| 6.0–6.99 | 4 (4) |
| 7.0–7.99 | 0 (0) |
| 8.0–8.99 | 1 (1) |
The number of additional tests undergone by patients after index CT ranged from 0 to 8 with mean of 3 (Table 2). There were only seven patients who had no additional studies after the index CT and before surgery. The most common additional imaging studies included CT, MRI, and PET. The most common procedural diagnostics included EUS, EUS with biopsy, ERCP and ERCP with biopsy. The total estimated costs of all studies after index CT in the study population was $340,504 or approximately $3,371 per patient (Table 3).
Table 2.
Frequency distribution of studies and interventions between the index CT and surgery
| Number of tests and interventions | N number of patients |
|---|---|
| 0 | 7 |
| 1 | 9 |
| 2 | 17 |
| 3 | 25 |
| 4 | 26 |
| 5 | 10 |
| 6 | 6 |
| 7 | 0 |
| 8 | 1 |
| Mean number of additional tests per patient | 3 |
Table 3.
Number of additional tests performed and associated costs
| Test | n | Cost Per Test ($, USD) | Total cost in study population ($, USD) |
|---|---|---|---|
| Additional CT | 78 | 547 | 42,666 |
| Additional CT (2nd) | 10 | 547 | 5,470 |
| MRI | 19 | 1,933 | 36,727 |
| MRI (2nd) | 1 | 1,933 | 1,933 |
| PET | 9 | 5,501 | 49,509 |
| Abdominal US | 7 | 819 | 5,733 |
| EGD | 5 | 1,251 | 6,255 |
| EUS | 44 | 1,509 | 66,396 |
| EUS (2nd) | 1 | 1,509 | 1,509 |
| EUS Bx | 43 | 225 | 9,675 |
| ERCP | 54 | 1,269 | 68,526 |
| ERCP (2nd) | 9 | 1,269 | 11,421 |
| ERCP (3rd) | 1 | 1,269 | 1,269 |
| ERCP Bx | 20 | 225 | 4,500 |
| ERCP Bx (2nd) | 2 | 225 | 450 |
| PTC | 7 | 3,530 | 24,710 |
| PTC Bx | 1 | 225 | 225 |
| Percutanous Bx | 1 | 3,530 | 3,530 |
| Aggregate costs in study population | $340,504 | ||
| Mean cost per patient | $3,371 |
EGD esophagogastroduodenoscopy, EUS endoscopic ultrasound, Bx biopsy, PTC percutaneous tube cholangiogram
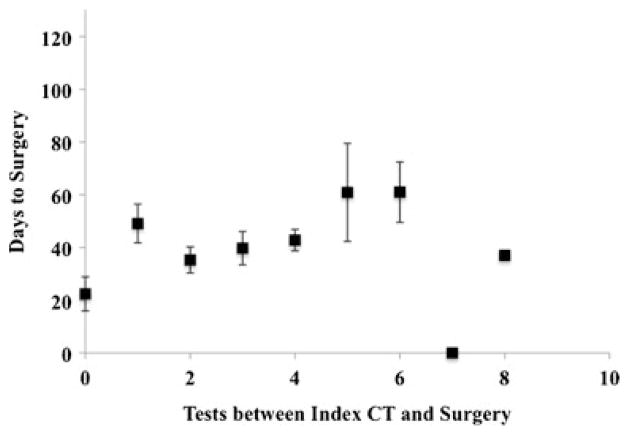
The mean number of days a patient waited for surgery from the first time a resectable pancreas mass was discovered on CT scan was 43 days. On average, patients with no additional tests (n=7) underwent surgical resection within 22 days, those with 1 additional test (n=9) in 49 days and those with 5 additional tests (n=10) within 61 days (Table 4). A t test analysis indicated that there was an increase in time to surgery with increasing the number of tests after the index CT from 0 to 1 test. Overall, there is a trend of increasing time to surgery with increasing number of tests (Fig. 1).
Table 4.
Time between the index CTand surgical treatment by number of interim tests
| Number of studies | Mean number of days between index CT and surgery |
|---|---|
| 0 | 22 |
| 1 | 49 |
| 2 | 35 |
| 3 | 40 |
| 4 | 42 |
| 5 | 61 |
| >5 | 57 |
Fig. 1.
Mean number of days to surgery from the index CT scan versus the number of tests performed between the index CT scan and surgery. The error bars are the standard error of the data
The mean number of days from the index CT to the patient’s first appointment in our multidisciplinary clinic was 24 days. On average, patients with no additional studies were seen in our clinic within 2 days, those with 1 additional test in 26 days and those with 5 additional tests in 32 days. A t test analysis indicated that there was an increase in time to being seen in our multidisciplinary clinic with increasing the number of tests after the index CT from 0 to 1 test. Overall, there is a trend of increasing time to being seen in our multidisciplinary clinic with increasing number of tests (Fig. 2).
Fig. 2.

Mean number of days to be seen in the Johns Hopkins multidisciplinary clinic from the index CT scan versus the number of tests performed between the index CT scan and surgery. The error bars are the standard error of the data
Discussion
CT is the most widely used imaging modality for the diagnosis and staging of pancreatic cancer. Over the past two decades, technological advances have revolutionized our ability to evaluate the pancreas. The introduction of multi-detector row helical CT scanners has markedly improved the speed and quality of cross-sectional imaging. Numerous studies have examined the efficacy of CT in the preoperative staging of pancreatic cancer. In determining resectability, positive predictive values of 90–100 % have consistently been reported.9–17. Bipat et al.16 published a meta-analysis of 1,823 patients, which showed the sensitivity and specificity of helical CT for determining resectability to be 81 and 82 %, respectively. It also demonstrated that CT was superior to both MRI and US. In a retrospective review correlating the CT, surgical, and pathological findings of 110 peri-pancreatic vessels in 22 patients with pancreatic adenocarcinoma, Vargas et al.17 reported a negative predictive value of 100 % and an accuracy of 99 % (one false-positive) in detecting vascular invasion. For these reasons, CT has become the standard imaging test for all patients prior to undergoing surgical exploration for pancreas masses. At Johns Hopkins, we use CT as the single best test to determine resectability of a solid pancreas mass and based on this study alone, we choose to offer patients a surgical exploration. PET imaging has not been shown to impact clinical management for pancreatic disease given its relatively low sensitivity9–11 and it is not FDA approved for evaluating solid pancreas tumors.
ERCP and EUS are relatively safe endoscopic procedures; however, there is the potential for complications. The most common complications are pancreatitis, hemorrhage, and bowel perforation.20–25 Eloubeidi et al. reported a 2.5 % incidence of immediate complications following EUS-FNA.20 Another study reported an immediate complication rate of 6.85 % for ERCP with a mortality rate of 0.33 %.21 With these life-threatening complications in mind, the potential value added from these studies should be considered. Preoperative tests should only be obtained if the result will change management decisions. In our study of 101 patients over 4 years, we found that patients who had clearly a resectable pancreatic mass on CT scan underwent an average of 3 additional studies prior to resection. On average, patients with no additional tests following an index CT underwent surgical resection within 22 days while those with four additional tests waited 43 days. There was an increase in time to surgery with undergoing no studies after the index CT scan compared to undergoing one study after the index CT. With greater than one additional study, although it was not significant, there was a trend of increasing time to surgery with each additional study. In a cancer that has one of the fastest doubling times of any type of cancer,5 this additional time to surgery could theoretically have a clinical impact. Additionally, there was an increase in time to being seen in our multidisciplinary clinic with undergoing no studies after the index CT scan compared to undergoing to one study after the index CT scan with a trend of increasing time to be seen in clinic with each additional study. If patients were referred to a specialty center early in the work-up for a solid pancreas mass, this could also theoretically also have a clinical impact.
In addition to the possible health consequences of extra testing, the financial costs of cancer care are a burden to people diagnosed with cancer, their families, and society as a whole. National cancer care expenditures have been steadily increasing in the United States. Cancer care accounted for an estimated $104.1 billion in medical care expenditures in the United States in 2006 of which pancreas cancer care accounted for $1.9 billion.18 Overall, approximately 33.6 % of expenditures are in the first year after diagnosis.18,19 Despite the high cost to Medicare in the first year following diagnosis, there is limited information on particular categories of cancer-related expenditures, especially costs of the preoperative work-up. As health care expenditures continue to rise, understanding associated cost trends and which components of treatment are contributing unnecessarily will be important in planning for future health budgets and adopting standards of care. In our study, we found that the mean added cost per patient of additional studies was $3,371, which is 6 % of the median household income.26 Moreover, this mean added cost was only the cost of the Medicare facility fees. This excludes any additional costs of the test such as professional fees, pharmaceutical costs, lab costs, as well as costs to the patients in terms of time lost from work. Therefore, our added cost value is likely an underestimate. This could have a significant impact on any family, especially one where one member is not able to work due to illness, as well as a significant impact on the rising costs in our healthcare system. Figure 3 shows an algorithm for work-up of a patient with a clinical suspicion of pancreas cancer to prevent testing that may be unnecessary.
Fig. 3.
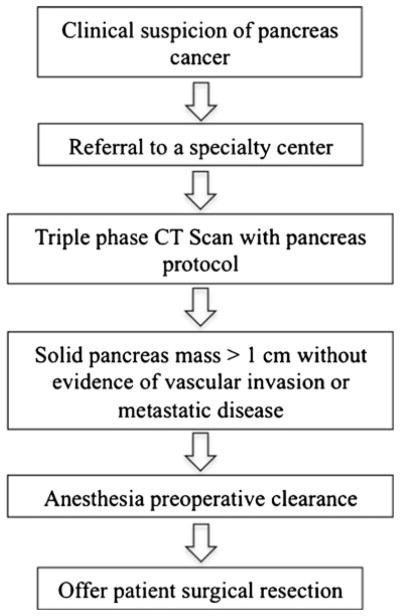
Algorithm for work-up of a patient with a clinical suspicion of pancreas cancer to prevent testing that may be unnecessary
While this study highlights overtreatment in the work-up of a subset of patients with pancreas solid tumors, we recognize some important limitations to this study. Cancer care should be individualized to the patient. On occasion patients with atypical appearing masses may benefit from such additional tests. For example, if a high-risk patient has a solid mass that appears indolent, then a biopsy to establish the diagnosis can be helpful in surgical decision-making. Also, a referral bias likely skews the data towards increased testing. Furthermore, we did not include those patients who presented with an index CT but ultimately did not undergo surgery at our institution. Another limitation is that this study does not account for the heterogeneity of patients presenting with solid pancreas masses with a history of pancreatitis that may warrant further investigation. Similarly, rare patients with liver impairment due to biliary obstruction may benefit from a temporizing biliary stent. Finally, costs are derived from estimates based on charge to cost ratios used at our institution.
Conclusion
Disparities in quality of care may be represented in the wide variations we observed for a highly standardized presentation of a solid, resectable pancreas mass. This variation is sometimes associated with long delays to surgical intervention and added costs. Any patient with a localized solid mass in the pancreas that is clearly resectable by CT should have a surgical exploration offered to them, provided there are no other contraindications. Standardized guidelines and education for providers who are not pancreas specialists could spare patients the delays, risks, and costs of unnecessary studies and interventions.
Contributor Information
Michol Cooper, Email: mcoope41@jhmi.edu, Department of Surgery, Johns Hopkins Hospital, Baltimore, MD, USA. General Surgery Resident, Johns Hopkins Hospital, Halsted 610, 600 N. Wolfe St, Baltimore, MD 21287, USA.
Naeem A. Newman, Department of Surgery, Johns Hopkins Hospital, Baltimore, MD, USA
Andrew M. Ibrahim, Department of Surgery, Johns Hopkins Hospital, Baltimore, MD, USA
Edwin Lam, Department of Radiation Oncology, Johns Hopkins Hospital, Baltimore, MD, USA.
Joseph M. Herman, Department of Radiation Oncology, Johns Hopkins Hospital, Baltimore, MD, USA
Vikesh K. Singh, Division of Gastroenterology, Johns Hopkins Hospital, Baltimore, MD, USA
Christopher L. Wolfgang, Department of Surgery, Johns Hopkins Hospital, Baltimore, MD, USA
Timothy M. Pawlik, Department of Surgery, Johns Hopkins Hospital, Baltimore, MD, USA
John L. Cameron, Department of Surgery, Johns Hopkins Hospital, Baltimore, MD, USA
Martin A. Makary, Department of Surgery, Johns Hopkins Hospital, Baltimore, MD, USA
References
- 1.Wilson CB. Adoption of new surgical technology. BMJ. 2006 Jan 14;332(7533):112–114. doi: 10.1136/bmj.332.7533.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barkun JS, Aronson JK, Feldman LS, Maddern GJ, Strasberg SM. Evaluation and stages of surgical innovations. Lancet. 2009;374:1089–96. doi: 10.1016/S0140-6736(09)61083-7. [DOI] [PubMed] [Google Scholar]
- 3.Ergina PL, Cook JA, Blazeby JM, Boutron I, Clavien P, Reeves BC, Seiler CM. Challenges in evaluating surgical innovation. Lancet. 2009;374:1097–104. doi: 10.1016/S0140-6736(09)61086-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McCulloch P, Altman DG, Campbell WB, Flum DR, Glasziou P, Marshall JC, Nicholl J, Aronson JK, Barkun JS, Blazeby JM, Boutron IC, Campbell WB, Clavien PA, Cook JA, Ergina PL, Feldman LS, Flum DR, Maddern GJ, Nicholl J, Reeves BC, Seiler CM, Strasberg SM, Meakins JL, Ashby D, Black N, Bunker J, Burton M, Campbell M, Chalkidou K, Chalmers I, de Leval M, Deeks J, Ergina PL, Grant A, Gray M, Greenhalgh R, Jenicek M, Kehoe S, Lilford R, Littlejohns P, Loke Y, Madhock R, McPherson K, Meakins J, Rothwell P, Summerskill B, Taggart D, Tekkis P, Thompson M, Treasure T, Trohler U, Vandenbroucke J Balliol Collaboration. No surgical innovation without evaluation: the IDEAL recommendations. Lancet. 2009;374:1105–12. doi: 10.1016/S0140-6736(09)61116-8. [DOI] [PubMed] [Google Scholar]
- 5.Vincent A, Herman J, Schulick R, Hruban RH, Goggins M. Pancreatic cancer. Lancet. 2011;378(9791):607–20. doi: 10.1016/S0140-6736(10)62307-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yeo CJ, Cameron JL, Lillemoe KD, Sitzman JV, Hruban RH, Goodman SN, Dooley WC, Coleman J, Pitt HA. Pancreaticoduodenectomy for cancer of the head of the pancreas. 201 patients. Ann Surg. 1995;221:721–733. doi: 10.1097/00000658-199506000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Soriano A, Castells A, Ayuso C, Ayuso JR, de Caralt MT, Gines MA, Real MI, Gilabert R, Quinto L, Trilla A, Feu F, Montaya X, Fernandez-Cruz L, Navarro S. Preoperative staging and tumor resectability assessment of pancreatic cancer: prospective study comparing endoscopic ultrasonography, helical computed tomography, magnetic resonance imaging, and angiography. Am J Gastroenterol. 2004 Mar;99(3):492–501. doi: 10.1111/j.1572-0241.2004.04087.x. [DOI] [PubMed] [Google Scholar]
- 8.Evans DB, Farnell MB, Lillemoe KD, Vollmer C, Jr, Strasberg SM, Schulick RD. Surgical treatment of resectable and borderline resectable pancreas cancer: expert consensus statement. Ann Surg Oncol. 2009 Jul;16(7):1736–44. doi: 10.1245/s10434-009-0416-6. [DOI] [PubMed] [Google Scholar]
- 9.Karmazanovsky G, Federov V, Kubyshkin V, Kotchatkov A. Pancreatic head cancer: accuracy of CT in determination of resectability. Abdom Imaging. 2005;30(4):488–500. doi: 10.1007/s00261-004-0279-z. [DOI] [PubMed] [Google Scholar]
- 10.Kinney T. Evidence-based imaging of pancreatic malignancies. Surg Clin North Am. 2010 Apr;90(2):235–49. doi: 10.1016/j.suc.2009.12.003. [DOI] [PubMed] [Google Scholar]
- 11.Clarke DL, Thomson SR, Madiba TE, Sanyika C. Preoperative imaging of pancreatic cancer: a management-oriented approach. J Am Coll Surg. 2003;196 (1):119–29. doi: 10.1016/s1072-7515(02)01609-5. [DOI] [PubMed] [Google Scholar]
- 12.Diehl SJ, Lehmann KJ, Sadick M, Lachmann R, Georgi M. Pancreatic cancer: value of dual phase helical CT in assessing resectability. Radiology. 1998;206:373–8. doi: 10.1148/radiology.206.2.9457188. [DOI] [PubMed] [Google Scholar]
- 13.Parsons CM, Sutcliffe JL, Bold RJ. Preoperative evaluation of pancreatic adenocarcinoma. J Hepatobiliary Pancreat Surg. 2008;15:429–435. doi: 10.1007/s00534-007-1240-7. [DOI] [PubMed] [Google Scholar]
- 14.Legmann P, Vignaux O, Dousset B, Baraza AJ, Palazzo L, Dumontier I, Coste J, Louvel A, Roseau G, Couturier D, Bonnin A. Pancreatic tumors: comparison of dual-phase helical CT and endoscopic sonography. AJR Am J Roentgenol. 1998;170:1315–22. doi: 10.2214/ajr.170.5.9574609. [DOI] [PubMed] [Google Scholar]
- 15.Lu DS, Reber HA, Krasny RM, Kadell BM, Sayre J. Local staging of pancreatic cancer: criteria for unresectability of major vessels as revealed by pancreatic-phase, thin-section helical CT. AJR Am J Roentgenol. 1997;168(6):1439–43. doi: 10.2214/ajr.168.6.9168704. [DOI] [PubMed] [Google Scholar]
- 16.Bipat S, Phoa SS, van Delden OM, Bossuyt PM, Gouma DJ, Lameris JS, Stoker J. Ultrasonography, computed tomography and magnetic resonance imaging for diagnosis and determining respectability of pancreatic adenocarcinoma: a meta-analysis. J Comput Assist Tomogr. 2005;29(4):438–435. doi: 10.1097/01.rct.0000164513.23407.b3. [DOI] [PubMed] [Google Scholar]
- 17.Vargas R, Nino-Murcia M, Trueblood W, Jeffrey RB., Jr MDCT in pancreatic adenocarcinoma: prediction of vascular invasion and resectability using a multiphasic technique with curved planar reformations. AJR Am J Roentgenol. 2004;182 (2):419–25. doi: 10.2214/ajr.182.2.1820419. [DOI] [PubMed] [Google Scholar]
- 18.Brown ML, Riley GF, Schussler N, Etzioni R. Estimating health care costs related to cancer treatment from SEER-Medicare data. Med Care. 2002;40(8 Suppl):IV-104-17. doi: 10.1097/00005650-200208001-00014. [DOI] [PubMed] [Google Scholar]
- 19.Cancer Trends Progress Report-2009/2010 Update. Bethesda, MD: National Cancer Institute, NIH, DHHS; Retrieved Nov 1, 2010 from http://progressreport.cancer.gov. [Google Scholar]
- 20.Eloubeidi MA, Tamhane A, Varadarajulu S, Wilcox CM. Frequency of major complications after EUS-guided FNA of solid pancreatic masses: a prospective evaluation. Gastrointest Endosc. 2006;63(4):622– 29. doi: 10.1016/j.gie.2005.05.024. [DOI] [PubMed] [Google Scholar]
- 21.Andriulli A, Loperfido S, Napolitano G, Niro G, Valvano MR, Spirito F, Pilotto A, Foriano R. Incidence rates of post-ERCP complications: a systematic survey of prospective studies. Am J Gastroenterol. 2007 Aug;102(8):1781–7. doi: 10.1111/j.1572-0241.2007.01279.x. [DOI] [PubMed] [Google Scholar]
- 22.Paquin SC, Gariepy G, Lepanto L, Bourdages R, Raymond G, Sahai AV. A first report of tumor seeding because of EUS-guided FNA of a pancreatic adenocarcinoma. Gastrointest Endosc. 2005 Apr;61(4):610–611. doi: 10.1016/s0016-5107(05)00082-9. [DOI] [PubMed] [Google Scholar]
- 23.Shah JN, Fraker D, Guerry D, Feldman M, Kochman ML. Melanoma seeding of an EUS-guided fine needle track. Gastrointest Endosc. 2004 Jun;59(7):923–924. doi: 10.1016/s0016-5107(04)00340-2. [DOI] [PubMed] [Google Scholar]
- 24.Fisher L, Segarajasingam DS, Stewart C, Deboer WB, Yussof IF. Endoscopic ultrasound guided fine needle aspiration of solid pancreatic lesions: performance and outcomes. J Gastroenterol Hepatol. 2009 Jan;24(1):90–96. doi: 10.1111/j.1440-1746.2008.05569.x. [DOI] [PubMed] [Google Scholar]
- 25.Al-Haddad M, Wallace MB, Woodward TA, Gross SA, Hodgens CM, Toton RD, Raimondo M. The safety of fine-needle aspiration guided by endoscopic ultrasound: a prospective study. Endoscopy. 2009 Mar;40(3):204–208. doi: 10.1055/s-2007-995336. [DOI] [PubMed] [Google Scholar]
- 26.United States Census Bureau. 2010 Retrieved July 1, 2012 from www.census.gov/hhes/www/income/