Abstract
OBJECTIVE.
The objective of our study was to determine the prevalence and CT appearance of cystlike changes of pancreatic neuroendocrine tumor (NET), particularly of small (≤3 cm) tumors.
MATERIALS AND METHODS.
The clinical records, images, and pathologic reports of 74 consecutive patients (average age, 55.5 years) with surgically resected pancreatic NETs who underwent preoperative CT were retrospectively reviewed. The size and location of the pancreatic NETs were recorded. The tumors were classified on the basis of CT appearance as small (≤3 cm) or large (> 3 cm) and as solid, partially (≤50% or > 50%) cystic, or purely (≈100%) cystic. Peripheral contrast enhancement on CT was characterized, and lymph node and liver metastases found by pathologic examination were recorded.
RESULTS.
A total of 78 pancreatic NETs were reviewed. Five were not visualized on CT, leaving 73 pancreatic NETs in 69 patients (multiple tumors were visualized on CT of three patients) for analysis. The mean size of the 73 tumors was 3.0 ± 2.6 (SD) cm (range, 0.7–13.1 cm); 52 tumors were 3 cm or smaller and 21 tumors were larger than 3 cm. Gross pathologic results confirmed that 13 of the 73 (17.8%) tumors were predominantly (> 50% or ≤ 100%) cystic: 10 of the 52 (19.2%) tumors 3 cm or smaller and three of the 21 (14.3%) tumors larger than 3 cm. Peripheral contrast enhancement was seen in 11 of the 13 (85%) predominantly cystic pancreatic NETs. Compared with solid pancreatic NETs, predominantly cystic pancreatic NETs were less commonly associated with lymph node and liver metastases.
CONCLUSION.
Cystic pancreatic NETs are not rare and should be included in the differential diagnosis of a cystic pancreatic mass, particularly if the cystic mass is associated with peripheral contrast enhancement. A minority of cystic pancreatic NETs can present with no peripheral enhancement.
Keywords: cystic pancreatic neuroendocrine tumor (NET), MDCT, pancreatic NET, peripheral contrast enhancement
Pancreatic neuroendocrine tumors (NETs) are uncommon, with an incidence rate of approximately 5–10 cases per 1 million persons per year, and represent fewer than 3% of all pancreatic neoplasms [1–3]. The incidence of pancreatic NETs has increased two- to three-fold in the past 16 years, whereas the age-adjusted incidence of pancreatic adenocarcinoma has remained relatively stable [3, 4]. Pancreatic NETs can display a variety of histopathologic appearances and can present with a wide range of clinical symptoms [2]. Most cases are sporadic, but some are associated with familial syndromes such as multiple endocrine neoplasia type 1 (MEN1), von Hippel–Lindau (VHL) syndrome, and neurofibromatosis type 1.
Pancreatic NETs are typically seen as hyperattenuating masses on arterial and portal venous phase CT images because of their rich blood supply [5]. Cystic pancreatic NET is considered uncommon and often is a component of a large tumor with cystic degeneration or necrosis [5, 6]. However, small NETs with a cystic change are occasionally encountered on CT. Cystic pancreatic NETs can mimic other cystic pancreatic masses on imaging studies, posing a diagnostic challenge to radiologists. Limited data are available for a detailed analysis of the CT appearance of cystic pancreatic NETs.
The purposes of this study were to determine the prevalence of cystic pancreatic NETs on CT, particularly the prevalence of small (≤3 cm) cystic pancreatic NETs, and to characterize the CT appearance of these tumors.
Materials and Methods
Patients
This retrospective study was performed after study exemption was granted by our institutional review board. Informed consent was not required.
The records of 74 consecutive patients who underwent surgical resection of pancreatic NET from February 2008 to March 2010 and whose preoperative CT scans were available were retrospectively reviewed. This group of patients consisted of 36 men and 38 women, with an age range of 24–83 years (average age, 55.5 ± 12.9 [SD] years). The interval between CT and surgical resection of the neoplasm was 1–159 days (average,32.0 ± 32.0 [SD] days). The clinical records and pathologic reports were reviewed.
CT Technique
The CT examinations were performed on a 64-MDCT scanner (Sensation 64, Siemens Healthcare) or a dual-source 64-MDCT scanner (Definition or Definition Flash, Siemens Healthcare) using a detector collimation of 64 × 0.6 or 128 × 0.6 mm. The effective tube current–exposure time product was 250 mAseff for the Sensation 64 scanner, and 290 mAs was used as the quality reference for an online dose modulation system (CareDose 4D, Siemens Healthcare) for the Definition and Definition Flash scanners. Other parameters were 120 kVp and a 0.5-second rotation speed.
After fasting for at least 2–3 hours, each patient ingested 750–1000 mL of water over a 15- to 20-minute period before scanning began. Then, arterial and portal venous phase images were acquired at approximately 25–30 seconds and 50–60 seconds, respectively, from the start of an IV contrast material injection. The contrast infusion protocol included 100–120 mL of either iohexol (Omnipaque 350, GE Healthcare) or iodixanol (Visipaque 320, GE Healthcare) through a peripheral venous line at 4–5 mL/s.
All image data were reconstructed using the body soft-tissue algorithm. The data were reconstructed at a 0.75-mm slice thickness at 0.5-mm intervals (0.25-mm overlap) for 3D and multiplanar reformation (MPR) imaging. For diagnostic reading, axial sections were generated with a 3-mm reconstruction thickness and 3-mm intervals. All scanning data were reviewed on a PACS system (Advanced Visualization, version 5.30.7.26, Emageon).
Image Analysis
The following CT features were evaluated. First, the size of the tumor was determined by measuring the largest diameter on axial images. Second, the location of the tumor was recorded as the head/uncinate, neck, body, or tail. If the tumor involved more than one segment (head/uncinate, neck, body, or tail), it was recorded. Third, tumors were subjectively classified on the basis of the visually estimated volume of the cystic component within the mass as solid, partially (> 50% or ≤ 50%) cystic, or purely (≈100% except hairline thin wall) cystic. The term “cystic component” was defined as a hypodense lesion less than 30 HU in both the arterial and the portal venous phases with no contrast enhancement between the two phases. Fourth, the presence of peripheral contrast enhancement around the cystic component of the tumor greater than the surrounding normal pancreatic parenchyma was subjectively determined. The attenuation of the peripheral enhancing component of tumor was obtained by averaging the attenuations of three regions of interest (ROIs) within the most enhancing component of the tumor when measurable. The degree of peripheral enhancement was quantitatively graded into one of five categories by obtaining the difference of average attenuation of an enhancing component and of normal pancreatic parenchyma at the arterial phase and portal venous phase: up to 10 HU, between 10 and up to 20 HU, between 20 and up to 40 HU, between 40 and up to 60 HU, or greater than 60 HU. Peripheral enhancement was classified as smooth or irregular and as thin (≤ 3 mm), medium thickness (> 3 and ≤ 6 mm), or thick (> 6 mm). Fifth, the internal structures within the cystic region (septations, nodules) were recorded if present. Sixth, the average attenuation of normal pancreatic parenchyma excluding the region of tumor was obtained using ROIs in three separate areas of the pancreas in the head, body, and tail. Seventh, the diagnosis or differential diagnoses of the cystic pancreatic NET provided in the CT report at the time of the clinical examination were recorded.
CT images were reviewed by two body CT fellowship-trained radiologists who had more than 5 years’ and more than 10 years’ experience in abdominal CT, respectively, at the time of the study. The reviewers used axial images as well as MPR and 3D display images. Discrepancies between the two radiologists’ findings were resolved by discussion, and a consensus was reached. The size of each tumor, attenuation of pancreatic parenchyma, and peripheral contrast enhancement were measured by one radiologist based on consensus findings.
Data Analyses
Pancreatic NETs were divided into two groups on the basis of size: 3 cm or smaller and larger than 3 cm. The prevalence of cystic pancreatic NETs was obtained for each group. The relations of predominantly (≈ 100% and > 50%) cystic pancreatic NETs and tumor location and the presence of liver and lymph node metastases confirmed by pathologic specimen were statistically analyzed. Statistical analysis was performed by the chi-square test using statistics software (StatView-J, version 5.0, SAS Institute). A pvalue of less than 0.05 was considered significant.
Results
Surgical Results
A total of 78 surgically resected pancreatic NETs from 74 patients were reviewed. In five of these patients, the pancreatic NET was not visualized on CT, leaving 73 pancreatic NETs in 69 patients for inclusion in this study.
Three patients underwent enucleation of the mass or segmental pancreatectomy; 41 patients, distal pancreatectomy without or with splenectomy; 24 patients, pancreaticoduodenectomy; and one patient, total pancreatectomy.
Pathologic and Clinical Findings
The pathologic diagnosis of the resected specimens of all 69 patients was pancreatic NET. Sixty-one patients had unifocal tumor in the surgical specimen, including one patient with VHL syndrome. Two patients with MEN1 had unifocal pancreatic NET arising in a background of multifocal neuroendocrine microadenomas. Six patients had multifocal tumors in the surgical specimen, four patients with a history of MEN1 and two without a history of hereditary syndromes. In three of these six patients with multifocal pancreatic NETs, one tumor was visualized on CT. In two patients, two tumors were visualized on CT. In one patient, three tumors were visualized on CT. Therefore, a total of 73 pancreatic NETs in 69 patients were visualized on CT and used for analysis.
In 64 of the 69 patients who had a tumor or tumors visible on CT, lymph nodes were resected at the time of surgery and submitted for pathologic examination. Twenty-five patients had lymph nodes positive for metastatic disease on pathologic examination, and 39 patients had lymph nodes negative for metastatic disease. In five patients, lymph nodes were not submitted for pathologic examination. Liver biopsy was performed at the time of surgery in 15 patients and was positive for metastasis in eight patients; seven of the eight patients also had lymph node metastasis, and one patient with liver metastasis had no lymph node metastasis. In addition, two other patients had a suspicious-appearing liver mass on CT and biopsy before surgery revealed liver metastasis. These two patients also had lymph node metastasis by surgical specimen. Therefore, a total of 26 of the 69 (38%) patients had lymph node metastasis, liver metastasis, or both by pathologic examination.
In nine patients, tumor was documented to be functional: six insulinomas, one gastrinoma, and two glucagonomas. In the other 60 patients, the presenting symptoms included abdominal symptoms (pain, cramping, discomfort, burning, nausea, vomiting, diarrhea) (n = 21), palpable epigastric mass (n = 1), pancreatitis (n = 2), new-onset diabetes mellitus (n = 1), jaundice due to pancreatic mass (n = 2), and systemic symptoms (weight loss, anemia, fatigue) (n = 4). In three patients with VHL syndrome or MEN1 with nonfunctional tumors, a pancreatic mass was noted at imaging surveillance. In 26 patients, the neoplasm was found incidentally on imaging studies performed because of nonspecific or unrelated symptoms or on imaging studies performed during workup for another disease process.
CT Findings With Pathologic Correlation
The size of the tumors and prevalence of cystic pancreatic NET are shown in Table 1. Fifty-two of 73 (71%) tumors were small (≤ 3 cm) and 21 (29%) tumors were large (> 3 cm).
TABLE 1.
Size of Pancreatic Neuroendocrine Tumors (NETs) and Prevalence of Cystic-Appearing Tumors on CT
| Pancreatic NETs |
All Pancreatic NETs (n = 73) |
||
|---|---|---|---|
| Characteristic | ≤ 3cm (n = 52) | >3cm (n = 21) | |
|
| |||
| Size (cm) | |||
| Mean ± SD | 1.7 ± 0.7 | 6.3 ± 2.7 | 3.0 ± 2.6 |
| Range | 0.7–13.1 | ||
| Cystic appearance on CT, no. (%) of lesions | |||
| ≈100% Cystic appearance on CT | 3 (4.1) | 0 | 3 (4.1) |
| > 50% Cystic appearance on CT | 7 (9.6) | 3 (4.1) | 10 (13.7) |
| ≤ 50% Cystic appearance on CT | 5 (6.8) | 4 (5.5) | 9 (12.3) |
| Solid appearance on CT | 37 (50.7) | 14 (19.2) | 51 (69.9) |
The degree of cystic appearance on CT, size, location, peripheral contrast enhancement, CT diagnosis or differential diagnoses of cystic-appearing tumors, and gross pathologic findings of small (≤ 3 cm) tumors are summarized in Table 2 and those of large (> 3 cm) tumors are summarized in Table 3. Grossly, cystic pancreatic NETs typically contained clear to straw-colored fluid that was sometimes noted to be hemorrhagic. Necrosis was uncommon (Tables 2 and 3).
TABLE 2.
Characteristics of Small (≤ 3 cm) Cystic Pancreatic Neuroendocrine Tumors (NETs)
| Case No. |
Cystic Change on CT |
Size (cm) |
Location | Peripheral Enhancementa |
CT Diagnosis or Differential Diagnosis |
Gross Pathology | ||
|---|---|---|---|---|---|---|---|---|
| Arterial Phaseb |
Venous Phaseb |
CT Appearance (Internal Enhancing Structures) |
||||||
|
| ||||||||
| 1c | ≈ 100% | 0.9 | Head | − | − | Indeterminate, possible IPMN | Contained cystic component | |
| 2 | ≈ 100% | 1.7 | Tail | ± | − | Equivocal, thin, smooth | Known pancreatic NET | Contained cystic component |
| 3 | ≈ 100% | 0.7 | Tail | − | − | Indeterminate, possible IPMN | Contained cystic component | |
| 4 | > 50% | 2.4 | Tail | ++ | + | Thin and thick, crescentic | Pancreatic NET, MCN | Contained cystic component |
| 5 | > 50% | 1.4 | Tail | + | ± | Medium thickness, smooth | Pancreatic NET | Contained cystic component, focal necrotic component |
| 6 | > 50% | 0.9 | Body | + | − | Thin, smooth | Known pancreatic NET | Contained cystic component |
| 7 | > 50% | 1.8 | Tail | + | + | Medium thickness, irregular | Pancreatic NET, primary cystic neoplasm |
Contained cystic component |
| 8 | > 50% | 1.8 | Neck | + | ++ | Medium thickness, smooth | Pancreatic NET | Contained cystic component |
| 9 | > 50% | 1.5 | Tail | + | ± | Thin and medium thickness, smooth |
IPMN, cystadenoma, pseudocyst |
Contained cystic component, hemorrhagic component |
| 10 | > 50% | 1.5 | Tail | + | − | Medium thickness, smooth | Indeterminate, possible IPMN | Contained cystic component |
| 11 | ≤50% | 2.8 | Head | + | ++ | Medium thickness, irregular (predominantly solid) |
Pancreatic NET | Contained cystic component, hemorrhagic component |
| 12c | ≤50% | 2.3 | Tail | + | ± | Thin and thick, smooth (predominantly solid) |
Pancreatic NET | Contained cystic component |
| 13 | ≤50% | 3.0 | Head | ++ | + | Thin and thick, smooth (predominantly solid) |
Indeterminate cystic lesion | Solid |
| 14 | ≤50% | 1.4 | Head | ++ | − | Thin and medium thickness, smooth (nodular) |
Pancreatic NET, other neoplasm |
Contained cystic component, hemorrhagic component |
| 15 | ≤50% | 2.5 | Head | ++ | +++ | Medium thickness and thick, irregular (predominantly solid) |
Pancreatic NET | Contained cystic component |
Note—IPMN = intraductal papillary mucinous neoplasm, MCN = mucinous cystic neoplasm.
Peripheral enhancement was classified as smooth or irregular and as thin (≤ 3 mm), medium (> 3 and ≤ 6 mm), or thick (> 6 mm).
Degree of peripheral enhancement was quantitatively graded by obtaining the difference of average attenuation of an enhancing component and of normal pancreatic parenchyma at the arterial phase and portal venous phase: − = ≤ 10 HU, ± = between 10 and ≤ 20 HU, + = between 20 and ≤ 40 HU, ++ = between 40 and ≤ 60 HU, or +++ = >60 HU.
Multiple endocrine neoplasia type 1.
TABLE 3.
Characteristics of Large (> 3 cm) Cystic Pancreatic Neuroendocrine Tumors (NETs)
| Case No. |
Cystic Change on CT |
Size (cm) |
Location | Peripheral Enhancementa |
CT Diagnosis or Differential Diagnosis |
Gross Pathology | ||
|---|---|---|---|---|---|---|---|---|
| Arterial Phaseb |
Venous Phaseb |
CT Appearance (Internal Enhancing Structures) |
||||||
|
| ||||||||
| 12c | > 50% | 7.9 | Neck | ++ | ++ | Medium thickness and thick, irregular (thin and medium thickness septations) |
Pancreatic NET | Contained cystic component |
| 16 | > 50% | 4.7 | Tail | ++ | + | Thin and thick, crescentic, smooth (thin septations) |
Pancreatic NET, mucinous cystic neoplasm |
Contained cystic component |
| 17 | > 50% | 7.0 | Body | +++ | ++ | Thin and thick, irregular (thin and thick septations) |
Pancreatic NET | Contained cystic component, hemorrhagic component |
| 18c | ≤50% | 3.8 | Head | ++ | + | (Predominantly solid) | Pancreatic NET | Contained cystic component |
| 19 | ≤50% | 13.1 | Body and tail |
Hypodense | Hypodense | (Predominantly solid, hypodense) |
Pancreatic NET, GIST, sarcoma |
Solid |
| 20 | ≤50% | 3.3 | Body | ++ | + | Medium thickness, irregular (predominantly solid) |
Pancreatic NET | Contained cystic component, hemorrhagic component |
| 21 | ≤50% | 11.1 | Body and tail |
+ | ± | Thin and thick, irregular (thin and thick septations) |
Known pancreatic NET | Contained cystic component |
Note—GIST = gastrointestinal stromal tumor.
Peripheral enhancement was classified as smooth or irregular and as thin (≤ 3 mm), medium (> 3 and < 6 mm), or thick (> 6 mm).
Degree of peripheral enhancement was quantitatively graded by obtaining the difference of average attenuation of an enhancing component and of normal pancreatic parenchyma at the arterial phase and portal venous phase: − = ± 10 HU, ± = between 10 and ≤ 20 HU, + = between 20 and ≤ 40 HU, ++ = between 40 and ≤ 60 HU, or +++ = >60 HU.
Multiple endocrine neoplasia type 1.
Three of the 73 (4.1%) tumors visualized on CT were nearly 100% cystic in appearance on CT (Figs. 1 and 2), and all three were small (average, 1.1 cm) (Table 2). Histologic sections of the lesions revealed tumor cells lining the cystic wall (Fig. 2C).
Fig. 1.
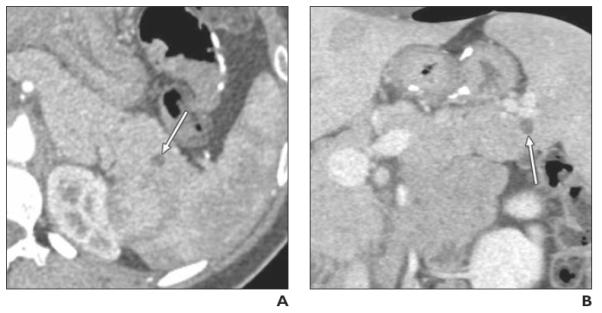
Well-differentiated pancreatic neuroendocrine tumor (NET), nonfunctioning, found on CT performed at another institution in 24-year-old woman with abdominal pain (case 3 in Table 2). Endoscopic ultrasound and fine-needle aspiration showed pancreatic NET.
A and B, Axial arterial phase (A) and coronal venous phase (B) multiplanar reformation images show purely cystic, unilocular cystic lesion (arrow) in tail of pancreas. CT differential diagnosis included intraductal papillary mucinous neoplasm and other cystic lesions.
Fig. 2.
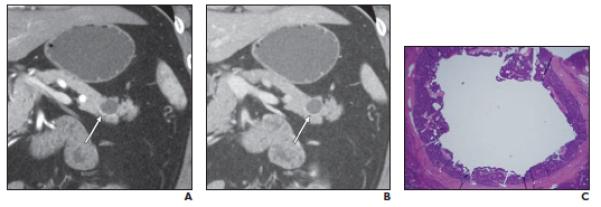
Well-differentiated neuroendocrine tumor (NET), nonfunctioning, in 56-year-old man. Cystic mass was initially found on CT performed for abdominal pain; mass had increased in size during follow-up. Fine-needle aspiration performed at another institution revealed well-differentiated NET (case 2 in Table 2).
A,Coronal arterial phase multiplanar reformation (MPR) image shows purely cystic mass in tail of pancreas with equivocal minimal smooth rim of enhancement along inferior border (arrow).
B,Coronal venous phase MPR image shows purely cystic mass (arrow) without detectable peripheral enhancement.
C,Photomicrograph (H and E, 2×) shows there is unilocular cyst in center of tumor. Cyst is lined by neuroendocrine cells.
Ten of 73 (13.7%) tumors were more than 50% cystic in appearance on CT (Tables 2 and 3): Seven were small (Fig. 3) and three were large. Cystic changes were confirmed by gross pathology in these 10 tumors. Overall, 13 of all 73 (17.8%) tumors were predominantly (≈ 100% and >50%) cystic.
Fig. 3.
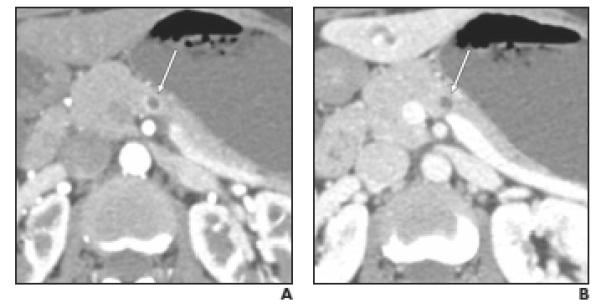
Well-differentiated neuroendocrine tumor (NET), nonfunctioning, in 47-year-old woman. Small cystic lesion was incidentally found on CT performed for evaluation of hematuria. Endoscopic ultrasound and fine-needle aspiration revealed pancreatic NET (case 6 in Table 2).
A,Axial arterial phase image shows partially (> 50%) cystic mass with thin, smooth peripheral enhancement (arrow) greater than that of pancreatic parenchyma.
B,Axial venous phase image shows small cystic mass (arrow) with no detectable peripheral enhancement.
Nine of the 73 (12.3%) tumors including five small tumors and four large tumors were 50% or less cystic in appearance on CT (Tables 2 and 3). In seven tumors, cystic changes were confirmed by pathologic examination. In two of nine tumors, no cystic changes were found by pathologic examination; one small tumor (case 13 in Table 1) had a small oval central hypodense component (13 HU in arterial phase, 22 HU in portal venous phase). This finding was thought to represent a slowly enhancing solid component. One large tumor (case 19 in Table 3) had central ill-defined nodular and stellate-shaped linear hypodense areas (21 HU in arterial phase, 24 HU in portal venous phase) that corresponded to a central scar on pathologic examination. Fifty-one pancreatic NETs were entirely solid in appearance on CT.
The average CT attenuation of the cystic component in 20 pancreatic NETs in which cystic changes were confirmed by pathologic examination was 15.8 ± 7.7 HU on arterial phase (range, 0–28 HU) and 16.8 ± 7.0 HU on venous phase (range, 8–29 HU). Cystic pancreatic NETs (including ≈ 100%, > 50%, and ≤ 50% cystic) and their solid counterparts were similar in size (mean ± SD, 3.1 ± 2.7 vs 3.0 ± 2.6 cm, respectively; p = 0.9749).
Peripheral contrast enhancement greater than normal pancreatic parenchyma was observed in 11 of 13 (85%) predominantly (≈ 100% and > 50%) cystic tumors (Figs. 2–4) including one case of equivocal peripheral enhancement (Fig. 2). Peripheral contrast enhancement was more conspicuous on arterial phase images in 14 cystic appearing tumors (Figs. 2 and 3), the venous phase in three tumors, and similar degrees on both arterial and venous phases in two tumors (Tables 2 and 3).
Fig. 4.

Well-differentiated neuroendocrine tumor (NET), nonfunctioning, in 43-year-old man (case 16 in Table 3).
A and B, Arterial phase (A) and venous phase (B) coronal multiplanar reformation images show large partially (>50%) cystic mass with thin-to-thick, smooth peripheral enhancement. Note focal crescentic thickening (arrow) along inferior border with intense contrast enhancement best seen on arterial phase. CT differential diagnosis included pancreatic NET and mucinous cystic neoplasm.
The mean attenuation value of the normal pancreatic parenchyma in 13 patients with predominantly (≈ 100% and > 50%) cystic pancreatic NETs was 87.1 HU in the arterial phase and 85.3 HU in the portal venous phase. The mean attenuation difference between the normal pancreatic tissue and peripheral enhancing component in 11 predominantly (≈ 100% and > 50%) cystic pancreatic NETs was 36.3 HU in the arterial phase and 25.9 HU in the portal venous phase.
An internal nodular structure within the cystic component was seen in one small tumor with a cystic component of 50% or less. Thin-to-thick septations were seen in three large tumors with more than 50% cystic component and one large tumor with 50% or less cystic component. On pathologic examination, these tumors contained an irregular unilocular cyst with tongues of cyst wall extending into the tumor or had large macrocysts in the center of the mass with multiple small peripheral cysts, which may be communicated.
Two small cystic tumors and one large cystic tumor had a preoperative diagnosis of pancreatic NET before CT based on endoscopic ultrasound and fine-needle biopsy. For other cases of cystic tumors, pancreatic NET was suspected or considered as a differential diagnosis on the basis of the CT findings in eight of 13 (62%) small (≤ 3 cm) cystic pancreatic NETs and six of six (100%) large (> 3 cm) cystic pancreatic NETs, all of which showed peripheral contrast enhancement.
Predominantly (≈ 100% and >50%) cystic pancreatic NETs were more commonly located in the neck, body, or tail of the pancreas than in the head or uncinate compared with solid pancreatic NETs (p = 0.0435 for ≤ 3 cm tumors, p = 0.0165 for tumors of all sizes) [5, 6].
Twenty-five of 51 (49%) solid pancreatic NETs were associated with lymph node or liver metastasis; one of 13 (8%) predominantly (≈ 100% and > 50%) cystic pancreatic NETs was associated with lymph node or liver metastasis. Lymph node and liver metastases were less commonly associated with predominantly (≈ 100% and > 50%) cystic pancreatic NETs than solid pancreatic NETs (p = 0.0402 for ≤ 3 cm tumors, p = 0.0066 for tumors of all sizes).
Discussion
Our study reports the largest analysis of CT findings of cystic pancreatic NETs. Eighteen percent of these NETs were predominantly cystic in appearance. The CT findings discussed in this article have been described previously in the literature; however, the number of cases in this study is larger than in previous studies. The most compelling observations are of the larger-than-anticipated incidence of cystic pancreatic NETs and the importance of the arterial phase in detecting the presence of the enhancing rim—the CT finding that would enable a diagnosis of cystic pancreatic NET and differentiation from other cystic lesions.
Pancreatic NETs typically appear as solid tumors on imaging, and most are hyper- attenuating on arterial and venous phase CT because of their rich vascularity [5]. Cystic pancreatic NETs were classically considered very rare; however, recent published studies suggest that cystic pancreatic NETs may be more common than previously thought [7]. Four of 38 (10.5%) pancreatic NETs were cystic in a study by Ahrendt et al. [8]. Goh et al. [9] reported six of 38 (15.8%) pancreatic NETs were cystic on radiologic imaging including CT, ultrasound, and MRI. Bordeianou et al. [10] recently reported that 29 of 170 (17%) pancreatic NETs were cystic using gross pathology as the gold standard. Baker et al. [11] reported that 13 of 62 (21%) resectable pancreatic NETs were cystic. In studies using endoscopic ultrasound, pancreatic NETs were cystic in eight of 86 (9.3%) cases, as reported by Figueiredo et al. [12], and in eight of 81 (9.9%) cases, as reported by Atiq et al. [13].
In some prior studies, cystic pancreatic NETs were larger than their solid counterparts [6, 9, 10]. For example, Buetow et al. [6] reported pancreatic NETs with areas of necrosis or cystic change found pathologically on imaging studies (56/133) were larger (mean, 8.4 cm) than homogeneous solid lesions (mean, 2.9 cm) and were predominantly non–insulin producing and nonhyperfunctioning. However, in other studies, solid and cystic pancreatic NETs were reported to be similar in size [8, 12]. In our current study, 17.8% of tumors were predominantly (≈ 100% and > 50%) cystic on CT, and cystic pancreatic NETs were similar to their solid counterparts in size.
Almost completely cystic pancreatic NETs are nonspecific in appearance on CT and were impossible to further characterize using CT findings. Three of 69 (3.4%) pancreatic NETs in this study were nearly purely cystic on CT and were seen as a unilocular cystic mass with a hairline-thin wall (Figs. 1 and 2). These nearly purely cystic pancreatic NETs were impossible to differentiate from other cystic masses such as branch duct–type intraductal papillary mucinous neoplasm, mucinous cystic neoplasm, or oligocystic serous cystadenoma. In our series, all three tumors of the purely cystic pancreatic NETs were small, with an average size of 1.1 cm. All other cystic pancreatic NETs in this study had some degree of peripheral enhancing component. Most of the small, more-than-50% cystic tumors in this study had thin-to–medium thickness smooth peripheral enhancement and one had a focal crescentic thick enhancing rim. Among three large, more-than-50% cystic pancreatic NETs, all had thin-to-thick peripheral enhancement and one had a focal crescentic thick enhancing rim (Fig. 4). Peripheral enhancement is often smooth in predominantly cystic pancreatic NETs but may be thicker in some parts and occasionally may be focally thickened with a crescentic appearance. An internal nodular or solid component or septations may enhance intensely similar to peripheral contrast enhancement (Fig. 5).
Fig. 5.
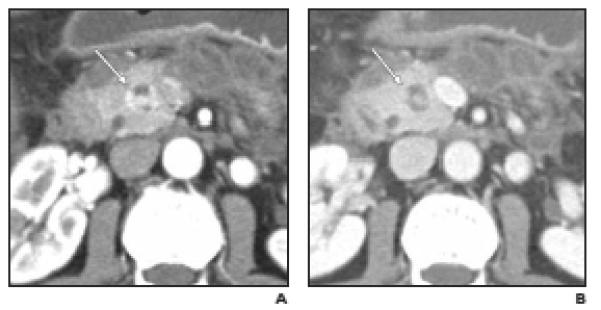
Well-differentiated neuroendocrine tumor (NET), nonfunctioning, in 51-year-old woman. Pancreatic head mass was found on CT performed for evaluation of pancreatitis (case 14 in Table 2).
A and B,Arterial phase (A) and venous phase (B) axial images show partially (≤50%) cystic small mass in head of pancreas (arrow). Note that intense contrast enhancement of peripheral and internal nodular components is visible only on arterial phase. Peripancreatic inflammation and fluid collections are due to pancreatitis. CT differential diagnosis included pancreatic NET and other tumors.
A peripheral hypervascular rim is considered the radiologic feature most suggestive of cystic pancreatic NETs [14]. Ligneau et al. [14] reported that in all 10 of 10 patients with a cystic pancreatic NET who were evaluated with CT, a marked hypervascular rim surrounding the cyst was observed. However, Baker et al. [11] reported that CT showed a hypervascular rim in the arterial phase in only three of 13 cystic pancreatic NETs. The remaining patients had bland, homogeneous, uniformly hypoenhancing lesions [11]. However, in that study, the technique of CT scanning was not specifically described, and it is not certain whether all patients underwent a dedicated CT protocol including arterial phase imaging.
A potential pitfall for the diagnosis of cystic pancreatic NET on CT is that peripheral contrast enhancement may be seen only transiently. Solid pancreatic NETs are known to, in general, enhance to a greater degree than normal pancreatic parenchyma during the arterial and portal venous phases of bolus contrast administration [15–17]. However, occasionally, small tumors are seen only on either arterial or portal venous phase images [15, 16]. The enhancing characteristic of the solid components of predominantly cystic pancreatic NETs in our study was similar to solid pancreatic NETs. Peripheral contrast enhancement was often observed more clearly on the arterial phase than on the venous phase and occasionally was seen only on the arterial phase. No cystic pancreatic NETs in this study showed peripheral contrast enhancement not visualized on the arterial phase and only visualized on the venous phase. If the tumor is not scanned during the phase of predominant contrast enhancement, the peripheral enhancing component may appear isodense to surrounding pancreatic parenchyma and these tumors may be mischaracterized as a nonspecific cystic mass (Figs. 2 and 3). A dedicated pancreas protocol including arterial phase scanning is essential to detect peripheral contrast enhancement and to avoid this misinterpretation. In this study, however, the specificity of peripheral contrast enhancement was not evaluated.
The cause of cyst formation of cystic pancreatic NET remains controversial, and several hypotheses have been proposed [9]. Infarction and liquefaction necrosis of large tumor were proposed to account for the cystic appearance, given the observation that the presence of cystic change correlated with increasing tumor size [6]. Internal hemorrhage has been reported within the cystic space of pancreatic NETs [18]. In some reports, cyst formation in pancreatic NETs does not appear to be caused by necrosis; rather, the cysts are typically filled with a serosanguineous fluid instead of necrotic debris and lined by well-preserved NET [19, 20]. In our patients, typically cystic pancreatic NETs contained clear to straw-colored fluid, and some were hemorrhagic. Necrosis was uncommon.
In our study, predominantly cystic tumors were more likely located in the neck, body, or tail of the pancreas as opposed to the head or uncinate. In prior studies, cystic pancreatic NETs were predominantly located in the body and tail of the pancreas. Ahrendt et al. [8] reported that three of four cystic pancreatic NETs were located in the body or tail of the pancreas and that cystic non-NETs were significantly (p < 0.05) more likely to be located in the head of the pancreas than pancreatic NETs. Ligneau et al. [14] reported that eight of 13 cystic pancreatic NETs were located in the body or tail. In a study by Goh et al. [9], four of six cystic pancreatic NETs were located in the body or tail; Bordeianou et al. [10] reported 22 of 29 cystic pancreatic NETs were also located in the body or tail of the pancreas. However, no significant difference was found in the location of the cystic pancreatic NETs versus the solid pancreatic NETs [9, 10].
Among the cases in our series, cystic pancreatic NETs were less likely associated with liver or lymph node metastasis, liver or lymph node metastasis, although not all patients with pancreatic NETs underwent regional lymphadenectomy. Our results differ from those reported in the existing literature. Figueiredo et al. [12] evaluated 86 pancreatic NETs including eight cystic tumors and found there was no significant difference in the presence of metastasis between solid pancreatic NETs and cystic pancreatic NETs. Bordeianou et al. [10] also reported there was no propensity for hepatic or lymph node metastasis in cystic versus solid pancreatic NETs. In the study by Bordeianou et al., however, the cystic pancreatic NETs were larger than the solid pancreatic NETs. In our institution, a clinicopathologic study of a larger number of patients with cystic pancreatic NETs was recently completed; it also showed a tendency similar to our current study regarding the location and presence of regional lymph node metastasis and synchronous distant metastasis of cystic pancreatic NETs compared with solid pancreatic NETs [21]. Singhi et al. [21] also found that neoplastic cells of the cystic pancreatic NETs were more likely well differentiated with a low mitotic rate and low Ki-67 proliferation index. Additional studies are necessary regarding these issues.
In the past, nonfunctioning pancreatic NETs were usually discovered only if they grew large enough to cause symptoms because of their size or metastases [7]. However, increasing numbers of nonfunctioning pancreatic NETs have been detected when they are small and therefore are more likely to be curable as a result of improved diagnostic methods including the widespread use of abdominal CT [22] and an increased resection rate [23]. Nonfunctioning pancreatic NETs were reported to be more prevalent than functioning pancreatic NETs and accounted for approximately 85% of all pancreatic NETs [4, 24]. Most of the cases in our study were clinically nonfunctioning tumors and were found because of nonspecific abdominal symptoms or were found incidentally on imaging studies.
There are several limitations in our study. First, this study is retrospective. Second, “cystic” tumor was defined by CT criteria: We defined “cystic” as when we observe fluid density (< 30 HU on both arterial and venous phases) within a mass. “Necrotic” and “cystic” changes are probably difficult to differentiate from one another on the basis of CT findings. Third, the mean interval between CT and surgery was 32 days, and the disease may have progressed during that interval. However, pancreatic NETs grow slowly, and this possibility is probably of no significance in data analysis. Fourth, quantitative analyses of peripheral enhancement of cystic masses may not be accurate because of the small size of the enhancing component.
In conclusion, cystic pancreatic NETs are not rare and occasionally can appear entirely cystic on CT. Cystic pancreatic NET should be considered as part of the differential diagnosis of a cystic pancreatic mass particularly if a cystic lesion is associated with peripheral contrast enhancement. A dedicated CT protocol including arterial phase imaging is essential to detect peripheral contrast enhancement.
Footnotes
WEB
This is a Web exclusive article.
References
- 1.Jensen RT. Pancreatic neuroendocrine tumors: overview of recent advances and diagnosis. J Gastrointest Surg. 2006;10:324–326. doi: 10.1016/j.gassur.2005.08.022. [DOI] [PubMed] [Google Scholar]
- 2.Verbeke CS. Endocrine tumours of the pancreas. Histopathology. 2010;56:669–682. doi: 10.1111/j.1365-2559.2010.03490.x. [DOI] [PubMed] [Google Scholar]
- 3.Metz DC, Jensen RT. Gastrointestinal neuroendocrine tumors: pancreatic endocrine tumors. Gastroenterology. 2008;135:1469–1492. doi: 10.1053/j.gastro.2008.05.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bilimoria KY, Tomlinson JS, Merkow RP, et al. Clinicopathologic features and treatment trends of pancreatic neuroendocrine tumors: analysis of 9,821 patients. J Gastrointest Surg. 2007;11:1460–1467. doi: 10.1007/s11605-007-0263-3. discussion, 1467-1469. [DOI] [PubMed] [Google Scholar]
- 5.Lewis RB, Lattin GE, Jr, Paal E. Pancreatic endocrine tumors: radiologic-clinicopathologic correlation. RadioGraphics. 2010;30:1445–1464. doi: 10.1148/rg.306105523. [DOI] [PubMed] [Google Scholar]
- 6.Buetow PC, Parrino TV, Buck JL, et al. Islet cell tumors of the pancreas: pathologic-imaging correlation among size, necrosis and cysts, calcification, malignant behavior, and functional status. AJR. 1995;165:1175–1179. doi: 10.2214/ajr.165.5.7572498. [DOI] [PubMed] [Google Scholar]
- 7.Patel KK, Kim MK. Neuroendocrine tumors of the pancreas: endoscopic diagnosis. Curr Opin Gastroenterol. 2008;24:638–642. doi: 10.1097/MOG.0b013e32830bf7fb. [DOI] [PubMed] [Google Scholar]
- 8.Ahrendt SA, Komorowski RA, Demeure MJ, Wilson SD, Pitt HA. Cystic pancreatic neuroendocrine tumors: is preoperative diagnosis possible? Gastrointest Surg. 2002;6:66–74. doi: 10.1016/s1091-255x(01)00020-8. [DOI] [PubMed] [Google Scholar]
- 9.Goh BK, Ooi LL, Tan YM, et al. Clinico-pathological features of cystic pancreatic endocrine neoplasms and a comparison with their solid counterparts. Eur J Surg Oncol. 2006;32:553–556. doi: 10.1016/j.ejso.2006.02.017. [DOI] [PubMed] [Google Scholar]
- 10.Bordeianou L, Vagefi PA, Sahani D, et al. Cystic pancreatic endocrine neoplasms: a distinct tumor type? J Am Coll Surg. 2008;206:1154–1158. doi: 10.1016/j.jamcollsurg.2007.12.040. [DOI] [PubMed] [Google Scholar]
- 11.Baker MS, Knuth JL, DeWitt J, et al. Pancreatic cystic neuroendocrine tumors: preoperative diagnosis with endoscopic ultrasound and fine-needle immunocytology. J Gastrointest Surg. 2008;12:450–456. doi: 10.1007/s11605-007-0219-7. [DOI] [PubMed] [Google Scholar]
- 12.Figueiredo FA, Giovannini M, Monges G, et al. EUS-FNA predicts 5-year survival in pancreatic endocrine tumors. Gastrointest Endosc. 2009;70:907–914. doi: 10.1016/j.gie.2009.05.020. [DOI] [PubMed] [Google Scholar]
- 13.Atiq M, Bhutani MS, Bektas M, et al. EUS-FNA for pancreatic neuroendocrine tumors: a tertiary cancer center experience. Dig Dis Sci. 2012;57:791–800. doi: 10.1007/s10620-011-1912-7. [DOI] [PubMed] [Google Scholar]
- 14.Ligneau B, Lombard-Bohas C, Partensky C, et al. Cystic endocrine tumors of the pancreas: clinical, radiologic, and histopathologic features in 13 cases. Am J Surg Pathol. 2001;25:752–760. doi: 10.1097/00000478-200106000-00006. [DOI] [PubMed] [Google Scholar]
- 15.Ichikawa T, Peterson MS, Federle MP, et al. Islet cell tumor of the pancreas: biphasic CT versus MR imaging in tumor detection. Radiology. 2000;216:163–171. doi: 10.1148/radiology.216.1.r00jl26163. [DOI] [PubMed] [Google Scholar]
- 16.King AD, Ko GT, Yeung VT, Chow CC, Griffith J, Cockram CS. Dual phase spiral CT in the detection of small insulinomas of the pancreas. Br J Radiol. 1998;71:20–23. doi: 10.1259/bjr.71.841.9534694. [DOI] [PubMed] [Google Scholar]
- 17.Stafford-Johnson DB, Francis IR, Eckhauser FE, Knol JA, Chang AE. Dual-phase helical CT of nonfunctioning islet cell tumors. J Comput Assist Tomogr. 1998;22:335–339. doi: 10.1097/00004728-199803000-00034. [DOI] [PubMed] [Google Scholar]
- 18.Takeshita K, Furui S, Makita K, et al. Cystic islet cell tumors: radiologic findings in three cases. Abdom Imaging. 1994;19:225–228. doi: 10.1007/BF00203512. [DOI] [PubMed] [Google Scholar]
- 19.Adsay NV. Cystic neoplasia of the pancreas: pathology and biology. J Gastrointest Surg. 2008;12:401–404. doi: 10.1007/s11605-007-0348-z. [DOI] [PubMed] [Google Scholar]
- 20.Adsay NV, Klimstra DS. Cystic forms of typically solid pancreatic tumors. Semin Diagn Pathol. 2000;17:81–88. [PubMed] [Google Scholar]
- 21.Singhi AD, Chu LC, Tatsas AD, et al. Cystic pancreatic neuroendocrine tumors: a clinicopathologic study. Am J Surg Pathol. 2012;36:1666–1673. doi: 10.1097/PAS.0b013e31826a0048. [DOI] [PubMed] [Google Scholar]
- 22.Vagefi PA, Razo O, Deshpande V, et al. Evolving patterns in the detection and outcomes of pancreatic neuroendocrine neoplasms: the Massachusetts General Hospital experience from 1977 to 2005. Arch Surg. 2007;142:347–354. doi: 10.1001/archsurg.142.4.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Klöppel G. Tumour biology and histopathology of neuroendocrine tumours. Best Pract Res Clin Endocrinol Metab. 2007;21:15–31. doi: 10.1016/j.beem.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 24.Jani N, Khalid A, Kaushik N, et al. EUS-guided FNA diagnosis of pancreatic endocrine tumors: new trends identified. Gastrointest Endosc. 2008;67:44–50. doi: 10.1016/j.gie.2007.07.046. [DOI] [PubMed] [Google Scholar]


