Abstract
Background
Little is known about current surveillance patterns after treatment of colorectal liver metastasis (CRLM) or whether the intensity of surveillance correlates with outcome. We sought to define current population-based patterns of surveillance and investigate whether intensity of surveillance impacted outcome.
Methods
We queried the Surveillance, Epidemiology, and End Results–linked Medicare database for patients with CRLM diagnosed between 1991 and 2005 who underwent liver resection and/or tumor ablation. Frequency of post-treatment abdominal computed tomography (CT), magnetic resonance imaging (MRI), or positron emission tomography (PET) was recorded for ≤5 years after treatment. The association between frequency of imaging with secondary interventions and long-term survival were analyzed.
Results
We identified 1,739 patients with CRLM treated with surgery; median age was 73 years, and the majority were male (52.6%). CRLM treatment consisted of liver resection (61%), ablation (32%), or both simultaneously (6%). CT (97%) was utilized more often for post-treatment surveillance compared with MRI (7%) and PET (18%). A temporal trend was noted with more frequent surveillance imaging obtained in post-treatment year 1 (2.4 scans/year) versus year 5 (0.6 scans/year; P = .01); 66% of living patients had no imaging after 2 years. Frequency of surveillance imaging correlated with procedure type (total number of scans/5 years: resection, 5.0; ablation, 4.6; resection and ablation, 6.2; P = .01). Other factors associated with a greater frequency of surveillance included younger age at diagnosis, geographic location in the South, and CRLM directed surgery in 2000 through 2005 (all P < .05). Overall survival did not differ by intensity of surveillance imaging (3–4 scans/yr, 43 months vs 2 scans/yr, 57 months vs 1 scan/yr, 54 months; P = .08).
Conclusion
Marked heterogeneity exists in how often surveillance imaging is obtained after treatment of CRLM. Intensity of imaging does not affect time to second procedure or median survival duration. Surveillance guidelines for CRLM need to be refocused to provide the best value for healthcare resources. (Surgery 2013;154:256–65.)
Colorectal cancer (CRC) is among the most common primary malignancies in both men and women. Approximately 140,000 new cases of CRC are diagnosed every year in the United States resulting in >50,000 deaths per year, making CRC the third most common cause of cancer-related mortality.1 The liver is the most common site for CRC metastasis (CRLM); 15–25% of patients present with CRLM at the time of diagnosis of their primary cancer and an additional 15–40% of patients develop metachronous CRLM.2–4 Operative management in the form of resection or ablation provides the best hope for cure among patients with CRLM.5 Although 5-year survival has significantly improved over the last decade with the introduction of more effective systemic chemotherapy, recurrence after curative intent surgery for CRLM remains common.6,7 In fact, despite a 5-year survival of 50–55%, about two thirds of patients with CRLM have a recurrence.6
Given the relatively high incidence of recurrence, surveillance with postoperative cross-sectional imaging to detect asymptomatic recurrent disease is a widespread practice. Numerous different organizations and associations have, in fact, published various “best-practice” guidelines on follow-up management of patients with CRLM.8–11 Despite these guidelines and the ubiquitous use of postoperative surveillance imaging, there is a paucity of evidence on the effect of postoperative imaging on outcomes. Although intense, close surveillance with cross-sectional imaging may intuitively seem appealing, there has been concern about unnecessary exposure to radiation,12 as well as associated avoidable, high healthcare costs.13
Most previous investigations have not specifically examined imaging utilization in relation to specific malignant disease processes. Witkowski et al14 did report on utilization of imaging after pancreatic cancer resection and noted no survival benefit among patients who underwent scans on a routine basis. For most patients, survival after curative intent surgery for pancreatic cancer is short; in addition, the options to treat recurrent pancreatic cancer are very limited and associated with a very high case mortality. In contrast, patients with CRLM have a much greater survival, and therefore a greater period for possible surveillance. In addition, there are a number of effective therapeutic options for recurrent CRLM that may advantage early detection of recurrent disease by cross-sectional imaging.15–17 Little is known about surveillance patterns after treatment of CRLM or whether the intensity of surveillance correlates with outcome. As such, we sought to define the pattern of utilization, as well as intensity of postoperative abdominal imaging after CRLM surgery using the population-based Surveillance, Epidemiology and End Results (SEER)-linked Medicare database. In addition, we investigated whether the intensity/frequency of surveillance impacted long-term survival.
METHODS
Data source and study population
This retrospective study analyzed prospectively collected data from the linked SEER–Medicare database. The SEER–Medicare database represents the unique linkage of 2 large, population-based sources of data that provide detailed information about Medicare beneficiaries with cancer.4,18 SEER data derive from 18 cancer registries, representing approximately 26% of the United States population, and is maintained by the National Cancer Institute.18 In brief, all Medicare-enrolled patients aged ≥66 years diagnosed with incident malignant primary colorectal adenocarcinoma between 1991 and 2005 in SEER were identified using International Classification of Diseases for Oncology topography, behavior, and histology codes (8140, 8210, 8261, 8263, 8480, 8481, 8141, 8144, 8211, 8260, 8262, 8490). Identification of patients with primary CRC who had hepatic metastasis was accomplished using an established algorithm, as described previously.4 The study cohort included only patients enrolled in Medicare parts A and B who were not enrolled in a managed care plan during the study period. In addition, given that the focus of the current study was on postoperative surveillance imaging, only patients with ≥1 year of follow-up data were included in the study cohort.
Data collection
Data on perioperative procedures, treatments, and complications were selected a priori based on clinical relevance and then identified from the Medicare database using both ICD-9-CM diagnosis and procedure codes, as well as CPT codes.4,19 Information on chemotherapy was designated as preoperative (within 6 months prior) and adjuvant (within 3 months after) liver-directed operation. Information on age, gender, race, marital status, and geographic region were obtained from the SEER portion of the database. Variables were transformed into categorical and indicator variables where appropriate. The Elixhauser comorbidity index, a comprehensive set of 30 comorbidity measures, was used to identify and adjust for comorbid conditions.20
Using the inpatient, outpatient, and provider billing records, data on abdominal computed tomography (CT), magnetic resonance imaging (MRI), and positron emission tomography (PET) were extracted for ≤5 years after the operative procedure. CPT codes were identified and imaging recorded based on claims made to Medicare.14 Scans performed within 3 months of the operative procedure were excluded, because these scans may have been related to staging or perioperative complication management. Data on the mean and median number of scans per year for a given patient were calculated and trends in utilization of imaging were examined over time. Proportion of imaging obtained for any given year were calculated based on the number of patients alive in that year of follow-up. As described previously, to estimate an average annual rate the total number of scans for each patient was divided by the number of months of available follow-up data and multiplied by 12.14
Statistical analyses
Mean and median values were used to describe continuous data, with discrete variables displayed as totals and frequencies. Cells with <11 cases per variable cell were relabeled as “<11 (<%)” in compliance with the National Cancer Institute regulation for reporting of SEER–Medicare data. Univariate comparisons were assessed using the 2-sample Student t-test for continuous variables and the Chi-square test for dichotomous and categorical variables. For purposes of analyses, the distribution of the total number of comorbid conditions per patient was divided into quartiles: 0, 1–2, or ≥3 comorbidities. Cumulative event rates were calculated using the Kaplan–Meier method, and survival curves were compared using the log-rank test. Overall survival time was calculated from the date of the liver-directed operation for CRLM to the date of last follow-up. To assess the impact of imaging on long-term survival, we performed a more restricted analysis on patients who had had survived for ≥2 years after the operative procedure. This mitigated against the effect of differential scanning frequency owing to varying time of follow-up due to early death. In case of differences in distribution of covariates among comparison groups, propensity score matching was used to create comparable cohorts. Univariate comparisons of survival were performed using the log-rank test. Multivariate modeling of survival was performed using Cox proportional hazards models. Covariates were included in the multivariate Cox model based on statistical significance in the univariate models (P≤.20). The overall fit of the multivariate models were assessed using the likelihood ratio test. Relative risks were expressed as hazard ratios (HR) with a 95% confidence interval (CI). Adherence to the proportional hazards assumption was assessed using Schoenfeld residuals and log–log plots. All reported P values are 2-tailed. All statistical analyses were performed using SAS 9.3 (SAS Corp., Cary, NC).
RESULTS
Patient, disease, and treatment characteristics
Utilizing the SEER database, 1,739 patients with CRLM who underwent operative management and who met study criteria were identified. Most patients were diagnosed between 2000 and 2005 (n = 1,072; 62%); the remaining cohort of 667 patients were diagnosed between 1991 and 1999. The demographic and clinical characteristics of the patients are outlined in Table I. The median patient age was 73 years (interquartile range, 68–78). The majority of patients were white (n = 1,510 [87%]) and slightly more than half of the patients were male (n = 914 [53%]). Most patients lived in an urban location (n = 1,466 [84%]), were from the Western United States (n = 813 [47%]), and belonged to top 2 quintiles of socioeconomic status (n = 1,068 [61%]). At the time of operation, a subset of patients had no comorbidities (n = 769 [44%]), whereas 632 patients (36%) had 1–2 comorbidities and 338 (19%) had ≥3 comorbidities. The most common noted comorbidities were hypertension (n = 594 [34%]), deficiency anemias (n = 367 [21%]), diabetes mellitus (n = 171 [10%]), and chronic pulmonary disease (n = 165 [10%]).
Table I.
Demographic and clinical characteristics of patients at index admission
| Characteristic | n (%) |
|---|---|
| Total | 1,739 (100) |
| Age | |
| 66–69 | 541 (31.1) |
| 70–74 | 498 (28.6) |
| 75–79 | 375 (21.6) |
| ≥80 | 325 (18.7) |
| Male gender | 914 (52.6) |
| Race | |
| White | 1,510 (86.8) |
| Black | 103 (5.9) |
| Other | 126 (7.3) |
| Urban location | 1,466 (84.3) |
| Top 2 quintiles of SES | 1,068 (61.4) |
| Geographic location | |
| Northeast | 329 (18.9) |
| West | 813 (46.8) |
| Midwest | 375 (21.6) |
| South | 222 (12.8) |
| Location of primary | |
| Colon | 1,318 (75.8) |
| Rectum | 421 (24.2) |
| SEER historic stage of CRC primary | |
| Localized/in situ | 379 (21.8) |
| Regional | 677 (38.9) |
| Distant | 683 (39.3) |
| Comorbidities | |
| 0 | 769 (44.2) |
| 1–2 | 632 (36.3) |
| ≥3 | 338 (19.4) |
| First procedure after diagnosis | |
| Hepatectomy | 1,066 (61.3) |
| Ablation | 564 (32.4) |
| Hepatectomy + ablation | 109 (6.3) |
| All procedures within 3 months of diagnosis | |
| Hepatectomy alone | 1,030 (59.2) |
| Ablation alone | 544 (31.3) |
| Hepatectomy + ablation | 165 (9.5) |
CRC, Colorectal cancer; SEER, Surveillance, Epidemiology, and End Results; SES, socioeconomic status.
A majority of patients had the colon as the primary tumor site (n = 1,318 [76%]), whereas 421 (24%) had a primary rectal neoplasm. Lymph node metastases were associated with the primary colorectal tumor in 750 patients (43%). Presentation of the hepatic metastasis was synchronous in 960 (55%). Among the 779 patients (45%) with metachronous disease, the median disease-free interval between diagnosis of the primary CRC and the CRLM was 18.6 months (interquartile range, 10.8–32.3). At the time of liver-directed operation, most patients underwent hepatectomy only (n = 1,066 [61%]); fewer underwent only ablation (n = 564 [32%]); 109 patients (6%) underwent concurrent hepatectomy and tumor ablation. Operative resection consisted of at least a hemihepatectomy in 438 patients (37%), whereas 757 (63%) underwent partial/wedge resection.
After the index liver-directed surgery, the overwhelming majority of patients (n = 1,665 [96%]) did not undergo a second liver-directed procedure. A repeat liver-directed procedure (ie, second hepatic resection, ablation) was undertaken, however, in a small subset of patients (n = 74 [4%]).
Intensity and factors associated with follow-up imaging
Among the 1,739 patients with CRLM who underwent surgery, a total of 5,707 imaging procedures were performed from the time of operation until being censored at 5 years after surgery or death. Overall utilization of surveillance abdominal imaging after surgery for CRLM increased over time (Fig 1). The median number of scans performed annually was 1 for patients operated from 1991 through 1999 compared with 3 for patients undergoing surgery from 2000 through 2005. Of note, although 1,151 patients (66%) underwent cross-sectional abdominal imaging after operation, a large subset of patients (n = 588 [34%]) did not have any recorded instance of abdominal imaging after operation. Among those 1,151 patients who had an imaging scan, most patients had a CT (n = 1,112 [97%]); fewer had an MRI (n = 85 [7%]) or PET (n = 201 [18%]). The overall yearly utilization of MRI and PET scanning increased slightly over time, but the greatest increase in imaging was largely attributable to an increase in CT (Fig 2).
Fig 1.
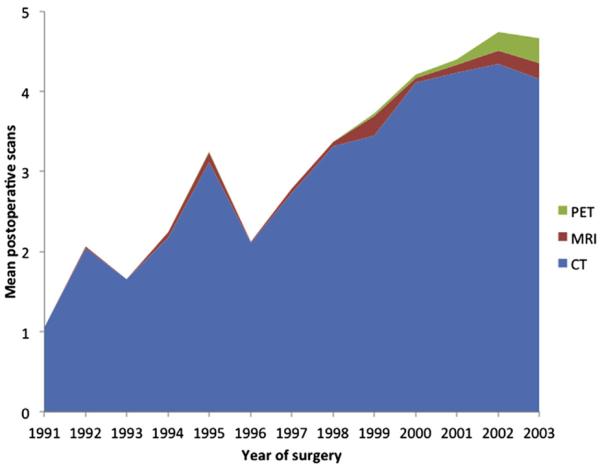
Mean number of imaging scans per patient within 5 years of surgery for colorectal liver metastasis.
Fig 2.
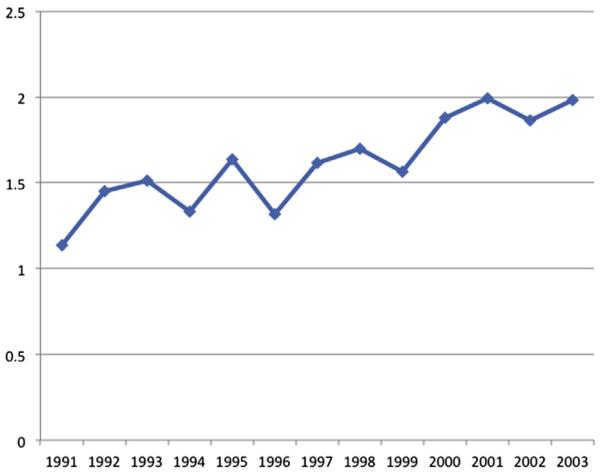
Annual rate of imaging utilization per patient, adjusted for postoperative survival.
In the first year after surgery, 1,068 patients (61%) had ≥1 abdominal scan; with each subsequent year of follow-up the proportion of patients having ≥1 imaging scan decreased (year 2, n = 729 [44%]; year 3, n = 366 [30%]; year 4, n = 207 [25%]; year 5, n = 96 [16%]). After adjusting for postoperative survival time, among the subset patients who had ≥1 scan per year, the intensity of scanning similarly decreased (year 1, 2.4 scans/year vs year 2, 1.5 scans/year vs year 3, 1.0 scan/year vs year 4, 0.8 scan/year vs year 5, 0.6 scan/year; P < .001 for trend). Overall, 66% of patients (n = 788) who were alive at 2 years post-procedure did not receive any abdominal imaging scans during subsequent follow-up. A number of factors were associated with receipt of any postoperative surveillance imaging (Table II). Specifically, there were differences in both patient and non-patient factors between individuals who had ≥1 imaging procedure recorded and those who did not. Patients who had postoperative surveillance imaging were more likely to be younger (66–69 years vs ≥80 years: odds ratio [OR], 2.18; 95% CI, 1.38–3.44), have lymph node metastasis associated with the primary colorectal tumor (N1 vs N0/Nx: OR, 1.64; 95% CI, 1.25–2.15), live in the Midwest or South region (Midwest vs West: OR, 2.18; 95% CI, 1.43–3.31; South vs West: OR, 1.87; 95% CI, 1.13–3.11), have synchronous CRLM (OR, 9.58; 95% CI, 6.26–14.66), and have undergone a hepatectomy only (OR, 2.28; 95% CI, 1.62–3.22; all P < .05). Patients undergoing surgery in 2000 or later were also more likely to have had postoperative surveillance imaging (OR, 4.02; 95% CI, 2.89–5.60; P < .001). Socioeconomic status was not associated with receipt of follow-up imaging either on univariate or multivariate analysis (univariate OR high vs low SES: 0.93; 95% CI, 0.76–1.14; P = .46). Although race showed an association with receipt of imaging on univariate analysis (white vs non-white: OR, 1.40; 95% CI, 1.06–1.86; P = .02), the association did not remain significant in the multivariate adjusted model (OR, 1.30; 95% CI, 0.95–1.80; P = .07).
Table II.
Characteristics of patients by scanning intensity*
| Regular, periodic scanning, n (%) |
|||||
|---|---|---|---|---|---|
| No scans (n = 466), n (%) | 1 scan in 2 y (n = 251), n (%) | 1–2 scans/y (n = 412) | 3–4 scans/y (n = 94) | P value | |
| Age | |||||
| 66–69 | 124 (26.6) | 82 (32.7) | 133 (32.3) | 39 (41.5) | <.001 |
| 70–74 | 120 (25.8) | 81 (32.3) | 143 (34.7) | 33 (35.1) | |
| ≥75 | 222 (47.6) | 88 (35.1) | 136 (33.0) | 22 (23.4) | |
| Male gender | 206 (44.2) | 129 (51.4) | 238 (57.8) | 53 (56.4) | .01 |
| White race | 383 (82.2) | 218 (86.6) | 374 (90.8) | 83 (88.3) | .03 |
| Urban location | 395 (84.8) | 210 (83.7) | 346 (84.0) | 83 (88.3) | .73 |
| Top 2 quintiles of SES | 283 (60.7) | 152 (60.6) | 265 (64.3) | 63 (67.0) | .49 |
| Geographic location | |||||
| Northeast | 75 (16.1) | 50 (19.9) | 83 (20.2) | 20 (21.3) | <.001 |
| West | 250 (53.7) | 105 (41.8) | 178 (43.2) | 41 (43.6) | |
| Midwest | 96 (20.6) | 61 (24.3) | 102 (24.8) | 12 (12.8) | |
| South | 54 (9.7) | 35 (13.9) | 49 (11.9) | 21 (22.3) | |
| Location of primary | |||||
| Colon | 371 (79.6) | 183 (72.9) | 300 (72.8) | 69 (73.4) | .07 |
| Rectum | 95 (20.4) | 68 (27.1) | 112 (27.2) | 25 (26.6) | |
| SEER historic stage of CRC primary | |||||
| Localized/in situ | 193 (41.4) | 42 (16.7) | 55 (13.4) | 14 (14.9) | <.001 |
| Regional | 199 (42.7) | 103 (41.0) | 148 (35.9) | 30 (31.9) | |
| Distant | 74 (15.9) | 106 (42.2) | 209 (50.7) | 50 (53.2) | |
| Any comorbidity | 232 (49.8) | 142 (56.6) | 244 (59.2) | 49 (52.1) | .04 |
| First procedure after diagnosis | |||||
| Hepatectomy | 283 (60.7) | 177 (70.5) | 285 (69.2) | 50 (53.2) | <.001 |
| Ablation alone or with hepatectomy | 183 (39.3) | 74 (29.5) | 127 (30.8) | 44 (46.8) | |
| Period of treatment | |||||
| 1991–1999 | 256 (54.9) | 71 (28.3) | 150 (36.4) | 17 (18.1) | <.001 |
| 2000–2005 | 210 (45.1) | 180 (71.7) | 252 (63.6) | 77 (81.9) | |
Among patients who survived for >2 years and did not undergo a second procedure during these years.
CRC, Colorectal cancer; SEER, Surveillance, Epidemiology, and End Results; SES, socioeconomic status.
Among patients who had ≥1 imaging event after surgery for CRLM and who had ≥2 years of follow-up (n = 757), factors associated with intensity of surveillance imaging were examined (≤2 vs ≥3 scans/year) (Table III). On both univariate and multivariate analyses, younger age, geographical region, and being diagnosed with CRLM after 2000 were associated with higher intensity of scanning. Patients age 66–69 years were 1.7 times more likely to be followed with more frequent imaging than patients ≥80 years (OR, 2.74; 95% CI, 1.13–6.78; P = .03). Patients in the Southern United States were also more likely to have more frequent surveillance imaging compared with patients in the Midwest, which had the lowest proportion of patients who underwent frequent scanning (OR, South vs Midwest: 2.76; 95% CI, 1.28–5.97; P = .02). In addition, patients diagnosed with CRLM in 2000 or later were twice as likely to have a high frequency of imaging compared with patients diagnosed between 1991 and 1999 (OR, 2.06, 95% CI 1.17–3.62; P = .01). Although disease stage at initial diagnosis of CRC and presence of medical comorbidities were not associated with intensity of surveillance, frequency of imaging did correlate with the index procedure type. The mean number of scans performed within 5 years of surgery was greater among patients undergoing resection and ablation (6.5) compared with resection (5.0) or ablation (4.6) only (P = .01).
Table III.
Factors associated with higher intensity of imaging on univariate and multivariate logistic regression
| Univariate |
Multivariate |
|||
|---|---|---|---|---|
| OR (95% CI) | P value | OR (95% CI) | P value | |
| Age (yrs) | ||||
| 66–69 | 2.66 (1.09–6.51) | .03 | 2.74 (1.13–6.78) | .03 |
| 70–74 | 2.16 (0.88–5.34) | .30 | 2.24 (0.94–5.82) | .21 |
| 75–79 | 1.73 (0.65–4.58) | .90 | 1.76 (0.66–4.70) | .86 |
| ≥80 | Reference | |||
| Male gender | 1.04 (0.68–1.61) | .85 | — | |
| Race | ||||
| White | Reference | |||
| Black | 0.86 (0.30–2.50) | .62 | — | |
| Other | 1.31 (0.57–3.04) | .48 | — | |
| Urban location | 1.45 (0.75–2.82) | .27 | — | |
| Top 2 quintiles of SES | 1.19 (0.76–1.90) | .44 | — | |
| Geographic location | ||||
| Northeast | 2.04 (0.96–4.33) | .77 | 1.86 (0.86–4.01) | .60 |
| West | 1.97 (1.01–3.86) | .89 | 1.95 (0.99–3.85) | .74 |
| Midwest | Reference | Reference | ||
| South | 3.40 (1.59–7.24) | .01 | 2.76 (1.28–5.97) | .04 |
| Location of primary | ||||
| Colon | 0.97 (0.60–1.58) | .91 | — | |
| Rectum | Reference | |||
| SEER historic stage of CRC primary | ||||
| Localized/in situ | Reference | |||
| Regional | 0.83 (0.42–1.63) | .34 | — | |
| Distant | 1.10 (0.58–2.08) | .41 | — | |
| Any comorbidity | 0.78 (0.51–1.21) | .26 | — | |
| Hepatectomy vs ablation alone | 0.78 (0.48–1.27) | .31 | — | |
| CRLM diagnosis in or after 2000 | 2.27 (1.31–3.93) | .01 | 2.06 (1.17–3.62) | .01 |
CI, Confidence interval; CRC, colorectal cancer; CRLM, colorectal liver metastasis; OR, odds ratio; SEER, Surveillance, Epidemiology, and End Results; SES, socioeconomic status.
Impact of imaging surveillance on survival
The overall median survival of patients who underwent operative management of CRLM was 47.0 months (95% CI, 43.2–50.3) with 3- and 5-year survivals of 61.4%, and 42.0%, respectively. A number of well-described factors were associated with survival such as age (HR 1.40; 95% CI, 1.19–1.68), preoperative medical comorbidities (HR 1.19; 95% CI, 1.01–1.40), synchronous disease (HR 1.17; 95% CI, 1.03–1.34), and treatment with ablation alone (HR 0.88; 95% CI, 0.77–0.99; all P <.05). Receipt of postoperative imaging was also associated with survival. Paradoxically, patients who had no record of postoperative imaging had a greater median survival (71.4 months; 95% CI, 64.7–86.5) compared with patients who had ≥1 postoperative imaging event recorded (39.3 months; 95% CI, 36.4–41.8; P < .01). Recognizing that there may be baseline differences among patients who did and did not undergo postoperative surveillance imaging, a matched propensity score analysis was then performed to account for these differences. After propensity score matching, the 507 patients who had no postoperative surveillance still had a survival advantage compared with patients who had undergone imaging (HR 1.73; 95% CI, 1.46–2.04; P ≤ .001).
To further evaluate the potential relationship between survival and imaging surveillance, and to avoid patients who had fewer scans owing to early death, we compared survival among the 757 patients who had survived for ≥2 years after the index procedure. Overall median survival was not associated with frequency of postoperative imaging (3–4 scans/year, 43.1 months vs 2 scans/year, 56.7 months vs 1 scan/year, 53.7 months; P = .08; Fig 3). After propensity score matching, patients who had 1 postoperative scan per year still had no survival advantage compared with patients who had multiple imaging (≥2 scans/year; HR, 1.30; 95% CI, 0.98–1.70; P = .05). Of note, among the 429 patients who survived to 5 years, only 44 (10.3%) had received imaging at intervals of ≤1 year. In addition, among the small subset of 74 patients who underwent a repeat liver-directed procedure, the median time interval to the second procedure was not associated with the frequency of postoperative imaging surveillance (3–4 scans/year, 11.5 months vs 2 scans/year, 13.0 months vs 1 scan/year, 13.0 months; P = .69).
Fig 3.
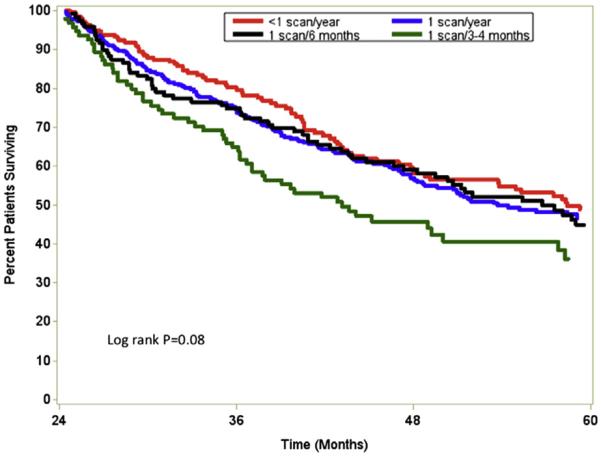
Overall survival stratified by intensity of postoperative imaging surveillance after surgery for colorectal liver metastasis.
DISCUSSION
Following patients after cancer surgery is a long-standing element of an oncology practice.21 As more patients survive longer after operations for cancer, the topic of survivorship and surveillance has become increasingly important.22 Depending on the site and histology of the primary tumor, surveillance may involve clinic visits, serum tumor makers, and cross-sectional imaging. In fact, postoperative surveillance imaging is almost universally employed in the follow-up of most patients with a history of a gastrointestinal malignancy. The use of routine, repeat imaging is driven not only by the provider's wish to identify recurrence early, but also by the patient's desire for testing to confirm that they remain disease free.23,24 Although several studies have attempted to investigate the impact of surveillance on outcomes, most previous work has focused on the impact of surveillance after surgery of the primary CRC.25–28 The current study is important, because we used national, population-based data to evaluate the utilization of postoperative surveillance imaging among patients undergoing surgery for metastatic CRC. Specifically, we demonstrated a significant increase in the use of surveillance imaging over time after surgery for CRLM. Perhaps as important, we noted dramatic variations in the use of surveillance imaging that were associated with not only patient-level characteristics, but also non-patient factors, such as geographic location of the hospital. In addition, a higher intensity of imaging did not affect time to second procedure, nor was it associated with improved median survival duration (Fig 3).
The general increase utilization of imaging, and CT in particular, has been well-documented. Several authors have noted a dramatic increase in noninvasive diagnostic radiology imaging in the United States over the last couple decades.29,30 In fact, Bhargavan et al30 reported an “across-the-board” increase in the utilization of high-technology modalities. MRI and nuclear medicine increased by >10% per year on average, and that of CT approximately of 8% annually, between 1992 and 2001. In a separate study, Dinan et al13 examined a nationally representative 5% sample of claims from the Centers for Medicare and Medicaid Services. These authors similarly noted an increase in utilization of radiologic studies, with the most pronounced increase in the use of PET and MRI. In the current study, we similarly noted a dramatic increase in the overall utilization and performance of radiologic imaging after surgery of CRLM. In fact, the median number of scans performed per year after CRLM tripled when comparing 1991 through 1999 versus 2000 through 2005. Although there was clearly an increased use of MRI and PET over time, the most notable increase in radiologic imaging was with CT (Fig 1). The increased use of CT scanning has not only been a cause of concern owing to the associated costs to the healthcare system, but also owing to the associated increased radiation exposure.12,31 In turn, an increasing number of providers and patients have begun to question the need for intense, repeat surveillance imaging in the postoperative period.
Unlike in metastatic setting, surveillance after surgery of primary CRC has been relatively well studied. A meta-analysis by Renehan et al32 reported on aggregate data from 5 clinical trials that examined the impact of frequent measurements of serum and carcinoembryonic antigen and intensive cross-sectional imaging. The authors concluded that intensive follow-up resulted in earlier detection of recurrences and an increased detection rate of isolated local recurrences. Jeffrey et al33 reported more recently a Cochrane Review on follow-up strategies for patients treated for non-metastatic CRC. Based on the 8 studies included in the review, the authors concluded that there was an overall survival benefit for intensifying the follow-up of patients after curative surgery for CRC. The analyses demonstrated a mortality benefit for performing more tests versus fewer tests (OR, 0.64) and liver imaging versus no liver imaging (OR, 0.64). There were also more curative operative procedures attempted in the intensively follow-up arm (OR, 2.41). Importantly, however, the authors commented that current data are lacking to guide the definition of “intensive” follow-up as well as what constitutes the exact optimal interval for follow-up. In addition, data on surveillance after resection of primary CRC may not necessarily be appropriate to extrapolate to the metastatic setting.
The current study begins to address the need for data on the impact of follow-up strategies for patients with CRLM. We found that a large subset of patients—about one third—did not have any recorded instance of abdominal imaging after surgery for CRLM. Perhaps not surprisingly, an important factor associated with receipt and intensity of imaging was patient age. As patient age advanced, provider utilization of surveillance imaging declined, perhaps suggesting the belief that detection of early recurrence was less important among the very elderly. Another factor that was strongly associated with receipt and intensity of surveillance imaging was geographic location of the hospital. Bhargavan et al30 had noted previously substantial variation in radiology services among states and census regions. In the current study, we found that patients in the Southern United States were more likely to have more frequent surveillance imaging compared with patients in the Midwest, which had the lowest proportion of patients who underwent frequent scanning. Although variation was noted in the intensity of surveillance imaging after CRLM surgery, we failed to detect any positive impact of surveillance intensity on long-term survival. In fact, we found that more frequent annual scanning was not associated with a survival benefit (Fig 3). In their paper looking at surveillance imaging after pancreatic cancer resection, Witkowski et al14 similarly demonstrated no survival benefit among patients who underwent scans on a routine basis. Although patients with recurrent CRLM may have more therapeutic options, it was interesting to note that only a very small subset of patients (4%) underwent a repeat liver-directed procedure, and the time to the second procedure did not seem to be associated with the intensity of follow-up surveillance imaging performed.
The present study had several limitations. The use of large administrative datasets, although providing data with a greater generalizability, suffers from a number of potential shortcomings. Specifically, despite utilizing a linked dataset such as SEER-Medicare, detailed data on characteristics of the CRLM were not available. The use of an algorithm approach to determine existence of CRLM poses the difficulty of not having detailed data on the characteristics of the CRLM, as opposed to the primary CRC for which SEER provides detailed information. Factors associated with the metastatic disease may have influenced patient selection for surveillance imaging, but we could not investigate this. Trends in procedure utilization are limited by the reporting biases of the administrative processes involved with billing codes and should, therefore, be viewed in broad terms and not exact values. Like most large administrative databases, information on indication for postoperative imaging was not available to us; however, we excluded patients undergoing periprocedural imaging in the early postoperative surveillance period because imaging in this time period may not be indicative of surveillance. In addition, there may have been some selection bias in assessing the impact of surveillance imaging on outcome. For example, patients undergoing no or limited surveillance may have been different from patients who underwent more intense follow-up. Although these differences can never be fully accounted for in a retrospective study, we did attempt to control for potential differences using multivariate and propensity matched analyses. The use of SEER-linked Medicare data restricted our study cohort to those ≥65 years of age. Even though CRC, and consequently CRLM, are predominantly diseases of the elderly population, the patterns and impact of imaging surveillance will need to be explored more fully in a younger population of patients. Finally, the regional distribution of the SEER data, owing to the varying locations of the reporting cancer registries, needs to be taken into consideration when considering the data.
In conclusion, utilization of surveillance imaging after surgery for CRLM has increased. Although an increase in MRI and PET imaging was noted over time, the predominant reason for the overall increase in imaging utilization was owing to an increase in the use of CT. Despite the dramatic increase in surveillance imaging, we noted that one third of Medicare beneficiaries had no imaging event recorded in the postoperative period after CRLM surgery. Among patients who did undergo postoperative surveillance imaging performed, there was significant variation in the intensity/frequency of annual scans obtained. Receipt and intensity of postoperative surveillance imaging after CRLM surgery was associated with both patient- and non-patient factors. Receipt and higher intensity of surveillance imaging was not, however, associated with an improved overall long-term survival. Similar to primary CRC, prospective trials are needed to define the role of postoperative surveillance imaging in the metastatic setting. Such data are needed to help refocus surveillance guidelines for CRLM to provide the best value for healthcare resources, as well as avoid unnecessary testing for the patient.
Footnotes
Presented at the Academic Surgical Congress, New Orleans, LA, 2013.
REFERENCES
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62:10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 2.Norstein J, Silen W. Natural history of liver metastases from colorectal carcinoma. J Gastrointest Surg. 1997;1:398–407. doi: 10.1016/s1091-255x(97)80126-6. [DOI] [PubMed] [Google Scholar]
- 3.Blumgart LH, Allison DJ. Resection and embolization in the management of secondary hepatic tumors. World J Surg. 1982;6:32–45. doi: 10.1007/BF01656371. [DOI] [PubMed] [Google Scholar]
- 4.Mayo SC, Heckman JE, Shore AD, et al. Shifting trends in liver-directed management of patients with colorectal liver metastasis: a population-based analysis. Surgery. 2011;150:204–16. doi: 10.1016/j.surg.2011.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pawlik TM, Schulick RD, Choti MA. Expanding criteria for resectability of colorectal liver metastases. Oncologist. 2008;13:51–64. doi: 10.1634/theoncologist.2007-0142. [DOI] [PubMed] [Google Scholar]
- 6.de Jong MC, Pulitano C, Ribero D, et al. Rates and patterns of recurrence following curative intent surgery for colorectal liver metastasis: an international multi-institutional analysis of 1669 patients. Ann Surg. 2009;250:440–8. doi: 10.1097/SLA.0b013e3181b4539b. [DOI] [PubMed] [Google Scholar]
- 7.Nathan H, de Jong MC, Pulitano C, et al. Conditional survival after surgical resection of colorectal liver metastasis: an international multi-institutional analysis of 949 patients. J Am Coll Surg. 2010;210:755–64. doi: 10.1016/j.jamcollsurg.2009.12.041. [DOI] [PubMed] [Google Scholar]
- 8.Anthony T, Simmang C, Hyman N, et al. Practice parameters for the surveillance and follow-up of patients with colon and rectal cancer. Dis Colon Rectum. 2004;47:807–17. doi: 10.1007/s10350-004-0519-x. [DOI] [PubMed] [Google Scholar]
- 9.Richard CS, McLeod RS. Follow-up of patients after resection for colorectal cancer: a position paper of the Canadian Society of Surgical Oncology and the Canadian Society of Colon and Rectal Surgeons. Can J Surg. 1997;40:90–100. [PMC free article] [PubMed] [Google Scholar]
- 10.Tveit KM, Kataja VV. ESMO Minimum Clinical Recommendations for diagnosis, treatment and follow-up of rectal cancer. Ann Oncol. 2005;16(Suppl 1):i20–1. doi: 10.1093/annonc/mdi827. [DOI] [PubMed] [Google Scholar]
- 11.Benson AB, 3rd, Desch CE, Flynn PJ, et al. 2000 update of American Society of Clinical Oncology colorectal cancer surveillance guidelines. J Clin Oncol. 2000;18:3586–8. doi: 10.1200/JCO.2000.18.20.3586. [DOI] [PubMed] [Google Scholar]
- 12.Brenner DJ, Hall EJ. Computed tomography–an increasing source of radiation exposure. N Engl J Med. 2007;357:2277–84. doi: 10.1056/NEJMra072149. [DOI] [PubMed] [Google Scholar]
- 13.Dinan MA, Curtis LH, Hammill BG, et al. Changes in the use and costs of diagnostic imaging among medicare beneficiaries with cancer, 1999–2006. JAMA. 2010;303:1625–31. doi: 10.1001/jama.2010.460. [DOI] [PubMed] [Google Scholar]
- 14.Witkowski ER, Smith JK, Ragulin-Coyne E, Ng SC, Shah SA, Tseng JF. Is it worth looking? Abdominal imaging after pancreatic cancer resection: a National Study. J Gastrointest Surg. 2012;16:121–8. doi: 10.1007/s11605-011-1699-z. [DOI] [PubMed] [Google Scholar]
- 15.de Jong MC, Mayo SC, Pulitano C, et al. Repeat curative intent liver surgery is safe and effective for recurrent colorectal liver metastasis: results from an international multi-institutional analysis. J Gastrointest Surg. 2009;13:2141–51. doi: 10.1007/s11605-009-1050-0. [DOI] [PubMed] [Google Scholar]
- 16.Andreou A, Brouquet A, Abdalla EK, Aloia TA, Curley SA, Vauthey JN. Repeat hepatectomy for recurrent colorectal liver metastases is associated with a high survival rate. HPB. 2011;13:774–82. doi: 10.1111/j.1477-2574.2011.00370.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Govindarajan A, Arnaoutakis D, D'Angelica M, et al. Use of intraoperative ablation as an adjunct to surgical resection in the treatment of recurrent colorectal liver metastases. J Gastrointest Surg. 2011;15:1168–72. doi: 10.1007/s11605-011-1470-5. [DOI] [PubMed] [Google Scholar]
- 18.Warren JL, Klabunde CN, Schrag D, Bach PB, Riley GF. Overview of the SEER-Medicare data: content, research applications, and generalizability to the United States elderly population. Med Care. 2002;40:IV-3–IV18. doi: 10.1097/01.MLR.0000020942.47004.03. [DOI] [PubMed] [Google Scholar]
- 19.Anaya DA, Becker NS, Richardson P, Abraham NS. Use of administrative data to identify colorectal liver metastasis. J Surg Res. 2012;176:141–6. doi: 10.1016/j.jss.2011.07.022. [DOI] [PubMed] [Google Scholar]
- 20.Elixhauser A, Steiner C, Harris DR, Coffey RN. Comorbidity measures for use with administrative data. Med Care. 1998;36:8–27. doi: 10.1097/00005650-199801000-00004. [DOI] [PubMed] [Google Scholar]
- 21.Edelman MJ, Meyers FJ, Siegel D. The utility of follow-up testing after curative cancer therapy. A critical review and economic analysis. J Gen Intern Med. 1997;12:318–31. doi: 10.1046/j.1525-1497.1997.012005318.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McCabe MS, Bhatia S, Oeffinger KC, et al. American Society of Clinical Oncology statement: achieving high-quality cancer survivorship care. J Clin Oncol. 2013;31:631–40. doi: 10.1200/JCO.2012.46.6854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Audisio RA, Robertson C. Colorectal cancer follow-up: perspectives for future studies. Eur J Surg Oncol. 2000;26:329–37. doi: 10.1053/ejso.1999.0894. [DOI] [PubMed] [Google Scholar]
- 24.Kievit J. Colorectal cancer follow-up: a reassessment of empirical evidence on effectiveness. Eur J Surg Oncol. 2000;26:322–8. doi: 10.1053/ejso.1999.0893. [DOI] [PubMed] [Google Scholar]
- 25.Kjeldsen BJ, Kronborg O, Fenger C, Jorgensen OD. A prospective randomized study of follow-up after radical surgery for colorectal cancer. Br J Surg. 1997;84:666–9. [PubMed] [Google Scholar]
- 26.Ohlsson B, Breland U, Ekberg H, Graffner H, Tranberg KG. Follow-up after curative surgery for colorectal-carcinoma - randomized comparison with no follow-up. Dis Colon Rectum. 1995;38:619–26. doi: 10.1007/BF02054122. [DOI] [PubMed] [Google Scholar]
- 27.Rodriguez-Moranta F, Salo J, Arcusa A, et al. Postoperative surveillance in patients with colorectal cancer who have undergone curative resection: a prospective, multicenter, randomized, controlled trial. J Clin Oncol. 2006;24:386–93. doi: 10.1200/JCO.2005.02.0826. [DOI] [PubMed] [Google Scholar]
- 28.Mortazavi A, Shaukat A, Othman E, et al. Postoperative computed tomography scan surveillance for patients with stage II and III colorectal cancer: worthy of further study? Am J Clin Oncol. 2005;28:30–5. doi: 10.1097/01.coc.0000139188.46296.d0. [DOI] [PubMed] [Google Scholar]
- 29.Maitino AJ, Levin DC, Parker L, Rao VM, Sunshine JH. Nationwide trends in rates of utilization of noninvasive diagnostic imaging among the medicare population between 1993 and 1999. Radiology. 2003;227:113–7. doi: 10.1148/radiol.2272020617. [DOI] [PubMed] [Google Scholar]
- 30.Bhargavan M, Sunshine JH. Utilization of radiology services in the United States: levels and trends in modalities, regions, and populations. Radiology. 2005;234:824–32. doi: 10.1148/radiol.2343031536. [DOI] [PubMed] [Google Scholar]
- 31.Smith-Bindman R, Miglioretti DL, Johnson E, et al. Use of diagnostic imaging studies and associated radiation exposure for patients enrolled in large integrated health care systems, 1996–2010. JAMA. 2012;307:2400–9. doi: 10.1001/jama.2012.5960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Renehan AG, Egger M, Saunders MP, O'Dwyer ST. Impact on survival of intensive follow up after curative resection for colorectal cancer: systematic review and meta-analysis of randomised trials. BMJ. 2002;324:813. doi: 10.1136/bmj.324.7341.813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jeffery M, Hickey BE, Hider PN. Follow-up strategies for patients treated for non-metastatic colorectal cancer. Cochrane Database Syst Rev. 2007;24:CD002200. doi: 10.1002/14651858.CD002200.pub2. [DOI] [PubMed] [Google Scholar]


