SUMMARY
IL-17 cytokine production by the Th17 T-cell subset is regulated by intestinal commmensals. We show microbial colonization also regulates innate IL-17 production. A population of CD62L− γ/δ T cells, in particular a lineage expressing the IL-1 receptor 1 (IL-1R1), can be quickly activated by microbes to produce IL-17. Antibiotic-treatment and monocolonization of mice suggest specific commensals—but not metronidazole-sensitive anaerobes like Bacteroides species—are required for maintaining IL-1R1+ γ/δ T cells. Signaling through the guanine nucleotide exchange factor VAV1 but not through Toll-like receptors or antigen presentation pathways is essential for inducing IL-1R1+ γ/δ T cells. Furthermore, IL-1R1+ γ/δ T cells are a potential source of IL-17 that can be activated by IL-23 and IL-1 in both infectious and noninfectious settings in vitro and in vivo. Thus, commensals orchestrate the expansion of phenotypically distinct γδ T cells and innate immunity is a three-way interaction between host, pathogens and microbiota.
Keywords: gamma delta, IL-17, IL-1R1, IL-23, Bacteroides fragilis
INTRODUCTION
Mammals are born sterile and are colonized by commensal bacteria during and after birth. The interaction of commensal bacteria with their host is complex, with contrasting outcomes: colonization both induces tolerance to certain antigens, such as lipopolysaccharide (LPS), and facilitates the immunologic development and subsequent health of the host (Mazmanian and Kasper, 2006; Mazmanian et al., 2008). The various immunologic functions of indigenous commensal bacteria in the mammalian gut include the exertion of a profound effect on CD4+ T cells. Specifically, microbial colonization expands the population of CD4+ T cells (Macpherson and Harris, 2004); enhances the differentiation of Th17 cells [interleukin 17 (IL-17)–producing CD4+ T cells)] (Atarashi et al., 2008); and corrects an imbalance among different CD4+ T cell subsets (Mazmanian et al., 2005; Ivanov et al., 2008).
Although γ/δ T cells are only a minor subset of T lymphocytes, their deletion by genetic knockout or by treatment with a specific antibody renders mice more susceptible to infection with a variety of microbes (Hayday, 2000; Nakasone et al., 2007). The features that may make γ/δ T cells of special interest in host defense at selected sites include (1) earlier maturation in the fetal thymus than α/β T cells; (2) possession of a relatively restricted repertoire of the variable region of the T cell receptor (TCR); (3) frequent association with epithelium; and (4) ability to accumulate quickly at sites of infection (Hayday, 2000). One important function of γ/δ T cells in defense against certain pathogens and tumors is the production of various cytokines, chemokines, and antibacterial compounds (Skeen and Ziegler, 1995; Hayday, 2000; Shibata et al., 2007). Despite an expanding base of knowledge about γ/δ T cells, the importance of the commensal microflora in the regulation of this T cell subset remains largely unexplored.
IL-17, which has been linked to a growing list of infectious, autoimmune, and inflammatory diseases, is produced mainly by CD4+ T cells (Th17 cells) and CD8+ T cells. However, in some cases, other T cells, such as γ/δ T cells and invariant natural killer T cells, have also been identified as important sources of IL-17 (Matsuzaki and Umemura, 2007). γ/δ T cells have been considered ideally suited to provide an innate source of IL-17 in the earliest stages of an inflammatory response—i.e., before this cytokine is produced by antigen-specific α/β T cells (Roark et al., 2008). These IL-17 producing γ/δ T cells possess similar features to Th17 cells, such as expression of chemokine receptor 6 (CCR6), retinoid orphan receptor (RORgt), aryl hydrocarbon receptor (AhR), and IL-23 receptor (Martin et al., 2009). In an animal model of experimental autoimmune encephalomyelitis, IL-17-expressing γ/δ T cells can serve in an amplification loop as an additional source of IL-17 to supplement that produced by Th17 cells (Sutton et al., 2009).
Herein we show that colonization with commensal bacteria is a key factor in the expansion of CD62L− γ/δ T cells and in particular of a γ/δ T cell lineage carrying CD121a (IL-1 receptor 1, or IL-1R1). It appears that specific commensal bacteria are required for maintenance of these IL-1R1+ γ/δ T cells. By examining various mouse strains with specific genetic mutations (VAV1, CD1, MHCI, MHCII, TLR3, and MyD88), we have found that the guanine nucleotide exchange factor VAV1 is essential for IL-1R1 expression on γ/δ T cells. Furthermore, we have identified IL-1R1+ γ/δ T cells as a potential source of IL-17 that can be activated by IL-23 and IL-1 in both infectious and noninfectious settings in vitro and in vivo.
RESULTS
Microbial colonization of germ-free mice drives the expansion of CD62L− and IL-1R1+ γ/δ T cells
Peritoneal γ/δ T cells are thought to be an important component of innate responses to gram-positive and gram-negative bacterial infections in the peritoneum (Skeen and Ziegler, 1993; Skeen and Ziegler, 1995; Shibata et al., 2007). Although the total population of peritoneal CD3ε T cells from germ-free (GF) mice [(13 ± 3.5) ×104; mean ± SEM for 8 mice, 6–7 weeks old] is numerically comparable to that from age-matched specific pathogen–free (SPF) mice [(12 ± 2.9)×104; mean ± SEM for 12 mice; p = 0.56], the total number of γ/δ T cells is reduced ~3-fold in GF mice [(8.1 ± 1.0)×103] from that in SPF mice [(25.2 ± 8.7)×103; p = 0.0008].
To examine whether microbial colonization plays a role in the activation of γ/δ T cells, we compared CD62L expression on γ/δ T cells from GF and SPF mice. CD62L, a leukocyte homing receptor and activation marker, is down-regulated after the activation of α/β or γ/δ T cells through their TCRs or by mitogens (Chao et al., 1997). The great majority of γ/δ T cells in the peritoneum of SPF mice are CD62L− (Fig. 1A), an observation consistent with the concept that the peritoneal cavity is a repository of activated/memory T cells (Skeen and Ziegler, 1993). However, compared with SPF mice, GF mice have far fewer CD62L− γ/δ T cells (Figure 1A) (p < 0.0001).
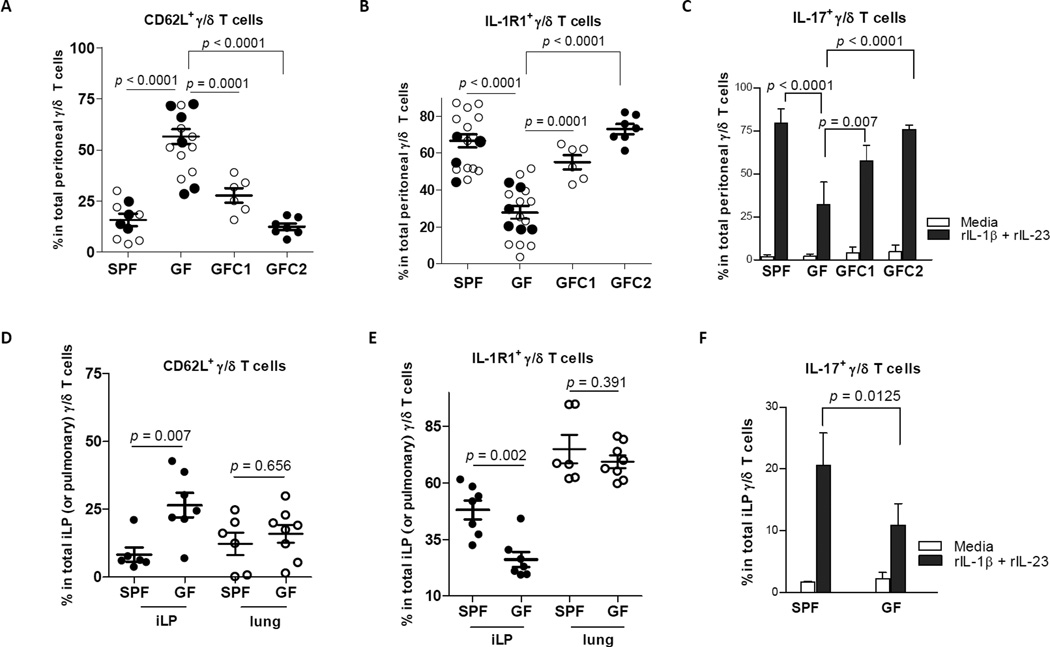
Figure 1. Microbial colonization is a key driving force in the expansion of CD62L− and IL-1R1+ γ/δ T cells.
Cells from the peritoneum (A and B), lung, and small-intestinal lamina propria (iLP) (D and E) of individual SPF mice (Swiss Webster), GF mice, GFC1 mice (GF mice reconstituted with complex flora for 7 weeks, and GFC2 mice (GF mice reconstituted with complex flora for 12 weeks) were analyzed by FACS after staining with mAbs to CD3ε, γ/δ TCR, and CD62L (or IL-1R1) as well as isotype controls. (C and F) Cells from the peritoneum and iLP of individual mice, as indicated, were cultured with medium only or with medium containing rIL-1β and rIL-23 (1 ng/mL) for 7 h. Percentages of CD62L− (or IL-1R1+ or IL-17+) γ/δ T cells are shown as mean ± SEM values. Each data point represents an individual mouse, and all collected data pooled from at least five independent experiments are shown. ○ (open dots) refer to data from SPF and GF mice at 7 weeks age; ● (closed dots) refer to data from SPF and GF mice at ~12 weeks age.
We took mice born to GF mothers and colonized them shortly after delivery with an SPF microbiota. Seven weeks after colonization (GFC1 mice), the proportion of CD62L− γ/δ T cells was higher than that in GF mice (p = 0.0001). Twelve weeks after SPF microbial colonization (GFC2 mice), the percentage of CD62L− γ/δ T cells in the peritoneum approached levels found in this site of SPF mice (Figure 1A; p < 0.0001, GFC2 versus GF mice).
IL-1 exerts pleotropic effects on a variety of tissues through binding to IL-1R1 (Dinarello, 1996). The IL-1R1 protein was detected on γ/δ T cells from the peritoneum, lung, and small-intestinal lamina propria (iLP) of SPF mice (Figure S1). Compared with SPF mice, GF mice had significantly fewer peritoneal IL-1R1+ γ/δ T cells (p < 0.0001). However, GFC1 mice (p = 0.0001) and GFC2 mice (p < 0.0001) had more IL-1R1+ γ/δ T cells than GF mice (Figure 1B). By intracellular staining, we examined IL-17 in γ/δ T cells after in vitro stimulation of peritoneal exudate cells (PECs) with recombinant IL-1β (rIL-1β) and rIL-23. GF mice had fewer IL-17+ γ/δ T cells than SPF mice (p < 0.0001). However, after reconstitution with an SPF microbiota, the percentage of IL-17+ cells was increased in the GFC1 group and was totally restored in the GFC2 group (Figure 1C).
In iLP, GF mice had fewer CD62L− γ/δ T cells (Figure 1D) and IL-1R1+ γ/δ T cells (Figure 1E) than did SPF mice. Again, there were fewer IL-17+ γ/δ T cells in this site from GF mice (Fig. 1F). In contrast, the percentages of CD62L− or IL-1R1+ γ/δ T cells in the lung are independent of colonization status in the gut lumen (Figures 1D and 1E). These results reflect the importance of commensal bacteria in maintenance of CD62L− as well as IL-1R1+ γ/δ T cells from some but not all sites.
Effect of treatment with different antibiotics on the IL-1R1+ γ/δ T cell population
Our laboratory has been working with Bacteroides fragilis (B. fragilis), a ubiquitous gram-negative anaerobe that colonizes the mammalian lower gastrointestinal tract (Mazmanian et al., 2005). We tested whether monocolonization of previously GF mice with B. fragilis would be sufficient to rescue the impaired IL-1R1+ γ/δ T cell population in the iLP. We had previously shown that such monocolonization was sufficient to correct the Th2 skew in GF mice. However, even heavy colonization of these mice with B. fragilis (Mazmanian et al., 2005) was not sufficient to restore the deficient IL-1R1+ γ/δ T cell population in the iLP (Figure S2).
Using Gram’s staining of cecal contents as a marker of anticipated antibiotic activity, we first examined SPF mice, which, as expected, harbored a diverse and complex microbial flora (Figure 2A). In mice and humans, gram-positive species of the phylum Firmicutes and gram-negative species of the phylum Bacteroidetes (Cytophaga-Flavobacterium-Bacteroides) account for >90% of commensal bacteria (Lupp et al., 2007; Ley et al., 2008; Ivanov et al., 2008). In an attempt to determine whether specific antibiotic sensitive bacteria are responsible for IL-1R1+ γ/δ T cell expansion, we treated SPF mice from birth to 6 weeks of age with one of three antibiotics: neomycin sulfate, vancomycin, or metronidazole. Although the activity spectra of these agents overlap to some degree, neomycin is primarily active against facultative gram-negative species, vancomycin predominantly against gram-positive species, and metronidazole against major groups of anaerobes like the Bacteroidetes. As other investigators have reported, antibiotic treatment was associated with relatively swollen ceca (data not shown), a finding that has previously been attributed to bacterial death (Ivanov et al., 2008).
Figure 2. Effects of different antibiotic treatments on IL-1R1+ γ/δ T cell population.
From birth onward, Swiss Webster mice received water containing no drugs (CTRL, SPF mice), metronidazole (METRO), neomycin sulfate (NEO), or vancomycin hydrochloride (VANCO), as described in Experimental Procedures. (A) Gram's staining of cecal contents from SPF mice and antibiotic-treated mice; (B) Cells from the peritoneum and small-intestinal lamina propria (iLP) of individual mice were analyzed by FACS after staining with mAbs to CD3ε, γ/δ TCR, and IL-1R1. (C) Cells from the peritoneum and iLP from individual mice were cultured with medium only or with rIL-1β and rIL-23 (1 ng/mL) for 7 h. Percentages of IL-1R1- and IL-17-expressing γ/δ T cells are shown as mean ± SEM values. Each data point represents an individual 6 weeks old mouse living in the same housing, and all collected data pooled from at least six independent experiments are shown.
By examining feces with Gram's stain, we found that the bacterial communities were quite different in SPF mice, mice treated with neomycin sulfate, and mice treated with vancomycin (Figure 2A). In the peritoneum and iLP of each group of both antibiotic-treated mice, numbers of IL-1R1+ γ/δ T cells and IL-1/IL-23-stimulated IL-17+ γ/δ T cells were lower than those in SPF mice (Figures 2A and 2B). Consistent with our B. fragilis monocolonization data, the metronidazole treatment had no impact on γ/δ T cell numbers. The commensal bacterial population in metronidazole-treated mice (which includes very few gram-negative anaerobes) would be expected to be quite different from that in SPF mice (in which gram-negative anaerobes are the predominant constituent of the flora) (Figure 2A).
Taken together, our results from antibiotic-treated and monocolonized mice suggest that specific components of the microbiota excluding the great majority of anaerobic organisms belonging to the phylum Bacteroidetes (rather than all bacteria or simply the presence of bacteria) are a requisite for expansion of systemic and local IL-1R1+ γ/δ T cells.
Signaling pathways used in the expansion of IL-1R1+ γ/δ T cells
The possible signaling pathways used for induction of IL-1R1+ γ/δ T cells were examined. Two classes of pathways were potentially involved: (1) those that stimulate γ/δ T cells via antigen-presenting cell (APC)–derived cytokines and (2) those that activate the γ/δ TCR through antigen presentation.
Recognition of signals induced by microbial pathogen–associated molecular patterns (PAMPs) is responsible for the activation of genes important in mounting an effective innate host defense, especially that mediated by proinflammatory cytokines (Netea et al., 2006). Toll-like receptors (TLRs) are among the most important classes of PAMPs (Akira, 2003). The adaptor molecule MyD88 is essential for proinflammatory cytokine production in response to all TLR ligands except TLR3. In contrast, type I interferons can be generated via MyD88-independent TLR signaling (Akira, 2003).The percentages of peritoneal IL-1R1+ γ/δ T cells in MyD88−/− mice, TLR3−/− mice, and their respective age-matched controls was not statistically different (Figure 3A). This observation suggested that inflammatory cytokines from TLR signaling pathways are not required for homeostatic regulation of IL-1R1+ γ/δ T cells in the peritoneum. At the same time, these data ruled out the possible role of IL-1 and IL-18 in maintenance of peritoneal IL-1R1+ γ/δ T cells, since MyD88 mediated-signaling is required for cellular responses to these two cytokines (Adachi et al., 1998).
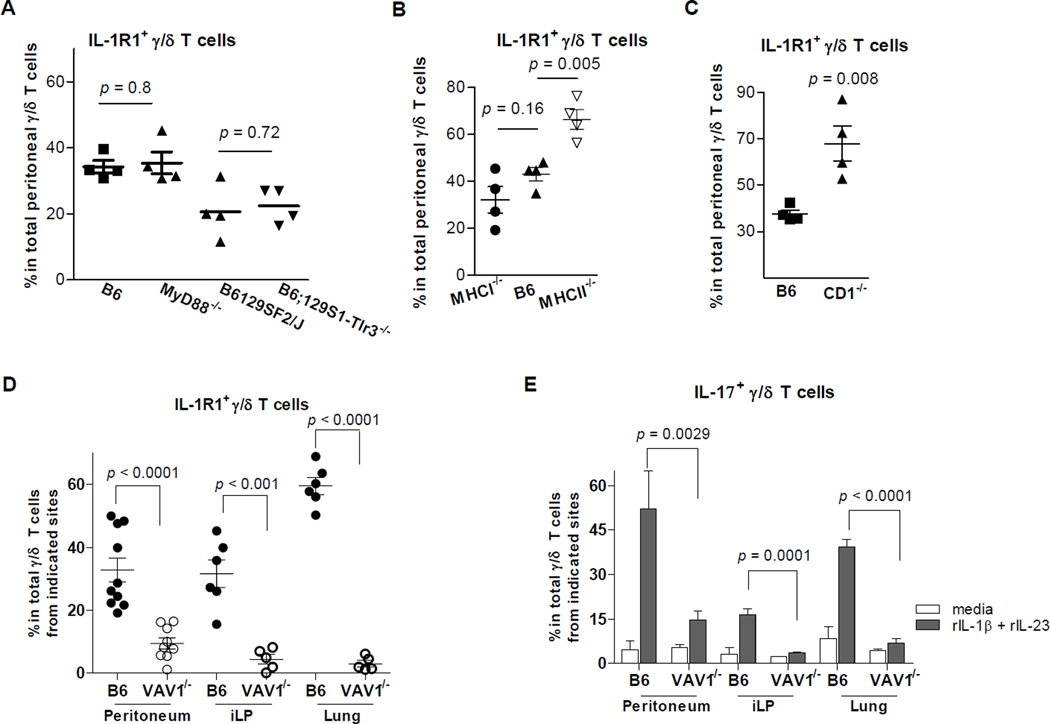
Figure 3. Role of signalings through adaptive and innate pathways in IL-1R1+ γ/δ T cell expansion.
IL-1R1 expression was measured by FACS on γ/δ T cells from the peritoneumof C57BL/6 (B6) and MyD88−/− mice, B6129SF1/J and B6;129S1-Tlr3tm1Flv/J (B6;129S1-Tlr3−/−) mice (A); B6, MHCI−/−, and MHCII−/− mice (B); B6 and CD1−/− mice (C); and B6 and VAV1−/− mice (D). For VAV1−/− mice and their controls, IL-1R1 expression on γ/δ T cells from cells from the lung and small-intestinal lamina propria (iLP) (D) was also analyzed. (E) Cells from the peritoneum and iLP from VAV1−/− mice and their controls were cultured with medium only or with rIL-1β and rIL-23 (1 ng/mL) for 7 h. Each data point represents an individual mouse. Genetically deficient mice of each strain and their respective age-matched controls were housed in the same conditions before use.
Stimulation of murine and human γ/δ T cells by peptides in the context of major histocompatibility class I (MHCI) or MHC class II (MHCII) molecules demonstrates conventional antigen recognition by γ/δ T cells (Kaufman, 1996). MHCI-like CD1 receptors are widely expressed on the surface of epithelial and professional APCs and present exogenous, self-lipid, and glycolipid antigens to γ/δ T cells (Russano et al., 2007). The percentages of peritoneal IL-1R1+ γ/δ T cells didn’t decrease in MHCI−/− and MHCII−/− mice compared with that in age-matched control mice (Figure 3B). This result indicated that MHCI- or MHCII-mediated antigen presentation pathways are not required for the induction of IL-1R1+ γ/δ T cells. The percentage of peritoneal IL-1R1-expressing γ/δ T cells were higher in CD1−/− mice than in control mice (Figure 3C), a result suggesting that CD1 molecules may help decrease the number of peritoneal IL-1R1+ γ/δ T cells. This observation is consistent with a report that γ/δ T cells expand after deletion of NK T cells, since CD1 molecules are essential for NK T cell development (Chen et al., 1997; French et al., 2005).
VAV1, a guanine nucleotide exchange factor for Rho-family GTPases, is essential for both activation and development of α/β T cells. In γ/δ T cells, VAV1 is required for activation via γ/δ TCR ligation but not for development (Swat et al., 2003). The proportion of IL-1R1+ γ/δ T cells in the peritoneum was much lower in VAV1−/− mice than in control mice (Figure 3D). We also looked at γ/δ T cells from other sites, particularly the lung and the iLP. We found there were far fewer IL-1R1+ γ/δ T cells in these tissues from VAV1−/− mice than from control mice. After stimulation with rIL-1β and rIL-23, fewer γ/δ T cells produced IL-17 from these sites in VAV1−/− mice than in control mice (Figure 3E). These data indicated that the signaling pathway through VAV1 plays an important role in the expansion of IL-1R1+ γ/δ T cells and IL-1/IL-23-stimulated IL-17+ γ/δ T cells.
IL-1R1 expression facilitates IL-17 production by γ/δ T cells from mucous sites
γ/δ T cells don’t produce IL-17 when stimulated transforming growth factor β and IL-6 in vitro (Shibata et al., 2007). Instead, IL-23 alone is sufficient to stimulate peritoneal or pulmonary γ/δ T cell production of IL-17 (Shibata et al., 2007; Umemura et al., 2007). In a recent study, γ/δ T cells from spleens and lymph nodes produced IL-17, IL-21, and IL-22 in response to IL-1β and IL-23 (Sutton et al., 2009). The exact role of IL-1 signaling in IL-17 production by γ/δ T cells from different anatomic sites has remained unclear.
rIL-23 alone induced the production of IL-17 by γ/δ T cells in PEC culture, as described in a previous report (Shibata et al., 2007). Similarly, rIL-1β alone induced very low levels of IL-17 production by γ/δ T cells (Figures S3A and S3B). However, a combination of rIL-1β and rIL-23 had an additive effect on IL-17 production by γ/δ T cells. Since IL-1β is known to be generated spontaneously in cultures of APCs (Okamoto and Nakano, 1990; Gorczynski et al., 1997), we measured spontaneous IL-1β levels in PEC cultures after 7 h of incubation with no stimulation. We found that high levels of spontaneous IL-1β were released by PECs with or without serum in the medium (Figure S3C). Therefore, the reported dramatic IL-17 production elicited in γ/δ T cells by rIL-23 alone in the presence of APCs (Shibata et al., 2007; Umemura et al., 2007) probably actually reflects the effects of a combination of exogenous rIL-23 and spontaneously generated IL-1β, the latter contributed by APCs.
To better dissect the role of IL-1 and/or IL-23 on early IL-17 production by γ/δ T cells, we stimulated purified T cells from PECs, lungs, and spleens for 7 h with rIL-1β alone, rIL-23 alone, or a combination of rIL-1β and rIL-23. As shown in Figures 4A and 4B, neither rIL-1β nor rIL-23 alone induced IL-17 production in γ/δ T cells. In contrast, the combination of rIL-1β and rIL-23 synergistically promoted IL-17 production in γ/δ T cells from all three sites. To a lesser extent, these same two cytokines also promoted IL-17 production in peritoneal and pulmonary α/β T cells (γ/δ negative) after 7 h of stimulation (Figures 4A and 4B).
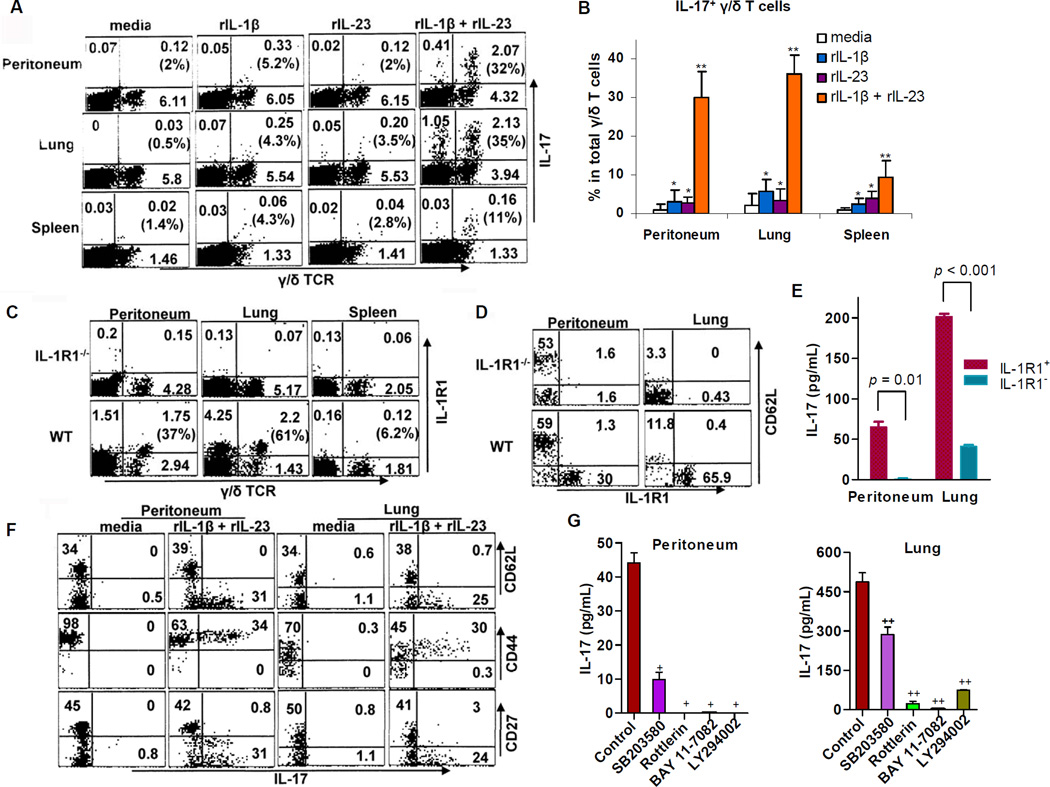
Figure 4. IL-1 acts synergistically with IL-23 to promote IL-1R1-associated IL-17 production by CD44+CD62L−CD27− γ/δ T cells in a p38-, PKC-, NF-kB-, and PI3K-dependent manner.
(A and B) CD90.2+ cells (~1.5 × 105/mL) from the peritoneum, lungs, and spleen of 6–8 WT mice (C57BL/6J) were cultured for 7 h with medium only or with medium containing rIL-1β alone (1 ng/mL), rIL-23 alone (1 ng/mL), or both rIL-1β and rIL-23 (1 ng/mL). (A) FACS plots gated on CD90.2+ cells are representative of four independent experiments, and the numbers in parentheses indicate the percentages of IL-17-producing γ/δ T cells. (B) Data from four experiments are combined as mean ± SEM values. *p > 0.1 and **p < 0.03 relative to medium alone. (C) A representative FACS plot demonstrating IL-1R1 expression on γ/δ T cells from 4 individual WT (C57BL/6J) or IL-1R1−/− mice is shown. Numbers in quadrants refer to percentages of CD3ε+ cells. Numbers in parentheses indicate percentages of IL-1R1+ γ/δ T cells. (D) A representative FACS plot gated on TCRγ/δ+ from 4 individual mice indicates that IL-1R1-expressing γ/δ T cells are CD62L−. (E) Sorted IL-1R1+ and IL-1R1− γ/δ T cells from the peritoneum (~1.5 × 104/mL) or lungs (~5 × 104/mL) of 6 WT mice were stimulated with rIL-1β and rIL-23 (1 ng/mL) for 24 h. The supernatants were analyzed for IL-17 concentrations by ELISA. (F) T cells from the peritoneum and lungs of 4–6 WT mice were cultured for 7 h with or without rIL-1β and rIL-23 (1 ng/mL). The FACS plots gated on TCRγ/δ+ represent one of three experiments. (G) γ/δ T cells were sorted from the peritoneum (~5 × 104/mL) and lungs (~1 × 105/mL) of 8–12 WT mice and were stimulated with rIL-1β and rIL-23 (1 ng/mL) in the absence (control) or presence (30-min pretreatment) of SB203580 (p38 inhibitor at 5 µM), rottlerin (PKC inhibitor at 10 µM), BAY 11-7082 (NF-κB inhibitor at 8 µM), or LY294002 (PI3K inhibitor at 40 µM). After 24 h, supernatants were collected for analysis of IL-17 concentrations by ELISA. +p < 0.047 and ++p < 0.011 relative to control. Results are mean ± SEM values from two independent experiments with similar results.
Notably, flow cytometry showed that the IL-1R1 protein was present on both peritoneal and pulmonary CD62L− γ/δ T cells but was rarely detectable on splenic γ/δ T cells (Figures 4C and 4D). In response to rIL-1β and rIL-23, IL-1R1+ γ/δ T cells produce more IL-17 than IL-1R1− γ/δ T cells (Figure 4E), a difference indicating that IL-1R1 expression is associated with γ/δ T cell production of IL-17. These data highlight a critical role of IL-1R1 signaling in IL-17 production by γ/δ T cells from different anatomic sites in the absence of APCs.
Promotion of IL-17 production by IL-1 and IL-23 in CD44+CD62L−CD27− γ/δ T cells depends upon intact p38, PKC, NF-kB, and PI3K pathways
Upon IL-1β and IL-23 stimulation, we found that IL-17-producing γ/δ T cells originate from CD44+CD62L− γ/δ T cell subsets (Figure 4F). Moreover, only CD27− γ/δ T cells are capable of generating IL-17; this observation is consistent with one recent report that tumor necrosis factor receptor family member CD27 is a thymic determinant of interferon γ (IFN-γ)–producing γ/δ T cells, whereas IL-17 production is restricted to CD27− γ/δ T cells (Ribot et al., 2009).
To address the intracellular signaling pathways used for IL-17 production by γ/δ T cells, we exposed purified γ/δ T cells to rIL-1β and rIL-23 for 24 h in the presence of a specific inhibitor of p38 (SB203580), PKC (rottlerin), NF-κB (BAY 11-7082), or PI3K (LY294002), as described elsewhere (Sutton et al., 2006). All inhibitors suppressed γ/δ T cell induction of IL-17. The implication is that IL-1 and IL-23 stimulate IL-17 production by γ/δ T cells in a p38-, PKC-, NF-κB-, and PI3K-dependent manner (Figure 4G).
Critical role of IL-1 signaling in optimal IL-17 production by γ/δ T cells in infection
Monoclonal antibody (mAb) to IL-1R1 dramatically suppressed E. coli LPS–induced IL-17 production in γ/δ T cells (Figures 5A and 5B). To determine whether this suppressive effect was due to blocking of IL-1R1 signaling in γ/δ T cells, we labeled PECs from wild-type (WT) or IL-1R1-deficient (IL-1R1−/−) mice with carboxyfluorescein succinimidyl ester (CFSE). CFSE-labeled WT PECs were mixed (1:1) with unlabeled IL-1R1−/− PECs; CFSE-labeled IL-1R1−/− PECs were mixed (1:1) with WT PECs. The cell mixtures were co-cultured with and without LPS. Using this approach, we compared IL-17 production in WT and IL-1R1−/− γ/δ T cells when identical cytokines were being produced by APCs in response to LPS. As shown in Figure 5C, IL-17 production was significantly impaired in IL-1R1−/− γ/δ T cells compared with that in WT γ/δ T cells, a result indicating that IL-1R1 signaling in γ/δ T cells is essential for LPS-induced γ/δ T cell production of IL-17.
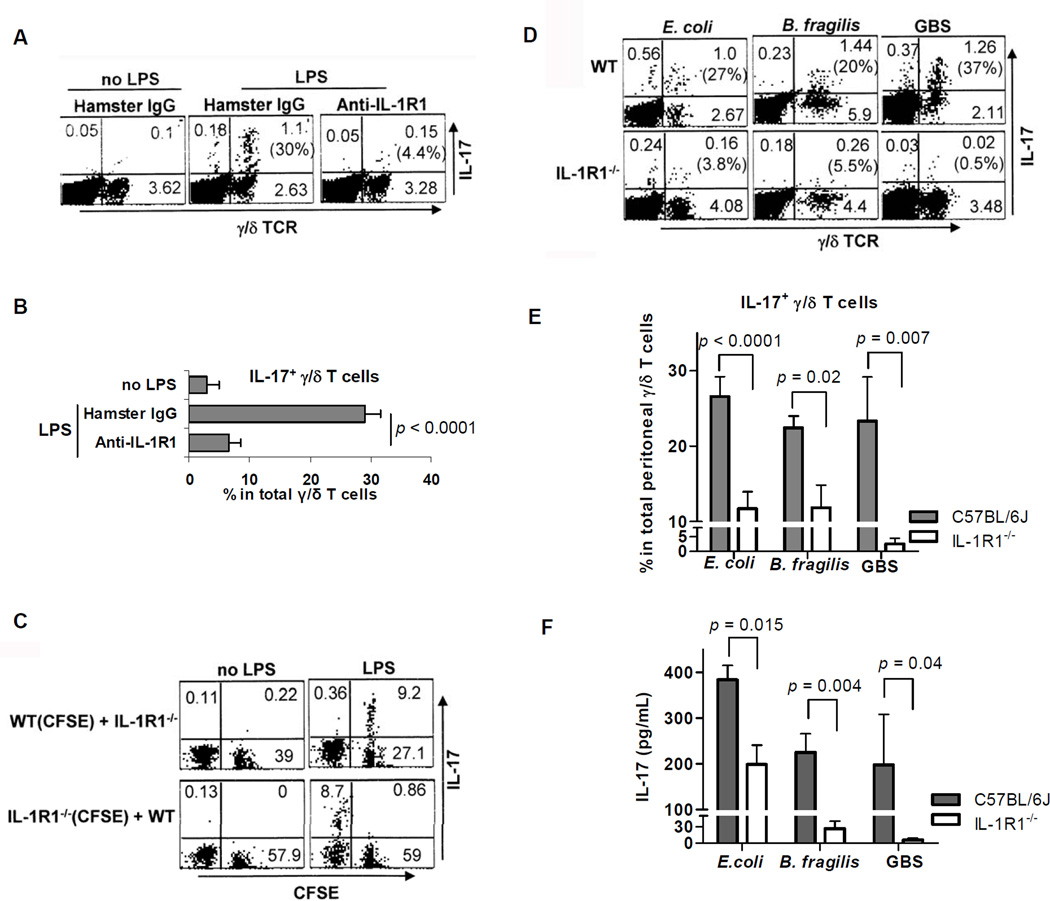
Figure 5. IL-1 signaling is required for optimal IL-17 production by γ/δ T cells in response to microbial products and microbes.
(A and B) PECs (6×106/mL) from WT mice were pretreated with Armenian hamster IgG or mAbs (10 µg/mL) to IL-1R1 for 30 min and subsequently stimulated with E. coli LPS (1 µg/mL) for 7 h. FACS plots (A) are gated on CD3ε+ cells. Numbers in parentheses, indicating percentages of IL-17-producing γ/δ T cells, are summarized as mean ± SEM values (B) from three independent experiments. (C) CFSE-labeled PECs from WT (or IL-1R1−/−) mice were mixed (1:1) with unlabeled IL-1R1−/− (or WT) PECs. The resulting PECs (4×106/mL/tube) were incubated for 7 h with and without LPS (1 µg/mL). The FACS plots are gated on TCRγ/δ+ and represent one of three experiments. (D and E) Brefeldin A was injected intraperitoneally into WT mice (8–10 per group) and IL-1R1−/− mice (5 or 6 per group) 2 h after ip infections with E. coli (ATCC 26, 1 × 108 CFU/ mouse), B. fragilis NCTC 9343 (6 × 108 CFU/mouse), or group B Streptococcus serotype Ia 515 (GBS, 1 × 108 CFU/ mouse). After 5 h, PECs were tested for intracellular IL-17. FACS plots (D) are gated on CD3ε+ cells. Numbers in parentheses indicate percentages of IL-17-producing γ/δ T cells. All collected data pooled from individual mice are summarized as mean ± SEM values (E). (F) WT mice (6 per group) and IL-1R1−/− mice (5 per group) were infected ip with E. coli (1 × 108 CFU/mouse), B. fragilis NCTC 9343 (6 × 108 CFU/ mouse), or GBS serotype Ia 515 (2 × 107 CFU/mouse). After 24 h, IL-17 in peritoneal fluid was measured by ELISA. Data are representative of three independent experiments.
IL-23-dependent γ/δ T cell production of IL-17 in the peritoneum is known to be key for neutrophil-mediated influx in defense against Escherichia coli infection (Shibata et al., 2007), yet it has remained unresolved whether IL-1R1 signaling is important at that site in IL-17 production by γ/δ T cells. After establishing an intraperitoneal E. coli infection in mice, we found that both the percentage of IL-17-producing γ/δ T cells and the IL-17 concentration in IL-1R1−/− mice were significantly lower than those in WT mice (Figures 5D, 5E, and 5F). These findings prompted us to ask whether the IL-1R1 signaling required for optimal IL-17 production by γ/δ T cells is restricted to E. coli infection or is more generally required for response to infection by other microbes.
When the intestinal symbiotic microbe B. fragilis escapes into the peritoneal cavity from its normal niche in the gastrointestinal tract, it can cause peritonitis and anaerobic sepsis (Tzianabos et al., 2000; Cobb et al., 2004; Duan et al., 2008). After 7 h and 24 h of in vitro infection of PECs with B. fragilis, γ/δ T cells produced more IL-17 than α/β T cells (Figure S4). In mice deficient in IL-1R1, the percentage of IL-17-producing γ/δ T cells and the IL-17 concentration were significantly lower after intraperitoneal infection with B. fragilis than in WT mice (Figures 5D, 5E, and 5F).
Group B Streptococcus (GBS) is a gram-positive coccus that is a major cause of pneumonia, bacterial meningitis, and neurologic morbidity in newborn infants (Charrel-Dennis et al., 2008). We challenged WT mice (C57BL/6J, 6–7 weeks old) and age-matched IL-1R1−/− mice intraperitoneally with GBS, stained PECs intracellularly for IL-17 for 7 h, and measured IL-17 in the peritoneal fluid at 24 h. After GBS challenge—as in E. coli and B. fragilis infections—γ/δ T cells produced predominantly IL-17 in the early phase, and optimal IL-17 production also required IL-1R1 signaling (Figures 5D, 5E, and 5F). Taken together, these data indicated that IL-1R1-dependent IL-17 production by γ/δ T cells is an innate source of IL-17 in response to gram-negative and gram-positive pathogens.
DISCUSSION
The peritoneum is a commonly infected site in certain groups of patients, and experimental inoculation of infectious agents, drugs, or chemicals into the peritoneal cavity is widely used in a variety of research (Steward et al., 1968). It has been suggested that γ/δ T cells in the peritoneum play a key role in early responses to various bacterial infections (Skeen and Ziegler, 1993; Shibata et al., 2007).
In the present study, we demonstrate that microbial colonization is a key driving factor in the expansion of CD62L− and IL-1R1+ γ/δ T cells in the peritoneum (Fig. 1). The association between the presence of the colonizing microbiota and a distinct phenotype of peritoneal IL-1R1+ γ/δ T cell raises the question of whether γ/δ T cells at other sites express IL-1R1. That might be expected to be the case in the gut, where γ/δ T cells coexist adjacent to a significant antigenic load of microorganisms (Kawaguchi et al., 1993). It is surprising, then, that no significant IL-1R1 expression (<~5%) is detectable on γ/δ T cells from intestinal intraepithelial lymphocytes, Peyer’s patches, mesenteric lymph nodes, or blood of either SPF (Swiss Webster) or C57 BL/6J mice (Figure S1). However, iLP γ/δ T cells from SPF mice express IL-1R1 (Figure S1). Far fewer γ/δ T cells expressing IL-1R1 are found in the iLP from GF mice than in that from SPF mice (Figure 1E). Consistent with this finding, the higher proportion of CD62L− iLP γ/δ T cells in SPF mice than in GF mice (Figure 1D) suggests more antigen experience of iLP γ/δ T cells in SPF mice. Surprisingly, though, microbial colonization does not alter the percentage of CD62L− or IL-1R1+ γ/δ T cells in the lung (Figures 1D and 1E). These data indicate that commensal bacteria orchestrate the phenotype of local and systemic γ/δ T cells from particular sites.
Treatment of SPF mice with three antibiotics (neomycin sulfate, vancomycin, and metronidazole) dramatically disturbs the composition of the bacterial community in the gastrointestinal tract (Ivanov et al., 2008), and our studies yielded results consistent with earlier reports. Compared with that in conventionally colonized mice, the number of peritoneal or iLP IL-1R1+ γ/δ T cells was low in mice treated with neomycin sulfate or vancomycin but was similar in metronidazole-treated mice (Figure 2B). These data suggest that specific microbes among the commensal bacteria (as opposed to the mere presence of bacteria) are required for maintenance of IL-1R1+ γ/δ T cells. Although the activity spectra of these three antibiotics overlap to some extent, the most likely interpretation of these data is that facultative gram-positive and/or gram-negative organisms—and not metronidazole sensitive anaerobes—maintain this T cell population. This point is interesting in view of the fact that >99% of the intestinal flora is made up of strict anaerobes. In support of this observation, mice heavily monocolonized with B. fragilis, a metronidazole-sensitive anaerobe, had iLP IL-1R1+ γ/δ T cell numbers similar to those in completely germ-free mice (Figure S2) (Mazmanian et al., 2005).
We investigated the signaling pathways required for maintenance of the IL-1R1+ γ/δ T cell population. Inflammatory cytokines released through MyD88- or TLR3- mediated TLR signaling did not play a role in maintaining these cells (Figure 3A). The expansion of IL-1R1+ γ/δ T cells was independent of TCR activation by CD1-, MHCI-, or MHCII-mediated antigen presentation, given that no decrease in the population of IL-1R1+ γ/δ T cells was seen in MHCI−/−, MHCII−/− or CD1−/− mice (Figures 3B and 3C). The antigens recognized by γ/δ T cells remain unclear; likewise, it is not yet certain whether the γ/δ TCR is required for recognition (Chien et al., 1996). However, murine γ/δ T lymphocytes are known to recognize surface-expressed proteins or phospholigands directly, independent of antigen processing and MHC presentation (Kaufman, 1996; Chien et al., 1996).
The guanine nucleotide exchange factor VAV1 encodes a protein that is believed to play a role in tyrosine-mediated signal transduction, functioning differently in different cell types (Swat et al., 2003; Malhotra et al., 2009). For instance, VAV1 is required for B cell receptor (BCR) endocytosis and BCR-induced Rac-GTP loading but not for B cell development and maturation (Malhotra et al., 2009). In contrast, whereas VAV1 is indispensable in both α/β T cell activation and development, it is involved only in activation of γ/δ T cells (via γ/δ TCR ligation) and not in their development (Swat et al., 2003). In VAV1−/− mice, γ/δ T cells are markedly impaired in signaling through the γ/δ TCR, as evidenced by a lack of proliferation and cytokine production in response to stimulation with antibodies to the γ/δ TCR (Swat et al., 2003). In this study, we identified a role for VAV1 signaling in restricting the IL-1R1+ γ/δ T cell population in the periphery (Figure 3D).
Our study consistently demonstrates that a lower percentage of IL-1R1+ γ/δ T cells corresponds with a smaller proportion of IL-1β/IL-23-stimulated, IL-17-producing γ/δ T cells (Figures. 1, 2, and 3). This correlation indicates that IL-1R1 expression on γ/δ T cells is likely associated with IL-17. This association was confirmed by the observation that IL-1R1+ γ/δ T cells produce much more IL-17 than IL-1R1− γ/δ T cells (Figure 4E). However, γ/δ T cells themselves do not produce detectable IL-17 —i.e., without rIL-1β and rIL-23 activation (Figures 4A and 4B). It appears that IL-1R1+ γ/δ T cells serve as a resting IL-17-producing γ/δ T cell pool that can be activated by IL-1β and IL-23. In fact, without PMA/INO or IL-1β/IL-23 stimulation, the percentage of IL-17+ γ/δ T cells is undetectable (Figure S3D). The percentages of IL-17+ γ/δ T cells are ~ 35% with PMA/INO, or IL-1β and IL-23. A combination of these two stimulations doesn’t increase the percentages of IL-17+ γ/δ T cells, suggesting IL-1β and IL-23 don’t promote the differentiation of IL-17-producing γ/δ T cells. However, the combination results in significant increase of IL-17 intensity, as indicated by Y Geo Mean in IL-17+ γ/δ T cells. These data indicated IL-1β and IL-23 promote IL-17 production by in situ differentiated IL-17-producing γ/δ T cells. IL-1- and IL-23-mediated IL-17 production appears to be restricted to CD44+CD62L−CD27− γ/δ T cells and relies upon intracellular signaling through p38, PKC, NF-kB, and PI3K (Figures 4F and 4G). The same requirement for p38, PKC, NF-kB, and PI3K in IL-1- and IL-23-mediated IL-17 production by α/β T cells (Sutton et al., 2003) suggests that a similar mechanism is probably involved in initiating IL-17 production by these two T cell subsets upon IL-1 and IL-23 stimulation.
Three lines of evidence make it apparent that IL-1R1 signaling is important for optimal IL-17 production by γ/δ T cells in vitro and in vivo in both infectious and noninfectious settings: (1) Antibody blocking of IL-1R1 greatly suppresses E. coli LPS–induced IL-17 production (Figures 5A and 5B). (2) In a mixed co-culture of IL-1R1−/− and WT PECs, more LPS-induced IL-17 is secreted by WT γ/δ T cells than by IL-1R1−/− γ/δ T cells (Figure 5C). (3) The proportion of IL-17-producing γ/δ T cells and the concentration of IL-17 are much lower in IL-1R1−/− mice than in WT mice after intraperitoneal injection of gram-negative and gram-positive pathogens, including E. coli, B. fragilis, and GBS serotype Ia (Figures 5D, 5E, and 5F).
Recently, it has been shown that γ/δ T cells from spleen and lymph nodes express IL-23R and the transcription factor RORγt and produce IL-17, IL-21, and IL-22 in response to IL-1β and IL-23 (Sutton et al., 2009). Although this study pointed out the critical role of IL-1 signaling in the induction of IL-17 by γ/δ T cells from spleen and lymph nodes, the association between IL-1R1 expression and γ/δ T cell production of IL-17 was not described. In addition, it remained unknown whether IL-1 signaling plays a role in the early IL-17 response by γ/δ T cells in infection. In another recent study (Martin et al., 2009), particular PAMP receptors on peritoneal γ/δ T cells have been shown to act synergistically with IL-23 to produce IL-17 in response to specific PAMPs and certain pathogens. However, this study didn’t dissect the role of IL-1 signaling in γ/δ T cell production of IL-17 in vitro and in vivo. Our study bridges the remaining gap showing IL-1 signaling contributes significantly to optimal IL-17 production by γ/δ T cells in response to both gram-positive and gram-negative bacterial infections.
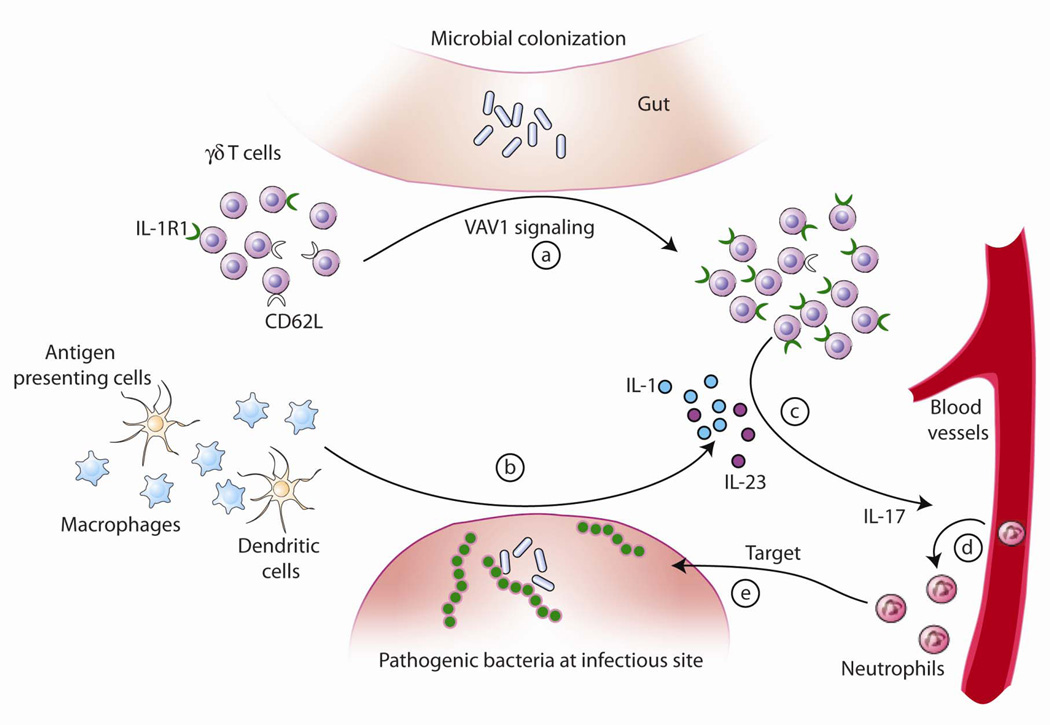
IL-17 is a proinflammatory cytokine that induces differentiation and migration of neutrophils. Accumulating evidence has established that IL-23-mediated IL-17 production by γ/δ T cells is essential in the resolution of infections by several organisms such as E. coli, BCG strain of Mycobacterium bovis, and Mycobacterium tuberculosis (Matsuzaki and Umemura, 2007). It seems that γ/δ T cells provide a default source of innate IL-17 production in response to bacterial pathogens. Moreover, IL-1R1 signaling is required for an optimal IL-17 response in this response. Taken together, our study suggests a possible model for IL-17 production by γ/δ T cells (Figure 6), in which specific commensal components of the intestinal microbiota and VAV1 signaling drive expansion of systemic and local γ/δ T cells bearing IL-1R1 (a). In the setting of infection with potentially pathogenic microbes, both macrophages and dendritic cells are stimulated to produce IL-1 and IL-23 (b); thus IL-1R1-bearing γ/δ T cells are activated and produce IL-17 (c), which mediates a protective immune response to the pathogenic bacteria by recruiting neutrophils from blood vessels (d) to the site of infection (e). Therefore, our study uncovers the importance of commensals on innate IL-17 production by γ/δ T cells, and highlights that innate immunity is not just a two-way interaction between host and pathogens, but that a third player is also intimately involved: the endogenous bacterial flora resident within the gastrointestinal tract.
Figure 6. A proposed model for how the commensal microbiota facilitates IL-17-mediated protective immune responses of γ/δ T cells to pathogenic bacteria.
Commensal bacteria and VAV1 signaling drive expansion of γ/δ T cells bearing IL-1R1 (a). In the setting of infection with potentially pathogenic microbes, macrophages and dendritic cells are stimulated to produce IL-1 and IL-23 (b), thus activating IL-1R1-bearing γ/δ T cells to produce IL-17 (c). IL-17 mediates a protective immune response to the pathogenic bacteria by recruiting neutrophils from blood vessels (d) to the site of infection (e).
EXPERIMENTAL PROCEDURES
Mice
WT mice (C57BL/6J), IL-1R1−/− C57BL/6J mice, B6129SF1/J mice, and B6;129S1-Tlr3tm1Flv/J mice were obtained from Jackson Laboratory. Swiss Webster SPF mice, Swiss Webster GF mice, MHCI−/− C57BL/6 (B6) mice, MHCII−/− B6 mice, and control B6 mice were purchased from Taconic Farms. CD1−/− mice, MyD88−/− mice, and VAV1−/− mice (on a B6 background) and their respective control mice were generous gifts from Drs. Michael B. Brenner (Harvard Medical School), Douglas T. Golenbock (University of Massachusetts Medical School), and Wojciech Swat (Washington University), respectively. All genetically deficient mice and their respective controls were age-matched males (6–8 weeks old) and were co-housed under SPF conditions.
Cell isolation and culture conditions
PECs were harvested by lavage with PBS. Pulmonary mononuclear cells (MNCs) were obtained as follows: The lung was directly incubated at 37°C for 1 h in 25 mL of 5% FBS-RPMI 1640 containing 37.5 mg of collagenase and 12.5 mg of dispase (Invitrogen); the tissue was then pressed through cell strainers, and MNCs were separated by Ficoll-Hypaque gradient. Splenic MNCs were likewise separated from spleens by Ficoll-Hypaque gradient. MNCs from intraepithelial lymphocytes, Peyer’s patches, and iLP were isolated as described elsewhere (Weigmann et al., 2007). For flow sorting, PECs, splenic MNCs, and pulmonary MNCs were stained with APC-anti mouse CD90.2, PE-anti mouse γ/δ TCR and Alexa 488-conjugated anti-mouse IL-1R1 (clone 12A6) to obtain purified T cells, γ/δ T cells and IL-1R1+ γ/δ T cells, respectively. The final purity of the T cells was 85–98% and was achieved with a BD FACSAria cell sorter. The cell medium was composed of RPMI-1640 (Gibco) supplemented with 10% fetal bovine serum, 1% penicillin-streptomycin, 1 mM sodium pyruvate, and 0.1 mM MEM Nonessential Amino Acids Solution.
Intracellular staining
Cells were cultured with medium alone, LPS (E. coli K-235 LPS, Sigma), or cytokines in a CO2 incubator at 37°C for 7 h. For the last 5 h of incubation, brefeldin A (10 µg/mL) and GolgiStop™ (3 µL/mL) were added. Cells were washed, blocked with Fcγ blocker (1 µg/mL; BD Biosciences) for 15 min at 4°C, and then stained with surface markers or isotype controls for 30 min at 4°C. Thereafter, BD Cytofix/Cytoperm solution (100 µL; BD Biosciences) was added, and the mixture was maintained at 4°C overnight. Cells were fixed, permeabilized, and stained with anti-mouse APC (or PE)–conjugated IL-17 mAb, anti-mouse PE-conjugated IFN-γ mAb, or isotype controls for 30 min at 4°C.
Ex vivo intracellular IL-17 staining was performed as described previously (Shibata et al., 2007). In brief, 500 µL of brefeldin A (0.5 mg/mL) was injected intraperitoneally 2 h after injection of E. coli (ATCC 26, 1 × 108 CFU/mouse), B. fragilis NCTC 9343 (ATCC 25285, 6 × 108 CFU/mouse), or GBS serotype Ia 515 (1 × 108 CFU/mouse). PECs were collected 5 h after infection. Cells were stained with surface markers and intracellular IL-17 as described above.
Analysis of IL-1R1 expression by flow cytometry
Cells from different anatomic sites were blocked with Fcγ blocker (1 µg/mL) for 15 min at 4°C and then stained with APC-conjugated anti-mouse CD3ε mAb, FITC-conjugated anti-mouse γ/δ TCR mAb, and PE-conjugated anti-mouse CD121a mAb (1 µg/mL) for 30 min at 4°C. After being washed twice, cells were directly analyzed with the FACSCalibur™ system.
IL-17 measurement by ELISA
For inhibition experiments, different concentrations of SB203580 (p38 inhibitor at 5 µM), rottlerin (PKC inhibitor at 10 µM), BAY 11-7082 (NF-κB inhibitor at 8 µM), or LY294002 (PI3K inhibitor at 40 µM) were added to sorted γ/δ T cell cultures 30 min before rIL-1β and rIL-23 (1 ng/mL), as described previously (Sutton et al., 2003). For measurement of IL-17 in vivo, peritoneal contents were washed with PBS (1 mL for E. coli; 0.6 mL for B. fragilis or GBS) 24 h after intraperitoneal infection of WT mice (C57BL/6J, 6–7 weeks old) and age-matched IL-1R1−/− mice with E. coli (1 × 108 CFU/mouse), B. fragilis (6 × 108 CFU/mouse), or GBS (2 × 107 CFU/mouse). The supernatants or lavage fluids were assayed for IL-17 by DuoSet ELISA kits (R&D Systems).
Microbiota reconstitution
Pregnant female GF mice were purchased from Taconic Farms. One day before the birth of pups, each pregnant GF mouse was co-housed for 24 h with one female SPF mouse under SPF conditions. The female SPF mouse was removed from the cage, and the male pups were housed for 7 weeks or 12 weeks under SPF conditions. For monocolonization of GF Swiss Webster mice, B. fragilis NCTC 9343 (1 × 108 CFU) was grown in BHI medium and spread on food and bedding in a GF isolator. Mice were colonized for 6–7 weeks before they were sacrificed.
Antibiotic treatment and bacterial analysis
Swiss Webster mice were given metronidazole (1 g/L), neomycin sulfate (1 g/L), or vancomycin hydrochloride (0.5 g/L) in drinking water for 6 weeks after birth, as described previously (Ivanov et al., 2008). Antibiotic efficiency was evaluated according to measurement of the swollen cecum and Gram's staining of the cecal contents.
Statistics
Values for p were calculated by unpaired t test. Differences with p values of < 0.05 were considered statistically significant.
Supplementary Material
HIGHLIGHTS.
Commensal bacteria are responsible for expanding IL-1R1+ γ/δ T cells
The guanine nucleotide exchange factor VAV1 is required for IL-1R1+ γ/δ T cell expansion
IL-1R1 expression correlates with γ/δ T cell production of the cytokine IL-17
IL-1R1 signaling is essential for optimal innate IL-17 production by γ/δ T cells in response to pathogens
ACKNOWLEDGEMENT
We thank Dr. Michael B. Brenner for providing CD1−/− mice and controls, Dr. Douglas T. Golenbock for providing MyD88−/− mice and controls, and Dr. Wojciech Swat for providing VAV1−/− mice and controls. We thank Dr. Suryasarathi Dasgupta and Dr. Rachel M. McLoughlin for reading this manuscript. We appreciate Ms. Julie McCoy for editing this paper. This work was supported by an NIH NIAID (RO1 AI039576) grant to DLK. The authors have no conflict of interest related to this work.
Footnotes
SUPPLEMENTAL DATA
Supplemental data includes 4 figures.
REFERENCES
- Adachi O, Kawai T, Takeda K, Matsumoto M, Tsutsui H, Sakagami M, Nakanishi K, Akira S. Targeted disruption of the MyD88 gene results in loss of IL-1- and IL-18-mediated function. Immunity. 1998;9:143–150. doi: 10.1016/s1074-7613(00)80596-8. [DOI] [PubMed] [Google Scholar]
- Akira S. Toll-like receptor signaling. J. Biol. Chem. 2003;278(40):38105–38108. doi: 10.1074/jbc.R300028200. [DOI] [PubMed] [Google Scholar]
- Atarashi K, Nishimura J, Shima T, Umesaki Y, Yamamoto M, Onoue M, Yagita H, Ishii N, Evans R, Honda K, Takeda K. ATP drives lamina propria T(H)17 cell differentiation. Nature. 2008;455(7214):808–812. doi: 10.1038/nature07240. [DOI] [PubMed] [Google Scholar]
- Chao CC, Jensen R, Dailey MO. Mechanisms of L-selectin regulation by activated T cells. J. Immunol. 1997;159(4):1686–1694. [PubMed] [Google Scholar]
- Charrel-Dennis M, Latz E, Halmen KA, Trieu-Cuot P, Fitzgerald KA, Kasper DL, Golenbock DT. TLR-independent type I interferon induction in response to an extracellular bacterial pathogen via intracellular recognition of its DNA. Cell Host Microbe. 2008;4(6):543–554. doi: 10.1016/j.chom.2008.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen YH, Chiu NM, Mandal M, Wang N, Wang CR. Impaired NK1+ T cell development and early IL-4 production in CD1-deficient mice. Immunity. 1997;6(4):459–467. doi: 10.1016/s1074-7613(00)80289-7. [DOI] [PubMed] [Google Scholar]
- Chien Y, Jores R, Crowley MP. Recognition by γδ T Cells. Annu. Rev. Immunol. 1996;14:511–532. doi: 10.1146/annurev.immunol.14.1.511. [DOI] [PubMed] [Google Scholar]
- Cobb BA, Wang Q, Tzianabos AO, Kasper DL. Polysaccharide processing and presentation by the MHCII pathway. Cell. 2004;117:677–687. doi: 10.016/j.cell.2004.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan J, Avci FY, Kasper DL. Microbial carbohydrate depolymerization by antigen-presenting cells: deamination prior to presentation by the MHCII pathway. Proc. Natl. Acad. Sci. U. S. A. 2008;105(13):5183–5188. doi: 10.1073/pnas.0800974105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- French JD, Roark CL, Born WK, O'Brien RL. {gamma}{delta} T cell homeostasis is established in competition with {alpha}{beta} T cells and NK cells. Proc. Natl. Acad. Sci. U. S. A. 2005;102(41):14741–14746. doi: 10.1073/pnas.0507520102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorczynski RM, Cinader B, Ramakrishna V, Terzioglu E, Waelli T, Westphal O. An antibody specific for interleukin-6 reverses age-associated changes in spontaneous and induced cytokine production in mice. Immunology. 1997;92(1):20–25. doi: 10.1046/j.1365-2567.1997.00327.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayday AC. [gamma][delta] cells: a right time and a right place for a conserved third way of protection. Annu. Rev. Immunol. 2000;18:975–1026. doi: 10.1146/annurev.immunol.18.1.975. [DOI] [PubMed] [Google Scholar]
- Ivanov II, Frutos Rde L, Manel N, Yoshinaga K, Rifkin DB, Sartor RB, Finlay BB, Littman DR. Specific microbiota direct the differentiation of IL-17-producing T-helper cells in the mucosa of the small intestine. Cell Host Microbe. 2008;4:337–349. doi: 10.1016/j.chom.2008.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman SH. gamma/delta and other unconventional T lymphocytes: what do they see and what do they do? Proc. Natl. Acad. Sci U. S. A. 1996;93(6):2272–2279. doi: 10.1073/pnas.93.6.2272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawaguchi M, Nanno M, Umesaki Y, Matsumoto S, Okada Y, Cai Z, Shimamura T, Matsuoka Y, Ohwaki M, Ishikawa H. Cytolytic activity of intestinal intraepithelial lymphocytes in germ-free mice is strain dependent and determined by T cells expressing gamma delta T-cell antigen receptors. Proc. Natl. Acad. Sci. U. S. A. 1993;90(18):8591–8594. doi: 10.1073/pnas.90.18.8591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koninck PR, Kennedy SH, Barlow DH. Endometriotic disease: the role of peritoneal fluid. Hum. Reprod. Update. 1998;4(5):741–751. doi: 10.1093/humupd/4.5.741. [DOI] [PubMed] [Google Scholar]
- Ley RE, Lozupone CA, Hamady M, Knight R, Gordon JI. Worlds within worlds: evolution of the vertebrate gut microbiota. Nat. Rev. Microbiol. 2008;6(10):776–788. doi: 10.1038/nrmicro1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupp C, Robertson ML, Wickham ME, Sekirov I, Champion OL, Gaynor EC, Finlay BB. Host-mediated inflammation disrupts the intestinal microbiota and promotes the overgrowth of Enterobacteriaceae. Cell Host Microbe. 2007;2:119–129. doi: 10.1016/j.chom.2007.06.010. [DOI] [PubMed] [Google Scholar]
- Macpherson AJ, Harris NL. Interactions between commensalintestinal bacteria and the immune system. Nat. Rev. Immunol. 2004;4:478–485. doi: 10.1038/nri1373. [DOI] [PubMed] [Google Scholar]
- Malhotra S, Kovats S, Zhang W, Coggeshall KM. B cell antigen receptor endocytosis and antigen presentation to T cells require Vav and dynamin. J. Biol. Chem. 2009;284(36):24088–24097. doi: 10.1074/jbc.M109.014209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin B, Hirota K, Cua DJ, Stockinger B, Veldhoen M. Interleukin-17-producing gammadelta T cells selectively expand in response to pathogen products and environmental signals. Immunity. 2009;31(2):321–330. doi: 10.1016/j.immuni.2009.06.020. [DOI] [PubMed] [Google Scholar]
- Matsuzaki G, Umemura M. Interleukin-17 as an effector molecule of innate and acquired immunity against infections. Microbiol. Immunol. 2007;51:1139–1147. doi: 10.1111/j.1348-0421.2007.tb04008.x. [DOI] [PubMed] [Google Scholar]
- Roark CL, Simonian PL, Fontenot AP, Born WK, O'Brien RL. gammadelta T cells: an important source of IL-17. Curr. Opin. Immunol. 2008;20(3):353–357. doi: 10.1016/j.coi.2008.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazmanian SK, Liu CH, Tzianabos AO, Kasper DL. An immunomodulatory molecule of symbiotic bacteria directs maturation of the host immune system. Cell. 2005;122:107–118. doi: 10.1016/j.cell.2005.05.007. [DOI] [PubMed] [Google Scholar]
- Mazmanian SK, Round JL, Kasper DL. A microbial symbiosis factor prevents intestinal inflammatory disease. Nature. 2008;453:620–625. doi: 10.1038/nature07008. [DOI] [PubMed] [Google Scholar]
- Mazmanian SK, Kasper DL. The love-hate relationship between bacterial polysaccharides and the host immune system. Nat. Rev. Immunol. 2006;6(11):849–858. doi: 10.1038/nri1956. [DOI] [PubMed] [Google Scholar]
- Nakasone C, Yamamoto N, Nakamatsu M, Kinjo T, Miyagi K, Uezu K, Nakamura K, Higa F, Ishikawa H, O'brien RL, Ikuta K, Kaku M, Fujita J, Kawakami K. Accumulation of gamma/delta T cells in the lungs and their roles in neutrophil-mediated host defense against pneumococcal infection. Microbes. Infect. 2007;9(3):251–258. doi: 10.1016/j.micinf.2006.11.015. [DOI] [PubMed] [Google Scholar]
- Netea MG, Van der Meer JW, Kullberg BJ. Role of the dual interaction of fungal pathogens with pattern recognition receptors in the activation and modulation of host defence. Clin. Microbiol. Infect. 2006;12(5):404–409. doi: 10.1111/j.1469-0691.2006.01388.x. [DOI] [PubMed] [Google Scholar]
- Okamoto H, Nakano K. Regulation of interleukin-1 synthesis by histamine produced by mouse peritoneal macrophages per se. Immunology. 1990;69(1):162–165. [PMC free article] [PubMed] [Google Scholar]
- Ribot JC, deBarros A, Pang DJ, Neves JF, Peperzak V, Roberts SJ, Girardi M, Borst J, Hayday AC, Pennington DJ, Silva-Santos B. CD27 is a thymic determinant of the balance between interferon-gamma- and interleukin 17-producing gammadelta T cell subsets. Nat. Immunol. 2009;10(4):427–436. doi: 10.1038/ni.1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russano AM, Bassotti G, Agea E, Bistoni O, Mazzocchi A, Morelli A, Porcelli SA, Spinozzi F. CD1-restricted recognition of exogenous and self-lipid antigens by duodenal gammadelta+ T lymphocytes. J. Immunol. 2007;178(6):3620–3626. doi: 10.4049/jimmunol.178.6.3620. [DOI] [PubMed] [Google Scholar]
- Skeen MJ, Ziegler HK. Induction of murine peritoneal gamma/delta T cells and their role in resistance to bacterial infection. J. Exp. Med. 1993;178(3):971–984. doi: 10.1084/jem.178.3.971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinarello CA. Biologic basis for interleukin-1 in disease. Blood. 1996;87(6):2095–2147. [PubMed] [Google Scholar]
- Skeen MJ, Ziegler HK. Activation of gamma delta T cells for production of IFN-gamma is mediated by bacteria via macrophage-derived cytokines IL-1 and IL-12. J. Immunol. 1995;154(11):5832–5841. [PubMed] [Google Scholar]
- Shibata K, Yamada H, Hara H, Kishihara K, Yoshikai Y. Resident Vdelta1+ gammadelta T cells control early infiltration of neutrophils after Escherichia coli infection via IL-17 production. J. Immunol. 2007;178:4466–4472. doi: 10.4049/jimmunol.178.7.4466. [DOI] [PubMed] [Google Scholar]
- Steward JP, Ornellas EP, Beernink KD, Northway WH. Errors in the technique of intraperitoneal injection of mice. Appl. Microbiol. 1968;16(9):1418–1419. doi: 10.1128/am.16.9.1418-1419.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutton C, Brereton C, Keogh B, Mills KH, Lavelle EC. A crucial role for interleukin (IL)-1 in the induction of IL-17-producing T cells that mediate autoimmune encephalomyelitis. J. Exp. Med. 2006;203(7):1685–1691. doi: 10.1084/jem.20060285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutton CE, Lalor SJ, Sweeney CM, Brereton CF, Lavelle EC, Mills KH. Interleukin-1 and IL-23 induce innate IL-17 production from gammadelta T cells, amplifying Th17 responses and autoimmunity. Immunity. 2009;31(2):331–341. doi: 10.1016/j.immuni.2009.08.001. [DOI] [PubMed] [Google Scholar]
- Swat W, Xavier R, Mizoguchi A, Mizoguchi E, Fredericks J, Fujikawa K, Bhan AK, Alt FW. Essential role for Vav1 in activation, but not development, of gammadelta T cells. Int. Immunol. 2003;15(2):215–221. doi: 10.1093/intimm/dxg021. [DOI] [PubMed] [Google Scholar]
- Tzianabos AO, Finberg RW, Wang Y, Chan M, Onderdonk AB, Jennings HJ, Kasper DL. T cells activated by zwitterionic molecules prevent abscesses induced by pathogenic bacteria. J. Biol. Chem. 2000;275(10):6733–6740. doi: 10.1074/jbc.275.10.6733. [DOI] [PubMed] [Google Scholar]
- Umemura M, Yahagi A, Hamada S, Begum MD, Watanabe H, Kawakami K, Suda T, Sudo K, Nakae S, Iwakura Y, Matsuzaki G. IL-17-mediated regulation of innate and acquired immune response against pulmonary Mycobacterium bovis bacille Calmette-Guérin infection. J. Immunol. 2007;178:3786–3796. doi: 10.4049/jimmunol.178.6.3786. [DOI] [PubMed] [Google Scholar]
- Weigmann B, Tubbe I, Seidel D, Nicolaev A, Becker C, Neurath MF. Isolation and subsequent analysis of murine lamina propria mononuclear cells from colonic tissue. Nat. Protoc. 2007;2(10):2307–2311. doi: 10.1038/nprot.2007.315. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.