Abstract
Anterior cruciate ligament (ACL) injuries most frequently occur under the large loads associated with a unipedal jump landing involving a cutting or pivoting maneuver. We tested the hypotheses that internal tibial torque would increase the anteromedial (AM) bundle ACL relative strain and strain rate more than would the corresponding external tibial torque under the large impulsive loads associated with such landing maneuvers.
Twelve cadaveric female knees [mean (SD) age: 65.0 (10.5) years] were tested. Pretensioned quadriceps, hamstring and gastrocnemius muscle-tendon unit forces maintained an initial knee flexion angle of 15°. A compound impulsive test load (compression, flexion moment and internal or external tibial torque) was applied to the distal tibia while recording the 3-D knee loads and tibofemoral kinematics. AM-ACL relative strain was measured using a 3mm DVRT. In this repeated measures experiment, the Wilcoxon Signed-Rank test was used to test the null hypotheses with p<0.05 considered significant.
The mean (± SD) peak AM-ACL relative strains were 5.4±3.7 % and 3.1±2.8 % under internal and external tibial torque, respectively. The corresponding mean (± SD) peak AM-ACL strain rates reached 254.4±160.1 %/sec and 179.4±109.9 %/sec, respectively. The hypotheses were supported in that the normalized mean peak AM-ACL relative strain and strain rate were 70% and 42% greater under internal than external tibial torque, respectively (p=0.023, p=0.041).
We conclude that internal tibial torque is a potent stressor of the ACL because it induces a considerably (70%) larger peak strain in the AM-ACL than does a corresponding external tibial torque.
Keywords: cruciate ligament, strain, rate, torque
INTRODUCTION
An estimated 250,000 anterior cruciate ligament (ACL) injuries occur annually in the United States, 70 % of which are termed “non-contact”.1,2 Regardless of the treatment, an ACL rupture increases the risk of developing degenerative arthritis in that knee ten-fold compared to an age-matched uninjured population.3–5 Non-contact ACL injuries frequently occur while landing unipedally from a jump or during a plant-and-cut or pivot maneuver.6 A current knowledge gap concerns the unknown interaction between lower extremity configuration, external loading direction, and muscle recruitment patterns in causing an ACL rupture. Insights could lead to improved prevention programs.
Previous studies, performed quasi-statically in vivo and in vitro, suggest higher ACL strains occur under an internally-directed tibial torque than under an externally-directed tibial torque.7–9 It is known that an internal tibial rotation increases a coupled anterior tibial translation, thereby increasing ACL strain.10 Post hoc video analyses suggested that ACL injury can occur under a forceful knee valgus loading and internal or external tibial rotation at or near a fully extended knee. 11–12 But the relative contribution of transverse plane tibial rotation to ACL injury remains unclear.15 Despite controversy 13–15 (see Discussion), ACL injury prevention programs have focused over the last decade on reducing valgus loading on the knee during jump landings.16–19 This may be because an apparent valgus knee posture is often observed on injury video tapes of athletes who sustained an ACL injury. However, while post hoc injury video analyses can provide valuable information on the timing of gross body and limb postures and movements, they cannot provide the detailed kinematics of the tibia and femur, the direction of the net external load from the ground reaction force and/or moment that act(s) on the tibia, or the concomitant knee muscle forces acting on the knee to cause the ACL injury. Krosshaug et al. showed that the accuracy of a simple visual inspection of injury video is poor 20 and the injury video analysis can be improved by using their model-based image-matching techniques 21. However, they also commented that their method predicted less accurate axial rotation of the thigh and shank compared to hip and knee flexion and abduction angles. 21 Hence, an investigation of the effect of axial tibial torque on ACL strain and strain rate during a realistic pivot landing seems warranted, particularly in the presence of direct measurements of the impulsive tibial compressive force, knee flexion moment, muscle forces and tibiofemoral joint kinematics.
The goal of the present study, therefore, was to investigate the effects of both internal and external tibial torque on AM-ACL relative strain and strain rate under large compound impulsive loads applied to an instrumented cadaveric knee. The first primary hypothesis we tested was that peak AM-ACL relative strain should be larger under large internal than external tibial torque during a simulated pivot landing. We also tested the corresponding hypothesis involving strain rate instead of strain. This is because the viscoelastic nature of the ACL causes its resistance to stretch to depend on the strain rate22, yet we are not aware of any data on the magnitude of the ACL strain rate during a pivot landing.
METHODS
Specimen Procurement and Knee Testing Apparatus
Since a full description of the methods has been published elsewhere23, only the most pertinent information will be given in what follows. Twelve fresh cadaveric limbs [mean (SD) age: 65.0 (10.5) years; eight female donors] were obtained from Anatomical Donation Program. Prior to the procurement, assessment of any indication of surgery and severe deformities was performed. In order to standardize specimen length, the lower extremities were cut ~8 in (~20 cm) proximal and distal to the knee joint line. The specimens were then dissected with the ligamentous knee structures and the tendons of the quadriceps, medial and lateral hamstring, and medial and lateral gastrocnemius muscles left intact. The dissected specimens were kept frozen at −20°C until they were needed and were thawed at room temperature for at least twelve hours before testing commenced. During the testing, isotonic saline solution was regularly sprayed on the soft tissues to prevent drying.
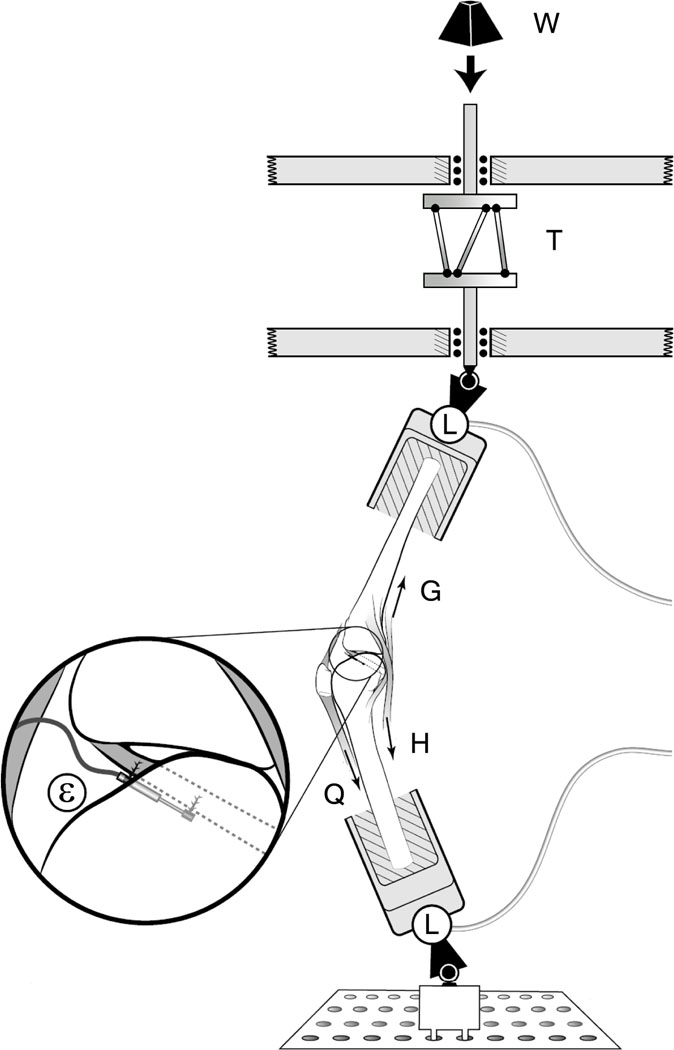
Each knee specimen was mounted in a testing apparatus to simulate the position of a single extremity as it strikes the ground while landing onto one leg during a plant-and-cut or pivot maneuver.23–25 The quadriceps and medial and lateral gastrocnemius muscles were represented by elastic muscle-equivalent structures (~2 mm diameter woven nylon cord, tensile stiffness ~2 kN/cm) pre-tensioned to 180 N and 70 N each, respectively. Two custom-made constant force springs (pre-tensioned to 70 N each) were used to represent the medial and lateral hamstring muscle forces. In each case the muscle tendon was gripped by a cryoclamp to attach it to the muscle-equivalent along the anatomic line of action, thereby representing its in vivo dynamic resistance to sudden stretch. In all trials, an initial knee flexion angle of 15 degrees was maintained by the pretension in the muscle-equivalents. An impulsive jump landing ground reaction force was simulated by releasing a drop weight onto a custom instrumented fixture holding the distal tibia of the inverted knee so as to generate a 2*BW impulsive force peaking in ~50 msec, where BW denotes each donor’s postmortem body-weight. In a new departure from the original Withrow et al. apparatus, a specially-designed adjustable torsional transformer device (Figure 1) was mounted in series with the distal tibial fixture so that the linear momentum of the drop-weight at impact was transformed into the combination of an axial compressive force and an impulsive axial torque component applied to the tibia. The torsional transformer device consists of two circular plates between which three palls are mounted equidistantly from one another and tangentially to an imaginary cylinder lying orthogonal to and within the two plates. The top circular plate can only translate vertically while the bottom plate can both translate vertically and rotate axially. The direction of the torque could be preselected by setting the inclination of each pall relative to the bottom plate.
Figure 1.
Schematic of testing apparatus. A weight (W) is dropped through a standard height onto an impact rod in series with a torsional device (T). Six--axis load cells (L) are located on distal tibia and proximal femur to measure knee input and output loads. Quadriceps (Q), hamstrings (H) and gastrocnemius (G) muscle forces are simulated. Inset shows the DVRT attached to the AM-ACL.
Two 6 degree-of-freedom load cells (MC3A-1000, AMTI, Watertown, MA) measured the 3-D tibial forces and moments delivered to the construct, as well as the 3-D femoral reaction forces and moments. A 3-mm DVRT (Microstrain, Burlington, VT) was mounted on the anteromedial (AM) bundle of the ACL to record relative strain. The anterior knee joint capsule was opened so as to identify the AM bundle and its fiber direction. The transducer was placed under direct vision parallel to the fiber direction at the first quartile of the AM-ACL length measured from the tibial insertion. The knee joint capsule was then closed prior to the testing. Five single degree-of-freedom load cells (TLL-1K, Transducer Techniques, Temecula, CA) measured simulated muscle tensions. Impulsive forces, the five muscle forces and ACL strain data were recorded at 2 kHz, while tibiofemoral kinematic data, defined in accordance with Grood and Suntay26, were recorded at 400 Hz to the nearest degree and mm using bone screws, infrared diodes and an Optotrak Certus system (Northern Digital, Inc., Waterloo, Canada).
Testing Protocol (Table 1)
TABLE 1.
Repeated measures experiment protocol proceeded from trial block in top row to bottom row. Two blocks of experimental trials were interposed between the two baseline trial blocks.
| Protocol | Loading direction |
|---|---|
| BASE1 | Compression + Flexion moment |
| INT† | Compression + Flexion moment + Internal tibial torque |
| EXT† | Compression + Flexion moment + External tibial orque |
| BASE2 | Compression + Flexion moment |
The order of the experimental blocks was randomized (see text for detail).
During the first five pre-conditioning trials, the height of the weight drop was varied to find the drop height that best simulated a two times body-weight (BW) impulsive ground reaction force for the baseline loading condition. That drop height was then maintained throughout all trials to apply the same kinetic energy to the knee specimens. After the five pre-baseline trials (‘BASE1’), three blocks of six trials were run on each ACL-Intact specimen in a ‘BASE1– B – C – BASE2’ repeated measures design, where the blocks ‘B’ and ‘C’ were randomized to be either an internally-directed (‘INT’) or externally-directed (‘EXT’) tibial torque superposed on the standard baseline compression force and flexion moment, followed by the post-baseline trial block (‘BASE2’).
The baseline loading conditions, ‘BASE1’ and ‘BASE2’, were designed to simulate a drop landing where the impulsive ground reaction force provides the compressive force on the knee joint and induces the knee flexion, thereby causing sudden stretch of the quadriceps muscle-tendon unit. This stretch of the quadriceps muscle-tendon unit resulted in anterior tibial displacement, thereby increasing the ACL strain via the patellofemoral mechanism.
Statistical Analysis
The primary outcomes were AM-ACL relative strain and strain rate in the simulated pivot landing scenario. The peak AM-ACL relative strain for each loading trial was normalized by dividing by the mean peak AM-ACL relative strain obtained during the baseline loading conditions (i.e., ‘BASE1’ and ‘BASE2’). This was done for the last five trials of each loading condition. Then, the five normalized peak AM-ACL relative strain values were averaged to find a representative strain value for each loading condition. In this repeated measures experiment, a non-parametric Wilcoxon Signed-Rank test was used to test the two hypotheses. An alpha level of 0.05 was considered significant.
RESULTS
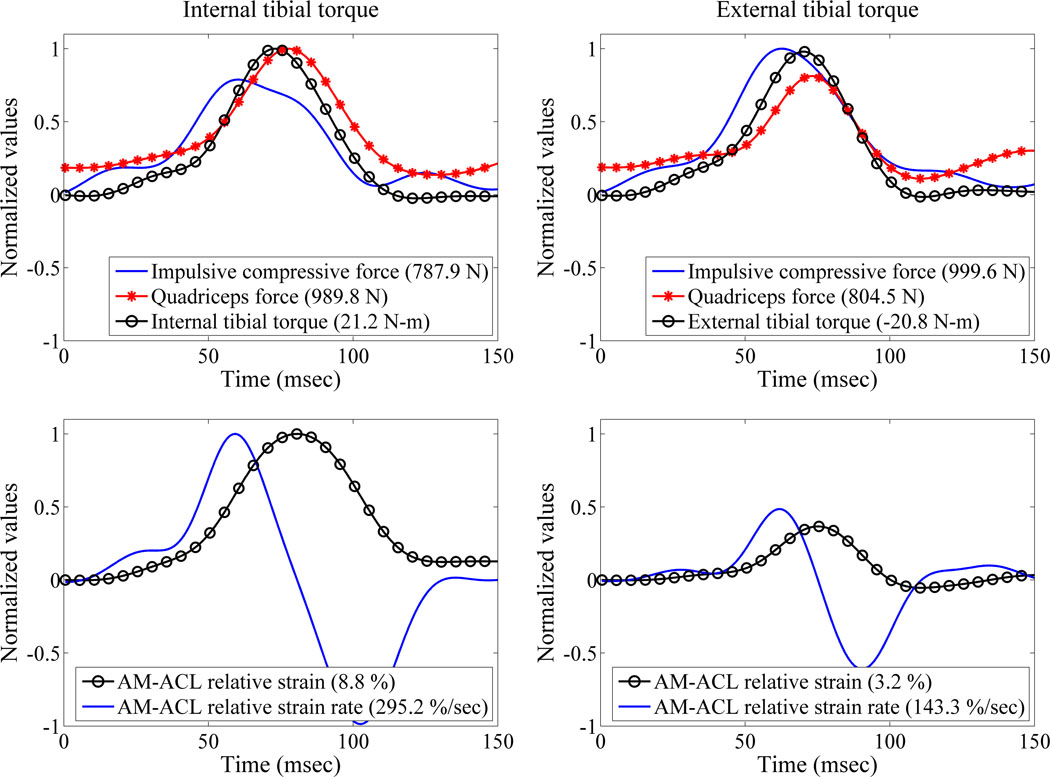
Ten of the twelve knee specimens exhibited significantly greater peak AM-ACL relative strain under the impulsive internal tibial torque than under a similar magnitude of external tibial torque. No differences were found between two baseline loading conditions (‘BASE1’ and ‘BASE2’), thereby confirming the knee specimens were not damaged during the testing. The increases in the AM-ACL relative strain and strain rate under the internal tibial torque were significantly different from the baseline (i.e., p=0.005 and p=0.021, respectively), while the corresponding values under the external tibial torque were not significantly different. The impulsive compressive force, input moments, and primary and secondary outcome measurements for each loading condition are summarized in Table 2. Sample temporal behavior from a single representative specimen and trials are shown in Figure 2.
TABLE 2.
Mean (± SD) value for the input force and moment, as well as the primary and secondary outcome measurements by testing block (N=12 specimens)
| BASE1 | Internal Tibial Torque | External Tibial Torque | BASE2 | ||
|---|---|---|---|---|---|
| Input Force | Impulsive Compressive Force (N) | 1,286.9 ± 203.4 | 852.4 ± 98.5 | 991.9 ± 123.0 | 1,256.5 ± 193.3 |
| Input Moment | Axial Tibial Torque (N-m)a | - | 17.3 ± 3.7 | −18.0 ± 2.1 | - |
| Primary Outcomes | AM-ACL Relative Strain (%) | 3.0 ± 2.0 | 5.4 ± 3.7 | 3.1 ± 2.8 | 2.9 ± 1.7 |
| AM-ACL Relative Strain Rate (%/sec) | 184.2 ± 112.0 | 252.4 ± 160.1 | 179.4 ± 109.9 | 196.1 ±101.3 | |
| Secondary Outcomes | Quadriceps Force (N) | 1,091.4 ± 305.5 | 1,093.5 ± 253.7 | 1,089.2 ± 349.8 | 1,181.3 ± 344.8 |
| Knee Flexion Angle (deg) | 4.6 ± 1.4 | 4.8 ± 1.3 | 2.8 ± 1.3 | 4.5 ± 1.2 | |
| Anterior Tibial Translation (mm) | 1.3 ± 1.0 | 3.6 ± 2.6 | 0.8 ± 0.6 | 1.3 ± 1.0 | |
| Axial Tibial Rotation (deg)a | 1.8 ± 1.5 | 12.2 ± 3.1 | −11.8 ± 3.7 | 1.7 ± 1.2 | |
Positive value represents internal tibial torque or rotation.
Figure 2.
Sample temporal behaviors of the impulsive compressive force, quadriceps force, axial tibial torque, and AM-ACL relative strain and strain rate (Specimen ID: F32933R). Measurements are normalized to their maximum values to ease comparison and the pertinent peak values are shown in the legend.
Effect of axial tibial torque direction on the peak AM-ACL relative strain
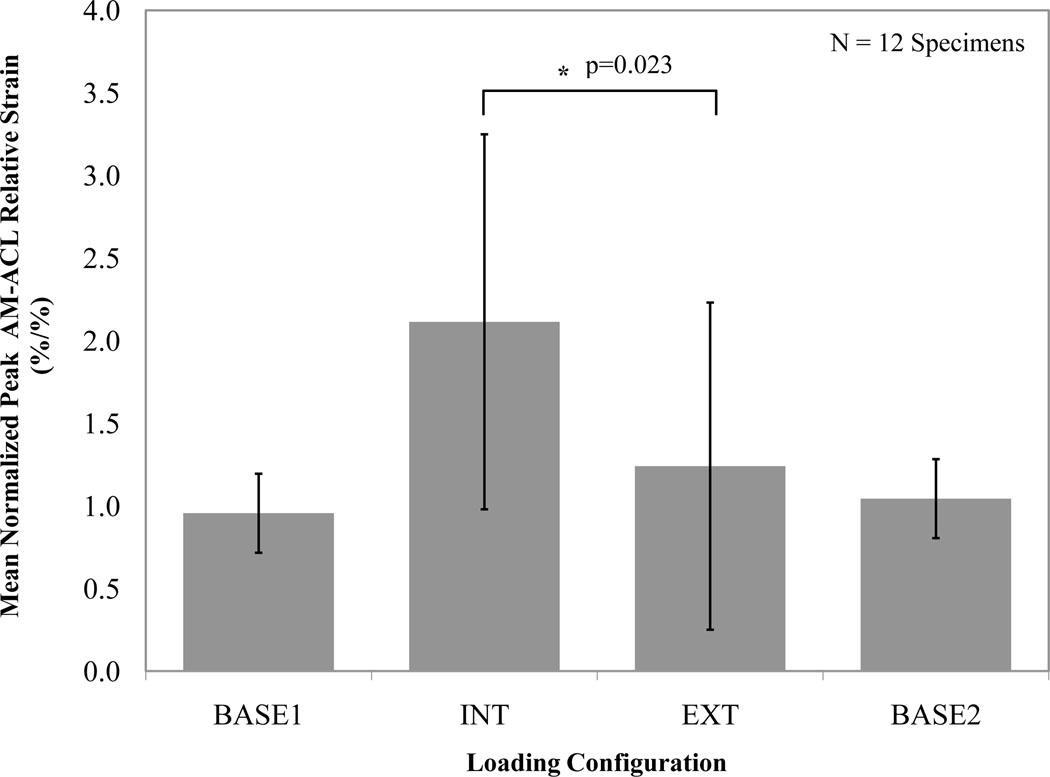
In testing the first primary hypothesis, across all 12 specimens, the normalized mean peak AM-ACL relative strain was significantly greater (70 % increase, p=0.023; Figure 3) under internal than external tibial torque. The internally-directed loading condition (‘INT’) caused the normalized mean peak AM-ACL relative strain to be 117 % greater than the baseline loading condition (‘BASE1’), whereas the corresponding increase for the externally-directed loading condition (‘EXT’) was 30 % (Figure 3).
Figure 3.
Mean (SD, represented by error bars) normalized peak AM-ACL relative strain values under each loading condition. In this and the following figure, the asterisk indicates a significant difference.
Effect of axial tibial torque direction on the peak AM-ACL relative strain rate
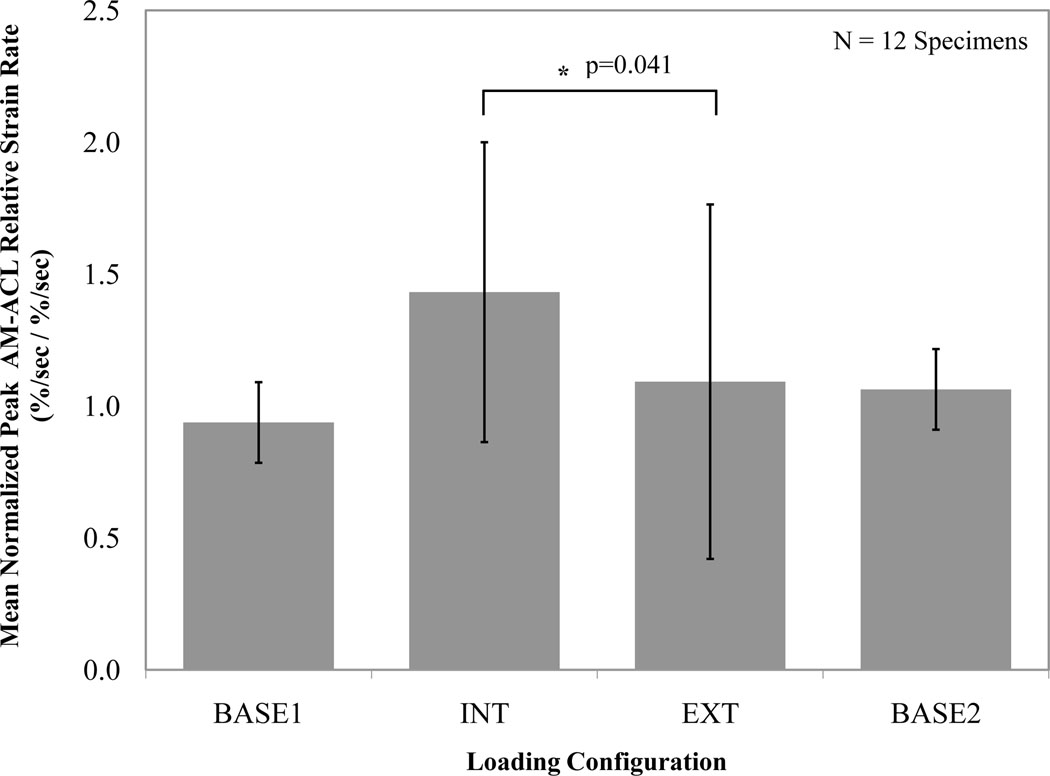
In testing the secondary hypothesis, the normalized mean peak AM-ACL relative strain rate was significantly greater (42 % increase, p=0.041; Figure 4) under internal than external tibial torque. The internally-directed loading condition (‘INT’) caused the normalized mean peak AM-ACL relative strain rate to increase 51 % when compared to the baseline loading condition (‘BASE1’), whereas the corresponding increase for the externally-directed loading condition (‘EXT’) was 16 % (Figure 4).
Figure 4.
Effect of tibial torque direction on mean (SD, represented by error bars) normalized peak AM-ACL relative strain rate values across all specimens.
DISCUSSION
The results demonstrate that the AM-ACL relative strain and strain rate increased significantly more under the internal than external tibial torque in the presence of the realistic impulsive compressive force, flexion moment and muscle forces.27 Considering the fact that daily mobility task and sports maneuvers induce large dynamic loads on the knee28, the present study provides useful insights into how the ACL is loaded when such loads include large axial tibial torques.
Our results corroborate and extend the earlier studies, which employed loads that were much less than one body-weight in magnitude and quasi-static in nature.7–9 For example, Arms et al. found that a quasi-static 13.6 N-m internal tibial torque combined with a simulated quadriceps contraction (~400 N) caused higher ACL strain than a corresponding external tibial torque.7 Similarly an in vivo study performed by Fleming et al. found that the ACL strain was higher when the knee was placed under a 10 N-m internal tibial torque than under a 10 N-m external tibial torque.8 Furthermore, Markolf et al. measured the ACL tension under a constant 5 N-m of internal or external tibial torque with and without a constant 100 N quadriceps force.9 They found that the ACL forces were significantly higher under the internal tibial torque than the external tibial torque near full extension. Recently, Meyer et al. showed that the ACL failure occured at about 58 degrees of internal tibial rotation under an average of 33 N-m internal tibial torque.29 They obtained quite large internal tibial rotations, which might not be observed in actual isolated ACL injury incidents. The large internal tibial rotation likely occurred because they did not incorporate the muscle forces. The qualitative findings, however, are consistent with our findings. Under more physiological loading conditions, the present results unequivocally demonstrate that the ACL relative strain is significantly larger under simulated landing conditions involving an internal tibial torque than an external tibial torque.
It is interesting that post hoc injury video analyses have suggested that the ACL injury can occur under both internal and external tibial rotation, often combined with knee valgus loading. For example, Olsen et al. analyzed 20 videotaped ACL handball injuries and found that two-thirds (12 of 19) of the injuries occurred in apparent external tibial rotation during a plant-and-cut, one-leg landing, and/or deceleration maneuver with the knee near full extension; the remainder were injured in a movement that appeared to generate a torque producing internal tibial rotation.11 On the other hand, Meyer et al. showed that the tibiofemoral joint compression caused ACL failure by inducing internal tibial rotation combined with anterior tibial translation. Interestingly, after the ACL failure, external tibial rotation was observed under the tibiofemoral joint compression. This observation suggests that after ACL failure is actually observed in post hoc injury video analyses, any subsequent motions are not representative of loading conditions that caused ACL failure.29 Our results showed that ten of twelve knee specimens exhibited significantly greater ACL relative strain under the internal tibial torque than the similar magnitude of the external tibial torque. One of the remaining two knee specimens in which the peak ACL relative strains were larger under the external tibial torque than the internal tibial torque exhibited the smallest notch height of any knee on frontal plane x-ray images. Thus it is possible that the notch interfered with the DVRT causing an artifact. It is known that ACL impingement can occur under external tibial rotation and knee abduction30 and a narrow femoral notch width is one risk factor for non-contact ACL injury31. In the case of the other knee, there was no obvious morphological difference from the other knees on radiographs or by visual inspection, so we are at a loss to explain why its ACL strain was greater under the external tibial torque. We can speculate that it might have been caused by a lateral movement of the patella, causing it to apply a greater posteriorly-directed force to the lateral condyle than the medial condyle.
A valgus posture has previously been identified as a primary ACL injury mechanism.17 Thus, many ACL injury prevention programs have focused on minimizing valgus loading to the knee during jump landings.16–19 However, there exist some controversies over the valgus loading mechanism. It is theorized that knee valgus (or knee abduction) loads during landing can cause ACL injury by inducing medial knee joint opening. However, the medial collateral ligament (MCL), considered as a primary restraint to knee valgus moment, is injured in only 4–27 % of all ACL injuries.32–33 In a systematic review by Quatman et al., the authors explain the relative lack of combined ACL/MCL injuries as being due to the failure load of MCL being greater than the corresponding value of ACL (i.e., 2,300 N vs. 640–2,100 N).13 However, it appears that the difference is not really large enough to explain the relative infrequency of combined ACL/MCL injuries. The lateral compartment bone bruise patterns also suggest that either a knee abduction or anterolateral tibial subluxation resulting from internal tibial rotation may be involved under large axial compressive joint loading.34–37 Unfortunately, it remains unclear which of these is the most crucial loading pattern during ACL injury. Additionally, Fleming et al. reported that the weightbearing condition significantly increased ACL strains compared to the non-weightbearing condition, while the ACL strains remained relatively consistent over the range of valgus moments tested. This finding suggests that the ACL is not a restraint to valgus loading.8 Previously, Withrow et al. investigated the effect of valgus loading on the peak AM-ACL relative strain: the normalized peak AM-ACL relative strain was 30% larger in the dynamic valgus loading (i.e., 132.5 ± 29.0 N-m) compared to the sagittally-symmetric baseline loading condition where an impulsive compressive force exceeding two body-weights (~1,500 N) and flexion moment were applied.26 Using a similar, but improved, testing apparatus to that used by Withrow et al.26, the present study found that the peak AM-ACL relative strain was 117% greater under the internal tibial torque (i.e., 17.3 ± 3.7 N-m) than the baseline loading condition. This indirect comparison implies that the internal tibial rotation might play a more important role in increasing the ACL strain than knee valgus loading. This would seem to be a role that cannot be assessed by injury videotape analyses.
As discussed in our recent article24, our in vitro methods include several limitations. First, only one initial knee flexion angle (15°) was tested. However, the knee flexion angle at injury is estimated to be 16° in injury video analysis and Li et al. reported the ACL strain to be highest with the knee in 15° of flexion.11,38 Thus, the initial 15° knee flexion angle used in this study seems reasonable. Second, the ACL strain was measured only in anteromedial (AM) region. The AM-ACL strain may not reflect the strain within the posterolateral (PL) region of the ACL. However, according to the results reported by Gabriel et al., the in situ force in the AM bundle was greater than the corresponding value in the PL bundle in response to a rotatory load (i.e., 10 N-m valgus and 5 N-m internal tibial torque) at 15° of knee flexion angle.39 Moreover, at 30° of knee flexion angle, the in situ force in the AM bundle increased in response to the same rotatory load, while the in situ force in the PL bundle decreased. A similar load sharing pattern was observed in response to a 134 N anterior tibial force for both the knee flexion angles. Thus, measuring the AM-ACL strain under axial tibial torque seems reasonable. The third limitation is that we tested knee specimens from older donors, so our results cannot necessarily be generalized to younger populations. Strocchi et al. reported that in adults and elderly subjects, the maximum diameter of the ACL collagen fibril is significantly decreased compared to younger (<20 years) subjects.40 The decreased diameter may or may not reduce elastic ACL stiffness. However, this does not necessarily mean that the ACL strain characteristics would be qualitatively different. Although knee specimens from young donors might show smaller ACL strain values for each loading condition, we expect that the general trend of the normalized AM-ACL strain and strain rate should be maintained.
This study clearly suggests that pivot landing or plant-and-cut maneuvers that apply large impulsive internal tibial torques to the knee are risky from the point-of view of causing excessive AM-ACL strain. It has been shown that a higher coefficient of friction between the shoe-ground interfaces is associated with a greater axial tibial torque transmitted to the knee joint, thereby leading a greater risk of ACL injury.41 Taken together with existing literature, the present study suggests the necessity for limiting the maximum axial torque that can be applied to a tibia perhaps by changing regulations to limit the maximum frictional torque that can be developed between a shoe sole and a playing surface.
ACKNOWLEDGMENTS
In support of this research for or preparation of this manuscript, one or more of the authors received grants from the National Institute of Health. The authors thank for their technical assistance in rebuilding the Withrow test apparatus and adding the torsional transformer device.
REFERENCES
- 1.Griffin LY, Albohm MJ, Arendt EA, et al. Understanding and preventing noncontact anterior cruciate ligament injuries - A review of the Hunt Valley II Meeting, January 2005. Am J Sports Med. 2006;34(9):1512–1532. doi: 10.1177/0363546506286866. [DOI] [PubMed] [Google Scholar]
- 2.McNair PJ, Marshall RN, Matheson JA. Important features associated with acute anterior cruciate ligament injury. N Z Med J. 1990;103:537–539. [PubMed] [Google Scholar]
- 3.Gillquist J, Messner K. Anterior cruciate ligament reconstruction and the long-term incidence of gonarthrosis. Sports Med. 1999;27:143–156. doi: 10.2165/00007256-199927030-00001. [DOI] [PubMed] [Google Scholar]
- 4.Myklebust G, Holm I, Maehlum S, Engebretsen L, Bahr R. Clinical, functional, and radiologic outcome in team handball players 6 to 11 years after anterior cruciate ligament injury. Am J Sports Med. 2003;31:981–989. doi: 10.1177/03635465030310063901. [DOI] [PubMed] [Google Scholar]
- 5.Lohmander LS, Englund PM, Dahl LL, Roos EM. The long-term consequence of anterior cruciate ligament and meniscus injuries - Osteoarthritis. Am J Sports Med. 2007;35:1756–1769. doi: 10.1177/0363546507307396. [DOI] [PubMed] [Google Scholar]
- 6.Kirkendall DT, Garrett WE. The anterior cruciate enigma: Injury mechanisms and prevention. Clin Orthop Rel Res. 2000;372:64–68. doi: 10.1097/00003086-200003000-00008. [DOI] [PubMed] [Google Scholar]
- 7.Arms SW, Pope MH, Johnson RJ, Fischer RA, Arvidsson I, Eriksson E. The biomechanics of anterior cruciate ligament rehabilitation and reconstruction. Am J Sports Med. 1984;12(1):8–18. doi: 10.1177/036354658401200102. [DOI] [PubMed] [Google Scholar]
- 8.Fleming BC, Renstrom PA, Beynnon BD, et al. The effect of weightbearing and external loading on anterior cruciate ligament strain. J Biomech. 2001;34(2):163–170. doi: 10.1016/s0021-9290(00)00154-8. [DOI] [PubMed] [Google Scholar]
- 9.Markolf KL, O'Neill G, Jackson SR, McAllister DR. Effects of applied quadriceps and hamstrings muscle loads on forces in the anterior and posterior cruciate ligaments. Am J Sports Med. 2004;32(5):1144–1149. doi: 10.1177/0363546503262198. [DOI] [PubMed] [Google Scholar]
- 10.Kanamori A, Woo SL, Ma CB, Zeminski J, Rudy TW, Li G, Livesay GA. The forces in the anterior cruciate ligament and knee kinematics during a simulated pivot shift test: a human cadaveric study using robotic technology. Arthroscopy. 2000;16:633–639. doi: 10.1053/jars.2000.7682. [DOI] [PubMed] [Google Scholar]
- 11.Olsen OE, Myklebust G, Engebretsen L, Bahr R. Injury mechanisms for anterior cruciate ligament injuries in team handball a systematic video analysis. Am J Sports Med. 2004;32(4):1002–1012. doi: 10.1177/0363546503261724. [DOI] [PubMed] [Google Scholar]
- 12.Teitz CC. Video analysis of ACL injuries. In: Griffin LY, editor. Prevention of noncontact ACL injuries. Rosemont, IL: American Association of Orthopaedic Surgeons; 2001. pp. 87–92. [Google Scholar]
- 13.Quatman CE, Quatman-Yates CC, Hewett TE. A 'plane' explanation of anterior cruciate ligament injury mechanisms - A systematic review. Sports Medicine. 2010;40(9):729–746. doi: 10.2165/11534950-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 14.Markolf KL, Burchfield DM, Shapiro MM, Shepard MF, Finerman GA, Slauterbeck JL. Combined knee loading states that generate high anterior cruciate ligament forces. J Orthop Res. 1995;13:930–935. doi: 10.1002/jor.1100130618. [DOI] [PubMed] [Google Scholar]
- 15.Boden BP, Torg JS, Knowles SB, Hewett TE. Video analysis of anterior cruciate ligament injury: abnormalities in hip and ankle kinematics. Am J Sports Med. 2009;37:252–259. doi: 10.1177/0363546508328107. [DOI] [PubMed] [Google Scholar]
- 16.Hewett TE, Lindenfeld TN, Riccobene JV, Noyes FR. The effect of neuromuscular training on the incidence of knee injury in female athletes - A prospective study. Am J Sports Med. 1999;27:699–706. doi: 10.1177/03635465990270060301. [DOI] [PubMed] [Google Scholar]
- 17.Hewett TE, Myer GD, Ford KR, et al. Biomechanical measures of neuromuscular control and valgus loading of the knee predict ACL injury risk in female athletes: A prospective study. Am J Sports Med. 2005;33:492–501. doi: 10.1177/0363546504269591. [DOI] [PubMed] [Google Scholar]
- 18.Lloyd DG. Rationale for training programs to reduce anterior cruciate ligament injuries in Australian football. J Orthop Sports Phys Ther. 2001;31:645–654. doi: 10.2519/jospt.2001.31.11.645. [DOI] [PubMed] [Google Scholar]
- 19.Myer GD, Ford KR, Hewett TE. Rationale and clinical techniques for anterior cruciate ligament injury prevention among female athletes. J Athl Training. 2004;39:352–364. [PMC free article] [PubMed] [Google Scholar]
- 20.Krosshaug T, Nakamae A, Boden B, Engebretsen L, et al. Estimating 3D joint kinematics from video sequences of running and cutting maneuvers--assessing the accuracy of simple visual inspection. Gait Posture. 2007;26(3):378–385. doi: 10.1016/j.gaitpost.2006.10.010. [DOI] [PubMed] [Google Scholar]
- 21.Krosshaug T, Slauterbeck JR, Engebretsen L, Bahr R. Biomechanical analysis of anterior cruciate ligament injury mechanisms: three-dimensional motion reconstruction from video sequences. Scand J Med Sci Sports. 2007;17(5):508–519. doi: 10.1111/j.1600-0838.2006.00558.x. [DOI] [PubMed] [Google Scholar]
- 22.Grood ES, Noyes FR. Cruciate ligament prosthesis – strength, creep, and fatigue properties. J Bone Joint Surg AM. 1976;58(8):1083–1088. [PubMed] [Google Scholar]
- 23.Oh YK, Kreinbrink JL, Ashton-Miller JA, Wojtys EM. Effect of ACL transaction on internal tibial rotation in an in vitro simulated pivot landing. J Bone Joint Surg AM. 2011;93(4):372–380. doi: 10.2106/JBJS.J.00262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Withrow TJ, Huston LJ, Wojtys EM, Ashton-Miller JA. The relationship between quadriceps muscle force, knee flexion, and anterior cruciate ligament strain in an in vitro simulated jump landing. Am J Sports Med. 2006;34(2):269–274. doi: 10.1177/0363546505280906. [DOI] [PubMed] [Google Scholar]
- 25.Withrow TJ, Huston LJ, Wojtys EM, Ashton-Miller JA. The effect of an impulsive knee valgus moment on in vitro relative ACL strain during a simulated jump landing. Clin Biomech. 2006;21(9):977–983. doi: 10.1016/j.clinbiomech.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 26.Grood ES, Suntay WJ. A joint coordinate system for the clinical description of three- dimensional motions: application to the knee. J Biomech Eng. 1983;105:136–144. doi: 10.1115/1.3138397. [DOI] [PubMed] [Google Scholar]
- 27.Pflum MA, Shelburne KB, Torry MR, Decker MJ, Pandy MG. Model prediction of anterior cruciate ligament force during drop-landings. Med Sci Sports Exerc. 2004;36:1949–1958. doi: 10.1249/01.mss.0000145467.79916.46. [DOI] [PubMed] [Google Scholar]
- 28.Besier TF, Lloyd DG, Ackland TR, Cochrane JL. Anticipatory effects on knee joint loading during running and cutting maneuvers. Med Sci Sports Exerc. 2001;33:1176–1181. doi: 10.1097/00005768-200107000-00015. [DOI] [PubMed] [Google Scholar]
- 29.Meyer EG, Haut RC. Anterior cruciate ligament injury induced by internal tibial torsion or tibiofemoral compression. J Biomech. 2008;41(16):3377–3383. doi: 10.1016/j.jbiomech.2008.09.023. [DOI] [PubMed] [Google Scholar]
- 30.Fung DT, Zhang LQ. Modeling of ACL impingement against the intercondylar notch. Clin Biomech. 2003;18(10):933–941. doi: 10.1016/s0268-0033(03)00174-8. [DOI] [PubMed] [Google Scholar]
- 31.Uhorchak JM, Scoville CR, Williams GN, Arciero RA, St Pierre P, Taylor DC. Risk factors associated with noncontact injury of the anterior cruciate ligament: A prospective four-year evaluation of 859 west point cadets. Am J Sports Med. 2003;31(6):831–842. doi: 10.1177/03635465030310061801. [DOI] [PubMed] [Google Scholar]
- 32.Miyasaka K, Daniel DM, Stone ML, et al. The incidence of knee ligament injuries in the general population. Am J Knee Surg. 1991;4:3–8. [Google Scholar]
- 33.LaPrade RF, Wentorf FA, Fritts H, et al. A prospective magnetic resonance imaging study of the incidence of posterolateral and multiple ligament injuries in acute knee injuries presenting with a hemarthrosis. Arthroscopy. 2007;23(12):1341–1347. doi: 10.1016/j.arthro.2007.07.024. [DOI] [PubMed] [Google Scholar]
- 34.Graf BK, Cook DA, Desmet AA, et al. Bone bruises on magnetic-resonance-imaging evaluation of anterior cruciate ligament injuries. Am J Sports Med. 1993;21(2):220–223. doi: 10.1177/036354659302100210. [DOI] [PubMed] [Google Scholar]
- 35.Viskontas DG, Giuffre BM, Duggal N, et al. Bone bruises associated with ACL rupture: correlation with injury mechanism. Am J Sports Med. 2008;36(5):927–933. doi: 10.1177/0363546508314791. [DOI] [PubMed] [Google Scholar]
- 36.Johnson DL, Urban WP, Caborn DNM, Vanarthos WJ, Carlson CS. Articular cartilage changes seen with magnetic resonance imaging-detected bone bruises associated with acute anterior cruciate ligament rupture. Am J Sports Med. 1998;26:409–414. doi: 10.1177/03635465980260031101. [DOI] [PubMed] [Google Scholar]
- 37.Spindler KP, Schils JP, Bergfeld JA, et al. Prospective study of osseous, articular, and meniscal lesions in recent anterior cruciate ligament tears by magnetic resonance imaging and arthroscopy. Am J Sports Med. 1993;21:551–557. doi: 10.1177/036354659302100412. [DOI] [PubMed] [Google Scholar]
- 38.Li G, Rudy TW, Sakane M, Kanamori A, et al. The importance of quadriceps and hamstring muscle loading on knee kinematics and in-situ forces in the ACL. J Biomech. 1999;32:395–400. doi: 10.1016/s0021-9290(98)00181-x. [DOI] [PubMed] [Google Scholar]
- 39.Gabriel MT, Wong EK, Woo SLY, et al. Distribution of in situ forces in the anterior cruciate ligament in response to rotatory loads. J Orthop Res. 2004;22(1):85–89. doi: 10.1016/S0736-0266(03)00133-5. [DOI] [PubMed] [Google Scholar]
- 40.Strocchi R, De Pasquale V, Facchini A, et al. Age-related changes in human anterior cruciate ligament (ACL) collagen fibrils. Ital J Anat Embryol. 1996;101(4):213–220. [PubMed] [Google Scholar]
- 41.Drakos MC, Hillstrom H, et al. The Effect of the Shoe-Surface Interface in the Development of Anterior Cruciate Ligament Strain. J Biomech Eng-T Asme. 2010;132(1):011003. doi: 10.1115/1.4000118. [DOI] [PubMed] [Google Scholar]