Abstract
This study describes the current situation and projects dynamic trends for HIV prevalence in a highly endemic area of China, Liangshan Prefecture, Sichuan Province. Epidemiological, behavioral, and population census data from multiple sources were analyzed to extract input for an Asian Epidemic Model (AEM). Fitting curves to historical trends in HIV prevalence were used as a baseline, and future intervention scenarios were explored using the AEM. For 2007, modeled data suggested ≈0.5% adult HIV prevalence in Liangshan, with an estimated 17,450 people living with HIV/AIDS and 3,400 new infections. With current high risk behaviors, the model predicts that adult prevalence will rise to 1.5% by 2020. Increased condom use and clean needle exchange among injection drug users (IDUs) have slowed the epidemic. The source of new HIV infections will change from a preponderance of IDU-related infections in 2007 (65.9%) to a mixed epidemic in 2020 (general population heterosexuals 45.2%, IDU 38.6%, homosexual transmission between men 12.7%, female sex workers and their clients 3.5%). We anticipate rising prevalence, stable incidence, and higher representation of sexual transmission over time. Prevention investments should target specific interventions toward sub-groups at highest risk, given that both IDUs and men who have sex with men will likely represent a majority of cases and serve as a bridge population.
Keywords: HIV, Asian Epidemic Model, epidemiologic modeling, substance abuse, homosexuality, China
INTRODUCTION
The first large outbreak of HIV in China was identified in 1989 among injection drug users (IDUs) in Dehong Prefecture, Yunnan Province, on the Myanmar (Burma) border in southwest China [1-3]. The majority of reported HIV infections in China are among rural people living in the southwest and among ethnic minorities. IDU and unhygienic plasma collecting practices have been the two major transmission routes of HIV/AIDS in various regions of China since 1989 [2, 4-6]. In recent years, however, the risk of sexual transmission of HIV has grown, either through female sex workers (FSWs) and their clients or men who have sex with men (MSM) [4]. Among the estimated 700,000 people living with HIV/AIDS in China in 2007, IDU (38.1%) and unprotected heterosexual sex (40.6%) were the most common transmission routes. Incidence estimates suggest that 11.0% of estimated new HIV cases in 2007 occurred among MSM, a group that also has a rising rate of syphilis infection [7].
Liangshan Yi Autonomous Prefecture in Sichuan Province, China, is located along one of the major drug trafficking routes to northwest and central China from the “Golden Triangle,” one of the world’s largest illicit heroin production and distribution centers [8-11]. The specific HIV subtypes first seen in Dehong spread to IDUs along drug trafficking routes, including Liangshan [12-14]. Autonomous regions are a political administrative designation for an entity that has a higher proportion of non-Han minority ethnic groups. There are 5 autonomous regions in China that have more local authority in finance, economic planning, arts, science, culture, organization of local police, and use of local languages. Liangshan is the largest autonomous prefecture of Yi nationality in China, with 48% of non-Han minority ethnic groups (44.4% Yi ethnic group, 1.5% Tibetan, and 2.1% other non-minority ethnic groups). Liangshan has 1 county-level city, 15 counties, and 1 autonomous county, with a population of 4.3 million persons living in an area of 60,423 km2 (23,329 square miles, about twice the size of Massachusetts and slightly larger than Denmark or the Netherlands). Its people confront an HIV epidemic fueled by IDU and, more recently, MSM [10, 11, 15].
The HIV epidemic in Liangshan has become one of the worst in southwest China, with 7,809 cumulative reported HIV/AIDS cases (including deceased persons) reported by the end of 2007 [8, 9, 16]. An HIV sentinel surveillance, in place in Liangshan since late 1995, has recorded a disturbing rise in HIV rates among both high-risk and general populations, including IDUs, sexually transmitted disease (STD) clinic visitors (an indicator group for the clients of FSWs), pregnant women attending antenatal clinics (ANCs), and hospital patients [8, 9, 16, 17]. HIV behavioral and biological surveillance in Liangshan has been incorporated into the existing system since 2002 [8, 15, 18]. Expanded screening for HIV has been conducted in 17 of Liangshan’s counties or cities. IDUs, FSWs, male clients of FSWs (as identified in STD clinic surveys), and MSM are the notable high-risk groups in Liangshan. Unsafe blood collection practices have not contributed substantially to HIV transmission in this prefecture, unlike patterns seen in central or eastern China [15].
To date, several modeling methods are commonly employed to estimate the dynamic trends of HIV/AIDS epidemics, such as the Asia Epidemic Model (AEM) [19, 20], the UNAIDS Workbook [21, 22], the Estimation and Projection Package (EPP) [23-26], and others [1, 27]. In contrast to the UNAIDS Workbook and the EPP, the AEM is a full-process model that mathematically replicates the key processes driving HIV transmission in Asia, and therefore needs more epidemiological and behavioral inputs. AEM permits examination of future scenarios in which prevention and care efforts induce behavior change [19]. The AEM has been applied in India, Thailand and Cambodia, and the agreement between modeled and observed HIV prevalence values suggests the potential validity of the AEM [28-31]. We used the AEM with high quality data from a variety of sources to describe the current situation and to project dynamic trends for subgroup-specific HIV prevalence in Liangshan Prefecture, Sichuan Province.
METHODS
The AEM considers HIV transmission within a population aged ≥15 years. People enter the population at age 15 and depart by either AIDS-related or non-AIDS-related death. Pediatric dynamics are calculated after the fact based on fertility data and female infection levels. We divided the Liangshan population into 8 modeling compartments: male IDUs, injection female sex workers (ISWs), FSWs, male clients of FSWs, MSM, male sex workers (MSWs), a general male group (without recognized risk), and a general female group (without recognized risk). All female IDUs were considered to be ISWs. Infection occurs either through unprotected sexual behavior or needle sharing with an infected partner. The number of new infections is calculated based on the prevalence in the partner population, the frequency of sex or injecting acts, and the probability of transmission of HIV. The model considers both protective behaviors (e.g., levels of condom use and clean needle use) and increased HIV transmission probability due to the presence of other STDs and lack of male circumcision. Details of the AEM are described elsewhere [19, 32]. Three steps were taken to complete the AEM.
First, multiple data sources were collected, including behavioral and biological surveillance, community-based surveys, epidemiological and behavioral studies, and census data. A total of 12 sentinel surveillance sites (including 3 for drug detoxification centers, 1 for community-based IDUs, 1 for ANCs, 5 for FSWs, and 2 for MSM), 12 community-based surveys, and 33 published or unpublished behavioral and epidemiological studies contributed data to the estimation and projection [1, 8-11, 33-42]. Nine surveys and epidemiological studies with poor methodology were excluded, e.g., unclear methods, inadequate description of population, case report data without adjustments, and biased samples within a given group. The population census data were obtained from the Liangshan Prefecture Statistical Bureau.
Second, the collected data were evaluated and analyzed; those with better quality were used to calculate the input parameters for the model. Bias elimination and adjustments were made to increase the representativeness of the data. Each input parameter for the model was calculated using multiple data sources. (1) The sizes of key populations (male IDUs, ISWs, FSWs, male clients of FSWs, MSM, MSWs, general male and general female groups) were calculated using surveillance data, census data, and previous estimates from the Sichuan Center for Disease Control and Prevention (unpublished data). (2) The input parameters of sexual risk behaviors (e.g., frequency of sexual contacts between different partner types and levels of condom use with different partners) were estimated using behavioral surveys and epidemiological studies. For example, the prevalence of condom use among FSWs with their clients was adjusted for socio-demographic variations in how prevention and care efforts induce behavior change. The traditional sexual culture among Yi ethnic group may contribute to HIV risk in Liangshan [8-11, 15, 18]. Casual sexual behavior among Yi males is more frequent than among Han males. Casual sex is more frequent among unmarried Yi females than among married Yi females. Thus, adjustments regarding the prevalent casual sex were made when calculating the frequency of sexual contacts of males and females who had casual sex in the last year. (3) The input parameters of injecting risk behaviors (e.g., frequency of injection and levels of needle sharing) were calculated using data from behavioral surveillance. Data from both community-based and facility-based sentinel sites (drug use detoxification centers and detention centers) were analyzed and used for these estimates. (4) The rates of movement into or out of each subgroup and the average duration in different key populations were calculated using the behavioral and epidemiological data and population census data. (5) HIV prevalence rates among each subgroup were calculated using data from the biological surveillance and epidemiological studies [19]. For example, in more highly endemic counties, the prevalence rates for the general low-risk population were estimated using data from biological and behavioral surveillance, community-based surveys, and epidemiological studies. Downward adjustment of the estimates was made to eliminate “double-counting” infections caused by high risk men and women. In the lower prevalence areas, military conscript and ANC-based data were applied to estimate the prevalence among the general low-risk male and female populations, respectively (Sichuan CDC, unpublished data). The AEM software was used to fit parameters into the model following the previously published procedures [8, 9, 15, 18].
Third, the model was used to generate prevalence curves for each subgroup. We expected these prevalence curves to be consistent with the observed prevalence curves pre-2007, which were estimated using multiple data sources. The fitted curves to historical trends in HIV prevalence among each subgroup between the epidemic start year 1994 and 2007 served as a baseline projection. Future scenarios of the epidemic post-2007 were predicted using the AEM under different rates of condom use, needle sharing, and casual sex as appropriate for each subgroup. Baseline rates of condom use, needle sharing, and casual sex were estimated based on unpublished and published data sources: 56.8% of condom use for the FSWs and ISWs with non-IDU clients, 12.3% of condom use for FSWs and ISWs with IDU clients, 5.0% of condom use during casual sex among the low-risk general population, 1.0% of condom use during sex among spouses and permanent sex partners, 52.3% of needle sharing among IDUs, 9.0% of condom use between IDUs and their spouses or steady sex partners, 37.5% of condom use among MSM and 33.3% among MSWs, and 26% of casual sex for male groups and 7% for female [8, 9, 16, 18]. Estimates are rounded to the nearest 50.
RESULTS
Output fitting
The final curves of model outputs for HIV prevalence were fitted with the estimated prevalence rates from data in all 8 populations in Liangshan. This served as the baseline fitted model for projecting under varying assumptions.
HIV dynamic trends with baseline prevention efforts induced behavior change
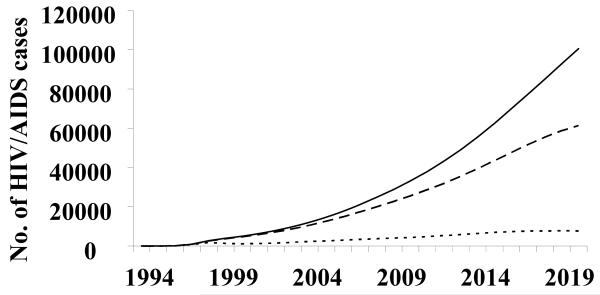
The HIV epidemic in Liangshan is thought to have begun in the mid-1990s when the first HIV cases in this prefecture were identified in 1995 among IDUs. The HIV prevalence of adults living with HIV/AIDS (PLWHA) in Liangshan increased to 0.5% (0.8% for male and 0.2% for female) in 2007, such that an estimated 17,450 people were PLWHA. There were an estimated 3,400 new infections, 1,050 cases of AIDS, and 900 deaths in that year. If behaviors were to be maintained at current baseline levels, the prevalence of PLWHA will rise to 1.1% in 2015 (1.6% for males and 0.7% for females) and to 1.5% in 2020 (2.0% for males and 1.0% for females). There will be 54,500 projected PLWHA; 6,600 new infections, 5,250 cases of AIDS, and 5,050 deaths in 2020 (Fig. 1). The cumulative number of HIV/AIDS cases was estimated at 21,100 by the end of 2007; this number is projected to rise to 94,500 in 2020 (Fig. 1).
Figure 1.
The estimated and projected number of current, new and cumulative HIV/AIDS infections in Liangshan Yi Autonomous Prefecture, Sichuan Province, China. — —, currently living with HIV/AIDS; - - - -, new infections; ——, cumulative infections (included deaths).
HIV prevalence rates among IDUs started to increase significantly in 1998 and reached an estimated 29.4% in 2007. With current prevention efforts, the prevalence is predicted to rise to 38.3% in 2020; the model predicts that the number of IDUs with HIV/AIDS will rise from 9,400 in 2007 to 14,300 IDUs in 2020; the incidence rates among IDUs will be stable at 7.0% from 2007 to 2020. The epidemic trends among ISWs were similar to those among IDUs until the prevalence reached 8.6% in 1998. HIV prevalence among ISWs gradually dropped to 3.5% in 2007, and then leveled off.
The number of FSWs and clients with HIV/AIDS is projected to grow from 150 FSWs and 1,100 clients in 2007 to 300 FSWs and 1,250 clients in 2020. With current rates of condom use, the prevalence rates for FSWs will rise from 2.6% in 2007 to 3.9% in 2020, but the rates for clients will be similar: 1.7% in 2007 and 1.5% in 2020.
HIV cases were first found among MSM in 2005 in Liangshan. In 2007, the rates for HIV infections were estimated at 2.1% for MSM and 2.0% for MSWs. With the current rate of condom use, the rates of HIV infections are projected to rise rapidly to the impossibly high level of 78.6% for MSM and 60.1% for MSWs in 2020, suggesting a failure of AEM to accommodate biological asymptotes when limited data are available. Hence, we have capped the HIV prevalence estimates for MSM and MSWs at 40% to provide the baseline estimates for this study. We also conducted a sensitivity analysis for our prevalence estimate using the capped and uncapped rates. Given an estimated 15,450 MSM in Liangshan (1.0% of the male population), MSM/MSWs are predicted to emerge as a major transmission route in Liangshan, accounting for 11% (capped) to 21.2% (uncapped) of PLWHA in 2020.
IDU has been the predominant transmission route for HIV/AIDS, contributing two-thirds of new HIV cases (65.9%) in 2007. The other major transmission routes are heterosexual transmission among the low-risk general population (26.8%), homosexual transmission between men (3.7%), and sexual transmission between FSWs and clients (3.6%). In 2020, the major transmission route for the new infections is predicted to be heterosexual transmission among the low-risk general population (45.2%), followed by IDU (38.6%), homosexual transmission between men (12.7%), and female sex workers and their clients (3.5%).
Future scenarios with increased prevention efforts (condom use and needle sharing)
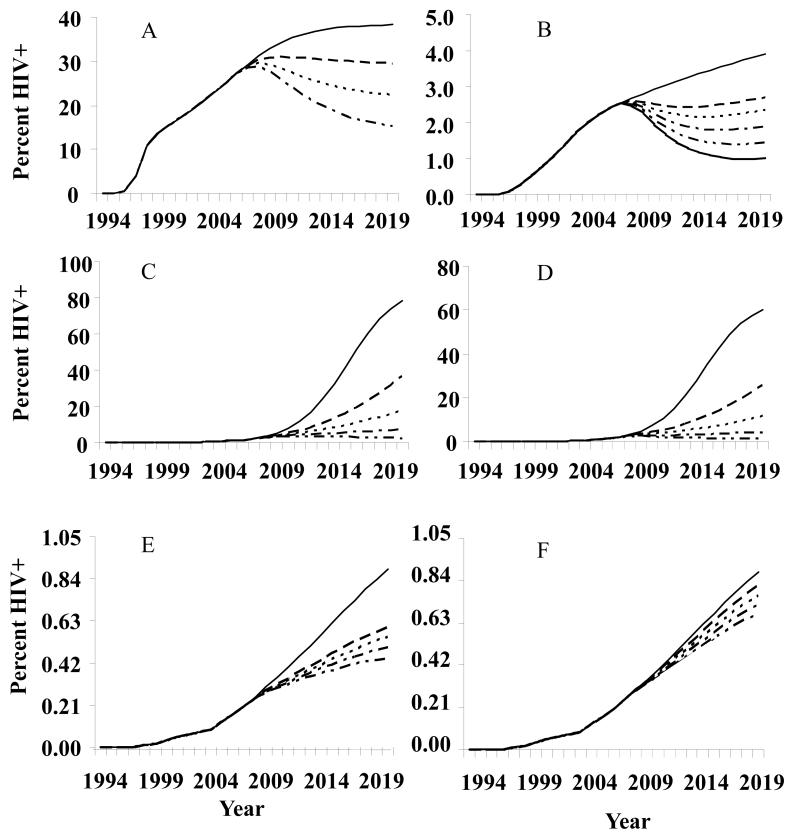
With 60% of clean needle use (40% needle sharing), the model predicts that HIV prevalence among IDUs will level off from 2007 to 2020 (Fig.2A). With ≥70% clean needle use, the prevalence among IDUs will decline dramatically (Fig.2A).
Figure 2.
Hypothetical scenarios for Liangshan.
A. HIV prevalence rates with different rates of needle sharing among injection drug users (IDUs). ——, 52.3% needle sharing (baseline); — —, 40%; - - - -, 30%; — - –, 20%.
B. HIV prevalence rates among female sex workers (FSWs) and female injection sex workers (ISWs), with different rates of condom use for FSWs and ISWs with non-IDU clients and IDU clients. ——, 56.8% for non-IDU clients, 12.3% for IDU clients (baseline rates of condom use); — —, 57%, 50%; - - - -, 60%, 60%; — - –, 70%, 70%; — - -, 80%, 80%; ----------, 90%, 90%.
C. HIV prevalence rates among men who have sex with men (MSM) with different rates of condom use. ——, 37.5% condom use (baseline); — —, 60%; - - - -, 70%; — - –, 80%; — - -, 90%. Baseline projection to 67.3% prevalence in 2020 (see note in results as to model lower bound adjustment).
D. HIV prevalence rates among male sex workers (MSWs) with different rates of condom use. ——, 33.3% condom use (baseline); — —, 60%; - - -, 70%; — - –, 80%; — - -, 90%. Baseline projection to 46.0% prevalence in 2020 (see note in results as to model lower bound adjustment).
E. HIV prevalence rates among low-risk general male and female populations with different rates of condom use. ——, 5% condom use for the people that ever engaged in casual sex, 1% for regular sex, baseline rates of condom use; — —, 60%; - - - -, 70%; — - –, 80%; — - -, 90%.
F. HIV prevalence rates among low-risk general male and female populations with different rates of casual sex. ——, 26% of males that ever engaged in casual sex, 7% of females that ever engaged in casual sex, baseline rates of casual sex; — —, 20%, 6%; - - - -, 15%, 6%; — - –, 10%, 5%; — - -, 5%, 4%.
Condom use alone can reduce the prevalence among FSWs (including ISWs). The condom use rate among FSWs (including ISWs) with IDU clients and non-IDU clients must be increased to 60% in order to maintain stable prevalence; increasing the rate of condom use to ≥90% will have a further significant impact on the epidemic (Fig. 2B).
The model predicts a continuing rise of HIV prevalence for MSM/MSWs even with 60% condom use rates. Condom use frequency must be increased to ≥90% for MSM/MSWs in order to turn the tide substantially (Fig. 2C, 2D).
With 90% condom use rates in the low-risk general population, the epidemic will decline (Fig. 2E). Reducing frequency of casual sex will also reduce the epidemic (Fig. 2F). With both condom use and reduced frequency of casual sex acts, the model predicts a slowing down in the rise of the epidemic but not enough to reverse the trend (data not shown).
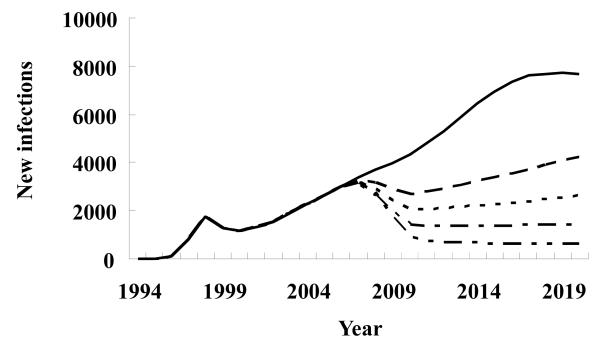
With current baseline prevention efforts, new infections in the entire population (all 8 subgroups) will increase rapidly. However, 60% of the coverage of both condom use and clean needle use would lead to a drop in the overall prevalence between 2008 and 2010; afterward, the prevalence starts to rise again (Fig. 3). Only >90% coverage for both clean needle use and condom use will result in a sustained decline in the overall prevalence.
Figure 3.
Hypothetical scenarios for the new HIV infections with different rates of needle sharing and condom use among highest risk groups, and low-risk general male and female populations in Liangshan Yi Autonomous Prefecture, Sichuan Province, China. ——, baseline; — —, 60%; - - - -, 70%; — - –, 80%; — - -, 90%.
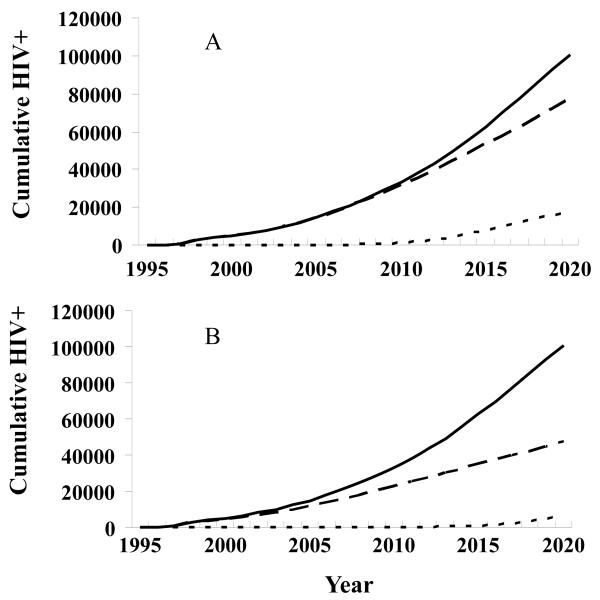
If no MSM/MSW epidemic occurred, the epidemic now spreading through IDUs, FSWs, and clients would still increase under current baseline prevention efforts (Fig. 4A). If no IDU epidemic occurred, the epidemic now spreading through FSWs, clients, and MSM/MSWs would remain at a low level, with the overall HIV prevalence among adults rising slightly to 0.2% in 2020. Unprotected male-male sex among MSM/MSWs and unprotected casual sex among low-risk populations would be the principal transmission routes in 2020, if substantial progress were made to reduce IDU incidence (Fig. 4B).
Figure 4.
Hypothetical scenarios for the HIV prevalence.
A. If no epidemic occurred among men who have sex with men (MSM)/male sex workers (MSWs), the epidemic now spreading through injection drug users (IDUs), female sex workers (FSWs), and clients would still increase under current baseline prevention efforts. ——, All infections; — —, With no infections among MSM/MSW; - - - -, infection among MSM/MSW.
B. If no IDU epidemic occurred, the epidemic now spreading through FSWs, clients, and MSM/MSWs would remain at a low level with the overall HIV prevalence among adults rising slightly to 0.2% in 2020. MSM/MSWs would be the predominant transmission route. ——, All infections; — —, infections among IDUs; - - - -, With no infection among IDUs.
DISCUSSION
The growing availability of high quality epidemiological and behavioral data from sentinel surveillance and community-based surveys makes site projection of the HIV epidemic feasible in many venues in China, such as Liangshan Prefecture [4]. Under baseline coverage rates of condom use and clean needle use, the model predicted that the HIV epidemic among adults (15-64 years of age) would continue to rise from 0.5% in 2007 to 1.5% in 2020. Thus, we provide concrete projections to authorities as to the importance of expanding prevention efforts now. Liangshan continues to be one of China’s most severely affected locales and we think the epidemic will worsen [15, 43].
Application of the AEM to Liangshan accurately reproduced current trends in all key populations. Epidemiological data indicate that the HIV epidemic started with IDUs in the mid-1990s. A high prevalence of needle sharing and a low level of condom use fueled rapid HIV spread among IDUs, subsequently infecting FSWs and clients through sexual contact. The local health departments initiated intervention efforts for IDUs and FSWs, e.g., needle exchange and condom promotion programs in the late 1990s. HIV spread among these two groups may have slowed down under current prevention efforts, but causality cannot be inferred. In contrast, sex among MSM/MSWs and casual sex transmission among low-risk populations have gradually emerged as major HIV transmission routes.
Since HIV cases were first identified among MSM in Liangshan in 2005, prevalence rates among MSM and MSWs have increased rapidly in the past 3 years [18]. With the current rate of condom use (<40%) among local MSM [8, 9], the HIV prevalence is projected to rise rapidly. The AEM projects levels of 78.6% among MSM and 60.10% among MSWs in 2020, both far higher than plausible. These predictions are incompatible with observed rates among MSM even in the highest prevalence venues. Modeling takes year by year values for many important parameters, including all years from epidemic starting year forward. Many assumptions must be made for early years or for later years, different hypothetical scenarios can be projected. The key inputs and sources for MSM/MSWs were based on the limited data in the past 3-year data in Liangshan, so unstable projection is expected. Thus, we ran our model with lower plausible MSM/MSW estimate to an overall range for 2020 population prevalence estimates. Our model suggests that the epidemic could level off if 90% condom use were achieved for both MSM and MSWs, an impossibly high level indeed.
The increased HIV spread among the “low risk” general population was driven by the higher prevalence of unprotected casual heterosexual sex in the Yi ethnic group [9]. The frequency of unprotected casual sex acts among Yi adolescents and adults, along with the frequency of casual sex acts with low condom use among the Han ethnic group, make the low-risk general populations more vulnerable in this part of China. Prevention intervention among the low-risk general population faces huge challenges due to the low awareness of risk and the unwillingness to seek an HIV test.
So far, IDU has remained the leading route for HIV transmission and HIV continues to spread rapidly among IDUs in Liangshan; the rate of needle sharing has remained >50% in this group [43]. Our model demonstrated the HIV incidence at the high level of 7.0% from 2007 to 2020 with current intervention effort among IDUs. The projected incidence rate in 2008 is similar to the BED-incidence of 8.3% (95%CI: 5.3-11.2%) estimated from a community-based cross sectional survey of 1,098 IDUs (unpublished data, Sichuan Center for Disease Control and Prevention). Our model indicated that the spread of HIV among IDUs will not be reduced unless needle sharing is reduced to ≤30%. With current prevention efforts among FSWs, a substantial behavioral change has occurred; the rate of condom use increased from 29.1% in 2003 to 56.8% in 2007 [18, 35]. Other studies suggested that condom use alone can reduce the prevalence among FSWs, but high coverage is the key. If Thailand had seen condom use drop from 85% to 60% in 1998, its epidemic would have taken off again[32]. Crack-downs on illegal commercial sex activities launched by the local Department of Public Security have forced FSWs from more visible places, e.g., entertainment establishments, to more underground venues, e.g., rented residence houses [4]. This makes it harder to reach this group with prevention measures.
The dynamic trends of the HIV epidemic in Liangshan reflect the epidemic trends also seen at a national level. China’s population faces a growing risk of contracting HIV through sexual transmission, either heterosexual sex (through casual or commercial sex) or homosexual sex (among MSM) [4, 44, 45]. Studies have found HIV prevalence among MSM ranging from 1.5% to 10.5% in certain cities in China [4, 6, 15, 46]. The nation recognizes the HIV/AIDS needs of more easily identifiable risk groups (e.g., IDUs, FSWs, and former blood/plasma donors), but neglects the needs of the estimated 10-20 million MSM [4]. The traditional Chinese culture does not openly endorse MSM behaviors[47]. Under social pressure, many MSM conceal their sexual orientation and do not inform their female sex partners about their MSM behaviors; nearly one-third of MSM are married in China, and an even higher proportion reported having had sex with women [7, 46, 48-50]. Therefore, MSM may play a critical bridging role in spreading HIV and other STDs from their high-risk male sexual partners to low-risk female partners, such as their wives. Low coverage of AIDS-related intervention programs (13.7%) and HIV testing was shown in the present study, as reported elsewhere [22, 27]. Without action in a timely fashion, MSM could become one of the major transmission routes for HIV infection in Liangshan.
China reported that in 2008, HIV/AIDS was the nation’s leading infectious disease killer and the epidemic is still growing. Although the government has committed resources and energy to tackling the epidemic, including its nationwide “Four Frees, One Care” program (free HIV testing, counseling, antiretroviral treatment, and schooling [for HIV orphans and vulnerable children], and care for AIDS [2, 51]), the low uptake of prevention, care and treatment programs remain an immense challenge [2, 51-54]. The government has also begun testing harm reduction strategies beyond needle exchange and condom promotion in recent years to tackle the dual epidemics of drug use, especially heroin, and HIV/AIDS [55]. It is well known that use of methadone and buprenorphine decrease opioid use, decrease transmission of HIV, and improve adherence to treatment in addition to needle exchange. Studies have demonstrated the effectiveness of MMT for reducing risk of HIV infection, illicit opiate use, illegal activities, morbidity and mortality, and improving overall functioning [56-58]; however, the major challenges for MMT are the high relapse rate and limited ability to reach the majority of heroin users due to various barriers [55]. Alternative substitution therapies are also being tested in China. For example, more than 10 clinical trials of buprenorphine have been conducted in China since 1990. Despite the demonstrated efficacy of buprenorphine, it had a minimal influence on policy [59].
Although the AEM provides a dynamic and realistic picture of an epidemic, the above intervention impacts of HIV prevalence have not been incorporated, the model has its limitations. (1) The AEM internal model may not be suitable to all settings. Users must fully understand the local epidemic patterns and make sure that the key modes of transmission in the study site are already included in the model. If not, modifications to the AEM may be required before use; (2) The more complex set of inputs limits the use of the model. The reliable estimation and projection fully relies on good quality data. The user must take great caution in evaluating the quality and validity of epidemiological and behavioral inputs in order to avoid a “garbage in – garbage out” situation; (3) The impacts of antiretroviral and substitution therapy on HIV prevalence as well as the potential behavioral inhibition have not been considered; (4) The model does not offer automatic input and output validity checks along with review of the user selected parameters for fitting; (5) A sensitivity analysis module is not available to better assess the impact of specific changes and to determine what changes will have the greatest impact on the rates of HIV infections.
Estimation and projection of HIV prevalence play an increasingly important role in the directing and evaluation of the prevention efforts. Few studies have applied these models to examine the status and/or dynamic trends of HIV prevalence in different areas in China [8, 9]. There are no studies that have reported implementing the AEM to assess the dynamics of HIV prevalence in China. Better estimation and projection of the dynamics of the HIV/AIDS epidemic among different populations in Liangshan can help guide interventions and may serve as a model for improving estimates elsewhere in China.
The HIV surveillance system that collects behavioral and epidemiologic data has improved as the geographical and population coverage has increased in the past few years. The number of sentinel surveillance sites increased to 14 in 2007 (up from 2 in 1998) and now cover the highest risk populations more extensively. While sentinel surveillance provided valuable information in terms of the trend in the epidemic, HIV prevalence measured in population-based surveys, adjusted for non-response and other errors or biases, provided improved data for estimation and projection.
Our modeled data emphasize the importance of accelerating prevention programs like needle exchange and drug treatment to reduce needle sharing among IDUs and condom distribution and promotion to reduce unprotected sex among FSWs and clients, MSM/MSWs, and the general population. Our model suggests the magnitude of the care and treatment program needed for the people living with HIV/AIDS in Liangshan Prefecture, Sichuan Province. Finally, we present the value of a small area analysis for long-term planning and action when data quality improves to permit a coherent modeling exercise.
ACKNOWLEDGEMENTS
The study was supported, in part, by China HIV/AIDS Roadmap Tactical Support Project (CHARTS), the grants from the National Institute of Health (# R03AI067349, R03AI073134 and # D43 TW001035), and a 2008 developmental core award from the Vanderbilt-Meharry Center for AIDS Research, an NIH-funded program #P30 AI 54999. We thank the Institute of Dermatitis Control and Prevention of Xichang City, Liangshan Prefecture,for their help with the data collection. We are grateful to the individuals who shared their insights, particularly, Drs. Zhongqiang Ming, Yajia Lan, Jianxin Zhang, Dunzhi Wang, Xiaodong Wang, Baohua Xu, and Chongxing Li. We thank Dr. Elizabeth Pisani for the critical assessments of draft manuscripts and Ms. Meredith Bortz for manuscript assistance.
REFERENCES
- [1].Jia Y, Sun J, Fan L, Song D, Tian S, Yang Y, et al. Estimates of HIV prevalence in a highly endemic area of China: Dehong Prefecture, Yunnan Province. International journal of epidemiology. 2008 Dec;37(6):1287–96. doi: 10.1093/ije/dyn196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Jia Y, Lu F, Sun X, Vermund SH. Sources of data for improved surveillance of HIV/AIDS in China. Southeast Asian J Trop Med Public Health. 2007 Nov;38(6):1041–52. [PMC free article] [PubMed] [Google Scholar]
- [3].Ma Y, Li Z, Zhang K, Yang W, Ren X, Yang Y, et al. HIV was first discovered among IDUs in China. Zhonghua Liu Xing Bing Xue Za Zhi (Chinese Journal of Epidemiology) 1990 Apr;11:184–5. [Google Scholar]
- [4].State Council AIDS Working Committee Office. UN theme Group on HIV/AIDS in China [Accessed on December 25, 2008];A joint assessment of HIV/AIDS prevention, treatment and care in China in 2007. 2008 Available at: http://www.chinaids.org.cn/n443289/n443292/6438.html.
- [5].Wu Z, Rou K, Cui H. The HIV/AIDS epidemic in China: history, current strategies and future challenges. AIDS Educ Prev. 2004 Jun;16(3 Suppl A):7–17. doi: 10.1521/aeap.16.3.5.7.35521. [DOI] [PubMed] [Google Scholar]
- [6].China CDC . National surveillance report for HIV/AIDS,1990-2007. Beijing: 2008. [Google Scholar]
- [7].Xiao Y, Li C, Lu F, Tang H, Zhang D, Wang L, et al. Prevalence and risk factors of syphilis infection in men who have sex with men in 16 cities of China. Chin J Demerol. 2008;41(6):353–6. [Google Scholar]
- [8].Sichuan Provincial CDC . Surveillance Report of HIV/AIDS in Sichuan Province. 2007. [Google Scholar]
- [9].Liangshan Prefecture CDC . Surveillance report of HIV/AIDS in Liangshan Yi Autonomous Prefecture, 1990-2007. 2008. [Google Scholar]
- [10].Ruan Y, Chen K, Hong K, He Y, Liu S, Zhou F, et al. Community-based survey of HIV transmission modes among intravenous drug users in Sichuan, China. Sex Transm Dis. 2004 Oct;31(10):623–7. doi: 10.1097/01.olq.0000140018.24262.4a. [DOI] [PubMed] [Google Scholar]
- [11].Ruan Y, Qin G, Liu S, Qian H, Zhang L, Zhou F, et al. HIV incidence and factors contributed to retention in a 12-month follow-up study of injection drug users in Sichuan Province, China. J Acquir Immune Defic Syndr. 2005 Aug 1;39(4):459–63. doi: 10.1097/01.qai.0000152398.47025.0f. [DOI] [PubMed] [Google Scholar]
- [12].Xiao Y, Jiang Y, Feng J, Xu W, Wang M, Funkhouser E, et al. Seroincidence of recent human immunodeficiency virus type 1 infections in China. Clin Vaccine Immunol. 2007 Oct;14(10):1384–6. doi: 10.1128/CVI.00356-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Lu L, Jia M, Zhang X, Luo H, Ma Y, Fu L. Analysis for epidemic trend of acquired immunodeficiency syndrome in Yunnan Province of China. Zhonghua Yu Fang Yi Xue Za Zhi (Chinese Journal of Preventive Medicine) 2004;38(5):309–12. [PubMed] [Google Scholar]
- [14].Wu Z. Recent trends of injecting drug use and related HIV infection in China; Global Research Network Meeting on HIV Prevention in Drug-Using Populations, Inauguration Meeting Report; Geneva, Switzerland: National Institute on Drug Abuse. June, 25-26, 1998.1999. [Google Scholar]
- [15].Sichuna Provincial CDC . HIV/AIDS Epidemics in Sichuan Province. 2. Vol. 6. Sichuan Provincial AIDS Prevention and Care Information; Jul, 2007. pp. 1–6. [Google Scholar]
- [16].Sichuan China-UK HIV/AIDS Prevention and Care Project Office . Exploration and practice: Completion report of Sichuan China-UK HIV/AIDS prevention and care project 2000-2006. Chengdu: 2006. [Google Scholar]
- [17].China Global Fund on AIDS Sichuan Provincial Office . Impact of comprehensive prevention intervention programs on the HIV transmission among risk groups in Sichuan Province. Chendu: 2007. [Google Scholar]
- [18].Sichuan Provincial CDC . Work progress Report on the Sichuan AIDS prevention and treatment. 2. Vol. 6. Sichuan Provincial AIDS Prevention and Care Information; Jul, 2007. pp. 7–39. [Google Scholar]
- [19].Brown T, Peerapatanapokin W. The Asian Epidemic Model: a process model for exploring HIV policy and programme alternatives in Asia. Sexually transmitted infections. 2004 Aug;80(Suppl 1):i19–24. doi: 10.1136/sti.2004.010165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Tobi JS, Jarlais Don Des, Peerapatanapokin Wiwat. Potential impact of HIV among IDUs on heterosexual transmission in Asian settings: scenarios from the Asian Epidemic Model. International Journal of Drus Policy. 2003;14(1):63–74. [Google Scholar]
- [21].Walker N, Stover J, Stanecki K, Zaniewski AE, Grassly NC, Garcia-Calleja JM, et al. The workbook approach to making estimates and projecting future scenarios of HIV/AIDS in countries with low level and concentrated epidemics. Sexually transmitted infections. 2004 Aug;80(Suppl 1):i10–3. doi: 10.1136/sti.2004.010207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Lu F, Wang N, Wu Z, Sun X, Rehnstrom J, Poundstone K. Estimating the number of people at risk for and living with HIV in China in 2005: methods and results. Sexually transmitted infections. 2006;82(Suppl 3):iii87–91. doi: 10.1136/sti.2006.020404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Somi GR, Matee MI, Swai RO, Lyamuya EF, Killewo J, Kwesigabo G, et al. Estimating and projecting HIV prevalence and AIDS deaths in Tanzania using antenatal surveillance data. BMC Public Health. 2006;6:120. doi: 10.1186/1471-2458-6-120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Rehle Thomas M, Shisana Olive. Epidemiological and demographic HIV/AIDS projections: South Africa. African Journal of AIDS Research. 2003;2(1):1–8. doi: 10.2989/16085906.2003.9626554. [DOI] [PubMed] [Google Scholar]
- [25].Ghys PD, Brown T, Grassly NC, Garnett G, Stanecki KA, Stover J, et al. The UNAIDS Estimation and Projection Package: a software package to estimate and project national HIV epidemics. Sexually transmitted infections. 2004 Aug;80(Suppl 1):i5–9. doi: 10.1136/sti.2004.010199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Hladik W, Shabbir I, Jelaludin A, Woldu A, Tsehaynesh M, Tadesse W. HIV/AIDS in Ethiopia: where is the epidemic heading? Sexually transmitted infections. 2006 Apr;82(Suppl 1):i32–5. doi: 10.1136/sti.2005.016592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Bacaer N, Abdurahman X, Ye J. Modeling the HIV/AIDS epidemic among injecting drug users and sex workers in Kunming, China. Bull Math Biol. 2006 Apr;68(3):525–50. doi: 10.1007/s11538-005-9051-y. [DOI] [PubMed] [Google Scholar]
- [28].Phoolcharoen W, Ungchusak K, Sittitrai W, Brown T. Thailand: lessons from a strong national response to HIV/AIDS. Aids. 1998;12(Suppl B):S123–35. [PubMed] [Google Scholar]
- [29].Thai Working Group on HIV/AIDS Projection . Projections for HIV/AIDS in Thailand: 2000-2020. Department of Communicable Disease Control, Ministry of Public Health; Bangkok: 2001. [Google Scholar]
- [30].Phalla T, Leng HB, Mills S, Bennett A, Wienrawee P, Gorbach P, et al. HIV and STD epidemiology, risk behaviors, and prevention and care response in Cambodia. Aids. 1998;12(Suppl B):S11–8. [PubMed] [Google Scholar]
- [31].Cambodia Working Group on HIV/AIDS Projection . Projections for HIV/AIDS in Cambodia: 2000-2010. Family Health International Cambodia; Phnom Penh: 2002. [Google Scholar]
- [32].Cohen J. The Asian epidemic model’s provocative curves. Science. 2004 Jun 25;304(5679):1934. doi: 10.1126/science.304.5679.1934. [DOI] [PubMed] [Google Scholar]
- [33].Sun Q, Zhang JX, Li XS, Lan YJ, Che XG, Li NX, et al. Study on AIDS related risk behaviors and the correlated factors among three groups of population in Sichuan province. Zhonghua liu xing bing xue za zhi = Zhonghua liuxingbingxue zazhi. 2004 Sep;25(9):761–5. [PubMed] [Google Scholar]
- [34].Yin L, Qin GM, Ruan YH, Zhang L, Hao QN, Chen XH, et al. A prospective cohort study on human immunodeficiency virus and syphilis seroconversion among injecting drug users. Zhonghua liu xing bing xue za zhi = Zhonghua liuxingbingxue zazhi. 2006 Apr;27(4):293–7. [PubMed] [Google Scholar]
- [35].Sichuan University School of Public Health . Comprehensive assessment report for HIV/AIDS control and prevention in Sichuan Province. Sichuan University; Chengdu: 2007. [Google Scholar]
- [36].Gu J, Wang R, Chen H, Lau JT, Zhang L, Hu X, et al. Prevalence of needle sharing, commercial sex behaviors and associated factors in Chinese male and female injecting drug user populations. AIDS care. 2009 Jan;21(1):31–41. doi: 10.1080/09540120802068787. [DOI] [PubMed] [Google Scholar]
- [37].Lau JT, Choi KC, Tsui HY, Zhang L, Zhang J, Lan Y, et al. Changes in HIV-related behaviours over time and associations with rates of HIV-related services coverage among female sex workers in Sichuan, China. Sexually transmitted infections. 2008 Jun;84(3):212–6. doi: 10.1136/sti.2007.029256. [DOI] [PubMed] [Google Scholar]
- [38].Lau JT, Tsui HY, Zhang Y, Cheng F, Zhang L, Zhang J, et al. Comparing HIV-related syringe-sharing behaviors among female IDU engaging versus not engaging in commercial sex. Drug and alcohol dependence. 2008 Sep 1;97(1-2):54–63. doi: 10.1016/j.drugalcdep.2008.03.024. [DOI] [PubMed] [Google Scholar]
- [39].Yin L, Qin G, Qian HZ, Zhu Y, Hu W, Zhang L, et al. Continued spread of HIV among injecting drug users in southern Sichuan Province, China. Harm reduction journal. 2007;4:6. doi: 10.1186/1477-7517-4-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Ma X, Ge J, Zhang JY, Wen W. Survey of behaviors and knowledge about HIV/AIDS among intravenous drug users at a city in Sichuan Province. Sichuan Da Xue Xue Bao Yi Xue Ban. 2004 May;35(3):376–8. [PubMed] [Google Scholar]
- [41].Lau JT, Zhang L, Zhang Y, Wang N, Lau M, Tsui HY, et al. Changes in the prevalence of HIV-related behaviors and perceptions among 1832 injecting drug users in Sichuan, China. Sex Transm Dis. 2008 Apr;35(4):325–35. doi: 10.1097/OLQ.0b013e3181614364. [DOI] [PubMed] [Google Scholar]
- [42].Ruan YH, Hong KX, Liu SZ, He YX, Zhou F, Qin GM, et al. Community-based survey of HCV and HIV coinfection in injection drug abusers in Sichuan Province of China. World J Gastroenterol. 2004 Jun 1;10(11):1589–93. doi: 10.3748/wjg.v10.i11.1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Chengdu Tongle Consulting Center . Report of Need Assessment for HIV/AIDS prevention and control among MSM in Sichuan Province. Chengdu Tongle Consulting Center; Chengdu: 2007. [Google Scholar]
- [44].Li X, Shi W, Li D, Ruan Y, Jia Y, Vermund SH, et al. Predictors of unprotected sex among men who have sex with men in Beijing, China. Southeast Asian J Trop Med Public Health. 2008 Jan;39(1):99–108. [PMC free article] [PubMed] [Google Scholar]
- [45].Lu F, Jia Y, Vermund SH. Predictors for casual sex and/or infection among sexually transmitted disease clinic attendees in China. Int J STD AIDS. 2008 doi: 10.1258/ijsa.2008.008290. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Ruan Y, Luo F, Jia Y, Li X, Li Q, Liang H, et al. Risk Factors for Syphilis and Prevalence of HIV, Hepatitis B and C among Men Who Have Sex with Men in Beijing, China: Implications for HIV Prevention. AIDS and behavior. 2008 Dec 12; doi: 10.1007/s10461-008-9503-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Liu JX, Choi K. Experiences of social discrimination among men who have sex with men in Shanghai, China. AIDS Behav. 2006 Jul;10(4 Suppl):S25–33. doi: 10.1007/s10461-006-9123-5. [DOI] [PubMed] [Google Scholar]
- [48].Xiao Y, Li C, Lu F, Tang H, Zhang D, Jin C, et al. Predictors and changes of condom use among men who have sex with men in 16 cities in China. Chin J AIDS STD. 2008;14(2):133–6. [Google Scholar]
- [49].Zhang B, Liu D, Li X, Hu T. A survey of men who have sex with men: mainland China. Am J Public Health. 2000 Dec;90(12):1949–50. doi: 10.2105/ajph.90.12.1949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Choi KH, Gibson DR, Han L, Guo Y. High levels of unprotected sex with men and women among men who have sex with men: a potential bridge of HIV transmission in Beijing, China. AIDS Educ Prev. 2004 Feb;16(1):19–30. doi: 10.1521/aeap.16.1.19.27721. [DOI] [PubMed] [Google Scholar]
- [51].Wu Z, Sullivan SG, Wang Y, Rotheram-Borus MJ, Detels R. Evolution of China’s response to HIV/AIDS. Lancet. 2007 Feb 24;369(9562):679–90. doi: 10.1016/S0140-6736(07)60315-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Wu Z, Sun X, Sullivan SG, Detels R. Public health. HIV testing in China. Science. 2006 Jun 9;312(5779):1475–6. doi: 10.1126/science.1120682. [DOI] [PubMed] [Google Scholar]
- [53].Wu Z, Luo W, Sullivan S. Evaluation of a needle social marketing strategy to control HIV among injecting drug users in PR China. AIDS. 2007 doi: 10.1097/01.aids.0000304706.79541.ef. in press. [DOI] [PubMed] [Google Scholar]
- [54].Jia Y, Lu F, Zeng G, Sun X, Xiao Y, Lu L, et al. Predictors of HIV infection and prevalence for syphilis infection among injection drug users in China: Community-based surveys along major drug trafficking routes. Harm Reduct J. 2008;5:29. doi: 10.1186/1477-7517-5-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Sullivan SG, Wu Z. Rapid scale up of harm reduction in China. The International journal on drug policy. 2007 Mar;18(2):118–28. doi: 10.1016/j.drugpo.2006.11.014. [DOI] [PubMed] [Google Scholar]
- [56].Metzger DS, Woody GE, McLellan AT, O’Brien CP, Druley P, Navaline H, et al. Human immunodeficiency virus seroconversion among intravenous drug users in- and out-of-treatment: an 18-month prospective follow-up. Journal of acquired immune deficiency syndromes. 1993 Sep;6(1999)(9):1049–56. [PubMed] [Google Scholar]
- [57].Kleber HD. Methadone maintenance 4 decades later: thousands of lives saved but still controversial. Jama. 2008 Nov 19;300(19):2303–5. doi: 10.1001/jama.2008.648. [DOI] [PubMed] [Google Scholar]
- [58].Spire B, Lucas GM, Carrieri MP. Adherence to HIV treatment among IDUs and the role of opioid substitution treatment (OST) The International journal on drug policy. 2007 Aug;18(4):262–70. doi: 10.1016/j.drugpo.2006.12.014. [DOI] [PubMed] [Google Scholar]
- [59].Tang YL, Zhao D, Zhao C, Cubells JF. Opiate addiction in China: current situation and treatments. Addiction. 2006 May;101(5):657–65. doi: 10.1111/j.1360-0443.2006.01367.x. [DOI] [PubMed] [Google Scholar]