Abstract
Background
Focal nodular hyperplasia (FNH) is a common benign disease of the liver with no recognized potential for malignant transformation. The term describes an entity of lobular proliferation of normally differentiated hepatocytes, frequently around a central fibrous scar. Two key issues influence surgical decision making in FNH: diagnostic certainty, and symptomatic assessment.
Methods
A systematic review of studies reporting hepatic resections of FNH was performed. Indications and outcomes in adult populations were examined with a focus on diagnostic workup, patient selection and operative mortality and morbidity.
Results
Diagnostic modalities in the majority of studies involved ultrasound and computed tomography. Fewer than half employed magnetic resonance imaging (MRI). In instances in which MRI was not available, diagnostic accuracy was inferior.
Conclusions
Percutaneous biopsy should be avoided to prevent the risk for tumour seeding. Patients presenting with asymptomatic definitive FNH can be safely managed conservatively. In symptomatic patients surgical resection is a safe and effective treatment for which acceptable rates of morbidity (14%) and zero mortality are reported. However, evidence of symptom resolution is reported with conservative strategies. Diagnostic uncertainty remains the principal valid indication for FNH resection, but only in patients in whom contrast-enhanced MRI forms part of preoperative assessment.
Introduction
Focal nodular hyperplasia (FNH) is a common benign disease of the liver. Its incidence in females is reported to be eight times higher than in males and is weakly associated with reproductive age and use of oral contraceptives. The term describes an entity of lobular proliferation of normally differentiated hepatocytes, frequently around a central fibrovascular scar.1 The natural history of FNH has been well studied and there is a sound evidence base for its benign classification. No instances of confirmed malignant transformation of FNH are reported in the literature.2
The profile of the typical FNH patient has changed as a result of the modern widespread availability and application of cross-sectional imaging. Previously, a significant proportion of patients diagnosed with FNH presented with vague symptoms of abdominal pain or discomfort. Such patients then proceeded to imaging and diagnosis. Today, the converse is true and the prevalent scenario concerns the incidental detection of liver lesions through high-resolution imaging performed for unrelated clinical reasons. As a result there has been broad international recognition of a substantial increase in the number of referrals for the characterization and subsequent management of suspected FNH.
There exists considerable controversy with respect to the absolute indications and contraindications for surgery in benign liver disease,3 and the available literature is unclear regarding the surgical management of FNH. The management spectrum ranges from simple observation to major hepatic resection, with two key issues influencing clinical decision making. The first is diagnostic certainty and the second symptomatic assessment. The lack of malignant potential in FNH means that when relative certainty is achieved, the asymptomatic patient might be safely managed conservatively. However, in patients in whom indeterminate features exist, the differential diagnosis includes more concerning pathologies, such as hepatic adenoma (HA) or fibrolamellar hepatocellular carcinoma (FHCC). For such lesions resection is justified to prevent the development or under-treatment of malignancy. In symptomatic patients in whom FNH lesions are plausibly deemed to be causal, resection has been performed and reported for palliation.
This systematic review focuses on studies that report outcomes of FNH resections and give details of the preoperative diagnostic and patient selection modalities employed. Outcomes assessed are the accuracy of preoperative diagnostic investigations, stated indications for surgery, operative procedure, mortality, morbidity and patient satisfaction. Thus the indications for conservative versus surgical management of suspected FNH might be more clearly understood.
Materials and methods
Literature search strategy
A systematic review of the literature was undertaken according to the principles of the PRISMA (preferred reporting items for systematic reviews and meta-analyses) guidelines.4 No randomized studies precluding the application of relevant PRISMA items were identified.
An electronic search of the PubMed and MEDLINE databases was performed for the period 2001 to 2012 inclusive using the MeSH (medical subject headings) terms: ‘focal nodular hyperplasia’; ‘liver tumours’; ‘liver resection’, and ‘hepatectomy’. The search was limited to English-language publications and studies on adult human subjects. All titles and abstracts were reviewed, and appropriate papers assessed for inclusion. The reference sections of all papers initially included were also assessed to ensure the identification of all relevant studies.
Inclusion and exclusion criteria
Studies were included if they described outcomes following hepatic resection in patients with FNH. Data collated included preoperative diagnostic methods used, indications for surgery, magnitude of hepatic resection and postoperative outcomes. The minimal dataset eligible for inclusion was required to refer to patients with FNH treated with surgery and to present diagnostic modality and patient outcome data. Series of hepatic resections for general benign disease were required to present specific FNH subgroup data. All series satisfying these criteria were included regardless of the size of the study population. Case reports, editorials, unpublished data from conference abstracts and review articles were excluded. The characteristics of excluded studies are shown in Fig. 1.
Figure 1.
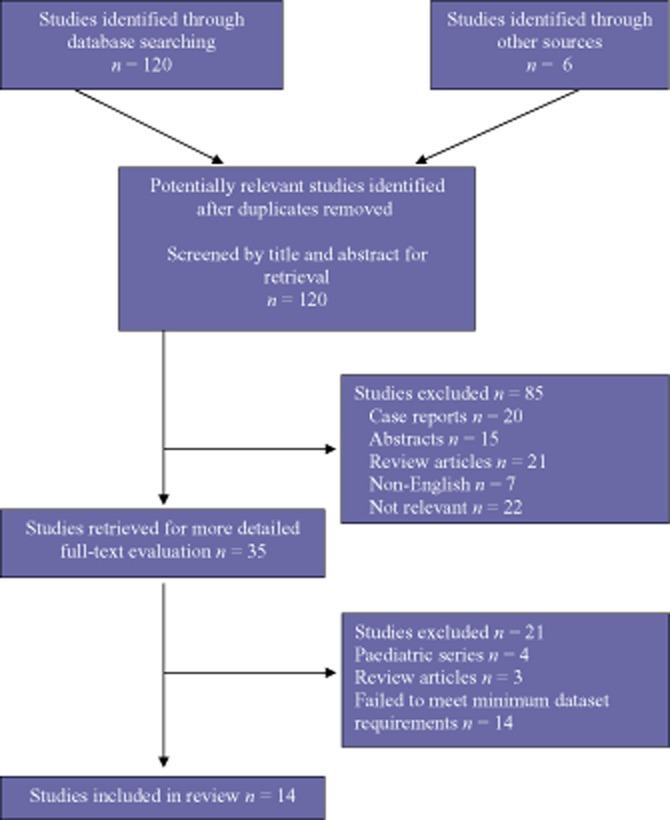
Flow diagram demonstrating search criteria and subsequent inclusion of relevant studies
Results
Characteristics of included studies
Of the 14 studies reviewed, three focused exclusively on patients with FNH.5–7 Nine further studies can be described as case series of hepatic resections performed for benign disease, including FNH.8–16 The remaining two studies reported on resections of both benign and malignant liver lesions.17,18 In total, the 14 studies involved 885 patients submitted to surgery for benign liver lesions, 37% (n = 331) of whom had a proven histological diagnosis of FNH (Table 1).
Table 1.
Published studies of patients with focal nodular hyperplasia (FNH) treated with hepatic resection
| Study | Patients, n | Benign resections, n | FNH resections, n | Overall morbidity, n (%) | FNH-specific morbidity, n (%) | Major resectiona, n (%) | Minor resectiona, n (%) | Resection indication: symptomatic, n (%) | Resection indication: diagnostic uncertainty, n (%) | Follow-up, months, median (range) |
|---|---|---|---|---|---|---|---|---|---|---|
| Charny et al. (2001)8 (USA) | 155 | 68 | 18 | 14 (21%) | N/A | 8 (44%) | 10 (55%) | 8 (44%) | 13 (72%) | 16 |
| Descottes et al. (2003)11 (France) | 87 | 87 | 48 | 4 (5%) | N/A | 0b | 51 (100%)b | 23 (45%)b | 28 (55%)b | 10 (2–72) |
| Clarke et al. (2004)9 (UK) | 49 | 49 | 12 | 15 (31%) | N/A | N/A | N/A | 10 (83%) | 2 (17%) | N/A |
| Liu et al. (2004)18 (China) | 107 | 45 | 17 | 7 (16%) | N/A | N/A | N/A | 0 | 17 (100%) | N/A |
| Fioole et al. (2005)12 (Netherlands) | 28 | 28 | 6 | 8 (29%) | N/A | 2 (33%) | 4 (67%) | 1 (17%) | 5 (83%) | N/A |
| Skalicky et al. (2005)16 (Czech Republic) | 43 | 43 | 14 | 3 (7%) | N/A | 4 (29%) | 10 (71%) | N/A | N/A | N/A |
| Hsee et al. (2005)6 (New Zealand) | 8 | 8 | 8 | 4 (50%) | 4 (50%) | 2 (25%) | 6 (75%) | 7 (88%) | 1 (12%) | N/A |
| Shen et al. (2007)7 (China) | 86 | 86 | 86 | 6 (7%) | 6 (7%) | 17 (20%) | 69 (80%) | N/A | N/A | 45 (6–60) |
| Bonney et al. (2007)5 (UK) | 52 | 15 | 15 | 3 (20%) | 3 (20%) | 6 (40%) | 9 (60%) | 8 (53%) | 7 (47%) | 23 (3–84) |
| Petri et al. (2008)15 (Hungary) | 132 | 112 | 21 | 31 (28%) | 7 (33%) | 4 (19%)c | 17 (81%) | N/A | N/A | N/A |
| Lordan et al. (2009)14 (UK) | 79 | 79 | 16 | 7 (9%) | N/A | 7 (44%) | 9 (56%) | 6 (38%) | 12 (75%) | N/A |
| Erdogan et al. (2009)17 (Netherlands) | 70 | 70 | 12 | 15 (21%) | N/A | N/A | N/A | 10 (83%) | 2 (17%) | N/A |
| Dardenne et al. (2010)10 (Belgium) | 132 | 49 | 13 | 7 (14%) | 0 | 0 | 13 (100%) | 3 (23%) | 10 (77%) | 115 (2–233) |
| Kamphues et al. (2011)13 (Germany) | 146 | 146 | 45 | 25 (17%) | N/A | N/A | N/A | N/A | N/A | 50 (3–95) |
Major resection: more than three Couinaud segments; minor resection: up to three Couinaud segments.
48 patients with FNH were grouped with three patients in whom histology showed hamartoma.
Includes one liver transplant.
N/A, not available.
Diagnostic modalities
Seven studies provided details of the diagnostic modalities employed preoperatively in a total of 195 FNH patients.5–7,9,11,12,18 The reference-standard method of diagnosis of FNH is histological analysis.
Lesional biopsy
Only two studies included data on the use of biopsy in the context of the management of presumed FNH. Descottes and colleagues11 reported preoperative liver investigations performed in 87 patients with benign lesions, 48 (55%) of whom were ultimately histologically diagnosed as having FNH. Within the subgroup of patients with FNH, 23% (n = 11) subsequently underwent either percutaneous (n = 3) or laparoscopic (n = 8) tumour biopsy. Biopsy-derived histological diagnoses were poorly correlated with resection specimen histology. Six (55%) true positive results were seen. The remaining findings included four false positive results referring to the misdiagnosis of three adenomas and one hepatocellular carcinoma (HCC) and one inconclusive result.
Bonney and colleagues5 reported a series of 52 patients with FNH who were either treated operatively (n = 15, 29%) or managed conservatively (n = 37, 71%). Seven patients in the conservatively managed group were diagnosed with FNH following histopathological analysis of percutaneous biopsies. Of the 15 patients with FNH managed with resection, five patients underwent preoperative percutaneous biopsy. A definitive diagnosis of FNH was achieved in only two (13%) patients.
Use and accuracy of preoperative imaging for FNH diagnosis
Ultrasound
Shen and colleagues7 reported the use of contrast-enhanced ultrasound (US) in 79 of 86 (92%) patients undergoing resection for lesions that were histologically diagnosed as FNH. Ultrasound achieved an accurate diagnosis of FNH in 33% (n = 26) of patients. Of the remaining 53 patients, 28 had an equivocal US diagnosis and 25 were over-diagnosed as having malignant lesions.
Bonney and colleagues5 performed an US examination in 27 (52%) patients and achieved an unequivocal diagnosis in eight (30%) of them. Descottes and colleagues11 reported using US in 85 (98%) patients, but did not present data on its accuracy. The small FNH series presented by Clarke et al.9 (n = 12), Fioole et al.12 (n = 6) and Liu et al.18 (n = 17) all employed preoperative US routinely, but did not present accuracy data. In the latter study,18 the patient cohort was heterogeneous with respect to histological diagnosis and details of investigations by histological subgroup were not provided. Similarly, accuracy data were not available.
Computed tomography
Shen and colleagues7 reported the use of multiphase computed tomography (CT) in 67% (n = 58) of their patients and described it as diagnostic in 60% (n = 35). Bonney and colleagues5 performed CT in 34 (65%) patients, in 24 (70%) of whom the method achieved a diagnosis of FNH. Descottes and colleagues11 reported using CT in 74 (85%) patients but did not present information on its diagnostic accuracy. Computed tomography (with US) was used routinely in all FNH patients reported by Clarke et al.,9 Fioole et al.12 and Liu et al.18 However, as with their US data, these studies did not present information on accuracy.
Magnetic resonance imaging
Magnetic resonance imaging (MRI) was employed sparingly by Shen and colleagues7 (n = 31, 36%) but produced an accurate diagnosis of FNH in 77% (n = 24) of patients. Twenty-two patients in this study underwent both preoperative CT and MRI. This combined approach yielded an accurate FNH diagnosis in 20 (91%) cases.7
Bonney et al.5 used MRI in 46 (88%) patients. Their findings with respect to diagnostic accuracy were broadly similar to those of Shen and colleagues.7 Bonney et al.5 reported that MRI achieved a definitive diagnosis of FNH in 40 (87%) patients. An interesting feature of the study by Bonney et al.5 is its inclusion of a majority of conservatively managed patients (n = 37/52, 71%). This may have resulted from the availability of MRI and hence a potential reduction in the number of resections performed for uncertain diagnoses. However, conclusions on diagnostic accuracy are limited as subsequent histological confirmation of the diagnosis does not apply in patients managed conservatively. In six of the 15 patients resected (40%), a definitive preoperative diagnosis of FNH was made using imaging alone. These patients were resected for symptoms. In the remaining seven (47%) patients, preoperative investigations were inconclusive. The remaining two patients were diagnosed following lesional biopsy.
Descottes and colleagues11 reported the use of MRI in 44 (51%) patients. Clarke et al. did not have access to MRI during the assessment of 12 FNH cases.9 Liu et al. did not state whether preoperative liver MRI was employed in 17 FNH patients.18 Similarly, Fioole and colleagues did not perform MRI or preoperative biopsy in any of the six cases of FNH reported.12
The studies by Charny et al.8 and Dardenne et al.10 failed to provide complete information on preoperative diagnostic methodology. However, Charny et al. did report that a preoperative diagnosis was achieved in seven (39%) of the 18 patients who proceeded to resection for FNH.8 Dardenne et al. discussed the characteristic features of FNH used for diagnosis on CT and MRI cross-sectional imaging and reported a preoperative diagnosis rate of 48% (n = 29) using these modalities.10
Summary
Across the seven studies analysed herein,5–7,9,11,12,18 preoperative US was performed in 84% (n = 163), CT in 76% (n = 149) and MRI in 48% (n = 93) of patients. The remaining patients (n = 19, 10%) underwent both imaging and either percutaneous or laparoscopic lesional biopsy. None of the studies reviewed provided detailed information on specific imaging protocols or the contrast agents used in MRI or CT.
Many clinicians believe that, using the modalities described above, an FNH diagnosis can be made with a high level of confidence in the majority of patients without requiring resection. However, as is apparent here, the literature-based support for such an assertion is sparse.
Indications for surgery
Nine studies reported data regarding indications for surgery for FNH.5,6,8–10,12,14,17,18 Although the semantics of the indications described vary among studies, all can be categorized as relating to either patient symptoms or diagnostic uncertainty and suspicion of cancer.
Charny and co-workers8 performed 18 hepatic resections for FNH. In 61% (n = 11) of these cases, the indication for surgery referred to diagnostic uncertainty on preoperative imaging, although a further two (11%) patients were resected following an increase in lesion size during follow-up.8 Presumably diagnostic uncertainty represented the rationale for surgery in these cases also. The remaining reported indication referred to symptomatic lesions (n = 8, 44%). Three patients had dual indications for resection (i.e. diagnostic uncertainty and symptoms). The nature of the symptoms experienced was not reported.8
Descottes and colleagues11 reported a series of 51 hepatic resections in 48 patients with FNH and three patients with hamartoma. Indications for resection were symptoms in 23 (45%) patients and diagnostic uncertainty or atypical radiological appearance in 18 (35%). In 10 (20%) patients, resection was performed for hepatic lesions detected during laparoscopic procedures performed for separate indications. It is presumed that these lesions were incidental findings as no information regarding preoperative imaging was reported.
Clarke and co-workers9 published a series of 49 cases of hepatic resection for benign non-cystic lesions. Twelve patients had a postoperative histological diagnosis of FNH. The authors recommended conservative management for asymptomatic patients with a radiological diagnosis of FNH.9 Patients with symptomatic lesions, diagnostic uncertainty or enlarging lesions were offered hepatic resection. Symptomatic FNH was the stated indication for resection in 10 (83%) of the 12 patients. No clear definition of ‘symptomatic FNH’ was described in this study. In two (17%) FNH cases, preoperative investigations had suggested a diagnosis of liver cell adenoma. Resection had subsequently been performed and provided a final histological diagnosis of FNH.9
Bonney and co-workers5 presented data for 15 patients with FNH submitted to hepatic resection between 1997 and 2006. In eight (53%) patients abdominal pain was the stated indication for surgery. However, the majority (n = 13, 87%) of the FNH patients reported moderate or severe abdominal pain. The indication for resection in the remaining seven patients was diagnostic uncertainty on preoperative imaging or suspicion of malignancy on biopsy (n = 3, 43%).5
Within these nine studies,5,6,8–10,12,14,17,18 97 (58%) patients underwent hepatic resection without a definitive preoperative diagnosis of FNH; the primary indication for surgery in these patients was therefore diagnostic uncertainty. Resection was performed in 76 (43%) patients to palliate abdominal symptoms attributable to a hepatic lesion. The data presented across all of these studies did not allow for assessment of the aetiology of pain, which may derive from mass effect, impingement on other structures or from the lesion itself.
Operative data
Ten studies presented data on the extent of resection performed for FNH in a total of 248 patients.5–8,10–12,14–16 Six studies provided data on the sizes of the hepatic lesions removed and the anatomical extent of resection.5,7,8,10,11,15 Major versus minor hepatic resection was inferred from the data presented, and the techniques employed are reviewed where presented.
Charny and colleagues8 performed right or left lobectomy in six patients (33%), trisegmentectomy in two patients (11%), segmentectomy in three patients (17%) and tumour enucleation in seven patients (39%). The median size of the resected tumours was 7.1 cm (range: 2–30 cm). Lesions resected from symptomatic patients demonstrated a trend towards a larger size compared with those from asymptomatic individuals (median diameter: 7.3 cm versus 4.4 cm).8
All of the 48 FNH and three hamartoma patients reported in the multinational series published by Descottes et al. underwent minor laparoscopic resections.11 The procedures performed included 14 (27%) left lateral sectionectomies, 12 (24%) non-left lateral segmentectomies and 25 (49%) non-anatomic resections. In six (12%) patients, a laparoscopic procedure was converted to open surgery as a result of significant bleeding (n = 2, 33%), a tumour location unfavourable to laparoscopic resection (n = 3, 50%) or instrument dysfunction (n = 1, 17%). The median diameter of the resected lesions was larger in the symptomatic group than in the asymptomatic group [5 cm (range: 2–11 cm) versus 4 cm (range: 1–6 cm)], but the difference was not significant.11
Shen and co-workers7 performed liver resections in 86 patients with FNH between 1996 and 2006. Hemi-hepatectomy was carried out in 17 (20%) patients, although most patients underwent non-anatomic wedge resection of FNH lesions (n = 68, 79%). One (1%) patient had 23 separate foci of FNH throughout both liver lobes; three foci with diameters of > 2 cm were enucleated and the remainder treated with electrocautery. The mean diameter of resected lesions in all patients was 3 cm (range: 0.3–15 cm).7
Bonney and colleagues5 retrospectively examined 52 cases of FNH, 15 of which had been managed with surgery. Trisectionectomy was performed in three (20%) patients, lobectomy in three (20%), segmentectomy in one (7%) and non-anatomic resection in eight (53%). The median size of resected FNH lesions was 6.1 cm. Across the entire FNH study population, data derived from preoperative imaging demonstrated a significant correlation between increased tumour size and symptoms of pain (P = 0.006).5
Across the 10 studies reviewed,5–8,10–12,14–16 minor resections of three or fewer Couinaud segments were performed in 79% (n = 198) of patients. Four (2%) patients did not undergo resection, but instead submitted to operative biopsy (n = 2) or radiofrequency ablation (RFA) (n = 2) of FNH lesions. Major resections of greater than three Couinaud segments were performed in 20% (n = 49) of patients. One patient (0.4%) underwent a liver transplant.
Outcomes
Five studies included follow-up data for 225 patients with benign disease including FNH.8,9,12,13,17 Five further studies, involving 143 patients, reported FNH-specific morbidity and mortality statistics.5–7,10,15
Charny and colleagues8 followed 18 patients resected for FNH for a median period of 16 months. All symptomatic patients (n = 8) reported resolution of symptoms. No late complications were reported.
Shen et al.7 reported a morbidity rate of 7% (n = 6) in their series of FNH resections. All of the reported complications were sub-phrenic collections with associated pleural effusions. During the follow-up period (0.5–10 years), no recurrence of symptoms or deaths were reported.
Bonney and colleagues5 provided follow-up data for 15 patients for a median of 24 months (range: 6–72 months). Three (20%) patients suffered postoperative complications, comprising one case of pneumonia, one sub-hepatic abscess requiring percutaneous drainage, and one wound infection. One death (7%) during follow-up was reported in a patient who was found to have concomitant HCC in the resection specimen. The cause of death was not given. Two (13%) patients developed recurrent FNH at, respectively, 24 months and 48 months post-resection. The imaging method used to assess recurrence was not described. One of these patients also developed mild recurrent symptoms of pain.5
In the series reported by Petri et al.,15 morbidity was observed in 29% (n = 6) of FNH resections and comprised postoperative bleeding (n = 2, 10%), postoperative jaundice (n = 2, 10%), fever of unknown origin (n = 1, 5%) and cerebrovascular accident (n = 1, 5%). One death (5%) occurred postoperatively following liver transplantation and was caused by bleeding with consumptive coagulopathy.
Dardenne and co-workers10 provided follow-up data on 13 FNH patients as part of a broader series of individuals undergoing operative or conservative management of benign liver disease. The authors reported no 30-day postoperative mortality. One patient in the operative FNH group died of an unrelated cardiac cause during the follow-up period (median: 115 months; range: 2–233 months). Instances of morbidity were not observed, although only severe morbidity requiring invasive treatment was recorded.
Kamphues and colleagues13 used the European Organization for Research and Treatment of Cancer (EORTC) Quality of Life (QLQ) C30 questionnaire to evaluate patient satisfaction with liver resection for benign disease at a median of 50 months postoperatively (range: 3–95 months). This study represents the only objective evidence of patient-derived outcomes in this area. Patients with FNH made up 33% (n = 27) of the sample; however, no subgroup analysis was performed. Results from the group as a whole demonstrated highly significant improvements in global health status (P = 0.001), social functioning (P = 0.03) and emotional functioning (P = 0.007) following resection. No significant impact on physical well-being or cognitive functioning was seen. Significant improvements were also seen in symptoms of pain (P = 0.001) and fatigue (P = 0.004). A significant majority of the patients questioned (n = 78, 96%) stated that they would undergo resection for benign disease again if offered the choice. A major flaw of this study13 concerns the heterogeneity of pathological indications for resection. In addition, the lack of evidence for a causal link between lesions and symptoms leaves potential for confounding.
In summary, the rate of morbidity experienced by patients submitted to resection for FNH was 14% (n = 20). No cases of mortality following hepatic resection of FNH lesions alone were reported. One case of 30-day mortality (0.7%) was observed; this occurred in a patient submitted to liver transplant for FNH.
Discussion
The aims of this review were to examine the indications for conservative versus surgical management of FNH in contemporary hepatobiliary practice and to better elucidate the roles of preoperative assessment in ensuring that selected strategies result in benefit to the FNH population. In fact, much of the debate uncovered stems from the ‘indeterminate lesion’, which frequently appears as the preoperatively suspicious lesion diagnosed as FNH on postoperative histology. Indeed, the majority of studies discussed cite ‘diagnostic uncertainty’ as the principal indicator for surgery.
Current methods of investigation for the diagnosis and characterization of liver lesions are radiological and principally comprise US, CT and MRI. The modalities employed in the majority of patients involved US and CT, and fewer than half of patients received basic MRI. This is remarkable given the number of studies showing that MRI has the highest sensitivity in the diagnosis of FNH. Current data suggest that a combination of imaging modalities which includes MRI allows for greater accuracy in diagnosing FNH, with a sensitivity and specificity for FNH of > 90%.5,7,19–23 It is therefore imperative that MRI is employed in situations of diagnostic doubt.
Focal nodular hyperplasia may be definitively non-histologically diagnosed in circumstances in which specific characteristic features are present on multiphase CT and contrast-enhanced MRI. In CT, a lesion exhibiting homogeneous late arterial phase enhancement with a hypodense central scar is diagnostic of FNH. The FNH enhancement pattern on MRI is similar to that seen in multiphase CT and the central scar will demonstrate high signal on T2 weighted images.21 The use of hepatobiliary MRI contrast agent allows for the definitive description of a hyperintense FNH lesion with low signal in the central scar. Indeed, the use of gadolinium-based MRI contrast agents significantly increases the accuracy of diagnosis of FNH, as well as improving the differentiation of FNH from malignant lesions.19,22,23 None of the studies considered in this review confirmed the use of gadolinium contrast-enhanced MRI in their preoperative imaging protocols, although they may have done so. Equally, the purportedly high rates of diagnostic accuracy of contrast MRI are not uniformly internally validated through histological confirmation of the FNH diagnosis.19,22,23
The studies included in this review report a high proportion of patients undergoing resection of indeterminate hepatic lesions. A notable historical study performed prior to the inclusion period of this analysis was published by Belghiti and colleagues in 1993.24 It described 51 patients submitted to resection for presumed benign lesions, in three (6%) of whom malignancy was proven. In 22 of the 51 patients, the indication for resection was diagnostic uncertainty.24 The obvious conclusion is that despite the availability of better imaging, later observations continue to reflect a prevalence of suboptimal preoperative imaging and diagnostic uncertainty. Clearly, it is sensible to offer surgery to patients in whom it has not been possible to reliably exclude malignancy. However, the discussed potential of the use of contrast-enhanced MRI for the accurate diagnosis of FNH would suggest that its universal use might result in a significant reduction in the numbers of asymptomatic FNH patients proceeding to unnecessary resection.
In patients in whom diagnosis remains uncertain, options include resection, as discussed and with concomitant mortality and morbidity, biopsy with histological analysis, and conservative management with repeated imaging. Biopsy can be achieved in various ways; there is some dichotomy between image-guided percutaneous and laparoscopic techniques. The aim of lesional biopsy is to achieve a definitive diagnosis and hence to avoid surgery for benign lesions. Biopsy of these indeterminate lesions is controversial because of two factors. The first is further diagnostic inaccuracy. Some of the series reviewed herein show evidence of sampling error with poor sensitivity and specificity seen in the context of retrospective analyses.5,11 The difficulty of distinguishing large FNH lesions from well-differentiated or fibrolamellar HCC on biopsy histology is also well reported.25 A second issue concerns the risk for needle track seeding of tumour cells from what may be a potentially operable malignant lesion.
Laparoscopic biopsy with intraoperative US guidance is an alternative to the percutaneous route and is potentially advantageous in terms of both procuring representative tissue and reducing the risk for tumour seeding. These perceived advantages have yet to be demonstrated in the literature. However, quantitative data regarding the observed risk for tumour seeding following percutaneous biopsy of malignant hepatic lesions are available in the literature and the risk is small. Azoulay and colleagues26 reported an incidence of < 2%, which suggests this issue to be of minor importance. In addition, it should be remembered that the majority of lesions biopsied will prove to be benign and hence seeding is not a concern.
There are several published algorithms which advise that where diagnostic doubt exists after imaging, percutaneous biopsy should be avoided in operable disease.5,8,9,18,27 In most cases, patients with a suspicious liver lesion are offered liver resection. If indeed future studies suggest biopsy should be performed laparoscopically, some would argue that, given the very low rates of morbidity recorded, resection could preferentially be performed to give the added benefit of a definitive outcome. However, in patients in whom individual factors favouring conservative approaches to management pertain (including patient choice), a robust biopsy protocol properly performed and repeat follow-up imaging both represent safe practice.
Knowledge regarding the prevalence and natural history of FNH is evolving as a result of the expanding use of modern, high-resolution, cross-sectional imaging. Prospective cohort studies suggest that around a quarter of FNH detected incidentally will be symptomatic and that the majority will remain stable in terms of size. In 2001, D'Halluin and colleagues reported outcomes in 44 cases of FNH followed prospectively in France over a median of 45 months.28 Ten (23%) patients were symptomatic at the beginning of the study period, but by the end all symptoms had resolved.28 No other complications of conservative management, including bleeding or rupture, were reported. In addition, the findings of a recent cohort study suggest that the majority of conservatively managed FNH lesions remain stable after diagnosis and a proportion regress over time.29
In patients with symptomatic FNH, several studies have reported that surgical resection is an effective treatment that provides for favourable levels of patient satisfaction and a low incidence of symptom recurrence.6,7,12 However, the two studies5,10 reviewed herein that assessed medium- and longterm outcomes of conservative FNH management reported that 80% of 25 symptomatic patients experienced the resolution of their symptoms with conservative management.
Hepatic resection for benign pathology is associated with acceptably low incidences of morbidity and mortality. In all of the 14 case series concerning surgical management of benign liver disease discussed in the present paper, the only case of perioperative mortality occurred following liver transplantation. The levels of morbidity observed are also acceptable and compare favourably with those seen after resections for malignant disease. This may in part reflect the fact that FNH is more commonly seen in a younger population than is malignant disease and that major resections are less likely to be necessary.17,18
The numbers of liver resections performed worldwide have increased dramatically in the recent past. The reasons for these increases relate to many factors, including the expansion of hepatic resection for colorectal liver metastases and the introduction of low-morbidity laparoscopic resection techniques. As a result, many centres will feel comfortable in performing large numbers of minor resections for benign disease.30 There is no direct evidence that the development and expansion of laparoscopic liver resection have increased rates of resection for benign disease. However, an analysis of published patterns of resection for benign liver lesions conducted by Toro and colleagues suggests a possible increase in the rate of FNH resection associated with the laparoscopic era.31 It might be argued that the availability of well-tolerated, low-risk procedures has reduced the drive to deliver reference-standard imaging and reduced resection rates.
The existing literature relating specifically to the management of FNH is incomplete. In the last 10 years, only three case series, involving a total of 109 patients, focusing on the surgical management of FNH have been published.5–7 Shen and colleagues have published the largest series to date; their information regarding preoperative imaging and post-resection outcome was compiled retrospectively using postoperative histology to identify FNH patients.7 Indeed, the majority of studies including data relevant to the management of FNH retrospectively report outcomes from single-centre ‘benign disease’ case series. Thus Level III evidence from small subgroup analysis is the rule. There is currently no Level I or II evidence to assist clinical decision making in the management of FNH.
The aim of this systematic review was to assess the indications for and outcomes of the operative management of FNH. Potential modalities not assessed include radiological embolization and RFA. The successful management of FNH employing these techniques either as primary treatment32–35 or as adjunctive to surgical resection36 has been reported. There are currently no published accounts of randomized controlled trials (RCTs) comparing the outcomes of embolization or RFA with those of either open resection or conservative management. The major limitation of local embolization or ablation techniques is the lack of post-procedural histology. This is particularly relevant as the accuracy of preoperative imaging and biopsy-derived diagnosis of FNH is fallible. Embolization, RFA and any other techniques employing similar principles would necessarily be limited to symptomatic patients in whom pre-procedural investigations allow a definitive FNH diagnosis.
Conclusions
Patients who present with potential FNH lesions should be imaged with multiphase CT and contrast-enhanced MRI. In patients in whom contrast-enhanced MRI is not employed, diagnostic accuracy is inferior and the rate of unnecessary resection may be high. Further study of this modality and its correlation to reference-standard histological FNH confirmation would strengthen this recommendation. Patients in whom state-of-the-art imaging studies have been inconclusive should be offered a range of options. They must be made fully aware of the potential for malignancy, but assured that a benign diagnosis is more likely. Principles of informed consent and patient choice should be exercised when discussing options of resection, percutaneous or laparoscopic biopsy, repeat imaging to observe for lesional change and conservative management.
Patients presenting with asymptomatic definitive FNH can be safely managed conservatively. In symptomatic patients surgical resection is a safe and effective treatment. However, reports of the spontaneous improvement or resolution of FNH-related symptoms with conservative management suggest this also remains a viable option (Fig. 2).
Figure 2.
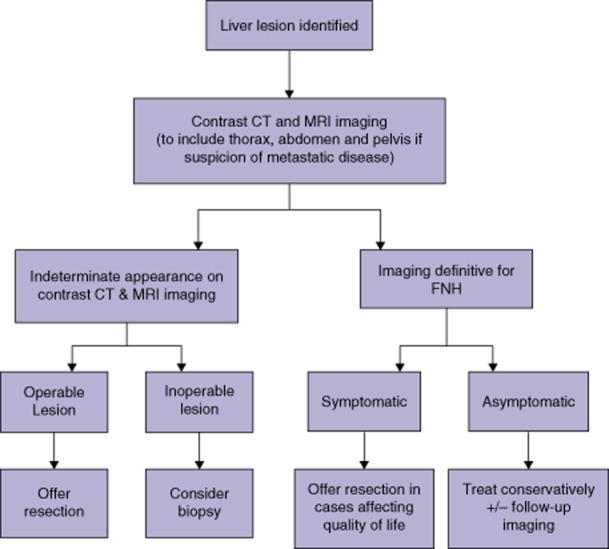
Suggested algorithm for the treatment of focal nodular hyperplasia (FNH). CT, computed tomography; MRI, magnetic resonance imaging
The evidence base for the management of FNH is weak. For symptomatic patients, a multicentre RCT comparing operative with conservative management strategies would provide the first Level I evidence for the effect of surgery on symptoms presumed to be caused by the FNH tumour. However, issues of diagnostic uncertainty may cause accrual problems for such a study. In addition, the optimal management of symptomatic FNH is subordinate to the issue of diagnostic uncertainty.
In recent years the hepatobiliary surgical community appears to have responded to the oncological risk for diagnostic uncertainty by performing an increasing number of safe hepatic resections in which the final histological diagnosis confirms the presence of FNH. By limiting diagnostic uncertainty through the use of state-of-the-art imaging, the individual and societal burdens imposed by the subjection of patients to surgical treatment for an entirely benign entity can be minimized. Therefore, the clear message of this review is that diagnostic uncertainty remains the principal valid indication for FNH resection, but only in patients in whom contrast-enhanced MRI forms part of the preoperative assessment.
Conflicts of interest
None declared.
References
- 1.Nguyen BN, Flejou JF, Terris B, Belghiti J, Degott C. Focal nodular hyperplasia of the liver: a comprehensive pathologic study of 305 lesions and recognition of new histologic forms. Am J Surg Pathol. 1999;23:1441–1454. doi: 10.1097/00000478-199912000-00001. [DOI] [PubMed] [Google Scholar]
- 2.Trotter JF, Everson GT. Benign focal lesions of the liver. Clin Liver Dis. 2001;5:17–42. doi: 10.1016/s1089-3261(05)70152-5. [DOI] [PubMed] [Google Scholar]
- 3.Colli A, Fraquelli M, Massironi S, Colucci A, Paggi S, Conte D. Elective surgery for benign liver tumours. Cochrane Database Syst Rev. 2007;(1) doi: 10.1002/14651858.CD005164.pub2. CD005164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA Statement. Open Med. 2009;3:e123–e130. [PMC free article] [PubMed] [Google Scholar]
- 5.Bonney GK, Gomez D, Al-Mukhtar A, Toogood GJ, Lodge JP, Prasad R. Indication for treatment and longterm outcome of focal nodular hyperplasia. HPB. 2007;9:368–372. doi: 10.1080/13651820701504173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hsee LC, McCall JL, Koea JB. Focal nodular hyperplasia: what are the indications for resection? HPB. 2005;7:298–302. doi: 10.1080/13651820500273624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shen YH, Fan J, Wu ZQ, Ma ZC, Zhou XD, Zhou J, et al. Focal nodular hyperplasia of the liver in 86 patients. Hepatobiliary Pancreat Dis Int. 2007;6:52–57. [PubMed] [Google Scholar]
- 8.Charny CK, Jarnagin WR, Schwartz LH, Frommeyer HS, DeMatteo RP, Fong Y, et al. Management of 155 patients with benign liver tumours. Br J Surg. 2001;88:808–813. doi: 10.1046/j.0007-1323.2001.01771.x. [DOI] [PubMed] [Google Scholar]
- 9.Clarke DL, Currie EJ, Madhavan KK, Parks RW, Garden OJ. Hepatic resection for benign non-cystic liver lesions. HPB. 2004;6:115–119. doi: 10.1080/13651820410026326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dardenne S, Hubert C, Sempoux C, Annet L, Jouret-Mourin A, Horsmans Y, et al. Conservative and operative management of benign solid hepatic tumours: a successful stratified algorithm. Eur J Gastroenterol Hepatol. 2010;22:1337–1344. doi: 10.1097/MEG.0b013e32833db907. [DOI] [PubMed] [Google Scholar]
- 11.Descottes B, Glineur D, Lachachi F, Valleix D, Paineau J, Hamy A, et al. Laparoscopic liver resection of benign liver tumours. Surg Endosc. 2003;17:23–30. doi: 10.1007/s00464-002-9047-8. [DOI] [PubMed] [Google Scholar]
- 12.Fioole B, Kokke M, van Hillegersberg R, Rinkes IH. Adequate symptom relief justifies hepatic resection for benign disease. BMC Surg. 2005;5:7. doi: 10.1186/1471-2482-5-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kamphues C, Engel S, Denecke T, Bova R, Hippler-Benscheidt M, Puhl G, et al. Safety of liver resection and effect on quality of life in patients with benign hepatic disease: single centre experience. BMC Surg. 2011;11:16. doi: 10.1186/1471-2482-11-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lordan JT, Worthington TR, Quiney N, Fawcett W, Karanjia ND. Early postoperative outcomes following hepatic resection for benign liver disease in 79 consecutive patients. HPB. 2009;11:321–325. doi: 10.1111/j.1477-2574.2009.00049.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Petri A, Hohn J, Kokai EL, Savanya GK, Lazar G. Surgery of benign liver tumours: indications for treatment: twenty years’ experience. Hepatogastroenterology. 2008;55:592–595. [PubMed] [Google Scholar]
- 16.Skalicky T, Treska V, Sutnar A, Liska V, Mirka H, Ohlidalova K, et al. Surgical treatment of benign liver tumours. Bratisl Lek Listy. 2005;106:330–332. [PubMed] [Google Scholar]
- 17.Erdogan D, Busch OR, Gouma DJ, van Gulik TM. Morbidity and mortality after liver resection for benign and malignant hepatobiliary lesions. Liver Int. 2009;29:175–180. doi: 10.1111/j.1478-3231.2008.01806.x. [DOI] [PubMed] [Google Scholar]
- 18.Liu CL, Fan ST, Lo CM, Chan SC, Tso WK, Ng IO, et al. Hepatic resection for incidentaloma. J Gastrointest Surg. 2004;8:785–793. doi: 10.1016/j.gassur.2004.06.001. [DOI] [PubMed] [Google Scholar]
- 19.Ahn SS, Kim MJ, Lim JS, Hong HS, Chung YE, Choi JY. Added value of gadoxetic acid-enhanced hepatobiliary phase MR imaging in the diagnosis of hepatocellular carcinoma. Radiology. 2010;255:459–466. doi: 10.1148/radiol.10091388. [DOI] [PubMed] [Google Scholar]
- 20.Buell JF, Tranchart H, Cannon R, Dagher I. Management of benign hepatic tumours. Surg Clin North Am. 2010;90:719–735. doi: 10.1016/j.suc.2010.04.006. [DOI] [PubMed] [Google Scholar]
- 21.Choi BY, Nguyen MH. The diagnosis and management of benign hepatic tumours. J Clin Gastroenterol. 2005;39:401–412. doi: 10.1097/01.mcg.0000159226.63037.a2. [DOI] [PubMed] [Google Scholar]
- 22.Grazioli L, Bondioni MP, Haradome H, Motosugi U, Tinti R, Frittoli B, et al. Hepatocellular adenoma and focal nodular hyperplasia: value of gadoxetic acid-enhanced MR imaging in differential diagnosis. Radiology. 2012;262:520–529. doi: 10.1148/radiol.11101742. [DOI] [PubMed] [Google Scholar]
- 23.Purysko AS, Remer EM, Coppa CP, Obuchowski NA, Schneider E, Veniero JC. Characteristics and distinguishing features of hepatocellular adenoma and focal nodular hyperplasia on gadoxetate disodium-enhanced MRI. AJR Am J Roentgenol. 2012;198:115–123. doi: 10.2214/AJR.11.6836. [DOI] [PubMed] [Google Scholar]
- 24.Belghiti J, Pateron D, Panis Y, Vilgrain V, Flejou JF, Benhamou JP, et al. Resection of presumed benign liver tumours. Br J Surg. 1993;80:380–383. doi: 10.1002/bjs.1800800340. [DOI] [PubMed] [Google Scholar]
- 25.Hanaoka J, Shimada M, Utsunomiya T, Imura S, Morine Y, Ikemoto T, et al. Huge focal nodular hyperplasia difficult to distinguish from well-differentiated hepatocellular carcinoma. Hepatol Res. 2012;42:727–731. doi: 10.1111/j.1872-034X.2012.00974.x. [DOI] [PubMed] [Google Scholar]
- 26.Azoulay D, Johann M, Raccuia JS, Castaing D, Bismuth H. ‘Protected’ double needle biopsy technique for hepatic tumours. J Am Coll Surg. 1996;183:160–163. [PubMed] [Google Scholar]
- 27.Kammula US, Buell JF, Labow DM, Rosen S, Millis JM, Posner MC. Surgical management of benign tumours of the liver. Int J Gastrointest Cancer. 2001;30:141–146. doi: 10.1385/IJGC:30:3:141. [DOI] [PubMed] [Google Scholar]
- 28.D'Halluin V, Vilgrain V, Pelletier G, Rocher L, Belghiti J, Erlinger S, et al. Natural history of focal nodular hyperplasia. A retrospective study of 44 cases. Gastroenterol Clin Biol. 2001;25:1008–1010. [PubMed] [Google Scholar]
- 29.Kuo YH, Wang JH, Lu SN, Hung CH, Wei YC, Hu TH, et al. Natural course of hepatic focal nodular hyperplasia: a longterm follow-up study with sonography. J Clin Ultrasound. 2009;37:132–137. doi: 10.1002/jcu.20533. [DOI] [PubMed] [Google Scholar]
- 30.Koffron AJ, Auffenberg G, Kung R, Abecassis M. Evaluation of 300 minimally invasive liver resections at a single institution: less is more. Ann Surg. 2007;246:385–392. doi: 10.1097/SLA.0b013e318146996c. discussion 392–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Toro A, Gagner M, Di Carlo I. Has laparoscopy increased surgical indications for benign tumours of the liver? Langenbecks Arch Surg. 2013;398:195–210. doi: 10.1007/s00423-012-1012-y. [DOI] [PubMed] [Google Scholar]
- 32.Amesur N, Hammond JS, Zajko AB, Geller DA, Gamblin TC. Management of unresectable symptomatic focal nodular hyperplasia with arterial embolization. J Vasc Interv Radiol. 2009;20:543–547. doi: 10.1016/j.jvir.2009.01.001. [DOI] [PubMed] [Google Scholar]
- 33.Huang D, Chen Y, Zeng Q, Zhao J, Wu R, Wu X, et al. Transarterial embolization using pingyangmycin lipiodol emulsion and polyvinyl alcohol for the treatment of focal nodular hyperplasia of the liver. Hepatogastroenterology. 2011;58:1736–1741. doi: 10.5754/hge11174. [DOI] [PubMed] [Google Scholar]
- 34.Terkivatan T, Hussain SM, Lameris JS, IJzermans JN. Transcatheter arterial embolization as a safe and effective treatment for focal nodular hyperplasia of the liver. Cardiovasc Intervent Radiol. 2002;25:450–453. doi: 10.1007/s00270-002-1929-6. [DOI] [PubMed] [Google Scholar]
- 35.Vogl TJ, Own A, Hammerstingl R, Reichel P, Balzer JO. Transarterial embolization as a therapeutic option for focal nodular hyperplasia in four patients. Eur Radiol. 2006;16:670–675. doi: 10.1007/s00330-005-2885-8. [DOI] [PubMed] [Google Scholar]
- 36.Wilhelm L, Albrecht L, Kirsch M, Heidecke CD. Preoperative application of selective angiographic embolization in the treatment of focal nodular hyperplasia. Surg Laparosc Endosc Percutan Tech. 2006;16:177–181. doi: 10.1097/00129689-200606000-00014. [DOI] [PubMed] [Google Scholar]


