Abstract
Background
Currently, resection criteria for colorectal cancer liver metastases (CRCLM) are only limited by remnant liver function. Morbidity and survival after a partial hepatectomy with limited or extended indication criteria were compared.
Methods/Design
Between 1991 and 2010, patients undergoing a liver resection for CRCLM with limited (n = 169) or extended indication criteria (n = 129) were retrospectively identified in a prospectively collected single-centre database. Limited indication criteria were defined as less than three unilateral, not centrally located liver metastases in the absence of extra hepatic metastases. The extended criteria were only limited by predicted remnant liver volume and patients fitness. Data on co-morbidity, resection margin, short- and long-term morbidity, disease-free (DFS) and overall survival were compared.
Results
Patients with limited indications had less major complications (19.5% vs. 33.1%, P < 0.01), longer overall survival of 68.8 months [confidence interval (CI) 46.5–91.1] vs. 41.4 months (CI 33.4–49.0, P ≤ 0.001) and longer median DFS of 22.0 months [confidence interval (CI) 15.8–28.2] vs 10.2 months (CI 8.4–11.9, P < 0.001) compared with the extended indication group. Cure rates, defined as 10-year DFS, were 35.5% and 15.8%, respectively. Fewer patients in the extended indication group underwent an R0 resection (92.9% vs. 77.5%, P < 0.001). Only 17% of all R1 resected patients had recurrences at the transection plane.
Conclusion
A partial hepatectomy for CRCLM with extended indications seems justified but is associated with higher complication rates, earlier recurrence and lower overall survival compared with limited indications. However, the median 5-year survival was substantial and a cure was achieved in 15.8% of patients.
Introduction
During the last decade, the limited criteria for a partial liver resection have been replaced by more extended indication criteria. Improvements in surgical technique, optimization of peri-operative care, improvements in diagnostic imaging, pre-operative liver remnant volume modulation and effectiveness of modern chemotherapy regimens have boosted the widening of resectability criteria. Traditionally, only patients with a maximum of three colorectal cancer liver metastases, located peripherally at one side of the liver with an anticipated resection margin greater than 10 mm and without signs of extrahepatic disease were considered eligible for a partial liver resection.1–4 Based on these limited criteria, only 10–20% of patients with colorectal cancer liver metastases were eligible for a resection. Recent studies have shown that a liver resection in patients with multiple and/or bilateral colorectal liver metastases results in overall 5-years survival rates between 23% and 51%.3,5 Moreover, centrally located liver metastases are no longer a contraindication for liver surgery. In patients with a normal functioning liver, extended hemihepatectomies can be performed safely and mesohepatectomy or a central liver resection is an alternative for an extended hemihepatectomy when parenchymal loss needs to be minimized.6 If resectable extrahepatic metastases are present, a resection can be offered with 5-year overall survival rates up to 28%.6,7 Thus, liver resection criteria for colorectal cancer liver metastases (CRCLM) are at present only limited by an anticipated R0 status and an adequate functional liver remnant. Patients in good general health, with technically resectable metastatic disease limited to the liver, regional lymph nodes or/and lungs, are considered for resection regardless of associated clinical predictive factors.8–10
Parallel to the expanding indications for a liver resection new strategies to improve resectability have also been popularized.11–13 A liver resection combined with ablation of metastases14–16 and/or induction chemotherapy to reduce the hepatic tumour size or tumour load may render unresectable metastases resectable or may help reduce the extent of liver resections.17 Staged resections, with or without portal vein embolization/ligation, can be used for a two-step clearance of liver metastases, to increase future remnant liver volume and to achieve a definitive R0 status.
Expanding the indications and application of the aforementioned strategies has increased the number of patients becoming resectable.18 The objective of the present study was to evaluate the post-operative clinical outcome and long-term survival in patients undergoing a liver resection for colorectal cancer liver metastases based on limited compared with extended indications.
Material and methods
Patients
A prospective database became operational in the Maastricht University Medical Centre HPB unit in 2001. All patients that underwent liver surgery before this date were included retrospectively. Patients undergoing liver surgery for CRCLM between 1991 and 2010 were included in the present study and assigned to a group with limited indication criteria or a group with extended indication criteria for resection. Patients were staged using a four-phase contrast enhanced abdominal CT scan. All patients with either primary or secondary liver tumours were discussed at a multidisciplinary liver meeting. Patient-specific co-morbidities and diagnostic procedures were assessed and the definitive treatment strategy was decided in consensus. Induction chemotherapy in irresectable patients, a liver resection combined with tumour ablation, pre-operative portal vein embolization, liver first policy in rectal cancer and a repeat hepatectomy were all among potential surgical strategies. Obviously some of these strategies became available only in more recent years. Vascular reconstructions were occasionally used. In recent years, patients not undergoing liver surgery were assigned to stereotactic radiotherapy, percutaneous tumour ablation, Y90- selective internal radiotherapy or palliative chemotherapy.
Study groups
Pre-operative CT-, MRI- or PET-CT-scans were used to determine the number and location of liver metastases. Operation notes gave insight in the specific type of resection, relation of metastases to the transection line, duration of surgery, the amount of blood loss and complications during surgery. Patients were, using predefined criteria, retrospectively assigned to either the extended indications group or to the limited indications group. Criteria for limited and extended indications are depicted in Table 1. Only patients with colorectal cancer liver metastases and a follow-up of at least 6 months were included in the present study.
Table 1.
Indication criteria for resection of colorectal liver metastases
| Limited indication criteria | Extended indication criteria |
|---|---|
| 1. Three or less liver metastases | 1. Four or more liver metastases |
| 2. Located at one side of liver only | 2. Bilateral metastases |
| 3. No signs of extra hepatic metastases | 3. Presence of resectable extra hepatic metastases |
| 4. Anticipated resection margin more than 10 mm. | 4. Centrally located metastases |
Liver resections
All liver resections were classified in accordance with the IHPBA Brisbane nomenclature (Table 2).19 A liver resection was performed as described previously.20 To determine the definitive extent of hepatic metastases and transection line, intra-operative ultrasound was used routinely.
Table 2.
Clinical characteristics
| Variables | No. of patients | P | ||
|---|---|---|---|---|
| All patients | Limited indication criteria | Extended Indication criteria | ||
| N = 298 | N = 169 | N = 129 | ||
| Patient characteristics | ||||
| Median age [range], y | 64 [24–88] | 64 [28–88] | 64 [24–82] | 0.183 |
| Gender, male (%) | 177 (59.4) | 110 (65.1) | 67 (51.9) | 0.022 |
| Co-morbidities, present (%) | 100 (33.6) | 56 (33.1) | 44 (34.1) | 0.860 |
| ASA classification | ||||
| Percentage with ASA 1 | 12.9 | 11.2 | 14.8 | 0.488 |
| Percentage with ASA 2 | 60.8 | 59.2 | 62.5 | 0.604 |
| Percentage with ASA 3 | 26.3 | 29.6 | 22.7 | 0.270 |
| Primary tumour | ||||
| Location, colon (%) | 177 (59.4) | 98 (58.0) | 79 (61.2) | 0.571 |
| AJCC T-stage, T3/T4 disease (%) | 87.9 | 84.8 | 92.0 | 0.082 |
| Nodal status, positive nodes (%) | 65.9 | 64.6 | 67.6 | 0.618 |
| Hepatic metastases | ||||
| Median size of largest lesion [range], cm | 3.0 [0.0–20.0] | 3.0 [0.4–13.0] | 3.0 [0.0–20.0] | 0.877 |
| Larger than 5 cm (%) | 18.7 | 17.6 | 20.2 | 0.622 |
| Median number [range] | 2 [1–12] | 1 [1–3] | 4 [1–12] | <0.001 |
| Bilateral metastases (%) | 74 (24.8) | 0 | 74 (57.4) | – |
| Concomitant extrahepatic disease (%) | 30 (10.1) | 0 | 30 (23.3) | – |
| Pre-operative management | ||||
| Pre-operative chemotherapy (%) | 42.1 | 34.5 | 52.0 | 0.003 |
| Operative details | ||||
| Two-stage procedure planned | 14 | 0 | 14 | – |
| PVE during first stage | 9 | 0 | 9 | – |
| Second stage performed | 10 | 0 | 10 | – |
| Combined procedure (%) | 13 (4.2) | 7 (4.1) | 6 (4.3) | 0.940 |
| Liver first procedure (%) | 26 (8.4) | 11 (6.5) | 15 (10.8) | 0.573 |
| Type of liver resection (%) | ||||
| Major (> 3 segments) | 174 (56.5) | 81 (47.9) | 97 (69.8) | <0.001 |
| Left hemihepatectomy | 18 (5.8) | 9 (5.3) | 9 (6.5) | 0.669 |
| Right hemihepatectomy | 103 (33.4) | 55 (32.5) | 48 (34.5) | 0.713 |
| Left extended hemihepatectomy | 4 (1.3) | 0 (0.0) | 4 (2.9) | – |
| Right ext. hemihepatectomy | 12 (3.9) | 2 (1.2) | 10 (7.2) | 0.007 |
| Central liver resection | 15 (4.9) | 0 (0.0) | 15 (10.8) | – |
| ≤1 segmentectomy or (multi) metastasectomy | 91 (29.5) | 65 (38.5) | 26 (18.7) | <0.001 |
| Multisegmentectomies (≥ 2) | 64 (20.8) | 38 (22.5) | 26 (18.7) | 0.416 |
| Only PVE | 1 (0.3) | 0 (0.0) | 1 (0.7) | – |
| Concomitant local ablation* (%) | 10 (3.2) | 1 (0.6) | 9 (6.7) | 0.004 |
| Post-operative management | ||||
| Percentage with post-operative chemotherapy | 37.2 | 36.6 | 37.8 | 0.841 |
ASA, American Society of Anesthesiologists; PVE, portal vein embolization.
Peri-operative care
In February 2005, the Enhanced Recovery After Surgery (ERAS®) fast track peri-operative care programme was introduced in liver surgery at our centre. This programme enhances post-operative recovery and as a consequence reduces hospital length of stay (LOS).21 Before the introduction of the ERAS programme there was no standard peri-operative care protocol.
Oncological follow-up
Follow-up consisted of outpatient visits with plasma carcinoembryonic antigen levels, every 3 months, and liver imaging twice in the first 2 years and annually up to 5 years after surgery. The median follow-up was 33 months (range, 0–235). In case of recurrence, patients were assessed with PET-CT and the indication for repeat liver or lung surgery was discussed in the multidisciplinary oncology meeting.
Outcome parameters
The primary endpoint of the study was overall survival. Secondary endpoints were all complications: a liver surgery-specific composite endpoint,22 readmissions, post-operative mortality, hospital LOS and disease-free survival (DFS). Complications were registered daily using National Surgical Adverse Event Registration (LHCR) software23 of the Dutch Association of General Surgery before 2009 and in the hospital information system (SAP, Walldorf, Germany) thereafter. The post-operative course of all discharged patients was discussed at the surgeons’ morning meeting and the Clavien–Dindo classification was used to grade complications after surgery.24 Complications with a Clavien–Dindo score ≤2 were considered minor complications whereas complications ≥3a were considered major complications. Patient demographics were also registered and information on co-morbidity, location and TNM stage of the primary tumour and moment of occurrence of liver metastases were retrieved from patient charts. Size of the metastases and the resection margins were retrieved from pathology reports. R0 resections were defined as resections with a tumour-free resection surface. If invasion in the resection surface was present, the resection was considered R1. R1 resections with metastases reaching the resection surface were analysed separately for local recurrences and survival.
Overall survival (OS) and DFS after a liver resection were registered in months and calculated in percentages. Overall survival was calculated as the time period between the date of diagnosis and death. In the case of two-stage hepatectomies, DFS was calculated as the time period between the first stage operation and the recurrence of metastases after the second stage operation.
Statistical analysis
Data were analysed using SPSS version 18.0 (SPSS Inc., Chicago, IL, USA). Data are expressed as median and percentages or survival in months [95% confidence interval (CI)]. The chi-square test was used to analyse categorical data whereas continuous data were analysed using the t-test U-test. The time to recurrence and OS were calculated with the Kaplan–Meier (sensored) method using the date of liver surgery and date of diagnosis, respectively, as a reference date. A level of P < 0.05 was considered to be statistically significant.
Results
Patients
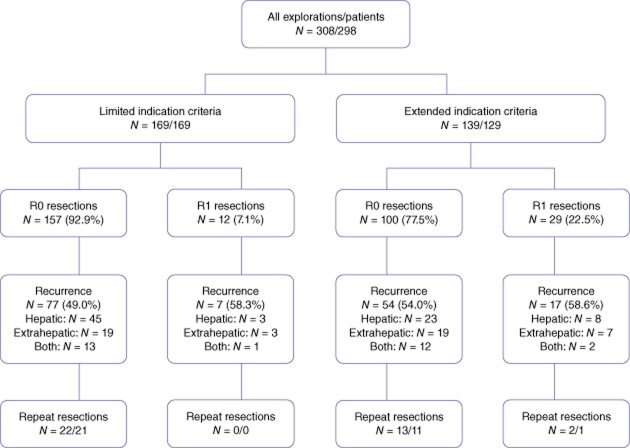
In total, 298 patients who underwent 308 liver resections for CRCLM between 1991 and 2010 were included. Ten patients had a two-stage strategy. Explorations with irresectable disease at laparotomy (n = 6) were excluded from further analysis (Fig. 1). In all patients with repeat resections for recurrent CRCLM (n = 41) results were analysed with the initial resection as a reference. The median age was 65 years (range 24–88). A total of 169 patients were included in the limited indications group compared with 129 patients in the extended indications group. Patient demographics, pre-operative characteristics, intra-operative details and pathology details are presented in Tables 2 and 3. Co-morbidities were present in 33.6% of patients, predominantly cardiovascular disease (28.2%), diabetes mellitus (7.7%) and pulmonary disease (4.0%). As expected through the allocation of patients, patients with extended indications had a more extensive spread of metastatic disease as is evidenced by the number of metastases, number of patients with bilateral disease and with concomitant extrahepatic disease. These patients underwent extended hepatectomies more often compared with the limited indications group (10.1% vs. 1.2%, P < 0.001). Surgical procedures are depicted in Table 2.
Figure 1.
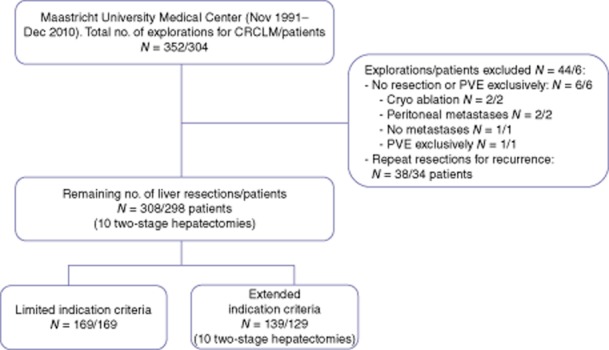
Flowchart study population. PVE, portal vein embolization
Table 3.
Comparison of surgical details, pathological details and hospital length of stay (LOS)
| Variables | No. of resections / patients | P | ||
|---|---|---|---|---|
| All resections / patients | Limited indication criteria | Extended indication criteria | ||
| N = 308 / 298 | N = 169 / 169 | N = 139 / 129 | ||
| Operative details | ||||
| Median estimated blood loss [range], ml | 800 [10–11600] | 775 [10–10000] | 800 [50–11600] | 0.592 |
| Median duration of operation [range], min | 217 [75–660] | 204.5 [75–660] | 240 [85–660] | <0.001 |
| Pathology | ||||
| Resection margin, | ||||
| Patients with R0 resections | 257 (86.2) | 157 (92.9) | 100 (77.5) | <0.001 |
| Patients with R1 resections | 41 (13.8) | 12 (7.1) | 29 (22.5) | <0.001 |
| Patients with R2 resections | 0.0 | 0.0 | 0.0 | – |
| Hospital LOS | ||||
| Median LOS [range], days | 8 [2–120] | 8 [3–92] | 8 [2–120] | 0.579 |
| Readmissions | ||||
| Number of patients readmitted (%) | 30 (9.7) | 14 (8.3) | 16 (11.5) | 0.342 |
| Repeat resections | ||||
| Number of patients undergoing repeat resections for recurrence (%) | 33 (11.1) | 21 (12.4) | 12 (9.3) | 0.190 |
| Number of patients with recurrence without resections (%) | 122 (40.6) | 62 (36.7) | 60 (46.5) | 0.190 |
Pathological analysis
The percentage of R0 resections was 92.9% in the limited indications group and 77.5% in the extended indications group (P < 0.001).
Surgical outcome
There were no significant differences in blood loss during surgery, length of hospital stay and number of readmissions between both the groups. However, there was a significant difference in the median operation time 205 [75–660] min in the limited indications group compared with 240 [85–660] min in the extended indications group (P < 0.001; Table 3). Major complications occurred in 19.5% of the patients with limited indications compared with 33.1% of the patients with extended indications (P = 0.007). The 90-day post-operative mortality rate was 3.6% in the limited indication group compared with 5.0% in the extended indications group (P = 0.519; Table 4).
Table 4.
Complications
| Variables | No. of resections / patients | P | ||
|---|---|---|---|---|
| All resections / patients | Limited indication criteria | Extended indication criteria | ||
| N = 308 / 298 | N = 169 / 169 | N = 139 / 129 | ||
| Complications | ||||
| Complications present | 113 (36.7) | 54 (32.0) | 59 (42.4) | 0.057 |
| Minor complications present | 34 (11.0) | 21 (12.4) | 13 (9.4) | 0.392 |
| Clavien–Dindo grade I | 10 (3.2) | 5 (3.0) | 5 (3.6) | 0.753 |
| Clavien–Dindo grade II | 24 (7.8) | 16 (9.5) | 8 (5.8) | 0.227 |
| Major complications present | 79 (25.6) | 33 (19.5) | 46 (33.1) | 0.007 |
| Clavien–Dindo grade IIIa | 48 (15.6) | 20 (11.8) | 28 (20.1) | 0.045 |
| Clavien–Dindo grade IIIb | 8 (2.6) | 2 (1.2) | 6 (4.3) | 0.085 |
| Clavien–Dindo grade Iva | 10 (3.2) | 5 (3.0) | 5 (3.6) | 0.753 |
| Clavien–Dindo grade Ivb | 0 (0.0) | 0 (0.0) | 0 (0.0) | – |
| Clavien–Dindo grade V | 13 (4.2) | 6 (3.6) | 7 (5.0) | 0.519 |
| Liver surgery specific composite endpoint (CEP) | ||||
| Liver surgery-specific CEP | 67 (21.8) | 29 (17.2) | 38 (27.3) | 0.031 |
| Ascites | 8 (2.6) | 2 (1.2) | 6 (4.3) | 0.085 |
| Post-resectional liver failure | 11 (3.6) | 4 (2.4) | 7 (5.0) | 0.209 |
| Bile leakage | 16 (5.2) | 5 (3.0) | 11 (7.9) | 0.051 |
| Intra-abdominal haemorrhage | 9 (2.9) | 4 (2.4) | 5 (3.6) | 0.524 |
| Intra-abdominal abscess | 35 (11.4) | 19 (11.2) | 16 (11.5) | 0.941 |
| 90-day post-operative mortality | 13 (4.2) | 6 (3.6) | 7 (5.0) | 0.519 |
| Other liver-related complications | ||||
| Hepatic encephalopathy | 5 (1.6) | 2 (1.2) | 3 (2.2) | – |
| Liver dysfunction | 2 (0.6) | 0 (0.0) | 2 (1.4) | – |
| Cholangitis | 2 (0.6) | 1 (0.6) | 1 (0.7) | – |
| Other complications | ||||
| Sepsis | 9 (2.9) | 5 (3.0) | 4 (2.9) | 0.943 |
| Cardiovascular | 13 (4.2) | 8 (4.7) | 5 (3.6) | 0.719 |
| Pulmonary | 28 (9.1) | 17 (10.1) | 11 (7.9) | 0.653 |
| Renal / urine tract | 12 (3.9) | 8 (4.7) | 4 (2.9) | 0.477 |
| Gastro-intestinal | 18 (5.8) | 7 (4.1) | 11 (7.9) | 0.115 |
| Haematological | 1 (0.3) | 0 (0.0) | 1 (0.7) | – |
| Neurological | 9 (2.9) | 1 (0.6) | 8 (5.8) | – |
| Wound infection | 7 (2.3) | 3 (1.8) | 4 (2.9) | – |
Overall survival
The median OS for all patients was 55.9 months (CI 48.5–63.3) with a 5-year OS rate of 48.3%. Patients in the limited indications group had a median OS of 68.8 months (CI 46.5–91.1) with a 5-year OS rate of 60.5%. Patients in the extended indications criteria group had a median OS of 41.2 months (CI 33.4–49.0) and a 5-year survival of 33.2% (P < 0.001). Ten-year OS rates in the limited indications group and the extended indications group were 35.5% and 15.8%, respectively.
365-day mortality
During the first post-operative year a steep drop in survival was observed in the Kaplan–Meier curve (Fig. 2) in both groups. We selected a group of all patients that died within 1 year after a partial hepatectomy and compared multiple patient characteristics with a group of all patients that survived at least 1 year. The only significant difference between the group that died within 1 year and the group that survived at least 1 year was age (median 67.7 [28.6–86.1] years and 63.7 [24.2–88.2], respectively and P = 0.025). Three more factors were borderline significant: combined oncological procedures, primary tumour positive lymph nodes and an American Society of Anesthesiologists’ (ASA) score of 3. 9.5% of the patients that died within 1 year had a liver resection combined with a resection of the primary tumour compared with 3.6% of the patients that survived more than 1 year, P = 0.077. Moreover, 77.5% of the patients that died within 1 year had primary colorectal positive lymph nodes compared with 63.7% of the patients surviving more than 1 year, P = 0.091. Finally, 44.4% of the patients that died within 1 year were considered ASA 3 whereas only 24.6% of the patients that survived more than 1 year were considered as ASA 3, P = 0.069.
Figure 2.
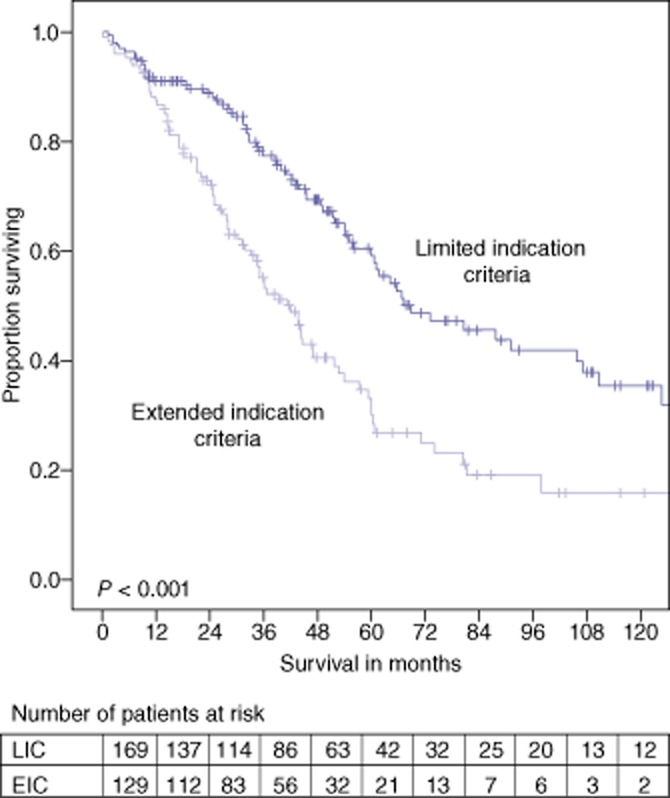
Overall survival
Disease-free survival
The median DFS for all patients was 15.7 months (CI 12.8–18.7) with a 5-year DFS rate of 23.7%. Patients in the limited indications group had a median DFS of 22.0 months (CI 15.8–28.2) and a 5-year DFS rate of 30.5%. Patients in the extended indications group had a median DFS of 10.2 months (CI 8.4–11.9) and a 5-year DFS rate of 14.2% (P < 0.001). Kaplan–Meier curves of both OS and DFS are presented in Figs 2 and 3.
Figure 3.
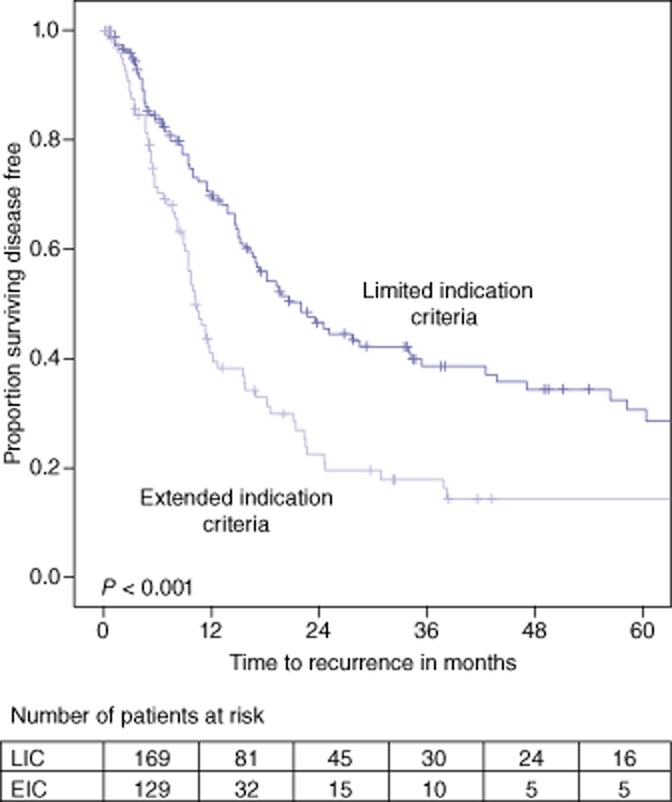
Disease-free survival
Thirty-three patients with a hepatic recurrence underwent a repeat liver resection. 14% of these patients did not suffer a recurrence during follow-up. 86% of patients, 82% in the limited indications group and 92% in the extended group, developed a second recurrence. The median time from the repeat resection to the second recurrence was 8.7 [0.0–18.9] months and 6.9 [1.9–11.9] months (P = 0.390) in both groups, respectively.
Recurrence after R1 liver resections
On histological examination, the resection specimen of 41 patients (13.3%) showed a microscopically positive resection margin (R1 resection). In these patients, recurrence of metastases in any location (i.e. liver and elsewhere) occurred in 23 (56%) out of 41 patients. Thirteen (32%) of these patients developed hepatic recurrence of which 3 (7%) had both intra- and extrahepatic recurrence. Only seven (17%) of patients with an R1 resection recurred at the former R1 resection surface: one patient in the limited indications group and six in the extended indications group. Ten (24%) patients showed extra hepatic recurrence only. Eighteen (44%) patients with an R1 resection stayed disease free during the follow-up period (median follow-up was 33.3 [0.2–165.9] months) (figure 4).
Figure 4.
R1 resections, recurrence and repeat resections
Overall survival in all patients with an R0 resection was 60.0 [53.0–67.0] months with a 5-year survival rate of 50.4%. Patients with an R1 resection showed a median OS of 44.3 [26.3–62.4] months and a 5-year survival rate of 34.9% (P = 0.040). Disease-free survival in all patients with an R0 resection was 16.9 [13.9–19.9] months (5-year DFS rate 25.2%) versus 9.4 [3.9–15.0] months (5-year DFS rate 17.4%) in patients with an R1 resection (P = 0.091).
Discussion
In this study, short- and long-term outcomes in patients undergoing a liver resection for colorectal cancer liver metastases with extended indications were assessed and compared with a group of patients operated according to limited indications criteria. We showed in the extended indications group a 5-year survival of 33.2% equivalent to a median OS of 41 months. In patients undergoing a liver resection according to the limited indications, a 5-year OS of 60.5% was observed, equivalent to a median OS of 69 months. In the latter group, 5-year DFS was 30.5% equivalent to a median DFS of 22 months. In patients with extended indications, 5-year DFS was 14.2% equivalent to a median DFS of 10 months, which is significantly lower than in patients with limited indications. When patients underwent a repeat liver resection for recurrence, the median DFS was prolonged by 8 months. Although the outcome was worse in the extended indication group, cure of a cure, defined as DFS longer than 10 years, was observed in 15.8% of patients.
Recently, short- and long-term outcomes in patients undergoing a liver resection for colorectal cancer liver metastases before and after 2000 have been published.25 In that study, two time periods with mixed indication criteria were compared. Although extended indication criteria were used in the patient groups before and after 2000, most patients operated after 2000 were still treated within the limited indication criteria. This may explain the relatively small difference observed in a 5-year OS of 47% and 58% between the two groups, respectively, in that study. Outcome results regarding patients undergoing a liver resection for colorectal cancer liver metastases within extended indication criteria are scarce. Recent publications report on the influence on outcome of only one single component of the former contraindications, e.g. number of metastases, resection margin or extra hepatic disease.3,5,7,26
The results of this study are in line with recent publications and are slightly better than the results of a recently published meta-analysis of studies on a liver resection for colorectal cancer liver metastases before 2007.9,25,27,28 From most published series, it transpires that over time survival after a liver resection of CRCLM improves probably as a consequence of improved effectiveness of modern chemotherapy regimens, improved peri-operative care, better patient selection and more aggressive resections. The OS of 56 months in this study may also be influenced by the long timeframe or modern chemotherapy regimens as well as additional treatment strategies in both patient groups with limited and extended indication criteria and seems to support an aggressive strategy towards CRCLM. From clinical practice, we observe that extending the indications for liver resection of colorectal cancer liver metastases renders more patients eligible. Our data show that extending the indications has its consequences for patients. It leads to more major complications, a shorter OS and a shorter DFS compared with patients with less extensive metastases. Major complications often require surgical, endoscopic, or radiological interventions. Although it may not be entirely legitimate to compare the median OS of 41 months in patients eligible for liver resection within extended indications to the median survival of 18–22 months in patients treated with chemotherapy alone, survival after a liver resection probably is substantially longer.29–32 The increase in OS of approximately one and a half years compared with the available literature data on outcome after palliative chemotherapy alone, seems to justify the acceptance of a higher complication rate after a resection.
Wiering et al.33 showed that quality of life recovered to baseline within 3 months in patients undergoing a potentially curative liver resection whereas a persistent decline in quality of life was demonstrated in patients immediately treated with palliative chemotherapy alone. If patients turned out to be irresectable at laparotomy, the quality of life was worst.34 It can therefore be hypothesized that the occurrence of complications after liver surgery with curative intent is an acceptable phenomenon for patients. Futile laparotomies should be avoided by using optimal pre-operative patient selection.
The morbidity and mortality results in the present study are consistent with other recently published series,25,35 although comparison is hampered to some extent as definitions used for severe or major morbidity vary across studies. In addition to standardized definitions of complications, the use of a composite endpoint for liver surgery-specific complications could enhance comparability of published series.22
Strikingly, the occurrence of more severe complications did not prolong the hospital LOS and did not lead to more readmissions. It can be hypothesized that the expected prolonged hospital LOS as a consequence of a higher percentage of major morbidity was compensated by the introduction of the Enhanced Recovery after Surgery (ERAS®) programme in our unit in 2005. A substantial proportion of patients deceased in the first year after a partial liver resection in both the extended indications and the limited indications group. In this study, high age, a ASA score of 3 and combined liver and colon resections proved to be risk factors for a high 365-day mortality. Hence, it should be possible to reduce post-operative mortality with better pre-operative patient selection. Unfortunately, our data, as well as other published data, did not show predictive factors usable to select patients who were at risk for mortality and consequently did not benefit from surgery.36,37
Compared with patients with limited indications, patients with extended indications more often underwent an R1 resection. In this series this did not lead to more repeat resections in the latter group. Moreover, we showed that only 17% of the patients with a R1 resection recurred at the level of the pathologically irradical resection surface. This might be a result of the use of the CUSA device (Integra LifeSiences, Plainsboro, NJ, USA), which does not allow the pathologist to evaluate the actual resection surface, as approximately 1 to 3 mm of hepatocytes disintegrate with the use of this device. The consequence of this observation might be that a close relation of metastases to vascular structures that cannot be resected is not an absolute contraindication for resection. Moreover, an R0 resection rate of 77.5% and a median survival of 41 months as well as the cure percentage of 15.8% seems to justify the use of extended indication criteria. As others have provided data to suggest these patients have a good quality of life, it also seems justified to be creative in future strategies to increase the resection rate in patients with colorectal cancer liver metastases. Suggestions might be to combine different treatment modalities that aim to remove or destroy metastases while functional liver capacity is maintained or enhanced. Open- or laparoscopic-resections combined with ablation, irreversible electroporation, peri-operative stereotactic radiotherapy or selective Internal Radio Therapy (SIRT) with Ytrium-bound microspheres,38 may help to increase the R0 resection rate and further improve survival with an acceptable quality of life.
The new technique of in situ liver partition and portal vein ligation for a two-stage hepatectomy with a short interval (ISLT or ALPPS) seems promising but the current morbidity and mortality of this procedure is still too high39–41
Strong points of this study are the selection of a series of patients with extended indication criteria and a standardized complication registration. Limitations are the relatively small sample size owing to the single-centre study design, the large time period during which the patients were included, the different peri-operative neo-adjuvant or adjuvant chemotherapy strategies and the retrospective analysis of prospectively collected outcome data.
Conclusion
Liver resection for colorectal cancer liver metastases with extended indication criteria seems justified. An R0 resection rate of 77%, median 5-year overall survival of over 33% and cure in 16% of patients was achieved with acceptable post-operative mortality and morbidity.
Conflicts of interest
None declared.
References
- 1.Nordlinger B, Guiguet M, Vaillant JC, Balladur P, Boudjema K, Bachellier P, et al. Surgical resection of colorectal carcinoma metastases to the liver. A prognostic scoring system to improve case selection, based on 1568 patients. Association Francaise de Chirurgie. Cancer. 1996;77:1254–1262. [PubMed] [Google Scholar]
- 2.Fong Y, Fortner J, Sun RL, Brennan MF, Blumgart LH. Clinical score for predicting recurrence after hepatic resection for metastatic colorectal cancer: analysis of 1001 consecutive cases. Ann Surg. 1999;230:309–318. doi: 10.1097/00000658-199909000-00004. discussion 318–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Altendorf-Hofmann A, Scheele J. A critical review of the major indicators of prognosis after resection of hepatic metastases from colorectal carcinoma. Surg Oncol Clin N Am. 2003;12:165–192. doi: 10.1016/s1055-3207(02)00091-1. [DOI] [PubMed] [Google Scholar]
- 4.Sharma S, Camci C, Jabbour N. Management of hepatic metastasis from colorectal cancers: an update. J Hepatobiliary Pancreat Surg. 2008;15:570–580. doi: 10.1007/s00534-008-1350-x. [DOI] [PubMed] [Google Scholar]
- 5.Pawlik TM, Abdalla EK, Ellis LM, Vauthey JN, Curley SA. Debunking dogma: surgery for four or more colorectal liver metastases is justified. J Gastrointest Surg. 2006;10:240–248. doi: 10.1016/j.gassur.2005.07.027. [DOI] [PubMed] [Google Scholar]
- 6.Mehrabi A, Mood ZA, Roshanaei N, Fonouni H, Muller SA, Schmied BM, et al. Mesohepatectomy as an option for the treatment of central liver tumors. J Am Coll Surg. 2008;207:499–509. doi: 10.1016/j.jamcollsurg.2008.05.024. [DOI] [PubMed] [Google Scholar]
- 7.Pulitano C, Bodingbauer M, Aldrighetti L, de Jong MC, Castillo F, Schulick RD, et al. Liver resection for colorectal metastases in presence of extrahepatic disease: results from an international multi-institutional analysis. Ann Surg Oncol. 2011;18:1380–1388. doi: 10.1245/s10434-010-1459-4. [DOI] [PubMed] [Google Scholar]
- 8.Fong Y. Surgical therapy of hepatic colorectal metastasis. CA Cancer J Clin. 1999;49:231–255. doi: 10.3322/canjclin.49.4.231. [DOI] [PubMed] [Google Scholar]
- 9.Pawlik TM, Schulick RD, Choti MA. Expanding criteria for resectability of colorectal liver metastases. Oncologist. 2008;13:51–64. doi: 10.1634/theoncologist.2007-0142. [DOI] [PubMed] [Google Scholar]
- 10.Shimada H, Tanaka K, Endou I, Ichikawa Y. Treatment for colorectal liver metastases: a review. Langenbecks Arch Surg. 2009;394:973–983. doi: 10.1007/s00423-009-0530-8. [DOI] [PubMed] [Google Scholar]
- 11.Adam R, Laurent A, Azoulay D, Castaing D, Bismuth H. Two-stage hepatectomy: a planned strategy to treat irresectable liver tumors. Ann Surg. 2000;232:777–785. doi: 10.1097/00000658-200012000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gallagher DJ, Kemeny N. Metastatic colorectal cancer: from improved survival to potential cure. Oncology. 2010;78:237–248. doi: 10.1159/000315730. [DOI] [PubMed] [Google Scholar]
- 13.Yang AD, Brouquet A, Vauthey JN. Extending limits of resection for metastatic colorectal cancer: risk benefit ratio. J Surg Oncol. 2010;102:996–1001. doi: 10.1002/jso.21701. [DOI] [PubMed] [Google Scholar]
- 14.Razafindratsira T, Isambert M, Evrard S. Complications of intraoperative radiofrequency ablation of liver metastases. HPB. 2011;13:15–23. doi: 10.1111/j.1477-2574.2010.00243.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Evrard S, Rivoire M, Arnaud J, Lermite E, Bellera C, Fonck M, et al. Unresectable colorectal cancer liver metastases treated by intraoperative radiofrequency ablation with or without resection. Br J Surg. 2012;99:558–565. doi: 10.1002/bjs.8724. [DOI] [PubMed] [Google Scholar]
- 16.Ruers T, Punt C, Van Coevorden F, Pierie JP, Borel-Rinkes I, Ledermann JA, et al. Radiofrequency ablation combined with systemic treatment versus systemic treatment alone in patients with non-resectable colorectal liver metastases: a randomized EORTC Intergroup phase II study (EORTC 40004) Ann Oncol. 2012;23:2619–2626. doi: 10.1093/annonc/mds053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Folprecht G, Gruenberger T, Bechstein WO, Raab HR, Lordick F, Hartmann JT, et al. Tumour response and secondary resectability of colorectal liver metastases following neoadjuvant chemotherapy with cetuximab: the CELIM randomised phase 2 trial. Lancet Oncol. 2010;11:38–47. doi: 10.1016/S1470-2045(09)70330-4. [DOI] [PubMed] [Google Scholar]
- 18.Lam VW, Spiro C, Laurence JM, Johnston E, Hollands MJ, Pleass HC, et al. A systematic review of clinical response and survival outcomes of downsizing systemic chemotherapy and rescue liver surgery in patients with initially unresectable colorectal liver metastases. Ann Surg Oncol. 2012;19:1292–1301. doi: 10.1245/s10434-011-2061-0. [DOI] [PubMed] [Google Scholar]
- 19.Strasberg SM. Nomenclature of hepatic anatomy and resections: a review of the Brisbane 2000 system. J Hepatobiliary Pancreat Surg. 2005;12:351–355. doi: 10.1007/s00534-005-0999-7. [DOI] [PubMed] [Google Scholar]
- 20.van de Poll MC, Siroen MP, van Leeuwen PA, Soeters PB, Melis GC, Boelens PG, et al. Interorgan amino acid exchange in humans: consequences for arginine and citrulline metabolism. Am J Clin Nutr. 2007;85:167–172. doi: 10.1093/ajcn/85.1.167. [DOI] [PubMed] [Google Scholar]
- 21.van Dam RM, Hendry PO, Coolsen MM, Bemelmans MH, Lassen K, Revhaug A, et al. Initial experience with a multimodal enhanced recovery programme in patients undergoing liver resection. Br J Surg. 2008;95:969–975. doi: 10.1002/bjs.6227. [DOI] [PubMed] [Google Scholar]
- 22.van den Broek MA, van Dam RM, van Breukelen GJ, Bemelmans MH, Oussoultzoglou E, Pessaux P, et al. Development of a composite endpoint for randomized controlled trials in liver surgery. Br J Surg. 2011;98:1138–1145. doi: 10.1002/bjs.7503. [DOI] [PubMed] [Google Scholar]
- 23.Marang-van de Mheen PJ, Stadlander MC, Kievit J. Adverse outcomes in surgical patients: implementation of a nationwide reporting system. Qual Saf Health Care. 2006;15:320–324. doi: 10.1136/qshc.2005.016220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205–213. doi: 10.1097/01.sla.0000133083.54934.ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.de Haas RJ, Wicherts DA, Andreani P, Pascal G, Saliba F, Ichai P, et al. Impact of expanding criteria for resectability of colorectal metastases on short- and long-term outcomes after hepatic resection. Ann Surg. 2011;253:1069–1079. doi: 10.1097/SLA.0b013e318217e898. [DOI] [PubMed] [Google Scholar]
- 26.Pawlik TM, Scoggins CR, Zorzi D, Abdalla EK, Andres A, Eng C, et al. Effect of surgical margin status on survival and site of recurrence after hepatic resection for colorectal metastases. Ann Surg. 2005;241:715–722. doi: 10.1097/01.sla.0000160703.75808.7d. discussion 722–724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.de Jong MC, Pulitano C, Ribero D, Strub J, Mentha G, Schulick RD, et al. Rates and patterns of recurrence following curative intent surgery for colorectal liver metastasis: an international multi-institutional analysis of 1669 patients. Ann Surg. 2009;250:440–448. doi: 10.1097/SLA.0b013e3181b4539b. [DOI] [PubMed] [Google Scholar]
- 28.Kanas GP, Taylor A, Primrose JN, Langeberg WJ, Kelsh MA, Mowat FS, et al. Survival after liver resection in metastatic colorectal cancer: review and meta-analysis of prognostic factors. Clin Epidemiol. 2012;4:283–301. doi: 10.2147/CLEP.S34285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Saltz LB, Clarke S, Diaz-Rubio E, Scheithauer W, Figer A, Wong R, et al. Bevacizumab in combination with oxaliplatin-based chemotherapy as first-line therapy in metastatic colorectal cancer: a randomized phase III study. J Clin Oncol. 2008;26:2013–2019. doi: 10.1200/JCO.2007.14.9930. [DOI] [PubMed] [Google Scholar]
- 30.van Iersel LB, Koopman M, van de Velde CJ, Mol L, van Persijn van Meerten EL, Hartgrink HH, et al. Management of isolated nonresectable liver metastases in colorectal cancer patients: a case-control study of isolated hepatic perfusion with melphalan versus systemic chemotherapy. Ann Oncol. 2010;21:1662–1667. doi: 10.1093/annonc/mdp589. [DOI] [PubMed] [Google Scholar]
- 31.Cassidy J, Clarke S, Diaz-Rubio E, Scheithauer W, Figer A, Wong R, et al. XELOX vs FOLFOX-4 as first-line therapy for metastatic colorectal cancer: NO16966 updated results. Br J Cancer. 2011;105:58–64. doi: 10.1038/bjc.2011.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Maughan TS, Adams RA, Smith CG, Meade AM, Seymour MT, Wilson RH, et al. Addition of cetuximab to oxaliplatin-based first-line combination chemotherapy for treatment of advanced colorectal cancer: results of the randomised phase 3 MRC COIN trial. Lancet. 2011;377:2103–2114. doi: 10.1016/S0140-6736(11)60613-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wiering B, Oyen WJ, Adang EM, van der Sijp JR, Roumen RM, de Jong KP, et al. Long-term global quality of life in patients treated for colorectal liver metastases. Br J Surg. 2011;98:565–571. doi: 10.1002/bjs.7365. discussion 571–572. [DOI] [PubMed] [Google Scholar]
- 34.Langenhoff BS, Krabbe PF, Peerenboom L, Wobbes T, Ruers TJ. Quality of life after surgical treatment of colorectal liver metastases. Br J Surg. 2006;93:1007–1014. doi: 10.1002/bjs.5387. [DOI] [PubMed] [Google Scholar]
- 35.Farges O, Goutte N, Bendersky N, Falissard B. Incidence and risks of liver resection: an all-inclusive French nationwide study. Ann Surg. 2012;256:697–704. doi: 10.1097/SLA.0b013e31827241d5. discussion 704–705. [DOI] [PubMed] [Google Scholar]
- 36.Schiesser M, Chen JW, Maddern GJ, Padbury RT. Perioperative morbidity affects long-term survival in patients following liver resection for colorectal metastases. J Gastrointest Surg. 2008;12:1054–1060. doi: 10.1007/s11605-007-0438-y. [DOI] [PubMed] [Google Scholar]
- 37.Abbott AM, Parsons HM, Tuttle TM, Jensen EH. Short-term outcomes after combined colon and liver resection for synchronous colon cancer liver metastases: a population study. Ann Surg Oncol. 2013;20:139–147. doi: 10.1245/s10434-012-2515-z. [DOI] [PubMed] [Google Scholar]
- 38.Vente MA, Wondergem M, van der Tweel I, van den Bosch MA, Zonnenberg BA, Lam MG, et al. Yttrium-90 microsphere radioembolization for the treatment of liver malignancies: a structured meta-analysis. Eur Radiol. 2009;19:951–959. doi: 10.1007/s00330-008-1211-7. [DOI] [PubMed] [Google Scholar]
- 39.Schnitzbauer AA, Lang SA, Goessmann H, Nadalin S, Baumgart J, Farkas SA, et al. Right portal vein ligation combined with in situ splitting induces rapid left lateral liver lobe hypertrophy enabling 2-staged extended right hepatic resection in small-for-size settings. Ann Surg. 2012;255:405–414. doi: 10.1097/SLA.0b013e31824856f5. [DOI] [PubMed] [Google Scholar]
- 40.Knoefel WT, Gabor I, Rehders A, Alexander A, Krausch M, Schulte am Esch J, et al. In situ liver transection with portal vein ligation for rapid growth of the future liver remnant in two-stage liver resection. Br J Surg. 2013;100:388–394. doi: 10.1002/bjs.8955. [DOI] [PubMed] [Google Scholar]
- 41.Neumann UP, Dejong CH. Split decision. Br J Surg. 2013;100:310–312. doi: 10.1002/bjs.9050. [DOI] [PubMed] [Google Scholar]