Abstract
Physiology and evolutionary biology have developed as two separated disciplines, a separation that mirrored the hypothesis that the physiological and evolutionary processes could be decoupled. We argue that non-genetic inheritance shatters the frontier between physiology and evolution, and leads to the coupling of physiological and evolutionary processes to a point where there exists a continuum between accommodation by phenotypic plasticity and adaptation by natural selection. This approach is also profoundly affecting the definition of the concept of phenotypic plasticity, which should now be envisaged as a multi-scale concept. We further suggest that inclusive inheritance provides a quantitative way to help bridging infra-individual (i.e. physiology) with supra-individual (i.e. evolution) approaches, in a way that should help building the long sough inclusive evolutionary synthesis.
Étienne Danchin is Director of research at the French CNRS. He leads a laboratory entitled Evolution & Diversité Biologique (EDB) in Toulouse regrouping 50 researchers and university teachers (from Université Paul Sabatier), plus about 50 PhD and postdoc students. He also co-leads a Laboratoire d'Excellence called TULIP that regroups five laboratories totalling 400 staff members. He is an expert in behavioural ecology for which he wrote a textbook published by Oxford University Press. His fields of interest range from social information use in habitat and mate choices to the evolution of coloniality in birds, as well as cultural evolution and information flows as determinants of ecological and evolutionary dynamics. He advocates the necessity to generalize the modern synthesis of evolution into an inclusive evolutionary synthesis by incorporating all the dimensions of non-genetic inheritance. He also studies costs of multiple mating in birds through sperm ageing and sperm-mediated bacterial transmission. Arnaud Pocheville is a theoretical biologist and a philosopher of biology, currently a Postdoctoral Fellow at the Center for Philosophy of Science, University of Pittsburgh. His research consists in questioning the need for new concepts and mathematical approaches in eco-evolutionary biology to deal with empirical cases that seem to escape current theorizations. He worked in particular on niche construction theory and ecological inheritance. He is also interested in theoretical medicine, collaborating on theoretical approaches to cancer and gene therapies.
Introduction
From their origin, physiology and evolution have mainly developed as two independent disciplines of biology. This separation can be justified by profound differences in concepts and methodologies, but such a division may also forbid more integrative approaches. Schematically (Fig. 1), physiology studies the mechanisms that govern the internal functioning of individual organisms in the context of their immediate environment. As such, it is clearly linked to adaptive phenotypic plasticity, a major mechanism of accommodation that allows organisms to adjust their state to the conditions that prevail in their environment. Furthermore, researchers in physiology most often work on pure lines to reduce genetic variation to be able to unravel the molecular mechanisms that govern the overall functioning of an organism. Physiology thus focuses on mechanisms occurring within individual organisms at the intragenerational time scale (Fig. 1). Evolutionary biology on the other hand, studies the transgenerational transformation of populations (i.e. collections of same species organisms) across generations, which involves the study of the mechanisms that generate the observed transgenerational dynamics. The origin and maintenance (or disappearance) of among individual organism variation are central topics of all evolutionary approaches as heritable variation constitutes the ‘raw material of evolution’. Thus, evolutionary biology mainly focuses on processes occurring at the populational level and at intergenerational time scales.
Figure 1. Scopes of physiology and evolution on the scales of life organization.
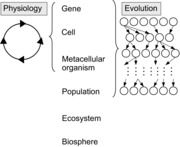
Physiology regroups phenomena taking place within the life cycle of a single organism, while evolution regroups populational phenomena of intergenerational change. The circle on the left side, as well as every circle on the right side represent the full life cycle of a single individual. Different lines of circles stand for different generations.
Here we propose that physiology and evolutionary biology still need to be better integrated, in particular as regards plasticity and adaptation by natural selection, because of the increasing recognition that epigenetic mechanisms are shared by physiological and evolutionary processes. Our main point is that the emergence of non-genetic inheritance is providing a unique way of bridging physiology and evolution, a link that remained quasi impossible as long as we persisted in reducing inheritance to its sole genetic dimension, i.e. to the sole transmission of the information encoded in the DNA sequence (Crick, 1958, 1970).
We organized this paper as follows. We first briefly review the rationale for thinking that the frontier between physiology and evolution is permeable. We then mention a possible way of articulating physiology and evolution, namely Evo-Devo, and argue that, though necessary, the articulation provided by this approach needs to be completed. We then briefly describe examples of non-genetic inheritance that shatter the frontier between physiological and evolutionary processes, and elaborate on the possible conceptual and theoretical consequences of such mechanisms. As this paper is meant to interest researchers of both disciplines, we provide a glossary of all the important terms that are central to our arguments. Terms in the glossary are in italics on their first appearance.
The permeable frontier between physiology and evolution
Historically, the hypothesis of a frontier between physiological and evolutionary processes partly developed because of the current interpretation of August Weismann's distinction between what he called germ-plasm and soma (Weismann, 1891). Though today this distinction is often equated with the modern distinction between germ cells and somatic cells, it was in fact more akin to the modern and Johannsen's (1911) distinction between the genotype and the phenotype (Haig, 2007). Weismann's rejection of the inheritance of acquired characters (the germ-plasm is supposed not to be directionally modifiable by the soma) probably greatly helped establishing genetics and the Modern Synthesis of evolution in which heredity boils down to gene transmission (Haig, 2007). In effect, the genocentric vision of heredity erected an impermeable barrier between physiology and evolution. Phenotypic plasticity and inheritance were thus confined in clearly separated domains of the functioning of living organisms and could not be thought of as interacting.
More recently, students of development have argued that the current version of the Modern Synthesis of evolution, which emerged from the merging of population and Mendelian genetics in the 1930s, needs to be expanded (Jablonka 1998; Avital & Jablonka, 2000; Mameli, 2004; Jablonka & Raz, 2009; Pigliucci & Muller, 2010). The discovery of genetics has been so fascinating – and yes it was and still is – that we have become oblivious to the accumulating evidence for non-genetic inheritance (Sapp, 1987). Evidence is coming from many fields of biology, including epigenetics (Henderson & Jacobsen, 2007; Jablonka & Raz, 2009; Jablonka & Lamb, 2010; Daxinger & Whitelaw, 2012), cultural (Danchin et al. 2004, 2010; Laland et al. 2010; Kruetzen et al. 2011; Mann et al. 2012) and ecological inheritance (Odling-Smee, 1988; Odling Smee et al. 2003; Odling-Smee & Laland, 2011), parental effects (Zeh & Zeh, 2008), the inheritance of gut and skin symbionts (reviews in Danchin et al. 2011; Fellous et al. 2011), as well as more esoteric aspects of inheritance such as prions (Shorter & Lindquist, 2005; Halfmann & Lindquist, 2010) and chaperone molecules (Saibil, 2013) that strongly affect and replicate specific configurations of major metabolic molecules.
The main claim of tenants of the expansion of the Modern Synthesis can be reformulated as stating that the frontier that current biology defines between physiology and evolution is so permeable that it is artificial, and needs to be abandoned or at least greatly faded. While tenants of the porosity of that frontier stress evidence coming from newly discovered mechanisms of development especially in the fields of culture and epigenetics, an avenue to establish the continuity between development and evolution lies in the acceptation that mechanisms of phenotypic plasticity percolate into inheritance. In other words, it is necessary to quantify the extent to which developmental mechanisms translate into inheritance (Danchin et al. 2004, 2011; Danchin & Wagner, 2010). Within evolutionary sciences, this approach has crystallized around concepts of non-genetic inheritance, which are reopening the concept of inheritance thus making it much more inclusive. We now briefly analyse a possible way of articulating physiology and evolution, namely Evo-Devo studies.
Evo-Devo and the concept of phenotypic plasticity
The term Evo-Devo was coined to label a research programme aiming at bridging developmental biology with both genetics and evolutionary theory. As Lamm & Jablonka (2008) concisely put it, Evo-Devo ‘focuses on the processes of evolutionary innovation, on the constraints and generic properties of developmental systems, on comparative studies of developmental genes with major effects, on the architecture of genetic developmental networks, and on the evolution of the ability to develop and learn’. In this sense, Evo-Devo is explicitly focused on the relation between evolution and development, on phylogenetic developmental and structural constraints, on the phylogenetic analysis between phylogeny and ontogeny (e.g. heterochrony), on the homology between pattern-associated genes such as the Hox genes in different lineages, and on the role of developmental plasticity in evolution. All these undoubtedly belong to evolutionary biology. However, the stress on phylogenetic patterns rather than on supra-individual processes of interactions within populations in their ecological context highlights the fact that Evo-Devo does not really incorporate evolutionary ecology processes. Thus, Evo-Devo has clearly started to build a bridge between physiology and evolution, but the central part of that bridge is still to be imagined.
The consequence is that today Evo-Devo only very occasionally deals with evolutionary questions framed in eco-evolutionary terms, namely, questions where the supra-individual (i.e. populational) aspects of evolutionary processes would be central (but see Gilbert, 2003). In effect, the emergence of Evo-Devo itself endorsed the fact that developmental biology was moving towards molecular biology rather than towards population biology. In addition, Evo-Devo has focused on the genetic side of inheritance (Gilbert, 2003), rarely discussing non-genetic inheritance per se (Lamm & Jablonka, 2008).
None the less, a major achievement of Evo-Devo is that it greatly helped unravelling the mechanisms of phenotypic plasticity, which is placed at the centre of the approach (West-Eberhard, 2003; Brakefield & Wijngaarden, 2006; Pigliucci & Muller, 2010). Despite the fact that the concept of plasticity has often been claimed to link genetics, developmental and evolutionary biology, it turned out to be more like a frontier under tension than a place of true articulation (Nicoglou, 2013). Furthermore, as phenotypic plasticity qualifies processes that unfold within an organism's lifespan (but see Lamm & Jablonka, 2008), it is unlikely to be a sufficient element to fully bridge physiology and evolution.
Ideally, to further bridge physiology and evolution we need a quantitative concept that clearly transfers the effect of processes occurring at the infra-individual level (i.e. development and physiology) to a populational and intergenerational level, as this is the level at which evolutionary processes occur.
The shattered frontier between physiology and evolution
The arousal of non-genetic inheritance in evolutionary approaches has literally shattered all the frontiers between physiology and evolution. All of a sudden, it appeared that many mechanisms of development, accommodation and adaptation, including cell differentiation, epigenetics, behaviour, cognition, etc. could transfer information across generations and thus participate in inheritance. In this section we use two examples to illustrate how processes involved in non-genetic inheritance are also processes of development (and vice versa), suggesting that physiology and evolution are tightly linked in ways that de facto challenge Neo-Darwinism.
Culture
One of the first challenges to the genocentric conception of heredity came from human sciences three decades ago, with the theoretical study of the consequences of cultural transmission on population evolutionary dynamics (Cavalli-Sforza & Feldman, 1981, 1983; Lunsden & Wilson, 1981; Boyd & Richerson, 1983; Feldman & Cavalli-Sforza, 1984). Culture clearly shows that social learning, which constitutes a major process of development and accommodation, also allows the transmission of key adaptive information across generations (Danchin et al. 2004, 2011; Danchin & Wagner, 2010; Thornton et al. 2010; Slagsvold & Wiebe, 2011). As a consequence, the transmission of acquired skills is at the heart of cultural transmission (Jablonka et al. 1998; Danchin et al. 2004; Danchin & Wagner, 2010), a process that has been, in addition, suggested to strongly affect the current genetic structuring of human populations (Laland et al. 2010). Social transmission also strongly affects major processes such as sexual selection (Laland, 1994). For instance, the whole of the literature on mate choice copying, which has been claimed to demonstrate cultural transmission (Galef & White, 1998; Witte & Noltemeier, 2002; Dubois, 2007; Mery et al. 2009) shows that social information participates in the building of sexual preferences in young organisms (Danchin et al. 2004). Consequently, such sexual preferences are socially transmitted across generations (Mesoudi & Lycett, 2008), thus drastically affecting the selection regime on the opposite sex over generations. Similarly, in humans, obesity is a complex trait with many potential causes, including the cultural transmission of diet. There is mounting evidence that the assortative mating among very obese humans increased in parallel with the obesity epidemics, which probably severely increased the offspring predisposition to obesity (Ajslev et al. 2012). The association of culturally transmitted diet and assortative mating may amplify the association between obesity-prone feeding habits and genetic predisposition to obesity, and affect the fate of the corresponding lineage over many generations.
Transgenerational epigenetics
Similarly, we now discover that the very same mechanisms that generate the epigenetic marks that participate in cell differentiation and the fine tuning of the phenotype to the environment are also responsible for transgenerational epigenetic inheritance (Lamm & Jablonka, 2008). For instance, it appears in germ line epigenetic inheritance that germ cells are much more exposed to environmental influences than evolutionary biologists usually claim (Danchin et al. 2011). New cases where germ cells are able to transfer epigenetic states across many generations are regularly documented (Anway et al. 2005; Ashe et al. 2012; Dias & Ressier, 2014; review in Jablonka & Lamb, 2005; Lamm & Jablonka, 2008; Danchin et al. 2011; Daxinger & Whitelaw, 2012).
Furthermore, experience-dependent epigenetic inheritance also demonstrates that epigenetic stages can be reconstructed, and thus transmitted through a variety of processes independent from germ cells (Francis et al. 1999; Champagne, 2008, review in Danchin et al. 2011). Variation in social interactions appears to reconstruct the same variation in gene expression in the next generation in a way that is maintained over many generations (Francis et al. 1999; Champagne, 2008; Curley et al. 2008, 2009).
Another process that affects development but that also generates non-genetic inheritance is genomic imprinting in which the epigenetic marks that are imposed on the chromosomes during male and female gametogenesis are different, and therefore, in the offspring, a gene's expression pattern depends on whether it was inherited from the father or from the mother (Wood & Oakey, 2006; Wilkinson et al. 2007; Hager et al. 2008; Daxinger & Whitelaw, 2012).
When physiology meets evolution
In the previous section, we very briefly provided evidence that inclusive inheritance whether genetic or non-genetic, do bridge physiology to evolution. In particular, the fact that many physiological and developmental mechanisms of accommodation are responsible for the transmission of characters across generations naturally links intra- and intergenerational processes.
The recent arousal of non-genetic inheritance has led to the concept of inclusive heritability (Danchin & Wagner, 2010; Danchin, 2013). This concept is grounded on the quantitative genetics framework, and generalizes narrow and broad sense heritability to quantify the part of phenotypic variation that is genetically or non-genetically transmitted to the next generation. New methods to estimate the relative importance of genetic and non-genetic components of inclusive heritability have been also proposed (Tal et al. 2010; Danchin et al. 2013), establishing the tractability of this concept. These quantifications are aimed at being a first step in establishing the quantitative importance of non-genetic inheritance in physiology and evolution, before dedicated studies can investigate the corresponding inheritance mechanisms.
From a theoretical point of view, non-genetic inheritance could affect the separation drawn by the Modern Synthesis between physiology and evolution in different ways. We wish here to clarify our own position concerning the debates on the modernization of the Modern Synthesis into the Inclusive Evolutionary Synthesis.
The first kind of bridge between physiology and evolution is that physiology determines how the variants function, i.e., physiology is an essential part of the relationship between genotypes, phenotypes and fitness. Should our vision of the bridge between physiology and evolution be limited to this approach, the Modern Synthesis would not be much challenged because physiological and evolutionary questions could still be treated in a decoupled way, as it is the case with the mainstream genocentric view of biology. This would be true even when taking into account non-genetic inheritance.
The second kind of bridge between physiology and evolution considers that physiology is central in determining how variation arises. In effect, physiology determines which phenotypic variants are possible, for instance because of mutational or physiological constraints. Stated like this, a bridge between physiology and evolution is still compatible with the genocentric view of inheritance, as well as with a decoupled view of physiology and evolution (as in the Modern Synthesis). However, the introduction of non-genetic inheritance can have more profound consequences as non-genetic mechanisms seem to be a hub causally linking the physiological history of an organism to the variation inherited by its offspring (see the section on ‘How phenotypic plasticity and inheritance interact’). This could give rise in particular to the heritability of physiological accommodations. The consequence is that the heritable variation produced at each generation may be non-blind relatively to changes in fitness resulting from changes in the environment (for blind variation see Sober, 1984; Merlin, 2010). This second vision of a bridge between physiology and evolution challenges not only the Modern Synthesis, but also the Neo-Darwinian principle of blind variation in general since its inception by Weismann (1891). This point deserves some clarification that we detail now.
A conservative argument can first be raised to save Neo-Darwinism. This argument is that mechanisms of non-genetic inheritance themselves are traits that are selected on evolutionary time scales (Dickins & Rahman, 2012 but see Mesoudi et al. 2013). The goal of this argument is to explain the part of non-genetic inheritance that seems to provide the offspring with adaptive phenotypic variations, and that can thus be considered as adaptive intergenerationally plastic traits (Lachmann & Jablonka, 1996; Haig, 2007; Bonduriansky et al. 2012; Sultan, 2011). Adaptive epigenetic variations would belong to an implicitly encoded repertoire (as could be genetic mutations, Caporale, 2003), enabling to deal with predictively changing environments. Central to this argument is an implicit hypothesis of time scale separation between supposedly fast and quickly changing non-genetic inheritance and apparently slow selective processes. In this conservative view, the slow selective processes are thought to operate only on the long-lasting genetic material, which in turn determines the mechanisms of non-genetic inheritance. Thus, in this conservative view, the non-genetically heritable non-blind variation would itself be explained by selection operating on blind variation occurring on the genetic material.
However, the conservative hypothesis of a clear separation between the time scales of non-genetic inheritance and selection might be much less trivial than usually supposed (Pocheville, 2010). An alternative hypothesis is that non-genetic variation (heritable or not) and genetic variation are coupled on developmental and selective time scales in essential ways. Selection can be faster than usually supposed (over tens of generations or fewer, Carroll et al. 2007), thus taking place within time scales that are commensurate with non-genetic inheritance (Braun & David, 2011; Stern et al. 2012). Furthermore, though supposed to be labile, non-genetic variation can have long-lasting impacts on genetic variation, both at the populational level and at the individual level. At the populational level, non-genetic variation can change the selection pressures perceived by a population. This is the case in models where plasticity enables a population to survive or diversify in a niche, before selection on potential genetic changes canalizes the new phenotype (see the model of genetic accommodation in chapter 6 of West-Eberhard, 2003). In the cultural domain, it has been shown that cultural variation in human populations durably affect the selection regime undergone by many genes within the genome (Laland et al. 2010). At the individual level, non-genetic variation (heritable or not) can also have mutagenic effects with, for example, adaptive regulatory epigenetic marks favouring local hypermutability of the genes they regulate (Wright et al. 1999; Wright, 2000). Whether the potentially induced genetic mutations can be considered as non-blind is debatable (Merlin, 2010), but in any case, the picture of evolution is now multi-scale, with non-genetic and genetic variations interacting at the physiological and evolutionary scales.
Still more radical, a ramification of the hypothesis is that quickly changing heritable non-blind variation (e.g. non-genetic) could not be fully explained by selection operating on the sole long-term blind variation (e.g. genetic). Contrary to the conservative hypothesis of an implicitly encoded repertoire of physiological responses, empirical results suggest that individual organisms can accommodate with previously non-encountered environmental challenges through physiological exploration and stabilization, and then pass on their physiological accommodations to their descendants for tens or hundreds of generations, involving non-genetic as well as genetic variation (Braun & David, 2011; Stern et al. 2012). This ability to adapt-through-accommodation may well have itself evolved through natural selection (though a complementary hypothesis is that the heritability of accommodations may be an exaptation of accommodation), but particular physiological responses would not necessarily be explained in terms of genetic variation.
Such a hypothesis of coupled non-blind and blind variation presents empirical and theoretical challenges that will be further discussed in a dedicated paper. For the moment, let us notice that under such a hypothesis, physiology cannot be black-boxed anymore in evolutionary studies (as is the case in the Modern Synthesis), because physiological responses (whether intra- or intergenerational) become dynamical determinants of evolution.
How phenotypic plasticity and inheritance interact
The emergence of non-genetic inheritance also deeply affects our vision of phenotypic plasticity (Fig. 2). In the Modern Synthesis, plasticity essentially qualifies the part of phenotypic variation that does not entirely result from genetic (i.e. DNA sequence) variation. The actual value of a given trait is determined by the interaction between the genotype and the environment and some random errors during development. In the case of phenotypic plasticity if the environment changes at the next generation the descendants are expected to develop another value of the trait, which is expected to be partly independent of that of the parents (Fig. 2, left side: trait TY in generation n + 1 in environment Y). This allows phenotypes to track environmental changes across generations.
Figure 2. From phenotypic plasticity to inclusive inheritance.

Non-genetic inheritance is affecting the classic vision of phenotypic plasticity. The Y axis quantifies any phenotypic trait, including morphological, physiological and behavioural, including language. The X axis depicts the environment in all its dimensions, including climate, food, safety, as well as competition and the social milieu (social context, potential mates, culture, etc.). See text.
The existence of parental effects, however, has shown for long that this is not necessarily the case, and more generally the emerging field of non-genetic inheritance shows that the trait value of the parents often channels the trait value of their offspring (right side of Fig. 2). Consequently, the trait value at generation n + 1 is influenced in a way that leads it to be closer to that of generation n, and so on across many generations. This may result from two contrasted groups of phenomena.
First, non-genetic inheritance can simply be due to characteristics of the environment being somehow transmitted (or reconstructed) and hence inherited across many generations (right side of Fig. 2). For instance, offspring often inherit the habitat patch of their parents or of some of its major characteristics (e.g. social environment). This is the case with language transmission where the language spoken by the offspring is learnt (i.e. reconstructed) from the parents’ language. In this context, what is inherited is not so much the parental trait than the environment itself (here the social component of it). This is also the case of parental effects, which occur for instance when female birds put antibodies against pathogens they have been confronted to in their eggs’ yolk (Gasparini et al. 2001, 2006). In doing so, they transmit resistance to the pathogen, which is adaptive because pathogens probably persist in the same environmental patch for several generations. Such an inheritance of the developmental environment can also occur in cases of niche construction where the parents modify and stabilize their and their offspring's environment, as is the case, for instance, when a lineage of beavers maintains a dam through generations (Odling Smee et al. 2003). Furthermore, offspring often actively choose habitats with characteristics that tightly match those of their natal habitat. This can result from early in life behavioural imprinting or habitat copying (Danchin et al. 1998; Wagner & Danchin, 2003; Parejo et al. 2005, 2006). In all these situations, the trait values of the parents can be transferred to the offspring independently from any supporting variation in genetic information because the developmental environment is inherited alongside genetic information (Ford & Lerner, 1992; Griffiths & Tabery, 2013). The parental trait could thus be considered either as a feature being inherited or as a developmental environment determining a plastic response in the offspring, which de facto couples heredity and plasticity. This process is at work mostly in cultural and ecological inheritance.
In the second group of phenomena, parents transfer molecules or macromolecular configurations that channel their offspring development in a way that leads to the same trait value TX to be realized in their offspring (Fig. 2) and over many generations. This can result from many processes such as genomic imprinting (Wood & Oakey, 2006; Wilkinson et al. 2007; Hager et al. 2008; Daxinger & Whitelaw, 2012) or in the case of epigenetic inheritance through germ cells (Anway et al. 2005; Daxinger & Whitelaw, 2012; Dias & Ressier, 2014). This group of processes is currently less understood because the study of the underlying mechanisms just became available with the development of high-throughput omics. These processes thus are mostly involved in transgenerational epigenetic inheritance.
In both cases, as with genetic variation, the part of phenotypic variation that is non-genetically inherited participates to heredity and is open to natural selection and evolution.
Towards a new conception of phenotypic plasticity
The classical definition of phenotypic plasticity makes it a highly genocentric concept and supports the mainstream vision that phenotypic variation should be decomposed into a genetic and an environmental components. However, the existence of non-genetic inheritance implies that we should rather decompose phenotypic variation into its transmitted versus non-transmitted components to estimate thoroughly the evolutionary potential of traits (Danchin et al. 2004, 2011, 2013; Mameli, 2004; Danchin & Wagner, 2010; Danchin, 2013). Historically, it is because we had reduced heredity to the DNA sequence that we adopted the current genocentric definition of phenotypic plasticity.
In the emerging inclusive evolutionary synthesis (Danchin, 2013), the concept of phenotypic plasticity becomes a more gradual concept (e.g. fig. 2 in Danchin, 2013) and an extreme alternative definition could be that phenotypic plasticity is the part of phenotypic variation that is not transmitted to the next generation. A more appropriate definition of phenotypic plasticity, however, should acknowledge that it is a multi-scale concept. At the scale of an organism's lifespan it encompasses the non-transmitted part of phenotypic variation. At longer time scales it also encompasses variation resulting from processes of non-genetic inheritance that affect gene expression. This new definition clearly links phenotypic plasticity to heredity.
Thus, in our view, non-genetic inheritance suggests that there is a continuum between intra- and intergenerational plasticity and inheritance: the more stable (e.g. genetic) inheritable determinants of physiology could correspond to the less plastic aspects of physiological responses to the environment, while the less stable (e.g. non-genetic) ones could correspond to more plastic aspects (Danchin, 2013). Furthermore, such a continuum could go beyond mere analogy, between physiological accommodation and adaptation by natural selection, as it appears that the same mechanisms can be recruited at the individual level for phenotypic accommodation and at the populational level for adaptation through natural selection. In such a new vision of evolutionary mechanisms, the limits of phenotypic plasticity would no longer be clearly defined, so that this concept would need to evolve further.
Conclusion
The main point here is that the emergence of non-genetic inheritance is providing a unique way of bridging physiology and evolution. This is because physiology can now percolate into non-blind heritable variation on which natural selection can act, and because fast processes occurring on the physiological time scale can have long-lasting impacts on the evolutionary time scale, such as in the case of gene–culture coevolution. Such a bridge between physiology and evolution remained quasi impossible as long as we persisted in reducing inheritance to its sole genetic dimension. This is because the Neo-Darwinian principle of blind variation decouples the physiological events from the generation of the heritable variation tracked on the evolutionary time scale, and because this evolutionary time scale is supposed only to concern stable, long-lasting entities, i.e. the genetic material. Non-blindness and time coupling are logically (though not necessarily physically) independent features that could each lead to major modifications of Neo-Darwinism and the Modern Synthesis.
The permeability of the frontier between physiology and evolution results from the fact that development and inheritance largely rest on the same mechanisms, both linked to the accommodation and adaptation, and diversification of the phenotype. The fact that developmental and selective processes are supposed to occur on different time scales should not obliterate the possibility for these time scales to be coupled.
The overlap between developmental and inheritance mechanisms also shatters other frontiers between various domains of biology usually considered as separate domains as many processes such as maternal effect appear to be largely mediated by epigenetic changes, for instance in the form of genomic imprinting (Bjorklund, 2006; Hager et al. 2008). The same reasoning holds for learning and culture where processes of long-term memory appear to be mediated by DNA methylation (Miller & Sweatt, 2007). Eventually, such shattering of frontiers between concepts and disciplines may result in the emergence of a kind of ‘intergenerational physiology’ approach in which non-genetic inheritance would play a pivotal role.
Here we thus proposed that the analogy between accommodation-through-phenotypic-plasticity and adaptation-through-natural-selection goes beyond mere analogy. The large overlap between inheritance and developmental mechanisms suggest that this analogy in fact reaches the level of a homology. There is no a priori reason why nature could not recycle mechanisms that enable inheritance and diversification of cells during development for evolutionary purposes, and inversely, plasticity at the level of the organism could result from mechanisms enabling evolvability. As a consequence, evolvability can well be an exaptation of plasticity, and vice versa, so that inheritance is where physiology meets evolution.
Glossary
Accommodation: the process of meeting environmental demands through plastic physiological responses.
Adaptation by natural selection: in this paper, we gloss over a plethora of literature and use the term adaptation to mean the transgenerational process of a population meeting environmental demands through natural selection (Darwin, 1859; Endler, 1986).
Development: the process of change of an organism through its lifespan. This inclusive definition includes growth, ageing and physiological responses.
Evolution: the process by which the frequencies of variants vary across generations.
Evolvability: ‘the ability to evolve’ (see Pigliucci, 2008) or ‘the ability to produce hereditary innovations’ (Lamm & Jablonka, 2008). These inclusive definitions depart from genocentric definitions of evolvability such as ‘the genome's ability to produce adaptive variants when acted upon by the genetic system’ (Wagner & Altenberg, 1996; Wagner, 2005).
Genetic (inheritance): the inheritance of the information that is encoded in the DNA sequence, i.e. the DNA's primary structure (Crick, 1970).
Heredity: in this paper, we use the term heredity to depict the pattern of parent offspring resemblance (Bonduriansky, 2012).
Inclusive Evolutionary Synthesis: a heredity centred version of the ‘Extended Synthesis’ of (Pigliucci & Muller, 2010). Stresses the necessity to incorporate all mechanisms of development into inheritance as a bridge between physiology and evolution.
Inclusive heritability: the heredity of differences, whatever the mechanism of transmission. It generalizes narrow and broad sense heritability to quantify the part of phenotypic variation that is genetically or non-genetically transmitted to the next generation (Danchin & Wagner, 2010; Danchin et al. 2011).
Inclusive inheritance: a broadened vision of inheritance that incorporates all processes of inheritance, whether genetic or non-genetic.
Inheritance: in this paper, we use the term inheritance to designate the processes of transmission underlying heredity (Danchin et al. 2011).
Modern Synthesis: research programme that emerged from the merging of population and Mendelian genetics in the 1930s (Huxley, 1942; Mayr & Provine, 1998).
Neo-Darwinism: the modification of the Darwinian theory rooted in Weismann's rejection of the inheritance of acquired characters (Weismann, 1891; Romanes, 1988).
Non-genetic inheritance: the inheritance of information other than the information encoded in the DNA sequence. Includes the inheritance of epigenetic marks, RNA-mediated inheritance, as well as cultural and ecological inheritance (Danchin & Wagner, 2010; Danchin et al. 2011).
Physiology: designates the mechanisms that govern the internal functioning of individual organisms in their environment. Because we do not restrict physiological responses to occur on a particular time scale (be they intra- or intergenerational), we use the term equivalently with development.
Plasticity: potential intra-individual variation. Plasticity may be active or passive, adaptive or non-adaptive, reversible or irreversible, continuous or discontinuous (see pp. 34–36 West-Eberhard, 2003).
Trangenerational epigenetic inheritance: the molecular processes by which genes expressions can be modified and inherited across generations of unicellular or multicellular organisms (adapted from Bateson & Gluckman, 2011).
Acknowledgments
We thank Antonine Nicoglou, Sébastien Dutreuil, Philippe Huneman, Francesca Merlin and two anonymous referees whose comments enabled to greatly improve earlier versions of the manuscript. Stimulating discussions with Benoit Pujol greatly helped crystallizing these ideas.
Additional information
Competing interests
The authors declare no competing interests.
Funding
This work was supported by the French Laboratory of Excellence project ‘TULIP’ (ANR-10-LABX-41; ANR-11-375 IDEX-0002-02) to E.D. A.P. benefits from a postdoctoral fellowship from the Center for Philosophy of Science, University of Pittsburgh.
References
- Ajslev TA, Ängquist L, Silventoinen K, Gamborg M, Allison DB, Baker JL, Sørensen TIA. Assortative marriages by body mass index have increased simultaneously with the obesity epidemic. Front Genet. 2012;3:125. doi: 10.3389/fgene.2012.00125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anway MD, Cupp AS, Uzumcu M, Skinner MK. Epigenetic transgenerational actions of endocrine disruptors and male fertility. Science. 2005;308:1466–1469. doi: 10.1126/science.1108190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashe A, Sapetschnig A, Weick E-M, Mitchell J, Bagijn MP, Cording AC, Doebley A-L, Goldstein LD, Lehrbach NJ, Le Pen J, Pintacuda G, Sakaguchi A, Sarkies P, Ahmed S, Miska EA. piRNAs can trigger a multigenerational epigenetic memory in the germline of C. elegans. Cell. 2012;150:88–99. doi: 10.1016/j.cell.2012.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avital E, Jablonka E. Animal Traditions. Behavioural Inheritance in Evolution. Cambridge: Cambridge University Press; 2000. [DOI] [PubMed] [Google Scholar]
- Bateson PPG, Gluckman P. Plasticity, Robustness, Development and Evolution. New York, NY: Cambridge University Press; 2011. [Google Scholar]
- Bjorklund DF. Mother knows best: Epigenetic inheritance, maternal effects, and the evolution of human intelligence. Develop Rev. 2006;26:213–242. [Google Scholar]
- Bonduriansky R. Rethinking heredity, again. Trends Ecol Evol. 2012;27:330–336. doi: 10.1016/j.tree.2012.02.003. [DOI] [PubMed] [Google Scholar]
- Bonduriansky R, Crean AJ, Day T. The implications of nongenetic inheritance for evolution in changing environments. Evol Appl. 2012;5:192–201. doi: 10.1111/j.1752-4571.2011.00213.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyd R, Richerson PJ. Why is culture adaptive? Q Rev Biol. 1983;58:209–214. [Google Scholar]
- Brakefield PM, Wijngaarden PJ. Phenotypic plasticity. In: Hall BK, Olson WM, editors. Keywords and Concepts in Evolutionary Developmental Biology. Cambridge, MA: Harvard University Press; 2006. pp. 288–297. [Google Scholar]
- Braun E, David L. The role of cellular plasticity in the evolution of regulatory novelty. In: Gissis SB, Jablonka E, editors. Transformations of Lamarckism From Fluids to Molecular Biology. Cambridge, MA: The MIT Press; 2011. [Google Scholar]
- Caporale LH. Natural selection and the emergence of a mutation phenotype: An update of the evolutionary synthesis considering mechanisms that affect genome variation. Annu Rev Microbiol. 2003;57:467–485. doi: 10.1146/annurev.micro.57.030502.090855. [DOI] [PubMed] [Google Scholar]
- Carroll SP, Hendry AP, Reznick DN, Fox CW. Evolution on ecological time-scales. Funct Ecol. 2007;3:387–393. [Google Scholar]
- Cavalli-Sforza LL, Feldman MW. Cultural Transmission and Evolution: a quantitative approach. Princeton NJ: Princeton University Press; 1981. [PubMed] [Google Scholar]
- Cavalli-Sforza LL, Feldman MW. Cultural versus genetic adaptation. Proc Natl Acad Sci U S A. 1983;80:4993–4996. doi: 10.1073/pnas.80.16.4993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Champagne FA. Epigenetic mechanisms and the transgenerational effects of maternal care. Front Neuroendocrinol. 2008;29:386–397. doi: 10.1016/j.yfrne.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crick FHC. Symposium of the Society for Experimental Biology XII. New York: Academic Press; 1958. On protein synthesis; pp. 138–163. [Google Scholar]
- Crick FHC. Central dogma of molecular biology. Nature. 1970;227:561–563. doi: 10.1038/227561a0. [DOI] [PubMed] [Google Scholar]
- Curley JP, Champagne FA, Bateson P, Keverne EB. Transgenerational effects of impaired maternal care on behaviour of offspring and grandoffspring. Anim Behav. 2008;75:1551–1561. [Google Scholar]
- Curley JP, Davidson S, Bateson P, Champagne FA. Social enrichment during postnatal development induces transgenerational effects on emotional and reproductive behaviour in mice. Front Behav Neurosci. 2009;3:1–14. doi: 10.3389/neuro.08.025.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danchin E. Avatars of information: towards an inclusive evolutionary synthesis. Trends Ecol Evol. 2013;28:351–358. doi: 10.1016/j.tree.2013.02.010. [DOI] [PubMed] [Google Scholar]
- Danchin É, Wagner RH. Inclusive heritability: combining genetic and nongenetic information to study animal behaviour and culture. Oikos. 2010;119:210–218. [Google Scholar]
- Danchin É, Boulinier T, Massot M. Conspecific reproductive success and breeding habitat selection: implications for the study of coloniality. Ecology. 1998;79:2415–2428. [Google Scholar]
- Danchin É, Giraldeau LA, Valone TJ, Wagner RH. Public information: from nosy neighbors to cultural evolution. Science. 2004;305:487–491. doi: 10.1126/science.1098254. [DOI] [PubMed] [Google Scholar]
- Danchin É, Blanchet S, Mery F, Wagner RH. Do invertebrates have culture? Commun Integr Biol. 2010;3:303–305. doi: 10.4161/cib.3.4.11970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danchin É, Charmantier A, Champagne FA, Mesoudi A, Pujol B, Blanchet S. Beyond DNA: integrating inclusive inheritance into an extended theory of evolution. Nat Rev Genet. 2011;12:475–486. doi: 10.1038/nrg3028. [DOI] [PubMed] [Google Scholar]
- Danchin E, Pujol B, Wagner RH. The double pedigree: a method for studying culturally and genetically inherited behaviour in tandem. PLoS One. 2013;8:e61254. doi: 10.1371/journal.pone.0061254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darwin C. On the Origin of Species by Means of Natural Selection. London: John Murray; 1859. [Google Scholar]
- Daxinger L, Whitelaw E. Understanding transgenerational epigenetic inheritance via the gametes in mammals. Nat Rev Genet. 2012;13:153–162. doi: 10.1038/nrg3188. [DOI] [PubMed] [Google Scholar]
- Dias BG, Ressier KJ. Parental olfactory experience influences behaviour and neural structure in subsequent generations. Nat Neurosci. 2014;17:89–96. doi: 10.1038/nn.3594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickins TE, Rahman Q. The extended evolutionary synthesis and the role of soft inheritance in evolution. Proc R Soc Lond B Biol Sci. 2012 doi: 10.1098/rspb.2012.0273. doi: 10.1098/rspb.2012.0273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubois F. Mate choice copying in monogamous species: should females use public information to choose extrapair mates? Anim Behav. 2007;74:1785–1793. [Google Scholar]
- Endler JA. Natural Selection in the Wild. Princeton University Press; 1986. [Google Scholar]
- Feldman MW, Cavalli-Sforza LL. Cultural and biological evolutionary processes: gene-culture disequilibrium. Proc Natl Acad Sci U S A. 1984;81:1604–1607. doi: 10.1073/pnas.81.5.1604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fellous S, Duron O, Rousset F. Adaptation due to symbionts and conflicts between heritable agents of biological information. Nat Rev Genet. 2011;12:663–663. doi: 10.1038/nrg3028-c1. [DOI] [PubMed] [Google Scholar]
- Ford DH, Lerner RM. Developmental Systems Theory: An Integrative Approach. Newbury Park, CA: Sage Publications; 1992. [Google Scholar]
- Francis D, Diorio J, Liu D, Meaney MJ. Nongenomic transmission across generations of maternal behaviour and stress responses in the rat. Science. 1999;286:1155–1158. doi: 10.1126/science.286.5442.1155. [DOI] [PubMed] [Google Scholar]
- Galef BG, White DJ. Mate-choice copying in Japanese quail, Coturnix coturnix japonica. Anim Behav. 1998;55:545–552. doi: 10.1006/anbe.1997.0616. [DOI] [PubMed] [Google Scholar]
- Gasparini J, McCoy KD, Haussy C, Tveraa T, Boulinier T. Induced maternal response to the Lyme disease spirochaete Borrelia burgdorferi senus lato in a colonial seabird, the kittiwake, Rissa tridactyla. Proc R Soc Lond B Biol Sci. 2001;268:647–650. doi: 10.1098/rspb.2000.1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasparini J, McCoy K, Staszewski V, Haussy C, Boulinier T. Dynamics of anti-borrelia antibodies in Black-legged Kittiwakes (Rissa tridactyla) chicks suggest a maternal educational effect. Can J Zool. 2006;84:623–627. [Google Scholar]
- Gilbert SF. Evo-Devo, Devo-Evo, and Devgen-Popgen. Biol Philos. 2003;18:347–352. [Google Scholar]
- Griffiths PE, Tabery J. Developmental systems theory: What does it explain, and how does it explain it? Adv Child Dev Behav. 2013;44:65–94. doi: 10.1016/b978-0-12-397947-6.00003-9. [DOI] [PubMed] [Google Scholar]
- Hager R, Cheverud JM, Wolf JB. Maternal effects as the cause of parent-of-origin effects that mimic genomic imprinting. Genetics. 2008;178:1755–1762. doi: 10.1534/genetics.107.080697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haig D. Weismann Rules! OK? Epigenetics and the Lamarckian temptation. Biol Philos. 2007;22:415–428. [Google Scholar]
- Halfmann R, Lindquist S. Epigenetics in the extreme: Prions and the inheritance of environmentally acquired traits. Science. 2010;330:629–632. doi: 10.1126/science.1191081. [DOI] [PubMed] [Google Scholar]
- Henderson IR, Jacobsen SE. Epigenetic inheritance in plants. Nature. 2007;447:418–424. doi: 10.1038/nature05917. [DOI] [PubMed] [Google Scholar]
- Huxley JS. Evolution: The Modern Synthesis. London: Allen and Unwin; 1942. [Google Scholar]
- Jablonka E, Lamb MJ. Evolution in Four Dimensions. Genetic, Epigenetic, Behavioural, and Symbolic Variation in the History of Life. Cambridge, MA: MIT Press; 2005. [Google Scholar]
- Jablonka E, Lamb MJ. Transgenerational epigenetic inheritance. In: Pigliucci M, Müller GB, editors. Evolution: The extended synthesis. Cambridge, MA: MIT Press; 2010. pp. 137–174. [Google Scholar]
- Jablonka E, Raz G. Transgenerational epigenetic inheritance: Prevalence, mechanisms, and implications for the study of heredity and evolution. Q Rev Biol. 2009;84:131–176. doi: 10.1086/598822. [DOI] [PubMed] [Google Scholar]
- Jablonka E, Lamb MJ, Avital E. ‘Lamarckian’ mechanisms in darwinian evolution. Trends Ecol Evol. 1998;13:206–210. doi: 10.1016/S0169-5347(98)01344-5. [DOI] [PubMed] [Google Scholar]
- Johannsen W. The genotype conception of heredity. Am Nat. 1911;45:129–159. doi: 10.1093/ije/dyu063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruetzen M, Willems EP, van Schaik CP. Culture and geographic variation in orangutan behavior. Curr Biol. 2011;21:1808–1812. doi: 10.1016/j.cub.2011.09.017. [DOI] [PubMed] [Google Scholar]
- Lachmann M, Jablonka E. The inheritance of phenotypes: An adaptation to fluctuating environments. J Theor Biol. 1996;181:1–9. doi: 10.1006/jtbi.1996.0109. [DOI] [PubMed] [Google Scholar]
- Laland KN. Sexual selection with a culturally transmitted mating preference. Theor Pop Biol. 1994;45:1–15. doi: 10.1006/tpbi.1994.1001. [DOI] [PubMed] [Google Scholar]
- Laland KN, Odling-Smee J, Myles S. How culture shaped the human genome: Bringing genetics and the human sciences together. Nat Rev Genet. 2010;11:137–148. doi: 10.1038/nrg2734. [DOI] [PubMed] [Google Scholar]
- Lamm E, Jablonka E. The nurture of nature: hereditary plasticity in evolution. Philos Psychol. 2008;21:305–319. [Google Scholar]
- Lunsden CJ, Wilson EO. Genes, Mind and Culture. Cambridge, MA: Harvard University Press; 1981. [Google Scholar]
- Mameli M. Nongenetic selection and nongenetic inheritance. Br J Philos Sci. 2004;55:35–71. [Google Scholar]
- Mann J, Stanton MA, Patterson EM, Bienenstock EJ, Singh LO. Social networks reveal cultural behaviour in tool-using using dolphins. Nature Commun. 2012;3:980. doi: 10.1038/ncomms1983. [DOI] [PubMed] [Google Scholar]
- Mayr E, Provine WB. The Evolutionary Synthesis: Perspectives on the Unification of Biology. Cambridge, MA: Harvard University Press; 1998. [Google Scholar]
- Merlin F. Evolutionary chance mutation: A defense of the Modern Synthesis’ consensus view. Philos Theor Biol. 2010;2:e103. [Google Scholar]
- Mery F, Varela SAM, Danchin É, Blanchet S, Parejo D, Coolen I, Wagner RH. Public versus personal information for mate copying in an invertebrate. Curr Biol. 2009;19:730–734. doi: 10.1016/j.cub.2009.02.064. [DOI] [PubMed] [Google Scholar]
- Mesoudi A, Lycett SJ. Random copying, frequency-dependent copying and culture change. 2008;30:41–48. [Google Scholar]
- Mesoudi A, Blanchet S, Charmantier A, Danchin E, Fogarty L, Jablonka E, Laland KN, Morgan TJH, Müller GB, Odling-Smee FJ, Pujol B. Is non-genetic inheritance just a proximate mechanism? Justifying the Extended Evolutionary Synthesis. Biol Theor. 2013 doi: 10.1007/s13752-13013-10091-13755. [Google Scholar]
- Miller CA, Sweatt JD. Covalent modification of DNA regulates memory formation. Neuron. 2007;53:857–869. doi: 10.1016/j.neuron.2007.02.022. [DOI] [PubMed] [Google Scholar]
- Nicoglou A. La Plasticité Du Vivant: Histoire D'un Concept et Enjeux Pour La Biologie. Paris: Université Paris 1 – Panthéon Sorbonne; 2013. [Google Scholar]
- Odling-Smee JF. Niche-constructing phenotypes. In: Plotkin HC, editor. The Role of Behaviour in Evolution. Cambridge, MA: MIT Press; 1988. [Google Scholar]
- Odling-Smee J, Laland KN. Ecological inheritance and cultural inheritance: What are they and how do they differ? Biol Theor. 2011;6:220–230. [Google Scholar]
- Odling Smee FJ, Laland KN, Feldman M. Niche Construction. Princeton, NJ: Princeton University Press; 2003. [Google Scholar]
- Parejo D, Danchin É, Avilés J. The heterospecific habitat copying hypothesis: can competitors indicate habitat quality. Behav Ecol. 2005;16:96–105. [Google Scholar]
- Parejo D, Oro D, Danchin É. Testing habitat copying in breeding habitat selection in a species adapted to variable environments. Ibis. 2006;148:146–154. [Google Scholar]
- Pigliucci M. Is evolvability evolvable. Nat Rev Genet. 2008;1:75–82. doi: 10.1038/nrg2278. [DOI] [PubMed] [Google Scholar]
- Pigliucci M, Muller GB. Evolution, the Extended Synthesis. Cambridge, MA: MIT Press; 2010. [Google Scholar]
- Pocheville A. La Niche Ecologique: Concepts, Modèles, Applications. 2010. Ecole Normale Supérieure Paris. http://hal.upmc.fr/tel-00715471/, Paris.
- Romanes GJ. Lamarckism versus Darwinism. Nature. 1988;38:413–413. [Google Scholar]
- Saibil H. Chaperone machines for protein folding, unfolding and disaggregation. Nat Rev Mol Cell Biol. 2013;14:630–642. doi: 10.1038/nrm3658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapp J. Beyond the Gene: cytoplasmic inheritance and the struggle for authority in genetics. New York, NY: Oxford University Press; 1987. [Google Scholar]
- Shorter J, Lindquist S. Prions as adaptive conduits of memory and inheritance. Nat Rev Genet. 2005;6:435–450. doi: 10.1038/nrg1616. [DOI] [PubMed] [Google Scholar]
- Slagsvold T, Wiebe KL. Social learning in birds and its role in shaping a foraging niche. Philos Trans R Soc Lond B. 2011;366:969–977. doi: 10.1098/rstb.2010.0343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sober E. The Nature of Selection: Evolutionary Theory in Philosophical Focus. London: University of Chicago Press; 1984. [Google Scholar]
- Stern S, Fridmann-Sirkis Y, Braun E, Soen Y. Epigenetically heritable alteration of fly development in response to toxic challenge. Cell Rep. 2012;1:5286542. doi: 10.1016/j.celrep.2012.03.012. [DOI] [PubMed] [Google Scholar]
- Sultan SE. Evolutionary implications of individual plasticity. In: Gissis SB, Jablonka E, editors. Transformations of Lamarckism: From Subtle Fluids to Molecular Biology. Cambridge, MA: MIT Press; 2011. [Google Scholar]
- Tal O, Kisdi E, Jablonka E. Epigenetic contribution to covariance between relatives. Genetics. 2010;184:1037–1050. doi: 10.1534/genetics.109.112466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thornton A, Samson J, Clutton-Brock T. Multi-generational persistence of traditions in neighbouring meerkat groups. Proc R Soc Lond B Biol Sci. 2010;277:3623–3629. doi: 10.1098/rspb.2010.0611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner A. Robustness, evolvability, and neutrality. FEBS Letters. 2005;579:1772–1778. doi: 10.1016/j.febslet.2005.01.063. [DOI] [PubMed] [Google Scholar]
- Wagner GP, Altenberg L. Perspective: Complex adaptations and the evolution of evolvability. Evolution. 1996;50:967–976. doi: 10.1111/j.1558-5646.1996.tb02339.x. [DOI] [PubMed] [Google Scholar]
- Wagner RH, Danchin É. Conspecific copying: a general mechanism of social aggregation. Behav Ecol. 2003;65:405–408. [Google Scholar]
- Weismann A. Essays upon Heredity and Kindred Biological Problems. Vol. 1. Oxford: Clarendon Press; 1891. [Google Scholar]
- West-Eberhard MJ. Developmental Plasticity and Evolution. Oxford: Oxford University Press; 2003. [Google Scholar]
- Wilkinson LS, Davies W, Isles AR. Genomic imprinting effects on brain development and function. Nat Rev Neurosci. 2007;8:832–843. doi: 10.1038/nrn2235. [DOI] [PubMed] [Google Scholar]
- Witte K, Noltemeier B. The role of information in mate-choice copying in female sailfin mollies (Poecilia latipinna. Behav Ecol Sociobiol. 2002;52:194–202. [Google Scholar]
- Wood AJ, Oakey RJ. Genomic imprinting in mammals: Emerging themes and established theories. Plos Genet. 2006;2:1677–1685. doi: 10.1371/journal.pgen.0020147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright BE. A biochemical mechanism for nonrandom mutations and evolution. J Bacteriol. 2000;182:2993–3001. doi: 10.1128/jb.182.11.2993-3001.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright BE, Longacre A, Reimers JM. Hypermutation in derepressed operons of Escherichia coli K12. Proc Natl Acad Sci U S A. 1999;96:5098–5094. doi: 10.1073/pnas.96.9.5089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeh JA, Zeh DW. Maternal inheritance, epigenetics and the evolution of polyandry. Genetica. 2008;134:45–54. doi: 10.1007/s10709-007-9192-z. [DOI] [PubMed] [Google Scholar]


