Abstract
Many forms of developmental plasticity have been observed and these are usually beneficial to the organism. The Predictive Adaptive Response (PAR) hypothesis refers to a form of developmental plasticity in which cues received in early life influence the development of a phenotype that is normally adapted to the environmental conditions of later life. When the predicted and actual environments differ, the mismatch between the individual's phenotype and the conditions in which it finds itself can have adverse consequences for Darwinian fitness and, later, for health. Numerous examples exist of the long-term effects of cues indicating a threatening environment affecting the subsequent phenotype of the individual organism. Other examples consist of the long-term effects of variations in environment within a normal range, particularly in the individual's nutritional environment. In mammals the cues to developing offspring are often provided by the mother's plane of nutrition, her body composition or stress levels. This hypothetical effect in humans is thought to be important by some scientists and controversial by others. In resolving the conflict, distinctions should be drawn between PARs induced by normative variations in the developmental environment and the ill effects on development of extremes in environment such as a very poor or very rich nutritional environment. Tests to distinguish between different developmental processes impacting on adult characteristics are proposed. Many of the mechanisms underlying developmental plasticity involve molecular epigenetic processes, and their elucidation in the context of PARs and more widely has implications for the revision of classical evolutionary theory.
 |
Professor Mark Hanson is the founding Director of the Institute of Developmental Sciences at the University of Southampton, Director of the Academic Unit of Human Development and Health in the University's Faculty of Medicine and British Heart Foundation Professor of Cardiovascular Science. Mark's research concerns several aspects of development and health, ranging from how the environment during our development (before and after birth) can affect the risk of chronic diseases – such as hypertension, heart disease, diabetes and obesity – to population studies aimed at the early identification of risk, so that timely preventative interventions can be made. The group are exploring the epigenetic processes which relate to such risks, and which may serve as valuable early life biomarkers. His Unit works on these problems in both developed and developing countries in many parts of the world. Mark has pioneered a hospital research lab based education programme for adolescents, LifeLab, in Southampton. Mark is also much involved in the wider public understanding of science through public lectures and popular science books. His recent books include Mismatch – the lifestyle diseases timebomb (2006), Principles of Evolutionary Medicine (2009) and Fat, Fate and Disease (2012) all published by OUP.
Introduction
The variation in structure, physiology and behaviour found between individuals of most, if not all, species commonly arises because each individual has the capacity to respond in more than one way according to the state of its past and present environments. Phenotypic variation generally arises during development because the processes of plasticity provide useful adaptations to the environment. Extreme or novel insults may, however, disrupt development to produce non-adaptive outcomes with which the individual must cope if it is to survive (Bateson et al. 2004; Gluckman et al. 2005; Ghalambor et al. 2007).
Adaptive developmental plasticity provides a means by which an organism can respond to environmental circumstances without undergoing a change in its genome. Many different mechanisms underlie such plasticity (Bateson & Gluckman, 2011) but often involve epigenetic changes in the expression of DNA (Godfrey et al. 2007). Research in this area has generally been in three domains: understanding the mechanisms leading to phenotypic variation, understanding their relevance to human health and understanding their role in evolutionary processes. In this paper we briefly review these domains.
The implication of many of the examples of adaptive developmental plasticity is that environmental induction provides a forecast about the future conditions of the external world that the individual will subsequently inhabit (Bateson, 2001). In mammals the best route for such a forecast may be via the mother. For example, vole pups (Microtus pennsylvanius) born in the autumn have much thicker coats than those born in spring; the cue to produce a thicker coat is provided by hormonal signals from the mother before birth, depending on day length (Lee & Zucker, 1988). The potential benefit of doing so, enabling the autumn-born voles to survive the winter better than they would with thinner coats, was termed the Predictive Adaptive Response (PAR) by Gluckman & Hanson (2004).
The PAR hypothesis was originally developed to explain why early life events are associated with an increased disease risk, especially non-communicable disease (NCD) in contemporary human populations (Bateson et al. 2004; Gluckman & Hanson, 2005). A range of epidemiological observations had found an association between low birth weight and greater risk of metabolic and cardiovascular diseases in later life. The suggestion was that developmental plasticity leads to a postnatal phenotype predicted by the conditions of early life and that a mismatch between prediction and subsequent reality leads to later health problems, for example in those human societies where economic circumstances and nutrition are rapidly improving. It is important to note that associations between early life events and later disease risk were found later to be independent of birth weight and occurred within the normative range of birth sizes and prenatal exposures (Gale et al. 2006; Godfrey et al. 2010).
Subsequent debate has been about the plausibility of such adaptive plasticity evolving in the first place. This debate has been complicated by the realization that many factors other than simply those reflecting a poor developmental environment, such as over-nutrition, maternal obesity, gestational diabetes or infant overfeeding, also lead to increased risk of later NCD in the offspring. These adverse outcomes are usually the products of recent Westernised lifestyles and are thus not relevant to an evolutionary argument. A further complication is that, if the nutritional plane in early life is very low, developmental disruption or immediate adaptive responses leading to intrauterine growth retardation (Gluckman & Hanson, 2005) may occur and, at best, the offspring is forced to ‘cope’, by trading off short-term survival against future reproductive success (see Fig. 1). Both immediate and predictive adaptive responses can coexist (Gluckman et al. 2010), and this can explain the association found experimentally and clinically between reduced birth size and later metabolic and cardiovascular compromise.
Figure 1. Relationship between nutritional plane and Darwinian fitness as an adult.
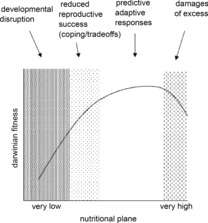
The diagram shows the proposed relationship between the environment (e.g. nutritional plane) to which the developing organism is exposed during the phase of developmental plasticity and the consequences for its Darwinian fitness in adult life. The relationship is determined by different mechanisms according to the degree of shift in the environmental state from the optimal, as suggested by the shaded columns. (From Gluckman et al. 2005.)
In terms of their biological functions, PARs are hypothesised to anticipate a future environmental condition that can vary over time. By doing so they increase the likelihood that the organism's phenotype will match the environmental conditions it encounters in its lifecourse up to reproduction (see Fig. 2). The delay between induction and phenotypic response implies that environmental cues during development provide a forecast about the future conditions of the external world that the individual will subsequently inhabit (Bateson, 2001; Gluckman et al. 2005). The induced phenotypic states may be dichotomous in some species and for some traits, as shown in the Fig. 2, or they may be graded from one extreme to the other and produce a continuous reaction norm.
Figure 2. A given genotype may give rise to different phenotypes depending on the state of the environment early in development.
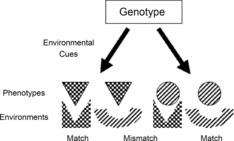
Cues from the environment may be used as predictors, determining which of a set of alternative developmental pathways is elicited. If the environment does not change, then the organism's phenotype will be well adapted to that environment, providing a close match, as is represented in the diagram by the pattern and shape of the phenotype and the pattern and shape of the environment. However, if the environment does change between the elicitation of the particular pattern and development, then the phenotype may be mismatched to the conditions of adult life. (From Bateson 2007.)
Clarifications
It must be emphasised that from an evolutionary point of view PARs are primarily seen as advantageous to survival until reproductive age, and for reproduction itself. Subsequent beneficial or deleterious effects, for example on health in the post-reproductive period, are not relevant to the individual's Darwinian fitness. Before considering possible instances of PARs, however, some clarification of terms and concepts is needed. Fundamental to this is the term ‘adaptation’, which is used in at least two different ways. The meanings, most commonly used in the ecological and evolutionary literature and relevant to the present discussion, are nonetheless often conflated. The first refers to a phenotypic characteristic that is well suited to meeting a requirement set by the environment and is measured by the effect of the characteristic on the organism's survival and reproductive success – its overall Darwinian fitness. The second meaning denotes the historical process by which the current state is achieved. This might be the evolutionary process of Darwinian selection acting on a trait's effectiveness in a particular context. Alternatively, or in addition, it might be the individual acquiring that trait during its own lifetime through plastic processes. The adjective ‘adaptive’ can apply to a particular adaptation, defined as a state, and ‘adaptability’ to describe the capacity of an individual to meet a new challenge set by the environment.
Tinbergen (1963) pointed out that biological mechanisms need not have the same effect on fitness in the present as they did when they first evolved see (Bateson & Laland, 2013). This is particularly likely where rapid environmental change has occurred, as has been the case with human populations, and it creates difficulties when interpreting human medical phenomena in a functional or evolutionary context (Gluckman et al. 2009). A problem for any study of current utility of a mechanism found in humans, therefore, is that modern Westernised human societies buffer offspring against the fitness costs that probably occurred in the past. Furthermore the nutritional environments of today, rich in carbohydrates and fats, are very recent in evolutionary terms. Hence an adaptation, which had potential fitness in the early decades of life in the past, nowadays can have adverse health consequences in later life.
Responses to dangerous environments
The benefits of a match between predicted and actual environments vary from case to case. East African Acridoid grasshoppers, usually green, develop into a black form after a savannah fire that blackens the environment (Rowell, 1972). Their developmental plasticity enables them to develop a camouflage that reduces the risk of being eaten by birds. In the small freshwater crustacean, Daphnia pulex, the benefits of the two alternative phenotypes are, for one, increased chances of survival and, for the other, increased reproductive success. When a predatory midge is present in the environment the young develop a spiked helmet and long pointed tail while still enclosed within the mother. The armour reduces the risks of predation. If the predators disappear before young are released from their mother's protection, the female offspring bear the cost of developing the armour and have lowered reproductive success relative to those without it (Laforsch et al. 2006). In somewhat similar cases, the larvae of the ringed salamander (Ambystoma annulatum), if exposed as an egg to the chemical cues of a predator's presence, show higher levels of shelter-seeking behaviour than individuals who were not so exposed (Mathis et al. 2008) and the crucian carp (Carassius carassius) develops a thickened body when predatory pike (Esox lucius) exist in the lake it inhabits, making it harder to catch (Brönmark et al. 1999).
In the domesticated rat (Rattus norvegicus) the role of the mother in affecting development of her pups by licking and grooming them has been studied extensively (Claessens et al. 2011). Mothers who are stressed groom their offspring less and have offspring that subsequently become more anxious as adults than those of high-grooming mothers. The induced behaviour is appropriate if the environment remains threatening when they are adults. Analogous responses may be seen in the wild: Belding's ground squirrels (Urocitellus beldingi) living in open grassland habitats develop into less fearful adults than those living in closed woodland habitats where the dangers of predation are higher (Mateo, 2007). The difference in this case might have arisen from Darwinian selection but similar striking differences are found within arctic species such as the lemming (Lemmus lemmus) and snowshoe hare (Lepus americanus), in which the induced phenotypes vary cyclically over generations and are likely to arise from developmental plasticity. In the hares, when the risk of predation is high the mother gives birth to offspring with a high level of fearfulness, which is thought to make them more alert and better able to escape from predators such as the lynx (Lynx Canadensis; Sheriff et al. 2009). The response is not perfect and high mortality leads to a decline in the hare population, quickly followed by a similar decline in the population of the predator species. Over the ensuing generations of hares, the threat of predation is low and offspring are less vigilant. Some carry-over from the grandmothers’ stressed state occurs (Sheriff et al. 2010), but eventually the offspring predict a safe environment and thus have a lower flight response setting when they are independent of their parents. When the predator population increases once again, the pregnant hares face increased stress and signal this danger to their fetuses, which develop a higher vigilance level. And so the cycle continues.
Maternal responses to aspects of the environment may act to integrate and coordinate responses across a range of body systems of their developing young (Del Giudice et al. 2011). For example, in the North American red squirrel (Tamiasciurus hudsonicus), maternal glucocorticoid levels are raised by high population density that affects their unborn offspring. When high population densities existed, or were simulated by playbacks of territorial calls from animals in high population settings, the offspring subsequently grew more rapidly than when the real or perceived population density was low (Dantzer et al. 2013). In many experimental models in which maternal nutrition is reduced or maternal glucocorticoids are elevated, the phenotype induced in the offspring involves their musculature, adiposity, insulin resistance, renal function, vascular function, neuroendocrine function and behaviour (McMillen & Robinson, 2005).
In a study of 22 small-scale non-Westernised human societies, Walker et al. (2006) found that the age at menarche in women was significantly correlated with probability of survival to the age of 15. This association might have been a consequence of Darwinian selection, but an alternative interpretation is that those individuals in greater danger responded to environmental cues that induced early menarche. Indeed, in modern Westernised human societies, a girl exposed to stressful conditions in early life, such as being orphaned or following separation of her parents, is likely to enter puberty at an earlier age than would otherwise have been the case (Belsky, 2012). She may express a strong wish to have children at an early age and become a mother in her early to mid-teens (Nettle et al. 2010). The potential biological benefit in a dangerous environment is that she has a child while she is still able to do so. If bad conditions in early life forecast bad conditions later on, her best chance of increasing her fitness is to breed early (Nettle et al. 2010).
Contradicting an earlier study by Lumey & Stein (1997), Painter et al. (2008) found that offspring of women who were pregnant during the 1944 Dutch famine had a higher reproductive success than those whose mothers were pregnant before or after the famine. Low birth weight is associated with accelerated menarche, particularly when nutrition before puberty is enhanced (Sloboda et al. 2007). If women experiencing an early age at menarche are more likely to reproduce, they may produce more children than other women developing under well-supported modern conditions.
Responses to nutritional cues
Nutrition during development can also have pronounced effects on the adult's preparedness for its nutritional environment. Saastamoinen et al. (2010) found that undernourished larvae of an East African butterfly (Bicyclus anynana) had more strongly developed thoracic musculature after pupation, enabling them to fly more strongly as adults and potentially to reach more favourable environments.
When pregnant rat dams were given restricted diets, their offspring were smaller at birth, but if these offspring were given plentiful food after weaning they became more obese than the offspring of dams given an unrestricted diet (Jones & Friedman, 1982). This early observation has been followed by extensive work on rodents in many laboratories. Offspring born to undernourished dams develop increased appetite (Vickers et al. 2000). A relatively poor prenatal nutritional environment induces a phenotype with less muscle mass, a higher set-point for satiety and a preference for fat in the diet. Such a phenotype can be seen to have survival advantage in a nutritionally poor environment. On the other hand obesity in such rats is associated with morbidities in a more plentiful nutritional environment.
Even though these offspring are more sedentary when kept in standard laboratory cages (Vickers et al. 2003), they are more likely to run in a wheel when given a choice between pressing a lever to obtain food and running in the wheel (Miles et al. 2009). This suggests that such offspring of under-nourished mothers may attempt to find more reliable sources of food in a natural environment. In addition to these behavioural consequences, the pups also had accelerated sexual maturation (Sloboda et al. 2009), once again showing that developmental plasticity has induced a set of changes in the life history of the animal.
When rats exposed to a poor level of maternal nutrition before birth were treated neonatally with leptin, the hyperphagia, insulin resistance, hypertension, gene expression and epigenetic changes were prevented (Vickers et al. 2005; Gluckman et al. 2007b). More recently similar results have been seen upon giving growth hormone to offspring of undernourished rats (Reynolds et al. 2013) showing that the leptin effect is not specific and suggesting that a variety of cues can induce the development of a PAR.
Nutritional PARs in human biology
In human biology extensive studies have shown that maternal body composition affects the offspring's body composition, metabolic control, neuronal reserve, kidney size, reproductive maturation, and behaviour (Godfrey, 2006; Moritz & Cullen-McEwen, 2006; Sloboda et al. 2007). The effects resemble those reported in animal studies in many ways. Thus children with lower birth weight, a proxy for poor intrauterine nutrition, enter puberty early, have a preference for high-fat foods, a higher set-point for satiety and a smaller stature – a suite of characteristics that are well adapted to limited food resources in adult life.
This adaptation favours the laying down of fat during the period of breast-feeding, thus providing lipid stores to buffer the developing brain against potentially inadequate nutrition (Kuzawa, 2010) or episodes of postnatal gastrointestinal disease around the time of weaning (Kuzawa, 1998). By the age of three, a switch to insulin resistance takes place which will be relatively advantageous in a poor post-weaning nutritional environment if the mother's nutritional plane has been relatively low (Mericq et al. 2005). The individual benefits, it is argued, by adjusting the trajectory of development so that his or her phenotype is most likely to match the anticipated environment.
The most direct contemporary data in support of the PAR hypothesis in humans comes from the studies of children with marasmus and kwashiorkor in Jamaica. Children who develop marasmus have different settings of intermediary metabolism, both during the illness and after recovery, compared with those who develop kwashiorkor. The metabolism of the children with kwashiorkor appears to be metabolically profligate whereas in those with marasmus metabolism appears to be more metabolically thrifty (Jahoor et al. 2006). As a result, the kwashiorkor children are more likely than marasmic children to die during the episode of acute malnutrition. A study of over 1000 children who developed marasmus or kwashiorkor showed a large difference in birth weight between these two phenotypes – marasmic infants had a 330 g lower median birth weight (Forrester et al. 2012). This has been interpreted as suggesting that marasmic children had both reduced birth size reflecting an immediate adaptive response and had induced PAR in utero appropriate to the low nutritional conditions that presumably induced their lower birth weight, whereas the kwashiorkor infants were adapted to a higher plane of nutrition. Studies in adults from this cohort show the persistence of differences in metabolic control into adulthood, with those individuals who had formerly been marasmic showing both insulin resistance and glucose intolerance (Francis-Emmanuel et al. 2014).
Other studies from around the world have shown that NCDs such as hypertension and type 2 diabetes in adulthood are more likely to be exhibited by people who were born small but subsequently lived in environments of abundant nutrition (Patel et al. 2006; Ebrahim et al. 2010). The long-term effects on health occur after the peak period of reproduction and may not reflect conditions that operated during evolution. Even so, fitness in the past might have been affected by a mismatch between the predicted and the actual environment if parental and grandparental care of offspring were compromised. The opposite side of the mismatch coin is that people who enjoyed a plentiful environment in early life may be at greater risk during periods of prolonged famine than those who experienced lower levels of nutrition during development. Children born to affluent parents are more likely to suffer adverse effects if they are starved in adulthood. In concentration camps and the worst prisoner-of-war camps, anecdotal evidence suggests that the physically large individuals died first while at least some of the small individuals survived (Bateson, 2001). In a famine-exposed Ethiopian population, high birth weight was associated with a 9-fold greater risk of rickets (Chali et al. 1998).
Whilst birth weight can be affected by prenatal environment, it is a side-effect of developing an adaptive response to relatively poor maternal nutrition and is not always well correlated with the adaptive suite of phenotypic characteristics . For example Gale et al. (2006) showed that the induction of effects on cardiovascular structure of children by unbalanced maternal nutrition was not accompanied by alterations in birth size. Similarly Godfrey et al. (2011) showed that epigenetic changes in children's DNA at birth were associated with changes in maternal nutrition in the first trimester, and in turn with childhood adiposity, but these epigenetic changes were independent of birth size.
Disagreements about the interpretation of the human evidence
While the PAR hypothesis has general application, the primary application in human biology has been in proposing that offspring born to mothers on a relatively low plane of nutrition develop a physiology associated with a suite of adaptations that suits them well to a low plane of nutrition. However, they are poorly adapted to an abundant nutritional environment. Conversely offspring born to mothers on a relatively high plane of nutrition are adapted to a plentiful nutritional environment. Developmental forecasting in the offspring by induction of a specific developmental trajectory is, therefore, thought by many researchers to be important in human biology e.g. (Bateson, 2001; Gluckman & Hanson, 2004; Sandman et al. 2012). A cue from the mother suggesting a future environment with relatively scarce resources leads to a more economical form and a bias towards insulin resistance, thereby capturing the higher-energy and fat-dense foods when they are available (Gluckman et al. 2010).
The PAR hypothesis as applied in this context has received considerable support, but it has been criticised on the grounds that the evidence could also be explained in other ways. Wells (2007) has argued that in humans maternal interests dominate and the fetal adaptive response is secondary to protecting maternal fitness. He is correct in stating that, ultimately, the mother is in control. If conditions are dire, she can spontaneously abort her fetus or abandon her infant, but this reflects extreme conditions under which mechanisms other than PARs are likely to operate (see Fig. 1). At a more subtle level, the mother can trade-off the benefits of fully supporting her current offspring against those of holding herself in readiness to produce another. The long-term interests of the mother are not identical with those of her offspring. This well-known principle, first developed by Trivers (1974), is widely accepted by evolutionary biologists and led to the idea of a conflict between the interests of parent and offspring. Yet the conflict principle can easily be overstated. Empirical studies of parent–offspring relationships in mammals suggest that both parties adjust their behaviour to the state of the other. Mothers both monitor and respond to the progress of their current offspring and delay breeding again if an offspring is developing slowly. If the mother is on a relatively low level of nutrition or is pregnant from an early postpartum oestrus, the offspring will take the initiative and wean earlier onto solid food. Two-way communication between mother and offspring benefits both parties (Bateson & Laland, 2013). In any event the argument that the fetus is making changes in response primarily to assist maternal fitness is questionable. Long-term effects in human offspring can be induced without any manifest change in birth size or gestational length. As no obvious change in phenotype is apparent at birth or in infancy in such situations, but the phenotypic effects occur later in childhood and young adulthood when the dependence on the mother has declined, the primary beneficiary must surely be the offspring.
Wells (2007) argued that a forecast is unlikely to be correct in such a long-lived species as humans in which environmental change is likely between generations. Lachmann & Jablonka (1996) modelled anticipation in fluctuating environments and pointed out that the anticipation would be advantageous provided the change in environment predicted was sustained for at least half a generation. In their model, reproduction was sustained through life, but a more appropriate extrapolation in humans would be to peak reproduction. Thus for a human the relative fidelity of the prediction of the environment need only last a few years and the main advantage of a PAR in humans would be to ensure survival to reproductive age, as illustrated in elasticity curves for human fitness (Jones, 2009).
In support of Wells’ argument, Nettle et al. (2013) suggested on the basis of a computer simulation over one generation that the PAR is only likely to evolve if environmental conditions are stable, which is contrary to the conclusion reached by Lachmann and Jablonka (1996) using a different modelling approach. In an extension of the model of Nettle et al., Del Guidice (1996) also found that environmental stability was important but fluctuations in conditions were less constraining to the evolution of a PAR. Baig et al. (2011) observed that stability across time would be more likely in a hierarchically organised society in which rank tends to be inherited, a point that Nettle et al. (2013) also accepted. A different issue is that members of the hominin lineage have manifestly been migratory, travelling into very different climatic regions of the globe. Fluctuations in conditions in one climatic zone would often have been smaller than the differences between zones. Darwinian selection would have led to genetic differences in populations living in different climates for a prolonged period. However, after migration into a new climatic zone any mechanism that protected individuals from changes in living conditions from those in which previous generations had lived would be highly beneficial before natural selection could act (Bateson & Gluckman, 2011). If a mother can transmit to her developing offspring cues that will affect its stature, body composition, metabolism and a host of life-history characteristics, she will be at an advantage in fitness terms over a mother who cannot.
In order to assess the PAR hypothesis in relation to the nutrition of humans, Hayward et al. (2013) analysed data from pre-industrial Finland. In 1887–8 the harvests failed and the poorest people suffered most in terms of their mortality and reproductive success. Poor individuals who were born in years of plenty were less affected than those born in lean years. Hayward et al. argued that according to the PAR hypothesis, the poorest people born in lean years should have been better able to withstand the effects of the famine. Since this was not the case, they claimed to have falsified the PAR hypothesis. However, the poorest people may have been debilitated by their poverty, infection and low resources so that their physical condition did not represent a PAR to low nutrition (see Gluckman et al. 2005). The richer people may well have been better provisioned and thus buffered against starvation after a poor harvest. The confounding issue of economic stratification was recognised by Hayward et al. and also highlighted in a study of the Dutch potato famine of 1846–7 (Lindeboom et al. 2010).
Tests of the PAR hypothesis
In general, tests of the current utility of an adaptation are easier to devise than those of the possible historical processes that led to the evolution of underlying mechanisms. These are not necessarily the same, as originally pointed out by Tinbergen (1963) and discussed further by Bateson & Laland (2013).
One of the tests of the current utility of alternative phenotypes requires data for survival and reproductive success for individuals that were exposed to an environment that was either stressful or non-stressful in early life and an environment that was either stressful or not stressful when the individuals were adult, providing four combinations of conditions for developmental responses to safe and risky environments. A possible candidate species for such tests might be the snowshoe hare. If the early environment was stressful and the adult environment was relatively free of stress, individuals that remained unnecessarily watchful for predators would disrupt their opportunities for feeding. Conversely individuals reared in a non-stressful environment would be at greater risk from predation if the number of predators had increased in the meantime.
A different form of test is possible when the response to an environmental condition varies. For example, some individuals exposed to a stressful environment in early life might not respond in anticipation of a stressful adult life. Do they suffer a higher cost in terms of survival and reproductive success than the more responsive individuals if the environment turned out to be stressful?
Under extremely adverse conditions for the mother, surviving offspring are likely to have had a disrupted development whilst offspring of mothers living in more favourable conditions will benefit from what has been called the ‘silver spoon’ effect (Monaghan, 2008). Under conditions of nutritional excess for the mother, her offspring is likely to be born overweight and may develop type 2 diabetes at an early age. As is shown in Fig. 3 the PAR hypothesis is only likely to be relevant in conditions that vary from a moderately low plane of maternal nutrition to a moderately high plane. Gradations at the margins are depicted by dashed lines in the figure. The justification for distinguishing between a very poor and a relatively poor nutritional environment is that the latter may not affect growth by means of an immediate adaptive response but does induce a suite of predictive responses that constitute adaptations to a relatively low plane of nutrition.
Figure 3. Different factors that may affect the survival and reproductive success of an individual.
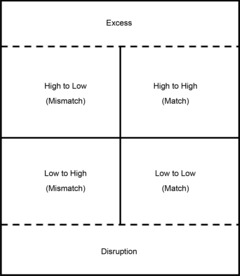
On the lowest plane of nutrition the individual may suffer developmental disruption or be forced to sacrifice future reproductive success in order to survive. On a plane of nutritional excess individuals are likely to suffer health problems. On intermediate levels of nutrition the degree of the match between the predicted and the actual environment is crucial, according to the PAR hypothesis.
The risks of making an incorrect decision may be asymmetrical. An individual adapted to environment A but living in environment B may be less severely affected than an individual adapted to environment B and living in environment A (Moran, 1992; Sultan & Spencer, 2002). For example, a human who is set as a fetus on a trajectory towards a phenotype adapted to a poor nutritional environment later, but who then lives in a rich environment, may suffer little or no health damage during childhood and through the reproductive phase and thus have relatively little effect on his or her fitness, but may experience deleterious effects on health later in life (Gluckman et al. 2005). The resulting insulin resistance in young people is likely to be a feature of contemporary rather than historical conditions and even the associated obesity does not affect human fertility unless it is very severe.
By contrast, an individual with a phenotype adapted to a high nutritional plane but living in a poor environment may be severely affected – his or her nutritional requirements might be more rapidly compromised in famine, and in women fertility is reduced under such circumstances (Chali et al. 1998). The general point is that a human who is mildly or moderately obese is generally healthy through the reproductive years. Therefore to predict a poor postnatal environment and live in a high-nutrition environment may be less deleterious to reproductive success than developing a body that requires high-energy support and living in a poor nutritional environment.
Given the difficulties for testing the utility of a PAR in modern Westernised humans, the best tests may have to be conducted on small-scale non-Westernised societies. The tests should not be conducted simply on individuals subjected to poor nutritional conditions in early life and those subjected to rich nutritional conditions. Instead, the groups should be divided into very poor, relatively poor, relatively rich and, if they exist in small scale societies, excessively rich conditions.
The distinctions between very poor and relatively poor environments imply that different developmental processes are involved. If that is so, an important line of evidence will be to examine the epigenetic profiles of people with different types of early experience.
Adaptive developmental plasticity and evolution
Two evolutionary issues are raised by the PAR hypothesis. First, if the phenomena exist – as we have argued that they do – how did they evolve? Secondly, how does plasticity impact on evolution? On the first issue, developmental plasticity is conserved across all multicellular taxa. Given that evolutionary change in response to Darwinian selection can be rapid, this suggests that a fitness advantage exists to sustaining plasticity in some systems such as those affected by normal variation in levels of nutrition, predation and stress. Admittedly, the costs of evolving PARs are not known, even though the costs of maintaining them are likely to be low (Van Buskirk & Steiner, 2009).
In a meta-analysis of studies of PARs, Uller et al. (2013) found only weak evidence for greater offspring fitness when parental and offspring environments were matched compared with when they were mismatched. However, most of the invertebrates and plants they considered produce large numbers of progeny. In such cases Darwinian selection would be expected to act quickly, rendering a PAR unnecessary unless conditions fluctuate markedly from one generation to the next. Indeed, in modelling conditions that might give rise to a PAR, Marshall & Uller (2007) found that the life-history characteristics of the trait were key determinants of the type of response that might be anticipated. PARs are much more likely to be found in a slow reproducing species with high parental investment, such as humans, many other mammals and birds. Stable maintenance of environmentally induced epigenetic states over an organism's lifetime is most likely to be favoured when the organism accurately responds to a single environmental change that subsequently remains constant, or when the environmental change cues an irreversible developmental transition (Herman et al. 2013). Stable transmission of adaptive epigenetic states from parents to offspring may be selectively favoured when environments vary across generations and the parental environment predicts the offspring environment.
On the second issue, many authors have argued that developmental plasticity provides a substrate of phenotypic variation on which Darwinian selection can act (Pigliucci, 2001; West-Eberhard, 2003; Noble et al. 2014). Evidence for transgenerational environmental effects mediated via direct and indirect epigenetic inheritance is increasing (Gluckman et al. 2007a; Jablonka & Raz, 2009). A wide variety of changes in endocrine regulation following developmental stresses are mediated by epigenetic mechanisms in experimental animals (Bateson & Gluckman, 2011). Induced epigenetic changes have also been described in naturally occurring plants (Pigliucci & Muller, 2010). The evidence for transmission across generations in both animals and plants continues to grow (Gissis & Jablonka, 2011). Epigenetic inheritance over at least eight generations has been reported in the plant Arabidopsis (Johannes et al. 2009). One research programme on mice examined individuals possessing a Kit paramutation (a heritable, meiotically stable epigenetic modification resulting from an interaction between alleles in a heterozygous parent) that results in a white-spotted phenotype. Injection of RNA from sperm of heterozygote mice into wild-type embryos led to the white-spotted phenotype in the offspring, which was in turn transmitted to their progeny (Rassoulzadegan, 2011). In another study, mouse embryos were injected with a microRNA that targets an important regulator of cardiac growth. In adulthood, these mice developed hypertrophy of the cardiac muscle, which was passed on to descendants through at least three generations without loss of effect (Wagner et al. 2007). Furthermore, the microRNA was detected in the sperm of at least the first two generations, thus implicating sperm RNA as the likely means by which the pathology is inherited. The possible involvement of sperm is also supported by observations that transgenerational genetic effects on body weight and appetite can be passed epigenetically through the mouse paternal germline for at least two generations (Yazbek et al. 2010).
Male rats were exposed in utero to the endocrine disruptor vinclozolin during the sensitive period for testis sex differentiation and morphogenesis. Lowered spermatogenic capacity and several adult-onset diseases were observed over four successive generations; these were accompanied by altered DNA methylation patterns in the germline (Anway et al. 2005; Jirtle & Skinner, 2007). Further analysis of these male offspring revealed that vinclozolin decreased methylation levels of two paternally imprinted genes and increased that of three maternally imprinted genes (Stouder & Paoloni-Giacobino, 2010). The work on Arabidopsis and mice suggests that micro-RNA may provide the means for transmission of methylation marks from one generation to the next (Teixeira et al. 2009; Rassoulzadegan, 2011).
In most experimental studies, the environmental stimulus producing an epigenetic change is only applied in one generation. This might be enough since work on yeast suggests that an environmental challenge can permanently alter regulation of genes (Braun & David, 2011). In natural conditions, the environmental cues that induce epigenetic change may be recurrent and repeat what has happened in previous generations. This recurring effect might stabilise the phenotype until genetic accommodation and fixation have occurred (Jablonka & Raz, 2009; Bateson & Gluckman, 2011). Alternatively, DNA silencing may be stable as, for example, in Linaria (Cubas et al. 1999) in which the epigenetically induced phenotype does not change from one generation to the next.
A central question in considering evolutionary change driven by the environment is whether the transmitted epigenetic markers could facilitate genomic change (Johnson & Tricker, 2010). The answer is that, in principle, they could if (a) they were transmitted from one generation to the next, (b) they increased the fitness of the individual carrying the markers, and (c) genomic reorganisation enabled some individuals to develop the same phenotype at lower cost (Bateson, 2012). Epigenetic inheritance would serve to protect the well-adapted phenotypes within the population until spontaneous fixation occurred. Developmentally induced phenotypic influences can persist for more than one generation without becoming fixed in the lineage. Grandparental effects mediated by an intermediate female generation may simply reflect exposure of the ovum for the second generation to the grandparental environment. Nevertheless, recent evidence suggests that some epigenetic marks can be passed from one generation to the next in many species (Jablonka & Raz, 2009; Noble et al. 2014), providing a mechanism by which environmental influences may be sustained over generations. In itself this does not explain why evolutionary changes in the genome have occurred. However, the situations under which genetic assimilation and accommodation are possible and plausible mechanisms by which the phenotype could have become genetically fixed are much discussed (West-Eberhard, 2003; Bateson, 2013). On one hand the phenotype may have been canalised by persistent intergenerational environmental change acting via developmental plasticity until chance mutations reduced or obviated the need for the environmental stimulus. Alternatively the induced epigenetic changes that mediate adaptive plasticity might have biased the sites of subsequent mutation (Pfeifer, 2006; Misawa et al. 2008; Bateson & Gluckman, 2011; Bateson, 2012). Variation at these sites may throw up phenotypes some of which are adaptive and subject to Darwinian selection. In this way adaptive developmental plasticity, as represented by PARs, might lead to evolutionary change.
Conclusion
Evidence for PARs in animals is strong and provides a valuable basis for new physiological work. The evidence from humans is more controversial. To conduct research in this area, the multiple mechanisms of developmental plasticity must be disentangled from developmental disruption and the adverse long-term effects of coping. Distinctions should be made between developmental disruption produced by lack of necessary resources during development, immediately adaptive responses with long-term adverse consequences, and those adaptive responses such as PARs induced early in development which confer long-term advantage. The empirical data, whether clinical or experimental, need to be interpreted in the light of these considerations, calling for a more nuanced approach to the effects of early experience on human variation than has been apparent in some of the recent literature. Doing so will greatly improve resolution of the important issue of the relative weight of different developmental factors in the origins of health and disease. Moreover, the advent of epigenetic profiling should facilitate the teasing apart of the different processes involved in development. We believe, therefore, that it will soon be possible to resolve some of the remaining disagreements about the importance of PARs based on nutritional cues in humans. Moreover the evidence from animal studies provides a framework for interpreting many health problems relating to human stress and nutrition.
Acknowledgments
We are grateful to Daniel Nettle and Melissa Bateson for their comments on an earlier version of this paper and to Jane Kitcher and Felicia Low for assistance with preparing the manuscript.
Additional information
Competing interests
None declared.
Funding
P.D.G. is supported by Gravida: The National Centre for Growth and Development, New Zealand; M.A.H. is supported by the British Heart Foundation.
References
- Anway MD, Cupp AS, Uzumcu M, Skinner MK. Epigenetic transgenerational actions of endocrine disruptors and male fertility. Science. 2005;308:1466–1469. doi: 10.1126/science.1108190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baig U, Belsare P, Watve M, Jog M. Can thrifty gene(s) or predictive fetal programming for thriftiness lead to obesity. J Obes. 2011;2011:861049. doi: 10.1155/2011/861049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bateson P. Fetal experience and good adult design. Int J Epidemiol. 2001;30:928–934. doi: 10.1093/ije/30.5.928. [DOI] [PubMed] [Google Scholar]
- Bateson P. Developmental plasticity and evolutionary biology. The Journal of Nutrition. 2007;137(4):1060–1062. doi: 10.1093/jn/137.4.1060. [DOI] [PubMed] [Google Scholar]
- Bateson P. The impact of the organism on its descendants. Genet Res Int. 2012;2012:640612. doi: 10.1155/2012/640612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bateson P. New thinking about biological evolution. Biol J Linn Soc. 2013 DOI: 10.1111/bij.12125. [Google Scholar]
- Bateson P, Barker D, Clutton-Brock T, Deb D, D'Udine B, Foley RA, Gluckman P, Godfrey K, Kirkwood T, Lahr MM, McNamara J, Metcalfe NB, Monaghan P, Spencer HG, Sultan SE. Developmental plasticity and human health. Nature. 2004;430:419–421. doi: 10.1038/nature02725. [DOI] [PubMed] [Google Scholar]
- Bateson P, Gluckman P. Plasticity, Robustness, Development and Evolution. Cambridge, UK: Cambridge University Press; 2011. [Google Scholar]
- Bateson P, Laland KN. Tinbergen's four questions: an appreciation and an update. Trends Ecol Evol. 2013;28:712–718. doi: 10.1016/j.tree.2013.09.013. [DOI] [PubMed] [Google Scholar]
- Belsky J. The development of human reproductive strategies progress and prospects. Curr Direc Psychol Sci. 2012;21:310–316. [Google Scholar]
- Braun E, David L. The role of cellular plasticity in the evolution of regulatory novelty. In: Jablonka E, Gissis S, editors. Transformations of Lamarckism: From Subtle Fluids to Molecular Biology. Cambridge, MA, USA: MIT Press; 2011. pp. 181–191. [Google Scholar]
- Brönmark C, Pettersson L, Nilsson A. Predator-induced defense in crucian carp. In: Tollrian R, Harvell CD, editors. The Evolution of Inducible Defenses. Princeton, NJ, USA: Princeton University Press; 1999. pp. 203–217. [Google Scholar]
- Chali D, Enquselassie F, Gesese M. A case-control study on determinants of rickets. Ethiop Med J. 1998;36:227–234. [PubMed] [Google Scholar]
- Claessens SE, Daskalakis NP, van der Veen R, Oitzl MS, de Kloet ER, Champagne DL. Development of individual differences in stress responsiveness: an overview of factors mediating the outcome of early life experiences. Psychopharmacology. 2011;214:141–154. doi: 10.1007/s00213-010-2118-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cubas P, Vincent C, Coen E. An epigenetic mutation responsible for natural variation in floral symmetry. Nature. 1999;401:157–161. doi: 10.1038/43657. [DOI] [PubMed] [Google Scholar]
- Dantzer B, Newman AE, Boonstra R, Palme R, Boutin S, Humphries MM, McAdam AG. Density triggers maternal hormones that increase adaptive offspring growth in a wild mammal. Science. 2013;340:1215–1217. doi: 10.1126/science.1235765. [DOI] [PubMed] [Google Scholar]
- Del Giudice M. Life history plasticity in humans: the predictive value of early cues depends on the temporal structure of the environment. Proc R Soc B. 2014;281:20132995. doi: 10.1098/rspb.2013.2222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Giudice M, Ellis BJ, Shirtcliff EA. The Adaptive Calibration Model of stress responsivity. Neurosci Biobehav Rev. 2011;35:1562–1592. doi: 10.1016/j.neubiorev.2010.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebrahim S, Kinra S, Bowen L, Andersen E, Ben-Shlomo Y, Lyngdoh T, Ramakrishnan L, Ahuja RC, Joshi P, Das SM, Mohan M, Davey Smith G, Prabhakaran D, Reddy KS Indian Migration Study Group. The effect of rural-to-urban migration on obesity and diabetes in India: a cross-sectional study. PLoS Med. 2010;7:e1000268. doi: 10.1371/journal.pmed.1000268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forrester TE, Badaloo AV, Boyne MS, Osmond C, Thompson D, Green C, Taylor-Bryan C, Barnett A, Soares-Wynter S, Hanson MA, Beedle AS, Gluckman PD. Prenatal factors contribute to the emergence of kwashiorkor or marasmus in severe undernutrition: evidence for the predictive adaptation model. PLoS One. 2012;7:e35907. doi: 10.1371/journal.pone.0035907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francis-Emmanuel PM, Thompson DS, Barnett AT, Osmond C, Byrne CD, Hanson MA, Gluckman PD, Forrester TE, Boyne MS. Glucose metabolism in adult survivors of severe acute malnutrition. J Clin Endocrinol Metab. 2014 doi: 10.1210/jc.2013-3511. DOI: http://dx.doi.org/10.1210/jc.2013-3511. [DOI] [PubMed] [Google Scholar]
- Gale CR, Jiang B, Robinson SM, Godfrey KM, Law CM, Martyn CN. Maternal diet during pregnancy and carotid intima-media thickness in children. Arterioscler Thromb Vasc Biol. 2006;26:1877–1882. doi: 10.1161/01.ATV.0000228819.13039.b8. [DOI] [PubMed] [Google Scholar]
- Ghalambor CK, McKay JK, Carroll SP, Reznick DN. Adaptive versus non-adaptive phenotypic plasticity and the potential for contemporary adaptation in new environments. Funct Ecol. 2007;21:394–407. [Google Scholar]
- Gissis S, Jablonka E. In: Transformations of Lamarckism: From Subtle Fluids to Molecular Biology. Jablonka E, Gissis S, editors. Cambridge, MA, USA: MIT Press; 2011. pp. 181–191. [Google Scholar]
- Gluckman P, Beedle A, Hanson M. Principles of Evolutionary Medicine. Oxford, UK: Oxford University Press; 2009. [Google Scholar]
- Gluckman P, Hanson M. The Fetal Matrix: Evolution, Development and Disease. Cambridge, UK: Cambridge University Press; 2005. [Google Scholar]
- Gluckman PD, Hanson MA. The developmental origins of the metabolic syndrome. Trends Endocrinol Metab. 2004;15:183–187. doi: 10.1016/j.tem.2004.03.002. [DOI] [PubMed] [Google Scholar]
- Gluckman PD, Hanson MA, Beedle AS. Non-genomic transgenerational inheritance of disease risk. Bioessays. 2007a;29:145–154. doi: 10.1002/bies.20522. [DOI] [PubMed] [Google Scholar]
- Gluckman PD, Hanson MA, Buklijas T. A conceptual framework for the developmental origins of health and disease. J Dev Orig Health Dis. 2010;1:6–18. doi: 10.1017/S2040174409990171. [DOI] [PubMed] [Google Scholar]
- Gluckman PD, Hanson MA, Spencer HG, Bateson P. Environmental influences during development and their later consequences for health and disease: implications for the interpretation of empirical studies. Proc R Soc B. 2005;272:671–677. doi: 10.1098/rspb.2004.3001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gluckman PD, Lillycrop KA, Vickers MH, Pleasants AB, Phillips ES, Beedle AS, Burdge GC, Hanson MA. Metabolic plasticity during mammalian development is directionally dependent on early nutritional status. Proc Natl Acad Sci U S A. 2007b;104:12796–12800. doi: 10.1073/pnas.0705667104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godfrey KM. The “developmental origins” hypothesis: epidemiology. In: Gluckman PD, Hanson MA, editors. Developmental Origins of Health and Disease. Cambridge, UK: Cambridge University Press; 2006. pp. 6–32. [Google Scholar]
- Godfrey KM, Gluckman PD, Hanson MA. Developmental origins of metabolic disease: life course and intergenerational perspectives. Trends Endocrinol Metabol. 2010;21:199–205. doi: 10.1016/j.tem.2009.12.008. [DOI] [PubMed] [Google Scholar]
- Godfrey KM, Lillycrop KA, Burdge GC, Gluckman PD, Hanson MA. Epigenetic mechanisms and the mismatch concept of the developmental origins of health and disease. Pediatr Res. 2007;61:5R–10R. doi: 10.1203/pdr.0b013e318045bedb. [DOI] [PubMed] [Google Scholar]
- Godfrey KM, Sheppard A, Gluckman PD, Lillycrop KA, Burdge GC, McLean C, Rodford J, Slater-Jefferies JL, Garratt E, Crozier SR, Emerald BS, Gale CR, Inskip HM, Cooper C, Hanson MA. Epigenetic gene promoter methylation at birth is associated with child's later adiposity. Diabetes. 2011;60:1528–1534. doi: 10.2337/db10-0979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayward AD, Rickard IJ, Lummaa V. Influence of early-life nutrition on mortality and reproductive success during a subsequent famine in a preindustrial population. Proc Natl Acad Sci U S A. 2013;110:13886–13891. doi: 10.1073/pnas.1301817110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman JJ, Spencer HG, Donohue K, Sultan SE. How stable ‘should’ epigenetic modifications be? Insights from adaptive plasticity and bet hedging. Evolution. 2013;68:632–643. doi: 10.1111/evo.12324. [DOI] [PubMed] [Google Scholar]
- Jablonka E, Raz G. Transgenerational epigenetic inheritance: prevalence, mechanisms, and implications for the study of heredity and evolution. Q Rev Biol. 2009;84:131–176. doi: 10.1086/598822. [DOI] [PubMed] [Google Scholar]
- Jahoor F, Badaloo A, Reid M, Forrester T. Unique metabolic characteristics of the major syndromes of severe childhood malnutrition. In: Picou D, Forrester T, Walker SP, editors. The Tropical Metabolism Research Unit, the University of the West Indies Jamaica 1956–2006. The House that John Built. Kingston, Jamaica: Ian Randle Publishers Ltd; 2006. pp. 25–60. [Google Scholar]
- Jirtle RL, Skinner MK. Environmental epigenomics and disease susceptibility. Nat Rev Genet. 2007;8:253–262. doi: 10.1038/nrg2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johannes F, Porcher E, Teixeira FK, Saliba-Colombani V, Simon M, Agier N, Bulski A, Albuisson J, Heredia F, Audigier P, Bouchez D, Dillmann C, Guerche P, Hospital F, Colot V. Assessing the impact of transgenerational epigenetic variation on complex traits. PLoS Genet. 2009;5:e1000530. doi: 10.1371/journal.pgen.1000530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson LJ, Tricker PJ. Epigenomic plasticity within populations: its evolutionary significance and potential. Heredity. 2010;105:113–121. doi: 10.1038/hdy.2010.25. [DOI] [PubMed] [Google Scholar]
- Jones AP, Friedman MI. Obesity and adipocyte abnormalities in offspring of rats undernourished during pregnancy. Science. 1982;215:1518–1519. doi: 10.1126/science.7063860. [DOI] [PubMed] [Google Scholar]
- Jones JH. The force of selection on the human life cycle. Evol Hum Behav. 2009;30:305–314. doi: 10.1016/j.evolhumbehav.2009.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuzawa CW. Adipose tissue in human infancy and childhood: an evolutionary perspective. Am J Phys Anthropol. 1998;(Suppl. 27):177–209. doi: 10.1002/(sici)1096-8644(1998)107:27+<177::aid-ajpa7>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- Kuzawa CW. Beyond feast-famine: brain evolution, human life history, and the metabolic syndrome. In: Muehlenbein MP, editor. Human Evolutionary Biology. Cambridge, UK: Cambridge University Press; 2010. pp. 518–527. [Google Scholar]
- Lachmann M, Jablonka E. The inheritance of phenotypes: an adaptation to fluctuating environments. J Theoret Biol. 1996;181:1–9. doi: 10.1006/jtbi.1996.0109. [DOI] [PubMed] [Google Scholar]
- Laforsch C, Beccara L, Tollrian R. Inducible defenses: the relevance of chemical alarm cues in Daphnia. Limnol Oceanogr. 2006;51:1466–1472. [Google Scholar]
- Lee TM, Zucker I. Vole infant development is influenced perinatally by maternal photoperiodic history. Am J Physiol Regul Integr Comp Physiol. 1988;255:R831–R838. doi: 10.1152/ajpregu.1988.255.5.R831. [DOI] [PubMed] [Google Scholar]
- Lindeboom M, Portrait F, van den Berg GJ. Long-run effects on longevity of a nutritional shock early in life: the Dutch Potato famine of 1846–1847. J Health Econ. 2010;29:617–629. doi: 10.1016/j.jhealeco.2010.06.001. [DOI] [PubMed] [Google Scholar]
- Lumey LH, Stein AD. In utero exposure to famine and subsequent fertility: The Dutch Famine Birth Cohort Study. Am J Public Health. 1997;87:1962–1966. doi: 10.2105/ajph.87.12.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMillen IC, Robinson JS. Developmental origins of the metabolic syndrome: prediction, plasticity, and programming. Physiol Rev. 2005;85:571–633. doi: 10.1152/physrev.00053.2003. [DOI] [PubMed] [Google Scholar]
- Marshall DJ, Uller T. When is a maternal effect adaptive? Oikos. 2007;116:1957–1963. [Google Scholar]
- Mateo JM. Ecological and hormonal correlates of antipredator behavior in adult Belding's ground squirrels (Spermophilus beldingi. Behav Ecol Sociobiol. 2007;62:37–49. doi: 10.1007/s00265-007-0436-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathis A, Ferrari MC, Windel N, Messier F, Chivers DP. Learning by embryos and the ghost of predation future. Proc R Soc B. 2008;275:2603–2607. doi: 10.1098/rspb.2008.0754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mericq V, Ong KK, Bazaes R, Pena V, Avila A, Salazar T, Soto N, Iniguez G, Dunger DB. Longitudinal changes in insulin sensitivity and secretion from birth to age three years in small- and appropriate-for-gestational-age children. Diabetologia. 2005;48:2609–2614. doi: 10.1007/s00125-005-0036-z. [DOI] [PubMed] [Google Scholar]
- Miles JL, Landon J, Davison M, Krageloh CU, Thompson NM, Triggs CM, Breier BH. Prenatally undernourished rats show increased preference for wheel running v. lever pressing for food in a choice task. Br J Nutr. 2009;101:902–908. doi: 10.1017/S0007114508043353. [DOI] [PubMed] [Google Scholar]
- Misawa K, Kamatani N, Kikuno RF. The universal trend of amino acid gain-loss is caused by CpG hypermutability. J Mol Evol. 2008;67:334–342. doi: 10.1007/s00239-008-9141-1. [DOI] [PubMed] [Google Scholar]
- Monaghan P. Early growth conditions, phenotypic development and environmental change. Phil Trans R Soc B. 2008;363:1635–1645. doi: 10.1098/rstb.2007.0011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran NA. The evolutionary maintenance of alternative phenotypes. Am Nat. 1992:971–989. [Google Scholar]
- Moritz KM, Cullen-McEwen LA. Kidney development and fetal programming. In: Wintour-Coghlan EM, Owens J, editors. Early Life Origins of Health and Disease. Springer; 2006. pp. 130–144. [Google Scholar]
- Nettle D, Coall DA, Dickins TE. Birthweight and paternal involvement predict early reproduction in British women: evidence from the National Child Development Study. Am J Hum Biol. 2010;22:172–179. doi: 10.1002/ajhb.20970. [DOI] [PubMed] [Google Scholar]
- Nettle D, Frankenhuis WE, Rickard IJ. The evolution of predictive adaptive responses in human life history. Proc R Soc B. 2013;280:20131343. doi: 10.1098/rspb.2013.1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noble D, Jablonka E, Joyner M, Muller G, Omholt S. The integration of evolutionary biology with physiological science. J Physiol. 2014;592:2237–2244. doi: 10.1113/jphysiol.2014.273151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Painter RC, Westendorp RG, de Rooij SR, Osmond C, Barker DJ, Roseboom TJ. Increased reproductive success of women after prenatal undernutrition. Hum Reprod. 2008;23:2591–2595. doi: 10.1093/humrep/den274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel JV, Vyas A, Cruickshank JK, Prabhakaran D, Hughes E, Reddy KS, Mackness MI, Bhatnagar D, Durrington PN. Impact of migration on coronary heart disease risk factors: comparison of Gujaratis in Britain and their contemporaries in villages of origin in India. Atherosclerosis. 2006;185:297–306. doi: 10.1016/j.atherosclerosis.2005.06.005. [DOI] [PubMed] [Google Scholar]
- Pfeifer GP. Mutagenesis at methylated CpG sequences. Curr Top Microbiol Immunol. 2006;301:259–281. doi: 10.1007/3-540-31390-7_10. [DOI] [PubMed] [Google Scholar]
- Pigliucci M. Phenotypic Plasticity: Beyond Nature and Nurture. Baltimore, MD, USA: Johns Hopkins University Press; 2001. [Google Scholar]
- Pigliucci M, Muller GB. Evolution – The Extended Synthesis. Cambridge, MA, USA: MIT Press; 2010. [Google Scholar]
- Rassoulzadegan M. An evolutionary role for RNA-mediated epigenetic variation. In: Jablonka E, Gissis S, editors. Transformations of Lamarckism: From Subtle Fluids to Molecular Biology. Cambridge, MA, USA: MIT Press; 2011. pp. 227–235. [Google Scholar]
- Reynolds CM, Li M, Gray C, Vickers MH. Pre-weaning growth hormone treatment ameliorates bone marrow macrophage inflammation in adult male rat offspring following maternal undernutrition. PLoS One. 2013;8:e68262. doi: 10.1371/journal.pone.0068262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowell C. The variable coloration of the acridoid grasshoppers. Adv In Insect Phys. 1972;8:145–198. [Google Scholar]
- Saastamoinen M, van der Sterren D, Vastenhout N, Zwaan BJ, Brakefield PM. Predictive adaptive responses: Condition-dependent impact of adult nutrition and flight in the tropical butterfly Bicyclus anynana. Am Nat. 2010;176:686–698. doi: 10.1086/657038. [DOI] [PubMed] [Google Scholar]
- Sandman CA, Davis EP, Buss C, Glynn LM. Exposure to prenatal psychobiological stress exerts programming influences on the mother and her fetus. Neuroendocrinology. 2012;95:7–21. doi: 10.1159/000327017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheriff MJ, Krebs CJ, Boonstra R. The sensitive hare: sublethal effects of predator stress on reproduction in snowshoe hares. J Anim Ecol. 2009;78:1249–1258. doi: 10.1111/j.1365-2656.2009.01552.x. [DOI] [PubMed] [Google Scholar]
- Sheriff MJ, Krebs CJ, Boonstra R. The ghosts of predators past: population cycles and the role of maternal programming under fluctuating predation risk. Ecology. 2010;91:2983–2994. doi: 10.1890/09-1108.1. [DOI] [PubMed] [Google Scholar]
- Sloboda DM, Hart R, Doherty DA, Pennell CE, Hickey M. Age at menarche: Influences of prenatal and postnatal growth. J Clin Endocrinol Metab. 2007;92:46–50. doi: 10.1210/jc.2006-1378. [DOI] [PubMed] [Google Scholar]
- Sloboda DM, Howie GJ, Pleasants A, Gluckman PD, Vickers MH. Pre- and postnatal nutritional histories influence reproductive maturation and ovarian function in the rat. PLoS One. 2009;4:e6744. doi: 10.1371/journal.pone.0006744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stouder C, Paoloni-Giacobino A. Transgenerational effects of the endocrine disruptor vinclozolin on the methylation pattern of imprinted genes in the mouse sperm. Reproduction. 2010;139:373–379. doi: 10.1530/REP-09-0340. [DOI] [PubMed] [Google Scholar]
- Sultan SE, Spencer HG. Metapopulation structure favors plasticity over local adaptation. Am Nat. 2002;160:271–283. doi: 10.1086/341015. [DOI] [PubMed] [Google Scholar]
- Teixeira FK, Heredia F, Sarazin A, Roudier F, Boccara M, Ciaudo C, Cruaud C, Poulain J, Berdasco M, Fraga MF, Voinnet O, Wincker P, Esteller M, Colot V. A role for RNAi in the selective correction of DNA methylation defects. Science. 2009;323:1600–1604. doi: 10.1126/science.1165313. [DOI] [PubMed] [Google Scholar]
- Tinbergen N. On aims and methods of ethology. Z Tierpsychol. 1963;20:410–433. [Google Scholar]
- Trivers RL. Parent-offspring conflict. Am Zool. 1974;14:249–264. [Google Scholar]
- Uller T, Nakagawa S, English S. Weak evidence for anticipatory parental effects in plants and animals. J Evol Biol. 2013;26:2161–2170. doi: 10.1111/jeb.12212. [DOI] [PubMed] [Google Scholar]
- Van Buskirk J, Steiner UK. The fitness costs of developmental canalization and plasticity. J Evol Biol. 2009;22:852–860. doi: 10.1111/j.1420-9101.2009.01685.x. [DOI] [PubMed] [Google Scholar]
- Vickers MH, Breier BH, Cutfield WS, Hofman PL, Gluckman PD. Fetal origins of hyperphagia, obesity, and hypertension and postnatal amplification by hypercaloric nutrition. Am J Physiol Endocrinol Metab. 2000;279:E83–E87. doi: 10.1152/ajpendo.2000.279.1.E83. [DOI] [PubMed] [Google Scholar]
- Vickers MH, Breier BH, McCarthy D, Gluckman PD. Sedentary behavior during postnatal life is determined by the prenatal environment and exacerbated by postnatal hypercaloric nutrition. Am J Physiol Regul Integr Comp Physio. 2003;285:R271–R273. doi: 10.1152/ajpregu.00051.2003. [DOI] [PubMed] [Google Scholar]
- Vickers MH, Gluckman PD, Coveny AH, Hofman PL, Cutfield WS, Gertler A, Breier BH, Harris M. Neonatal leptin treatment reverses developmental programming. Endocrinology. 2005;146:4211–4216. doi: 10.1210/en.2005-0581. [DOI] [PubMed] [Google Scholar]
- Wagner GP, Pavlicev M, Cheverud JM. The road to modularity. Nat Rev Genet. 2007;8:921–931. doi: 10.1038/nrg2267. [DOI] [PubMed] [Google Scholar]
- Walker R, Gurven M, Hill K, Migliano A, Chagnon N, De Souza R, Djurovic G, Hames R, Hurtado AM, Kaplan H, Kramer K, Oliver WJ, Valeggia C, Yamauchi T. Growth rates and life histories in twenty-two small-scale societies. Am J Hum Biol. 2006;18:295–311. doi: 10.1002/ajhb.20510. [DOI] [PubMed] [Google Scholar]
- Wells JC. Flaws in the theory of predictive adaptive responses. Trends Endocrinol Metab. 2007;18:331–337. doi: 10.1016/j.tem.2007.07.006. [DOI] [PubMed] [Google Scholar]
- West-Eberhard MJ. Developmental Plasticity and Evolution. Oxford, UK: Oxford University Press; 2003. [Google Scholar]
- Yazbek SN, Spiezio SH, Nadeau JH, Buchner DA. Ancestral paternal genotype controls body weight and food intake for multiple generations. Hum Mol Genet. 2010;19:4134–4144. doi: 10.1093/hmg/ddq332. [DOI] [PMC free article] [PubMed] [Google Scholar]


