Abstract
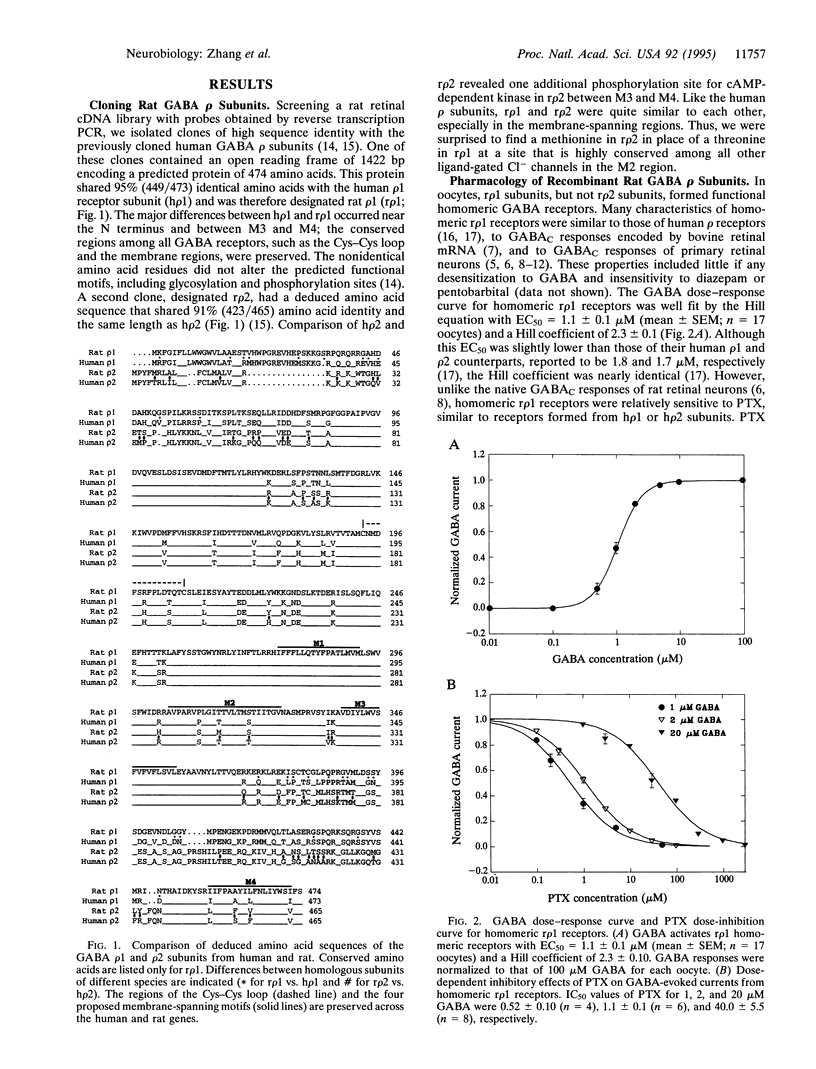
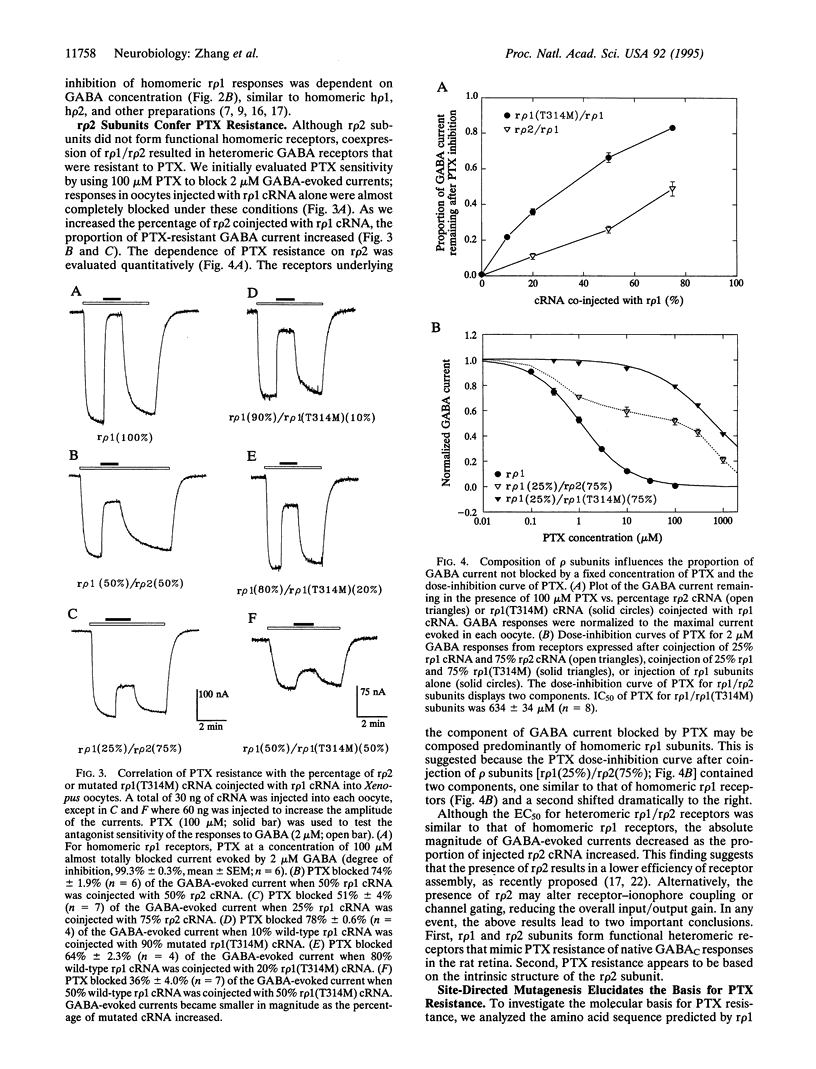
Ionotropic receptors for gamma-aminobutyric acid (GABA) are important to inhibitory neurotransmission in the mammalian retina, mediating GABAA and GABAC responses. In many species, these responses are blocked by the convulsant picrotoxinin (PTX), although the mechanism of block is not fully understood. In contrast, GABAC responses in the rat retina are extremely resistant to PTX. We hypothesized that this difference could be explained by molecular characterization of the receptors underlying the GABAC response. Here we report the cloning of two rat GABA receptor subunits, designated r rho 1 and r rho 2 after their previously identified human homologues. When coexpressed in Xenopus oocytes, r rho 1/r rho 2 heteromeric receptors mimicked PTX-resistant GABAC responses of the rat retina. PTX resistance is apparently conferred in native heteromeric receptors by r rho 2 subunits since homomeric r rho 1 receptors were sensitive to PTX; r rho 2 subunits alone were unable to form functional homomeric receptors. Site-directed mutagenesis confirmed that a single amino acid residue in the second membrane-spanning region (a methionine in r rho 2 in place of a threonine in r rho 1) is the predominant determinant of PTX resistance in the rat receptor. This study reveals not only the molecular mechanism underlying PTX blockade of GABA receptors but also the heteromeric nature of native receptors in the rat retina that underlie the PTX-resistant GABAC response.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cutting G. R., Curristin S., Zoghbi H., O'Hara B., Seldin M. F., Uhl G. R. Identification of a putative gamma-aminobutyric acid (GABA) receptor subunit rho2 cDNA and colocalization of the genes encoding rho2 (GABRR2) and rho1 (GABRR1) to human chromosome 6q14-q21 and mouse chromosome 4. Genomics. 1992 Apr;12(4):801–806. doi: 10.1016/0888-7543(92)90312-g. [DOI] [PubMed] [Google Scholar]
- Cutting G. R., Lu L., O'Hara B. F., Kasch L. M., Montrose-Rafizadeh C., Donovan D. M., Shimada S., Antonarakis S. E., Guggino W. B., Uhl G. R. Cloning of the gamma-aminobutyric acid (GABA) rho 1 cDNA: a GABA receptor subunit highly expressed in the retina. Proc Natl Acad Sci U S A. 1991 Apr 1;88(7):2673–2677. doi: 10.1073/pnas.88.7.2673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong C. J., Picaud S. A., Werblin F. S. GABA transporters and GABAC-like receptors on catfish cone- but not rod-driven horizontal cells. J Neurosci. 1994 May;14(5 Pt 1):2648–2658. doi: 10.1523/JNEUROSCI.14-05-02648.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feigenspan A., Wässle H., Bormann J. Pharmacology of GABA receptor Cl- channels in rat retinal bipolar cells. Nature. 1993 Jan 14;361(6408):159–162. doi: 10.1038/361159a0. [DOI] [PubMed] [Google Scholar]
- Ffrench-Constant R. H., Rocheleau T. A., Steichen J. C., Chalmers A. E. A point mutation in a Drosophila GABA receptor confers insecticide resistance. Nature. 1993 Jun 3;363(6428):449–451. doi: 10.1038/363449a0. [DOI] [PubMed] [Google Scholar]
- Hill D. R., Bowery N. G. 3H-baclofen and 3H-GABA bind to bicuculline-insensitive GABA B sites in rat brain. Nature. 1981 Mar 12;290(5802):149–152. doi: 10.1038/290149a0. [DOI] [PubMed] [Google Scholar]
- Inoue M., Akaike N. Blockade of gamma-aminobutyric acid-gated chloride current in frog sensory neurons by picrotoxin. Neurosci Res. 1988 Jun;5(5):380–394. doi: 10.1016/0168-0102(88)90024-7. [DOI] [PubMed] [Google Scholar]
- King R. G., Nielsen M., Stauber G. B., Olsen R. W. Convulsant/barbiturate activity on the soluble gamma-aminobutyric acid-benzodiazepine receptor complex. Eur J Biochem. 1987 Dec 15;169(3):555–562. doi: 10.1111/j.1432-1033.1987.tb13645.x. [DOI] [PubMed] [Google Scholar]
- Kuhse J., Laube B., Magalei D., Betz H. Assembly of the inhibitory glycine receptor: identification of amino acid sequence motifs governing subunit stoichiometry. Neuron. 1993 Dec;11(6):1049–1056. doi: 10.1016/0896-6273(93)90218-g. [DOI] [PubMed] [Google Scholar]
- Lukasiewicz P. D., Maple B. R., Werblin F. S. A novel GABA receptor on bipolar cell terminals in the tiger salamander retina. J Neurosci. 1994 Mar;14(3 Pt 1):1202–1212. doi: 10.1523/JNEUROSCI.14-03-01202.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macdonald R. L., Olsen R. W. GABAA receptor channels. Annu Rev Neurosci. 1994;17:569–602. doi: 10.1146/annurev.ne.17.030194.003033. [DOI] [PubMed] [Google Scholar]
- Matthews G., Ayoub G. S., Heidelberger R. Presynaptic inhibition by GABA is mediated via two distinct GABA receptors with novel pharmacology. J Neurosci. 1994 Mar;14(3 Pt 1):1079–1090. doi: 10.1523/JNEUROSCI.14-03-01079.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newland C. F., Cull-Candy S. G. On the mechanism of action of picrotoxin on GABA receptor channels in dissociated sympathetic neurones of the rat. J Physiol. 1992 Feb;447:191–213. doi: 10.1113/jphysiol.1992.sp018998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan Z. H., Lipton S. A. Multiple GABA receptor subtypes mediate inhibition of calcium influx at rat retinal bipolar cell terminals. J Neurosci. 1995 Apr;15(4):2668–2679. doi: 10.1523/JNEUROSCI.15-04-02668.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polenzani L., Woodward R. M., Miledi R. Expression of mammalian gamma-aminobutyric acid receptors with distinct pharmacology in Xenopus oocytes. Proc Natl Acad Sci U S A. 1991 May 15;88(10):4318–4322. doi: 10.1073/pnas.88.10.4318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian H., Dowling J. E. Novel GABA responses from rod-driven retinal horizontal cells. Nature. 1993 Jan 14;361(6408):162–164. doi: 10.1038/361162a0. [DOI] [PubMed] [Google Scholar]
- Qian H., Dowling J. E. Pharmacology of novel GABA receptors found on rod horizontal cells of the white perch retina. J Neurosci. 1994 Jul;14(7):4299–4307. doi: 10.1523/JNEUROSCI.14-07-04299.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimada S., Cutting G., Uhl G. R. gamma-Aminobutyric acid A or C receptor? gamma-Aminobutyric acid rho 1 receptor RNA induces bicuculline-, barbiturate-, and benzodiazepine-insensitive gamma-aminobutyric acid responses in Xenopus oocytes. Mol Pharmacol. 1992 Apr;41(4):683–687. [PubMed] [Google Scholar]
- Sivilotti L., Nistri A. GABA receptor mechanisms in the central nervous system. Prog Neurobiol. 1991;36(1):35–92. doi: 10.1016/0301-0082(91)90036-z. [DOI] [PubMed] [Google Scholar]
- Strata F., Cherubini E. Transient expression of a novel type of GABA response in rat CA3 hippocampal neurones during development. J Physiol. 1994 Nov 1;480(Pt 3):493–503. doi: 10.1113/jphysiol.1994.sp020378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan J. M., Traynelis S. F., Chen H. S., Escobar W., Heinemann S. F., Lipton S. A. Identification of two cysteine residues that are required for redox modulation of the NMDA subtype of glutamate receptor. Neuron. 1994 Oct;13(4):929–936. doi: 10.1016/0896-6273(94)90258-5. [DOI] [PubMed] [Google Scholar]
- Verrall S., Hall Z. W. The N-terminal domains of acetylcholine receptor subunits contain recognition signals for the initial steps of receptor assembly. Cell. 1992 Jan 10;68(1):23–31. doi: 10.1016/0092-8674(92)90203-o. [DOI] [PubMed] [Google Scholar]
- Villarroel A., Sakmann B. Threonine in the selectivity filter of the acetylcholine receptor channel. Biophys J. 1992 Apr;62(1):196–208. doi: 10.1016/S0006-3495(92)81805-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang T. L., Guggino W. B., Cutting G. R. A novel gamma-aminobutyric acid receptor subunit (rho 2) cloned from human retina forms bicuculline-insensitive homooligomeric receptors in Xenopus oocytes. J Neurosci. 1994 Nov;14(11 Pt 1):6524–6531. doi: 10.1523/JNEUROSCI.14-11-06524.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu M., Akabas M. H. Amino acids lining the channel of the gamma-aminobutyric acid type A receptor identified by cysteine substitution. J Biol Chem. 1993 Oct 15;268(29):21505–21508. [PubMed] [Google Scholar]
- Yoon K. W., Covey D. F., Rothman S. M. Multiple mechanisms of picrotoxin block of GABA-induced currents in rat hippocampal neurons. J Physiol. 1993 May;464:423–439. doi: 10.1113/jphysiol.1993.sp019643. [DOI] [PMC free article] [PubMed] [Google Scholar]




