Abstract
Chronic myelogenous leukemia (CML) is driven by the Bcr-Abl fusion protein which is a result of a (9;22) chromosomal translocation. Imatinib, dasatinib, and nilotinib (tyrosine kinase inhibitors, TKIs) have revolutionized how CML is treated. While the majority of patients respond to these kinase inhibitors, a subset become resistant to these therapeutics. Synribo (omacetaxine mepesuccinate) was recently FDA approved for Philadelphia-positive CML either in chronic or accelerated phase whose disease failed two prior TKIs. With omacetaxine 1.25 mg/m2 twice daily for 14 days during induction and for 7 days during maintenance, a major cytogenetic response occurred in 20% of patients in chronic phase and major hematologic response in 27% of patients in accelerated phase. Laboratory investigations unraveled the mechanism of action and effectiveness of this agent. Bcr-Abl protein is intrinsically programmed to turn over with a short half-life which makes it susceptible to protein translation inhibitors. Omacetaxine (homoharringtonine) inhibits total protein biosynthesis by binding to A-site cleft of ribosomes. As a corollary to this action, there is a diminution of short-lived proteins such as Bcr-Abl followed by cell death. Approval of this first-in-class protein translation inhibitor opens up new avenues for its use in other diseases as well as mechanism-based combinations.
Introduction
On October 26, 2012, Omacetaxine mepesuccinate (Synribo for injection, for subcutaneous use, Teva Oncology) was approved by the U. S. Food and Drug Administration (FDA) for treatment of patients with chronic or accelerated phase chronic myelogenous leukemia (CML) whose cancer has progressed during treatment with at least two tyrosine kinase inhibitors. This drug originally received orphan-product designation and was approved under the accelerated drug approval program.
This is a first protein translation inhibitor approved by the FDA. The drug's effectiveness in CML resistant to tyrosine kinase inhibitor (TKI) therapy is considered to be due to a decrease in the target i.e. the Bcr-Abl fusion protein. This protein, a tyrosine kinase, is intrinsically programmed to turn over with a short half-life and hence is vulnerable to transient inhibition of protein translation.
Clinical Studies Resulting in Approval
CML is identified by the Philadelphia chromosome which is generated by a reciprocal translocation of chromosomes 9 and 22, resulting in fusion of two genes Bcr and Abl, creating the Bcr-Abl oncogene which codes for the oncoprotein. The disease has three phases; chronic, accelerated, and blastic-phase. There are five recently approved TKIs for this disease; Gleevec (imatinib mesylate), Sprycel (dasatinib), Tasigna (nilotinib hydrochloride monohydrate), Bosulif (bosutinib), and Iclusig (ponatinib). For the FDA accelerated approval of omacetaxine, data were combined from two open label single-arm trials enrolling patients with CML in chronic phase or in accelerated phase: one for patients with CML with the mutation T315I (1) and the other for patients who had developed resistance or intolerance to at least two prior TKIs (2). The populations of these two studies were combined to select all patients in chronic or accelerated phase that had confirmation of resistance or intolerance to at least two TKIs. All were treated with the approved dose and schedule for omacetaxine mepesuccinate. For the induction phase this was 1.25 mg/m2 subcutaneous injection twice daily for 14 days of a 28 day cycle. For the maintenance phase, the dose was the same but the duration was reduced (1.25 mg/m2 subcutaneous injection twice daily for 7 days of a 28 day cycle). A total of 81 patients with chronic phase were included in the registration analysis; for patients in this phase major cytogenetic response (MCyR) i.e. decrease in the Philadelphia chromosome to 35% or fewer metaphases, was the primary response endpoint (3). Sixteen of the 81 patients (20%) achieved a MCyR (8 a partial cytogenetic response and 8 a complete cytogenetic response) with an additional 12 patients achieving a minor cytogenetic response. The median duration of response was 17.7 months. The median failure-free survival for the overall population was 9.6 months and overall survival was 9.6 months; for patients who achieved a MCyR median failure-free survival and overall survival had not been reached after a median follow-up of 19.5 months. There were 41 patients with accelerated phase of CML in the registration cohort. For these patients, a major hematologic response was the primary endpoint which was achieved in 27% of patients with a median response duration of 9 months. The median overall survival was 16 months.
For safety evaluation, data were combined from 163 patients (108 chronic phase + 55 accelerated phase). The most common (20% or more) adverse events included hematological toxicity (thrombocytopenia, anemia, neutropenia, lymphopenia), gastrointestinal (diarrhea, nausea) toxicity, weakness and fatigue, as well as reaction at the injection site. In the chronic phase thrombocytopenia grade 3 or 4 occurred in 67% of patients, neutropenia in 45% and anemia in 36%. Corresponding rates for patients in accelerated phase were 49%, 18%, and 36%, respectively. Non-hematologic adverse events were mostly grade 1-2 with the most common grade 3-4 events (occurring in more than 2 patients) being infections in 11% and fatigue in 5% in chronic phase, and infections (20%), fatigue (9%), diarrhea (7%) and nausea (4%) in accelerated phase. Seven patients in the chronic phase cohort had discontinued therapy because of adverse events (pancytopenia in 2, and aplasia, gout, sepsis, diplopia and tachyarrhythmia in 1 each).
What is Omacetaxine?
There is a long history (~ 40 years) to the development of omacetaxine. Anticancer activity was originally observed in association with Chinese herbal medicine practices using extracts from the bark of species of the Chinese plum yew, Cephalotaxus (4). It was brought to the western world by the Earl of Harrington and the species name was designated Cephalotaxus harringtonii, from which the names of the pharmacologically active forms were derived. Homoharringtonine (HHT) as well as the harringtonine and isoharringtonine isoforms were isolated and biological activities were characterized by Powell (5, 6). Chemically, homoharringtonine is 4-methyl (2R)-2-hydroxy-2-(4-hydroxy-4-methylpentyl) butanedioate ester, also known as Cephalotaxine ester or Ceflatonin (Figure 1).
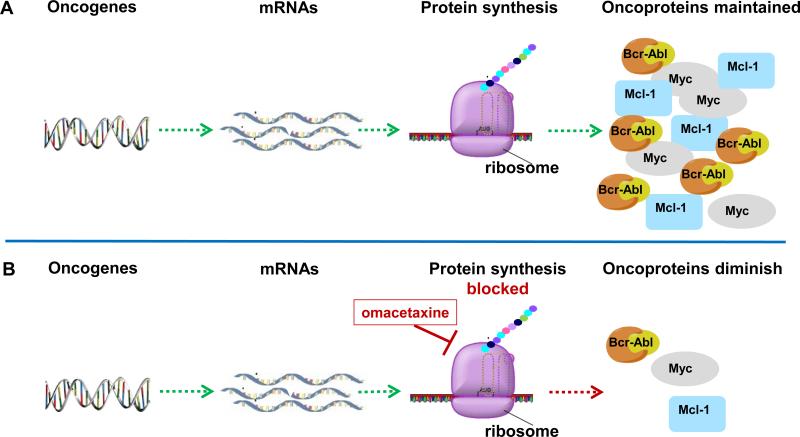
Figure 1. Oncoprotein synthesis and mechanism of action of omacetaxine mepesuccinate.
A. Oncogenes are transcribed to mRNAs followed by protein synthesis on ribosomes to produce oncoproteins such as Bcr-Abl, Mcl-1, Myc.
B. Oncogenes are transcribed but protein synthesis on ribosomes is blocked by action of omacetaxine. This results in overall inhibition of protein synthesis. Proteins with short half-lives such as Bcr-Abl, Mcl-1, and Myc diminish. If cells are dependent on these proteins for survival, they undergo apoptosis.
Initial clinical studies were conducted in China using mixtures of uncertain proportions of the active compounds. Clinical activity was observed in patients with both acute and chronic myeloid leukemias (7, 8). Subsequently, the National Cancer Institute generated highly purified HHT for early phase investigations, but investigations were not sustained due to the lack of a reliable source of purified HHT. Subsequently, ChemGenex took over development of this agent in the USA as Omacetaxine (OP1384) but was then acquired by Cephalon where a new name, CEP-41443, was assigned. Cephalon merged with Teva Pharmaceuticals in 2011, which designated the drug as Omacetaxine mepesuccinate with a trade name of Synribo. Omacetaxine mepesuccinate is slightly different than the prior formulation, as this is a semisynthetic highly purified homoharringtonine compound (99.7% purity) and is the FDA approved agent that impacts protein synthesis.
Protein synthesis in general and components of protein translation machinery are deregulated in cancer. This deregulation provides a new avenue for development of novel therapeutics (9). Mechanistically, omacetaxine (homoharringtonine) is a first in class of protein translation inhibitors. While the drug does not target specific proteins, proteins with rapid turnover rates (short half-life) are most impacted. The key for successful use of the agent is a transient use of this drug so as to not affect long-lived proteins. Deleterious effects of oncogenes are delivered by their respective oncoproteins; which generally have short half-lives (10, 11). The most studied oncoproteins that are evaluated with this inhibitor include Bcr-Abl, the etiologic lesion of CML. Other short-lived proteins affected by omacetaxine include Myc which is involved in driving many hematological and solid tumors, and Mcl-1 which plays a major role in sustaining the viability of several leukemias.
Protein Translation: The Process
The level of proteins in a given cell is determined by several processes such as translation of mRNA, diminution of proteins after ubiquitylation and proteasomal degradation, and protection from protein degradation by other mechanisms such as heat-shock proteins. Omacetaxine, which inhibits mRNA translation at the level of the ribosome, is the first agent that is FDA approved that targets this process. Inhibitors of the proteasomal degradation process, bortezomib and carfilzomib; both FDA approved and the heat shock protein inhibitor (17-AAG) have been tested in clinic.
Because omacetaxine acts on protein biosynthesis, it is essential to understand protein translation. Translation of mRNA to create protein is a multi-step complex process (12, 13). Protein synthesis occurs on ribosomes which are made up of a large 60s subunit and smaller 40s subunit. The translation process consists of four steps, activation, initiation, elongation, and termination. The activation process brings tRNAs charged with the correct amino acids to the ribosomes. Amino-acyl-tRNAs (tRNAs with an amino acid linked to it, also known as charged tRNA) first come to the acceptor or A-site on the 40S ribosome subunit. This is followed the chemical reaction of transferring the nascent peptide chain to the incoming amino acid and a move of the complex to the P-site (peptidyl-tRNA) and subsequently to the E-site where the de-acylated tRNA is removed from the ribosome. The initiation process is now studied in detail as the onset of this occurs after growth factors and or nutrient stimulation of cells (14). Several eukaryotic initiation proteins (eIF family) have been identified with specific roles and this process that brings the tRNA for the first amino acid residue (methionine) at the start codon (AUG) of the mRNA. The third step, elongation, results in assembly of polypeptides based on the open reading frame of the mRNA sequence. In the final step, termination, the ribosome encounters a stop codon in the mRNA sequence which completes the process.
Mechanisms of Action of Omacetaxine
Early studies of homoharringtonine indicated that it was an inhibitor of protein synthesis. Investigations in model systems demonstrated that HHT interacted preferentially with free ribosomes, and to a much lesser extent with polysomes. This was consistent with the other data that suggested that the alkaloid blocked peptide bond formation. This supported the conclusion that the primary effect of HHT was on nascent peptide chain elongation (15). Recent investigations by Steitz and colleagues (16) of the crystal structures of HHT in its interactions with the ribosome provided a structural basis for these findings by demonstrating that HHT becomes embedded in the A-site of the large ribosomal subunit, an action that would block access by the charged tRNA. Together, these findings support a model of HHT binding within the free ribosome in a position that interferes with the elongation process.
Pharmacokinetics of Omacetaxine
Previous investigations of the pharmacokinetics of this agent focused on iv formulations of homoharringtonine (17). However, regarding pharmacokinetics only one report is available (18) for the subcutaneous (sc) omacetaxine. Two inactive drug metabolites have been identified, however, for omacetaxine, after sc 1.25 mg/m2 b.i.d. dosing, a peak level of 25 ng/ml (~50 nM) was achieved after one hour. The drug was eliminated with a half-life of 7 hr. Additional dosing resulted in a slight increase in Cmax levels. This plasma concentration achieved was sufficient to inhibit protein synthesis during in vitro investigations.
Other Inhibitors of Protein Synthesis
The initial descriptions of the activity of HHT in hematological malignancies were accompanied by descriptions of other inhibitors of protein synthesis. As with the alkaloid HHT, investigations of the quassinoid compound, bruceantin indicated that it acted by translation inhibition, which was supported by crystallographic studies that showed it to act similarly to HHT by binding the ribosomal A-site (16). Initial clinical studies in solid tumors failed to demonstrate promising clinical activities. More recently, there has been renewed interest in investigations in models of hematological malignancies (19). In contrast to inhibitors of elongation, compounds have been identified that block initiation of translation by targeting the RNA helicase, eIF4A. Three such compounds, pateamine A (Pat A), silvestrol and hippuristanol had previously been isolated from different sources and characterized independently. However, a screen for translation inhibition identified all three as inhibitors of cap dependent initiation of translation (reviewed in ref 20). As is the case with omacetaxine (10), silvestrol treatment reduced the level of Mcl-1, the short-lived pro-survival protein, in cell lines derived from hematological malignancies.
Clinical Activity of Omacetaxine (and Homoharringtonine)
There is a long track record of the clinical efficacy of omacetaxine and its parent drug, homoharringtonine, in the treatment of CML. Homoharringtonine was first used to treat patients with CML in late chronic phase that had resistance or intolerance to interferon alpha. Homoharringtonine was used initially as an intravenous continuous infusion for 7 to 14 days which lowered the cardiovascular toxicity that could be observed when administered as a short infusion (21). With this schedule, cytogenetic responses were reported among 31% of patients, which was major in 15% (complete in 7%; ref 22). This established homoharringtonine as the treatment of choice for patients with inadequate response to interferon (the standard of care at the time) who were unable to receive a stem cell transplant. Homoharringtonine was also used as initial therapy for CML in chronic phase in combination with interferon. In one study, patients received an “induction” phase with homoharringtonine, followed by maintenance with interferon (23). A cytogenetic response of 60% was reported (major in 27%). Other studies combined homoharringtonine with low-dose cytarabine, first in patients with interferon failure reporting a major cytogenetic response rate of 15% (24), and then as initial therapy for CML in chronic phase (major cytogenetic response rate 17%) (25). A triple-drug combination of homoharringtonine, interferon and cytarabine was also investigated in patients with newly diagnosed CML in chronic phase with a reported cytogenetic response rate of 75% (major in 45%; ref 26).
Following the introduction of TKIs, the focus of the use of homoharringtonine shifted to its use in patients who had failed to maintain a response to these agents. The first step was to develop a subcutaneous formulation that would facilitate its administration. A phase I/II study of this formulation confirmed the adequate safety while maintaining efficacy (27).
The availability of the semisynthetic derivative omacetaxine made production and administration more predictable. Preclinical studies demonstrating efficacy of homoharringtonine in cells carrying the T315I mutation (10), a variant that is unresponsive to all TKI available at the time (later ponatinib was introduced with significant activity in vitro and in the clinic against this mutant) prompted the interest in administering omacetaxine to patients with this mutation. Among 62 patients with chronic phase disease with such characteristics, a major cytogenetic response was achieved in 23% of patients (complete in 16%, ref 1). Omacetaxine was also investigated in patients who had received at least 2 prior TKIs. Among 46 such patients treated in chronic phase, 58% of whom had already received 3 or more TKIs, 22% achieved a MCyR (complete in 4%, ref 2). The combined analysis of these two studies for those with confirmed resistance or intolerance to at least 2 TKI (described above) resulted in the regulatory approval of omacetaxine for patients who had failed at least 2 prior TKIs (3).
The potential of adding low-dose omacetaxine to improve the outcome of patients with partial or complete cytogenetic response to imatinib was explored. The drug was administered at a dose of 1.25 mg/m2 twice daily for one day only. Seven of the 10 patients treated achieved an improvement in BCR-ABL transcript levels (28).
Future Investigations
FDA approval was only for patients with CML who had failed two or more TKI treatments. With that respect, a recent study demonstrated that omacetaxine is also effective in ponatinib resistant CML cells (29). Preclinical as well as clinical literature suggests several opportunities for this agent used either as single drug or in mechanism-based combinations.
Single agent
This drug could be used for malignancies and cancer stem-cells that rely on short-lived proteins for survival (30). For CML, omacetaxine-induced decreases in Mcl-1 (a fast turn-over protein) have been shown to make a major contribution to the effectiveness of this agent in cell lines (10, 19) although other proteins have also been suggested (31). Reliance on short-lived oncoproteins for existence has been shown for several hematological malignancies. For example, both in chronic lymphocytic leukemia and acute myelogenous leukemia, Mcl-1 has been shown as a factor that provides survival benefit (11). In fact, early studies, mostly from China, have suggested clinical activity in AML and MDS (7), and ongoing clinical trials are investigating this potential application further. Myc, which is among the most labile proteins in the genome, and has been associated with several malignancies. This is consistent with findings that protein translation inhibitors, including homoharringtonine, were highly effective in Myc-driven mouse tumors (19). Tumors dependent upon cMet, another short-lived oncoprotein that has been shown to provide subsistence to myeloma cells, could be and additional target for such strategies.
Combinations
Within CML, several clinical trials have combined omacetaxine with cytarabine, interferon, and imatinib (Table 1). Studies adding low-dose omacetaxine to the treatment with TKI for patients with persistent minimal residual disease are being initiated. There is also interest in combining omacetaxine with TKIs for the treatment of patients with blast phase CML, a scenario in which single agent TKI treatment is clearly inadequate for sustained remissions.
Table 1.
Clinical studies with homoharringtonine and omacetaxine in patients with CML (focus on chronic phase).
| Setting / Study | Drug | Regimen | No. | Major cytogenetic response rate | Reference |
|---|---|---|---|---|---|
| Post interferon therapy | |||||
| O'Brien | HHT | Single agent | 71 | 15 | 21 |
| Kantarjian | HHT | With LDAC | 100 | 15 | 23 |
| First-line therapy | |||||
| O'Brien | HHT | Single agent | 90 | 27 | 22 |
| O'Brien | HHT | With IFN | 37 | 43 | 22 |
| Ernst | HHT | With LDAC | 14 | 84 | In 23 |
| Stone | HHT | With LDAC | 44 | 17 | 24 |
| O'Brien | HHT | With LDAC and IFN | 90 | 46 | 25 |
| Post TKI therapy | |||||
| Cortes (T315I) | Omace | Single agent | 62 | 23 | 1 |
| Cortes (≥2 TKI) | Omace | Single agent | 46 | 22 | 2 |
| Cortes (≥2 TKI) | Omace | Single agent | 81 | 20 | 3 |
| Marin (MCyR on imatinib) | SS HHT | With imatinib | 10 | NA | 27 |
NA = not applicable; patients entered the study with MCyR. The objective was to achieve an improvement in transcript levels. Thus was achieved in 7 of 10 patients.
Omace = Omacetaxine; SS HHT = Semisynthetic HHT; LDAC = low-dose cytarabine; IFN = interferon
Overall Conclusions
The approval of omacetaxine identifies agents that primarily target mRNA translation as a new class of cancer therapeutics. Several aspects of its activity still remain to be determined for short-lived oncoproteins or survival proteins. In addition to mechanistic investigations, limited pharmacokinetic studies that have been conducted should be expanded to identify the optimal use (dose, schedule, infusion site) of the agent. Omacetaxine has demonstrated clinical efficacy and plays a role in patients who have experienced resistance or intolerance to multiple TKI. Further studies will explore alternative uses of this drug, including combinations with TKI. Preclinical and clinical investigations of this compound indicate opportunities for its use in hematological malignancies in addition to CML. Furthermore, mechanism-based combination strategies need to be evaluated both in CML and other neoplasm during preclinical and clinical investigations. Finally, activities of this agent with novel actions are guiding us to explore other inhibitors of protein translation.
Acknowledgments
Grant Support
This work was supported in part by CLL Global Research Foundation Alliance grant to VG and WP and a P01 CA049639 from the NIH to JEC.
Footnotes
Conflict of interest:
Dr. Gandhi has received research funding from Teva (Cephalon) for bendamustine, another oncology drug, has received honoraria from Teva, and is a consultant for Teva.
Dr. Plunkett has nothing to disclose.
Dr. Cortes has received research grants from ChemGenex and is a consultant for Teva.
Authors’ Contributions
Conception and design: V. Gandhi, W. Plunkett, J. E. Cortes
Development of methodology: V. Gandhi
Analysis and interpretation of data: V. Gandhi, W. Plunkett, J. E. Cortes
Writing, review, and/or revision of the manuscript: V. Gandhi, W. Plunkett, J. E. Cortes
References
- 01.Cortes J, Lipton JH, Rea D, Digumarti R, Chuah C, Nanda N, et al. Phase 2 study of subcutaneous omacetaxine mepesuccinate after TKI failure in patients with chronic-phase CML with T315I mutation. Blood. 2012;120:2573–80. doi: 10.1182/blood-2012-03-415307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 02.Cortes J, Digumarti R, Parikh PM, Wetzler M, Lipton JH, Hochhaus A, et al. Phase 2 study of subcutaneous omacetaxine mepesuccinate for chronic-phase chronic myeloid leukemia patients resistant to or intolerant of tyrosine kinase inhibitors. Am J Hematol. 2013;88:350–4. doi: 10.1002/ajh.23408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 03.Cortes JE, Nicolini FE, Wetzler M, Lipton JH, Akard L, Craig A, et al. Subcutaneous omacetaxine mepesuccinate in patients with chronic-phase chronic myeloid leukemia previously treated with 2 or more tyrosine kinase inhibitors including imatinib. Clin Lympho Myelo Leukemia. 2013;13:584–91. doi: 10.1016/j.clml.2013.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 04.Kantarjian HM, Talpaz M, Santini V, Murgo A, Cheson B, O'Brien SM. Homoharringtonine. History, current research, and future directions. Cancer. 2001;92:1591–605. doi: 10.1002/1097-0142(20010915)92:6<1591::aid-cncr1485>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 05.Powell RG, Weisleder D, Smith CR, Jr, Rohwedder WK. Structures of harringtonine, isoharringtonine, and homoharringtonine. Tetrahedron Lett. 1970;11:815–8. doi: 10.1016/s0040-4039(01)97839-6. [DOI] [PubMed] [Google Scholar]
- 06.Powell RG, Weisleder D, Smith CR. Antitumor alkaloids from Cephalotaxus harringtonia: structure and activity. J Pharm Sci. 1972;61:1227–30. doi: 10.1002/jps.2600610812. [DOI] [PubMed] [Google Scholar]
- 07.Grem JL, Cheson BD, King SA, Leyland-Jones B, Suffness M. Cephalotaxine esters: antileukemic advance or therapeutic failure? JNCI. 1988;80:1095–103. doi: 10.1093/jnci/80.14.1095. [DOI] [PubMed] [Google Scholar]
- 08.Huang MT. Harringtonine, an inhibitor of protein synthesis. Molec Pharmacol. 1975;11:511–9. [PubMed] [Google Scholar]
- 09.Hagner PR, Schneider A, Gartenhaus RB. Targeting the translational machinery as a novel treatment strategy for hematologic malignancies. Blood. 2010;115:2127–35. doi: 10.1182/blood-2009-09-220020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen R, Gandhi V, Plunkett W. A sequential blockade strategy for the design of combination therapies to overcome oncogene addiction in chronic myelogenous leukemia. Cancer Res. 2006;66:10959–66. doi: 10.1158/0008-5472.CAN-06-1216. [DOI] [PubMed] [Google Scholar]
- 11.Chen R, Guo L, Chen Y, Jiang Y, Wierda WG, Plunkett W. Homoharringtonine reduced Mcl-1 expression and induced apoptosis in chronic lymphocytic leukemia. Blood. 2011;117:156–64. doi: 10.1182/blood-2010-01-262808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jackson RJ, Hellen CUT, Pestova TV. The mechanism of eukaryotic translation initiation and principles of its regulation. Nature Rev Mol Cell Biol. 2010;10:113–27. doi: 10.1038/nrm2838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Scheper GC, van der Knaap MS, Proud CG. Translation matters: protein synthesis defects in inherited disease. Nature Rev Genetics. 2007;8:711–23. doi: 10.1038/nrg2142. [DOI] [PubMed] [Google Scholar]
- 14.Nawijn MC, Alendar A, Berns A. For better or for worse: the role of Pim oncogenes in tumorigenesis. Nature Reviews Cancer. 2011;11:23–34. doi: 10.1038/nrc2986. [DOI] [PubMed] [Google Scholar]
- 15.Fresno M, Gonzales A, Vazquez D, Jimenez A. Bruceantin, a novel inhibitor of peptide-bond formation. Biochim Biophys Acta. 1978;518:104–12. doi: 10.1016/0005-2787(78)90120-x. [DOI] [PubMed] [Google Scholar]
- 16.Gürel G, Blaha G, Moore PB, Steitz TA. U2504 determines the species specificity of the A-site cleft antibiotics: the structures of tiamulin, homoharringtonine, and bruceantin bound to the ribosome. J Mol Biol. 2009;389:146–56.. doi: 10.1016/j.jmb.2009.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Savaraj N, Lu K, Dimery I, Feun LG, Burgess M, Keating M, et al. Clinical pharmacology of homoharringtonine. Cancer Treat Report. 1986;70:1403–7. [PubMed] [Google Scholar]
- 18.Nemunaitis J, Mita A, Stephenson J, Mita MM, Sarantopoulos J, Padmanabhan-Iyer S, et al. Pharmacokinetic study of omacetaxine mepesuccinate administered subcutaneously to patients with advanced solid and hematologic tumors. Cancer Chemother Pharmacol. 2013;71:35–41. doi: 10.1007/s00280-012-1963-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Robert F, Carrier M, Rawe S, Chen S, Lowe S, Pelletier J. Altering chemosensitivity by modulating translation elongation. PLoS ONE. 2009;4:e5428. doi: 10.1371/journal.pone.0005428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Malina A, Mills JR, Pelletier J. Emerging therapeutics targeting mRNA translation. Cold Spring Harb Perspect Biol. 2012 doi: 10.1101/cshperspect.a012377. doi: 10.1101/cshperspect.a012377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kantarjian HM, Keating MJ, Walters RS, Smith TL, O'Brien S, Estey EH, et al. Phase II study of low-dose continuous infusion homoharringtonine in refractory acute myelogenous leukemia. Cancer. 1989;63:813–7. doi: 10.1002/1097-0142(19890301)63:5<813::aid-cncr2820630502>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 22.O'Brien S, Kantarjian H, Keating M, Beran M, Koller C, Robertson LE, et al. Homoharringtonine therapy induces responses in patients with chronic myelogenous leukemia in late chronic phase. Blood. 1995;86:3322–6. [PubMed] [Google Scholar]
- 23.O'Brien S, Kantarjian H, Koller C, Feldman E, Beran M, Andreeff M, et al. Sequential homoharringtonine and interferon-alpha in the treatment of early chronic phase chronic myelogenous leukemia. Blood. 1999;93:4149–53. [PubMed] [Google Scholar]
- 24.Kantarjian HM, Talpaz M, Smith TL, Cortes J, Giles FJ, Rios MB, et al. Homoharringtonine and low-dose cytarabine in the management of late chronic-phase chronic myelogenous leukemia. J Clin Oncol. 2000;18:3513–21. doi: 10.1200/JCO.2000.18.20.3513. [DOI] [PubMed] [Google Scholar]
- 25.Stone RM, Donohue KA, Stock W, Hars V, Linker CA, Shea T, et al. Cancer and Leukemia Group B, A phase II study of continuous infusion Homoharringtonine and cytarabine in newly diagnosed patients with chronic myeloid leukemia: CALGB study 19804. Cancer Chemother Pharmacol. 2009;63:859–64. doi: 10.1007/s00280-008-0805-8. [DOI] [PubMed] [Google Scholar]
- 26.O'Brien S, Giles F, Talpaz M, Cortes J, Rios MB, Shan J, et al. Results of triple therapy with interferon-alpha, cytarabine, and Homoharringtonine, and the impact of adding imatinib to the treatment sequence in patients with Philadelphia chromosome-positive chronic myelogenous leukemia in early chronic phase. Cancer. 2003;98:888–93. doi: 10.1002/cncr.11620. [DOI] [PubMed] [Google Scholar]
- 27.Quintas-Cardama A, Kantarjian H, Garcia-Manero G, O'Brien S, Faderl S, Estrov Z, et al. Phase I/II study of subcutaneous homoharringtonine in patients with chronic myeloid leukemia who have failed prior therapy. Cancer. 2007;109:248–55. doi: 10.1002/cncr.22398. [DOI] [PubMed] [Google Scholar]
- 28.Marin D, Kaeda JS, Andreasson C, Saunders SM, Bua M, Olavarria E, et al. Phase I/II trial of adding semisynthetic homoharringtonine in chronic myeloid leukemia patients who have achieved partial or complete cytogenetic response on imatinib. Cancer. 2005;103:1850–5. doi: 10.1002/cncr.20975. [DOI] [PubMed] [Google Scholar]
- 29.Okabe S, Tauchi T, Tanaka Y, Katagiri S, Kitahara T, Ohyashiki K. Activity of omacetaxine mepesuccinate against ponatinib-resistant BCR-ABL-positive cells. Blood. 2013;122:3086–8. doi: 10.1182/blood-2013-04-494773. [DOI] [PubMed] [Google Scholar]
- 30.Allan EK, Holyoake TL, Craig AR, Jørgensen HG. Omacetaxine may have a role in chronic myeloid leukaemia eradication through down-regulation of Mcl-1 and induction of apoptosis in stem/progenitor cells. Leukemia. 2011;25:985–94.. doi: 10.1038/leu.2011.55. [DOI] [PubMed] [Google Scholar]
- 31.Lindqvist LM, Vikström I, Chambers JM, McArthur K, Ann Anderson M, Henley KJ, et al. Translation inhibitors induce cell death by multiple mechanisms and Mcl-1 reduction is only a minor contributor. Cell Death Dis. 2012;11:3, e409. doi: 10.1038/cddis.2012.149. [DOI] [PMC free article] [PubMed] [Google Scholar]