Abstract
Background
There are two types of diabetes. Type 1 diabetes affects younger people and needs treatment with insulin injections. Type 2 diabetes affects older people and can usually be treated by diet and oral drugs. Diabetic neuropathy affects 10% of patients with diabetes mellitus at diagnosis and 40% to 50% after 10 years. Enhanced glucose control is the best studied intervention for the prevention of this disabling condition but there have been no systematic reviews of the evidence.
Objectives
To examine the evidence for enhanced glucose control in the prevention of distal symmetric polyneuropathy in people with type 1 and type 2 diabetes.
Search methods
We searched the Cochrane Neuromuscular Disease Group Specialized Register (30 January 2012), CENTRAL (2012, Issue 1), MEDLINE (1966 to January 2012) and EMBASE (1980 to January 2012) for randomized controlled trials of enhanced glucose control in diabetes mellitus.
Selection criteria
We included all randomized, controlled studies investigating enhanced glycemic control that reported neuropathy outcomes after at least one year of intervention. Our primary outcome measure was annual development of clinical neuropathy defined by a clinical scale. Secondary outcomes included motor nerve conduction velocity and quantitative vibration testing.
Data collection and analysis
Two authors independently reviewed all titles and abstracts identified by the database searches for inclusion. Two authors abstracted data from all included studies with a standardized form. A third author mediated conflicts. We analyzed the presence of clinical neuropathy with annualized risk differences (RDs), and conduction velocity and quantitative velocity measurements with mean differences per year.
Main results
This review identified 17 randomized studies that addressed whether enhanced glucose control prevents the development of neuropathy. Seven of these studies were conducted in people with type 1 diabetes, eight in type 2 diabetes, and two in both types. A meta‐analysis of the two studies that reported the primary outcome (incidence of clinical neuropathy) with a total of 1228 participants with type 1 diabetes revealed a significantly reduced risk of developing clinical neuropathy in those with enhanced glucose control, an annualized RD of ‐1.84% (95% confidence interval (CI) ‐1.11 to ‐2.56). In a similar analysis of four studies that reported the primary outcome, involving 6669 participants with type 2 diabetes, the annualized RD of developing clinical neuropathy was ‐0.58% (95% CI 0.01 to ‐1.17). Most secondary outcomes were significantly in favor of intensive treatment in both populations. However, both types of diabetic participants also had a significant increase in severe adverse events including hypoglycemic events.
Authors' conclusions
According to high‐quality evidence, enhanced glucose control significantly prevents the development of clinical neuropathy and reduces nerve conduction and vibration threshold abnormalities in type 1 diabetes mellitus. In type 2 diabetes mellitus, enhanced glucose control reduces the incidence of clinical neuropathy, although this was not formally statistically significant (P = 0.06). However, enhanced glucose control does significantly reduce nerve conduction and vibration threshold abnormalities. Importantly, enhanced glucose control significantly increases the risk of severe hypoglycemic episodes, which needs to be taken into account when evaluating its risk/benefit ratio.
Keywords: Humans; Diabetes Mellitus, Type 1; Diabetes Mellitus, Type 1/complications; Diabetes Mellitus, Type 2; Diabetes Mellitus, Type 2/complications; Diabetic Neuropathies; Diabetic Neuropathies/prevention & control; Hyperglycemia; Hyperglycemia/prevention & control; Hypoglycemic Agents; Hypoglycemic Agents/therapeutic use; Insulin; Insulin/therapeutic use
Plain language summary
Enhanced glucose control for preventing and treating diabetic neuropathy
Diabetes is defined as high sugar levels in the blood. There are two forms of the disease. In type 1 diabetes, the body does not produce enough insulin. In type 2 diabetes, the body becomes less responsive to insulin. Regardless of the type of diabetes, many people develop a disabling neuropathy. Neuropathy is a condition that results in numbness, tingling, pain, or weakness that typically starts in the feet and progresses up the legs. The distribution is often described as a stocking glove pattern since the feet are affected first followed by the legs and fingers. The most common treatment for diabetes is control of blood sugar levels in an attempt to prevent the many complications, including neuropathy. This review identified 17 randomized studies that addressed whether more aggressive attempts to lower blood glucose levels prevent people from developing neuropathy. Seven of these studies were conducted in people with type 1 diabetes, eight in type 2 diabetes, and two in both types. However, only two studies in type 1 diabetes including 1228 participants and four studies in type 2 diabetes including 6669 participants investigated our primary outcome. In type 1 diabetes, there was a significant effect of more aggressive therapies in preventing neuropathy compared with standard treatment. In type 2 diabetes, more aggressive therapy was also beneficial in preventing symptoms and signs of clinical neuropathy, but the result was not statistically significant as measured by the primary method selected for this review. However, there was a significant positive effect on the amount of nerve damage measured with electrical nerve conduction tests and a special machine to measure the threshold of detection of vibration in both types of diabetes. Overall, the evidence indicates that more aggressive treatments of sugar levels delay the onset of neuropathy in both types of diabetes. No other treatments have proven effective to date. However, the beneficial effect has to be balanced against the significantly increased risk of dangerously low blood sugar levels that can occur in both types of diabetes and which can lead to brain injury amongst other issues.
Summary of findings
Summary of findings for the main comparison. Enhanced glucose control for diabetic neuropathy in type 1 diabetes.
| Enhanced glucose control for diabetic neuropathy | ||||||
| Patient or population: patients with diabetic neuropathy Settings: outpatients Intervention: enhanced glucose control | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Enhanced glucose control | |||||
| Incidence of clinical neuropathy after 5 years: risk ratio Follow‐up: 5 years | 173 per 1000 | 79 per 1000 (57 to 109) | RR 0.46 (0.33 to 0.63) | 1228 (3 studies) | ⊕⊕⊕⊕ high | Large significant difference in favor of enhanced glucose control. Annualized RD of ‐1.84% (95% CI ‐2.56 to ‐1.11) |
| Annual change in peroneal nerve motor conduction velocity m/sec | The mean annual change in peroneal nerve motor conduction velocity in the control groups was ‐0.33 m/sec | The mean annual change in peroneal nerve motor conduction velocity in the intervention groups was 0.61 higher (0.51 to 0.71 higher) | ‐ | 1371 (4 studies) | ⊕⊕⊕⊕ high | Small significant difference in favor of enhanced glucose control |
| Annual change in median nerve motor conduction velocity | The mean annual change in median nerve motor conduction velocity in the control groups was ‐0.25 m/sec | The mean annual change in median nerve motor conduction velocity in the intervention groups was 0.46 higher (0.36 to 0.57 higher) | ‐ | 1241 (2 studies) | ⊕⊕⊕⊕ high | Small significant difference in favor of enhanced glucose control |
| Annual change in ulnar nerve motor conduction velocity | The mean annual change in ulnar nerve motor conduction velocity in the control groups was ‐0.93 m/sec | The mean annual change in ulnar nerve motor conduction velocity in the intervention groups was 1.49 higher (0.74 lower to 3.71 higher) | ‐ | 134 (2 studies) | ⊕⊕⊕⊝ moderate1 | No significant difference |
| Annual change in vibration threshold in the feet | The mean annual change in vibration threshold in the feet in the control groups was ‐0.62 SMD | The mean annual change in vibration threshold in the feet in the intervention groups was 0.32 standard deviations higher (0.02 to 0.62 higher) | ‐ | 177 (3 studies) | ⊕⊕⊕⊕ high | Small significant difference in favor of enhanced glucose control |
| Adverse events | See comment | See comment | Not estimable | ‐ | ‐ | Hypoglycemic episodes significantly more common with enhanced glucose control: see text |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RD: risk difference; RR: risk ratio; SMD: standardized mean difference | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Wide 95% CI.
Summary of findings 2. Enhanced glucose control for diabetic neuropathy in type 2 diabetes.
| Enhanced glucose control for diabetic neuropathy | ||||||
| Patient or population: patients with diabetic neuropathy Settings: outpatients Intervention: enhanced glucose control | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Enhanced glucose control | |||||
| Annual incidence of clinical neuropathy: risk ratio | See comment | See comment | Not estimable | 6669 (4 studies) |
⊕⊕⊕⊕ high | Annualized RD ‐0.58% (95% CI ‐1.40 to 0.01) less with enhanced glucose control |
| Annual change in median nerve motor conduction velocity | The mean annual change in median nerve motor conduction velocity in the control groups was ‐0.125 m/sec | The mean annual change in median nerve motor conduction velocity in the intervention groups was 0.56 higher (0.53 to 0.6 higher) | ‐ | 99 (1 study) | ⊕⊕⊕⊝ moderate1,2 | Small significant difference in favor of enhanced glucose control |
| Annual change in vibration threshold in the feet | The mean annual change in vibration threshold in the feet in the control groups was ‐2.25 micrometers | The mean annual change in vibration threshold in the feet in the intervention groups was 1.63 higher (1.34 to 1.91 higher) | ‐ | 99 (1 study) | ⊕⊕⊕⊝ moderate1 | Small significant difference in favor of enhanced glucose control |
| Death | 40 per 1000 | 50 per 1000 (42 to 60) | RR 1.26 (1.06 to 1.51) | 10,251 (1 study) | ⊕⊕⊕⊕ high | Significantly more deaths with enhanced glucose control: led to termination of the trial |
| Weight gain | 141 per 1000 | 277 per 1000 (256 to 301) | RR 1.96 (1.81 to 2.13) | 10,078 (1 study) | ⊕⊕⊕⊕ high | Large significant difference indicating harm from enhanced glucose control |
| Other adverse events | See comment | See comment | Not estimable | ‐ | See comment | Hypoglycemic episodes significantly more common with enhanced glucose control: see text |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RD: risk difference; RR: risk ratio; SMD: standardized mean difference | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Single trial with small sample 2 Wide 95% CI includes benefit or harm
Background
Diabetes mellitus is a disease caused by an inability of the body to metabolize glucose properly, either through impaired insulin secretion, insulin resistance, or both. Before the introduction of insulin and oral medications targeting blood glucose levels, the diagnosis of diabetes heralded early death in type 1 (early onset, insulin requiring) disease. It also shortened lifespan in type 2 (late onset, not insulin requiring) disease. Furthermore, any of a large number of complications compromised general health and quality of life (Gale 2001).
Hyperglycemia is diagnostic for diabetes (Expert Committee 2003); however, the causality and association of hyperglycemia in the many observed complications remain to be fully established. A number of hypotheses have been advanced to explain how hyperglycemia may have its myriad effects on the vascular system and multiple organs. These include, but are not limited to, theories related to advanced glycation products, the polyol pathway, the hexosamine pathway, and the protein kinase C pathway (Brownlee 2005). The elucidation of metabolic disruptions related to hyperglycemia is providing new targets within metabolic pathways for treatment to reduce complications.
Peripheral neuropathy is one of the many complications of diabetes, resulting in significant morbidity and mortality. Diabetic neuropathy is considered to be “the presence of symptoms and/or signs of peripheral nerve dysfunction in people with diabetes after the exclusion of other causes” (Boulton 1998). A number of types of autonomic and somatic diabetic neuropathies are recognized (Boulton 2004; Vinik 2003), of which peripheral sensorimotor neuropathy is the most common. Diabetic peripheral neuropathy may be asymptomatic, clinically evident with either positive (painful) or negative (lack of sensation) symptoms, or both, or clinically evident and associated with further complications such as distal weakness (hands and feet), imbalance, foot ulcers, joint destruction (called Charcot arthropathy), and amputations of the lower limbs. Symptoms typically start in the feet and progress up the legs before the involvement of the hands in a length‐dependent manner.
The presence of peripheral diabetic neuropathy is suggested by complaints of numbness, pain, or both, usually in a symmetrical distribution and noticed first in the toes. Casual neurological exam performed in an office setting may reveal impairments in sensation to light touch, pinprick, vibration, or joint position sense. Quantitative measures of neuropathy may be obtained through quantitative sensory testing (of vibration, thermal, and pain thresholds) and nerve conduction studies. A severity staging system based on neurological exam and more formal testing has been developed by Dyck (Dyck 2003). Composite scores incorporating physical examination and sensory testing, such as the Neuropathy Impairment Score developed by Dyck (Dyck 2005), are predictors of foot ulceration (North‐West Diabetes Foot Care Study 2002).
The prevalence of peripheral neuropathy at the time of diagnosis of diabetes (diagnosis by abnormalities in blood glucose levels demonstrated through an abnormal oral glucose tolerance test or elevated levels of fasting or random blood glucose) is close to 10% and may be the presenting complaint that leads to the diagnosis of diabetes. The prevalence increases to 40% to 50% at 10 years after diagnosis. The highest prevalence of neuropathy is in those people with poorest blood glucose control as measured by hemoglobin A1c (HbA1c) or glycated hemoglobin (GHb) (Partanen 1995). The annual incidence of new foot ulcers yearly in a community‐based diabetic population in the UK was 2.2% (North‐West Diabetes Foot Care Study 2002).
Hyperglycemia has been the most visible target for intervention in preventing complications of diabetes. Before the development of insulin, there were trials of diet to control hyperglycemia and prolong life in people with type 1 diabetes. Insulin and oral hypoglycemic agents were developed to target hyperglycemia in type 1 and type 2 diabetics and are used with varying degrees of success. The perceived benefits of hyperglycemic control in general led to trials of strict glycemic control and evaluation of individual complications (including peripheral neuropathy). The first major trials were the Diabetes Control and Complications Trial (DCCT 1993a; DCCT 1993b) targeting type 1 diabetes and the UK Prospective Diabetes Study (UKPDS Study Group 1998) targeting type 2 diabetes. Control of hyperglycemia has to be balanced against the risk of hypoglycemic episodes which are associated with their own morbidity. Presence and progression of peripheral neuropathy was a secondary outcome in these and other randomized trials of glycemic control and a primary outcome in a number of observational studies and trials with regard to glycemic control. Although there have been non‐systematic reviews of glycemic control, we did not know of a systematic review.
Objectives
We set out to review the benefits and harms of enhanced glycemic control for preventing and treating distal symmetrical sensory and motor diabetic neuropathy.
Methods
Criteria for considering studies for this review
Types of studies
Randomized controlled trials (RCTs) of enhanced glycemic control for type 1 and type 2 diabetes in which the presence or severity of peripheral neuropathy has been measured.
Types of participants
Males or females of any age with type 1 or type 2 diabetes (diagnosed by the accepted standard at the time of the study with criteria stated in the publication).
Types of interventions
Any intervention that enhances glycemic control more than standard care for a period of 12 months or more. Interventions might include more frequent subcutaneous insulin administration, continuous insulin infusion, oral antidiabetic agents, lifestyle modifications such as diet and exercise, or pancreas transplant.
Types of outcome measures
We analyzed change in two ways. First, we dichotomized results into improved or unchanged versus worse by an amount which was predefined as being clinically significant for each scale. Where the clinical significance of a scale had been investigated, we used the definition of clinical significance proposed by the authors of the scale. For continuous scales, we took a clinically significant change as being half a standard deviation (SD) of the combined baseline values. Secondly, we treated the results from the two groups as continuous scales and presented the mean differences (MDs) and 95% confidence intervals (CIs).
Primary outcomes
The primary outcome was incidence of clinical neuropathy (in those without clinical neuropathy at baseline). If clinical neuropathy was not reported, then assessment of the primary outcome was not possible. Nevertheless, we still included studies in the review if data for any of the secondary outcomes were adequate.
Secondary outcomes
Secondary outcomes were change in:
neuropathic symptoms (measured by change in symptom scales including pain scales);
nerve conduction studies in the following order of preference: peroneal nerve motor conduction velocity (MCV), median nerve MCV, ulnar nerve MCV, peroneal nerve distal compound muscle action potential amplitude (CMAP), median CMAP, ulnar CMAP, and sural sensory nerve action potential amplitude (SNAP);
quantitative sensory testing (vibration, pain or temperature) in the lower extremities;
adverse events classified into foot ulcers, amputations, hypoglycemic episodes requiring hospitalization, serious adverse events, events which prevented continuation with the trial, and other events.
Timing of outcome assessments
The primary time for assessing outcome was 12 months. We did not consider outcomes measured after less than 12 months of treatment. Where outcomes had been measured after intervals longer than 12 months, we have presented the annual rates of worsening for dichotomous outcomes and annual rates of change for continuous outcomes.
'Summary of findings' tables
We prepared 'Summary of findings' tables including the outcomes: development of clinical neuropathy, change in motor nerve conduction velocity, change in vibration threshold in the feet, and serious adverse events.
Search methods for identification of studies
Electronic searches
We searched the Cochrane Neuromuscular Disease Group Specialized Register (30 January 2012), CENTRAL (The Cochrane Library 2012, Issue 1), MEDLINE (1966 to January 2012), and EMBASE (1980 to January 2012) for RCTs in all languages using the following search terms: (diabetic neuropathy or diabetic polyneuropathy or peripheral nervous system diseases) and (insulin infusion or enhanced glycemic control or pancreatic transplantation). We also searched the Current Controlled Trials register (www.controlled‐trials.com/) for ongoing and recently completed trials. The detailed search strategies are in the appendices: MEDLINE (Appendix 1), EMBASE (Appendix 2), CENTRAL (Appendix 3), and Current Controlled Trials Register (Appendix 4).
Searching other resources
We reviewed the bibliographies of the randomized trials identified, contacted the authors and known experts in the field and approached pharmaceutical companies to identify additional published or unpublished data.
Data collection and analysis
Two review authors (BCC, AAL, ELF, or RACH) inspected the titles and abstracts of all the references retrieved by the searches and decided upon selection independently. We resolved disagreement by discussion with a third author if necessary. We obtained the full papers of the selected references for further assessment and two review authors (BCC, AAL, ELF, or RACH) decided upon inclusion. We resolved disagreement by discussion with the third author (ELF or RACH) if necessary. We included only RCTs.
Data extraction and management
We designed a data extraction tool including the following.
Details of study quality (as above)
Details of study design (treatment duration, follow‐up duration)
Other study details (inclusion and exclusion criteria, number of participants, number of persons withdrawing or lost to follow‐up, reasons for withdrawal)
Details of intervention to control hyperglycemia
Baseline measurements of interest
Results of outcomes selected as of interest for this review (as above) outcomes including adverse events
Results of the primary outcome selected by the trial authors if not included above
Text entry for the conclusion of the trial authors
Two review authors extracted data independently.
Assessment of risk of bias in included studies
Two review authors (BCC, AAL, ELF, or RACH) independently assessed the risk of bias for the included studies with the Cochrane Collaboration’s tool for assessing risk of bias (Higgins 2011). In case of disagreement, the third review author (ELF or RACH) adjudicated. This tool considers sequence generation, allocation concealment, blinding of participants, personnel and outcome assessors, incomplete outcome data, selective outcome reporting, and other sources of bias. We used the quality ratings of high, low, or unclear risk. We made separate assessments for each main outcome (or class of outcomes). We completed a 'Risk of bias' table for each of the studies included in our review.
Measures of treatment effect
Our preferred methods of comparison for dichotomous outcomes were risk differences (RDs) and risk ratios (RRs) and, for continuous outcomes, mean differences (MDs). For each trial that measured our primary outcome, we calculated RDs and corresponding standard errors (SEs) over the period of follow‐up and then divided by the length of follow‐up. We report these results as annualized RDs. Of note these values are not true incidence rates because such rates can only be calculated if the total person‐years of follow‐up (allowing for censoring at the time of an event and for dropouts) were available. For continuous outcomes, if different scales were used and it was not possible to convert the data into the same scale we expressed the change in outcome in standard deviation (SD) units and reported standardized mean differences (SMDs). We calculated annual rates of change for continuous outcomes (MD per year). Uncertainty was expressed with 95% CIs.
Assessment of heterogeneity
We used the I2 statistic in the Cochrane statistical software Review Manager 5.1.2 (RevMan) to test for heterogeneity.
Assessment of reporting biases
We considered the possibility of publication and reporting biases and if there had been sufficient trials we would have constructed funnel plots to help detect bias.
Data synthesis
Where data from similar outcome measures were available for more than one trial of a similar intervention, we performed meta‐analysis with the RevMan software.
Subgroup analysis and investigation of heterogeneity
In our protocol we originally intended to include type 1 and type 2 diabetes together and then perform a subgroup analysis, but because of the known clinical and biological differences between the types we have collected data and performed the analyses for each separately and not combined.
If data had allowed, we would have performed the following subgroup analyses.
Glycemic control measured by HbA1c at randomization divided into strict: < 7.0%, versus moderate: 7.0% to 9.0%, versus poor: > 9.0%
'Early' (two years or less from diagnosis of diabetes) versus 'established' (more than two years from diagnosis of diabetes)
Age: 50 years or less versus more than 50 years
If heterogeneity was suggested by an I2 statistic > 50%, we inspected the forest plots and tried to explain the heterogeneity by differences between the trials in study populations, trial interventions, or trial methodological quality attributes. We performed initial analyses with a fixed‐effect model. Where no explanation was satisfactory, we would have repeated the analysis with a random‐effects model.
Sensitivity analysis
If trials differed in their risk of bias, we repeated any meta‐analyses omitting trials with a high risk of bias.
Results
Description of studies
Results of the search
The search retrieved 101 titles and abstracts from the Cochrane Neuromuscular Disease Group Specialized Register. We selected 36 for full‐text examination and of these we included 10. From the MEDLINE search, we identified 183 titles and abstracts, of which 34 were selected for full‐text examination. We included 14 of these articles. The search also retrieved 215 titles and abstracts from the CENTRAL database, 20 of which we examined in detail and from which we selected 13 for inclusion. From the EMBASE search, we identified 154 titles and abstracts. We selected four for examination and none for inclusion. Many of the included articles were identified in multiple databases so that there were only 14 unique trials. The authors included an additional three trials identified from personal knowledge (Accord 2010; Kawamori 1991; UKPDS Study Group 1998). Included studies are described in Characteristics of included studies.
Included studies
Type 1 diabetes
We included seven clinical trials that studied people with type 1 diabetes (median duration of follow‐up 2 to 7.5 years) and two that studied both subsets of diabetes (median duration of follow‐up three to four years). Of the seven studies exclusively of type 1 diabetes, all compared different levels of insulin regimens other than Reichard 1993 which investigated education versus standard care. Three of the seven compared the effectiveness of continuous insulin pumps to intermittent injections. The two studies including both types of diabetes both compared different insulin regimens.
Type 2 diabetes
Of the eight studies of type 2 diabetes (median duration of follow‐up 1 to 10 years), three directly compared different insulin regimens. The remaining five trials either investigated the effects of more aggressive glycemic goals through the use of diet and exercise, oral hypoglycemic agents, insulin, or oral hypoglycemic agents plus insulin.
The primary outcome for this review was reported in only two of the studies of type 1 diabetes (DCCT 1993a; DCCT 1993b; Linn 1996) and in four studies of type 2 diabetes (Accord 2010; Azad 1999; Duckworth 2009; Tovi 1998). Secondary outcomes measured in more than one trial included peroneal nerve motor conduction velocity (MCV), ulnar nerve MCV, median MCV, and vibration threshold in the feet.
Excluded studies
None.
Risk of bias in included studies
The risk of bias of the included studies is summarized in Figure 1.
1.
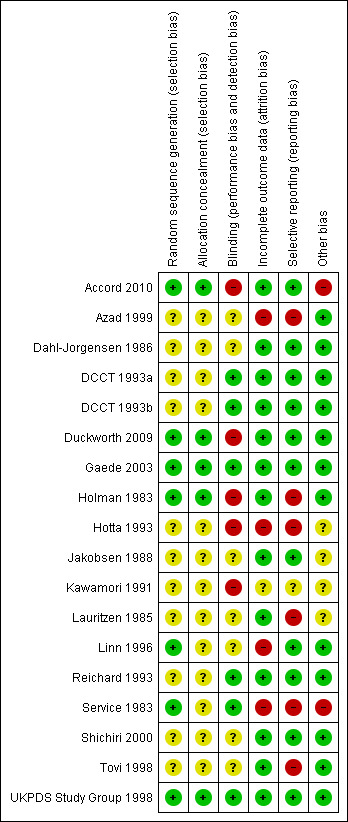
'Risk of bias' summary: review authors' judgments about each risk of bias item for each included study.
Allocation
Generation of the randomization sequence was adequate in seven out of 17 studies and unclear in 10. Similarly, the allocation concealment was sufficient in five of 17 studies and unclear in the remaining 12.
Blinding
Five studies had inadequate blinding, five were sufficient, and the remaining seven were unclear.
Incomplete outcome data
Four studies had incomplete outcome data, 12 were sufficient, and one was unclear.
Selective reporting
Six studies had selective reporting of data, 10 were sufficient, and one was unclear.
Other potential sources of bias
Two studies had other potential sources of bias, 11 were sufficient, and four were unclear.
Effects of interventions
Type 1 diabetes
Primary outcome: incidence of clinical neuropathy after at least one year
Seven studies investigated people with type 2 diabetes and two additional studies involved those with type 1 and type 2 diabetes. Only two of the included trials measured this outcome (DCCT 1993a; DCCT 1993b; Linn 1996). The DCCT trial (DCCT 1993a; DCCT 1993b) reported separately the results for the primary prevention participants ("IDDM of 1 to 5 years' duration, no detectable retinopathy on stereo fundus photography, and urinary albumin excretion less than 40 mg/24 hour") and the secondary prevention participants ("IDDM of 1 to 15 years' duration, very mild to moderate non‐proliferative retinopathy, and urinary albumin excretion less than 200 mg/24 hour"). Note that primary prevention refers not to neuropathy but to retinopathy. Definite clinical neuropathy was defined as the presence of two or more of the following: symptoms, sensory examination findings, and decreased or absent reflexes. Of the participants 1441 were randomized but only 1436 received baseline neuropathy assessment (primary cohort: 346 intensive, 376 conventional; secondary cohort: 362 intensive, 353 conventional). Of the 1243 participants that had an evaluation for neuropathy at five years, 1186 did not have neuropathy at baseline (primary cohort: 252 intensive, 292 conventional; secondary cohort: 327 intensive, 315 conventional) (DCCT 1995).
At five years in the primary prevention cohort, there was a decrease in the incidence of clinical neuropathy in the participants randomized to intensive treatment compared to standard treatment: annualized RD ‐1.53% (95% CI ‐2.54 to ‐0.51), RR at five years 0.47 (95% CI 0.27 to 0.80) (DCCT 1993a). Confidence in the significance of this result is reduced by major losses to follow‐up in both groups (25% and 21% respectively). In comparison, the retention of cases after five years in the secondary prevention cohort (DCCT 1993b) was nearly complete with only 4.1% and 3.4% lost to follow‐up in the two groups respectively. In this cohort, the annualized RD was ‐1.97% (95% CI ‐3.04 to ‐0.90) in favor of enhanced glucose control (RR at five years 0.48, 95% CI 0.32 to 0.73). The Linn 1996 trial defined definite neuropathy differently as the presence of three of the following: symptoms, examination signs, abnormal quantitative sensory testing, and peroneal motor nerve conduction velocity. They followed 49 consecutive participants with newly diagnosed IDDM, based on World Health Organization recommendations, admitted to their clinic. After five years, one participant in the intensive group and six participants in the conventional group developed neuropathy. In the absence of any measure of sensory or motor impairment we used the dichotomised composite scores of presence or absence of neuropathy as the basis for a meta‐analysis. The annualized RD was ‐5.45% (95% CI ‐9.95 to ‐0.95) in favor of enhanced glucose control (RR at five years 0.14, 95% CI 0.02 to 1.05). With the assumption that the two definitions in the different trials measure the same construct, we performed a meta‐analysis of all three trials (DCCT 1993a; DCCT 1993b; Linn 1996) which showed that the annualized RD of developing neuropathy was highly significantly reduced in those randomized to enhanced treatment compared with conventional management: ‐1.84% (95% CI ‐2.56 to ‐1.11) (Analysis 1.1; Analysis 1.2; Figure 2; Figure 3; Table 1).
1.1. Analysis.
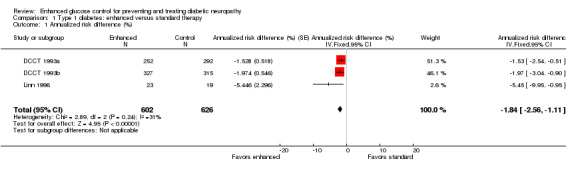
Comparison 1 Type 1 diabetes: enhanced versus standard therapy, Outcome 1 Annualized risk difference (%).
1.2. Analysis.
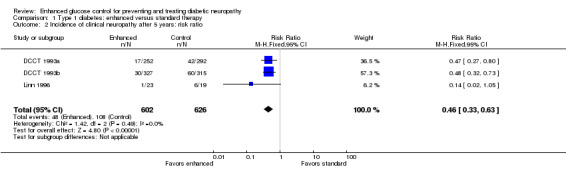
Comparison 1 Type 1 diabetes: enhanced versus standard therapy, Outcome 2 Incidence of clinical neuropathy after 5 years: risk ratio.
2.
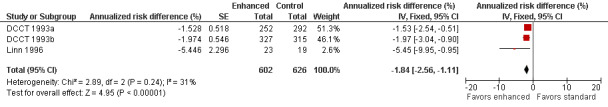
Forest plot of comparison: 1 Type 1 diabetes: enhanced versus standard therapy, outcome: 1.1 Annualized risk difference (%).
3.
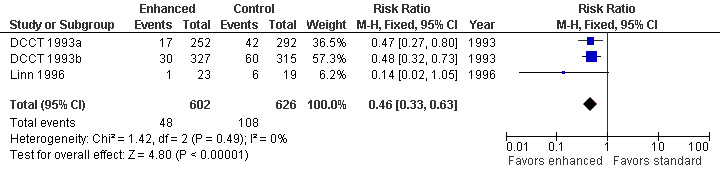
Forest plot of comparison: 1 Type 1 diabetes: enhanced versus standard therapy, outcome: 1.2 Incidence of clinical neuropathy after 5 years: risk ratio.
Secondary outcome: change in peroneal nerve motor conduction velocity
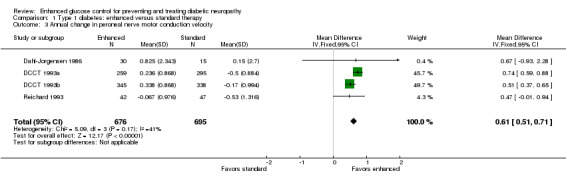
Four studies measured peroneal nerve motor conduction velocity (Dahl‐Jorgensen 1986; DCCT 1993a; DCCT 1993b; Reichard 1993; Service 1983). Service 1983 did not provide information on the variation in their measurements, and could not be included in the meta‐analysis. They did report that there was no significant difference between the two treatment groups. In Dahl‐Jorgensen 1986, 45 participants with type 1 diabetes were randomly assigned to three modes of treatment: continuous subcutaneous insulin infusion (CSII), multiple insulin injections, or continued conventional treatment with twice daily injections of insulin. In the continuous insulin group there was an annual increase of 1.45 (2.7) m/s compared to 0.2 (1.8) m/s in the multiple injection group and 0.15 (2.7) m/s in the conventional group (MD between intensive and conventional 0.67 m/s, 95% CI ‐0.93 to 2.28). In the meta‐analysis the two intensive treatments were combined and compared to the conventional treatment group. When the results of all the trials were combined, the annual MD was 0.61 m/s (95% CI 0.51 to 0.71) in favor of the intensive group (Analysis 1.3; Figure 4; Table 1).
1.3. Analysis.
Comparison 1 Type 1 diabetes: enhanced versus standard therapy, Outcome 3 Annual change in peroneal nerve motor conduction velocity.
4.
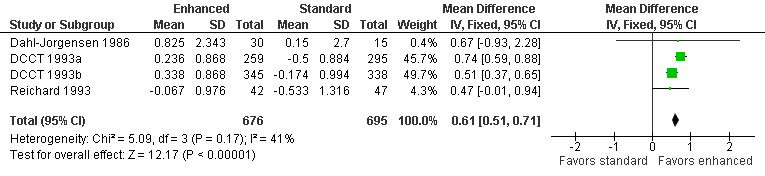
Forest plot of comparison: 1 Type 1 diabetes: enhanced versus standard therapy, outcome: 1.3 Annual change in peroneal nerve motor conduction velocity.
Secondary outcome: annual change in median nerve motor conduction velocity
Median nerve MCV was only measured in DCCT 1993a and DCCT 1993b. In the meta‐analysis of the two parts of this study, there was a significant improvement in median nerve MCV in the enhanced glucose compared to the control group: MD 0.46 m/s (95% CI 0.36 to 0.57) (Analysis 1.4; Table 1).
1.4. Analysis.
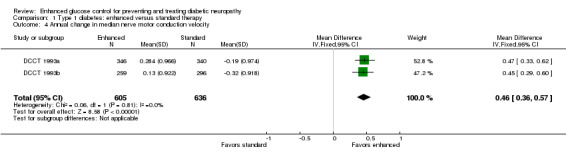
Comparison 1 Type 1 diabetes: enhanced versus standard therapy, Outcome 4 Annual change in median nerve motor conduction velocity.
Secondary outcome: annual change in ulnar nerve motor conduction velocity
In the two studies which included this measurement (Dahl‐Jorgensen 1986; Reichard 1993), a meta‐analysis showed a non‐significant difference in favor of enhanced glucose control in the annual change in ulnar nerve motor conduction velocity: MD 1.49 m/s (95% CI ‐0.74 to 3.71) (Analysis 1.5; Table 1).
1.5. Analysis.
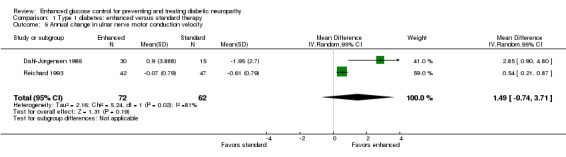
Comparison 1 Type 1 diabetes: enhanced versus standard therapy, Outcome 5 Annual change in ulnar nerve motor conduction velocity.
Secondary outcome: annual change in vibration threshold in the feet
Four studies reported this outcome (Holman 1983; Jakobsen 1988; Reichard 1993; Service 1983). Service 1983 did not report the variance of their measurements and was not included in the meta‐analysis. They did report that there was no significant difference between the two treatment groups. A meta‐analysis of the other three studies showed a marginally significant difference in favor of enhanced glucose control: SMD 0.32 (95% CI 0.02 to 0.62) (Analysis 1.6; Figure 5; Table 1).
1.6. Analysis.
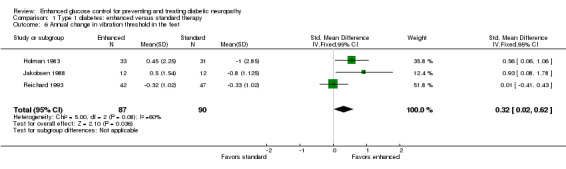
Comparison 1 Type 1 diabetes: enhanced versus standard therapy, Outcome 6 Annual change in vibration threshold in the feet.
5.
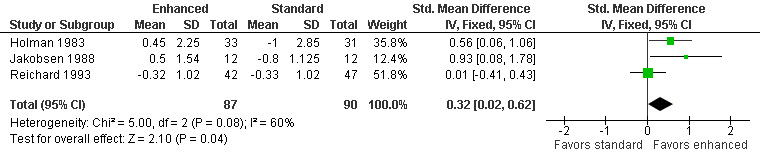
Forest plot of comparison: 1 Type 1 diabetes: enhanced versus standard therapy, outcome: 1.6 Annual change in vibration threshold in the feet.
Other outcomes
Lauritzen 1985 measured vibration quantitative sensory testing in the hands, feet, and legs but did not report any specific quantitative results. They did state that there were no significant differences between treatment groups for any of these three outcomes. Reichard 1993 also reported the number of participants with new symptoms of neuropathy including paresthesias, dulled sensation, and pain in the feet or legs after 7.5 years. They discovered one new participant out of 48 with neuropathic symptoms in the intensive group and five new participants out of 54 in the conventional group (P = 0.21, Fisher's exact test). Hotta 1993 followed 50 participants with type 1 and type 2 diabetes, but did not distinguish between these subgroups to allow for proper comparisons with other studies.
Adverse events
Five studies reported on the adverse events seen in the two treatment groups. The DCCT study (DCCT 1993a; DCCT 1993b) reported 62 episodes of hypoglycemia requiring assistance per 100 patient‐years in the enhanced glucose control group, compared with 19 in the conventional group (P < 0.001). Besides hypoglycemic episodes, the DCCT group (DCCT 1993a; DCCT 1993b) demonstrated more deaths (seven versus four) and hospitalizations (54 versus 36), but similar numbers of motor vehicle accidents (one versus one) and other accidents (20 versus 22) in the 711 intensive participants compared to the 730 conventional participants. The rate of coma/seizure (16 versus 5 per 100 patient‐years) and becoming overweight (12.7 versus 9.3 per 100 patient‐years) was also higher in the intensive group.
Reichard 1993 also reported more episodes of hypoglycemia with intensive treatment, 110 episodes per 100 patient‐years of severe hypoglycemia compared with 40 with standard treatment. On the other hand, Dahl‐Jorgensen 1986 reported similar numbers of symptomatic hypoglycemic episodes per week per participant in all three groups. Dahl‐Jorgensen 1986 revealed more participants with hypoglycemic coma in the standard (7 out of 15) and multiple injection (6 out of 15) arms compared with continuous insulin (2 out of 15) (P = 0.12, Fisher's exact test). In the same trial six participants developed a subcutaneous abscess in the continuous insulin group compared with none in the other two groups. Holman 1983 observed only two episodes of severe hypoglycemia in two years (one in each group). Linn 1996 reported that 3.9% of glucose measurements were in the hypoglycemic range (glucose < 3.5 mmol/L) in the intensive group compared with 2.2% in the standard group.
Type 2 diabetes
Primary outcome: incidence of clinical neuropathy after at least one year
Eight studies investigated people with type 2 diabetes and two additional studies involved those with type 1 and type 2 diabetes. Four studies investigated the primary outcome at least one year after the intervention was instituted (Accord 2010; Azad 1999; Duckworth 2009; Tovi 1998). Accord 2010 was a parallel‐group, randomized trial on 10,251 participants in 77 clinical sites in North America that included participants with high HbA1c concentrations (> 7.5%) and cardiovascular disease ( ≥ 2 cardiovascular risk factors). These participants were randomly assigned to intensive (target hemoglobin A1c (HbA1c) < 6.0%) or standard target HbA1c 7.0% to 7.9%) glycemic therapy and followed for a median of 3.7 years. They defined clinical neuropathy as a score on the Michigan Neuropathy Screening Instrument (MNSI) greater than 2. They found that 1277 of 2815 participants in the intensive arm and 1338 of 2791 in the conventional arm developed neuropathy; annualized RD ‐0.70% (95% CI ‐1.40 to 0.01) (RR at 3.7 years 0.95, 95% CI 0.89 to 1.00). Duckworth 2009 investigated 1791 military veterans (mean age 60.4 years), who had had a suboptimal response to therapy for type 2 diabetes, and randomized them to receive either intensive or standard glucose control for a median of 5.6 years. Participants in the intensive‐therapy group were started on maximal doses, and those in the standard‐therapy group were started on half the maximal doses. The definition of clinical neuropathy in this study was a diagnosis on a yearly physical examination (no other details provided). They discovered that 178 of 464 participants developed neuropathy in the intensive group compared with 199 of 498 in the conventional group (annualized RD ‐0.29%, 95% CI ‐1.39 to 0.82) (RR at 5.6 years 0.96, 95% CI 0.82 to 1.12). Azad 1999 undertook a two‐year trial of 153 participants over two years randomized to conventional versus enhanced treatment with insulin, which lowered the HbA1c by 2.1% lower than the standard arm. The only outcome comparable to those selected for this review was a composite score of neuropathic symptoms and neurological examination. There was no significant difference in the incidence of neuropathy between the treatment groups (annualized RD ‐1.43%, 95% CI ‐12.29 to 9.43) (RR at two years 0.921, 95% CI 0.47 to 1.79). Tovi 1998 followed 40 elderly type 2 diabetic participants who attended their healthcare center and had secondary failure of oral diabetic drug therapy but without symptoms of hyperglycemia. These participants were randomized to insulin versus oral hypoglycemic agents and followed for one year. The definition of clinical neuropathy used by these investigators was based on a composite score combining examination findings with electrodiagnostic findings. No participants developed neuropathy in either group (annualized RD 0%, 95% CI ‐11.72 to 11.72). With the assumption that the clinical neuropathy definitions in these four studies were measuring the same construct, we performed a meta‐analysis using the generic inverse variance method. The combined annualized RD was ‐0.58% (95% CI ‐1.17 to 0.01) (Analysis 2.1; Analysis 2.2; Figure 6; Table 2).
2.1. Analysis.
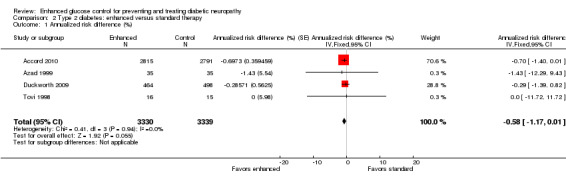
Comparison 2 Type 2 diabetes: enhanced versus standard therapy, Outcome 1 Annualized risk difference (%).
2.2. Analysis.
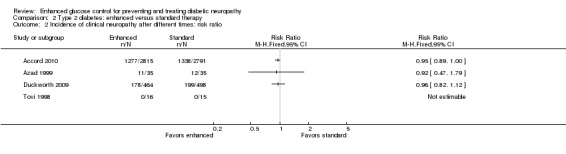
Comparison 2 Type 2 diabetes: enhanced versus standard therapy, Outcome 2 Incidence of clinical neuropathy after different times: risk ratio.
6.
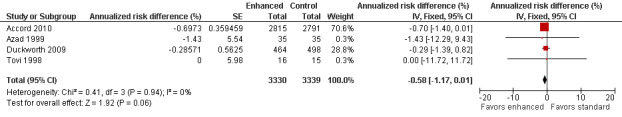
Forest plot of comparison: 2 Type 2 diabetes: enhanced versus standard therapy, outcome: 2.1 Annualized risk difference (%).
Secondary outcome: change in median nerve motor conduction velocity
Two studies reported this outcome (Kawamori 1991; Shichiri 2000) but Kawamori 1991 did not report this result for those participants randomized to conventional therapy. They state that those in the conventional group had no significant change with time compared with the intensive group which demonstrated improvement. Shichiri 2000 studied 110 participants for eight years with type 2 diabetes (55 with no retinopathy ‐ primary prevention cohort and 55 with simple retinopathy ‐ secondary intervention cohort) and randomly assigned them to multiple insulin injection therapy (three or more daily injections) or to conventional insulin therapy (one to two daily injections). This study showed an increase of 0.44 (0.09) m/s in the intensive group and a decline of 0.13 (0.08) m/s in the conventional group (MD 0.56, 95% CI 0.53 to 0.60) (Analysis 2.3; Table 2).
2.3. Analysis.
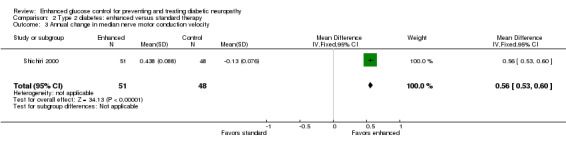
Comparison 2 Type 2 diabetes: enhanced versus standard therapy, Outcome 3 Annual change in median nerve motor conduction velocity.
Secondary outcome: change in vibration detection threshold in the legs
Two studies reported this outcome measure after at least one year of intervention (Service 1983; Shichiri 2000). As discussed previously, Service 1983 did not provide information on the variation in their measurements and could not be included in the meta‐analysis. They did report that there was no significant difference between the two treatment groups. Shichiri 2000 showed a mean annual decline of 0.625 (0.94) μm in the intensive arm and 2.25 (0.43) μm in the conventional arm, MD 1.63 μm (95% CI 1.34 to 1.91) (Analysis 2.4; Table 2).
2.4. Analysis.
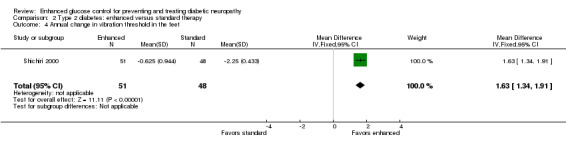
Comparison 2 Type 2 diabetes: enhanced versus standard therapy, Outcome 4 Annual change in vibration threshold in the feet.
Other outcomes
The largest study that reported neuropathy outcomes in this population was the UKPDS Study Group 1998 study that followed 3867 participants with newly diagnosed type 2 diabetes who were randomly assigned to an intensive policy with a sulphonylurea (chlorpropamide, glibenclamide, or glipizide) or with insulin, or conventional policy with diet. They reported neuropathy defined as a vibration threshold > 25 V on a biothesiometer. They found that there was a RR in favor of intensive treatment of 0.95 at three years (95% CI 0.76 to 1.18), 0.88 at six years (95% CI 0.72 to 1.08), 0.84 at nine years (95% CI 0.68 to 1.04), 0.92 at 12 years (95% CI 0.70 to 1.20), and 0.60 at 15 years (95% CI 0.39 to 0.94). Similarly, Gaede 2003 reported neuropathy based on a biothesiometer measurement. They investigated participants with persistent type 2 diabetes and microalbuminuria in an open, parallel‐group trial. Eighty participants were randomly assigned to receive conventional treatment in accordance with national guidelines and 80 to receive intensive treatment with a stepwise implementation of behavior modification and pharmacologic therapy that targeted hyperglycemia, hypertension, dyslipidemia, and microalbuminuria, along with secondary prevention of cardiovascular disease with aspirin. This study found a RR of 1.09 (95% CI 0.54 to 2.22) in favor of conventional treatment at a median follow‐up of 7.8 years. Accord 2010 reported on three additional neuropathy outcomes besides clinical neuropathy. They found hazard ratios in favor of intensive treatment of 0.95 (95% CI 0.86 to 1.05) for loss of vibration sensation, 0.94 (95% CI 0.87 to 1.01) for loss of ankle reflexes, and 0.88 (95% CI 0.77 to 1.00) for loss of sensation to light touch based on monofilament testing at a median follow‐up of 3.7 years.
Adverse events
Six of the nine studies reported adverse events, but only four (Accord 2010; Duckworth 2009; Gaede 2003; UKPDS Study Group 1998) provide information on serious hypoglycemic episodes. Accord 2010 reported 538 events in 3.7 years of follow‐up in intensively treated participants compared with 179 in the conventional group (P < 0.001). Similarly, Duckworth 2009 revealed 1333 episodes per 100 patient‐years in the intensive arm versus 383 in the standard arm (P < 0.001). The UKPDS Study Group 1998 group described a mean proportion of participants per year with one or more major hypoglycemic episodes of 1.0% of participants on chlorpropamide, 1.4% on glibenclamide, 1.8% on insulin, and 0.7% on diet. In contrast, Gaede 2003 reported fewer cases (5 out of 67 versus 12 out of 63) of major hypoglycemia in intensive participants over eight years of follow‐up (P = 0.07, Fisher's exact test). Gaede 2003 also reported a similar number of mild hypoglycemic events in the two treatment groups (42 out of 67 versus 39 out of 63). Shichiri 2000 describes 35 mild episodes per 100 patient‐years in the intensive group compared with 22 in the conventional group. Duckworth 2009 also reported more episodes (nine versus three per 100 patient‐years) of impaired consciousness in intensive participants (P < 0.001). Furthermore, Accord 2010 followed 5128 participants in the intensive arm and 5123 in the standard arm and revealed more non‐hypoglycemic serious adverse events (113 versus 82) (P = 0.03, Chi2 test), weight gain (1399 versus 713) (RR 1.96, 95% CI 1.81 to 2.13) (Analysis 2.6), and deaths (257 versus 203) (RR 1.26, 95% CI 1.06 to 1.51) in the intensive group (Analysis 2.5). The UKPDS Study Group 1998 also described more weight gain in the intensive group compared to the conventional group (MD 3.1 kg, 99% CI ‐0.9 to 7.0).
2.6. Analysis.
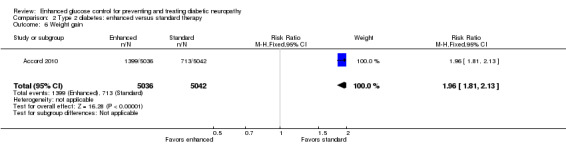
Comparison 2 Type 2 diabetes: enhanced versus standard therapy, Outcome 6 Weight gain.
2.5. Analysis.
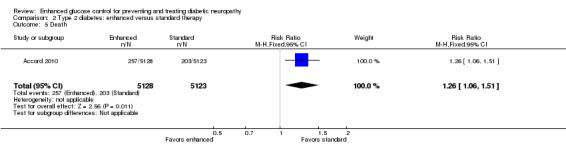
Comparison 2 Type 2 diabetes: enhanced versus standard therapy, Outcome 5 Death.
Discussion
Summary of main results
Type 1 diabetes
While there were seven randomized, controlled studies comparing intensive to conventional glycemic control in participants with type 1 diabetes, only two reported clinical neuropathy as an outcome. The DCCT study (DCCT 1993a; DCCT 1993b) was by far the largest study and demonstrated a 1.53% per year risk reduction (95% CI 0.51 to 2.54) and a relative risk reduction after five years of 53% in the primary prevention cohort. In the secondary prevention cohort, there was a 1.97% per year risk reduction (95% CI 0.90 to 3.04) and a 52% relative risk reduction. Taking these two cohorts together, there was a 1.74% per year (95% CI 1.00 to 2.48) risk reduction in the incidence of clinical neuropathy in the intensive treatment groups. Though the Linn 1996 study was much smaller in scope and utilized a different definition of clinical neuropathy, it revealed a 5.45% per year risk reduction (95% CI 0.95 to 9.95) and an 86% relative risk reduction in clinical neuropathy. In a meta analysis, these studies reveal a statistically significant 1.84% per year risk reduction (95% CI 1.11 to 2.56). These clinical trials taken together provide high‐quality evidence that intensive glycemic control prevents neuropathy in participants with type 1 diabetes.
Similarly, the secondary outcomes were all in favor of the intensive treatment groups. Specifically, three of the four studies that investigated peroneal nerve MCV revealed a significant annual MD between the randomized groups in favor of the intensively treated participants. The only study that did not show a significant difference was Service 1983. The authors studied only 15 participants with type 1 diabetes, and those in the intensive group did perform better on this outcome measure but the results were not statistically significant. This study was not powered to detect such a difference. The other three studies revealed an annual MD of 0.61 m/s (95% CI 0.51 to 0.71) in favor of the intensive group. Although only the DCCT study group (DCCT 1993a; DCCT 1993b) reported median nerve MCV they discovered a similar effect, annual MD of 0.46 (95% CI 0.36 to 0.57) in favor of the intensive group. In regards to ulnar nerve MCV, Reichard 1993 reported an annual MD of 0.54 m/s (95% CI 0.21 to 0.87) in favor of enhanced control whereas the Dahl‐Jorgensen 1986 team reported a much higher MD of 2.85 m/s. However, the Reichard 1993 study was 5.5 years longer in duration and studied twice the number of participants (89 versus 45). Taking all of these results together, multiple studies have demonstrated an annual MD in conduction velocity of between 0.4 to 0.6 m/s in three different motor nerves. These studies provide strong evidence of improvement in nerve function which complements the data on clinical neuropathy in this condition.
Further supporting evidence for the effect of intensive treatment in people with type 1 diabetes comes from quantitative vibration testing. While four studies measured this outcome only three reported enough information to allow meta‐analysis. These studies revealed an annual SMD of 0.32 (95% CI 0.02 to 0.43) in favor of intensively treated participants. Although all four studies showed improvement in quantitative vibration testing, the results were less consistent when compared to the other outcomes reported. The two studies using a biothesiometer demonstrated more convincing improvement compared with other forms of quantitative sensory testing.
On the other hand, there were substantially more episodes of serious hypoglycemia in those participants receiving intensive treatment. The two largest studies with the longest follow up, DCCT (DCCT 1993a; DCCT 1993b) and Reichard 1993, revealed a similar threefold increase in the rates of serious hypoglycemia. In contrast, three other smaller studies demonstrated no or insignificant increases in hypoglycemic events. Taken together, the overall evidence supports a significant increase in serious hypoglycemia in intensive participants. However, given the substantial benefit in not only neuropathy outcomes but in other clinical outcomes including nephropathy and retinopathy (DCCT 1993a; DCCT 1993b), the risk/benefit ratio is likely still in favor of treatment.
Type 2 diabetes
In contrast, in type 2 diabetes, the effect of intensive therapy on clinical neuropathy outcomes is less robust and not statistically significant. In the four studies that examined this outcome, none demonstrated a statistically significant difference between the groups. The largest study, Accord 2010, demonstrated a 0.70% per year risk reduction (95% CI ‐.01 to 1.40) and a 5% relative risk reduction at a median of 3.7 years of follow‐up (non‐significant) in those receiving intensive therapy. The second largest study, Duckworth 2009, revealed a 0.29% per year risk reduction (95% CI ‐0.82 to 1.39) and a 4% relative risk reduction at a median follow‐up of 5.6 years and these results were also not statistically significant. Of the two smaller studies, neither showed a statistically significant difference in favor of either group. The meta‐analysis of these four studies revealed a risk reduction of 0.58% per year (95% CI ‐0.01 to 1.17), which did not quite meet statistical significance. However, support for a positive effect of intensive therapy comes from the UKPDS Study Group 1998 that defined neuropathy based on a biothesiometer measurement. They followed 3867 participants for as many as 15 years of follow‐up and found that there was a modest risk reduction in favor of intensive treatment similar to that found by Accord 2010 and Duckworth 2009. However, this result was only statistically significant at 15 years. While this study did not include our primary outcome, it remains the largest study with the longest follow‐up to date in this patient population other than the Accord 2010 study. Overall, the evidence supports a potential but modest improvement in neuropathy outcomes in participants with enhanced glycemic control.
Despite the modest effects on clinical neuropathy outcomes in this type 2 population, there was a statistically significant effect on the median nerve MCV that was comparable to that seen in participants with type 1 diabetes. In the only study that reported results for both groups, there was an annual MD of 0.56 m/s (95% CI 0.53 to 0.60) in favor of the intensive group. Given the much smaller relative risk reduction in clinical neuropathy in participants with type 2 diabetes compared to type 1 diabetes, the similar effect on conduction velocity is surprising. However, in contrast to the studies on participants with type 1 diabetes, there was only one study investigating conduction velocity in this population. Another possible explanation is that there is a direct effect of reducing hyperglycemia on MCV. On the other hand, the reduced magnitude of effect on preventing neuropathy in those with type 2 diabetes may be due to the use of different definitions of neuropathy. Notably, a much higher incidence of neuropathy was reported in the trials in type 2 diabetes compared to those with type 1 diabetes.
Similar to studies in participants with type 1 diabetes, participants with type 2 diabetes also suffered from more adverse events in the intensive groups. In the three largest studies with the longest follow‐up (Accord 2010; Duckworth 2009; UKPDS Study Group 1998), there was an approximately threefold higher risk of a severe hypoglycemic episode in those receiving intensive therapy. Furthermore, two studies revealed more weight gain on intensive therapy, and Accord 2010 described significantly more deaths, with a RR of 1.26 (95% CI 1.06 to 1.51). Of note, the Accord 2010 trial investigated the most aggressive glucose control regimen with a target HA1C of less than 6 in the enhanced group. Only one smaller study (Gaede 2003) demonstrated a similar risk of hypoglycemia between groups. In contrast to participants with type 1 diabetes, the effect of intensive glycemic control on neuropathy is much less impressive. Unfortunately, the risk of hypoglycemia still remains substantial and needs to be taken into account along with the effect on other clinical outcomes in determining the risk/benefit ratio of enhanced glucose control.
Overall completeness and applicability of evidence
The overall completeness of the data gathered is quite strong. There were 17 RCTs identified from four clinical databases and the authors’ knowledge of trials in this area. However, there were only two trials in participants with type 1 diabetes that reported the primary outcome of development of clinical neuropathy. For type 2 diabetes, there were four trials that reported this outcome. The remainder of trials reported many of the pre‐identified secondary outcomes that corroborate the clinical neuropathy outcome measures. The evidence gathered for this systematic review is applicable to most people with diabetes. There were several articles pertaining to each subtype of diabetes. The two trials in type 1 diabetes that reported the primary outcome studied a total of 1228 participants and the four trials in type 2 diabetes followed a total of 6669 participants. These trials included many different geographic locations internationally and there were varied inclusion criteria that increase the generalizability of the results of this review.
Quality of the evidence
The quality of the evidence is presented in Table 1 and Table 2. In type 1 diabetes, the quality of evidence supporting the conclusion of a beneficial effect of enhanced glucose control on the development of clinical neuropathy was high. The quality of evidence for a significant improvement in peroneal and median nerve MCVs and improved vibration threshold in the feet with enhanced glucose control was also high, although the amount of absolute improvement was low. According to moderate‐quality evidence there was no significant change in ulnar nerve MCV. In type 2 diabetes, there was high‐quality evidence to support no significant difference in the primary endpoint, annual development of clinical neuropathy, but moderate‐quality evidence to support a small but significant difference in favor of enhanced glucose control for all available secondary outcomes, annual change in median nerve MCV, and annual change in vibration threshold in the feet. However, there were also high levels of evidence for a significant increase in death and weight gain with enhanced glucose control.
Potential biases in the review process
In type 1 diabetes, the conclusions of the review are heavily dependent on one trial (DCCT 1993a; DCCT 1993b) which accounted for 97.4% of the evidence. In type 2 diabetes the conclusions also depend heavily on one trial (Accord 2010) which accounted for 70.6% of the evidence. Both trials were considered to have low risk of bias.
Agreements and disagreements with other studies or reviews
There are no other systematic reviews on this subject but there are three non‐systematic reviews which have summarized portions of the literature. In 2001, Ratner 2001 described the results of the DCCT trial as showing that enhanced glucose control decreased the incidence of neuropathy in people with type 1 diabetes. He goes on to describe that the Kumomoto study (Shichiri 2000) revealed a similar effect in people with type 2 diabetes, which was later supported by the UKPDS Study Group 1998. In 2010, Habib 2010 and Stolar 2010 both emphasized the DCCT findings. Stolar 2010 go on to describe the UKPDS Study Group 1998 as well. All these reviews agreed with the assessment of these results described in this systematic review. However, none of these reviews incorporated the 14 other clinical trials on the effects of enhanced glucose control on diabetic neuropathy. Furthermore, no previous review has incorporated the diverse clinical and electrophysiologic outcomes in each of the studies.
Authors' conclusions
Implications for practice.
According to high‐quality evidence, enhanced glucose control in type 1 diabetes significantly reduces the annual development of clinical neuropathy and produces significant small improvements in peroneal and median motor nerve conduction velocity and vibration detection threshold. In type 2 diabetes, also according to high‐quality evidence, the reduction in annual development of neuropathy with enhanced glucose control was small and not statistically significant. However, a small improvement in motor nerve conduction velocity and vibration detection threshold was significant. Importantly, there was a large increased risk of adverse events with enhanced glucose control in both types of diabetes. In type 2 diabetes there was a significant large increase in the risk of weight gain and a significant increase in the risk of death in the one trial that targeted a hemoglobin A1C of less than 6%. While these results show clear improvement in the prevention of neuropathy in those with type 1 diabetes and potential benefits to those with type 2 diabetes, the precise glucose control target remains to be defined and potential adverse events must be weighed in the decision.
Implications for research.
In both types of diabetes there is a need for further research to discover the optimal target level which will reduce the development of neuropathy without increasing the risk of death, weight gain, hypoglycemia, and other adverse events. Since despite tight glucose control people with diabetes continue to develop neuropathy, additional treatments should be sought. Multinational agreement on simple measures of the presence and severity of neuropathy and their adoption in all future trials would enhance future meta‐analyses.
History
Protocol first published: Issue 1, 2009 Review first published: Issue 6, 2012
| Date | Event | Description |
|---|---|---|
| 4 June 2008 | Amended | Converted to new review format. |
Acknowledgements
CN Dang ‐ for sharing earlier protocol design and references. C Frost ‐ for biostatistical support that was instrumental to this manuscript.
The editorial base of the Cochrane Neuromuscular Disease Group is supported by the Medical Research Council (MRC) Centre for Neuromuscular Diseases.
Appendices
Appendix 1. MEDLINE (OvidSP) search strategy
Database: Ovid MEDLINE(R) <1946 to January Week 3 2012> Search strategy: ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ 1 randomized controlled trial.pt. (317022) 2 controlled clinical trial.pt. (83278) 3 randomized.ab. (222482) 4 placebo.ab. (127590) 5 clinical trials as topic.sh. (157231) 6 randomly.ab. (161036) 7 trial.ti. (95545) 8 or/1‐7 (736311) 9 exp animals/ not humans.sh. (3644792) 10 8 not 9 (679093) 11 exp diabetes mellitus/ (272093) 12 diabet$.tw. (323791) 13 11 or 12 (371544) 14 neuropath$.mp. (82137) 15 exp peripheral nervous system diseases/ (111799) 16 peripheral nervous system disease$.tw. (105) 17 polyneuropath$.mp. (11379) 18 or/14‐17 (162248) 19 13 and 18 (17606) 20 exp Diabetic Neuropathies/ (15138) 21 diabetic neuropath$.tw. (4410) 22 diabetic polyneuropath$.tw. (668) 23 or/19‐22 (21018) 24 Insulin Infusion Systems/ (3412) 25 "Islets of Langerhans Transplantation"/ (6799) 26 insulin infusion.tw. (4527) 27 (islets adj3 transplant$).tw. (1847) 28 improve$ glucose control.tw. (216) 29 improve$ metabolic control.tw. (525) 30 ((intensive therapy and diabet$) or (intensified therapy and diabet$)).tw. (420) 31 ((intensive treatment and diabet$) or (intensified treatment and diabet$)).tw. (537) 32 (intensified conventional adj3 treatment).tw. (39) 33 intensi$ glyc?emic control.tw. (232) 34 (intensively treated adj5 (patient$ or group$)).tw. (194) 35 (multiple adj3 insulin injection$).tw. (262) 36 optimal diabetes control.tw. (20) 37 (rigorous adj5 glucose control).tw. (13) 38 strict glyc?emic control.tw. (332) 39 (intensive insulin therapy or intensified insulin therapy).tw. (1279) 40 (intensive insulin treatment or intensified insulin treatment).tw. (328) 41 enhanced glyc?emic control.mp. (7) 42 or/24‐41 (16849) 43 10 and 23 and 42 (100)
Appendix 2. EMBASE (OvidSP) search strategy
Database: EMBASE <1980 to 2012 Week 03> Search strategy: ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ 1 crossover‐procedure/ (31692) 2 double‐blind procedure/ (102662) 3 randomized controlled trial/ (296049) 4 single‐blind procedure/ (14708) 5 (random$ or factorial$ or crossover$ or cross over$ or cross‐over$ or placebo$ or (doubl$ adj blind$) or (singl$ adj blind$) or assign$ or allocat$ or volunteer$).tw. (1053738) 6 or/1‐5 (1124940) 7 human/ (12790353) 8 6 and 7 (827600) 9 nonhuman/ or human/ (15866935) 10 6 not 9 (193480) 11 8 or 10 (1021080) 12 exp diabetes mellitus/ (459022) 13 diabet$.tw. (426766) 14 12 or 13 (534252) 15 neuropath$.tw. (95760) 16 exp peripheral neuropathy/ (41940) 17 peripheral nervous system disease$.tw. (128) 18 polyneuropath$.mp. (16924) 19 or/15‐18 (127906) 20 14 and 19 (25625) 21 exp Diabetic Neuropathy/ (15205) 22 diabetic neuropath$.tw. (5991) 23 diabetic polyneuropath$.tw. (1016) 24 or/20‐23 (25625) 25 insulin infusion/ (3905) 26 insulin infusion.tw. (5680) 27 pancreas islet transplantation/ (6536) 28 (islet$ adj5 transplant$).tw. (6221) 29 improve$ glucose control.tw. (313) 30 improve$ metabolic control.tw. (707) 31 (intensive therapy and diabet$).tw. (515) 32 (intensive treatment and diabet$).tw. (656) 33 (intensified conventional adj3 treatment).tw. (49) 34 intensi$ glyc?emic control.tw. (360) 35 (intensively treated adj5 (patient$ or group$)).tw. (231) 36 (multiple adj3 insulin adj3 injection$).tw. (552) 37 optimal diabetes control.tw. (32) 38 (rigorous adj5 glucose control).tw. (17) 39 strict glyc?emic control.tw. (474) 40 intensive insulin therapy.tw. (1565) 41 intensive insulin treatment.tw. (340) 42 enhanced glyc?emic control.mp. (18) 43 antidiabetic agent/ (24913) 44 antidiabetic.tw. (8678) 45 lifestyle modification.tw. or lifestyle modification/ (12844) 46 diet.mp. (381902) 47 exercise.mp. (260236) 48 or/25‐47 (667801) 49 11 and 24 and 48 (322)
Appendix 3. CENTRAL search strategy
#1 MeSH descriptor Diabetes Mellitus explode all trees #2 (diabet*):ti or (diabet*):ab #3 (#1 OR #2) #4 neuropath* #5 MeSH descriptor Peripheral Nervous System Diseases explode all trees #6 "peripheral nervous system disease" or "peripheral nervous system diseases" #7 polyneuropath* #8 (#4 OR #5 OR #6 OR #7) #9 (#3 AND #8) #10 MeSH descriptor Diabetic Neuropathies explode all trees #11 "diabetic neuropathy" or "diabetic neuropathies" #12 "diabetic polyneuropathy" or "diabetic polyneuropathies" #13 (#10 OR #11 OR #12) #14 MeSH descriptor Insulin Infusion Systems, this term only #15 MeSH descriptor Islets of Langerhans Transplantation, this term only #16 "insulin infusion" #17 (islets NEAR/3 transplant*) #18 (improve* glucose control):ti or (improve* glucose control):ab #19 (improve* metabolic control):ti or (improve* metabolic control):ab #20 (intensive therapy) NEAR diabet* or (intensified therapy) NEAR diabet* #21 (intensive treatment) NEAR diabet* or (intensified treatment) NEAR diabet* #22 (intensive conventional) NEAR/3 treatment or (intensiied conventional) NEAR/3 treatment #23 (intensi* glyc?emic control):ti or (intensi* glyc?emic control):ab #24 (intensively treated NEAR/5 patient*):ti or (intensively treated NEAR/5 patient*):ab #25 (intensively treated NEAR/5 group*):ti or (intensively treated NEAR/5 group*):ab #26 (multiple NEAR/3 insulin injection*):ti or (multiple NEAR/3 insulin injection*):ab #27 "optimal diabetes control" #28 (rigorous NEAR/5 glucose control):ti or (rigorous NEAR/5 glucose control):ab #29 (strict glyc?emic control):ti or (strict glyc?emic control):ab #30 (internsive insulin therapy):ti or (internsive insulin therapy):ab #31 (intensive insulin therapy):ti or (intensive insulin therapy):ab #32 (intensive insulin treatment):ti or (intensive insulin treatment):ab #33 (enhanced glyc?emic control) #34 MeSH descriptor Hypoglycemic Agents explode all trees #35 (hypoglyc?emic NEAR/3 agent*):ti or (hypoglyc?emic NEAR/3 agent*):ab #36 (hypoglyc?emic NEAR/3 drug*):ti or (hypoglyc?emic NEAR/3 drug*):ab #37 antidiabetic #38 MeSH descriptor Life Style, this term only #39 (lifestyle modification) #40 (life style modification) #41 diet or exercise #42 (#14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20 OR #21 OR #22 OR #23 OR #24 OR #25 OR #26 OR #27 OR #28 OR #29 OR #30 OR #31 OR #32 OR #33) #43 (#34 OR #35 OR #36 OR #37 OR #38 OR #39 OR #40 OR #41) #44 (#13 AND #42) #45 (#13 AND #43)
Appendix 4. www.controlled‐trials.com search strategy
"diabetic neuropathy”
“peripheral neuropathy”
"hyperglycemia"
“blood glucose”
Data and analyses
Comparison 1. Type 1 diabetes: enhanced versus standard therapy.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Annualized risk difference (%) | 3 | 1228 | Annualized risk difference (%) (Fixed, 95% CI) | ‐1.84 [‐2.56, ‐1.11] |
| 2 Incidence of clinical neuropathy after 5 years: risk ratio | 3 | 1228 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.46 [0.33, 0.63] |
| 3 Annual change in peroneal nerve motor conduction velocity | 4 | 1371 | Mean Difference (IV, Fixed, 95% CI) | 0.61 [0.51, 0.71] |
| 4 Annual change in median nerve motor conduction velocity | 2 | 1241 | Mean Difference (IV, Fixed, 95% CI) | 0.46 [0.36, 0.57] |
| 5 Annual change in ulnar nerve motor conduction velocity | 2 | 134 | Mean Difference (IV, Random, 95% CI) | 1.49 [‐0.74, 3.71] |
| 6 Annual change in vibration threshold in the feet | 3 | 177 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.32 [0.02, 0.62] |
Comparison 2. Type 2 diabetes: enhanced versus standard therapy.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Annualized risk difference (%) | 4 | 6669 | Annualized risk difference (%) (Fixed, 95% CI) | ‐0.58 [‐1.17, 0.01] |
| 2 Incidence of clinical neuropathy after different times: risk ratio | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3 Annual change in median nerve motor conduction velocity | 1 | 99 | Mean Difference (IV, Fixed, 95% CI) | 0.56 [0.53, 0.60] |
| 4 Annual change in vibration threshold in the feet | 1 | 99 | Mean Difference (IV, Fixed, 95% CI) | 1.63 [1.34, 1.91] |
| 5 Death | 1 | 10251 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.26 [1.06, 1.51] |
| 6 Weight gain | 1 | 10078 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.96 [1.81, 2.13] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Accord 2010.
| Methods | ACCORD was a parallel‐group, randomized trial done in 77 clinical sites in North America. People with diabetes, high HbA1c concentrations (> 7.5%), and cardiovascular disease (or ≥ 2 cardiovascular risk factors) were randomly assigned by central randomization to intensive (target hemoglobin A1c (HbA1c) of < 6.0%) or standard (7.0% to 7.9%) glycemic therapy. | |
| Participants | Volunteers who had type 2 diabetes mellitus, HbA1c concentrations of 7.5% or more, and were aged 40 to 79 years with history of cardiovascular disease or 55 to 79 years with anatomical evidence of significant atherosclerosis, albuminuria, left ventricular hypertrophy, or at least 2 risk factors for cardiovascular disease (dyslipidemia, hypertension, being a smoker, or obesity). Exclusion criteria included frequent or recent serious hypoglycemic events, unwillingness to monitor glucose at home or inject insulin, body mass index of more than 45 kg/m², serum creatinine more than 132.6 μmol/L, or other serious illness. Participants were recruited at 77 clinical centers (aggregated within 7 networks) in the USA and Canada. | |
| Interventions | Participants were randomly assigned to receive 1 of 2 glycemia control strategies: intensive treatment targeting a HbA1c concentration of < 6.0% or standard treatment targeting HbA1c of 7.0% to 7.9%. Participants were also assigned to 1 of 2 blood pressure interventions (intensive blood pressure target < 120 mm Hg, or standard < 140 mm Hg), or a lipid intervention (fenofibrate or placebo while maintaining good control of LDL cholesterol with simvastatin). | |
| Outcomes | New score of > 2.0 on Michigan Neuropathy Screening Instrument (MNSI) every year, new loss of vibratory sensation (tested with 128 Hz tuning fork) every year, new loss of ankle jerk during Jendrassik maneuver every year, new loss of light touch (10 g force monofilament test) every year | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Unique randomization sequences were computer‐generated for every clinical site centrally at the co‐ordinating center. Randomization was done by clinical staff via secure access to the ACCORD trial website. |
| Allocation concealment (selection bias) | Low risk | There was a central telephone allocation which concealed allocation from the randomizing physician. A web‐based randomization system did conceal lipid intervention allocation from the randomizing physician. However, the glycemia and blood pressure interventions were open label and treatment allocation for those interventions was revealed to the randomizing physician once randomization was complete, since that knowledge was required to implement the study protocol. Although information on previously randomized participants was available to study investigators for these two interventions, use of the web‐based randomization did help conceal the sequence of future allocations from study investigators. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Blinding participants was not possible and blinding assessors was not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All measures stated in the methods reported |
| Selective reporting (reporting bias) | Low risk | Provides detailed table of those who were not followed up and the reasons within each group |
| Other bias | High risk | Participants in the intensive therapy group attended monthly visits for the first 4 months and then every 2 months thereafter, with at least 1 interim phone call, with the aim of rapidly and safely reducing glycated hemoglobin levels to below 6.0%. Additional visits were scheduled as needed to achieve glycemic goals. Participants in the standard‐therapy group had glycemic management visits every 4 months. Thus the intensive group had more visits than the standard group. |
Azad 1999.
| Methods | RCT of conventional versus intensive glycemic control in a Veterans Administration population followed for 2 years | |
| Participants | Participants were males, between 40 and 69 years of age, with diabetes mellitus for 15 years or less on maximum dose of sulfonylurea and/or any dose of insulin. At entry, each participant had an HbA1c greater than 3 standard deviations above the mean of normal. Fasting C‐peptide levels were greater than 0.21 nmol/L. Criteria for exclusion were conditions that would have precluded intensive treatment, endpoints evaluation, or continuance into a proposed long‐term study. | |
| Interventions | Once daily injections versus a stepwise approach with (1) an evening insulin injection, (2) same injection adding daytime glipizide, (3) 2 injections of insulin alone and (4) multiple injections | |
| Outcomes | No primary outcome was specified. Assessments included a neuropathy score based on upper limb sensory, lower limb sensory symptoms, and neurological examination with 0 for normal and 1 for abnormal for each item; the numbers for each item were added, expressed as a proportion of all items and then multiplied by 1000. Neuropathy was considered present if there was any abnormality in any component of the neurological examination. RR variation, Valsalva ratio and erectile dysfunction were also collected. | |
| Notes | Nerve conduction velocities were not measured | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Participants were stratified by participating hospital (5 strata), and by presence or absence (2 strata) of any prior microvascular complication (myocardial infarction, angina pectoris, congestive heart failure, or cerebrovascular event). Within these 10 strata, participants were then randomized to intensive glycemic control or standard therapy. This stratification was done to insure that the 2 treatment arms would be balanced by participating hospital and macrovascular complications. However, the method of randomization was not stated. |
| Allocation concealment (selection bias) | Unclear risk | Methods not clearly stated in article |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Methods not clearly stated in article |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 4 participants in conventional group and 8 participants in intensive group unaccounted for and their fate was not described |
| Selective reporting (reporting bias) | High risk | Reports on some but not all subsets of the clinical neuropathy score |
| Other bias | Low risk | No other bias detected |
Dahl‐Jorgensen 1986.
| Methods | Participants with type 1 diabetes were randomly assigned to 3 modes of treatment: continuous subcutaneous insulin infusion (CSII), multiple insulin injections, or continued conventional treatment with twice daily injections of insulin (controls) | |
| Participants | Participants had type 1 diabetes mellitus with serum C peptide concentrations (after glucagon stimulation) below 0.1 nmol. During the 2 months before the study, home blood glucose monitoring was introduced and baseline results obtained. All participants used twice daily insulin injections. | |
| Interventions | In the control group a mixture of regular and isophane insulin was injected before breakfast and dinner. In the group receiving multiple injections, isophane insulin was given at bedtime. During CSII a constant basal rate of regular insulin was infused. In the groups treated by CSII and multiple injections, additional regular insulin was infused or injected, respectively, before each meal (4 to 6 times daily). Two different pumps were used for CSII: Nordisk infuser or Autosyringe AS6C. Only highly purified porcine insulin preparations were used. | |
| Outcomes | Motor nerve conduction velocities were measured in the ulnar, peroneal, and tibial nerves. To distinguish between acute "metabolic" and chronic "structural" neuropathy, measurements were performed every 3 to 6 months. The nerve was stimulated percutaneously with a bipolar surface electrode. Motor responses were recorded with surface electrodes, Medelec MS 92 equipment was used. Skin temperature was kept within narrow limits throughout the study. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Block randomization was performed to ensure comparable groups |
| Allocation concealment (selection bias) | Unclear risk | Methods not clearly stated |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Methods not clearly stated |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants completed study |
| Selective reporting (reporting bias) | Low risk | Reported all 3 motor nerve conduction velocities performed |
| Other bias | Low risk | None |
DCCT 1993a.
| Methods | A total of 1441 participants with insulin‐dependent diabetes mellitus (726 with no retinopathy at baseline (the primary‐prevention cohort) and 715 with mild retinopathy (the secondary‐intervention cohort)) were randomly assigned to intensive therapy administered either with an external insulin pump or by 3 or more daily insulin injections and guided by frequent blood glucose monitoring or to conventional therapy with 1 or 2 daily insulin injections. The participants were followed for a mean of 6.5 years, and the appearance and progression of retinopathy and other complications were assessed regularly. 1436 received baseline neuropathy assessment (primary cohort: 346 intensive, 376 conventional; secondary cohort: 362 intensive, 353 conventional). | |
| Participants | The major criteria for eligibility included insulin dependence, as evidenced by deficient C‐peptide secretion; an age of 13 to 39 years; and the absence of hypertension, hypercholesterolemia, and severe diabetic complications or medical conditions. To be eligible for the primary‐prevention cohort, participants were required to have had IDDM for 1 to 5 years, to have no retinopathy as detected by 7‐field stereoscopic fundus photography, and to have urinary albumin excretion of less than 40 mg per 24 hours. To be eligible for the secondary‐intervention cohort, the participants were required to have had IDDM for 1 to 15 years, to have very mild to moderate non‐proliferative retinopathy, and to have urinary albumin excretion of less than 200 mg per 24 hours. | |
| Interventions | Conventional therapy consisted of 1 or 2 daily injections of insulin, including mixed intermediate and rapid‐acting insulins, daily self‐ monitoring of urine or blood glucose, and education about diet and exercise. Intensive therapy included the administration of insulin 3 or more times daily by injection or an external pump. The dosage was adjusted according to the results of self monitoring of blood glucose performed at least 4 times per day, dietary intake, and anticipated exercise. The goals of intensive therapy included preprandial blood glucose concentrations between 70 and 120 mg per dL (3.9 and 6.7 mmol per L), postprandial concentrations of less than 180 mg per dL (10 mmol/L), a weekly 3 a.m. measurement greater than 65 mg/dL (3.6 mmol/L), and hemoglobin A1c (glycated hemoglobin), measured monthly, within the normal range (less than 6.05%). | |
| Outcomes | Definite clinical neuropathy was defined as the presence of abnormalities consistent with diabetic neuropathy in at least 2 of the following: physical symptoms, peripheral sensation, or decreased or absent reflexes. A single abnormality was labeled “possible” neuropathy. “Confirmed” definite clinical neuropathy also required the finding of unequivocal abnormality on nerve conduction studies or autonomic nervous system (ANS) testing. Nerve conduction evaluations were performed at baseline, at 5 years, and at study end. Nerve conduction evaluations included the dominant median (motor and sensory), peroneal (motor), and sural nerves. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomization was stratified according to the primary‐prevention and secondary‐intervention cohorts at each center. However, the process of randomization was not stated in the methods. |
| Allocation concealment (selection bias) | Unclear risk | Neither the investigators nor the participants were aware of the outcome data unless predetermined criteria, such as the development of severe retinopathy requiring laser therapy, were met. However, there is no mention of allocation concealment. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | All responses were recorded and available for independent review |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 1441 randomized, 1290 at least 4.5 years, 1243 had nerve conduction studies at baseline and at 5 years |
| Selective reporting (reporting bias) | Low risk | Authors report on all measures |
| Other bias | Low risk | None |
DCCT 1993b.
| Methods | See DCCT 1993a | |
| Participants | ||
| Interventions | ||
| Outcomes | ||
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomization was stratified according to the primary‐prevention and secondary‐intervention cohorts at each center. However, the process of randomization was not stated in the methods. |
| Allocation concealment (selection bias) | Unclear risk | Neither the investigators nor the participants were aware of the outcome data unless predetermined criteria, such as the development of severe retinopathy requiring laser therapy, were met. However, there is no mention of allocation concealment. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | All responses were recorded and available for independent review |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 1441 randomized, 1290 at least 4.5 years, 1243 had nerve conduction studies at baseline and at 5 years |
| Selective reporting (reporting bias) | Low risk | Authors report on all measures |
| Other bias | Low risk | None |
Duckworth 2009.
| Methods | 1791 military veterans (mean age, 60.4 years) who had a suboptimal response to therapy for type 2 diabetes randomly assigned to either intensive or standard glucose control | |
| Participants | Selection criteria included an inadequate response to maximal doses of an oral agent or insulin therapy. Exclusion criteria included a glycated hemoglobin level of less than 7.5%, the occurrence of a cardiovascular event during the previous 6 months, advanced congestive heart failure, severe angina, a life expectancy of less than 7 years, a body mass index (BMI, the weight in kg divided by the square of the height in meters) > 40, a serum creatinine level > 1.6 mg/dL (141 micromol per liter), and an alanine aminotransferase level > 3 times the upper limit of the normal range. | |
| Interventions | Participants in the intensive‐therapy group were started on maximal doses, and those in the standard‐therapy group were started on half the maximal doses. Before any change in oral medications, insulin was added for participants in the intensive‐therapy group who did not achieve a glycated hemoglobin level of less than 6% and for those in the standard‐therapy group with a level of less than 9%. Subsequent changes in medication were determined according to protocol guidelines and local assessment. The guidelines allowed for the use of any approved drug at the discretion of the investigator. The goal for glycated hemoglobin levels was an absolute reduction of 1.5% points in the intensive‐therapy group, as compared with the standard‐therapy group. | |
| Outcomes | The primary outcome was the time to the first occurrence of any one of a composite of cardiovascular events, adjudicated by an endpoint committee that was unaware of assignments to study groups. The cardiovascular events were documented myocardial infarction; stroke; death from cardiovascular causes; new or worsening congestive heart failure; surgical intervention for cardiac, cerebrovascular, or peripheral vascular disease; inoperable coronary artery disease; and amputation for ischemic gangrene. Secondary cardiovascular outcomes included new or worsening angina, new transient ischemic attacks, new intermittent claudication, new critical limb ischemia, and death from any cause. Secondary outcomes also included microvascular complications (retinopathy, nephropathy, and neuropathy). Adverse events, including hypoglycemia, were monitored. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomly assigned with the use of a permuted‐block design with a block size of 6 and stratified according to study site, the previous occurrence of a macrovascular event, and current insulin use |
| Allocation concealment (selection bias) | Low risk | The randomization codes were generated by the study’s biostatistician |
| Blinding (performance bias and detection bias) All outcomes | High risk | Open label |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Fully account for all follow‐up |
| Selective reporting (reporting bias) | Low risk | Reports on all outcomes |
| Other bias | Low risk | None |
Gaede 2003.
| Methods | The primary endpoint of this open, parallel trial was a composite of death from cardiovascular causes, nonfatal myocardial infarction, nonfatal stroke, revascularization, and amputation. 80 participants were randomly assigned to receive conventional treatment in accordance with national guidelines and 80 to receive intensive treatment, with a stepwise implementation of behavior modification and pharmacologic therapy that targeted hyperglycemia, hypertension, dyslipidemia, and microalbuminuria, along with secondary prevention of cardiovascular disease with aspirin. | |
| Participants | Participants with persistent type 2 diabetes and microalbuminuria were selected, since microalbuminuria is a well‐established independent risk factor for cardiovascular disease (the primary endpoint) as well as for nephropathy, retinopathy, and neuropathy (secondary endpoints) | |
| Interventions | The aim of dietary intervention was a total daily intake of fat that was less than 30% of the daily energy intake and an intake of saturated fatty acids that was less than 10% of the daily energy intake. Light‐to‐moderate exercise for at least 30 minutes 3 to 5 times weekly was recommended. If participants were unable to maintain glycated hemoglobin values below 6.5% by means of diet and increased physical activity alone after 3 months, an oral hypoglycemic agent was started. As the initial step, overweight participants (defined as those with a body mass index (the weight in kg divided by the square of the height in m above 25) received metformin (maximum, 1 g twice daily); lean participants, or overweight participants who had contraindications to metformin therapy, received gliclazide (maximum, 160 mg twice daily). As the second step, metformin was added to the regimen of lean participants and gliclazide to that of overweight participants if hyperglycemia was not controlled. If the glycated hemoglobin value exceeded 7.0 percent despite maximal doses of oral agents, the addition of neutral protamine Hagedorn (NPH) insulin at bedtime was recommended. When insulin was started, lean participants stopped metformin treatment and overweight participants stopped gliclazide therapy unless it was the only oral hypoglycemic agent given. The insulin dose was adjusted on the basis of the morning fasting blood glucose concentration. If the daily dose of insulin exceeded 80 IU at bedtime or there was no decrease in the glycated hemoglobin value, participants were switched to regimens in which regular and NPH insulin was given 2 to 4 times a day. | |
| Outcomes | Peripheral neuropathy was measured with a biothesiometer | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | 160 participants stratified according to urinary albumin excretion and then randomly assigned to treatment groups |
| Allocation concealment (selection bias) | Low risk | Randomization was performed with the use of sealed envelopes |
| Blinding (performance bias and detection bias) All outcomes | Low risk | The study was a randomized, open, parallel trial therefore participants and physicians were not blinded to treatment. However, the outcome assessors were blinded and there was an independent committee for adjudication |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 15 versus 12 participants died in the conventional versus intensive groups. 2 versus 1 withdrew. |
| Selective reporting (reporting bias) | Low risk | Report on clinical neuropathy as defined as symptoms + abnormal nerve conduction studies in one nerve |
| Other bias | Low risk | None |
Holman 1983.
| Methods | 74 insulin‐dependent diabetic participants with background retinopathy were randomized to continue with usual diabetic care (group U) or to a more intensive program (group A) using ultralente insulin as basal cover and soluble insulin at mealtimes | |
| Participants | Participants attending routine diabetic clinics were screened for retinopathy by ophthalmoscopy through dilated pupils. Reasons for exclusion were age over 60 years; proliferative retinopathy; renal impairment (plasma creatinine > 175 mol/L), more than one significant cardiovascular event (or one within the previous year); and other major disease processes. Opacities of the ocular media sufficient to impair detailed retinal observation precluded study. | |
| Interventions | The U group continued their usual therapy and attended the routine diabetic clinic. Participants in group A (alternative therapy) were treated more intensively; they were seen at least 6‐weekly in a special clinic set aside for the purpose. Individual dietary advice, given by a single dietitian, aimed to maintain ideal body weight and to adjust the timing and size of meals to help optimize control. Approximately 50% of total daily energy intake was derived from carbohydrate (predominantly fiber‐rich complex carbohydrate) and 30% to 35% of energy from fat. The use of polyunsaturated fat was encouraged. With the aid of a research nurse, all participants were intensively educated in the care of their diabetes. They were taught home blood glucose monitoring with an 'Autolet,16' and either 'BM Glycaemie 20‐800' sticks (Boehringer) or 'Dextrostix' (Ames) with a 'Hypocount' meter (Hypoguard). Participants were encouraged to test 4 times a day (before breakfast, lunch, dinner, and bed) at least twice a week. They were asked to aim for preprandial blood glucose values between 4 mmol/L and 7 mmol/L by adjusting insulin doses on the basis of results obtained. Advice was available over the telephone at any time. Each participant kept a logbook of glucose levels, hypoglycemic episodes, insulin doses, and other events which might relate to their diabetes. | |
| Outcomes | The vibration sensory threshold was assessed with a 'Biothesiometer' 17 (Biomedical Instrument Co., Newbury, Ohio); in each case the mean of 3 readings over both lateral malleoli and the medial border of the distal phalanx of both great toes was recorded. All readings were made by the same research nurse who was aware of the participant’s group but had no record of previous measurements. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Stratified by weight and blood pressure. |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes |
| Blinding (performance bias and detection bias) All outcomes | High risk | Research nurse was not blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 2 died (1 each), 3 conventional participants withdrew versus 0 intense participants |
| Selective reporting (reporting bias) | High risk | They report vibration perception threshold of the medial malleolus but not at the great toe |
| Other bias | Low risk | None |
Hotta 1993.
| Methods | 9 participants with insulin dependent diabetes mellitus (IDDM) and 41 participants with (non‐insulin dependent diabetes mellitus) were randomly assigned to either conventional insulin therapy or multiple doses of insulin | |
| Participants | 9 participants with IDDM and 41 participants with NIDDM. All with early microvascular complications (undefined). | |
| Interventions | Conventional insulin therapy (once daily injection of intermediate‐acting insulin) versus multiple insulin therapy. In both groups, insulin was frequently adjusted to maintain the strictest glycemic control possible. | |
| Outcomes | Right median and ulnar nerve motor conduction velocities (MCV) and the vibration perception threshold | |
| Notes | Results of the vibration perception threshold not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Methods not stated |
| Allocation concealment (selection bias) | Unclear risk | Methods not stated |
| Blinding (performance bias and detection bias) All outcomes | High risk | No attempt was made to blind the participants and no mention is made of blinding the assessors |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Only 22 of 28 participants on enhanced control and 21 of 22 on conventional treatment had results recorded |
| Selective reporting (reporting bias) | High risk | They recorded ulnar and median MCV and vibration perception threshold but only report the median MCV |
| Other bias | Unclear risk | Report is not sufficiently detailed to rule out other sources of bias |
Jakobsen 1988.
| Methods | 24 participants with insulin dependent diabetes mellitus (IDDM) were allocated to either continuous subcutaneous insulin (CSII) or conventional insulin therapy (CIT) at random | |
| Participants | 12 woman and 12 men with IDDM. None received any other medication. | |
| Interventions | Participants on CSII treatment used the Nordic Infusor. Highly purified crystalline U‐100 porcine insulin was infused subcutaneously in the abdominal wall. This delivered ˜50% of the total 24‐hour dose as basal continuous insulin with the remaining dose given before meals. CIT group received 2 daily subcutaneous injections of crystalline and NPH highly purified porcine insulin. | |
| Outcomes | Vibration perception threshold (VPT) was determined with a biothesiometer at the pulp of the second finger, the styloid process of the radial bone, the medial malleolus, and the pulp of the great toe | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Random, otherwise not stated |
| Allocation concealment (selection bias) | Unclear risk | Not stated in methods |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not stated in methods |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | One participant in the continuous group withdrew because of problems with the pump. One participant in each group did not finish the study because of pregnancy. |
| Selective reporting (reporting bias) | Low risk | Reported on all 4 sites of VPT |
| Other bias | Unclear risk | Methods section is extremely brief and therefore other sources of bias are unclear |
Kawamori 1991.
| Methods | A randomized, prospective study was undertaken to determine the glycemic threshold in 50 people treated with insulin who were showing an early stage of diabetic microangiopathies and who had been treated with once or twice daily intermediate‐acting insulin injection for an average period of 6.3 years. These were divided randomly into 2 groups. | |
| Participants | People with non‐insulin dependent diabetes who had been taking once to twice daily intermediate acting insulin for an average duration of 6.3 years | |
| Interventions | 22 participants, maintained on intermediate‐acting insulin (once daily injection) therapy, were used as the control group (CIT). The other 28 participants were given multiple insulin injection therapy (MIT). In the latter group, all participants were treated with multiple insulin injections, receiving either short‐, intermediate‐, or long‐acting insulin. During the experimental period, in both groups, insulin doses were frequent adjusted to accomplish as strict glycemic control as possible. | |
| Outcomes | Median nerve motor conduction velocity | |
| Notes | Report lacks many details | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Divided randomly into two groups” but method not stated |
| Allocation concealment (selection bias) | Unclear risk | Method not stated |
| Blinding (performance bias and detection bias) All outcomes | High risk | The different frequencies of injections made blinding participants and treating physicians impossible. No mention is made of blinding the neurophysiologist measuring the nerve conduction velocity. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | It is not said that all participants were followed up but no dropouts are mentioned |
| Selective reporting (reporting bias) | Unclear risk | The report is not sufficiently detailed to allow a judgment |
| Other bias | Unclear risk | The report is not sufficiently detailed to allow a judgment. There is not enough information to determine if baseline characteristics were equal between groups. |
Lauritzen 1985.
| Methods | 30 people with insulin dependent diabetes mellitus (IDDM) who had advanced background retinopathy were randomized to unchanged conventional treatment (UCT) or to continuous subcutaneous insulin infusion (CSII). They were prospectively followed for 2 years. | |
| Participants | Background retinopathy, postprandial C peptide < 0.2, Cr < 150, age 18 to 51, diabetes onset before 30, diabetes duration < 35 years. 40 consecutive patients fulfilling the above mentioned criteria were identified. | |
| Interventions | Unchanged conventional treatment (UCT) or to continuous subcutaneous insulin infusion (CSII) | |
| Outcomes | Vibration sense at the first phalanges of hands and feet plus the medial malleolus of the legs was measured by biothesiometer | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | 32 participants were randomly assigned |
| Allocation concealment (selection bias) | Unclear risk | Not stated in the methods |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Unclear if assessors were blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 1 participant with emigration issues (UCT), 1 participant excluded based on baseline retinal photos (SCII). 1 participant in UCT switched to SCII group (excluded). |
| Selective reporting (reporting bias) | High risk | Did not report values for vibration perception threshold |
| Other bias | Unclear risk | Methods section is limited in detail. There is not enough information to determine if the baseline characteristics between the groups are equal. |
Linn 1996.
| Methods | After informed consent had been obtained from the participants, randomization was performed with the use of computer‐selected random numbers. A total of 49 participants were randomized for intensive (I) or conventional (C) insulin therapy, and they were evaluated for 5 years after clinical diagnosis. | |
| Participants | People with newly diagnosed insulin dependent diabetes mellitus (IDDM) admitted to a clinic starting in 1988. IDDM defined on the basis of insulin dependency according to World Health Organization recommendations. | |
| Interventions | Intensive therapy included administration of insulin at least 3 times daily by injection. The dosage was adjusted by the participants or by healthcare professionals according to the results of self monitoring of blood glucose, dietary intake, and anticipated exercise. The mean (SD) frequency of glucose determinations was 4.2 (2.8) per participant per day. Target blood glucose in the I group was defined as self determined capillary glucose less than 6.8 mmol/L before meals and less than 10 mmol/L postprandially. Capillary blood glucose testing was validated with laboratory values at entry and every half‐year thereafter. The goals of intensive therapy also included glycated hemoglobin in the normal range (HbAlc < 6.5%). The I group contacted the diabetes educator by visit or telephone once per month to review and adjust the regimens. Conventional therapy consisted of one or two daily injections of insulin, including mixed intermediate and rapid‐acting insulins and variable self monitoring of blood glucose. Participants contacted the study center quarterly, and the mean (SD) contacts for glucose measurements was 2.3 (1.9) per participant and day. Conventional therapy did not always include daily adjustments in the insulin dosage. The goals of conventional therapy included the absence of symptoms attributable to glucosuria or hyperglycemia, and freedom from severe or frequent hypoglycemia. In both groups, I and C, a small amount of exogenous insulin was maintained even when C˜peptide secretion recovered significantly. | |
| Outcomes | Peripheral sensory neuropathy was diagnosed when at least 3 of the following categories were positive: clinical symptoms, signs, quantitative sensory testing, and peroneal motor nerve conduction velocity (following the San Antonio Consensus Statement) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random numbers generated by computer |
| Allocation concealment (selection bias) | Unclear risk | Methods not clearly stated |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Methods not clearly stated |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 7 participants did not complete study and were excluded from analysis (no specifics given in article) |
| Selective reporting (reporting bias) | Low risk | Reports on incidence of peripheral neuropathy |
| Other bias | Low risk | None |
Reichard 1993.
| Methods | 102 participants with insulin‐dependent diabetes mellitus, non‐proliferative retinopathy, normal serum creatinine concentrations, and unsatisfactory blood glucose control were randomly assigned to intensified insulin treatment (48 participants) or standard insulin treatment (54 participants). Evaluated for microvascular complications after 18 months and 3, 5, and 7.5 years. | |
| Participants | The participants enrolled had insulin‐dependent diabetes mellitus, non‐proliferative retinopathy, normal serum creatinine concentrations, and unsatisfactory blood glucose control (high blood glucose concentrations), according to their personal physicians. People with albuminuria were not excluded. | |
| Interventions | The treatment regimen of the intensified‐treatment group consisted of individual education and then continuous tutoring with frequent face‐to‐face and telephone contact, initially every second week and then at greater intervals. Education concerned the action of insulin, intermediary metabolism, home glucose monitoring, and how to interpret blood glucose tests to modify treatment. The notion of diabetes as a shortage of insulin correctable by injection treatment was reinforced. During tutoring the participants tried in daily life to use the knowledge achieved, and then discussed their experiences with the physician‐tutor. Most of the participants (82%) took at least 3 insulin injections daily. The participants in the standard‐treatment group continued with routine diabetes care, visiting the physician every 4 months. They were advised to measure their blood glucose concentrations, but their test results were discussed only at regular visits and were then used to improve treatment (to reduce blood glucose concentrations without increasing the frequency of hypoglycemia). During the first 5 years of the study a majority of the participants in the standard‐treatment group took 2 daily insulin injections, but thereafter more of them took at least 3 injections a day (more than 60% after 7.5 years). | |
| Outcomes | Peripheral neuropathy was assessed at baseline and after 7.5 years by questioning the participants about symptoms of neuropathy, including paresthesia, dulled sensation, and pain in the legs and feet. Hand and arm symptoms were not assessed because of the possibility that they were caused by median‐nerve compression. Conduction velocities of the ulnar (motor and sensory), tibial, peroneal, and sural nerves and sensory thresholds (vibratory and thermal) on the feet and hand were measured. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated in methods |
| Allocation concealment (selection bias) | Unclear risk | Not stated in methods |
| Blinding (performance bias and detection bias) All outcomes | Low risk | The other investigators (the ophthalmologist, clinical neurophysiologist, and others) were unaware of the participants' treatment assignments |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Initially, 102 participants were randomly assigned to receive either intensified insulin treatment (48 participants) or standard insulin treatment (54 participants). During the study 7 participants died (4 in the intensified‐treatment group and 3 in the standard‐treatment group). 2 participants in the intensified‐treatment group and 4 in the standard‐treatment group moved away, and their follow‐up was only partial. They were therefore excluded from some of the analyses of results after 7.5 years |
| Selective reporting (reporting bias) | Low risk | They report on all outcomes |
| Other bias | Low risk | None |
Service 1983.
| Methods | A prospective, stratified, randomized 3‐year clinical trial was conducted on the rigorous versus conventional glucose control on peripheral nerve function in 33 people who had diabetes treated with insulin, with a duration of diabetes of less than 2 years | |
| Participants | Recent onset (< 2 years) of diabetes requiring insulin stratified by IDDM versus NIDDM by clinical characteristics and basal/postprandial C peptide values (< 1 = IDDM) | |
| Interventions | Continued conventional insulin regiment which consisted of a single insulin injection in all but 3 participants versus an intensive insulin regimen in which all with IDDM received multiple injections. | |
| Outcomes | At entry and every 6 months, each participant was examined by the same neurologist who was unaware of treatment group. They performed a neurologic symptom score and a neurologic disability score, and each underwent a computer‐assisted sensation examination of the detection threshold of touch‐pressure, vibration, and thermal cooling and a comprehensive evaluation of amplitude, latencies, and conduction velocities of motor and sensory fibers of multiple limb nerves. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomly assigned using a book of random numbers |
| Allocation concealment (selection bias) | Unclear risk | Methods not clearly stated |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Neurologist was blinded to assigned group |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 7 participants were excluded after randomization because insulin was no longer required (2), followed (1), or because of early < 6 month dropouts (4). Only 5 participants completed 3 years. 7 participants only completed 6 months. |
| Selective reporting (reporting bias) | High risk | Only report 11 of 25 nerve conduction study variables. No reporting of neurologic symptom score. |
| Other bias | High risk | The treatment groups had different key baseline demographics variables |
Shichiri 2000.
| Methods | A total of 110 people with type 2 diabetes (55 with no retinopathy‐primary prevention cohort and 55 with simple retinopathy‐secondary intervention cohort) were randomly assigned to multiple insulin injection therapy (3 or more daily injections) or to conventional insulin therapy (1 to 2 daily injections) | |
| Participants | Participants with type 2 diabetes with 1 to 2 daily injection of insulin (outpatient clinic). Had no retinopathy or simple retinopathy, UER < 300, creatinine < 1.5, no somatic or autonomic neuropathy severe enough to require treatment, < 70, otherwise healthy, no history of DKA, negative islet cell Ab, and a C peptide > 20. | |
| Interventions | Multiple insulin injection therapy (MIT) (3 or more daily injections) or to conventional insulin therapy (CIT) (1 to 2 daily injections) | |
| Outcomes | Peripheral nerve functions were evaluated by median nerve conduction velocity and by vibration threshold on the radial styloid process of the arm and the medial malleolus of the leg on both sides | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomly assigned, otherwise not stated in methods |
| Allocation concealment (selection bias) | Unclear risk | Randomly assigned, otherwise not stated in methods |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Methods not stated for blinding outcome assessor |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 5 died (2 intense versus 3 conventional), 4 moved (2 versus 2) and 2 conventional changed to intense group |
| Selective reporting (reporting bias) | Low risk | They report all outcomes including median motor and sensory CV and VPT |
| Other bias | Low risk | None |
Tovi 1998.
| Methods | In 38 people with diabetes and 20 controls, symptoms and neurophysiological examinations including electroneurography, vibration perception and temperature discrimination thresholds were investigated. Participants were randomized to insulin (n = 18) or sulfonylurea (n = 16) treatment and were re‐investigated after 1 year. | |
| Participants | 40 elderly type 2 diabetic patients who attended a healthcare center. 22 women and 18 men (mean age 75.2 years, range 67 to 86 years and mean height 1.66 m, range 1.45 to 1.81 m) with secondary failure of oral antidiabetic drug therapy but without symptoms of hyperglycemia. | |
| Interventions | One group was put on insulin (insulin‐treated, n = 20) and a district nurse showed them how to monitor the blood glucose levels regularly and to administer injections. Adjustments in doses were made until the blood‐glucose reached levels of 6 to 12 mmol/L during the day. Participants received 0.52 (0.27) units (mean and SD) of insulin per kg body weight and day. The other group (sulfonylurea‐treated, n = I6), was kept on high doses of sulfonylurea, i.e. 7 to 10.5 mg glibenclamide or 10 to 15 mg glipizide per day. | |
| Outcomes | On the initial examination and after 12 months, participants were asked if they experienced numbness, weakness or pain in the legs or arms. Their feet were inspected for ulcers. The Achilles tendon jerks were assessed and vibration sensation was tested with a tuning fork (128 Hz) on each medial malleolus and great toe. Electroneurography in motor and sensory nerves were performed on the median, ulnar, peroneal, and sural nerves on one side. Vibration perception was tested at the dorsum of the second metacarpal bone of one hand and the first metatarsal bone of one foot with an electromagnetic vibrameter. Thresholds for temperature discrimination were determined with the method described by Fruhstorfer, employing a Peltier element placed on the palm of one hand and the dorsum of one foot. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomly divided otherwise not stated in methods |
| Allocation concealment (selection bias) | Unclear risk | Not clearly stated in methods |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not clearly stated in methods |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 2 participants were excluded prior to examination and 4 after (2 versus 2) |
| Selective reporting (reporting bias) | High risk | They report only differences between diabetics and non‐randomized controls but not between participants |
| Other bias | Low risk | None |
UKPDS Study Group 1998.
| Methods | 3867 people with newly diagnosed type 2 diabetes, median age 54 years (interquartile range 48 to 60 years), who after 3 months’ diet treatment had a mean of 2 fasting plasma glucose (FPG) concentrations of 6.1 to 15.0 mmol/L, were randomly assigned intensive policy with a sulphonylurea (chlorpropamide, glibenclamide, or glipizide) or with insulin, or conventional policy with diet | |
| Participants | Between 1977 and 1991, general practitioners in the catchment areas of the 23 participating UKPDS hospitals were asked to refer all patients with newly diagnosed diabetes aged 25 to 65 years. Participants generally attended a UKPDS clinic within 2 weeks of referral. Participants who had a fasting plasma glucose (FPG) greater than 6 mmol/L on 2 mornings, 1 to 3 weeks apart, were eligible for the study. An FPG of 6 mmol/L was selected because this was just above the upper limit of normal for our reference range. The exclusion criteria were: ketonuria more than 3 mmol/L; serum creatinine greater than 175 micromol/L; myocardial infarction in the previous year; current angina or heart failure; more than one major vascular event; retinopathy requiring laser treatment; malignant hypertension; uncorrected endocrine disorder; occupation that precluded insulin therapy (e.g. driver of heavy goods vehicle); severe concurrent illness that would limit life or require extensive systemic treatment; inadequate understanding; and unwillingness to enter the study. | |
| Interventions | Conventional group: the aim in this group was to maintain FPG below 15 mmol/L without symptoms of hyperglycemia. Participants attended UKPDS clinics every 3 months and received dietary advice from a dietician with the aim of maintaining near‐normal bodyweight. The aim of intensive treatment was FPG less than 6 mmol/L and, in insulin‐treated participants, pre‐meal glucose concentrations of 4 to 7 mmol/L. These participants also continued to receive dietary advice from a dietician. The daily doses of the sulphonylureas used were: chlorpropamide 100 to 500 mg; glibenclamide 2.5 to 20 mg; and glipizide 2.5 to 40 mg. Participants assigned insulin started on once daily ultralente insulin. | |
| Outcomes | The criteria for neuropathy were loss of both ankle or both knee reflexes or mean biothesiometer reading from both toes 25 V or greater | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomization was by means of centrally produced, computer‐generated therapy allocations in sealed, opaque envelopes which were opened in sequence. The numerical sequence of envelopes used, the dates they were opened, and the therapies stipulated were monitored. The trial was open once participants were randomized. No placebo treatments were given |
| Allocation concealment (selection bias) | Low risk | Computer‐generated therapy allocations in sealed, opaque envelopes which were opened in sequence |
| Blinding (performance bias and detection bias) All outcomes | Low risk | The trial was open once participants were randomized. No placebo treatments were given. However, the neurologic assessments were carried out by staff from whom the allocations and actual therapies were concealed. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | At the end of the trial, the vital status of 76 (2.0%) participants who had emigrated was not known; 57 and 19 in intensive and conventional groups, respectively, which reflects the 70/30 randomization. A further 91 (2.4%) participants (65 in the intensive group) could not be contacted in the last year of the study for assessment of clinical endpoints. The corresponding numbers for comparison of the individual intensive agents were 69 (2.7%) emigrated and 63 (2.1%) not contactable. |
| Selective reporting (reporting bias) | Low risk | They report ankle/knee reflexes and vibration perception test |
| Other bias | Low risk | None |
CIT: conventional insulin therapy CSII: continuous subcutaneous insulin infusion CV: conduction velocities DKA: diabetic ketoacidosis FPG: fasting plasma glucose IDDM: insulin dependent diabetes mellitus LDL: low‐density lipoprotein NIDDM: non‐insulin dependent diabetes mellitus NPH: neutral protamine Hagedorn RCT: randomized controlled trial VPT: vibration perception threshold
Differences between protocol and review
In our protocol we originally intended to include type 1 and type 2 diabetes together and then perform a subgroup analysis, but because of the known clinical and biological differences between the types, we have collected data and performed the analyses for both separately and not combined. We have provided 'Summary of findings' tables which were not originally envisaged and updated the 'Risk of bias' methodology.
Contributions of authors
AAL wrote and ELF and RACH edited the first draft of the protocol. BCC and AAL reviewed abstracts and determined which articles met inclusion criteria. BCC, AAL, ELF, and RACH performed abstraction of data from articles that met inclusion criteria. BCC and RACH wrote the manuscript. All authors reviewed and edited the final manuscript.
Sources of support
Internal sources
AA Little: University of Michigan Medical Center, Ann Arbor, MI, USA.
EL Feldman: University of Michigan Medical Center, Ann Arbor, MI, USA.
BC Callaghan: University of Michigan Medical Center, Ann Arbor, MI, USA.
External sources
EL Feldman: ELF is supported by the National Institutes of Health, the Juvenile Diabetes Research Foundation (JDRF), the American Diabetes Association (ADA) and the Program for Understanding Neurological Diseases (PFUND), USA.
-
BC Callaghan, USA.
BCC is supported by the American Diabetes Association (ADA), the Program for Neurology Research and Discovery (PNRD), the A. Alfred Taubman Medical Institute, and the Katherine Rayner Program
Declarations of interest
BC: none
AAL: none
RACH: after starting this review, RACH was invited to give expert testimony in a legal case in which an issue was whether treatment of diabetes would prevent or reduce neuropathy.
ELF: serves as the consulting neurologist to the National Institutes of Health on the Diabetes Control and Complications Trial (DCCT) and Epidemiology of Diabetes Interventions and Complications (EDIC) trials.
New
References
References to studies included in this review
Accord 2010 {published data only}
- Ismail‐Beigi F, Craven T, Banerji MA, Basile J, Calles J, Cohen RM, et al. ACCORD trial group. Effect of intensive treatment of hyperglycaemia on microvascular outcomes in type 2 diabetes: an analysis of the ACCORD randomised trial. Lancet 2010;376(9739):419‐30. [PUBMED: 20594588 ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Azad 1999 {published data only}
- Azad N, Emanuele NV, Abraira C, Henderson WG, Colwell J, Levin SR, et al. The effects of intensive glycemic control on neuropathy in the VA Cooperative Study on Type II Diabetes Mellitus (VA CSDM). Journal of Diabetes and Its Complications 1999;13(5‐6):307–13. [PUBMED: 10765007] [DOI] [PubMed] [Google Scholar]
Dahl‐Jorgensen 1986 {published data only}
- Amthor KF, Dahl‐Jørgensen K, Berg TJ, Skard Heier M, Sandvik L, Aagenæs Ø, et al. The effect of 8 years of strict glycaemic control on peripheral nerve function in IDDM patients: the Oslo study. Diabetologia 1994;37(6):579‐84. [PUBMED: 7926342 ] [DOI] [PubMed] [Google Scholar]
- Dahl‐Jørgensen K, Brinchmann‐Hansen O, Hanssen KF, Ganes T, Kierulf P, Smeland E, et al. Effect of near normoglycaemia for two years on progression of early diabetic retinopathy, nephropathy, and neuropathy: the Oslo study. British Medical Journal 1986;293(6556):1195‐9. [PUBMED: 3096429] [DOI] [PMC free article] [PubMed] [Google Scholar]
DCCT 1993a {published data only}
- Diabetes Control and Complications Trial (DCCT) Research Group. Effect of intensive diabetes treatment on nerve conduction in the Diabetes Control and Complications Trial. Annals of Neurology 1995;38(6):869‐80. [PUBMED: 8526459] [DOI] [PubMed] [Google Scholar]
- The Diabetes Control and Complications Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long‐term complications in insulin‐dependent diabetes mellitus. New England Journal of Medicine 1993;329(14):977‐86. [PUBMED: 8366922] [DOI] [PubMed] [Google Scholar]
DCCT 1993b {published data only}
- The Diabetes Control and Complications Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long‐term complications in insulin‐dependent diabetes mellitus. New England Journal of Medicine 1993;329(14):977‐86. [PUBMED: 8366922] [DOI] [PubMed] [Google Scholar]
Duckworth 2009 {published data only}
- Duckworth W, Abraira C, Moritz T, Reda D, Emanuele N, Reaven PD, et al. Glucose control and vascular complications in veterans with type 2 diabetes. New England Journal of Medicine 2009;360(2):129‐39. [PUBMED: 19092145] [DOI] [PubMed] [Google Scholar]
Gaede 2003 {published data only}
- Gaede P, Vedel P, Larsen N, Jensen GV, Parving HH, Pedersen O. Multifactorial intervention and cardiovascular disease in patients with type 2 diabetes. New England Journal of Medicine 2003;348(5):383‐93. [PUBMED: 12556541] [DOI] [PubMed] [Google Scholar]
Holman 1983 {published data only}
- Holman RR, Dornan TL, Mayon‐White V, Howard‐Williams J, Orde‐Peckar C, Jenkins L, et al. Prevention of deterioration of renal and sensory‐nerve function by more intensive management of insulin‐dependent diabetic patients: a two‐year randomised prospective study. Lancet 1983;321(8318):204‐8. [PUBMED: 6130244] [DOI] [PubMed] [Google Scholar]
Hotta 1993 {published data only}
- Hotta N, Kawamori R, Sano T, Kakuta H, Kamada T, Sakamoto N. Diabetic neuropathy: effects of intensified glycaemic control with multiple insulin injections. Diabetic Medicine 1993;10 (Suppl 2):91S‐4S. [PUBMED: 8334854] [DOI] [PubMed] [Google Scholar]
Jakobsen 1988 {published data only}
- Jakobsen J, Christansen J, Kristoffersen I, Christensen C, Hermansen K, Schmitz A, et al. Autonomic and somatosensory nerve function after 2 years of continuous subcutaneous insulin infusion in type I diabetes. Diabetes 1988;37(4):452‐5. [PUBMED: 3288533] [DOI] [PubMed] [Google Scholar]
Kawamori 1991 {published data only}
- Kawamori R, Kamada T. Determination of the glycemic threshold for the regression or prevention of diabetic microangiopathies, and the insulin injection regimen to establish strict glycemic control in NIDDM. Japanese Journal of Medicine 1991;30(6):618‐21. [PUBMED: 1798230] [DOI] [PubMed] [Google Scholar]
Lauritzen 1985 {published data only}
- Lauritzen T, Frost‐Larsen K, Deckert T. Two‐year experience with continuous subcutaneous insulin infusion in relation to retinopathy and neuropathy. Diabetes 1985;34(Suppl 3):74‐9. [PUBMED: 4018423] [DOI] [PubMed] [Google Scholar]
Linn 1996 {published data only}
- Linn T, Ortac K, Laube H, Federlin K. Intensive therapy in adult insulin‐dependent diabetes mellitus Is associated with improved insulin sensitivity and reserve: a randomized, controlled, prospective study over 5 years in newly diagnosed patients. Metabolism: Clinical and Experimental 1996;45(12):1508‐13. [PUBMED: 8969284] [DOI] [PubMed] [Google Scholar]
Reichard 1993 {published data only}
- Reichard P, Nilsson BY, Rosenqvist U. The effect of long‐term intensified insulin treatment on the development of microvascular complications of diabetes mellitus. New England Journal of Medicine 1993;329(5):304‐9. [PUBMED: 8147960] [DOI] [PubMed] [Google Scholar]
Service 1983 {published data only}
- Service FJ, Daube JR, O'Brien PC, Zimmerman BR, Swanson CJ, Brennan MD, et al. Effect of blood glucose control on peripheral nerve function in diabetic patients. Mayo Clinic Proceedings 1983;58(5):283‐9. [PUBMED: 6341720] [PubMed] [Google Scholar]
Shichiri 2000 {published data only}
- Shichiri M, Kishikawa H, Ohkubo Y, Wake N. Long‐term results of the Kumamoto study on optimal diabetes control in type 2 diabetic patients. Diabetes Care 2000;23 (Suppl 2):B21‐9. [PUBMED: 10860187] [PubMed] [Google Scholar]
Tovi 1998 {published data only}
- Tovi J, Svanborg E, Nilsson BY, Engfeldt P. Diabetic neuropathy in elderly type 2 diabetic patients: effects of insulin treatment. Acta Neurologica Scandinavica 1998;98(5):346‐53. [PUBMED: 9858106] [DOI] [PubMed] [Google Scholar]
UKPDS Study Group 1998 {published data only}
- UK Prospective Diabetes Study (UKPDS) Group. Intensive blood‐glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet 1998;352(9131):837‐53. [PUBMED: 9742976] [PubMed] [Google Scholar]
Additional references
Boulton 1998
- Boulton AJM, Gries FA, Jervell JA. Guidelines for the diagnosis and outpatient management of diabetic peripheral neuropathy. Diabetes Medicine 1998;15(6):508‐14. [DOI] [PubMed] [Google Scholar]
Boulton 2004
- Boulton AJM, Malik RA, Arezzo JC, Sosenko JM. Diabetic somatic neuropathies (technical review). Diabetes Care 2004;27(6):1458‐86. [DOI] [PubMed] [Google Scholar]
Brownlee 2005
- Brownlee M. The pathobiology of diabetic complications. Diabetes 2005;54(6):1615‐25. [DOI] [PubMed] [Google Scholar]
DCCT 1995
- Diabetes Control and Complications Trial (DCCT) Research Group. Effect of intensive diabetes treatment on nerve conduction in the Diabetes Control and Complications Trial. Annals of Neurology 1995;38:869‐80. [DOI] [PubMed] [Google Scholar]
Dyck 2003
- Dyck PJ. Severity and staging of diabetic polyneuropathy. In: Gries FA, Cameron NE, Low PA, Ziegler D editor(s). Textbook of Diabetic Neuropathy. Stuttgart: Thieme, 2003:170‐5. [Google Scholar]
Dyck 2005
- Dyck PJ, Hughes RAC, O’Brien PC. Quantitating overall neuropathic symptoms, impairments, and outcomes. In: Dyck PJ, Thomas PK editor(s). Peripheral Neuropathy. 4th Edition. Philadelphia: Elsevier Saunders, 2005:1038‐9. [Google Scholar]
Expert Committee 2003
- Genuth S, Alberti KG, Bennett P, Buse J, Defronzo R, Kahn R, et al. Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Follow‐up report on the diagnosis of diabetes mellitus. Diabetes Care 2003;26(11):3160‐7. [DOI] [PubMed] [Google Scholar]
Gale 2001
- Gale EAM. The discovery of type I diabetes. Diabetes 2001;50(2):217‐26. [DOI] [PubMed] [Google Scholar]
Habib 2010
- Habib AA, Brannagan TH 3rd. Therapeutic strategies for diabetic neuropathy. Current Neurology and Neuroscience Reports 2010;10(2):92‐100. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Altman DG, Sterne JAC (editors). Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
North‐West Diabetes Foot Care Study 2002
- Abbott CA, Carrington AL, Ashe H, Bath S, Every LC, Griffiths J, et al. North‐West Diabetes Foot Care Study. The North‐West Diabetes Foot Care Study: incidence of, and risk factors for, new diabetic foot ulceration in a community‐based patient cohort. Diabetic Medicine 2002;19(5):377‐84. [DOI] [PubMed] [Google Scholar]
Partanen 1995
- Partanen J, Niskanen L, Lehtinen J, Mervaala E, Siitonen O, Uusitupa M. Natural history of peripheral neuropathy in patients with non‐insulin‐dependent diabetes mellitus. New England Journal of Medicine 1995;333(2):89‐94. [DOI] [PubMed] [Google Scholar]
Ratner 2001
- Ratner R. Glycemic control in the prevention of diabetic complications. Clinical Cornerstone 2001;4(2):24‐37. [DOI] [PubMed] [Google Scholar]
Stolar 2010
- Stolar M. Glycemic control and complications in type 2 diabetes mellitus. American Journal of Medicine 2010;123(3 Suppl):S3‐11. [DOI] [PubMed] [Google Scholar]
Vinik 2003
- Vinik AI, Maser RE, Mitchell BD, Freeman R. Diabetic autonomic neuropathy. Diabetes Care 2003;26(5):1553‐79. [DOI] [PubMed] [Google Scholar]


