Abstract
In 2010, the National Institutes of Health (NIH) established the Therapeutics for Rare and Neglected Diseases (TRND) program within the National Center for Advancing Translational Science (NCATS), which was created to stimulate drug discovery and development for rare and neglected tropical diseases through a collaborative model between the NIH, academic scientists, nonprofit organizations, and pharmaceutical and biotechnology companies. This paper describes one of the first TRND programs, the development of 2-hydroxypropyl-β-cyclodextrin (HP-β-CD) for the treatment of Niemann-Pick disease type C1 (NPC1). NPC is a neurodegenerative, autosomal recessive rare disease caused by a mutation in either the NPC1 (about 95% of cases) or the NPC2 gene (about 5% of cases). These mutations affect the intracellular trafficking of cholesterol and other lipids, which leads to a progressive accumulation of unesterified cholesterol and glycosphingolipids in the CNS and visceral organs. Affected individuals typically exhibit ataxia, swallowing problems, seizures, and progressive impairment of motor and intellectual function in early childhood, and usually die in adolescence. There is no disease modifying therapy currently approved for NPC1 in the US. A collaborative drug development program has been established between TRND, public and private partners that has completed the pre-clinical development of HP-β-CD through IND filing for the current Phase I clinical trial that is underway. Here we discuss how this collaborative effort helped to overcome scientific, clinical and financial challenges facing the development of new drug treatments for rare and neglected diseases, and how it will incentivize the commercialization of HP-β-CD for the benefit of the NPC patient community.
Keywords: 2-hydroxypropyl-β-cyclodextrin, Niemann-Pick disease type C1, neurodegenerative rare disease, translational research
INTRODUCTION
Rare diseases are defined as those affecting less than 200,000 individuals in the US, or less than one in 2000 in Europe [1, 2]. There are approximately 7000 rare or orphan diseases, collectively affecting about 30 million people in the US, and only 250 of these diseases have an FDA approved therapy designed to address a particular rare disease [3]. The overall process of drug development to obtain regulatory approval for use in humans is lengthy, 12 years on average, expensive, and with a very low success rate [4–7]. As a consequence, pharmaceutical and biotechnology companies mostly focus on so-called “druggable” targets in clinically validated pathways and for diseases of large populations, in order to lower risk and increase the return on their financial investment [8]. The development of therapeutics for rare diseases therefore faces many scientific, clinical and economic challenges [9]. The biology underlying rare diseases is often complex and poorly understood, and therapeutic targets are, in general, less tractable, further increasing the difficulty of developing new drugs. The investment needed for pre-clinical and clinical studies remains as high as in common diseases, and because populations are small, the potential financial returns are perceived to be too low to justify investment. For rare diseases, it is necessary to carefully design clinical trials to support drug approval due to the small patient populations, varying ages of onset, and complex clinical symptoms [9]. Furthermore, the clinical trials often rely on multifaceted and lengthy natural history studies that monitor disease progression and identify biomarkers in order to determine beneficial clinical effects of a therapy in patients [10–14]. To overcome these challenges in drug development for rare diseases, an innovative collaborative drug development framework needs to be established where public and private partners work together towards a common effort [4–6, 9, 15]. In this model, each partner contributes resources and expertise to increase the probability of success to regulatory approval, and lower the financial risk to make the treatment viable for industry to take advantage of incentives and commercialize for the benefit of the patient community.
In 2010, the National Institutes of Health (NIH) established the Therapeutics for Rare and Neglected Diseases (TRND) program, within the National Center for Advancing Translational Science (NCATS), in order to stimulate drug discovery and development for rare and neglected tropical diseases through a collaborative model interlinking the NIH, academic scientists, nonprofit organizations, and pharmaceutical and biotechnology companies [16]. The goal of TRND is to create a new framework that brings partners together that can contribute resources and expertise to enable the development of drug treatments through pre-clinical studies for IND filing and Phase I studies. By helping fund the early stage drug development process, TRND hopes to incentivize industry to further develop, obtain approval, and commercialize these treatments. Eventually, TRND will share its best practices and lessons learned with the rare disease community to help others build successful collaborative paths to new therapeutic treatments.
One of the initial projects selected by TRND was the development of 2-hydroxypropyl-β-cyclodextrin (HP-β-CD) for the treatment of Niemann-Pick disease type C (NPC). NPC is a neurodegenerative, autosomal recessive rare disease caused by a mutation in either the NPC1 (about 95% of cases) or the NPC2 gene (about 5% of cases). These mutations affect the intracellular trafficking of cholesterol and other lipids, which leads to a progressive accumulation of unesterified cholesterol and sphingolipids in the CNS and visceral organs [17, 18]. NPC has an estimated incidence of 1 in 120,000 to 150,000 live births, and the majority of these cases are NPC1 mutations [19]. The disease becomes apparent in patients at varying ages, from infancy to adulthood, and with different neurological manifestations. Affected individuals typically exhibit ataxia, swallowing problems, seizures, and progressive impairment of motor and intellectual function in early childhood, and usually die in adolescence [18, 20]. There is no disease modifying therapy currently approved for NPC1 in the US. Miglustat (Zavesca), a small iminosugar molecule that partially and reversibly inhibits glucosylceramide synthase, a pivotal early enzyme in the glycosphingolipid pathway, is approved for NPC treatment in the European Union, Canada, Brazil, and other countries [21, 22]. While miglustat has been shown to have modest efficacy in delaying disease progression in NPC1 juvenile and adult patients, it does not mobilize intracellular cholesterol accumulation associated with this disorder [23–26]. New treatments for NPC are therefore urgently needed.
This paper discusses the formation of a scientific collaboration involving academia, industry, and government, and describes the elements of this public-private partnership that contributed to the successful translation of HP-β-CD from the laboratory setting to a Phase I clinical trial for the treatment of NPC1. We will describe the larger network of collaborative interactions that were established with the different groups to provide critical resources to this rare disease development program, including the NIH Office of Rare Disease Research (ORDR) [3], the US Food and Drug Administration (FDA) rare disease program [27], the FDA Office of Orphan Products Development (OOPD) [28], and disease advocacy groups, both in the US and Europe [29–35]. The value added by this collaborative preclinical development program will be discussed, as well as outlining the path for further implementation of the next phases of clinical development and continued support towards the end goal of FDA approval of HP-β-CD for NPC1 disease with a commercial partner.
PRE-CLINICAL LEAD COMPOUND 2-HYDROXYPROPYL-β-CYCLODEXTRIN
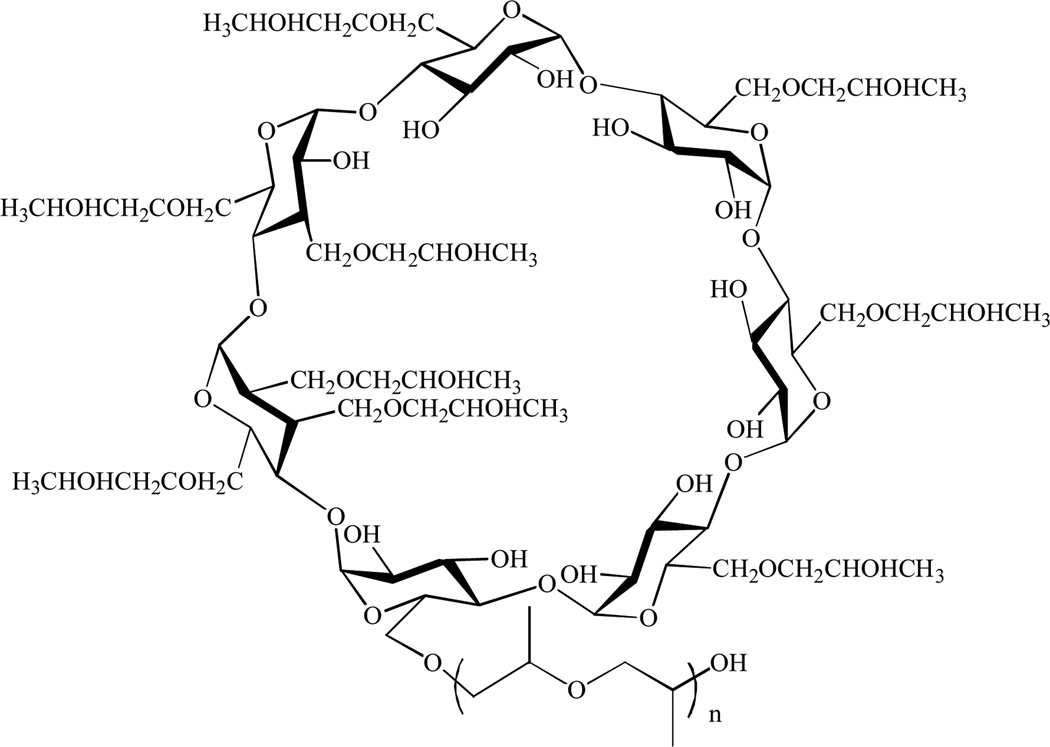
Cyclodextrins are a family of cyclic oligosaccharides with a relatively hydrophilic exterior surface and a hydrophobic interior cavity. Among other uses, cyclodextrins, particularly β-cyclodextrins, are widely utilized as pharmaceutical excipients due to their ability to increase the solubility and dissolution rate of applicable poorly water-soluble drugs [36–38]. 2-Hydroxypropyl-β-cyclodextrin consists of 7 cyclo-α-(1,4)-anhydroglucose units with hydroxypropyl groups randomly substituted onto the β-cyclodextrin molecule, Fig (1). Use of 2-hydroxypropyl-β-cyclodextrin in drug formulations was pioneered by Janssen Pharmaceuticals, (now Janssen Research and Development LLC, part of the Janssen Pharmaceutical Companies of Johnson & Johnson, JRD) with the marketed product Sporanox® (itraconazole), and cyclodextrins can now be found in over 35 commercially available drug products [39]. β-cyclodextrin is found in the FDA “generally recognized as safe” (GRAS) list [40] and its derivative, HP-β-CD is referenced in the USP/NF and EP and is cited in the FDA’s list of inactive pharmaceutical ingredients [41]. There are a great deal of data in the literature indicating that HP-β-CD is well tolerated in most species, and in IV infusion of HP-β-CD in human volunteers with doses of up to 470 mg/kg/day (30 g in 4 days), and up to 3.0 g in a single dose [42–49]. However, HP-β-CD has only been considered as an excipient and has not been FDA-approved as a pharmaceutical drug product for use as a therapeutic agent.
Fig. (1).
2-Hydroxypropyl-β-cyclodextrin.
HISTORY OF 2-HYROXYPROPYL-β-CYCLODEXTRIN AND NPC1 DISEASE
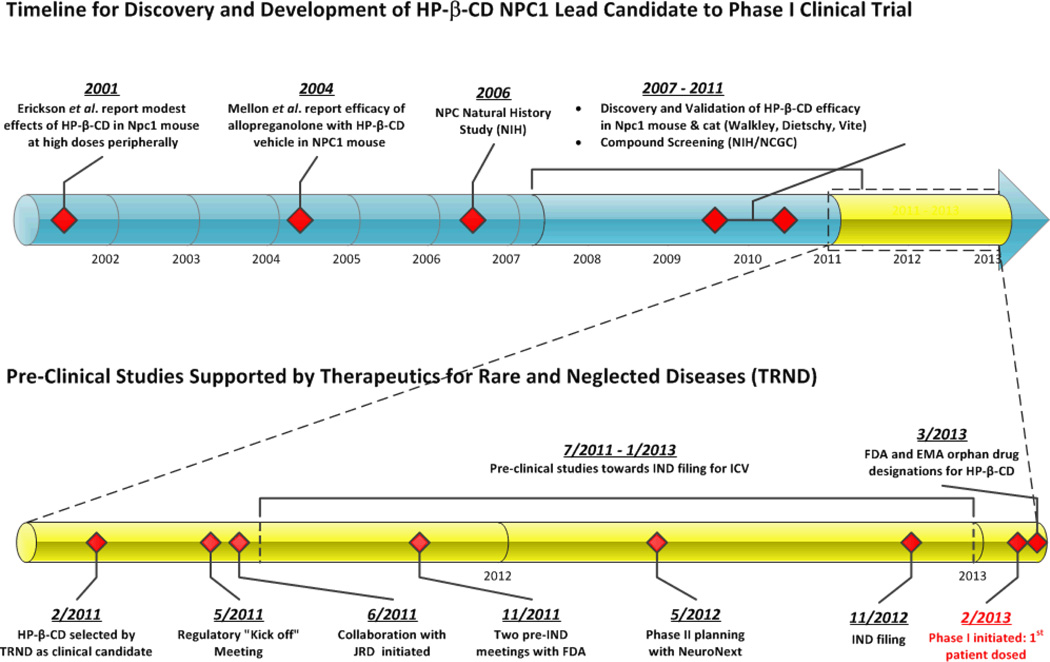
The NPC1 gene was cloned in 1997, and its protein product was found to contain a sterol-sensing domain, thus suggesting a role for NPC1 in cholesterol homeostasis [50]. Cells from NPC1 patients are defective in the release of cholesterol from late endosomes/lysosomes [17]. This excessive storage of cholesterol led investigators to study cholesterol-lowering drugs for the treatment of NPC1. Cyclodextrins are known to form complexes with cholesterol and in vitro studies with HP-β-CD have shown that it is able to remove cholesterol from a variety of cell lines and from macrophage foam cells [51, 52]. In 2001, Erikson and colleagues studied the cholesterol mobilizing effect of HP-β-CD in Npc1−/− knockout mice, and reported a decrease in liver cholesterol storage, but only a small effect on the delay of neurological symptoms when dosed peripherally, and no effect when dosed directly into the brain. Based on these results, it was initially concluded that HP-β-CD was not likely to be beneficial to NPC1 patients [53]. In 2004, Mellon, et al. reported that allopregnanolone, a neurosteroid, reduced cholesterol in NPC1 deficient mice, when dosed as a single injection subcutaneously at postnatal day 7, and almost doubled the lifespan of Npc1−/− mice [54]. However, in the Mellon study, the allopregnanolone was solubilized with HP-β-CD, which alone was subsequently shown to be efficacious in Npc1−/− mice [25, 55]. From 2007 to the present, there has been an extensive effort by the Walkley and Dietschy groups using the Npc1−/− mouse model [25, 55–58] and by the Vite lab using the Npc1 cat model [59], to test the efficacy of HP-β-CD in vivo when dosed ether parenterally or directly into the brain, intracerebroventricularly (ICV) or via intrathecal (IT) injection at the cerebellomedullary cistern. In both mouse and cat models of NPC1, HP-β-CD demonstrated the ability to mobilize cellular cholesterol, decrease GM2 and GM3 ganglioside storage, slow cerebellar Purkinje cell loss, reduce the clinical effects of the disease, and prolong survival of the affected animals. An overview of the timeline for the discovery and development of HP-β-CD for NPC1 is shown in Fig (2).
Fig. (2).
Timeline for HP-β-CD discovery and development.
The mechanism of action of HP-β-CD in NPC is not understood; however, it has been shown in vitro to be effective in reversing the intracellular accumulation of unesterified cholesterol and associated lipids in neuronal cell lines. HP-β-CD, at concentrations of 0.1 mM and 1 mM, reduced stored cholesterol in primary neurons and astrocytes [60]. The effect appeared to be dose-dependent; at lower concentrations (0.1 mM), HP-β-CD mobilized endolysosomal cholesterol and re-distributed to the endoplasmic reticulum, whereas at higher concentrations (1 mM), HP-β-CD appeared to function by extracting plasma membrane cholesterol. The precise mechanism by which HP-β-CD triggers release of endolysosomal cholesterol and gangliosides is uncertain, though studies in Npc1−/− mouse embryonic fibroblasts suggest involvement of lysosomal exocytosis [61].
HISTORY OF NPC1 CLINICAL STUDIES
In 2006, a single-center Natural History Study for the identification of clinical and biochemical disease progression markers in NPC1 patients was initiated at the NIH clinical center by Dr. Forbes Porter [11]. For rare diseases, natural history studies play an important role in supporting the development of therapeutic candidates because they can provide an understanding of the etiology, range of manifestations, and disease progression. Through natural history studies, tools such as disease severity scores or other correlates for clinical outcomes can be developed to show effectiveness and measure improvement in patients’ quality of life, which is often an important component of the efficacy assessment for regulatory approval of a drug. From the NPC natural history study, cholesterol oxidation products have been identified as potential specific blood and CSF-based biomarkers that can be utilized in clinical trials to monitor a response to therapy [13, 14, 62]. Natural history studies also help engage the different members of a disease community, including patients, patient families, scientists and clinicians, to establish strong collaborations to develop common goals and joined efforts that benefit the whole patient community. Through natural history studies, clinicians can not only learn how to better care for patients, but also can form critical networks of patients who may be eligible for enrollment in clinical trials for the systemic evaluation of potential therapies.
The in vitro cellular and in vivo animal studies validated HP-β-CD as a promising lead candidate for testing in NPC1 patients. In 2009, the physician of a family with twin daughters affected by NPC1 initiated individual compassionate use INDs for IV administration of HP-β-CD and, in 2010, these INDs were modified for IT route of administration [29]. In the US, individual use INDs can be submitted to the FDA for patients with a “serious or immediately life-threatening disease or condition for which there is no comparable or satisfactory therapy” where the possible benefit justifies the possible risks [9, 63–65]. Worldwide, there are also children in Brazil and Japan that have been reported to receive HP-β-CD on an individual basis [66]. The use of HP-β-CD in individual compassionate use INDs provided an initial indication of safety in NPC1 patients. However, well-controlled trials in a larger number of NPC1 patients are needed to meet the regulatory standards of safety and efficacy for FDA approval. As stated in the FDA guidelines, these individual INDs should not comprise the ability to perform clinical studies of the drug to determine safety and efficacy [63].
PRE-CLINICAL DEVELOPMENT OF HP-β-CD for NPC1
There is a small community of researchers studying NPC, and in order to further scientific advancements in the field, collaborations have been fostered to complement strengths and expertise. In 2010, the National Institute for Neurological Disorders and Stroke (NINDS) sponsored the meeting, “Promising Therapies for NPC,” organized by Drs. Steven Walkley and Danilo Tagle, to bring together NPC researchers and disease advocacy groups at NIH to discuss the current state of knowledge and possible further therapeutic development, focusing in part on cyclodextrin. Subsequently, in 2011, the TRND program, based on the promising in vitro cellular and in vivo animal efficacy studies, chose the development of the lead compound, HP-β-CD, for the treatment of NPC1 as one of its initial projects to provide the needed resources to support a Phase I clinical trial, Fig (2). Upon project acceptance, the first steps were 1) to form the collaborative team that would work together towards a common goal, and 2) evaluate studies needed for pre-clinical development. The initial scientific team was formed by an academic NPC1 disease group of experts some of whom had been already collaborating on HP-β-CD and other potential NPC therapies since 2008, and included the laboratories of Dr. Daniel Ory at Washington University School of Medicine with biomarker discovery and assay development expertise, Dr. Steven Walkley at the Albert Einstein School of Medicine with Npc1−/− mouse model and central nervous system (CNS) analysis expertise, and Dr. Charles Vite at University of Pennsylvania with expertise in the Npc1 cat model. These investigators also formed connections with researchers at the NIH/NCATS National Genomics Chemical Genomics Center (NCGC) with expertise in assay development and chemical compound library screening, and at the NIH Clinical Center with Dr. Forbes Porter, providing expertise in human NPC1 disease and patient care. In order to organize the TRND project team and facilitate the coordination of all the drug development activities, TRND assigned a project manager with industry experience to serve as the central point of contact through which all project communication flowed and ensured progression of the project milestones. This centralized project management was key to driving the project forward to the end goal, a Phase I clinical trial, by ensuring the necessary resources were available, tracking activities and studies, monitoring overall project costs, and fostering the team environment, Fig (3). A “kick off” regulatory meeting was organized in May 2011 to formulate a plan for submission of an Investigational New Drug (IND) application to the FDA. JRD had been collaborating with the Vite lab on pharmacokinetics and distribution studies of HP-β-CD in the Npc1 cat model, and provided their resources and expertise in HP-β-CD to help the NPC community. The scientific team met with JRD at NIH in June 2011, which resulted in Janssen becoming a key integral member of the TRND team, donating their expertise and resources in the pharmacokinetics, safety, and chemistry and manufacturing of HP-β-CD for toxicology and Phase I clinical studies.
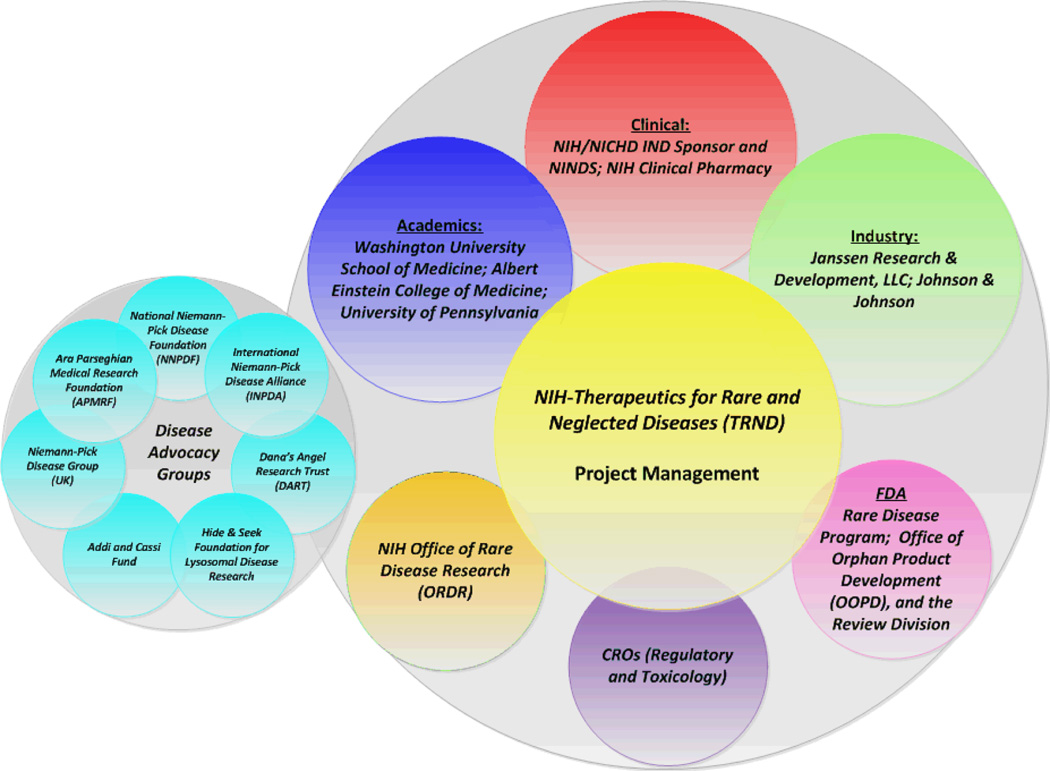
Fig. (3).
NPC1 collaboration network.
One of the first critical questions in the design of the development program was election of a route of administration for HP-β-CD. Results from nonclinical pharmacology studies investigating HP-β-CD as treatment for NPC1 indicated that administration directly into the CNS would likely be better to ameliorate the neurological symptoms of NPC than peripheral administration [25, 55–58]. Extremely high doses of HP-β-CD are required in the Npc1 mouse and cat animal models to normalize biochemical abnormalities and prevent neurodegeneration if administered peripherally and later studies established how little CD was able to cross the BBB following peripheral administration [67]. ICV administration in the mouse decreased the estimated effective dose (ED50) from 132,000 mg/kg body weight to 0.5 mg/kg body weight, normalized biochemical abnormalities, and prevented neurodegeneration [56]. Based on pharmacokinetic studies in the Npc1 mouse and cats [55] (Kao and Vite unpublished data), administration of HP-β-CD via ICV directly into the cerebral ventricle or the cerebellomedullary cistern of mouse and cat models of NPC1, respectively, overcame issues with BBB penetration [56] (Vite, unpublished data). In addition, in a quantitative whole-body radiography (QWBR) distribution study in cats, cerebellomedullary cistern injection of HP-β-CD also showed penetration of drug-derived radioactivity into the deeper parts of the CNS, suggesting that HP-β-CD administration via cerebellomedullary cistern injection in animals is an appropriate method of administration (Vite and Kao unpublished results). Pre-clinical studies were therefore directed towards supporting ICV administration of HP-β-CD in the Phase I trial using an Ommaya reservoir for delivery, Table (1).
Table 1.
Pre-clinical studies needed to support phase I study in NPC1 patients.
| Pre-Clinical Studies | Description |
|---|---|
| Npc1 Animal Studies | Efficacy and dose range finding in Npc1 mouse and cat models by intended route of administration |
| Pharmacokinetics (PK)/Pharmacodynamics (PD) | Brain distribution and correlation to dosing levels for Phase I Clinical Trial |
| Biomarker Development | Identification and development of assays to measure disease progression biomarkers in NPC1 patients |
| Bioanalytical Method Development | Assay development to measure HP-β-CD in plasma and CSF for toxicology studies and Phase I Clinical Trial |
| CMC Support | Development of formulation, development and validation of analytical methods, manufacturing of the clinical supply of HP-β-CD, stability and compatibility studies with the Ommaya reservoir |
| Toxicology Studies | Juvenile dog and cat studies to establish safe starting dose in humans |
| Regulatory Support | IND filing and FDA interactions |
| Clinical Studies | |
| Natural History Study | Identification and measurement of disease progression markers |
| Phase I Clinical Trial | Study of feasibility, safety, tolerability, pharmacokinetics (PK), and activity of intracerebroventricular (ICV) HP-β-CD administered via an Ommaya reservoir to patients with NPC1 |
Once the route of administration was chosen, the TRND team had two pre-IND meetings with the FDA in November and December of 2011 that provided critical feedback and guidance from the FDA on the development program that would support an IND application. Based on FDA guidance, safety studies were conducted in juvenile beagle dogs: safety parameters were collected in conjunction with ongoing observational studies conducted in a 2nd species, Npc1 cats, to provide support for administration of HP-β-CD directly into the CNS. Additional studies to support a Phase I trial included the development of a validated GLP assay to measure HP-β-CD in plasma and CSF for pharmacokinetic (PK) measurements, validated assays to measure disease progression biomarkers that may provide surrogate endpoints of efficacy, and formulation development and manufacturing of the clinical supply of HP-β-CD that was compatible with ICV administration via an Ommaya reservoir. Table (1) lists the pre-clinical IND enabling studies and clinical studies for this stage of the development. The total cost was estimated to be ~5 million US dollars; however, the preclinical cost for development of the Drug Master File was not included. The IND was submitted in December 2012, and the Phase I Clinical Trial began in February 2013. The Phase I trial is an adaptive, dose escalation study designed to systematically determine a safe and biochemically effective dose of HP-β-CD.
THE NPC COLLABORATION NETWORK
The strong commitment and passion of every member in the collaborative team was key for the successful translation of HP-β-CD from the laboratory setting to a Phase I clinical trial for the treatment of NPC. External and internal collaborators worked seamlessly, with each member providing their resources and expertise in a clearly outlined plan, with direct and clear points of contact from each partner. A cohesive team was established that met on a bi-weekly basis. Sub-teams were formed for specific activities, such as development of the HP-β-CD formulation, manufacturing of the clinical supply, stability and compatibility studies with the Ommaya reservoir with JRD in Beerse, Belgium, toxicology studies and regulatory activities with contract research organizations (CROs), which also met on a regular basis. It was essential that every member of the team was proactive, responsive, and followed through on action items to deliver assignments on time. The team members devoted time beyond their regular job responsibilities to actively participate in team meetings and respond to questions and program needs in a timely manner. The team dynamics fostered open and interactive information sharing, collaboration, and communication that was supported by a multiparty Confidential Disclosure Agreement (CDA) that was put in place early in the project. As needed, other agreements, such as Material Transfer Agreements (MTAs) and a Clinical Trial Agreement (CTA) were executed through close collaboration with Technology Transfer Departments. Lastly and critically, every partner respected and trusted the central management provided by TRND and the project manager to directly share all information at all times throughout the project.
In addition to the main scientific collaboration team, other collaborative partners, including other NIH individuals and groups enabled the advancement of HP-β-CD into the clinic. Dr. Bill Pavan at the National Human Genome Research Institute (NHGRI) was part of the team that first identified the NPC1 gene [50, 68]. Dr. Russell Lonser and Dr. John Heiss at NINDS, the NIH clinical center pharmacy, and other staff at the NIH clinical center are participating in the execution of the clinical protocol. The NIH/NCATS Office of Rare Disease Research (ORDR)[3], which supports rare diseases, for example, with granting programs for scientific meetings, the rare disease clinical research network (RDCRN), and patient registries provided some initial funding support for the NPC1 natural history trial and the cyclodextrin pre-clinical studies through several Bench to Bedside grants. The FDA rare disease program [27], while providing no formal IND review guidance, served as a resource on general advice on FDA processes and support of the NPC rare disease program, and was represented at the pre-IND meetings.
Patients and their advocates have played a very critical role in the support of rare disease research and the development of therapeutic interventions, Fig (3). Since rare diseases have not had the focused attention of pharmaceutical and biotechnology companies, rare disease advocacy organizations have been key to catalyzing the discovery and development of new treatments. Some of these organizations have made progress supporting research from basic through late stage development and approval of new therapeutics. Examples include the Cystic Fibrosis Foundation (CFF) [69], the Leukemia and Lymphoma Society’s Therapy Acceleration Program (LLS-TAP) [70], the Myelin Research Foundation’s Accelerated Research Collaboration Model [71], and the Multiple Myeloma Research Foundation [72]. For NPC, there are many disease patient advocacy groups founded by parents, including both individual families and larger umbrella non-profit organizations. Some of these organizations such as the Ara Parseghian Medical Research Foundation (APMRF) [30], the National Niemann-Pick Disease Foundation (NNPDF) [31], the International Niemann-Pick Disease Alliance (INPDA) [32], the Niemann-Pick Disease Group (UK) [33], the Addi and Cassi Fund [29], Dana’s Angel’s Research Trust (DART) [34], and the Hide & Seek Foundation for Lysosomal Disease Research [35] have had a major impact on NPC research and awareness. For example, several of the initial animal studies investigating HP-β-CD were funded by the Ara Parseghian Medical Research Foundation (APMRF) which was founded in 1994, and has subsequently raised nearly $40 million in support of NPC research. Over the last 20 years, several of the NPC organizations have supported the advancement of research, including the development of research tools, cell lines, animal models, biomarkers, and compound screening. In 2008, a collaborative research funding project was initiated, Support Of Accelerated Research for Niemann-Pick Disease Type C (SOAR-NPC), to also accelerate and contribute to the development of NPC therapies. This project is supported by DART and the Hide & Seek Foundation for Lysosomal Disease Research. SOAR-NPC facilitated connections between the scientific and patient advocacy communities that helped catalyze the development of the HP-β-CD program.
These patient organizations also support the NPC community in so many other ways and provide organized access to patients that are critical to clinical trials. The NPC natural history study was supported by APMRF, NNPDF and DART. Other forms of support include conferences, forums, and webinars to disseminate information, and advocate for policy changes that will benefit rare diseases with more public funding and flexibility in the FDA review process. Their interactive collaboration with the scientific community on these multiple levels has been an integral component of the progress towards new therapeutics.
FUTURE DEVELOPMENT PATH FOR HP-β-CD
If HP-β-CD is shown to be safe and efficacious for the treatment of NPC1 in clinical trials, the goal is to obtain drug approval by regulatory agencies in the US and other countries, which would enable wide availability of this treatment option to the NPC1 patient community. The continued development of HP-β-CD for regulatory approval will need support from a commercial partner that has the infrastructure and expertise for the late stages of the drug development process. A key driver for commercial viability is the ability of pharmaceutical and biotechnology companies to receive a return on their investment [8]. The TRND program is a collaborative development model that focuses on connection and coordination of a network of resources to gather early evidence of medical value. The stronger the pre-clinical and early phase clinical data, the more attractive the drug candidate should be to potential industry partners because there is lower likelihood for failure in late stage development and less overall costs to be incurred. The early stage development costs are shared amongst the collaborators, with both public and private funding, including government, academic, industry, and disease advocacy groups. In addition, the drug development and project management expertise provided by the TRND program provides additional value by ensuring that the completed pre-clinical studies meet the current regulatory standards to support clinical testing and FDA approval.
Another critical factor in transitioning the program to a pharmaceutical sponsor is the ability of the sponsor to obtain exclusive rights to all the development data, intellectual property (IP), and patents. Ownership of IP for a drug substance, its manufacturing process, formulation, or use in a particular indication, ensures the viable potential for commercialization of a product. In the case of cyclodextrin, HP-β-CD is commercially available as an excipient for use in drug formulations [39]. For rare diseases, however, the Orphan Drug Act passed in 1983 [73] provides additional incentives for clinical testing and regulatory approval to stimulate the development and commercialization of orphan drugs [74]. The Office of Orphan Products (OOPD) grants an orphan drug designation to products indicated for a rare disease or disorder affecting fewer than 200,000 persons [1]. For the decade prior to 1983, 10 drugs were approved for rare diseases. From 1983 to 2009, the number increased to 346 approvals and 2,112 orphan product designations [75–77], and from 2000–2009, 38 orphan pediatric drugs were approved [78]. The incentives provided include 7 years market exclusivity from the date of market approval, tax credits up to 50% for clinical development costs, ability to apply for grants for product development, and exemption from New Drug Application (NDA) application user fees [79]. Similar legislation for rare diseases is approved in Europe, with orphan drug designation providing 10 years exclusivity from the time of approval, protocol assistance, fee reduction for the European Medicine Agency (EMA) marketing authorization, and the ability to apply for specific grants for clinical trials [2, 77]. TRND received orphan drug designation for HP-β-CD from the FDA in January 2013, and EMA designation, in collaboration with a European sponsor, the International Niemann-Pick Disease Alliance (INPDA) in February 2013. These designations are easily transferrable to another development partner. More than one sponsor can hold orphan drug designation for the same molecule and same indication, however; the marketing exclusivity is only granted for the first party to gain marketing approval. Since HP-β-CD has not previously been approved by the FDA as an active ingredient for a therapeutic treatment, it also will not be easily used off-label through a doctor’s prescription. Therefore, the incentives provided by the orphan drug designation to take HP-β-CD to approval could be significant.
If safety is demonstrated in the Phase I clinical trial, a Phase II trial will be conducted to further test safety and efficacy in a larger number of patients. The NPC TRND team plans to establish collaborations with another NIH program, the Network for Excellence in Neuroscience Clinical Trials (NeuroNEXT), to support a multicenter, international Phase II trial [80]. The NeuroNEXT program was also created to conduct studies of treatments for neurological diseases using a collaborative framework, establishing partnerships with academia, private foundations, and industry, and providing the infrastructure needed to coordinate clinical sites, data, and a unified IRB/Ethics Committee approval process. For approval of any drug, including orphan drugs, the FDA requires “substantial evidence” of effectiveness derived from “adequate and well-controlled investigations”[76]. A recent review of orphan drug approvals suggests that the FDA has exercised flexibility in the designs and number of clinical studies to meet the statutory requirements for demonstration of a drug’s safety and efficacy [81]. The new provisions provided in the Food and Drug Administration Safety and Innovation Act of 2012 (FDASIA) will have further impact on rare pediatric diseases, and two provisions in particular, for NPC, a serious life-threatening rare pediatric disease and for which no FDA-approved therapy currently exists [82]. Upon approval of a New Drug Application for a treatment for a rare pediatric disease, provided the active ingredient has not previously been approved in the US and provided that the application meets certain data criteria, the FDA provides the sponsor with a voucher which entitles the sponsor to priority review by the FDA of one future drug application. The “Priority Review Voucher” can be applied to shorten the review time for a future new drug application submitted by the company. In addition, there is a new “Breakthrough Designation” to expedite the development of drugs to treat a serious life-threatening disease or condition where “preliminary clinical evidence indicates that the drug may demonstrate substantial improvement over existing therapies on one or more clinically significant endpoints, such as substantial treatment effects observed early in clinical development." [83]. This designation will provide more guided interaction with FDA that can lead to more innovative clinical trials and possible approval at earlier clinical phases of development.
Lastly, significant overarching value from investing and working in the rare disease community relates to the possible application of the science learned in the study of rare disorders to understand disease mechanisms of more common conditions. Understanding the underlying biology of NPC, involving neurodegeneration and lipid storage, can provide new mechanisms and targets for the treatment of other diseases such as Alzheimer’s and Huntington’s diseases, arteriosclerosis, and other lysosomal storage diseases [84–86].
CONCLUSIONS
The development of therapeutics for rare disorders is faced with the challenges that come with any drug discovery/development project as well as the lack of financial incentives due to the low return in investment from small patient populations. This article highlights the importance of innovative collaborative drug development models that de-risk the process of drug discovery for rare diseases so that industry is incentivized to engage in development opportunities for new treatments for these patient communities. The recently established NIH program Therapeutics for Rare and Neglected Diseases (TRND) enables collaboration between academic scientists, nonprofit organizations, and pharmaceutical and biotechnology companies to leverage resources, expertise and infrastructure from all partners to facilitate all stages of drug development, from early non-clinical to late phase clinical development and ideally, through to regulatory approval. Utilizing this collaborative model, in conjunction with strong centralized project management to ensure alignment and focus on the project goals and milestones, HP-β-CD has been successfully advanced from a laboratory setting to a Phase I clinical trial for the treatment of NPC1. Although many activities to develop HP-β-CD were on-going before the TRND Team was assembled, the TRND Team served to critically focus and accelerate the efforts towards an IND filing with the goal of regulatory approval for the benefit of the entire patient population. We believe this collaborative model can be extended to other rare diseases where academics and patient advocacy groups can benefit from partnerships, public and private, that help them through the process of regulatory requirements for drug discovery and commercialization strategies.
ACKNOWLEDGEMENTS
The authors express their appreciation to the NPC1 families and patients for their support in the research and development of therapies for NPC disease.
Funding support was provided by NIH [National Center for Advancing Translational Science (NCATS), Intramural Research Program of the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD), National Institute of Neurological Disorders & Stroke (NINDS), and National Human Genome Research Institute (NHGRI)], Janssen Research & Development, LLC part of the Janssen Pharmaceutical Companies of Johnson &Johnson, Ara Parseghian Medical Research Foundation (D.S.O., S.U.W., C.H.V., F.D.P.), National Niemann-Pick Disease Foundation (D.S.O., C.H.V., F.D.P.), Dana’s Angels Research Trust (D.S.O., S.U.W., F.D.P., C. H.V.), the Hide & Seek Foundation for Lysosomal Disease Research (D.S.O., S.U.W., C.H. V.), and the Race for Adam (C.H.V).
Footnotes
CONFLICT OF INTEREST
The author(s) confirm that this article content has no conflicts of interest.
REFERENCES
- 1.FDA Orphan Drug Designation. [Accessed April 1, 2013]; http://www.fda.gov/ForIndustry/DevelopingProductsforRareDiseasesConditions/HowtoapplyforOrphanProductDesignation/default.htm.
- 2.European Medicines Agency Orphan Drug Designation. [Accessed April 1, 2013]; http://www.emea.europa.eu/ema/index.jsp?curl=pages/regulation/general/general_content_000029.jsp&mid=WC0b01ac05800240ce.
- 3.Office of Rare Disease Research. [Accessed April 1, 2013]; http://www.rarediseases.info.nih.gov/Default.aspx.
- 4.Getz KA, Kaitin KI. Open innovation: the new face of pharmaceutical research and development. Expert Rev Clin Pharmacol. 2012;5(5):481–483. doi: 10.1586/ecp.12.44. [DOI] [PubMed] [Google Scholar]
- 5.Munos BH, Chin WW. A call for sharing: adapting pharmaceutical research to new realities. Sci Transl Med. 2009;1(9) doi: 10.1126/scitranslmed.3000155. 9cm8. [DOI] [PubMed] [Google Scholar]
- 6.Munos BH, Chin WW. How to revive breakthrough innovation in the pharmaceutical industry. Sci Transl Med. 2011;3(89) doi: 10.1126/scitranslmed.3002273. 89cm16. [DOI] [PubMed] [Google Scholar]
- 7.Adams CP, Brantner VV. Spending on new drug development1. Health Economics. 2010;19(2):130–141. doi: 10.1002/hec.1454. [DOI] [PubMed] [Google Scholar]
- 8.Deloitte Center for Health Solutions. Measuring Return from Pharmaceutical Innovation 2012: Is R&D Earning its Investment. [Accessed March 25, 2013]; http://www.deloitte.com/view/en_XB/xb/news/b47f30374ca4b310VgnVCM2000003356f70aRCRD.htm.
- 9.Rare Diseases and Orphan Products: Accelerating Research and Development. The National Academies Press; 2011. [PubMed] [Google Scholar]
- 10.Mehta A, Clarke JT, Giugliani R, Elliott P, Linhart A, Beck M, Sunder-Plassmann G. Natural course of Fabry disease: changing pattern of causes of death in FOS - Fabry Outcome Survey. J Med Genet. 2009;46(8):548–552. doi: 10.1136/jmg.2008.065904. [DOI] [PubMed] [Google Scholar]
- 11.Yanjanin NM, Velez JI, Gropman A, King K, Bianconi SE, Conley SK, Brewer CC, Solomon B, Pavan WJ, Arcos-Burgos M, Patterson MC, Porter FD. Linear clinical progression, independent of age of onset, in Niemann-Pick disease, type C. Am J Med Genet B Neuropsychiatr Genet. 2010;153B(1):132–140. doi: 10.1002/ajmg.b.30969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kishnani PS, Hwu W-L, Mandel H, Nicolino M, Yong F, Corzo D. A retrospective, multinational, multicenter study on the natural history of infantile-onset Pompe disease. The Journal of Pediatrics. 2006;148(5):671.e672–676.e672. doi: 10.1016/j.jpeds.2005.11.033. [DOI] [PubMed] [Google Scholar]
- 13.Porter FD, Scherrer DE, Lanier MH, Langmade SJ, Molugu V, Gale SE, Olzeski D, Sidhu R, Dietzen DJ, Fu R, Wassif CA, Yanjanin NM, Marso SP, House J, Vite C, Schaffer JE, Ory DS. Cholesterol oxidation products are sensitive and specific blood-based biomarkers for Niemann-Pick C1 disease. Sci Transl Med. 2010;2(56) doi: 10.1126/scitranslmed.3001417. 56ra81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cluzeau CV, Watkins-Chow DE, Fu R, Borate B, Yanjanin N, Dail MK, Davidson CD, Walkley SU, Ory DS, Wassif CA, Pavan WJ, Porter FD. Microarray expression analysis and identification of serum biomarkers for Niemann-Pick disease, type C1. Hum Mol Genet. 2012;21(16):3632–3646. doi: 10.1093/hmg/dds193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kaitin KI. Translational Research and the Evolving Landscape for Biomedical Innovation. Journal of Investigative Medicine. 2012;60(7):995–998. doi: 10.231/JIM.0b013e318268694f. 910.231/JIM.990b013e318268694f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Therapeutics for Rare and Neglected Diseases. [Accessed April 1, 2013]; http://www.ncats.nih.gov/research/rare-diseases/trnd/trnd.html.
- 17.Ory DS. Niemann-Pick type C: a disorder of cellular cholesterol trafficking. Biochim Biophys Acta. 2000;1529(1-3):331–339. doi: 10.1016/s1388-1981(00)00158-x. [DOI] [PubMed] [Google Scholar]
- 18.Vanier MT. Niemann-Pick disease type C. Orphanet J Rare Dis. 2010;5:16. doi: 10.1186/1750-1172-5-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vanier MT, Millat G. Niemann-Pick disease type C. Clin Genet. 2003;64(4):269–281. doi: 10.1034/j.1399-0004.2003.00147.x. [DOI] [PubMed] [Google Scholar]
- 20.Sevin M, Lesca G, Baumann N, Millat G, Lyon-Caen O, Vanier MT, Sedel F. The adult form of Niemann-Pick disease type C. Brain. 2007;130(Pt 1):120–133. doi: 10.1093/brain/awl260. [DOI] [PubMed] [Google Scholar]
- 21.European Medicine Agency Summary for Authorization of Zavasca (Miglustat) [Accessed April 13, 2013]; http://www.emea.europa.eu/docs/en_GB/document_library/EPAR_-_Summary_for_the_public/human/000435/WC500046724.pdf.
- 22.Patterson MC, Hendriksz CJ, Walterfang M, Sedel F, Vanier MT, Wijburg F. Recommendations for the diagnosis and management of Niemann-Pick disease type C: an update. Mol Genet Metab. 2012;106(3):330–344. doi: 10.1016/j.ymgme.2012.03.012. [DOI] [PubMed] [Google Scholar]
- 23.Patterson MC, Vecchio D, Jacklin E, Abel L, Chadha-Boreham H, Luzy C, Giorgino R, Wraith JE. Long-term miglustat therapy in children with Niemann-Pick disease type C. J Child Neurol. 2010;25(3):300–305. doi: 10.1177/0883073809344222. [DOI] [PubMed] [Google Scholar]
- 24.Wraith JE, Vecchio D, Jacklin E, Abel L, Chadha-Boreham H, Luzy C, Giorgino R, Patterson MC. Miglustat in adult and juvenile patients with Niemann-Pick disease type C: long-term data from a clinical trial. Mol Genet Metab. 2010;99(4):351–357. doi: 10.1016/j.ymgme.2009.12.006. [DOI] [PubMed] [Google Scholar]
- 25.Davidson CD, Ali NF, Micsenyi MC, Stephney G, Renault S, Dobrenis K, Ory DS, Vanier MT, Walkley SU. Chronic cyclodextrin treatment of murine Niemann-Pick C disease ameliorates neuronal cholesterol and glycosphingolipid storage and disease progression. PLoS One. 2009;4(9):e6951. doi: 10.1371/journal.pone.0006951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zervas M, Somers KL, Thrall MA, Walkley SU. Critical role for glycosphingolipids in Niemann-Pick disease type C. Curr Biol. 2001;11(16):1283–1287. doi: 10.1016/s0960-9822(01)00396-7. [DOI] [PubMed] [Google Scholar]
- 27.FDA Rare Disease Program. [Accessed April 1, 2013]; http://www.fda.gov/AboutFDA/CentersOffices/OfficeofMedicalProductsandTobacco/CDER/ucm221248.htm.
- 28.Office of Orphan Products Development. [Accessed April 1, 2013]; http://www.fda.gov/ForIndustry/DevelopingProductsforRareDiseasesConditions/HowtoapplyforOrphanProductDesignation/default.htm.
- 29.Addi and Cassi Fund. [Accessed April 1, 2013]; http://addiandcassi.com/
- 30.Ara Parseghian Medical Research Foundation. [Accessed April 1, 2013]; http://www.parseghian.org/aboutus.html.
- 31.National Niemann-Pick Disease Foundation. [Accessed April 1, 2013]; http://www.nnpdf.org/
- 32.International Niemann-Pick Disease Alliance. [Accessed April 1, 2013]; http://inpda.org/
- 33.Niemann-Pick Disease Group (UK) [Accessed April 1, 2013]; http://www.niemannpick.org.uk/
- 34.Dana’s Angel’s Research Trust. [Accessed April 1, 2013]; http://danasangels.org/
- 35.Hide & Seek Foundation for Lysosomal Disease Research. [Accessed April 1, 2013]; http://hideandseek.org/FindTheCure.html.
- 36.Loftsson T, Jarho P, Masson M, Jarvinen T. Cyclodextrins in drug delivery. Expert Opin Drug Deliv. 2005;2(2):335–351. doi: 10.1517/17425247.2.1.335. [DOI] [PubMed] [Google Scholar]
- 37.Loftsson T, Vogensen SB, Brewster ME, Konradsdottir F. Effects of cyclodextrins on drug delivery through biological membranes. J Pharm Sci. 2007;96(10):2532–2546. doi: 10.1002/jps.20992. [DOI] [PubMed] [Google Scholar]
- 38.Kurkov SV, Loftsson T. Cyclodextrins. Int J Pharm. 2012 doi: 10.1016/j.ijpharm.2012.06.055. [DOI] [PubMed] [Google Scholar]
- 39.Davis ME, Brewster ME. Cyclodextrin-based pharmaceutics: past, present and future. Nat Rev Drug Discov. 2004;3(12):1023–1035. doi: 10.1038/nrd1576. [DOI] [PubMed] [Google Scholar]
- 40.GRAS Notice Inventory. [Accessed April 1, 2013]; http://www.accessdata.fda.gov/scripts/fcn/fcnNavigation.cfm?filter=cyclodextrin&sortColumn=&rpt=grasListing.
- 41.FDA List of Inactive Pharmaceutical Ingredients. [Accessed April 1, 2013]; http://www.accessdata.fda.gov/scripts/cder/iig/getiigWEB.cfm.
- 42.Gould S, Scott RC. 2-Hydroxypropyl-beta-cyclodextrin (HP-beta-CD): a toxicology review. Food Chem Toxicol. 2005;43(10):1451–1459. doi: 10.1016/j.fct.2005.03.007. [DOI] [PubMed] [Google Scholar]
- 43.Stella VJ, He Q. Cyclodextrins. Toxicol Pathol. 2008;36(1):30–42. doi: 10.1177/0192623307310945. [DOI] [PubMed] [Google Scholar]
- 44.Brewster ME, Estes KS, Bodor N. An Intravenous Toxicity Study of 2-Hydroxypropyl-Beta- Cyclodextrin, a Useful Drug Solubilizer, in Rats and Monkeys. Int J Pharm. 1990;59(3):231–243. [Google Scholar]
- 45.Loftsson T, Brewster ME. Pharmaceutical applications of cyclodextrins: basic science and product development. Journal of Pharmacy and Pharmacology. 2010;62(11):1607–1621. doi: 10.1111/j.2042-7158.2010.01030.x. [DOI] [PubMed] [Google Scholar]
- 46.Brewster ME, Loftsson T. Cyclodextrins as pharmaccutical solubilizers. Adv Drug Deliver Rev. 2007;59(7):645–666. doi: 10.1016/j.addr.2007.05.012. [DOI] [PubMed] [Google Scholar]
- 47.Loftsson T, Brewster ME. Pharmaceutical applications of cyclodextrins .1. Drug solubilization and stabilization. J Pharm Sci. 1996;85(10):1017–1025. doi: 10.1021/js950534b. [DOI] [PubMed] [Google Scholar]
- 48.Rajewski RA, Stella VJ. Pharmaceutical applications of cyclodextrins. 2. In vivo drug delivery. J Pharm Sci. 1996;85(11):1142–1169. doi: 10.1021/js960075u. [DOI] [PubMed] [Google Scholar]
- 49.Irie T, Uekama K. Pharmaceutical applications of cyclodextrins. III. Toxicological issues and safety evaluation. J Pharm Sci. 1997;86(2):147–162. doi: 10.1021/js960213f. [DOI] [PubMed] [Google Scholar]
- 50.Carstea ED, Morris JA, Coleman KG, Loftus SK, Zhang D, Cummings C, Gu J, Rosenfeld MA, Pavan WJ, Krizman DB, Nagle J, Polymeropoulos MH, Sturley SL, Ioannou YA, Higgins ME, Comly M, Cooney A, Brown A, Kaneski CR, Blanchette-Mackie EJ, Dwyer NK, Neufeld EB, Chang TY, Liscum L, Strauss JF, 3rd, Ohno K, Zeigler M, Carmi R, Sokol J, Markie D, O’Neill RR, van Diggelen OP, Elleder M, Patterson MC, Brady RO, Vanier MT, Pentchev PG, Tagle DA. Niemann-Pick C1 disease gene: homology to mediators of cholesterol homeostasis. Science. 1997;277(5323):228–231. doi: 10.1126/science.277.5323.228. [DOI] [PubMed] [Google Scholar]
- 51.Liu SM, Cogny A, Kockx M, Dean RT, Gaus K, Jessup W, Kritharides L. Cyclodextrins differentially mobilize free and esterified cholesterol from primary human foam cell macrophages. J Lipid Res. 2003;44(6):1156–1166. doi: 10.1194/jlr.M200464-JLR200. [DOI] [PubMed] [Google Scholar]
- 52.Kilsdonk EP, Yancey PG, Stoudt GW, Bangerter FW, Johnson WJ, Phillips MC, Rothblat GH. Cellular cholesterol efflux mediated by cyclodextrins. J Biol Chem. 1995;270(29):17250–17256. doi: 10.1074/jbc.270.29.17250. [DOI] [PubMed] [Google Scholar]
- 53.Camargo F, Erickson RP, Garver WS, Hossain GS, Carbone PN, Heidenreich RA, Blanchard J. Cyclodextrins in the treatment of a mouse model of Niemann-Pick C disease. Life Sci. 2001;70(2):131–142. doi: 10.1016/s0024-3205(01)01384-4. [DOI] [PubMed] [Google Scholar]
- 54.Griffin LD, Gong W, Verot L, Mellon SH. Niemann-Pick type C disease involves disrupted neurosteroidogenesis and responds to allopregnanolone. Nat Med. 2004;10(7):704–711. doi: 10.1038/nm1073. [DOI] [PubMed] [Google Scholar]
- 55.Liu B, Ramirez CM, Miller AM, Repa JJ, Turley SD, Dietschy JM. Cyclodextrin overcomes the transport defect in nearly every organ of NPC1 mice leading to excretion of sequestered cholesterol as bile acid. J Lipid Res. 2010;51(5):933–944. doi: 10.1194/jlr.M000257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Aqul A, Liu B, Ramirez CM, Pieper AA, Estill SJ, Burns DK, Repa JJ, Turley SD, Dietschy JM. Unesterified cholesterol accumulation in late endosomes/lysosomes causes neurodegeneration and is prevented by driving cholesterol export from this compartment. J Neurosci. 2011;31(25):9404–9413. doi: 10.1523/JNEUROSCI.1317-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ramirez CM, Liu B, Aqul A, Taylor AM, Repa JJ, Turley SD, Dietschy JM. Quantitative role of LAL, NPC2, and NPC1 in lysosomal cholesterol processing defined by genetic and pharmacological manipulations. J Lipid Res. 2011;52(4):688–698. doi: 10.1194/jlr.M013789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ramirez CM, Liu B, Taylor AM, Repa JJ, Burns DK, Weinberg AG, Turley SD, Dietschy JM. Weekly cyclodextrin administration normalizes cholesterol metabolism in nearly every organ of the Niemann-Pick type C1 mouse and markedly prolongs life. Pediatr Res. 2010;68(4):309–315. doi: 10.1203/PDR.0b013e3181ee4dd2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ward S, O’Donnell P, Fernandez S, Vite CH. 2-hydroxypropyl-beta-cyclodextrin raises hearing threshold in normal cats and in cats with Niemann-Pick type C disease. Pediatr Res. 2010;68(1):52–56. doi: 10.1203/PDR.0b013e3181df4623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Peake KB, Vance JE. Normalization of cholesterol homeostasis by 2-hydroxypropyl-betacyclodextrin in neurons and glia from Niemann-Pick C1 (NPC1)-deficient mice. J Biol Chem. 2012;287(12):9290–9298. doi: 10.1074/jbc.M111.326405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chen FW, Li C, Ioannou YA. Cyclodextrin induces calcium-dependent lysosomal exocytosis. PLoS One. 2010;5(11):e15054. doi: 10.1371/journal.pone.0015054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jiang X, Sidhu R, Porter FD, Yanjanin NM, Speak AO, te Vruchte DT, Platt FM, Fujiwara H, Scherrer DE, Zhang J, Dietzen DJ, Schaffer JE, Ory DS. A sensitive and specific LCMS/ MS method for rapid diagnosis of Niemann-Pick C1 disease from human plasma. J Lipid Res. 2011;52(7):1435–1445. doi: 10.1194/jlr.D015735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Availability of Investigational Drugs for Compassionate Use. [Accessed April 1, 2013]; http://www.fda.gov/NewsEvents/Testimony/ucm115209.htm.
- 64.Physician Request for an Individual Patient IND under Expanded Access for Non-emergency or Emergency Use. [Accessed April 1, 2013]; http://www.fda.gov/Drugs/DevelopmentApprovalProcess/HowDrugsareDevelopedandApproved/ApprovalApplications/InvestigationalNewDrugINDApplication/ucm107434.htm.
- 65. Vol. 52 FR 19476, May 22, 1987, as amended at 57 FR 13248, Apr. 15, 1992. [Google Scholar]
- 66.Matsuo M, Togawa M, Hirabaru K, Mochinaga S, Narita A, Adachi M, Egashira M, Irie T, Ohno K. Effects of cyclodextrin in two patients with Niemann-Pick Type C disease. Mol Genet Metab. 2013;108(1):76–81. doi: 10.1016/j.ymgme.2012.11.005. [DOI] [PubMed] [Google Scholar]
- 67.Pontikis CC, Davidson CD, Walkley SU, Platt FM, Begley DJ. Cyclodextrin alleviates neuronal storage of cholesterol in Niemann-Pick C disease without evidence of detectable blood-brain barrier permeability. J Inherit Metab Dis. 2013 doi: 10.1007/s10545-012-9583-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Loftus SK, Morris JA, Carstea ED, Gu JZ, Cummings C, Brown A, Ellison J, Ohno K, Rosenfeld MA, Tagle DA, Pentchev PG, Pavan WJ. Murine model of Niemann-Pick C disease: mutation in a cholesterol homeostasis gene. Science. 1997;277(5323):232–235. doi: 10.1126/science.277.5323.232. [DOI] [PubMed] [Google Scholar]
- 69.Cystic Fibrosis Foundation Therapeutics. [Accessed April 1, 2013]; http://www.cff.org/research/CFFT/
- 70.Leukemia and Lymphoma Society’s Therapy Acceleration Program. [Accessed April 1, 2013]; http://www.lls.org/researchershealthcareprofessionals/drugdevelopment/therapyacceleration/
- 71.Mylein Research Foundation’s Accelerated Research Collaboration Model. [Accessed April 1, 2013]; http://www.myelinrepair.org/research_model/
- 72.Multiple Myeloma Research Consortium. [Accessed April 1, 2013]; http://themmrc.org/
- 73.FDA. Orphan Drug Act (as ammended) [Accessed April 1, 2013];2009 http://www.fda.gov/RegulatoryInformation/Legislation/FederalFoodDrugandCosmeticActFDCAct/SignificantAmendmentstotheFDCAct/OrphanDrugAct/default.htm.
- 74.Haffner ME, Torrent-Farnell J, Maher PD. Does orphan drug legislation really answer the needs of patients? Lancet. 2008;371(9629):2041–2044. doi: 10.1016/S0140-6736(08)60873-9. [DOI] [PubMed] [Google Scholar]
- 75.Cote T, Kelkar A, Xu K, Braun MM, Phillips MI. Orphan products: an emerging trend in drug approvals. Nat Rev Drug Discov. 2010;9(1):84. doi: 10.1038/nrd2546-c1. [DOI] [PubMed] [Google Scholar]
- 76.Cote TR, Xu K, Pariser AR. Accelerating orphan drug development. Nat Rev Drug Discov. 2010;9(12):901–902. doi: 10.1038/nrd3340. [DOI] [PubMed] [Google Scholar]
- 77.Joppi R, Bertele V, Garattini S. Orphan drugs, orphan diseases. The first decade of orphan drug legislation in the EU. Eur J Clin Pharmacol. 2013;69(4):1009–1024. doi: 10.1007/s00228-012-1423-2. [DOI] [PubMed] [Google Scholar]
- 78.Thorat C, Xu K, Freeman SN, Bonnel RA, Joseph F, Phillips MI, Imoisili MA. What the Orphan Drug Act has done lately for children with rare diseases: a 10-year analysis. Pediatrics. 2012;129(3):516–521. doi: 10.1542/peds.2011-1798. [DOI] [PubMed] [Google Scholar]
- 79.Orphan Product Designation FDA. [Accessed April 1, 2013]; http://www.fda.gov/ForIndustry/DevelopingProductsforRareDiseasesConditions/HowtoapplyforOrphan ProductDesignation/default.htm.
- 80.Network for Excellence in Neuroscience Clinical Trials (NeuroNEXT) [Accessed April 1, 2013]; doi: 10.1001/jamaneurol.2013.3663. http://www.neuronext.org/ [DOI] [PubMed]
- 81.Sasinowski FJ. Quantum of Effectiveness Evidence in FDA’s Approval of Orphan Drugs Cataloguing FDA’s Flexibility in Regulating Therapies for Persons with Rare Disorders. Drug Inf J. 2012;46(2):238–263. [Google Scholar]
- 82.Food and Drug Administration Safety and Innovation Act of 2012. [Accessed April 1, 2013]; http://www.fda.gov/RegulatoryInformation/Legislation/FederalFoodDrugandCosmeticActFDCAct/SignificantAmendmentstotheFDCAct/FDASIA/default.htm.
- 83.Breakthrough Designation. [Accessed March 25, 2013]; http://www.fda.gov/RegulatoryInformation/Legislation/FederalFoodDrugandCosmeticActFDCAct/SignificantAmendmentstotheFDCAct/FDASIA/ucm329491.htm.
- 84.Valenza M, Rigamonti D, Goffredo D, Zuccato C, Fenu S, Jamot L, Strand A, Tarditi A, Woodman B, Racchi M, Mariotti C, Di Donato S, Corsini A, Bates G, Pruss R, Olson JM, Sipione S, Tartari M, Cattaneo E. Dysfunction of the cholesterol biosynthetic pathway in Huntington's disease. J Neurosci. 2005;25(43):9932–9939. doi: 10.1523/JNEUROSCI.3355-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Yao J, Ho D, Calingasan NY, Pipalia NH, Lin MT, Beal MF. Neuroprotection by cyclodextrin in cell and mouse models of Alzheimer disease. J Exp Med. 2012;209(13):2501–2513. doi: 10.1084/jem.20121239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Dass CR, Jessup W, Apolipoprotein A-I. cyclodextrins and liposomes as potential drugs for the reversal of atherosclerosis. A review. J Pharm Pharmacol. 2000;52(7):731–761. doi: 10.1211/0022357001774606. [DOI] [PubMed] [Google Scholar]