Abstract
The first study aimed at determining the structural characteristics needed to prepare antibacterial 2-alkynoic fatty acids (2-AFAs) was accomplished by synthesizing several 2-AFAs and other analogues in 18-76% overall yields. Among all the compounds tested, the 2-hexadecynoic acid (2-HDA) displayed the best overall antibacterial activity against Gram-positive Staphylococcus aureus (MIC = 15.6 μg/mL), Staphylococcus saprophyticus (MIC = 15.5 μg/mL), and Bacillus cereus (MIC = 31.3 μg/mL), as well as against the Gram-negative Klebsiella pneumoniae (7.8 μg/mL) and Pseudomonas aeruginosa (MIC = 125 μg/mL). In addition, 2-HDA displayed significant antibacterial activity against methicillin-resistant S. aureus (MRSA) ATCC 43300 (MIC = 15.6 μg/mL) and clinical isolates of MRSA (MIC = 3.9 μg/mL). No direct relationship was found between the antibacterial activity of 2-AFAs and their critical micelle concentration (CMC) suggesting that the antibacterial properties of these fatty acids are not mediated by micelle formation. It was demonstrated that the presence of a triple bond at C-2 as well as the carboxylic acid moiety in 2-AFAs are important for their antibacterial activity. 2-HDA has the potential to be further evaluated for use in antibacterial formulations.
Keywords: 2-alkynoic fatty acids, antibacterial agents, synthesis, micelles, MRSA, nosocomial infections
1. Introduction
Nosocomial infection is defined as “an infection acquired in a hospital by a patient who was admitted for a reason other than that infection” (WHO, 2002). These infections are major source of morbidity and mortality affecting more than 1.7 million patients annually and generating over 99,000 death per year in the United States (Klevens et al., 2007). In addition to the human cost, nosocomial infections affect the quality costs in hospitals as well as the finances of the public health care system (Brachman et al., 1980; Pittet et al., 1994; Stone et al., 2002; Zhan and Miller, 2003). Mechanical ventilation, use of invasive procedures, and patients' immunocompromised status are the principal factors that propitiate nosocomial infections (Inweregbu et al., 2005). This situation is complicated when bacteria gain resistance towards those antibiotics commonly used in hospitals such as penicillins, polyenes, glycopeptides, macrolides, and cephalosporins (Inweregbu et al., 2005). Bacteria develop resistance when they acquire new genetic material as a result of an inadequate antibiotic therapy (Inweregbu et al., 2005; Klevens et al., 2007). Despite the fact that several compounds such as synthetic fluroquinolones (Bradbury and Pucci, 2008), aminocoumarins (Freitag et al., 2005; Heide, 2009), and cyclothialidines (Kampranis et al., 1999; Rudolph et al., 2001) are being evaluated as antibacterial agents, there is an urgent need to develop new antibiotics to replace those that are not effective.
Among those compounds that are being evaluated as antibacterial agents, unsaturated fatty acids have demonstrated to be biologically active towards Gram-positive bacteria, specifically against Staphylococcus aureus (Carballeira et al., 2002; Carballeira et al., 1998; Zheng et al., 2005)[DS1]. In the present study, we prepared a series of 2-alkynoic fatty acids (2-AFAs) and other synthetic analogues such as 2-tetrahydropyranyl protected alkynols and 2-alkynols aimed at establishing a structure activity relationship (SAR) with these compounds in order to find the fatty acid with better cytotoxicity against both Gram-positive and Gram-negative bacteria. 2-AFAs are acetylenic fatty acids which have the peculiarity of containing a triple bond (C≡C) at C-2 in their structures. Acetylenic fatty acids have been widely studied by medicinal chemists due to their interesting antimicrobial properties such as antifungal (Carballeira, 2008; Carballeira et al., 2006; Carballeira et al., 2005; Gershon and Shanks, 1978; Li et al., 2003; Li et al., 2008; Xu et al., 2012), antiprotozoal (Carballeira et al., 2012; Tasdemir et al., 2010), and antibacterial activities (Konthikamee et al., 1982; Morbidoni et al., 2006). Acetylenic fatty acids are mainly produced by certain plants as a chemical defense against microorganisms (Cahoon et al., 2003; Carballeira, 2008; Fatope et al., 2000; Li et al., 2003; Li et al., 2008; Xu et al., 2012). Among the acetylenic fatty acids, the 2-hexadecynoic acid (2-HDA) has received the most attention for its antimicrobial and cytotoxic properties (Carballeira et al., 2012; Carballeira et al., 2006; Gershon and Shanks, 1978; Morbidoni et al., 2006; Upreti et al., 1981; Wood and Lee, 1981). For example, Konthikamee et al. reported that 2-HDA was particularly active against the Gram-positive cocci, including penicillin-resistant Staphylococcus aureus[DS2], at concentrations of 21.4-56.8 μM (Konthikamee et al., 1982). In addition, it was reported that 2-HDA was active against the Gram-negative Neisseria gonorrhoeae, Neisseria meningitidis, and Bacteroides fragilis[DS3] at concentrations of 5.0-21.4 μM. More recently, Carballeira et al. determined that 2-HDA and its analog 2,6-hexadecadiynoic acid (2,6-HDA) were active against Mycobacterium tuberculosis displaying minimum inhibitory concentrations (MICs) of 141-145 μM (Carballeira et al., 2006). 2-Octadecynoic acid (2-ODA) was other acetylenic acid that was evaluated as an antimycobacterial agent (Morbidoni et al., 2006). According to that study, 2-ODA and its metabolites displayed the best antimycobacterial activity against Mycobacterium smegmatis and Mycobacterium bovis BCG through the inhibition of fatty acid biosynthesis, such as fatty acid degradation and mycolic acid biosynthesis, which are fundamental pathways for the subsistence of mycobacteria (Morbidoni et al., 2006).
The antifungal properties of 2-HDA against several fungal strains, including Aspergillus niger, Candida albicans, and Trichophyton mentagrophytes, were also studied (Gershon and Shanks, 1978). The antifungal properties of 2-HDA has been attributed to its ability to inhibit the elongation of saturated and unsaturated fatty acid as well as its potential to inhibit the fatty acid acylation process, particularly triacylglycerol synthesis (Carballeira et al., 2006; Wood and Lee, 1981). The biological properties of 2-HDA provoked for Carballeira and collaborators to evaluate the antifungal properties of 2,6-HDA (Carballeira et al., 2006). They found that this fatty acid displayed increased activity against C. albicans [DS4]and Cryptococcus neoformans compared to the parent compounds 2-HDA and 6-HDA. Carballeira et al. postulated that both the inhibition of fungal fatty acid biosynthesis and inhibition of sphingolipid biosynthesis are responsible for the enhanced antifungal activity of 2,6-HDA (Carballeira et al., 2006).
In addition to its antibacterial and antifungal properties, 2-HDA has also shown antiprotozoal activity and inhibitory properties against protozoal enzymes. For example, Tasdemir et al. reported that 2-HDA effectively inhibited plasmodial FAS-II enzymes (IC50's between 1.5 and 13.9 μM) and arrests erythrocytic and liver stage plasmodium infections (Tasdemir et al., 2010). In addition, they showed that 2-HDA displays antiprotozoal activity against Leishmania donovani amastigotes (IC50 = 17.8 μM), but no studies on key L. donovani enzymes amenable for therapeutic intervention were performed.
Aimed at studying the antiprotozoal properties of 2-HDA and other 2-AFAs, Carballeira and collaborators determined the antiprotozoal activity of a series of 2-AFAs, including 2-HDA (Carballeira et al., 2012). Results from this study revealed that 2-ODA and 2-HDA were the most potent antiprotozoal acids against L. donovani with IC50's of 11.0 and 17.8 μM, respectively. Moreover, it was reported that the antiprotozoal activity of 2-HDA and 2-ODA was associated with their inhibitory properties against the L. donovani DNA topoisomerase IB enzyme (LdTopIB). 2-ODA and 2-HDA were inhibitory against LdTopIB at IC50 of 5.3 and 28.7 μM, respectively (Carballeira et al., 2012). Although it has been suggested that the inhibition of LdTopIB could be a possible mechanism that explains the antileishmanial properties of the 2-alkynoic acids, Carballeira et al. did not discard the possibility that other mechanisms could be operative.
Despite the fact that the antimicrobial properties of 2-AFAs have been reported, further studies are needed to discover those structural characteristics that favor the antibacterial activity of 2-AFAs against multidrug-resistant bacteria. In this study, we synthesized four 2-AFAs by modifying the degree of unsaturation and carbon chain length. In addition, we prepared two alcohols and two tetrahydropyranyl ether analogues of 2-AFAs in order to determine whether the carboxylic group in 2-AFAs is essential for the antibacterial activity of these compounds. The 2-AFAs presented here, were evaluated against both Gram-positive and Gram-negative bacteria including some methicillin-resistant Staphylococcus aureus (MRSA) strains. Moreover, we investigated the relationship between antibacterial properties of 2-AFAs and their ability to form micelles. Finally, the cytotoxicity properties of 2-AFAs against normal peripheral blood mononuclear cells (PBMC) were also determined. Results from this study revealed that 2-AFAs were selective against bacteria and that there is no direct relationship between the antibacterial properties of these compounds and micelle formation. These results are important for future research in the area of the development of new antimicrobial agents that could be used to treat infections caused by multidrug-resistant bacteria in hospitals.
2. Materials and methods
2.1 Instrumentation
IR spectra were recorded on a Spectrum One Perkin Elmer FT-IR spectrophotometer. 1H-NMR and 13C-NMR spectra were recorded on a Bruker DRX 500 spectrophotometer. 1H-NMR chemical shifts are reported relative to internal tetramethyl silane (SiMe4) and 13C-NMR chemical shifts are reported in parts per million (ppm) relative to CDCl3 (77.00 pm). Mass spectra data was gathered by using a GC-MS (Agilent 5975C MS ChemStation; Agilent, Palo Alto, CA, USA) ate 70 eV equipped with 30 m × 0.25 mm special performance capillary column (HP-5MS) of polymethysiloxane cross-linked with 5% phenyl methylpolysiloxane. UV/Vis data were determined on a Beckman Coulter DU-530 spectrophotometer. Minimal inhibitory concentrations (MICs) were spectrophotometrically determined by using a Multiskan FC microplate reader (Fisher Scientific) at 620 nm.
2.2 Microorganisms
Staphylococcus aureus (ATCC 29213), Staphylococcus saprophyticus (ATCC 15305), Bacillus cereus (ATCC 10876), Escherichia coli (ATCC 25922), Klebsiella pneumoniae (ATCC 13883), Pseudomonas aeruginosa (ATCC 27853), and Methicillin-resistant S. aureus (MRSA, ATCC 43300) were obtained from the American Type Culture Collection (Manassas, VA). Clinical isolates of MRSA (CIMRSA) were kindly donated by a community hospital in San Juan, Puerto Rico. Stock cultures were kept on blood agar (TSA with 5% sheep blood, Remel and Oxoid Microbiology Products, Lenexa, KS). Subcultures were incubated for 18-24 h on TSA at 37°C. Suspension cultures were prepared by inoculation of single colonies in 5 mL Trypticase Soy Broth (TSB, BD Diagnostic Systems, Franklin Lakes, NJ). Prior to preparation of susceptibility assays, bacteria cells were re-suspended in TSB and visually standardized by using 0.5 McFarland standard solution, which provided an equivalent concentration of 1.0 x108 CFU/mL.
2.3 Diagnostic tests for identification of S. aureus
To confirm the identity of S. aureus, MRSA (ATCC 43300) and two CIMRSA strains were tested for fermentation on mannitol salt agar (MSA, BD Diagnostic System, Franklin Lakes, NJ). A positive fermentation result was recorded as growth of yellow colonies on MSA surrounded by yellow zones after 24 h of incubation at 37°C.
The coagulase test was performed with plasma rabbit following the recommendations of the manufacturer (Becton, Dickinson and Company, Sparks, MD). Results were recorded after 4 and 24 hours of incubation at 37°C using S. aureus (ATCC 25923) as positive control and S. saprophyticus (ATCC 15305) as negative control. Small organized clots were recorded as a positive result.
2.4 Susceptibility testing
A modified version of the microdilution method outlined by the CLSI was used (CLSI, 2006). 2-AFAs were dissolved in 95% ethanol, serially diluted with sterile TSB, and transferred to a flat-bottomed microplate wells that were previously inoculated with 10 μL of TSB solution containing 4-5 × 105 colony-forming units (CFU[DS5]). The wells were inspected spectrophotometrically (620 nm) using both a positive control well (containing the bacterial inoculated TSB but not the fatty acid solution) and a negative control well (containing only TSB) for comparison. The minimum inhibitory concentration (MIC) was considered to be the concentration at which 2-alkynoic acids prevented turbidity in the well after incubation for 18-24 h at 37°C.
2.5 Determination of bactericidal concentration (MBC)
Once the MIC experiments were performed, MBC was determined by removing approximately 10 μL of the TSB containing 2-AFA from four microplate wells whose turbidity was not observed (starting from the well where MIC was determined) and sub-culturing onto fresh TSA plate containing 5% sheep blood (Remel and Oxoid Microbiology Products, Lenexa, KS). TSA plates were incubated for a further 18-24 h at 37°C. Any growth observed from TSA plates was designated as an ineffective bactericidal concentration of 2-AFA[DS6].
2.6 Cytotoxicity testing
Peripheral blood mononuclear cells (PBMC) were used for this assay. The cells were cultured in culture medium supplemented with interleukin-2 (IL-2). PBMC was seeded into a 96-well microplate (5 × 104 cells/ 200 μL/ well) and fatty acids were added to the cell cultures. The final concentrations of fatty acids ranged from 25 to 400 μg/mL. The cells were incubated at 37°C for 3 days in humidified 5% CO2 incubator. The cytotoxicity of the cells was evaluated by MTT assay.
2.7 Synthesis of 2-HDA, 2-ODA , and 2-ICA
The synthesis of 2-HDA, 2-ODA, and 2-icosanoic acid (2-ICA) followed a previously published procedure(Carballeira et al., 2012; Tasdemir et al., 2010) In general, the above-mentioned AFAs were prepared by reacting the commercially available 1-pentadecyne, 1-heptadecyne, or 1-nonadecyne with n-BuLi in THF at -70°C, quenched with CO2 and subsequently protonated with NH4Cl (Figure 1). The 2-HDA, 2-ODA, and 2-ICA were obtained in 40-79% yield. The spectral data of 2-HDA and 2-ODA were in agreement with those previously reported (Morbidoni et al., 2006; Tasdemir et al., 2010).
Figure 1.
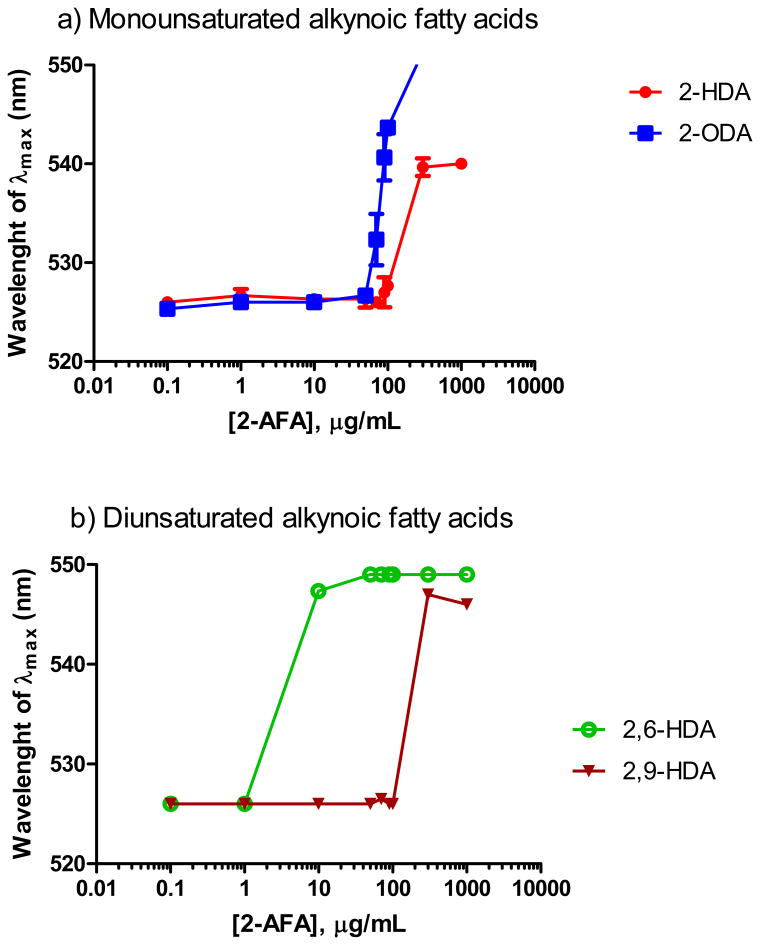
Wavelength of maximum absorption of rhodamine 6G with increasing concentrations of 2-AFAs in 1X PBS at 25°C. a) Monounsaturated alkynoic fatty acids; b) Diunsaturated alkynoic fatty acids. Values are reported as means ± SEM (N = 3[DS12]).
2.7.1 Synthesis of 2-ICA
2-ICA was obtained in a 40% yield as a white solid from the reaction of 0.550 g (2.08 mmol), n-BuLi (2.5 M, 3.75 mmol) in dry hexane (1.50 mL), and subsequent quenching with CO2 according to the general procedure described above. The spectral data of 2-ICA is described below: M.p. 54-56°C. IR (KBr) vmax: 3000-2840, 2963, 2916, 2844, 2255, 1671, 1463, 1432, 1317, 1281, 1083, 910, 796, 754, 713, 613 cm−1; 1H NMR (300 MHz, CDCl3) δ 2.35 (2H, t,), 1.58 (2H, m), 1.45-1.35 (2H, m) 1.35-1.18 (26H, m), 0.88 (3H, t,); 13C NMR (75 MHz, CDCl3) δ 157.96 (s, C-1), 92.53 (s, C-3), 72.61 (s, C-2), 31.31 (t), 29.68 (t) 29.65 (t), 29.62 (t), 29.56 (t), 29.39 (t), 29.35 (t), 28.99 (t), 28.81 (t), 23.37 (t), 22.68 (t), 18.74 (t), 14.11 (q, C-20). GC-MS (70ev) m/z (relative intensity) for the methyl ester derivative; 291(M+,12), 195(4), 181(4), 154(76), 151(6), 150(9), 149(9), 141(7), 140(35), 139(17), 136(13), 135(20), 126(7), 125(13), 123(12), 122(23), 121(31), 114(20), 113(13), 111(28), 109(24), 108(29), 107(34), 100(63), 97(19), 96(15), 95(44), 94(45), 93(47), 91(19), 87(7), 85(10), 83(22), 82(19), 81(58), 80(34), 79(81), 77(19), 71(19), 69(45), 68(22), 67(54), 66(23), 65(10), 57(71), 56(20), 55(100); HRMS (APCI) Calcd for C12H21O2 [M+H]+ 309.27881, found 309.27835.
2.8 Synthesis of 2,6-HDA and 2,9-HDA
2,6-HDA and 2,9-HDA were prepared following a previously published procedure (Carballeira et al., 2006) The above-mentioned diunsaturated AFAs were prepared by reacting the lithium acetylide of 1,5-hexadiyne with 1-bromononane and the lithium acetylide of 1,8-nonadiyne with 1-bromohexane to obtain both 1,5-pentadecadiyne and 1,8-pentadecadiyne (Figure 2). Coupling of the lithium acetylides of 1,5-pentadecadiyne and 1,8-pentadecadiyne with CO2 at -70°C and subsequent protonation with NH4Cl afforded the desired 2,6-HDA and 2,9-HDA. The spectral data of 2,6-HDA and 2,9-HDA were in agreement with those previously reported (Carballeira et al., 2006).
2.9 Synthesis of 2-(2-alkynyloxy)-tetrahydro-2H-pyran compounds
To a stirred solution of tetrahydro-2-(2-proponyloxy)-2H-pyran (7.13 mmol) in dry THF (20 mL), n-BuLi (2.5 M, 17.83 mmol) in dry hexane (7.10 mL) was added dropwise while keeping the temperature at -78°C. After 30 min, HMPA (5.0 mL) and 1-bromotridecane (7.13 mmol) or 1-bromopentadecane (7.13 mmol) was added dropwise to the reaction mixture while maintaining the temperature at 78°C. After 24 h, the reaction mixture was worked up by pouring a large volume of water and extracting with diethyl ether (2 × 20 mL). The organic layer was washed with brine (1 × 20 mL) before drying (MgSO4). Filtration, rotoevaporation of the solvent and column purification with hexane-diethyl ether (9:1 v/v) solution afforded 1.38 g (60% yield) of 2-(2-hexadecynyloxy)-tetrahydro-2H-pyran and 1.89 g (76% yield) of 2-(2-octadecynyloxy)-tetrahydro-2H-pyran.
2.9.1 2-(2-Hexadecynyloxy)-tetrahydro-2H-pyran (2-HDOTHP)
2-(2-hexadecynyloxy)-tetrahydro-2H-pyran was obtained in a 60% yield as colorless oil from the reaction of 1.00 mL of tetrahydro-2-(2-propynoloxy)-2H-pyran (1.00 g, 7.13 mmol) and 1.82 mL of 1-bromotridecane (7.13 mmol) according to the general procedure described above. IR (neat) vmax: 2926, 2854, 2225, 1466, 1345, 1202, 1118, 1133, 1079, 1054, 1025 cm−1; 1H NMR (300 MHz, CDCl3) δ 4.81 (1H, t, J = 3.3 Hz), 4.24 (2H, m), 3.84 (1H, m); 3.52 (1H, m), 2.20 (2H, m); 1.89-1.25 (33H, m), 0.87 (3H, t, J = 6.4 Hz); 13C NMR (75 MHz) δ 96.58, 86.78 (s, C-3), 75.64 (s, C-2), 61.96, 54.62 (t, C-1), 31.90, 30.27, 29.64 (x 3) , 29.61, 29.52, 29.34, 29.12, 28.87, 28.58, 25.36, 22.67, 19.11, 18.80, 14.10 (q, C-16). GC-MS (70ev) m/z (relative intensity); 322 (M+, 0.11), 279 (0.13), 209 (0.55), 149 (2), 135 (4), 121 (7), 111 (17), 95 (46), 85 (100), 81 (49), 77 (11), 67 (57), 55 (90).
2.9.2 2-(2-Octadecynyloxy)-tetrahydro-2H-pyran (2-ODOTHP)
2-(2-Octadecynyloxy)-tetrahydro-2H-pyran was obtained in a 76% yield as colorless oil from the reaction 1.00 mL of tetrahydro-2-(2-propynoloxy)-2H-pyran (1.00 g, 7.13 mmol) and 2.00 mL of 1-bromopentadecane (7.13 mmol) according to the general procedure described above. IR (neat) vmax: 2925, 2854, 2222, 1466, 1345, 1201, 1132, 1118, 1079, 1053, 1025 cm−1; 1H NMR (300 MHz, CDCl3) δ 4.81 (1H, t, J = 3.27 Hz), 4.24 (2H, m), 3.84 (1H, m), 3.52 (1H, m), 2.20 (2H, m); 1.89-1.25 (32H, m), 0.87 (3H, t, J = 6.69 Hz); 13C NMR (75 MHz) δ 96.57, 86.77 (s, C-3), 75.64 (s, C-2), 61.95, 54.62 (t, C-1), 31.90, 30.27, 29.67 (x 3) , 29.61, 29.52, 29.348, 29.12, 28.87, 28.75, 28.59, 28.16, 25.36, 22.67, 19.10, 18.80, 14.10 (q, C-18). GC-MS (70ev) m/z (relative intensity); 350 (M+, 0.55), 295 (0.68), 279 (1), 209 (2), 149 (5), 135 (14), 121 (17), 111 (39), 95 (59), 85 (100), 81 (55), 77 (9), 67 (47), 55 (81).
2.10 Synthesis 2-HDOH and 2-ODOH
2-(2-alkynyloxy)-tetrahydro-2H-pyran compounds (4.29-5.08 mmol) in methanol (35.0-40.0 mL), and catalytic amounts of p-toluenesulfonic acid (PTSA) were stirred at 45°C for 24 h. The excess PTSA was removed through a liquid-liquid extraction using a saturated solution of NaHCO3 and diethyl ether (2 × 30 mL). Drying of the organic phase (MgSO4) and subsequent rotoevaporation of the solvent gave 2-alkynols as white solids.
2.10.1 2-Hexadecyn-1-ol (2-HDOH)
2-HDOH was obtained as white solid in 67% yield from the reaction of 1.38 g (4.29 mmol) of 2-(2-hexadecynyloxy)-tetrahydro-2H-pyran and catalytic amounts of PTSA using the procedure described above. M.p. 40-43°C, IR (neat) vmax: 3314, 2925, 2854, 2218, 1466, 1378, 1022 cm−1; 1H NMR (300 MHz, CDCl3) δ 4.24 (2H, s), 2.20 (2H, tt, J = 7.05, J = 2.11), 1.72 (1H, s), 1.59-1.21 (22H, m), 0.87 (3H, t, J = 6.63 Hz); 13C NMR (75 MHz) δ 86.64 (s), 78.21 (s), 51.38 (t, C-1), 31.90, 29.66, 29.64 (x 3), 29.50, 29.34, 29.13, 28.86, 28.58, 22.67, 18.70, 14.11 (q, C-16). GC-MS (70ev) m/z (relative intensity); 238 (M+, 0.01), 223 (M+-15, 0.02), 207 (1), 191 (0.6), 177 (0.4), 149 (3), 135 (9), 121 (14), 111 (19), 107 (13), 93 (40), 81 (56), 67 (65), 55 (100). HRMS (APCI) Calcd for C16H31O [M+H]+ 239.23694, found 239.23661.
2.10.2 2-Octadecyn-1-ol (2-ODOH)
2-ODOH was obtained as white solid in 65% yield from the reaction of 1.78 g (5.08 mmol) and catalytic amounts of PTSA using the procedure described above. M.p. 45-47°C. vmax: 3184, 2917, 2850, 2219, 1471, 1367, 1022 cm−1; 1H NMR (300 MHz, CDCl3) δ 4.24 (2H, s), 2.20 (2H, tt, J = 7.06, J = 2.16 Hz), 1.82 (1H, s), 1.72-1.24 (26H, m), 0.87 (3H, t, J = 6.70 Hz); 13C NMR (75 MHz) δ 86.62 (s), 78.22 (s), 51.38 (C-1, t), 31.90, 29.67, 29.64 (x 3), 29.61, 29.51, 29.42, 29.34, 29.13, 28.86, 28.58, 22.67, 19.28, 14.11 (C-18, q). GC-MS (70ev) m/z (relative intensity); 266 (M+, 0.31); 251 (M+-15, 0.25), 235 (4), 219 (2), 205 (1), 149 (8), 135 (22), 121 (30), 111 (43), 107 (22), 93 (53), 81 (75), 67 (73), 55 (100); HRMS (APCI) Calcd for C18H35O [M+H]+ 267.26824, found 267.26791.
2.11 Determination of the critical micelle concentration (CMC)
The CMC of the fatty acids was assessed as previously described by Courtney et al (Courtney et al., 1986). In general, the fatty acids were dissolved in 95% ethanol[DS7] and serially diluted (0.1-1000 μg/mL) in 1X phosphate-buffered saline (1X PBS) containing rhodamine 6G at 2.5 × 10−6 M. The wavelength of maximum absorption was determined for each dilution using a Beckman Coulter DU-530 spectrophotometer and plotted as a function of the fatty acid concentration. The CMC value was described as the point at which the wavelength of maximum absorption first deviated from linearity.
2.12 Statistical analyses
All dose-response curves were carried out by using GraphPad Prism® (v 5.0) biostatistics software (San Diego, CA) to determine IC50. The IC50 was defined as the concentration of the 2-alkynoic acid that inhibits 50% of the bacterial growth.
3. Results and discussion
In a prior study, we synthesized novel 2-AFAs in order to study their antifungal properties (Carballeira et al., 2006). In that report, we determined that the addition of a triple bond at C-6 in 2-HDA increased its fungitoxicity, while the addition of a triple bond at C-9 was not effective in increasing its antifungal activity. By using a similar approach as described before (Carballeira et al., 2012; Carballeira et al., 2006; Tasdemir et al., 2010), we synthesized 2-AFAs and other synthetic analogues in order to determine those structural characteristics that favor the antibacterial activity of 2-AFAs. In this study, we prepared 2-HDA, 2-ODA, 2-ICA, 2,6-HDA, and 2,9-HDA according to Schemes 1 and 2. The syntheses of 2-HDA, 2-ODA, and 2-ICA were successfully completed in one step and in 40-79% yields (Scheme 1). The synthesis of these 2-AFAs was accomplished by reacting commercially available 1-pentadecyne, 1-heptadecyne or 1-nonadecyne with n-BuLi in THF followed by quenching with CO2 and final protonation with NH4Cl.
Scheme 1.
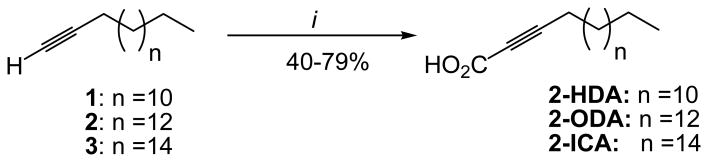
Total synthesis of 2-HDA, 2-ODA, and 2-ICA. i) n-BuLi, THF, -78°C, carbon dioxide(CO2), NH4Cl.
Scheme 2.
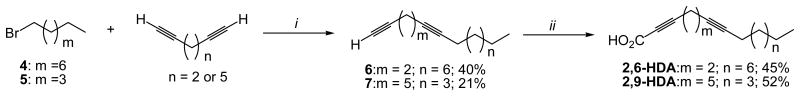
Total synthesis of 2,6-HDA and 2,9-HDA. i) n-BuLi, THF, -78°C, HMPA, 24 h; ii) n-BuLi, THF, -78°C, carbon dioxide (CO2), NH4Cl.
In the case of 2,6-HDA and 2,9-HDA, these AFAs were conveniently synthesized by using either 1,5-hexadiyne (50% in pentane) or 1,8-nonadiyne as starting materials (Scheme 2). The 2,6-HDA was prepared in two steps with an overall yield of 18%. The synthesis started with the coupling of 1,5-hexadiyne with 1-bromononane using n-BuLi in THF/HMPA at -78°C , subsequent coupling of the lithium acetylide of 1,5-pentadecadiyne with CO2, and final protonation with NH4Cl affording the desired dimethylene-interrupted 2,6-HDA. 2,9-HDA was prepared with an overall yield of 11% following a similar synthetic strategy described above, with the exception that this synthesis started with 1,8-nonadecyne. This diyne was coupled with 1-bromohexane using n-BuLi in THF/HMPA at -78°C, subsequently reacted with n-BuLi and CO2, and finally protonated with NH4Cl to afford the desired 2,9-HDA.
Other analogues of 2-AFAs including tetrahydropyranyl ethers and 2-alkynols were also prepared to establish a SAR aimed at determining the compound with better cytotoxicity towards bacteria. In this study, we prepared the tetrahydropyranyl ethers 2-HDOTHP and 2-ODOTHP, which served as starting materials for the preparation of the 2-alkynols 2-HDOH and 2-ODOH as depicted in Scheme 3. For the preparation of 2-HDOTHP and 2-ODOTHP, the 2-(2-propynyloxy)-tetrahydro-2H-pyran was coupled with either 1-bromotridecane or 1-bromopentadecane to produce 2-HDOTHP and 2-ODOTHP in 60 and 76% yields, respectively. The removal of the tetrahydropyranyl protecting group in both 2-HDOTHP and 2-ODOTHP was successfully accomplished with PTSA in methanol at 45°C, producing the desired 2-alkynols 2-HDOH and 2-ODOH in 67 and 65% yields, respectively.
Scheme 3.
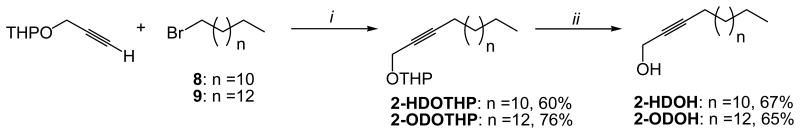
Total synthesis of 2-HDOH and 2-ODOH. i) n-BuLi, THF, -78°C, HMPA, 24 h; ii) PTSA, MeOH, 45°C, 24h.
The antibacterial activity of the 2-AFAs and their analogues against S. aureus, S. saprophyticus, B. cereus, E. coli, K. pneumoniae, and P. aeruginosa were determined by using a modified version of the microdilution method outlined by the CLSI (CLSI, 2006). The Kirby-Bauer method (CLSI, 2012) was also used for determining the antibacterial activity of 2-AFAs against several S. aureus strains (data not shown[DS8]). Altogether these bacteria were selected for this study because they are associated to nosocomial infections such as skin infections, urinary tract infections, food poisoning, respiratory tract infections, and cross-infections when using contaminated medical equipment. Table 1 shows the MICs and IC50s of 2-AFAs and their analogues against the above mentioned bacteria. Methicillin and ciprofloxacin were used as positive controls. Among the compounds tested, 2-HDA exhibited the lowest[DS9] MIC/IC50 values against Gram-positive S. aureus, S. saprophyticus, and B. cereus and against the Gram-negative K. pneumoniae [DS10]and P. aeruginosa with MICs between 7.8 and 125 μg/mL. 2-ODA also displayed good antibacterial activity against the bacteria mentioned above. It can be noted from the MICs/IC50 values in Table 1 that 2-HDA showed a broader antibacterial activity when compared to the other analogues. Also, it was noted that the increase in the carbon chain length in 2-AFA, decreases its antibacterial activity[DS11]. This is the case of 2-ICA, a 20-carbon chain length AFA, which did not show antibacterial activity against any bacteria presented in Table 1.
Table 1. Antibacterial activity of 2-AFAs and other analogues against Gram-positive and Gram-negative bacteria at 37°C after 18-24 ha.
| Compound | MIC/ IC50 ± SEM (μg/mL) | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| S. aureus (ATCC 29213) | S. saprophyticus (ATCC 15305) | B. cereus (ATCC 10876) | E. coli (ATCC 25922) | K. pneumoniae (ATCC 13883) | P. aeruginosa (ATCC 27853) | |
| 2-HDA | 15.6/6.2 ± 0.9 | 15.6/8.8 ± 0.1 | 31.3/17.2 ± 1.0 | >1,000 | 7.8/6.1 ± 0.1 | 125/87.8 ± 1.1 |
| 2-ODA | 15.6/8.1 ± 1.0 | 125/68.8 ± 3.5 | 15.6/11.9 ± 0.8 | >1,000 | 7.8/5.6 ± 0.2 | >1,000 |
| 2-ICA | >1,000 | >1,000 | >1,000 | >1,000 | >1,000 | >1,000 |
| 2,6-HDA | 31.3/14.1 ± 0.1 | 62.5/21.5 ± 0.6 | 62.5/46.1 ± 0.3 | >1,000 | 62.5/ 31.2 ± 0.5 | 250/132.3 ± 3.5 |
| 2,9-HDA | 250/94.0 ± 0.9 | 125/61.5 ± 1.2 | 62.5/34.2 ± 0.3 | >1,000 | 250/135.4 ± 0.7 | >1,000 |
| 2-HDOTHP | >1,000 | >1,000 | >1,000 | >1,000 | >1,000 | >1,000 |
| 2-ODOTHP | >1,000 | >1,000 | >1,000 | >1,000 | >1,000 | >1,000 |
| 2-HDOH | >1,000 | >1,000 | >1,000 | >1,000 | >1,000 | >1,000 |
| 2-ODOH | >1,000 | >1,000 | >1,000 | >1,000 | >1,000 | >1,000 |
| METH | 0.06/0.03 ± 0.01 | 0.12/0.06 ± 0.01 | 62.5/38.2 ± 0.7 | >1,000 | 0.98/0.36 ± 0.01 | >1,000 |
| CIPRO | 0.25/0.14 ± 0.01 | <0.002 | 0.05/0.03 ± 0.01 | <0.008 | 0.24/0.13 ± 0.01 | 0.24/0.11 ± 0.01 |
Experiments were performed in triplicate (N =3). Meth, Methicillin, Cipro, Ciprofloxacin.
Unlike our previous antifungal work, the addition of a second triple bond at C-6 in 2-HDA, decreases its antibacterial activity. Moreover, it can be noted from Table 1 that the addition of a second triple bond at C-9 decreases more notably the antibacterial activity of 2-HDA than the addition of a second triple bond at C-6. According to Morbidoni et al., 2-HDA is specific against bacteria because the acid inhibits the activity of InhA, the enoyl-ACP reductase of the type II fatty acid synthase (FASII) that catalyzes the NADH-specific reduction of 2-trans-enoyl-ACP (Morbidoni et al., 2006). Based on this finding, we believe that the addition of a second triple bond at either C-6 or C-9 in 2-HDA do not favor the interactions between either 2,6-HDA or 2,9-HDA, and the InhA enzyme.
The data in Table 1 also demonstrate that the presence of a carboxylic acid moiety in 2-AFAs is important for their antibacterial activity. For example, 2-HDA displayed good biological activity against several Gram-positive and Gram-negative bacteria, but the corresponding alcohol analog, 2-HDOH, was not active against any of the bacterial strains presented in Table 1. A similar pattern was observed with 2-ODA and 2-ODOH. Tetrahydropyranyl ethers, such as 2-HDOTHP and 2-ODOTHP, were also inactive against all tested bacteria (Table 1), which suggests that the presence of an ether functional group does not favor the antibacterial activity of 2-AFA analogues. In general, as antibacterial agents, the 2-AFAs followed the order: 2-HDA > 2-ODA > 2,6-HDA > 2,9-HDA > 2-ICA. Altogether these findings support that the carboxylic acid moiety as well as the triple bond at C-2 are needed for the antibacterial activity of 2-AFAs.
MRSA were included in our study, because the reduction of infections caused by this bacteria in hospital settings represents a priority for the Center for Control Diseases and Prevention (CDC), since this threat is responsible for the largest outbreak of hospital acquired infections (CDC, 2013; Klevens et al., 2007). As part of this study, we determined the antibacterial activity of 2-AFAs against three pathogenic MRSA strains: one commercial strain and two bacterial strains isolated from clinical patients. All three MRSA strains fermented mannitol and were coagulase positive, which confirmed them as S. aureus. Moreover, we confirmed the resistance pattern of the clinical isolates of MRSA (CIMRSA) to both methicillin and ciprofloxacin by using the Kirby-Bauer disk diffusion method (CLSI, 2012), where S. aureus ATCC 29213 was used as the standard (data not shown). Our disk diffusion results demonstrate that the two CIMRSA strains tested were both methicillin-resistant and ciprofloxacin-resistant, which supports that these bacteria strains are multidrug resistant organisms (MRO). Moreover, our disk diffusion results showed that all MRSA strains tested have different resistance pattern to both methicillin and ciprofloxacin, which suggest that these bacteria are not the same. Table 2, shows the antibacterial activity of several 2-AFAs against MRSA ATCC 43300 and the two CIMRSA strains. It can be observed from Table 2 that both 2-HDA and 2-ODA are more active against all MRSA strains tested. Moreover, these compounds demonstrated to be comparable or more effective as antibacterial compounds than either methicillin or ciprofloxacin (Table 2). Results in Table 2 also show that the addition of a second triple bond at either C-6 or C-9 do not favor the antibacterial activity of 2-AFAs, which is consistent with the results displayed in Table 1. The bactericidal effect of 2-AFAs against CIMRSA strains were also evaluated. Table 3 shows that 2-HDA and 2-ODA were bactericidal against CIMRSA 1 and 2 at concentrations of 7.8 and 15.6 μg/mL, respectively. In the case of 2,6-HDA and 2,9-HDA, these compounds displayed bactericidal effect against CIMRSA strains at concentrations of 125-500 μg/mL.
Table 2. Antibacterial activity of several 2-AFAs against MRSA ATCC 43300 and CIMRSA strains. a.
| Compound | MIC/IC50± SEM (μg/mL)/Therapeutic Index (TI) b | PBMC cytotoxicity, IC50± SEM (μg/mL) | ||
|---|---|---|---|---|
|
| ||||
| MRSA ATCC 43300 | CIMRSA 1c | CIMRSA 2c | ||
| 2-HDA | 15.6/9.4 ± 0.1/6.1 | 3.9/3.3 ± 0.1/17.3 | 3.9/3.4 ± 1.0/16.8 | 57.2 ± 0.1 |
| 2-ODA | 7.8/5.5 ± 1.0/10.7 | 7.8/4.3 ± 1.0/13.7 | 7.8 /5.5 ± 1.0/10.7 | 58.9 ± 0.1 |
| 2,6-HDA | 62.5/22.3 ± 0.3/2.8 | 62.5/39.5 ± 0.9/1.6 | 62.5/22.4 ± 0.4/2.6 | 63.4 ± 0.3 |
| 2,9-HDA | 500/203.5 ± 2.6/0.4 | 250/102.1 ± 2.4/0.9 | 250/ 76.0 ± 2.4/1.2 | 93.3 ± 0.4 |
| Methicillin | 1.95/0.98 ± 0.02/87.9 | 7.8/2.2 ± 0.7/39.1 | 31.3/11.8 ± 3.9/7.3 | 86.1 ± 0.5 |
| Ciprofloxacin | 0.50/0.20 ± 0.09/332 | 15.6/10.4 ± 3.7/6.4 | 3.9/2.9 ± 1.2/22.9 | 66.4 ± 0.6 |
Experiments were performed in triplicate (N =3). Methicillin and ciprofloxacin were used as positive controls.
TI was determined by dividing PBMC cytotoxicity by the inhibitory concentration (IC50) of the drug.
CIMRSA = Clinical isolate of methicillin-resistant S. aureus.
Table 3.
Bactericidal activity of 2-AFAs against CIMRSA strains.a
| Compound | MBC, μg/mL | |
|---|---|---|
|
| ||
| CIMRSA 1b | CIMRSA 2b | |
| 2-HDA | 7.8 | 7.8 |
| 2-ODA | 15.6 | 15.6 |
| 2,6-HDA | 125 | 250 |
| 2,9-HDA | 500 | 500 |
Experiments were carried out in triplicate (N = 3).
CIMRSA = Clinical isolate of methicillin-resistant S. aureus.
Cytotoxicity of 2-AFAs was determined against PBMC isolated from healthy volunteers. As shown in Table 2, 2-HDA was the most toxic of the four 2-AFAs towards PBMC. However, 2-HDA showed the highest selectivity towards CIMRSA strains, which make it a more therapeutically valuable compound. This result is in agreement with findings published by Carballeira et al., where they reported that 2-HDA showed the highest therapeutic index (TI) as antiprotozoal agent (Carballeira et al., 2012). The marked difference between the cytotoxicity of 2-AFAs towards PBMC and their antibacterial activities can be due to the fact that the FASI complex in microsomal systems is not inhibited by 2-AFAs (Morbidoni et al., 2006).
Since several fatty acids tend to form micellar aggregates in aqueous media (Courtney et al., 1986; Orellano et al., 2013), we determined the CMC of several 2-AFAs in order to investigate whether micellar formation is associated with their antibacterial activity against MRSA. To the best of our knowledge, the CMCs of 2-AFAs have not been reported before. Several studies have demonstrated a relationship between the CMC of amphiphilic compounds and their biological activity (Courtney et al., 1986; Smith et al., 1979). Recently, Carballeira and collaborators determined that the anticancer activities of α-methoxylated fatty acids are related to their CMCs (Orellano et al., 2013). In contrast to other studies, the results in Table 4 showed that the antibacterial activity of 2-HDA and 2-ODA is not mediated by micelle formation, suggesting that the free monomeric form of these 2-AFAs are responsible for their biological activity against MRSA. These results strongly support the findings reported by Wicken and collaborators (Wicken et al., 1986). In this report they demonstrated that the free monomer of lipoteichoic acids (LTAs), amphiphiles isolated from Gram-positive organisms, represents the major configuration of extracellular LTAs in bacterial culture fluids (Wicken et al., 1986). It can be noted from Table 4 that there is a relationship between the decline in the antibacterial activity of 2,6-HDA and its CMC. Wicken et al. concluded that it is important for micellar aggregates to be separated in their respective monomer units to allow the intercalation of the monomer into membranes (Wicken et al., 1986). Therefore, we hypothesize that the decline in the concentration of the monomer units of 2,6 -HDA in aqueous medium, do not favor the hydrophobic interactions between this 2-AFA and the bacterial cell wall. These membrane-fatty acid interactions would presumably be facilitated at high concentrations of the free fatty acid. On the other hand, it can be observed that the CMC of 2,9-HDA is ten-fold higher than the CMC of 2,6-HDA suggesting that the presence of a triple bond at C-9 in 2-HDA does not favor the micelle formation. Therefore, the antibacterial activity of 2,9-HDA can be mediated by a non-micelle-dependent mechanism.
Table 4.
| Compound | IC50 ± SEM (μg/mL) | CMC (μg/mL) | ||
|---|---|---|---|---|
|
| ||||
| MRSA ATCC 43300 | CIMRSA 1 c | CIMRSA 2c | ||
| 2-HDA | 9.4 ± 0.1 | 3.3 ± 0.1 | 3.4 ± 1.0 | > 90 |
| 2-ODA | 5.5 ± 1.0 | 4.3 ± 1.0 | 5.5 ± 1.0 | 50-70 |
| 2,6-HDA | 22.3 ± 0.3 | 39.5 ± 0.9 | 22.4 ± 0.4 | 1-10 |
| 2,9-HDA | 203.5 ± 2.6 | 102.1 ± 2.4 | 76.0 ± 2.4 | > 100 |
The CMC was determined as described in materials and methods.
Experiments were performed in triplicate (N = 3).
Clinical isolates of methicillin-resistant S. aureus (MRSA).
Further experiments are required to understand the mechanism of action of antibacterial 2-AFAs. Although the InhA enzyme has been proposed as a possible molecular target (Morbidoni et al., 2006), other possible mechanisms of action for the 2-AFAs have been explored to explain their antibacterial properties. For example, Konthikamee et al. demonstrated that the absorption and utilization of 2-AFAs in the synthesis of bacterial phospholipids is a possible mechanism of action of these compounds (Konthikamee et al., 1982). These authors explained that the mechanism above takes place as a result of the activation of 2-AFAs to the acyl coenzyme A thioester, which act as substrate for the acyl coenzyme A phospholipid acyl transferase of bacterial cells (Konthikamee et al., 1982).
In conclusion, we performed three synthetic strategies aimed at determining those structural characteristics that are needed to prepare 2-AFAs with better antibacterial activity. Our results demonstrated that the presence of a triple bond at C-2 as well as the carboxylic acid moiety are important functionalities for the antibacterial activity of 2-AFAs against Gram-positive and Gram-negative bacteria, including clinical isolates of methicillin-resistant S. aureus. Moreover, we determined that by increasing the carbon chain length in 2-AFAs, their antibacterial activity decreases. Our results also demonstrated that the antibacterial activity of 2-AFAs is not mediated by micellar aggregates formation. This study will surely impact our understanding regarding the antibacterial properties of 2-AFAs, and highlight 2-HDA as a novel agent against nosocomial bacterial infections.
Acknowledgments
This project was supported by the National Center for Research Resources and the National Institute of General Medical Sciences of the National Institutes of Health through Grant Number 8 P20 GM 103475 and the Inter American University of Puerto Rico. I. Domínguez and C. Ríos acknowledge the support of the PR-INBRE program for undergraduate fellowships. We also thank Mrs. Myrna Vázquez, Mrs. Zahiraliz Reyes, Mr. Luis López, Mr. Juan Ordein-Sánchez, Ms. Carmen Reyes, and Ms. Tania Torres for their technical support in performing antibacterial susceptibility tests. This project also was supported in part by the NIH-SCORE Program through Grant Number SC1GM084708, PRCTRC Grant through the Grant Numbers U54 RR026139 and 8U54MD 007587-03, and National Institute on Minority Health and Health Disparities of the National Institute of Health through the Grant Number 8G12MD007583-28.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Brachman PS, Dan BB, Haley RW, Hooton TM, Garner JS, Allen JR. Nosocomial surgical infections: incidence and cost. Surg Clin North Am. 1980;60:15–25. doi: 10.1016/s0039-6109(16)42030-x. [DOI] [PubMed] [Google Scholar]
- Bradbury BJ, Pucci MJ. Recent advances in antibacterial topoisomerase inhibitors. Curr Opin Pharm. 2008;8:574–581. doi: 10.1016/j.coph.2008.04.009. [DOI] [PubMed] [Google Scholar]
- Cahoon EB, Schnurr JA, Huffman EA, Minto RE. Fungal responsive fatty acid acetylenases occur widely in evolutionarily distant plant families. The Plant Journal. 2003;34:671–683. doi: 10.1046/j.1365-313x.2003.01757.x. [DOI] [PubMed] [Google Scholar]
- Carballeira NM. New advances in fatty acids as antimalarial, antimycobateial and antifungal agents. Prog Lipid Res. 2008;47:50–61. doi: 10.1016/j.plipres.2007.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carballeira NM, Betancourt JE, Orellano EA, González FA. Total synthesis and biological evaluation of (5Z, 9Z)-5,9-hexadecadienoic acid, an inhibitor of human topoisomeras. I J Nat Prod. 2002;65:1715–1718. doi: 10.1021/np0202576. [DOI] [PubMed] [Google Scholar]
- Carballeira NM, Cartagena M, Sanabria D, Tasdemir D, Prada CF, Reguera RM, Balana-Fouce R. 2-Alkynoic fatty acids inhibit topoisomerase IB from Leishmania donovani. Bioorg Med Chem Lett. 2012;22:6185–6189. doi: 10.1016/j.bmcl.2012.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carballeira NM, Emiliano A, Hernandez-Alonso N, Gonzalez FA. Facile total synthesis and antimicrobial activity of the marine fatty acids (Z)-2-methoxy-5-hexadecenoic acid and (Z)-2-methoxy-6-hexadecenoic acid. J Nat Prod. 1998;61:1543–1546. doi: 10.1021/np980274o. [DOI] [PubMed] [Google Scholar]
- Carballeira NM, Sanabria D, Cruz C, Parang K, Wan B, Franzblau S. 2,6-hexadecadiynoic acid and 2,6-nonadecadiynoic acid: Novel synthesized acetylenic fatty acids as potent antifungal agents. Lipids. 2006;41:507–511. doi: 10.1007/s11745-006-5124-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carballeira NM, Sanabria D, Parang K. Total synthesis and further scrutiny of the in vitro antifungal activity of 6-nonadecynoic acid. Arch Pharm Chem Life Sci. 2005;338:441–443. doi: 10.1002/ardp.200500102. [DOI] [PubMed] [Google Scholar]
- CDC. Antibiotic resitance threat in the United States. Technical Report. 2013:77–78. [Google Scholar]
- CLSI. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically, M7-A7. 2. 7th. Vol. 26. Clinical and Laboratory Standards Institute (Formerly NCCLS); Wayne, PA: 2006. [Google Scholar]
- CLSI. Performance standards for antimicrobial disk susceptibility tests, M02-A11. 1. Vol. 32. Clinical and Laboratory Standards Institute (Formerly, NCCLS); Wayne, PA: 2012. [Google Scholar]
- Courtney HS, Simpson WA, Beachey EH. Relationship of critical micelle concentrations of bacterial lipoteichoic acids to biological activities. Infect Immun. 1986;51:414–418. doi: 10.1128/iai.51.2.414-418.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fatope MO, Adoum OA, Takeda Y. C(18) acetylenic fatty acids of Ximenia americana with potential pesticidal activity. J Agric Food Chem. 2000;48:1872–1874. doi: 10.1021/jf990550k. [DOI] [PubMed] [Google Scholar]
- Freitag A, Rapp H, Heide L, Li SM. Metabolic engineering of aminocoumarins: inactivation of the methyltransferase gene cloP and generation of new clorobiocin derivatives in a heterologous host. Chembiochem. 2005;6:1411–1418. doi: 10.1002/cbic.200500019. [DOI] [PubMed] [Google Scholar]
- Gershon H, Shanks L. Antifungal properties of 2-alkynoic acids and their methyl esters. Can J Microbiol. 1978;24:593–597. doi: 10.1139/m78-096. [DOI] [PubMed] [Google Scholar]
- Heide L. Genetic engineering of antibiotic biosynthesis for the generation of new aminocoumarins. Biotechnology Advances. 2009;27:1006–1014. doi: 10.1016/j.biotechadv.2009.05.017. [DOI] [PubMed] [Google Scholar]
- Inweregbu K, Dave J, Pittard A. Nosocomial infections. Counting Education in Anaesthesia, Critical Care & Pain. 2005;5:14–17. [Google Scholar]
- Kampranis SC, Gormley NA, Tranter R, Orphanides G, Maxwell A. Probing the binding of coumarins and cyclothialidines to DNA gyrase. Biochemistry. 1999;38:1967–1976. doi: 10.1021/bi982320p. [DOI] [PubMed] [Google Scholar]
- Klevens MR, Morrison MA, Nadle J, Petit S, Gershman K, Ray S, Harrison LH, Lynfield R, Dumyati G, Townes JM, Graig AS, Zell ER, Fosheim GE, McDougal LK, Carey RB, Fridkin SK. Invasive methicillin-resistant Staphylococcus aureus infections in the United States. JAMA. 2007;298:1763–1771. doi: 10.1001/jama.298.15.1763. [DOI] [PubMed] [Google Scholar]
- Klevens RM, Edwards JR, Richards CL, Horan TC, Gaynes RP, Pollock PA, Cardo DM. Estimating health care-associated infections and deaths in U.S. hospitals, 2002. Public Health Reports. 2007;122:160–166. doi: 10.1177/003335490712200205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konthikamee W, Gilbertson JR, Langkamp H, Gershon H. Effect of 2-alkynoic acids on in vitro growth of bacterial and mammalian cells. Antimicrob Agents Chemother. 1982;22:805–809. doi: 10.1128/aac.22.5.805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li XC, Jacob MR, ElSohly HN, Nagle DG, Smillie TJ, Walker LA, Clark AM. Acetylenic acids inhibiting azole-resistant Candida albicans from Pentagonia gigantifolia. J Nat Prod. 2003;66:1132–1135. doi: 10.1021/np030196r. [DOI] [PubMed] [Google Scholar]
- Li XC, Jacob MR, Khan SI, Ashfaq MK, Babu KS, Agarwal AK, Elsohly HN, Manly SP, Clark AM. Potent in vitro antifungal activities of naturally occurring acetylenic acids. Antimicrob Agents Chemother. 2008;52:2442–2448. doi: 10.1128/AAC.01297-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morbidoni HR, Vilchèze C, Kremer L, Bittman R, Sacchettini JC, Jacobs WR. Dual Inhibition of mycobacterial fatty acid biosynthesis and degradation by 2-alkynoic acids. Chem Biol. 2006;13:297–307. doi: 10.1016/j.chembiol.2006.01.005. [DOI] [PubMed] [Google Scholar]
- Orellano EA, Cartagena MM, Rosado K, Carballeira NM. Synthesis of the novel (±)-2-methoxy-6-icosynoic acid-A fatty acid that induces death of neuroblastoma cells. Chem Phys Lipids. 2013:172–173. 14–19. doi: 10.1016/j.chemphyslip.2013.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pittet D, Tarara D, Wenzel RP. Nosocomial bloodstream infection in critically ill patients. Excess length of stay, extra costs, and attributable mortality. JAMA. 1994;271:1598–1601. doi: 10.1001/jama.271.20.1598. [DOI] [PubMed] [Google Scholar]
- Rudolph J, Theis H, Hanke R, Endermann R, Johannsen L, Geschke F. seco-Cyclothialidines: new concise synthesis, inhibitory activity toward bacterial and human DNA topoisomerases, and antibacterial properties. J Med Chem. 2001;44:619–626. doi: 10.1021/jm0010623. [DOI] [PubMed] [Google Scholar]
- Smith CM, Williams GC, Krivit W, White JG, Hanson RF. Micellar properties of 3 alpha, 7 alpha, 12 alpha-trihydroxy-5 beta-cholestan-26-oyl taurine and relationship to in vitro red cell disruption. J Lab Clin Med. 1979;94:624–632. [PubMed] [Google Scholar]
- Stone PW, Larson E, Kawar LN. A systematic audit of economic evidence linking nosocomial infections and infection control interventions: 1990-2000. Am J Infect Control. 2002;30:145–152. doi: 10.1067/mic.2002.121099. [DOI] [PubMed] [Google Scholar]
- Tasdemir D, Sanabria D, Lauinger IL, Tarun A, Herman R, Perozzo R, Zloh M, Kappe SH, Brun R, Carballeira NM. 2-Hexadecynoic acid inhibits plasmodial FAS-II enzymes and arrests erythrocytic and liver stage Plasmodium infections. Bioorg Med Chem. 2010;18:7475–7485. doi: 10.1016/j.bmc.2010.08.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Upreti GC, Matocha M, Wood R. Effect of 2-hexadecynoic acid on cultured 7288C hepatoma cells. Lipids. 1981;16:315–322. doi: 10.1007/BF02534955. [DOI] [PubMed] [Google Scholar]
- WHO. Prevention of hospital-acquired infections: A practical guide. 2nd. Geneva: 2002. pp. 4–6. [Google Scholar]
- Wicken AJ, Evans JD, Knox KW. Critical micelle concentrations of lipoteichoic acids. J Bacteriol. 1986;166:72–77. doi: 10.1128/jb.166.1.72-77.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood R, Lee T. Metabolism of 2-hexadecynoate and inhibition of fatty acid elongation. J Biol Chem. 1981;256:12379–12386. [PubMed] [Google Scholar]
- Xu T, Tripathi SK, Feng Q, Lorenz MC, Wright MA, Jacob MR, Mask MM, Baerson SR, Li XC, Clark AM, Agarwal AK. A potent plant-derived antifungal acetylenic acid mediates its activity by interfering with fatty acid homeostasis. Antimicrob Agents Chemother. 2012;56:2894–2907. doi: 10.1128/AAC.05663-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhan C, Miller MR. Excess length of stay, charges, and mortality attributable to medical injuries during hospitalization. JAMA : the journal of the American Medical Association. 2003;290:1868–1874. doi: 10.1001/jama.290.14.1868. [DOI] [PubMed] [Google Scholar]
- Zheng CJ, Yoo J, Lee T, Cho H, Kim Y, Kim W. Fatty acid synthesis is a target for antibacterial activity of unsaturated fatty acids. FEBS Lett. 2005;579:5157–5162. doi: 10.1016/j.febslet.2005.08.028. [DOI] [PubMed] [Google Scholar]