Abstract
Background
The variable incidence of gallbladder cancer (GBCA) suggests regional pathogenetic differences. This study compares cell cycle-regulatory, angiogenesis-related, and PI3K pathway protein expression in GBCAs from three continents.
Methods
Immunohistochemical expression of several proteins was assessed, correlated with clinicopathologic variables, and compared among centers from Chile (Fundación Arturo López Pérez [FALP]), Japan (Yokohama City University [YCU]), and the United States (Memorial Sloan-Kettering Cancer Center [MSKCC]). Hierarchical clustering was used to partition the data based on protein-expression and treatment center.
Results
Tissue from 117 patients (MSKCC = 76; FALP = 22; YCU = 19) was analyzed. Mdm2 overexpression was seen only at MSKCC (p < 0.0001). Absence of p21 (p = 0.03) and VEGFR2 (p = 0.018) were more common and p27 expression was less frequent (p = 0.047) in tumors from YCU. Ki-67 labeling index in YCU tumors (median = 10) was two-thirds lower than at other centers. On hierarchical clustering analysis, all YCU patients (p = 0.017) and those with early tumors (p = 0.017) clustered separately from MSKCC. Median disease-specific survival after curative intent (R0) resection was 27 months and was similar among centers (p = 0.9). Median disease-specific survival of patients with early tumors was 28.4 months and was higher at YCU (not reached, p = 0.06).
Conclusions
Cell cycle-regulatory protein expression patterns of YCU tumors differed from those treated at FALP and MSKCC. The differential clustering of protein expression and survival in patients with early tumors suggest regional differences in pathogenesis and disease biology.
Gallbladder cancer (GBCA) is an uncommon malignancy with poor prognosis and variable incidence worldwide, although the common feature among all patients is advanced disease at diagnosis.1–8 Epidemiological and molecular studies suggest that GBCA may arise and progress through different pathways, a hypothesis supported by a recent comparison among centers from Japan, the United States, and Chile revealing disease-related differences based on national origin.9–12
Whether these differences reflect regional variation in pathogenesis is uncertain but plausible based on prior work. For example, K-ras mutations are frequent in patients with anomalous pancreaticobiliary duct junction, a relatively common risk factor in Japan, but are rare in GBCA associated with adenomas.13–15 Additionally, deregulatory mutations in the TP53 gene are seen in both Japan and Chile, but the spectrum of mutations varies according to location.16–19
The expression patterns of certain cell cycle-regulatory, pro-angiogenic, and PI3K pathway proteins are important predictors of clinical behavior in biliary tract cancers.20–22 Most studies, however, include small numbers of patients, and few, if any, include a comprehensive assessment in the same cohort. This study examines expression of key elements of these pathways in GBCA patients treated at centers on three continents. The primary objective was to assess for differences in expression patterns among groups, which might suggest regional differences in pathogenesis.
METHODS
Subjects and Data Collection
After institutional review board approval from Instituto Oncológico Fundación Arturo López Pérez (FALP, Santiago, Chile), Yokohama City University (YCU, Yokohama, Japan), and Memorial Sloan-Kettering Cancer Center (MSKCC, New York, NY), patients with GBCA treated from 1994 to 2009 and with adequate archived tissue were identified. Demographics, laboratory values, procedures, perioperative outcome, histopathology and staging, follow-up, and survival data were recorded. The approach to evaluation and treatment of GBCA at each center has been described.12,23,24 Incidental gallbladder cancer (IGBCA) was defined as an unsuspected tumor from a specimen removed for presumed benign disease. All cases of IGBCA were re-reviewed to confirm diagnosis and T stage. All patients with tumor invasion to at least the muscularis propria layer (T1b) and without distant metastases were offered reoperation and definitive resection.23
Pathologic Examination
All archived hematoxylin and eosin (HE)-stained slides were reviewed by study pathologists: staging was based on the 7th edition of the American Joint Committee on Cancer, Cancer Staging Manual.25 Tissue microarrays were created by identifying tumor and nontumor tissue on HE-stained slides; an automated tissue arrayer (Beecher ATA-27) was used to procure triplicate 0.6-mm cores from the corresponding paraffin block.21 HE-stained sections were examined to ensure specimen integrity. Immunostaining was performed using standard streptavidin–biotin immunoperoxidase techniques. Primary antibodies were placed overnight at 4 °C. Mouse antihuman monoclonal antibodies to mutant or wild-type p53 (clone D07, 1:500; Dako), Bcl2 (clone 124, 1:200; Dako), Ki-67 (clone 30–9, 1:100; Ventana), p21Waf–1 (clone 57, 1:100; Oncogene Science), p27Kip–1 (clone SX53G8, 1:2,000; Dako), Mdm2 (clone IF2, 1:4,000; Calbiochem), CD1 (clone SP4, 1:25; Labvision), VEGF (clone sc-7269, 1:200; Santa Cruz), VEGFR2 (clone 55B11, 1:600; cell signaling), CD31 (clone JC70A, 1:700; Dako), CD34 (clone QBEnd 10, 1:1,000; Dako), PTEN (clone 6H2.1, 1:50: Dako), and polyclonal antibody phospho-S6 ribosomal protein (clone 2215L, 1:100; Cell Signaling) were used. After washing, a biotinylated antimouse secondary antibody (1:500; Vector) was added, followed by phosphate-buffered saline washing and a peroxidase-conjugated streptavidin (1:500; Dako). The secondary antibody and streptavidin were placed for 1 h at ambient temperature. Diaminobenzidine served as the chromogen, and hematoxylin was the nuclear counterstain. Positive controls were p53 (bladder transitional-cell carcinoma), Mdm2 (colon adenocarcinoma), Ki-67, Bcl2, and p21Waf–1 (lymphoma), p27Kip–1 (prostate adenocarcinoma), CD1 (breast adenocarcinoma), VEGF (colon carcinoma), VEGFR2 (clear cell carcinoma), CD34 (colon), CD31 and Phospho-S6 ribosomal protein (tonsil), and PTEN (ovarian carcinoma).
Immunostaining Analysis
Stained sections from tissue microarrays were evaluated and graded by three pathologists (JT, EV, and DK) using the same criteria. All sections were graded for intensity on a scale of 0–4 (0 = absence of staining at all magnifications; 1 = staining not observed at 10x magnification but positive on 40×; 2 = mild staining observed at 10× magnification; 3 = staining easily observed at 10×; 4 = intense staining observed at 49) and percentage of tumor cell staining (p53, p21, p27, Bcl2, Mdm2, CD1, VEGF, VEGFR2, and phospho-S6 ribosomal protein); for these antibodies, criteria for positive staining included intensity grade ≥ 2 or ≥ 20 % positive tumor cells. Ki-67 was graded only by the percentage of stained cells. Microvessel density was assessed by counting microvessels stained with CD31 and CD34. Nonneoplastic tissue was the internal control for each antibody. Only staining of GBCA cells was scored; nonspecific staining or staining of other cell types was discounted. Immunoreactivity of PTEN was evaluated semiquantitatively. The surrounding microenvironment was used as a positive internal control and internal reference for scoring. The cytoplasmic and/or nuclear immunoreaction score of tumor cells was based on intensity: a score of 2 was positive, a score of 1 was weak, and a score of 0 was negative. Loss of PTEN was considered only in cases defined as negative, whereas cases with no staining of surrounding tissue were discarded as not evaluable.26
The results were dichotomized into positive or negative for each antibody, except Ki-67, CD31, CD34, and PTEN. Values for Ki-67 represented the average percentage of positively stained cells; CD31 and CD34 were expressed as average number of positive microvessels identified; PTEN was considered either present or lost. For p53, p21, Bcl2, Mdm2, CD1, VEGF, VEGFR2, and phospho-S6 ribosomal protein, criteria for positive staining were an intensity of 2 and ≥ 20 % of cells reactive; p27 was positive if intensity was ≥ 1 and ≥ 10 % cells reactive. These reference values are consistent with those used in prior studies.21,27–32
Statistical Analysis
Results are presented as proportions for categorical variables or as mean ± SD and median (range) for continuous variables. Continuous and categorical variables were compared using the Student’s t test and the χ2 test, respectively. Surgical mortality was defined as death resulting from postoperative complications at any time after surgery. Survival analysis was performed in all patients and in patients with early GBCA (T1 + T2). Survival probabilities were calculated using the Kaplan–Meier method and compared with log-rank test. Disease-specific survival (DSS) was defined as the interval between the date of operation and the date of last follow-up or death. Curative intent (R0) resection was defined as free of microscopic disease at all margins, whereas R1- or R2-resection had microscopic or macroscopic disease after definitive resection, respectively, at one or more margin. Univariate proportional hazards regression identified clinicopathologic factors predictive of DSS after an R0-resection; a multivariate model was performed using proportional hazards regression and including variables significant at the 20 % level on univariate analysis. Only patients with tumor differentiation typically seen in GBCA (adenocarcinoma, squamous/adenosquamous carcinoma) were included in the survival, uni- and multivariate analyses. χ2 test was used to compare the prevalence of positive staining across centers (Student’s t test for Ki-67, CD31, and CD34, which were dichotomized according to median value). All tests were two-sided, p < 0.05. Hierarchical clustering with complete linkage and binary distance was performed to partition the data based on protein expression and then compared with center of origin, excluding patients with incomplete immunostaining data. The same analysis was repeated for T1/T2 versus T3/T4. The rationale for this is that differences in pathogenesis are likely to be more apparent in early stage disease. In each subset, partitions were redefined based on the subset by hierarchical clustering only on that subset. R functions hclust and dist (version 2.12) were used for analysis (www.r-project.org). Statistical analysis was performed with SPSS 19.0, SAS 9.2, and R 2.9.
RESULTS
Demographics and Treatment
The analysis included 117 patients treated for GBCA between 1994 and 2009 at MSKCC (N = 76), FALP (N = 22), or YCU (N = 19). Demographic, operative, and perioperative data are summarized in Table 1. Ethnic distribution differed among centers, with greater heterogeneity at MSKCC, although Caucasians still represented the large majority (N = 58, 76.3 %). Cholelithiasis was more common at FALP and MSKCC compared with YCU (p < 0.0001), and IGBCA (N = 46, 39.3 %) was far less common at YCU (p < 0.0001), consistent with prior observations.12
TABLE 1.
Patient demographics, treatment, and pathology results
| Total N = 117 (%) |
FALP N = 22 (%) |
YCU N = 19 (%) |
MSKCC N = 76 (%) |
p | |
|---|---|---|---|---|---|
| Gender | 0.9 | ||||
| Female | 75 (64.1) | 15 (68.2) | 12 (63.2) | 48 (63.2) | |
| Male | 42 (35.9) | 7 (31.8) | 7 (36.8) | 28 (36.8) | |
| Age (years) | 0.056 | ||||
| Median (range) | 65 (28–91) | 60 (41–91) | 63 (48–85) | 69 (28–90) | |
| Mean ± SD | 65.4 ± 11.4 | 60.3 ± 12.3 | 65.8 ± 10.2 | 66.8 ± 11.1 | |
| Race | <0.0001 | ||||
| Caucasian | 58 (49.6) | 0 | 0 | 58 (76.3) | |
| Hispanic | 26 (22.2) | 21 (95.5) | 0 | 5 (6.6) | |
| Asian-Japanese | 19 (16.2) | 0 | 19 (100) | 0 | |
| African-American | 3 (2.6) | 0 | 0 | 3 (3.9) | |
| Pacific Islander | 2 (1.7) | 0 | 0 | 2 (2.6) | |
| Asian-American | 3 (2.6) | 0 | 0 | 3 (3.9) | |
| Indian | 1 (0.9) | 0 | 0 | 1 (1.3) | |
| Native American | 1 (0.9) | 1 (4.5) | 0 | 0 | |
| Unknown | 4 (3.4) | 0 | 0 | 4 (5.3) | |
| Gallstones | <0.0001 | ||||
| Yes | 94 (80.3) | 22 (100) | 9 (47.4) | 63 (82.9) | |
| No | 23 (19.7) | 0 | 10 (52.6) | 13 (17.1) | |
| Incidental | <0.0001 | ||||
| Yes | 46 (39.3) | 12 (54.5) | 0 | 34 (44.7) | |
| No | 71 (60.7) | 10 (45.5) | 19 (100) | 42 (55.3) | |
| Curative resection | 0.027 | ||||
| Yes | 71 (60.7) | 8 (36.4) | 14 (73.7) | 49 (64.5) | |
| No | 46 (39.3) | 14 (63.6) | 5 (26.3) | 27 (35.5) | |
| Main procedure | 0.002 | ||||
| Segmentectomy 4B/5 | 49 (41.9) | 7 (31.8) | 9 (47.4) | 33 (43.4) | |
| Segmentectomy 4B/5 + PDD | 4 (3.4) | 1 (4.5) | 2 (10.5) | 1 (1.3) | |
| Extended hepatectomy | 28 (23.9) | 0 | 6 (31.6) | 22 (28.9) | |
| Cholecystectomy (noncurative) | 27 (23.1) | 12 (54.5) | 0 | 15 (19.7) | |
| Cholecystectomy (curative) | 5 (4.3) | 1 (4.5) | 2 (10.5) | 2 (2.6) | |
| Nonresection | 4 (3.4) | 1 (4.5) | 0 | 3 (3.9) | |
| Hepatic resection | <0.0001 | ||||
| Yes | 81 (69.2) | 8 (36.4) | 17 (89.5) | 56 (73.7) | |
| No | 36 (30.8) | 14 (63.6) | 2 (10.5) | 20 (26.3) | |
| Bile duct resection | <0.0001 | ||||
| Yes | 56 (47.9) | 3 (13.6) | 14 (73.7) | 39 (51.3) | |
| No | 61 (52.1) | 19 (86.4) | 5 (26.3) | 37 (48.7) | |
| Adjacent organ resection | 0.1 | ||||
| Yes | 15 (12.8) | 0 | 3 (15.8) | 12 (15.8) | |
| No | 102 (87.2) | 22 (100) | 16 (84.2) | 64 (84.2) | |
| Adjuvant therapy | 0.1 | ||||
| Yes | 53 (45.3) | 6 (27.3) | 11 (57.9) | 36 (47.4) | |
| No | 64 (54.7) | 16 (72.7) | 8 (42.1) | 40 (52.6) | |
| T stage | 0.001 | ||||
| 1 | 5 (4.3) | 1 (4.5) | 1 (5.3) | 3 (3.9) | |
| 2 | 41 (35) | 14 (63.6) | 5 (26.3) | 22 (28.9) | |
| 3 | 63 (53.8) | 6 (27.3) | 9 (47.4) | 48 (63.2) | |
| 4 | 5 (4.3) | 0 | 4 (21.1) | 1 (1.3) | |
| Unknown | 3 (2.6) | 1 (4.5) | 0 | 2 (2.6) | |
| Histology | 0.3 | ||||
| Adenocarcinoma | 106 (90.6) | 21 (95.5) | 17 (89.5) | 68 (89.5) | |
| Adenosquamous | 6 (5.1) | 1 (4.5) | 0 | 5 (6.6) | |
| Squamous | 2 (1.7) | 0 | 0 | 2 (2.6) | |
| Neuroendocrine | 2 (1.7) | 0 | 1 (5.3) | 1 (1.3) | |
| Undifferentiated | 1 (0.9) | 0 | 1 (5.3) | 0 | |
| Differentiation | <0.0001 | ||||
| Well | 16 (13.7) | 1 (4.5) | 10 (52.6) | 5 (6.6) | |
| Moderate | 61 (52.1) | 15 (68.2) | 5 (26.3) | 41 (53.9) | |
| Poor | 40 (34.2) | 6 (27.3) | 4 (21.1) | 30 (39.5) | |
| Lymphovascular invasion (n = 88) | 0.2 | ||||
| No | 42 (47.7) | 5 (45.5) | 6 (31.6) | 31 (53.4) | |
| Yes | 46 (52.3) | 6 (54.5) | 13 (68.4) | 27 (46.6) | |
| Perineural invasion (n = 81) | 0.8 | ||||
| No | 30 (37) | 4 (40) | 8 (42.1) | 18 (34.6) | |
| Yes | 51 (63) | 6 (60.0) | 11 (57.9) | 34 (65.4) | |
| Lymph node stage | 0.03 | ||||
| Negative | 39 (33.3) | 4 (18.2) | 4 (21.1) | 31 (40.8) | |
| Positive | 64 (54.7) | 12 (54.5) | 14 (73.7) | 38 (50) | |
| Unknown | 14 (12) | 6 (27.3) | 1 (5.3) | 7 (9.2) | |
| Lymph nodes resected | <0.0001 | ||||
| Median (range) | 3.5 (0–55) | 4 (0–13) | 13 (0–55) | 3 (0–17) | |
| Mean ± SD | 6.6 ± 9.5 | 4.6 ±4 | 19 ± 17 | 3.9 ± 3.7 | |
| Positive lymph nodes | 0.019 | ||||
| Median (range) | 1 (1–43) | 2 (1–7) | 2 (1–43) | 1 (1–6) | |
| Mean ± SD | 1.6 ± 4.2 | 1.3 ± 1.8 | 4 ± 9.6 | 1 ± 1.5 | |
| TNM stage | 0.02 | ||||
| 1 | 3 (2.6) | 0 | 1 (5.3) | 2 (2.6) | |
| 2 | 10 (8.5) | 4 (18.2) | 2 (10.5) | 4 (5.3) | |
| 3a | 21 (17.9) | 0 | 1 (5.3) | 20 (26.3) | |
| 3b | 37 (31.6) | 7 (31.8) | 7 (36.8) | 23 (30.3) | |
| 4a | 3 (2.6) | 0 | 2 (10.5) | 1 (1.3) | |
| 4b | 43 (36.8) | 11 (50) | 6 (31.6) | 26 (34.2) |
PDD pancreaticoduodenectomy
R0-resections (N = 71, 60.7 %) were more common at MSKCC and YCU compared with FALP (p = 0.027). R0-resection procedures included segmentectomy 4B/5 in 41 patients, extended hepatectomy in 22, pancreaticoduodenectomy combined with 4B/5 liver segmentectomy in 3, and cholecystectomy alone in 5. These procedures were combined with porta hepatis lymphadenectomy (except in patients treated with cholecystectomy alone), and in selected patients were combined with bile duct resection, adjacent organ resection, or vascular reconstruction. Fifty-three (45.3 %) patients received perioperative adjuvant therapy, which was not different among the centers (Table 1).
Pathologic Characteristics
A total of 109 patients had invasive disease; depth of invasion was unknown in three patients (Table 1). There were more T2 tumors in patients from FALP compared with the other centers (p = 0.001). One hundred three (88 %) patients had at least one lymph node resected, and this was more common at MSKCC (90.8 %) and YCU (94.8 %) compared with FALP (72.7 %; p = 0.03). The median number of lymph nodes resected was 3.5 (range, 0–55). Sixty-four (54.7 %) patients had positive nodes; 39 (33.3 %) had negative nodes; and 14 (12 %) had none evaluated histologically. The median number of positive nodes for all patients was 1 (range, 1–43) and was higher at YCU (p = 0.019). Note that two patients with neuroendocrine tumor were excluded from survival, uni-, and multivariate analyses.
Immunostaining Analysis
Immunostaining data are shown in Table 2. p21, Mdm2, p27, and VEGFR2 expression were significantly less common in tumors from YCU. In fact, neither p21, Mdm2, nor VEGFR2 was detectable in tumors from Japanese patients, whereas Mdm2 was detected only in tumors from patients treated at MSKCC (N = 50, 70.4 %; p < 0.0001). Additionally, the Ki67 index was strikingly lower in cases from YCU (median, 10; range, 0–65) compared with FALP (median, 30; range, 1–70) and MSKCC (median, 31; range, 1–94), although this difference did not reach significance (p = 0.07). There were no location-related differences in the staining patterns of the other proteins analyzed (Table 2).
TABLE 2.
Immunohistochemistry results
| Total N = 117 (%) |
FALP N = 22 (%) |
YCU N = 19 (%) |
MSKCC N = 76 (%) |
p | |
|---|---|---|---|---|---|
| BCL2 (n = 103) | 0.3 | ||||
| Negative | 95 (92.2) | 16 (100) | 16 (94.1) | 63 (90) | |
| Positive | 8 (7.8) | 0 | 1 (5.9) | 7(10) | |
| p53 (n = 104) | 0.5 | ||||
| Negative | 75 (72.1) | 12 (75) | 14 (82.4) | 49 (69) | |
| Positive | 29 (27.9) | 4 (25) | 3 (17.6) | 22 (31) | |
| p21 (n = 104) | 0.03 | ||||
| Negative | 80 (76.9) | 12 (80) | 17 (100) | 51 (70.8) | |
| Positive | 24 (23.1) | 3 (20) | 0 | 21 (29.2) | |
| p27 (n = 101) | 0.047 | ||||
| Negative | 70 (69.3) | 6 (42.9) | 14 (82.4) | 50 (71.4) | |
| Positive | 31 (30.7) | 8 (57.1) | 3 (17.6) | 20 (28.6) | |
| CD1 (n = 102) | 0.1 | ||||
| Negative | 64 (62.7) | 7 (46.7) | 14 (82.4) | 43 (61.4) | |
| Positive | 38 (37.3) | 8 (53.3) | 3 (17.6) | 27 (38.6) | |
| Mdm2 (n = 104) | <0.0001 | ||||
| Negative | 54 (51.9) | 16 (100) | 17 (100) | 21 (29.6) | |
| Positive | 50 (48.1) | 0 | 0 | 50 (70.4) | |
| VEGF (n = 102) | 0.5 | ||||
| Negative | 36 (35.3) | 6 (37.5) | 4 (23.5) | 26 (37.7) | |
| Positive | 66 (64.7) | 10 (62.5) | 13 (76.5) | 43 (62.3) | |
| VEGFR2 (n = 102) | 0.018 | ||||
| Negative | 97 (95.1) | 13 (81.3) | 17 (100) | 67 (97.1) | |
| Positive | 5 (4.9) | 3 (18.8) | 0 | 2 (2.9) | |
| PTEN (n = 88) | 0.7 | ||||
| Lost | 20 (19.4) | 3 (18.8) | 2 (11.8) | 15 (21.4) | |
| Present | 68 (66) | 12 (75) | 12 (70.6) | 44 (62.9) | |
| Noninterpretable | 15 (14.6) | 1 (6.2) | 3 (17.6) | 11 (15.7) | |
| P-S6 (n = 98) | 0.1 | ||||
| Negative | 66 (67.3) | 7 (46.7) | 12 (70.6) | 47 (71.2) | |
| Positive | 32 (32.7) | 8 (53.3) | 5 (29.4) | 19 (28.8) | |
| Ki 67 (n = 100) | 0.07 | ||||
| Median (range) | 23.5 (0–94) | 30 (1–70) | 10 (0–65) | 31 (1–94) | |
| Mean ± SD | 31.3 ± 25.9 | 28 ± 27 | 20 ± 22 | 35 ± 26 | |
| CD31 (n = 99) | 0.4 | ||||
| Median (range) | 17 (0–48) | 19 (5–46) | 19 (0–33) | 16 (0–48) | |
| Mean ± SD | 17.7 ± 10.3 | 20.7 ± 9.8 | 18 ± 9.5 | 17 ± 10.5 | |
| CD34 (n = 100) | 0.1 | ||||
| Median (range) | 17 (0–54) | 22 (10–36) | 21 (0–47) | 15 (0–57) | |
| Mean ± SD | 19 ± 11.5 | 22.3 ± 7.8 | 22 ± 13 | 17.5 ± 11.5 |
Protein Expression and Clinicopathologic Variables
PTEN was more commonly expressed in patients with IGBCA (p = 0.03) and in the absence of metastases (p = 0.04). Similarly, phospho-S6 ribosomal protein was more often expressed in patients with IGBCA (p = 0.04), T2 tumors (p = 0.009), and absence of perineural invasion (p = 0.02). p21 expression correlated with CD1 (p < 0.001) and Mdm2 (p = 0.01) expression. Ki-67 index correlated with expression of Mdm2 (p = 0.04), PTEN (p = 0.01), and phospho-S6 ribosomal protein (p = 0.04); CD34-positive microvessel count was associated with expression of phospho-S6 ribosomal protein (p = 0.002).
Hierarchical Clustering
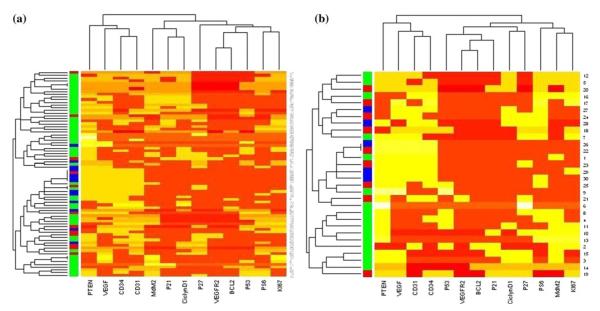
Notable differences in protein expression were observed among the groups when all patients (excluding 2 with neuroendocrine tumors) were analyzed; partition 1 included a significantly higher number of MSKCC patients (N = 30, 83.3 %) compared with partition 2 (N = 21, 52.5 %), and the majority of YCU patients (N = 10, 77 %) were located in partition 2 (p = 0.017). Most FALP patients (N = 9, 75 %) also were mainly located in partition 2 (Table 3; Fig. 1a).
TABLE 3.
Hierarchical clustering partitions
| Total N (%) | FALP N (%) | YCU N (%) | MSKCC N (%) | p * | |
|---|---|---|---|---|---|
| A) Center | |||||
| All patients | 0.017 | ||||
| Partition 1 | 36 | 3 (8.3) | 3 (8.3) | 30 (83.3) | |
| Partition 2 | 40 | 9 (22.5) | 10 (25) | 21 (52.5) | |
| R0-resection | 0.546 | ||||
| Partition 1 | 29 | 3 (10.3) | 10 (34.5) | 16 (55.2) | |
| Partition 2 | 18 | 2(11.1) | 1 (5.6) | 15 (83.3) | |
| B) T stage | |||||
| T1 + 2 | 0.017 | ||||
| Partition 1 | 20 | 8 (40) | 5 (25) | 7 (35) | |
| Partition 2 | 10 | 1 (10) | 0 | 9 (90) | |
| T3 + 4 | 0.859 | ||||
| Partition 1 | 29 | 2 (6.9) | 6 (20.7) | 21 (72.4) | |
| Partition 2 | 15 | 1 (6.7) | 2 (13.3) | 12 (80) |
Fisher’s exact test
FIG. 1.
Expression profile of all patients (a) and patients with T1/T2 GBCA (b). Each row represents 1 patient; columns indicate the immunostaining results (positive = yellow; negative = red). Center of origin is indicated in the shaded column (red FALP, blue YCU, green MSKCC). The column dendrogram indicates patterns of protein clustering, and the row dendrogram indicates clustering by center
To better assess for possible site-specific differences in pathogenesis, the staining patterns in patients with early lesions were compared with those with more advanced tumors (T1/T2 vs. T3/T4). Early T-stage tumors showed significant differences, with partition 1 including an equal distribution of patients treated at FALP (N = 8, 40 %), MSKCC (N = 7, 35 %), and YCU (N = 5, 25 %) compared with partition 2 where almost all patients (N = 9, 90 %) were treated at MSKCC and did not include patients treated at YCU (p = 0.017; Table 3; Fig. 1b). By contrast, the distribution of patients with advanced disease (T3/T4) did not differ between partitions when center of origin was analyzed (Table 3).
Survival
Median follow-up for all patients (excluding 2 with neuroendocrine tumors) was 14 months (range, 0.4–109) and was similar among centers (p = 0.2). Median DSS was 15.6 months (range, 1–109), with an actuarial DSS of 17.6 % at 5 years, and this was similar among sites (p = 0.6). Median DSS after complete resection (N = 69) was 27 months (range, 2–109; 30 % at 5 years) and was similar among centers: MSKCC (median, 28.1 months; range, 1–108), FALP (median, 21.1 months; range, 5.5–94.2), and YCU (median, 27 months; range, 3–60; p = 0.9).
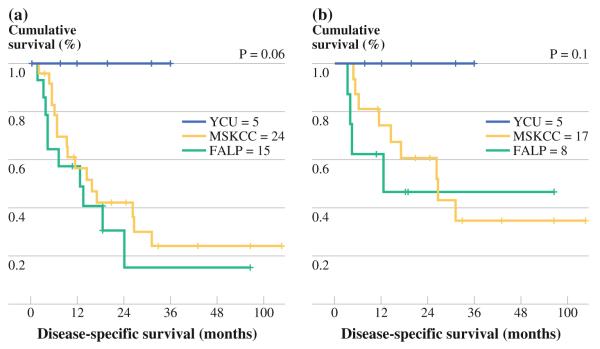
Median DSS of all T1/T2 patients (N = 44) was 28.4 months (95 % confidence interval (CI), 16.9–39.9) with an actuarial DSS of 71.6 % at 1 year and 28 % at 5 years. There was a trend toward significant difference in DSS in YCU patients (not reached) compared with MSKCC (median, 21.6 months) and FALP (median, 19.6 months; p = 0.06). Median DSS of T1/T2 patients treated with R0-resection (N = 30) was 52 months (95 % CI, 38.2–65.9) with an actuarial DSS of 79.4 % at 1 year and 43.1 % at 5 years: MSKCC (median, 37.8 months), FALP (median, 19.6 months), and YCU (not reached; p = 0.1; Fig. 2a, b).
FIG. 2.
a Disease-specific survival (DSS) among all patients with T1/T2 GBCA. b DSS among patients with T1/T2 GBCA treated with R0-resection. Survival analysis did not include two patients with neuroendocrine tumor
Univariate and Multivariate Analysis of DSS
Outcome analysis was limited to R0-resections. Univariate analysis of clinicopathologic variables is shown in Table 4. On multivariate analysis, higher T stage, lymphovascular invasion, and CD1 expression predicted worse survival. When the analysis was limited to T1/T2 tumors, the presence of gallstones was the only predictor of poor outcome (20.1 months with versus not reached without gallstones, p = 0.012). When treatment center was included as a variable, DSS was strikingly better in YCU patients, although this difference did not achieve significance (p = 0.1; Fig. 2b).
TABLE 4.
Uni- and multivariate analyses for clinicopathologic variables associated with DSS
| Variable | Total | Number failed |
1-year survival |
5-year survival |
Median survival |
p log- rank |
p multivariate | Hazard ratio |
95 % CI |
|---|---|---|---|---|---|---|---|---|---|
| Center | 0.9 | ||||||||
| FALP | 8 | 4 | 0.62 | 0.46 | 21.1 | ||||
| YCU | 13 | 7 | 0.69 | 0.35 | 25 | ||||
| MSKCC | 48 | 32 | 0.77 | 0.29 | 28.1 | ||||
| Gender | 0.19 | ||||||||
| Female | 46 | 28 | 0.77 | 0.37 | 28.1 | ||||
| Male | 23 | 15 | 0.66 | 0.09 | 25 | ||||
| Age (*, years) | 0.1 | ||||||||
| ≤68 | 34 | 19 | 0.78 | 0.36 | 43.8 | ||||
| >68 | 35 | 24 | 0.69 | 0.24 | 21.1 | ||||
| Race | 0.9 | ||||||||
| Caucasian | 37 | 25 | 0.76 | 0.29 | 28.2 | ||||
| Hispanic | 11 | 7 | 1 | 0 | 13.2 | ||||
| Asian-Japanese | 13 | 7 | 0.69 | 0.35 | 19 | ||||
| African-American | 1 | 1 | 1 | 0.50 | – | ||||
| Pacific Islander | 1 | 1 | 0.63 | 0.34 | 16.7 | ||||
| Asian-American | 2 | 1 | – | – | – | ||||
| Indian | 1 | 0 | 1 | 0 | 31.8 | ||||
| Unknown | 3 | 1 | 0.74 | 0.28 | 28.4 | ||||
| Gallstones | 0.09 | ||||||||
| Yes | 53 | 36 | 0.69 | 0.25 | 24.1 | ||||
| No | 16 | 7 | 0.87 | 0.52 | – | ||||
| Incidental diagnosis | 0.24 | ||||||||
| Yes | 27 | 17 | 0.84 | 0.32 | 44.3 | ||||
| No | 42 | 26 | 0.67 | 0.33 | 21.1 | ||||
| Hepatic resection | 0.19 | ||||||||
| Yes | 64 | 41 | 0.7 | 0.27 | 24.6 | ||||
| No | 5 | 2 | 1 | 0.66 | 88.8 | ||||
| Bile duct resection | 0.06 | ||||||||
| Yes | 41 | 29 | 0.68 | 0.21 | 19.0 | ||||
| No | 28 | 14 | 0.81 | 0.42 | 44.4 | ||||
| Extra organ resection | 0.7 | ||||||||
| Yes | 10 | 5 | 0.76 | 0.38 | 31.8 | ||||
| No | 56 | 35 | 0.74 | 0.30 | 28.1 | ||||
| Histology | <0.0001 | ||||||||
| Adenocarcinoma | 62 | 37 | 0.79 | 0.32 | 28.1 | ||||
| Adenosquamous | 5 | 4 | 0.40 | 0.20 | 11.8 | ||||
| Squamous | 1 | 1 | 0 | 0 | 4.7 | ||||
| Grade | 0.25 | ||||||||
| Well | 13 | 6 | 0.76 | 0.44 | 43.8 | ||||
| Moderate | 29 | 17 | 0.81 | 0.34 | 25 | ||||
| Poor | 27 | 20 | 0.64 | 0.19 | 17.7 | ||||
| T stage | 0.009 | 0.02 | 0.37 | 0.16–0.88 | |||||
| T1 + T2 | 30 | 13 | 0.79 | 0.43 | 52 | ||||
| T3 + T4 | 39 | 30 | 0.69 | 0.20 | 16.7 | ||||
| N stage | 0.48 | ||||||||
| Positive | 38 | 24 | 0.72 | 0.32 | 24.1 | ||||
| Negative | 30 | 18 | 0.75 | 0.30 | 31.8 | ||||
| M stage | 0.23 | ||||||||
| Yes | 12 | 10 | 0.58 | 0.25 | 17.2 | ||||
| No | 57 | 33 | 0.77 | 0.31 | 28.4 | ||||
| TNM stage | 0.17 | ||||||||
| 1 + 2 | 56 | 32 | 0.77 | 0.32 | 28.4 | ||||
| 3 + 4 | 13 | 11 | 0.61 | 0.23 | 19 | ||||
| Lymphovascular invasion |
0.04 | 0.01 | 0.38 | 0.17–0.82 | |||||
| Yes | 30 | 24 | 0.58 | 0.09 | 24.1 | ||||
| No | 26 | 14 | 0.91 | 0.42 | 31.8 | ||||
| Perineural invasion | 0.05 | ||||||||
| Yes | 31 | 23 | 0.65 | 0.15 | 19.0 | ||||
| No | 21 | 11 | 0.85 | 0.36 | 47.9 | ||||
| Adjuvant treatment | 0.9 | ||||||||
| Yes | 34 | 23 | 79 | 0.29 | 27.0 | ||||
| No | 35 | 20 | 0.68 | 0.31 | 24.6 | ||||
| BCL2 | 0.37 | ||||||||
| Positive | 5 | 3 | 0.8 | 0.6 | 67.9 | ||||
| Negative | 57 | 37 | 0.71 | 0.26 | 24.1 | ||||
| p53 | 0.92 | ||||||||
| Positive | 16 | 12 | 0.73 | 0.2 | 28.4 | ||||
| Negative | 47 | 29 | 0.73 | 0.33 | 19.1 | ||||
| p21 | 0.47 | ||||||||
| Positive | 11 | 8 | 0.63 | 0.27 | 13.3 | ||||
| Negative | 51 | 33 | 0.75 | 0.28 | 27 | ||||
| p27 | 0.13 | ||||||||
| Positive | 21 | 15 | 0.61 | 0.13 | 21.1 | ||||
| Negative | 40 | 25 | 0.78 | 0.34 | 28.1 | ||||
| Cyclin D1 | 0.01 | 0.03 | 2.57 | 1.07–6.1 | |||||
| Positive | 19 | 8 | 0.89 | 0.41 | 52 | ||||
| Negative | 43 | 32 | 0.65 | 0.22 | 19 | ||||
| Mdm2 | 0.2 | ||||||||
| Positive | 29 | 17 | 0.84 | 0.3 | 28.4 | ||||
| Negative | 34 | 24 | 0.63 | 0.27 | 21.1 | ||||
| VEGF | 0.5 | ||||||||
| Positive | 40 | 26 | 0.76 | 0.33 | 27 | ||||
| Negative | 20 | 13 | 0.61 | 0.22 | 13.3 | ||||
| PTEN | 0.16 | ||||||||
| Lost | 13 | 10 | 0.69 | 0.18 | 17.7 | ||||
| Present | 40 | 22 | 0.75 | 0.35 | 42.1 | ||||
| PS6 | 0.004 | ||||||||
| Positive | 16 | 4 | 0.79 | 0.68 | – | ||||
| Negative | 45 | 36 | 0.69 | 0.17 | 19.1 |
Dichotomized around the median (68; range, 28–86)
DISCUSSION
Gallbladder cancer is a rare and enigmatic disease with the highest incidence in India and some countries of South America and Asia, such as Chile and Japan, respectively.1,2,33 By contrast, the United States is a low-incidence area, although Native Americans of the Southwest have a high risk.34 The dysplasia-carcinoma sequence, related to chronic inflammation, and adenoma-carcinoma progression, after development of a polyp, are two distinct pathways associated with GBCA.11,19 It has been suggested that local factors may be linked with this typical demographic distribution. Specifically, a strong association between gallstones and GBCA has been found in Chile and in high-risk areas of the United States, whereas other risk factors appear uncommon.4 Also, while pancreaticobiliary maljunction is rarely reported in Chile or the United States, it is an important GBCA risk factor in Japan.6,13,35
We recently reported several differences in clinical and pathological variables in patients treated at three centers in the United States, Japan, and Chile.12 However, these data may not necessarily represent definitive evidence of differences between centers or countries of origin, and molecular studies are necessary to validate these disparities. The molecular markers used in this analysis were chosen to evaluate pathways possibly related with the pathogenesis of GBCA and to facilitate comparison among centers of origin.
The present study found several differences among the centers when expression of specific molecular markers and their pathways were compared. In particular, cell cycle-regulatory proteins, specifically expression of p21, p27, and Mdm2, were significantly different in Japanese patients; indeed, neither p21, nor Mdm2 were expressed in any patient in this subgroup, and immunohistochemistry detection of p53 also was lower. Mutations of TP53 gene and accumulation of its protein product lead to increased expression of p21 and CD1.36,37 Alternatively, wild-type p53 can be inhibited through direct interaction with the Mdm2 oncoprotein. Thus, for the Japanese patients in the present study, inactivation of p53—either directly or indirectly—was an uncommon pathogenetic event compared with patients from Chile and the United States. These disparities also may explain the clinical differences among the groups and the final survival rates. Patients treated at YCU had a significantly lower incidence of tumors associated with cholelithiasis but had more tumors arise from polyps. Indeed, Japanese patients had a higher incidence of well-differentiated tumors. Site-specific differences in angiogenesis-related and PIK3 pathway markers were less striking.38,39 Activation of the PI3K pathway is associated with aggressive tumor biology. Loss of the tumor suppressor PTEN, which is associated with PI3K activation, increased cell proliferation, and reduced apoptosis, was lowest in YCU patients and nearly 50 % lower compared with MSKCC.40 Overexpression of p-S6, a marker of AKT activation, was seen in one-third of patients, more than 50 % of FALP patients, compared with less than 30 % of patients from YCU and MSKCC.41 Although neither marker was significantly different with respect to treatment center, p-S6 was significantly associated with earlier T-stage tumors, suggesting that PI3K pathway activation is a proximal pathogenetic change.
The clustering analysis accounts for variations in protein expression across treatment centers, and the results suggest the possibility of different disease biology among Japanese patients in this study. When all patients were included, two groups emerged: the first partition included more than 80 % of patients treated at MSKCC, whereas the second included a variable proportion from three centers, but YCU patients were almost exclusively located in this second group. These differences persisted when the analyses were repeated in patients with early lesions, where one would expect any such differences would be more evident. The survival analysis, suggesting better DSS in patients with early GBCA (T1/T2) treated at YCU, and the markedly lower Ki67 labeling index in the entire Japanese cohort compared with the other two sites, both support this argument.
This analysis has several limitations, not the least of which is the small sample size, even though large in comparison to other published studies of this type. Furthermore, although several differences were identified in YCU patients, suggesting differences in pathogenesis and outcome compared with other sites, it cannot be assumed that they will extend to and reflect each center’s respective national population. Whereas this may be true, the possibility that the differences are related to regional differences within each country must be considered. Definitive conclusions must await confirmation in larger series.
In summary, the findings of this study suggest regional differences in GBCA pathogenesis. Differences in tumor biology were suggested when patients with early tumors treated in Japan and the United States were compared. These disparities are supported by differential clustering based on molecular marker expression and the suggestion of a survival difference in patients with early tumors. Additionally, inactivation of p53 was uncommon in Japanese patients.
Footnotes
Disclosures None.
REFERENCES
- 1.Andia ME, Hsing AW, Andreotti G, Ferreccio C. Geographic variation of gallbladder cancer mortality and risk factors in Chile: a population-based ecologic study. Int J Cancer. 2008;123(6):1411–6. doi: 10.1002/ijc.23662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hariharan D, Saied A, Kocher HM. Analysis of mortality rates for gallbladder cancer across the world. HPB. 2008;10(5):327–31. doi: 10.1080/13651820802007464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kumar JR, Tewari M, Rai A, Sinha R, Mohapatra SC, Shukla HS. An objective assessment of demography of gallbladder cancer. J Surg Oncol. 2006;93(8):610–4. doi: 10.1002/jso.20526. [DOI] [PubMed] [Google Scholar]
- 4.Lazcano-Ponce EC, Miquel JF, Munoz N, et al. Epidemiology and molecular pathology of gallbladder cancer. CA Cancer J Clin. 2001;51(6):349–64. doi: 10.3322/canjclin.51.6.349. [DOI] [PubMed] [Google Scholar]
- 5.Dutta U, Nagi B, Garg PK, Sinha SK, Singh K, Tandon RK. Patients with gallstones develop gallbladder cancer at an earlier age. Eur J Cancer Prev. 2005;14(4):381–5. doi: 10.1097/00008469-200508000-00011. [DOI] [PubMed] [Google Scholar]
- 6.Elnemr A, Ohta T, Kayahara M, et al. Anomalous pancreaticobiliary ductal junction without bile duct dilatation in gallbladder cancer. Hepatogastroenterology. 2001;48(38):382–6. [PubMed] [Google Scholar]
- 7.Kayahara M, Nagakawa T. Recent trends of gallbladder cancer in Japan: an analysis of 4,770 patients. Cancer. 2007;110(3):572–80. doi: 10.1002/cncr.22815. [DOI] [PubMed] [Google Scholar]
- 8.Tewari M, Kumar V, Mishra RR, Kumar M, Shukla HS. Is there a role for cholecystectomy in gallbladder carcinoma discovered to be unresectable for cure at laparotomy? World J Surg. 2008;32(12):2683–7. doi: 10.1007/s00268-008-9763-x. [DOI] [PubMed] [Google Scholar]
- 9.Wistuba II, Miquel JF, Gazdar AF, Albores-Saavedra J. Gall-bladder adenomas have molecular abnormalities different from those present in gallbladder carcinomas. Hum Pathol. 1999;30(1):21–5. doi: 10.1016/s0046-8177(99)90295-2. [DOI] [PubMed] [Google Scholar]
- 10.Moreno M, Pimentel F, Gazdar AF, Wistuba II, Miquel JF. TP53 abnormalities are frequent and early events in the sequential pathogenesis of gallbladder carcinoma. Ann Hepatol. 2005;4(3):192–9. [PubMed] [Google Scholar]
- 11.Pai RK, Mojtahed K. Mutations in the RAS/RAF/MAP kinase pathway commonly occur in gallbladder adenomas but are uncommon in gallbladder adenocarcinomas. Appl Immunohistochem Mol Morphol. 2011;19(2):133–40. doi: 10.1097/PAI.0b013e3181f09179. [DOI] [PubMed] [Google Scholar]
- 12.Butte JM, Matsuo K, Gonen M, et al. Gallbladder cancer: differences in presentation, surgical treatment, and survival in patients treated at centers in three countries. J Am Coll Surg. 2011;212(1):50–61. doi: 10.1016/j.jamcollsurg.2010.09.009. [DOI] [PubMed] [Google Scholar]
- 13.Nakayama K, Konno M, Kanzaki A, et al. Allelotype analysis of gallbladder carcinoma associated with anomalous junction of pancreaticobiliary duct. Cancer Lett. 2001;166(2):135–41. doi: 10.1016/s0304-3835(01)00436-0. [DOI] [PubMed] [Google Scholar]
- 14.Hanada K, Tsuchida A, Iwao T, et al. Gene mutations of K-ras in gallbladder mucosae and gallbladder carcinoma with an anomalous junction of the pancreaticobiliary duct. Am J Gastroenterol. 1999;94(6):1638–42. doi: 10.1111/j.1572-0241.1999.01155.x. [DOI] [PubMed] [Google Scholar]
- 15.Hanada K, Tsuchida A, Kajiyama G. Cellular kinetics and gene mutations in gallbladder mucosa with an anomalous junction of pancreaticobiliary duct. J Hepatobiliary Pancreat Surg. 1999;6(3):223–8. doi: 10.1007/s005340050111. [DOI] [PubMed] [Google Scholar]
- 16.Wistuba II, Gazdar AF, Roa I, Albores-Saavedra J. p53 protein overexpression in gallbladder carcinoma and its precursor lesions: an immunohistochemical study. Hum Pathol. 1996;27(4):360–5. doi: 10.1016/s0046-8177(96)90109-4. [DOI] [PubMed] [Google Scholar]
- 17.Yokoyama N, Hitomi J, Watanabe H, et al. Mutations of p53 in gallbladder carcinomas in high-incidence areas of Japan and Chile. Cancer Epidemiol Biomarkers Prev. 1998;7(4):297–301. [PubMed] [Google Scholar]
- 18.Hanada K, Itoh M, Fujii K, et al. TP53 mutations in stage I gallbladder carcinoma with special attention to growth patterns. Eur J Cancer. 1997;33(7):1136–40. doi: 10.1016/s0959-8049(97)00080-4. [DOI] [PubMed] [Google Scholar]
- 19.Goldin RD, Roa JC. Gallbladder cancer: a morphological and molecular update. Histopathology. 2009;55(2):218–29. doi: 10.1111/j.1365-2559.2008.03192.x. [DOI] [PubMed] [Google Scholar]
- 20.Harino Y, Imura S, Kanemura H, et al. Role of tumor angiogenesis in gallbladder carcinoma: with special reference to thymidine phosphorylase. Int J Clin Oncol. 2008;13(5):452–7. doi: 10.1007/s10147-008-0778-y. [DOI] [PubMed] [Google Scholar]
- 21.Jarnagin WR, Klimstra DS, Hezel M, et al. Differential cell cycle-regulatory protein expression in biliary tract adenocarcinoma: correlation with anatomic site, pathologic variables, and clinical outcome. J Clin Oncol. 2006;24(7):1152–60. doi: 10.1200/JCO.2005.04.6631. [DOI] [PubMed] [Google Scholar]
- 22.Deshpande V, Nduaguba A, Zimmerman SM, et al. Mutational profiling reveals PIK3CA mutations in gallbladder carcinoma. BMC Cancer. 2011;11:60. doi: 10.1186/1471-2407-11-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Butte JM, Gonen M, Allen PJ, et al. The role of laparoscopic staging in patients with incidental gallbladder cancer. HPB. 2011;13(7):463–72. doi: 10.1111/j.1477-2574.2011.00325.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.D’Angelica M, Dalal KM, DeMatteo RP, Fong Y, Blumgart LH, Jarnagin WR. Analysis of the extent of resection for adenocarcinoma of the gallbladder. Ann Surg Oncol. 2009;16(4):806–16. doi: 10.1245/s10434-008-0189-3. [DOI] [PubMed] [Google Scholar]
- 25.AJCC Cancer Staging Handbook. 7 ed American Joint Committee on Cancer; Chicago: 2010. [Google Scholar]
- 26.Sakr RA, Barbashina V, Morrogh M, et al. Protocol for PTEN expression by immunohistochemistry in formalin-fixed paraffin-embedded human breast carcinoma. Appl Immunohistochem Mol Morphol. 2010;18(4):371–4. doi: 10.1097/PAI.0b013e3181d50bd5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Della Torre G, Pasquini G, Pilotti S, et al. TP53 mutations and mdm2 protein overexpression in cholangiocarcinomas. Diagn Mol Pathol. 2000;9(1):41–6. doi: 10.1097/00019606-200003000-00007. [DOI] [PubMed] [Google Scholar]
- 28.Furubo S, Harada K, Shimonishi T, Katayanagi K, Tsui W, Nakanuma Y. Protein expression and genetic alterations of p53 and ras in intrahepatic cholangiocarcinoma. Histopathology. 1999;35(3):230–40. doi: 10.1046/j.1365-2559.1999.00705.x. [DOI] [PubMed] [Google Scholar]
- 29.Ito Y, Takeda T, Sasaki Y, et al. Bcl-2 expression in cholangiocellular carcinoma is inversely correlated with biologically aggressive phenotypes. Oncology. 2000;59(1):63–7. doi: 10.1159/000012139. [DOI] [PubMed] [Google Scholar]
- 30.Ito Y, Takeda T, Sasaki Y, et al. Expression and clinical significance of the G1-S modulators in intrahepatic cholangiocellular carcinoma. Oncology. 2001;60(3):242–51. doi: 10.1159/000055325. [DOI] [PubMed] [Google Scholar]
- 31.Tenjo T, Toyoda M, Okuda J, et al. Prognostic significance of p27(kip1) protein expression and spontaneous apoptosis in patients with colorectal adenocarcinomas. Oncology. 2000;58(1):45–51. doi: 10.1159/000012078. [DOI] [PubMed] [Google Scholar]
- 32.Wiksten JP, Lundin J, Nordling S, Kokkola A, von Boguslawski K, Haglund C. The prognostic value of p27 in gastric cancer. Oncology. 2002;63(2):180–4. doi: 10.1159/000063813. [DOI] [PubMed] [Google Scholar]
- 33.Randi G, Franceschi S, La Vecchia C. Gallbladder cancer worldwide: geographical distribution and risk factors. Int Cancer. 2006;118(7):1591–602. doi: 10.1002/ijc.21683. [DOI] [PubMed] [Google Scholar]
- 34.Lemrow SM, Perdue DG, Stewart SL, et al. Gallbladder cancer incidence among American Indians and Alaska Natives, US, 1999-2004. Cancer. 2008;113(5 Suppl):1266–73. doi: 10.1002/cncr.23737. [DOI] [PubMed] [Google Scholar]
- 35.Mori K, Nagakawa T, Ohta T, et al. Association between gall-bladder cancer and anomalous union of the pancreaticobiliary ductal system. Hepatogastroenterology. 1993;40(1):56–60. [PubMed] [Google Scholar]
- 36.Schafer KA. The cell cycle: a review. Vet Pathol. 1998;35(6):461–78. doi: 10.1177/030098589803500601. [DOI] [PubMed] [Google Scholar]
- 37.Vermeulen K, Van Bockstaele DR, Berneman ZN. The cell cycle: a review of regulation, deregulation and therapeutic targets in cancer. Cell Prolif. 2003;36(3):131–49. doi: 10.1046/j.1365-2184.2003.00266.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.da Rocha AO, Coutinho LM, Schall JG. The prognostic value of angiogenesis by Chalkley counting in gallbladder carcinoma. Hepatogastroenterology. 2009;56(89):34–8. [PubMed] [Google Scholar]
- 39.Conde E, Angulo B, Tang M, et al. Molecular context of the EGFR mutations: evidence for the activation of mTOR/S6K signaling. Clin Cancer Res. 2006;12(3 Pt 1):710–7. doi: 10.1158/1078-0432.CCR-05-1362. [DOI] [PubMed] [Google Scholar]
- 40.Yin Y, Shen WH. PTEN: a new guardian of the genome. Oncogene. 2008;27(41):5443–53. doi: 10.1038/onc.2008.241. [DOI] [PubMed] [Google Scholar]
- 41.Baba Y, Nosho K, Shima K, et al. Phosphorylated AKT expression is associated with PIK3CA mutation, low stage, and favorable outcome in 717 colorectal cancers. Cancer. 2011;117(7):1399–408. doi: 10.1002/cncr.25630. [DOI] [PMC free article] [PubMed] [Google Scholar]