Abstract
Objective: A range of immune system abnormalities have been associated with schizophrenia. Probiotic compounds modulate the immune response and offer a potential treatment strategy for schizophrenia. Probiotic compounds have also been observed to improve gastrointestinal dysfunction, which is a common problem in individuals with schizophrenia. We performed a randomized, double-blind, placebo-controlled trial to examine whether probiotic supplementation can reduce symptom severity in patients with schizophrenia receiving antipsychotic treatment and also whether probiotics are associated with bowel functioning.
Methods: Outpatients with schizophrenia (N = 65) meeting DSM-IV criteria and with at least moderately severe psychotic symptoms were enrolled in the study from December 2010–August 2012. Following a 2-week placebo run-in period, patients were randomly assigned to 14 weeks of double-blind adjunctive probiotic (combined Lactobacillus rhamnosus strain GG and Bifidobacterium animalis subsp. lactis strain Bb12) or placebo therapy. Psychiatric symptoms were assessed biweekly with the Positive and Negative Syndrome Scale (PANSS), and patients were queried weekly about their gastrointestinal functioning.
Results: Repeated-measures analysis of variance showed no significant differences in the PANSS total score between probiotic and placebo supplementation (F = 1.28, P = .25). However, patients in the probiotic group were less likely to develop severe bowel difficulty over the course of the trial (hazard ratio = 0.23; 95% CI, 0.09–0.61, P = .003).
Conclusions: Probiotic supplementation may help prevent a common somatic symptom associated with schizophrenia.
Trial Registration: ClinicalTrials.gov identifier: NCT01242371
Clinical Points
⊠ Probiotic supplementation is acceptable and well-tolerated in patients with schizophrenia.
⊠ Probiotic supplementation may help to prevent severe bowel difficulty in patients with schizophrenia.
⊠ Clinicians may consider probiotic supplementation for patients with schizophrenia to address gastrointestinal symptoms.
Probiotic microorganisms are nonpathogenic anaerobic bacteria such as Lactobacilli and Bifidobacteria, which are found in breast milk and have been shown to modulate the immune response and yield a health benefit.1–3 This clinical trial was undertaken to test the hypothesis that symptoms of schizophrenia may be reduced by the administration of probiotic microorganisms when used in addition to antipsychotic medications. The proposed mechanism of action of the probiotic compounds in individuals with schizophrenia is to modulate the inflammatory response,3,4 which has been found to be abnormally activated in many individuals with schizophrenia.5
The principle of probiotics is based on the fact that the immune response to external antigens is determined in part by the composition of the bacterial flora of the intestinal tract. Previous studies have indicated that individuals whose intestinal tract contains potentially pathogenic anaerobic bacteria such as Bacteroides fragilis and aerobic bacteria such as Escherichia coli are more likely to develop aberrant immune responses than individuals whose intestinal tract consists largely of nonpathogenic anaerobic bacteria such as lactic acid–producing bacteria (Lactobacilli) and nonmotile gram-positive bacteria known as Bifidobacteria.6 Since few adults living in the United States have an intestinal flora that contains predominantly Lactobacilli or Bifidobacteria, probiotic therapies have been devised that attempt to alter intestinal flora by the feeding of these organisms.7–9
Most probiotic products contain bacteria from the genera Lactobacillus or Bifidobacterium. The most commonly used organisms are Lactobacillus rhamnosus strain GG and a Bifidobacteria lactis strain Bb12. These microorganisms are similar to those found in breast milk and may contribute to the healthier status of infants who were breast fed compared to infants who were not breastfed.10 Probiotic microorganisms are also found in fermented dairy products such as yogurt as well as in several other fermented food products such as sausages. However, the concentration of probiotic organisms in these foods is not consistent, and the organisms are subject to degradation during food processing and storage.
Probiotic compounds have been reported to have positive effects on digestion and on gastrointestinal disorders such as chronic constipation,11,12 diarrhea,13,14 and celiac disease.15,16 Although the mechanism by which probiotic microorganisms achieve their beneficial effects is not known with certainty, it is likely that they alter the balance of the gut microbiota and prevent the aberrant immune response to food-derived and other harmful antigens.3 Since elevated levels of antibodies to food antigens are also found in individuals with schizophrenia,17 we postulate that probiotic microorganisms may also have beneficial effects for persons with this disorder.
Another reason to consider probiotic supplementation in individuals with schizophrenia is that gastrointestinal symptoms, particularly constipation, are highly prevalent in this population.18–20 Constipation is observed in as many as 50% of patients with schizophrenia and may have serious consequences.21 Patients taking clozapine are observed to be the most severely affected, but other antipsychotic medications are also associated with this side effect.19,21 Constipation may be further exacerbated by the lack of exercise and poor diet found in many individuals with schizophrenia.19,20 Probiotic supplementation has been found to be helpful for gastrointestinal symptoms in other populations11,12,16 but has not been studied in schizophrenia.
The primary aim of the current study was to evaluate the safety and efficacy of a supplemental probiotic therapy containing the microorganisms Lactobacillus rhamnosus strain GG and Bifidobacterium lactis strain Bb12 for individuals with schizophrenia who have residual psychotic symptoms of at least moderate severity. The secondary aim of the study was to assess the effect of probiotic treatment on patients’ gastrointestinal functioning.
METHOD
Participants
Patients were enrolled from psychiatric rehabilitation programs in the Baltimore, Maryland, area who met the following inclusion criteria: age 18–65 years; capacity for written informed consent; primary Axis I diagnosis (DSM-IV) of schizophrenia, any type, or schizoaffective disorder; an outpatient at the time of enrollment; residual psychotic symptoms that were at least moderately severe as evidenced by Positive and Negative Syndrome Scale (PANSS)22 positive symptom scores ≥ 1 and/or PANSS negative symptom scores ≥ 4 or a total PANSS score ≥ 50, containing at least 3 positive or negative items with scores ≥ 3 at screening; conformance to the Schizophrenia Patient Outcomes Research Team treatment recommendation about maintenance antipsychotic medication23; receipt of antipsychotic medication for at least 8 weeks prior to starting the study with no medication changes within the previous 21 days; and proficient in the English language.
Exclusion criteria were diagnosis of mental retardation; any clinically significant or unstable medical disorder as determined by the investigators, including congestive heart failure, abnormal liver function or disease, renal failure, acute pancreatitis, any diagnosis of cancer undergoing active treatment, HIV infection or other immunodeficiency condition; history of intravenous drug use; primary diagnosis of substance abuse or dependence according to DSM-IV criteria within the last 3 months; participated in any investigational drug trial in the past 30 days; pregnant or planning to become pregnant during the study period; receipt of antibiotic medication within the previous 14 days (as anaerobic organisms residing in the gastrointestinal tract may be minimally affected by antibiotics); and documented celiac disease (such persons should be on a gluten-free diet as this is the standard care).
Study Compound
The active study compound consisted of 1 tablet containing approximately 109 colony-forming units of the probiotic organism Lactobacillus rhamnosus strain GG and 109 colony-forming units of the probiotic organism Bifidobacterium animalis subsp. lactis Bb12. The probiotic microorganisms were obtained from Ferrosan A/S (Søborg, Denmark; Ferrosan A/S now operates as a subsidiary of Pfizer). The product containing the 2 microorganisms is sold commercially in Denmark as “Bifiform Balance.” The probiotic microorganisms were grown in a medium that does not contain casein, lactose, or other milk products or gluten. The probiotic microorganisms were provided as a tablet that can be chewed or swallowed. The control tablets appeared identical to the capsules containing the probiotic microorganisms and were also prepared for use in this study by Ferrosan A/S. Ferrosan A/S provided data to substantiate the composition and specification of the probiotic product. The study compounds were stored in aluminum tubes to maintain their stability. Each participant received a 2 weeks’ supply of the medication that could be stored at room temperature. Participants were asked to take their study drug once per day with a meal or with a snack.
Study Procedures
After a physical and psychiatric evaluation to establish eligibility, each participant was assessed at baseline with the PANSS,22 a standardized measure of psychiatric symptoms for individuals with schizophrenia. After a 2-week placebo run-in, participants were randomly assigned to 14 weeks of the adjunctive probiotic therapy or adjunctive placebo. All participants were maintained on a stable regimen of psychiatric medications prescribed by their treating psychiatrist. Participants were seen weekly during the trial by study staff to assess adherence to the study and also to monitor side effects and gastrointestinal functioning. Psychiatric symptoms were rated every 2 weeks with the PANSS. At each weekly visit, participants were asked to rate the difficulty of moving their bowels over the past week on a 4-point scale from “no difficulty” to “severe difficulty.” Participants were also asked about whether they had experienced any diarrhea and about their use of laxatives.
Written informed consent was obtained from all study participants, and the study was approved by institutional review boards of the Sheppard Pratt Health System and the Johns Hopkins School of Medicine (Baltimore, Maryland). The study was monitored by a Data Safety Monitoring Board. Patients were enrolled and data were collected during the period December 2010–August 2012 (ClinicalTrials.gov identifier: NCT01242371).
Data Analysis
Statistical analysis was performed by repeated-measures analysis of variance of PANSS scores for all participants during the 14 weeks of the randomized phase of the trial, using last observation carried forward for the small amount of missing data. We performed a Kaplan-Meier survival analysis looking at the development of severe bowel difficulties by group as reported by participants over the 14 weeks of the randomized phase of the trial. We also compared the prevalence of diarrhea and the use of laxatives between groups over the course of the trial. Because clozapine is particularly associated with constipation, we examined whether receipt of clozapine contributed to the outcomes. All statistical analyses were performed with STATA version 11 (StataCorp, College Station, Texas).
RESULTS
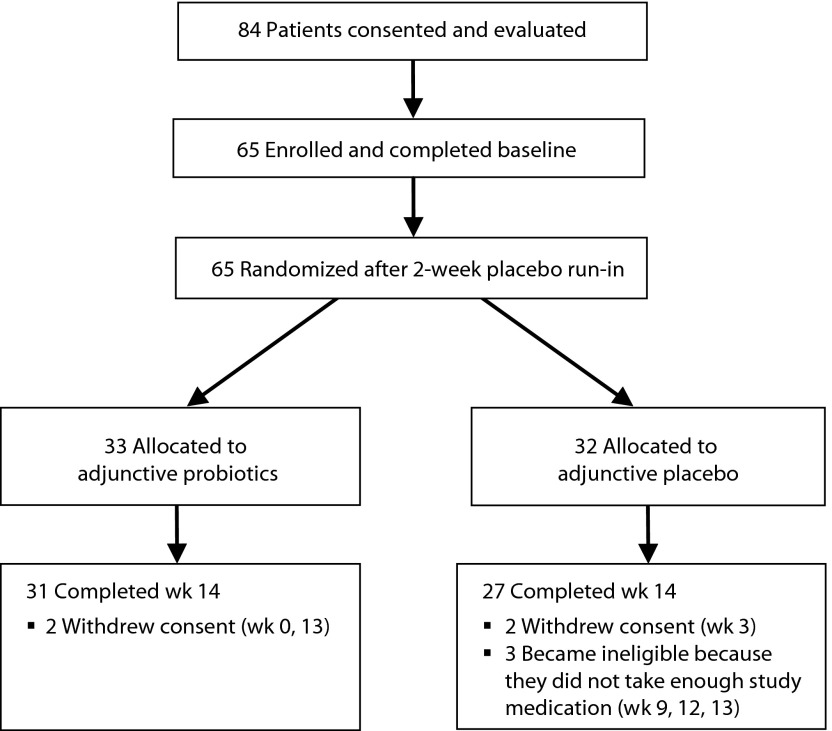
A total of 65 patients were enrolled and randomized to treatment: 33 to adjunctive probiotic therapy and 32 to adjunctive placebo. A total of 58 participants completed the trial, 89% of those randomized. The study flowchart is shown in Figure 1. As shown in Table 1, there were no significant differences in clinical or demographic characteristics between the groups at the beginning of the study.
Figure 1.
Flowchart of the Study
Table 1.
Clinical Characteristics of Trial Participants at Baseline by Group (N = 65)a
| Characteristic | Adjunctive Probiotic (n = 33) | Adjunctive Placebo (n = 32) |
| Age, mean (SD), y | 44.4 (11.0) | 48.1 (9.4) |
| Race (white), n (%) | 17 (51) | 22 (69) |
| Gender (male), n (%) | 23 (70) | 19 (59) |
| Education, mean (SD), y | 12.9 (2.6) | 11.3 (2.5) |
| Maternal education, mean (SD), y | 12.4 (2.3) | 12.7 (2.6) |
| Duration of illness, mean (SD), y | 25.2 (10.8) | 30.2 (8.8) |
| PANSS total symptom score, mean (SD) | 69.9 (11.0) | 72.3 (11.2) |
| PANSS positive score, mean (SD) | 18.2 (4.9) | 18.4 (4.7) |
| PANSS negative score, mean (SD) | 19.4 (3.6) | 20.6 (3.6) |
| PANSS general score, mean (SD) | 32.0 (6.2) | 33.3 (6.3) |
| Receipt of clozapine medication, n (%) | 7 (21) | 10 (31) |
Placebo vs probiotics, all nonsignificant (P ≥ .10).
Abbreviation: PANSS=Positive and Negative Syndrome Scale.
All participants were receiving antipsychotic medication. A total of 17 (26%) received clozapine, 15 (23%) received olanzapine, 15 (23%) received risperidone, 11 (17%) received aripiprazole, 9 (14%) received quetiapine, 7 (11%) received haloperidol, 5 (8%) received ziprasidone, and 1 (2%) received asenapine. The total is greater than 100% because some participants received more than 1 antipsychotic medication. In terms of additional medications, a total of 13 (20%) participants received valproic acid and 23 (36%) received an anticholinergic medication.
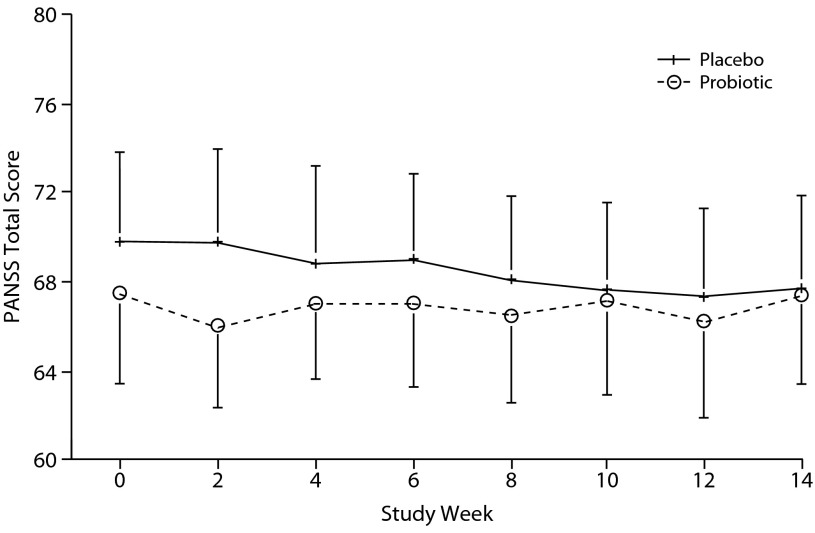
In terms of the primary aim, as shown in Figure 2, there was no significant difference between the groups in PANSS total symptom score over the course of the trial, ie, there was no significant interaction between group and time (F = 1.28, P = .25). There was also no significant interaction between group and time for the PANSS positive, negative, or general scale scores (F = 1.13, P = .90; F = 1.80, P = .08; F = 0.99, P = .44; respectively).
Figure 2.
Mean Total PANSS Scores in the Adjunctive Probiotic and Placebo Groups Over the Course of the Triala,b
aAdjunctive probiotic: n = 33; adjunctive placebo: n = 32.
bRepeated-measures analysis of variance, group × time interaction: F = 1.28, P = .25.
Abbreviation: PANSS=Positive and Negative Syndrome Scale.
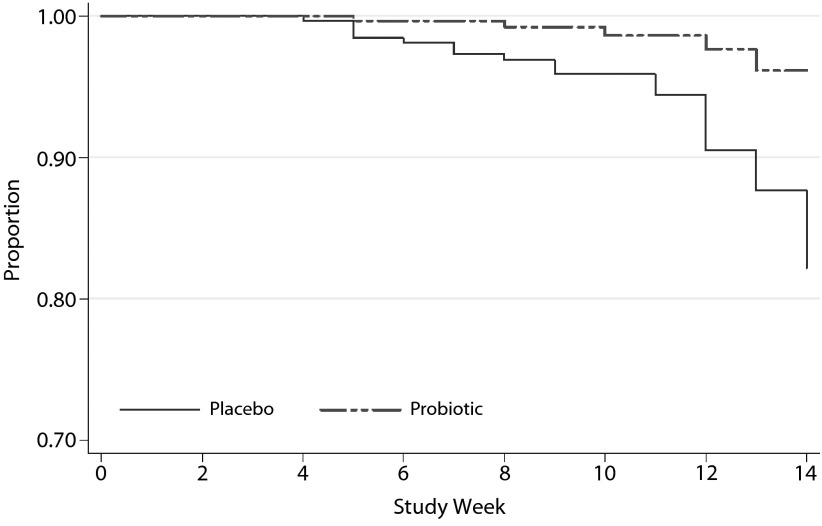
In terms of the secondary aim, we found that the probiotic group was less likely to report severe difficulty moving their bowels over the course of the trial as shown in the Mann-Whitney survival analysis (hazard ratio [HR] = 0.23; 95% CI, 0.09–0.61; P = .003) and in Figure 3.
Figure 3.
Survival Analysis for the Development of Severe Bowel Difficultya,b,c
aThe figure shows the proportion of the sample who did not develop severe bowel difficulty, by group, over the course of the trial.
bAdjunctive probiotic: n = 33; adjunctive placebo: n = 32.
cHazard ratio = 0.23; 95% CI, 0.09–0.61; P = .003.
Clozapine was not associated with the development of severe bowel difficulty over the course of the trial (HR = 0.53; 95% CI, 0.55–2.89; P = .59). There was also no significant interaction between clozapine and probiotic versus placebo supplementation on the development of severe bowel difficulty (HR = 1.07; 95% CI, 0.46–2.47; P = .87).
The probiotic and placebo groups did not differ significantly in the use of laxatives or in the experience of diarrhea (F = 1.05, P = .40; F = 1.07, P = .39; respectively).
Safety and Side Effects
The study was overseen by a Data Safety Monitoring Board that convened at regular intervals throughout the study. There were a total of 4 serious adverse events involving 3 participants during the course of the study: 3 in the probiotic group involving 2 participants and 1 in the placebo group. The 3 serious adverse events in the probiotic group were 2 psychiatric hospitalizations for an increase in psychotic symptoms and 1 medical hospitalization for dehydration. The 1 serious adverse event in the placebo group was a psychiatric hospitalization for an increase in psychotic symptoms.
The probiotic study medication was well-tolerated with no appreciable difference between groups in the number of persons with adverse events that were experienced by at least 5% of the overall sample. A total of 10 participants in the placebo group had an upper respiratory illness in the course of the trial compared with 12 in the probiotic group; 12 participants in each group reported new-onset gastrointestinal symptoms, which included constipation, diarrhea, heartburn, nausea, stomach cramps, and/or flatulence. Within the set of gastrointestinal adverse events, 6 participants in the placebo group and 9 in the probiotic group reported diarrhea. No participant who was allocated to the probiotic group discontinued the study because of a serious or nonserious adverse event.
DISCUSSION
This is the first study to investigate the effect of probiotic supplementation on schizophrenia symptoms and the association with gastrointestinal functioning. In this trial, 65 patients receiving ongoing antipsychotic treatment were randomized to 14 weeks of probiotic or placebo supplementation; 58 participants completed the trial. Although we did not observe a significant difference in psychiatric symptom scores between probiotic- and placebo-supplemented groups, we found that the probiotic group was significantly less likely to develop severe bowel difficulty over the course of the trial. These findings are of importance given the high prevalence of gastrointestinal problems, especially constipation, in this population.
Our findings are consistent with those of other trials in showing that probiotic supplementation may be helpful for gastrointestinal problems.11,12,16 Probiotic supplementation is highly acceptable to patients and generally regarded as safe. Such treatment may provide a helpful strategy to prevent some of the gastrointestinal symptoms associated with schizophrenia and its treatment.
To our knowledge, there are no other published studies of probiotic supplementation for patients with a primary psychiatric disorder. Numerous studies in animal models have shown that manipulations of the intestinal microbiome are associated with changes in brain development and cognition.24,25 In addition, the administration of probiotic organisms such as Bifidobacteria and Lactobacilli have been shown to alter behavior in animal model systems, largely through effects on γ-aminobutyric acid receptor expression.26,27 These studies suggest that probiotic compounds may have a role in treating psychiatric symptoms. While we did not find an effect on psychiatric symptoms in our patients with long-term schizophrenia, introducing probiotic supplementation earlier in the course of illness may be more effective and should be the focus of future clinical investigations.
Limitations of the study include that we did not perform more detailed measures of gastrointestinal functioning or obtain a more complete history of participants’ gastrointestinal symptoms and associated treatment. Further trials of probiotic compounds are indicated in patients with schizophrenia selected for those with bowel difficulty and studied over a longer time period.
Drug names: aripiprazole (Abilify), asenapine (Saphris), clozapine (Clozaril, FazaClo, and others), haloperidol (Haldol and others), olanzapine (Zyprexa), quetiapine (Seroquel), risperidone (Risperdal and others), valproic acid (Depakene, Stavzor, and others), ziprasidone (Geodon and others).
Potential conflicts of interest: Dr Yolken is a member of the Stanley Medical Research Institute Board of Directors and Scientific Advisory Board. The terms of this arrangement are being managed by Johns Hopkins University in accordance with its conflict of interest policies. Drs Dickerson, Goga, and Khushalani and Mss Stallings, Origoni, Katsafanas, Savage, and Schweinfurth report no conflicts of interest related to the subject of this article.
Funding/support: Funded by the Stanley Medical Research Institute, Bethesda, Maryland, grant #09T-1300. Study medication provided by Ferrosan A/S, Søborg, Denmark.
Role of the sponsor: The funding source did not have any role in the design or conduct of the study; collection, management, analysis, or interpretation of data; or in preparation, review, or approval of the manuscript.
References
- 1.Singh V, Singh K, Amdekar S, et al. Innate and specific gut-associated immunity and microbial interference. FEMS Immunol Med Microbiol. 2009;55(1):6–12. doi: 10.1111/j.1574-695X.2008.00497.x. [DOI] [PubMed] [Google Scholar]
- 2.Trebichavsky I, Rada V, Splichalova A, et al. Cross-talk of human gut with bifidobacteria. Nutr Rev. 2009;67(2):77–82. doi: 10.1111/j.1753-4887.2008.00141.x. [DOI] [PubMed] [Google Scholar]
- 3.Dinan TG, Stanton C, Cryan JF. Psychobiotics: a novel class of psychotropic. Biol Psychiatry. 2013;74(10):720–726. doi: 10.1016/j.biopsych.2013.05.001. [DOI] [PubMed] [Google Scholar]
- 4.Bested AC, Logan AC, Selhub EM. Intestinal microbiota, probiotics and mental health: from Metchnikoff to modern advances, part 3: convergence toward clinical trials. Gut Pathog. 2013;5(1):4. doi: 10.1186/1757-4749-5-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Suvisaari J, Mantere O. Inflammation theories in psychotic disorders: a critical review. Infect Disord Drug Targets. 2013;13(1):59–70. doi: 10.2174/18715265112129990032. [DOI] [PubMed] [Google Scholar]
- 6.Altamura AC, Pozzoli S, Fiorentini A, et al. Neurodevelopment and inflammatory patterns in schizophrenia in relation to pathophysiology. Prog Neuropsychopharmacol Biol Psychiatry. 2013;42:63–70. doi: 10.1016/j.pnpbp.2012.08.015. [DOI] [PubMed] [Google Scholar]
- 7.Niers L, Martín R, Rijkers G, et al. The effects of selected probiotic strains on the development of eczema (the PandA study) Allergy. 2009;64(9):1349–1358. doi: 10.1111/j.1398-9995.2009.02021.x. [DOI] [PubMed] [Google Scholar]
- 8.Ramakrishna BS. Probiotic-induced changes in the intestinal epithelium: implications in gastrointestinal disease. Trop Gastroenterol. 2009;30(2):76–85. [PubMed] [Google Scholar]
- 9.Tang ML. Probiotics and prebiotics: immunological and clinical effects in allergic disease. Nestle Nutr Workshop Ser Pediatr Program. 2009;64:219–235. doi: 10.1159/000235793. discussion 235–238, 251–257. [DOI] [PubMed] [Google Scholar]
- 10.Lara-Villoslada F, Olivares M, Sierra S, et al. Beneficial effects of probiotic bacteria isolated from breast milk. Br J Nutr. 2007;98(suppl 1):S96–S100. doi: 10.1017/S0007114507832910. [DOI] [PubMed] [Google Scholar]
- 11.Jayasimhan S, Yap NY, Roest Y, et al. Efficacy of microbial cell preparation in improving chronic constipation: a randomized, double-blind, placebo-controlled trial. Clin Nutr. 2013;32(6):928–934. doi: 10.1016/j.clnu.2013.03.004. [DOI] [PubMed] [Google Scholar]
- 12.Guerra PV, Lima LN, Souza TC, et al. Pediatric functional constipation treatment with Bifidobacterium-containing yogurt: a crossover, double-blind, controlled trial. World J Gastroenterol. 2011;17(34):3916–3921. doi: 10.3748/wjg.v17.i34.3916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Saulnier DM, Kolida S, Gibson GR. Microbiology of the human intestinal tract and approaches for its dietary modulation. Curr Pharm Des. 2009;15(13):1403–1414. doi: 10.2174/138161209788168128. [DOI] [PubMed] [Google Scholar]
- 14.Saavedra JM, Bauman NA, Oung I, et al. Feeding of Bifidobacterium bifidum and Streptococcus thermophilus to infants in hospital for prevention of diarrhea and shedding of rotavirus. Lancet. 1994;344(8929):1046–1049. doi: 10.1016/s0140-6736(94)91708-6. [DOI] [PubMed] [Google Scholar]
- 15.De Angelis M, Rizzello CG, Fasano A, et al. VSL#3 probiotic preparation has the capacity to hydrolyze gliadin polypeptides responsible for celiac sprue. Biochim Biophys Acta. 2006;1762(1):80–93. doi: 10.1016/j.bbadis.2005.09.008. [DOI] [PubMed] [Google Scholar]
- 16.Medina M, De Palma G, Ribes-Koninckx C, et al. Bifidobacterium strains suppress in vitro the pro-inflammatory milieu triggered by the large intestinal microbiota of celiac patients. J Inflamm (Lond) 2008;5(1):19. doi: 10.1186/1476-9255-5-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dickerson F, Stallings C, Origoni A, et al. Markers of gluten sensitivity and celiac disease in recent-onset psychosis and multi-episode schizophrenia. Biol Psychiatry. 2010;68(1):100–104. doi: 10.1016/j.biopsych.2010.03.021. [DOI] [PubMed] [Google Scholar]
- 18.De Hert M, Dockx L, Bernagie C, et al. Prevalence and severity of antipsychotic related constipation in patients with schizophrenia: a retrospective descriptive study. BMC Gastroenterol. 2011;11(1):17. doi: 10.1186/1471-230X-11-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.De Hert M, Hudyana H, Dockx L, et al. Second-generation antipsychotics and constipation: a review of the literature. Eur Psychiatry. 2011;26(1):34–44. doi: 10.1016/j.eurpsy.2010.03.003. [DOI] [PubMed] [Google Scholar]
- 20.Koizumi T, Uchida H, Suzuki T, et al. Oversight of constipation in inpatients with schizophrenia: a cross-sectional study. Gen Hosp Psychiatry. 2013;35(6):649–652. doi: 10.1016/j.genhosppsych.2013.06.007. [DOI] [PubMed] [Google Scholar]
- 21.Hibbard KR, Propst A, Frank DE, et al. Fatalities associated with clozapine-related constipation and bowel obstruction: a literature review and two case reports. Psychosomatics. 2009;50(4):416–419. doi: 10.1176/appi.psy.50.4.416. [DOI] [PubMed] [Google Scholar]
- 22.Kay SR, Fiszbein A, Opler LA. The Positive and Negative Syndrome Scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13(2):261–276. doi: 10.1093/schbul/13.2.261. [DOI] [PubMed] [Google Scholar]
- 23.Buchanan RW, Kreyenbuhl J, Kelly DL, et al. Schizophrenia Patient Outcomes Research Team (PORT). The 2009 schizophrenia PORT psychopharmacological treatment recommendations and summary statements. Schizophr Bull. 2010;36(1):71–93. doi: 10.1093/schbul/sbp116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Diaz Heijtz R, Wang S, Anuar F, et al. Normal gut microbiota modulates brain development and behavior. Proc Natl Acad Sci U S A. 2011;108(7):3047–3052. doi: 10.1073/pnas.1010529108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.O’Mahony SM, Marchesi JR, Scully P, et al. Early life stress alters behavior, immunity, and microbiota in rats: implications for irritable bowel syndrome and psychiatric illnesses. Biol Psychiatry. 2009;65(3):263–267. doi: 10.1016/j.biopsych.2008.06.026. [DOI] [PubMed] [Google Scholar]
- 26.Bravo JA, Forsythe P, Chew MV, et al. Ingestion of Lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve. Proc Natl Acad Sci U S A. 2011;108(38):16050–16055. doi: 10.1073/pnas.1102999108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bercik P, Park AJ, Sinclair D, et al. The anxiolytic effect of Bifidobacterium longum NCC3001 involves vagal pathways for gut-brain communication. Neurogastroenterol Motil. 2011;23(12):1132–1139. doi: 10.1111/j.1365-2982.2011.01796.x. [DOI] [PMC free article] [PubMed] [Google Scholar]