Abstract
The phenazine derivative 2-hydroxyphenazine (2-OH-PHZ) plays an important role in the biocontrol of plant diseases, and exhibits stronger bacteriostatic and fungistatic activity than phenazine-1-carboxylic acid (PCA) toward some pathogens. PhzO has been shown to be responsible for the conversion of PCA to 2-OH-PHZ, however the kinetics of the reaction have not been systematically studied. Further, the yield of 2-OH-PHZ in fermentation culture is quite low and enhancement in our understanding of the reaction kinetics may contribute to improvements in large-scale, high-yield production of 2-OH-PHZ for biological control and other applications. In this study we confirmed previous reports that free PCA is converted to 2-hydroxy-phenazine-1-carboxylic acid (2-OH-PCA) by the action of a single enzyme PhzO, and particularly demonstrate that this reaction is dependent on NADP(H) and Fe3+. Fe3+ enhanced the conversion from PCA to 2-OH-PHZ and 28°C was a optimum temperature for the conversion. However, PCA added in excess to the culture inhibited the production of 2-OH-PHZ. 2-OH-PCA was extracted and purified from the broth, and it was confirmed that the decarboxylation of 2-OH-PCA could occur without the involvement of any enzyme. A kinetic analysis of the conversion of 2-OH-PCA to 2-OH-PHZ in the absence of enzyme and under different temperatures and pHs in vitro, revealed that the conversion followed first-order reaction kinetics. In the fermentation, the concentration of 2-OH-PCA increased to about 90 mg/L within a red precipitate fraction, as compared to 37 mg/L within the supernatant. The results of this study elucidate the reaction kinetics involved in the biosynthesis of 2-OH-PHZ and provide insights into in vitro methods to enhance yields of 2-OH-PHZ.
Introduction
Phenazine compounds are of interest because of their broad spectrum activity against soil born root disease [1], [2]. They encompass a large family of natural heterocyclic nitrogen-containing compounds that are produced in late exponential and stationary growth phase of some strains. Over 100 natural phenazine compounds with the same basic structure are known, differing only in the derivatization of the heterocyclic core. These differences largely determine the physical properties of the phenazines and greatly influence their biological activity toward plant and animal pathogens. Natural phenazine derivatives are synthesized primarily by Pseudomonas spp. and Streptomyces spp. [3], [4], especially Pseudomonas chlororaphis spp. [5], [6], [7]. The most commonly identified and evaluated phenazine derivatives produced by P. chlororaphis spp. are phenazine-1-carboxylic acid (PCA), phenazine carboxamide (PCN) and a number of hydroxy-phenazines [8].
Pseudomonas chlororaphis GP72 [9], a plant-beneficial rhizobacterium that has shown broad-spectrum antifungal activity against various phytopathogens of agricultural significance, produces three phenazine compounds [9], [10], [11]: PCA, 2-hydroxy-phenazine-1-carboxylic acid (2-OH-PCA) [11] and 2-hydroxy-phenazine (2-OH-PHZ) [12], [13]. 2-OH-PHZ is derived from PCA by the unique modification of a key enzyme PhzO which belongs to a family of two-component nonheme flavin-diffusible bacterial aromatic monooxygenases. This enzyme was first discovered in Pseudomonas chlororaphis subsp. aureofaciens30-84 [14], then also identified in Pseudomonas chlororaphis GP72 which shares 98% gene similarity with strain 30-84 [11].
Previous studies have shown that 2-hydroxyphenazines including 2-OH-PHZ and 2-OH-PCA exhibit stronger bacteriostatic and fungistatic activity compared with PCA toward some pathogens such as Gaemannomyces. graminis var. tritici [14], [15], [16]. 2-OH-PHZ is produced primarily by P. chlororaphis except for the strain P. aurantiaca PB-St2 [17].
The biosynthetic pathway leading to the production of 2-OH-PHZ was first described in P. chlororaphis 30–84 [18]. In this early study, it was shown that when in trans, a cosmid containing 30–84 genomic DNA comprising the coding sequence of only 5 of the 7 genes in what was later shown to be the phenazine biosynthetic operon was sufficient to produce all three phenazines at low level in E.coli. It was hypothesized that only a part of phzC was needed to convert PCA to 2-OH-PCA in E.coli [19]. In later work Mavrodi et al. [20] suggested that other E.coli enzymes likely contribute to the small amount of phenazines made by E.coli when only 5 genes were introduced; the amount of phenazine produced was substantially increased when the entire 7 gene operon now known to be important for PCA synthesis was present. Subsequently Delaney et al. [14] verified the entire biosynthetic pathway leading to 2-OH-PHZ. Importantly, they showed that 2-OH-PHZ was produced from PCA by the action of a single enzyme PhzO, which catalyzed the conversion of PCA to 2-OH-PCA, and that 2-OH-PCA was then spontaneously decarboxylated to form 2-OH-PHZ. Knowledge of this biosynthesis pathway provides the possibility to enhance production of 2-OH-PHZ for its application in agriculture [15], [21].
However, the yield of 2-OH-PHZ using chemical methods or via biosynthetic production in Pseudomonas spp. is relatively low [9], [16] in contrast with the production of its precursor, PCA [22], [23]. This limitation on production has become the main obstacle to widespread application of 2-OH-PHZ. Thus, it is important to conduct systematic studies and understand the reaction kinetics of 2-OH-PHZ and explore methods to produce 2-OH-PHZ in high yield.Herein, the catalytic conditions of PhzO were studied, 2-OH-PCA was extracted and purified from the broth, and the kinetics of the conversion of 2-OH-PCA to 2-OH-PHZ was studied systematically in vitro.
Methods and Materials
Bacterial strains and plasmid construction
Strains and plasmids are listed in Table 1. Escherichia coli BL21, Pseudomonas chlororaphis GP72 and its mutants [11] were obtained from our lab stock preserved in 20% (vol/vol) glycerol at −70°C. Unless indicated otherwise, E. coli was routinely grown at 37°C in Luria-Bertani (LB) medium. P. chlororaphis GP72 and its mutants were incubated at 28°C in LB and King's B (KB) broth, respectively. LB medium supplemented with 50 µg/ml kanamycin was used for phzO gene over-expression.
Table 1. List of bacterial strains, PCR products, primers and plasmids used in this study.
| Strains | Characteristics | Reference |
| E. coli BL21 | recA1 endA1 gyrA96 thi1 hsdR17 (rk− mk+) supE44 re1A1 | Sambrook & Rossel |
| P. chlororaphis GP72 | PCA, 2-OH-PHZ producer | Liu et al. |
| P. chlororaphis GP72AN | PCA, 2-OH-PCA, 2-OH-PHZ producer, Gm r | Huang et al. |
| P. chlororaphis GP72ON | PCA producer, Cm r | Huang et al |
| P. chlororaphis GP72FN | No phenazine producer | Zhao et al |
| Plasmids | Characteristics | Source |
| pET28a | expression vector, T7 promoter, 6-His tag, Kanr | Xuping lab |
| pET28a-phzO | 1476-bp NdeI- BamHI PCR amplified fragment containing phzO cloned into pET28a | This study |
| Primers | Sequence (5' - 3') | Source |
| phzO-F(NdeI) | 5′-CCCGAACATATGCTAGATCTTCAAAACAAGCGT-3′ | This study |
| phzO-R(BamHI) | 5′-TTTGGATCCCTATTTGGCGTTGAGCCCCACCA-3′ | This study |
Cloning, expression, and purification of recombinant phzO
Plasmid construction: A pair of primers phzOF and phzOR was used (Table 1) to clone phzO at an annealing temperature of 58°C with the genome of strain GP72 as the template. The 1.5 kb PCR product was purified by agarose gel electrophoresis, and then digested with EcoRI and XhoI, the fragment was inserted into the multiple cloning site of pET28a, an expression vector, to obtain pET28a-phzO. Then the plasmid was introduced into E. coli BL21 to give E. coli BL21-phzO which was used for phzO gene over-expression.
For protein expression, the bacteria were cultured until the OD600 reached 0.4–0.6, and IPTG was added to a final concentration of 0.1 mM. After 8 h incubation, the bacteria were harvested and lysed by sonication in buffer A (20 mM Tris-HCl, 0.5 mM PMSF, 1 mM DTT). The lysate was cleared by centrifugation for 15 min at 10,000×g and then applied to a Ni-NTA-agarose column (Superflow Cartridge,QIAGEN) in buffer A. The recombinant PhzO was purified to homogeneity as previously described [24].
PhzO activity assay in vitro and in vivo
PhzO activity assay In vivo: E. coli BL21 harboring pET28a or pET28a-phzO was grown at 37°C in LB broth supplemented with 50 µg/ml kanamycin for about 13 h, and then was diluted to fresh LB broth containing the same kanamycin concentration at a ratio of 1∶40. PCA was added to a final concentration of 0.3–0.7 mg/ml from a 25 mM stock solution in 55% (wt/vol) NaHCO3. The strain was cultivated at 37°C and 180 rpm to an optical density of 0.6 at 600 nm, and then induced with 0.1 mM IPTG. Samples were extracted, and analyzed for phenazine composition by high-performance liquid chromatography (HPLC).
PhzO activity assay in vitro: Crude cell extracts were prepared by sonication treatment of recombinant E. coli cells and assayed for hydroxyl activity by adding free PCA under different conditions. The effects of different metal ions were determined in vitro in 0.1 M Tris-HCl buffer (Table 2).
Table 2. Results of the in vitro PhzO activity assay.
| NAD(H) | NADP(H) | Co2+ | Mn2+ | Mg2+ | Fe3+ | PCA | 2-OH-PCA | 2-OH-PHZ | |
| 1 | − | − | − | − | − | − | + | ND | ND |
| 2 | − | + | − | − | − | − | + | ND | ND |
| 3 | − | + | + | − | − | − | + | ND | ND |
| 4 | − | + | − | + | − | − | + | ND | ND |
| 5 | − | + | − | − | + | − | + | ND | ND |
| 6 | − | + | − | − | − | + | + | D | D |
| 7 | + | − | − | − | − | − | + | ND | ND |
| 8 | + | − | + | − | − | − | + | ND | ND |
| 9 | + | − | − | + | − | − | + | ND | ND |
| 10 | + | − | − | − | + | − | + | ND | ND |
| 11 | + | − | − | − | − | + | + | ND | ND |
| 12 | − | − | − | − | − | − | − | ND | ND |
+ represents the addition of the specified metal ion, the final concentration of metal ion was 1 mM; the final concentration of PCA was 200 mg/L.
D, detectable.
ND, not detectable.
The conversion of 2-OH-PCA to 2-OH-PHZ in cell extracts of Pseudomonas
To test the influence of enzymes in cell extracts of Pseudomonas on the reaction that the intermediate product 2-OH-PCA underwent spontaneous decarboxylation to form 2-OH-PHZ, 30 ul 100 mg/L purified 2-OH-PCA was added to 1 mL crude cell extracts of GP72FN and GP72ON at 28°C, respectively, and incubated for 18h in PBS (pH = 7.0). Crude cell extracts were prepared by sonication treatment of GP72FN and GP72ON after 24h incubation in KB at 28°C.
Kinetics at different temperatures
The kinetic experiments were carried out in polypropylene tubes containing 1 mg purified 2-OH-PCA and 20 mL of phosphate-buffered saline solutions (PBS) at pH of 7.0. The experiments were performed in a waterbath at 28°C, 37°C, 55°C and 70°C from 0 to 23 h.
Effect of initial pH
To evaluate the effect of pH on the spontaneous transformation of 2-OH-PCA to 2-OH-PHZ, 1 mg purified 2-OH-PCA was exposed to 10 mL of PBS in polypropylene tubes at pH from 3 to 11 at 70°C. The suspensions were shaken in a 20°C water bath for 5 min. For all of the experiments, concentrated NaOH and HCl solutions were used to adjust the pH of the mixtures.
Effect of different irradiation
To evaluate the effect of different irradiation on the reaction of 2-OH-PCA to 2-OH-PHZ, 1 mg purified 2-OH-PCA was added to 20 mL of phosphate-buffered saline solutions (PBS) at pH of 7.0, and then exposed the different light sources at room temperature.
Quantification of phenazine compounds
The fermentation broth was adjusted to pH 2.0 with 6 N HCl, before centrifugation at 10,000×g for 10 minutes using an Eppendorf Minispin centrifuge. Cellular debris was removed and the clear supernatant was collected and extracted with an equal volume of ethyl acetate with vigorously shaking [9],[11]. The collected organic layer was mixed with 1/10 volume distilled water and shaken rigorously. Finally, the organic phase containing 2-OH-PHZ was evaporated under vacuum pressure. The 2-OH-PHZ residue was dissolved in methanol for further analysis.
The HPLC analysis method for simultaneous analysis of the phenazine compounds PCA, 2-OH-PCA and 2-OH-PHZ was carried out using a 1260 Infinity HPLC apparatus (Agilent Technologies Group, Beijing, China)equipped with a UV detector and a C-18 reverse phase column (Agilent, USA) as described previously [11].
Reagents
PCA (>95%), 2-OH-PCA (99.4% purity) and 2-OH-PHZ (99.0% purity) were prepared by our laboratory (Laboratory of Microbial Resources and Metabolic Engineering). The red pigment precipitate from the GP72 culture was suspended in water, and extracted using a double volume of acetic acid/ether, then evaporated by rotary evaporation. Further purification was performed by preparative liquid chromatography (Shimadzu LC-20AP) using a previously described method [11]. 2-OH-PCA (99.4%purity) was prepared and used for the following experiments. HPLC grade methanol (Lingfeng Chemical Reagent Co. Ltd., Shanghai, China) and ammonium acetate (Sigma Chemicals Co.) were used for the HPLC analysis, and all other chemicals were reagent grade (Sinopharm Chemical Reagent Co. Ltd., Shanghai, China). The pure water was produced by a water purifier (Aquapro DZG-303A; Zhongqin, China).
Statistical analysis
Differences between treatments were determined by analysis of variance followed by Students test (P<0.05).
Results
Expression and purification of PhzO
Heterogenous expression of PhzO in E.coli BL21 was similar to the work done by Daleney et al [14]. PhzO was successfully expressed in E.coli BL21 (S1), and converted free PCA to 2-OH-PCA, then decarboxylated to form 2-OH-PHZ (S2). Other factors important for 2-OH-PHZ biosynthesis including different temperatures, IPTG concentrations and induction times of expression were examined, and the amount of PhzO in the soluble fraction and in inclusion bodies was studied by SDS-PAGE. Unfortunately, protein was detected in both soluble fraction and inclusion bodies under all conditions. The best results were achieved at 20°C after 8 h incubation induced with 0.1 mM IPTG as these conditions minimized the amount of PhzO found in inclusion bodies compared with 37°C.
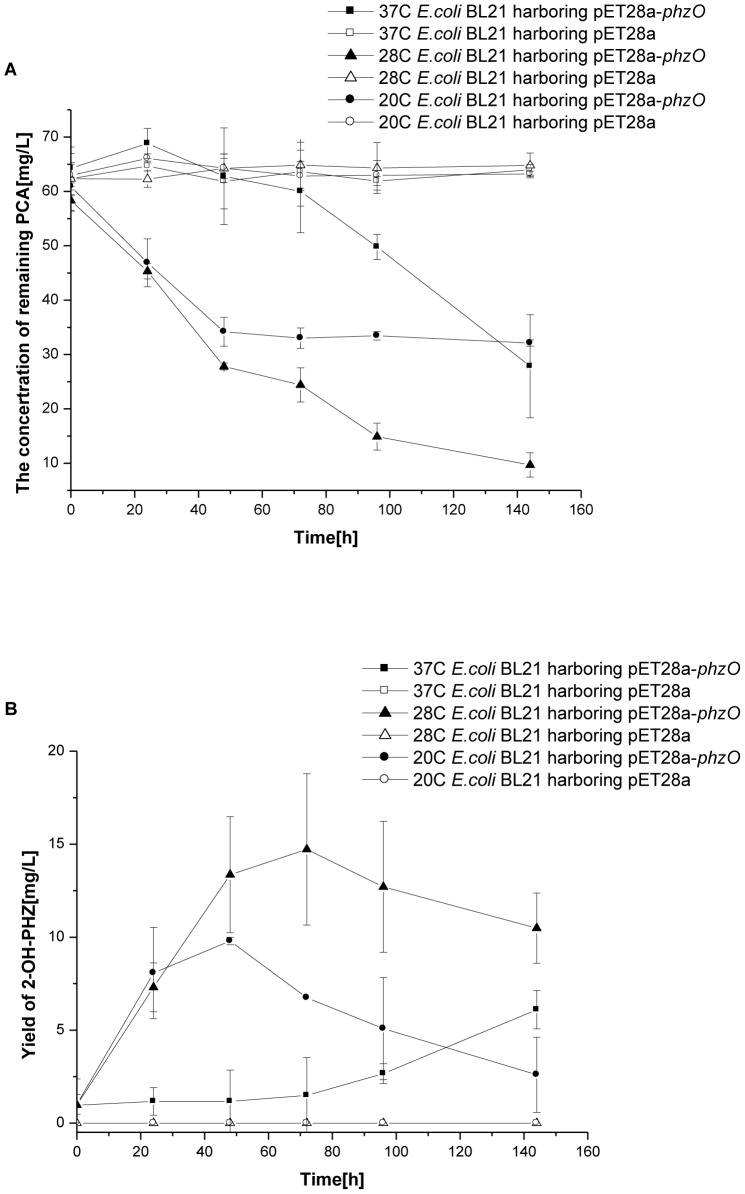
Temperature is one of the most important factors that can change the activity of the enzyme. Cells of strain E.coli BL21 harboring pET28a-phzO and strain E.coli BL21 harboring pET28a were grown at temperatures between 20 and 37°C. Although 20°C is the most suitable temperature for expression of PhzO (Fig. 1), when temperature raised from 20°C to 28°C, the transformation of substrate PCA and the yield of 2-OH-PHZ increased, but when the temperature raised from 28°C to 37°C, the yield of 2-OH-PHZ decrased as shown in Fig. 2.
Figure 1. The expression profile of protein PhzO in E.coli BL21 at different temperatures.
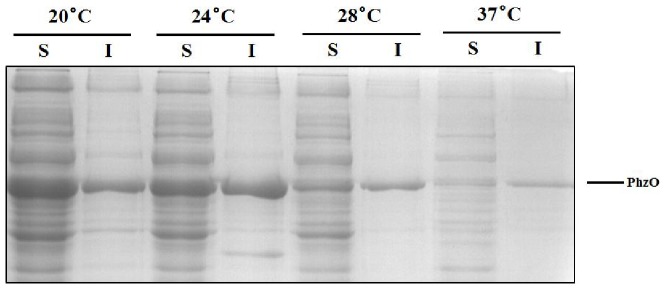
I represents Insoluble aggregates, S represents Soluble supernatants.
Figure 2. The conversion of PCA to 2-OH-PHZ at different temperatures.
A Degradation of PCA at different temperature over time. B The production of 2-OH-PHZ at different temperatures over time. The values are means±standard deviations of triplicate cultures.
PhzO was sufficient to hydroxylate PCA to produce 2-OH-PHZ in the presence of NADP(H) and Fe3+
Based on the PCA transformation assay and optimized conditions of HPLC analysis, the IPTG-induced cultures of E. coli BL21 harboring pET28a-phzO, converted PCA (0.3–0.5 mg/ml in 5% NaHCO3) [14] to 2-OH-PCA after 12 h, while no production of 2-OH-PHZ or 2-OH-PCA occurred in control cultures harboring only the respective pET28a vectors. The production of 2-OH-PHZ increased as that of 2-OH-PCA decreased in the culture of E. coli BL21 harboring pET28a-phzO. After 72 h, no 2-OH-PCA could be detected and the yield of 2-OH-PHZ reached a maximum (S1). The result confirmed the previous work done by Delaney et al.
We further tested the hydroxyl activity of purified PhzO. Significant activity was detected in the presence of NADP(H) and 1 mM Fe3+. The results indicated that PhzO was sufficient to hydroxylate PCA to produce 2-OH-PHZ in the presence of NADP(H) and Fe3+ (Table 2). The influence of Fe3+ on the levels of 2-OH-PHZ was investigated by adding 1 mM Fe3+ to the culture of E.coli BL21 harboring pET28a-phzO. More 2-OH-PHZ was produced in the cultures containing added Fe3+ than in the control groups. The control groups produced about 3 mg/L 2-OH-PHZ, while the groups with 1 mM Fe3+ added reached a final concentration of 11 mg/L 2-OH-PHZ after 96h incubation.
2-OH-PHZ production showed a steady increase for additions of PCA of 40–80 mg/L. However, PCA added in excess to the culture inhibited the production of 2-OH-PHZ. Above that amount, at 120 mg/LPCA, the production of 2-OH-PHZ was equivalent to that of 80 mg/L PCA. The quantity of 2-OH-PHZ even dropped when PCA exceeded 160 mg/L.
Low solubility of 2-OH-PCA in fermentation broth
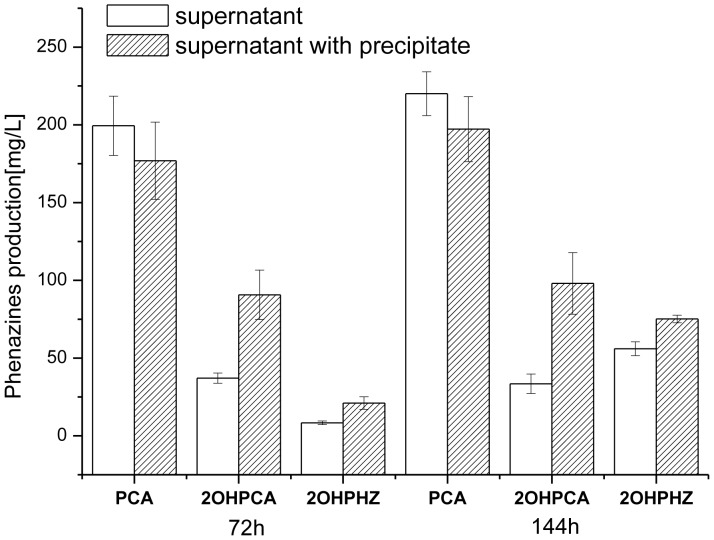
Pseudomonas GP72AN produced three main phenazines: PCA, 2-OH-PCA and 2-OH-PHZ. Previous work showed that the yield of 2-OH-PCA in Pseudomonas spp. GP72AN is relatively low (<50 ppm), only 10–20% of its precursor PCA. Interestingly, red pigment precipitate was observed in the fermentation broth of GP72AN after 72 h incubation, which was determined to be primarily 2-OH-PCA (75%) with lesser amounts of PCA & 2-OH-PHZ as determined by the same HPLC method described previously (Fig. 3). The yield of 2-OH-PCA in the fermentation broth with the red pigment increased to 97±15 mg/L, in comparison with 37±3 mg/L for the supernatant.
Figure 3. Phenazine derivative production in the supernatant and the precipitate after 72 h and 144 h incubation of GP72AN in KB medium.
The values are means±standard deviations of triplicate cultures. Different letters in columns indicate statistically significant differences between treatments (P = 0.05).
Kinetics at different temperatures
Previous experiments had investigated the stability of 2-OH-PCA in aqueous solution and an organic solvent. The results indicated that 2-OH-PCA was stable in organic solvent even over a week period. However, significant degradation of 2-OH-PCA was observed in aqueous solution while the concentration of 2-OH-PHZ increased over 1 day.
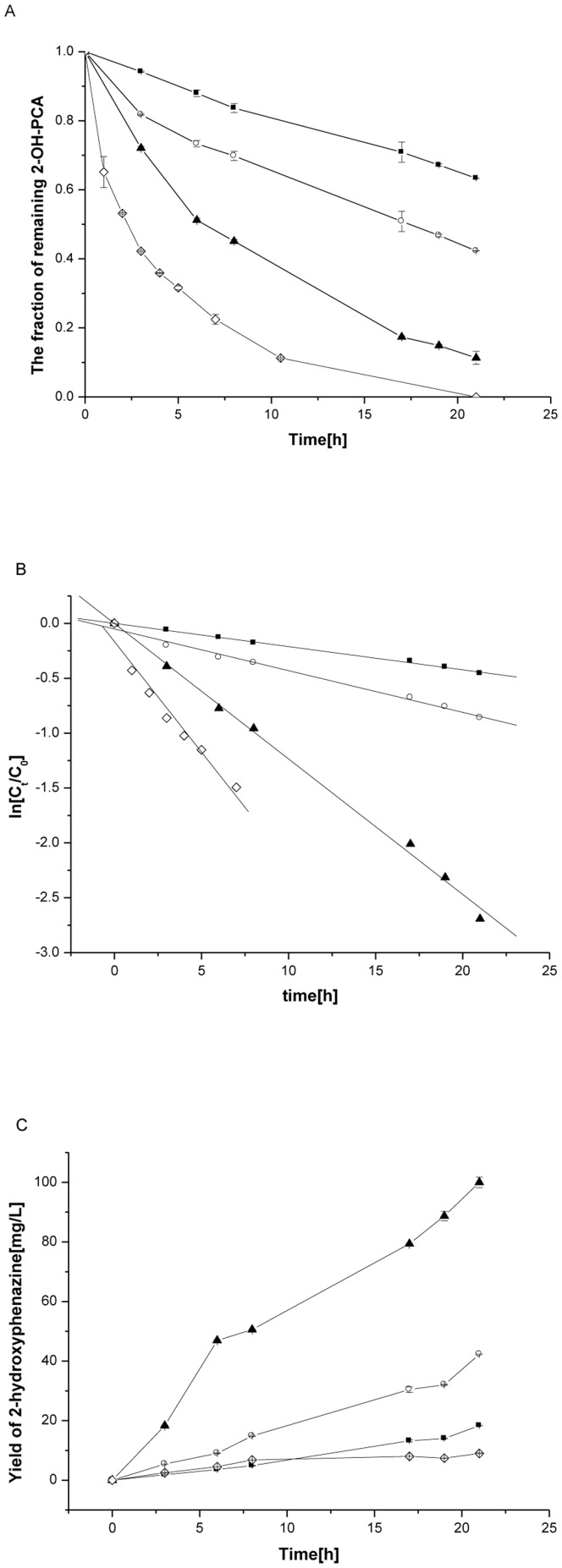
An investigation of the reaction kinetics in aqueous solution at different temperatures revealed that the conversion rate increased with increasing temperature (Fig. 4A). The logarithm concentration rate of 2-OH-PCA (ln [Ct/C0]) versus time plot was linear, and showed that the degradation of 2-OH-PCA follows first-order kinetics. The kinetic equation is represented mathematically as [25]:
Figure 4. The conversion of 2-OH-PCA to 2-OH-PHZ in phosphate buffer at different temperatures (PBS solution stored in complete darkness).
A and B. Degradation of 2-OH-PCA. C. The changes of 2-OH-PHZ over time. filled square 28°C; empty circle 37°C; filled triangle 55°C; empty diamond 70°C. Error bars represent standard deviations of data from triplicate samples. The coefficient of variation(standard deviation/mean×100) was below 7% in all cases.
| (1) |
or
| (2) |
Where k (h− 1) is the rate constant of the first order kinetic reaction, Ct is the concentration of 2-OH-PCA at time t, C0 is the initial concentration of 2-OH-PCA. According to Fig. 4B, the first-order rate constant and the corresponding half-lives were calculated and are listed in Table 3.
Table 3. Kinetic parameters of 2-OH-PCA degradation at different temperatures.
| Temperature °C | Reaction order | Rate constant (h− 1) | R2 | Half-life (h) |
| 28 | 1 | 0.0206 | 0.9979 | 33.64 |
| 37 | 1 | 0.0378 | 0.9898 | 18.33 |
| 55 | 1 | 0.1197 | 0.9993 | 5.79 |
| 70 | 1 | 0.1936 | 0.9818 | 3.58 |
The logarithm of the first-order constant versus the reciprocal of temperature (K) plots were linear and can be expressed by the Arrhenius equation [25]
| (3) |
or
| (4) |
where k (h− 1) is the rate constant of the first order kinetic reaction, T is the temperature in Kelvin (K), E is the activation energy (kJ mol− 1), A is the frequency factor and R is the molar gas constant (8.314 kJ mol− 1).
From Fig. 4B , the half-lives of 2-OH-PCA at various temperatures were calculated, and is an important consideration when planning the handling and storage of 2-OH-PCA. Unfortunately, less 2-OH-PHZ than expected was detected at 70°C, as 2-OH-PHZ is unstable at high temperatures (Fig. 4C).
Kinetics at different pHs
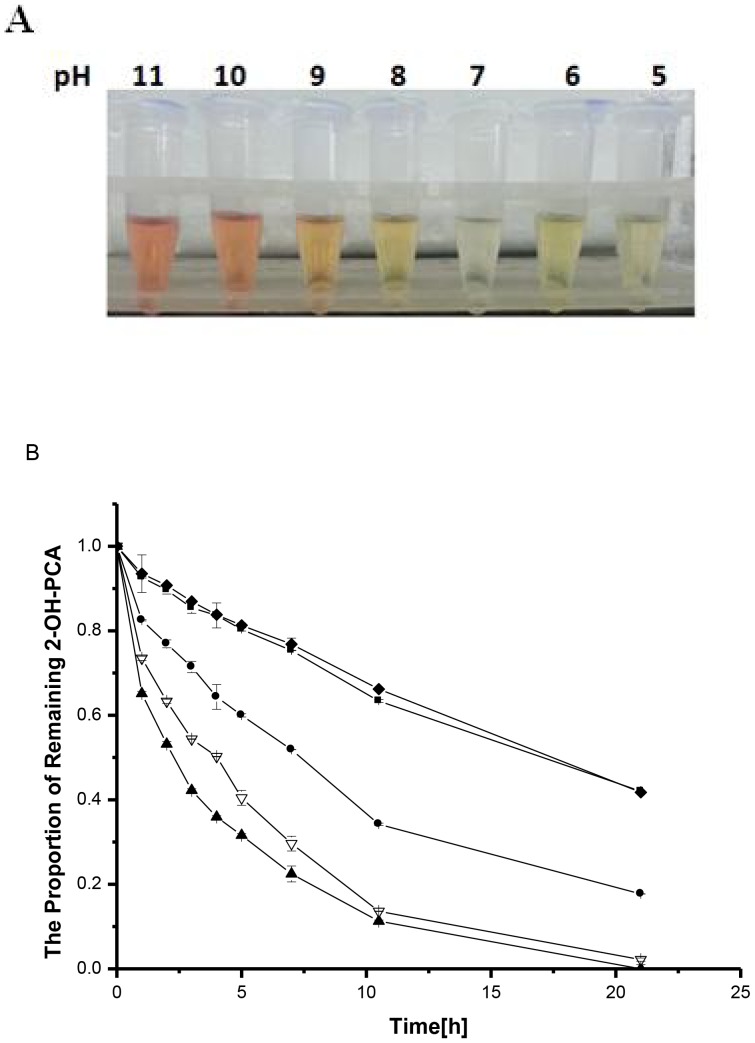
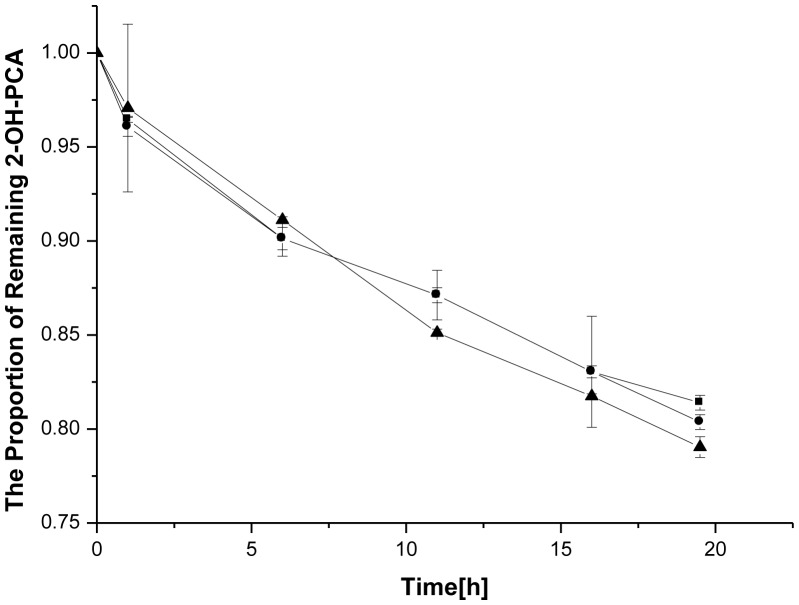
Previous study revealed that 2-OH-PCA was sensitive to changes in pH and it had distinctive coloration depending on its pH environment [14]. To understand the effect of pH on the reaction, the conversion of 2-OH-PCA to 2-OH-PHZ in PBS at pHs 3.0-11.0 was studied (Fig. 5A). The results showed that the optimal pH for the conversion was 7.0. As shown in Fig. 5B, the concentration of 2-OH-PCA was 42%, 17%, 0%, 2%, and 41% of the initial value after 21 h at pH 3.0, 6.0, 7.0, 9.0 and 11.0, respectively, and the first-order rate constant and the corresponding half-lives were calculated and are listed in Table 4.
Figure 5. The conversion of 2-OH-PCA to 2-OH-PHZ in phosphate buffer at different pHs (water solution stored at 70°C in complete darkness).
A: 2-OH-PCA with phosphate buffer at different pHs. B: Degradation of 2-OH-PCA at different pHs. filled diamond pH 11.0; filled square pH 3.0; filled circle pH 6.0; empty triangle pH 9.0; filled triangle pH 7.0. Error bars represent standard deviations of data from triplicate samples. The coefficient of variation (standard deviation/mean×100) was below 3% in all cases.
Table 4. Kinetic parameters of 2-OH-PCA degradation at different pH values.
| pH | Reaction order | Rate constant (h− 1) | R2 | Half-life (h) |
| 3 | 1 | 0.0407 | 0.9978 | 17.03 |
| 6 | 1 | 0.0813 | 0.985 | 8.53 |
| 7 | 1 | 0.1936 | 0.9818 | 3.58 |
| 9 | 1 | 0.1812 | 0.9974 | 3.82 |
| 11 | 1 | 0.0408 | 0.9965 | 16.99 |
Photodegradation
Many natural compounds are light sensitive, such as plt, aldicarb, parathion, mecoprop, linuron and chlorpyifos. Previous study in our lab discovered that PCA was light sensitive (data not shown). Here the stability of the conversion of 2-OH-PCA under different lights were studied. An ultraviolet lamp and a fluorescent lamp were used to measure the effects of different irradiation. The results showed that the light has less effect on the conversion of 2-OH-PCA to 2-OH-PHZ in contrast to the conversion of 2-OH-PCA to 2-OH-PHZ in the dark (Fig. 6).
Figure 6. The conversion of 2-OH-PCA to 2-OH-PHZ under different light sources at room temperature.
filled square dark; filled circle fluorescent lamp; filled triangle ultraviolet lamp. Error bars represent standard deviations of data from triplicate samples.
The conversion of 2-OH-PCA to 2-OH-PHZ in cell extracts of Pseudomonas chlororaphis and its mutants
As shown in Table 5, enzymes in cell extracts did not accelerate the reaction of intermediate product 2-OH-PCA that underwent spontaneous decarboxylation to form 2-OH-PHZ. With the activity of enzyme of GP72FN or GP72ON, 2-OH-PHZ accounts for 10% of the total phenazine present after 18h, while 14% of the initial 2-OH-PCA underwent decarboxylation to form 2-OH-PHZ without any enzyme in PBS, according with the results after 48h incubation.
Table 5. The influence of enzymes in cells extracts on the conversion of 2-OH-PCA to 2-OH-PHZ.
| Strains | Concentration of phenazines (mg/L) | |||||
| 0h | 18h | 48h | ||||
| 2-OH-PCA | 2-OH-PHZ | 2-OH-PCA | 2-OH-PHZ | 2-OH-PCA | 2-OH-PHZ | |
| GP72FN | 30 | 0 | 24.42±0.94 | 2.00±0.02 | 21.42±0.05 | 3.11±0.95 |
| GP72ON | 30 | 0 | 26.09±0.91 | 1.49±0.06 | 23.12±0.84 | 3.74±0.01 |
| Control1 | 30 | 0 | 25.24±0.75 | 1.31±0.02 | 21.42±1.25 | 3.61±0.32 |
The control group was 30 mg/L 2-OH-PCA in PBS without any enzyme.
The experiment was performed three times and means±SE of triplicate experiments from same culture are plotted.
Discussion
In recent studies, based on PCA as a precursor, several phenazine biosynthetic related genes were found to play roles in the synthesis of specific phenazine derivatives including phzH, phzM, phzS and phzO. PhzH [26] could convert PCA to PCN in P. aeruginosa PAO1 while PhzM could catalyze PCA to pyocyanin (PYO) with the help of PhzS [27]. PhzO in P. chlororaphis 30–84 and P. chlororaphis GP72 was found to hydroxylate PCA to 2-OH-PHZ [11], [14]. Both strains could produce 2-OH-PHZ and PCA, and GP72 and 30–84 were supposed to have similar genomic structures. Based on the genomic data of P. chlororaphis GP72 [10], the phenazine biosynthetic genes and regulators have been investigated in an effort to improve the production of phenazines.
As PhzO belongs to the family of flavin-diffusible monooxygenase NAD(P)H-dependent flavoproteins, a lack of NADP (H) will also result in the inactivation of PhzO, which would block the conversion of PCA to 2-OH-PCA. In this study, PhzO was heterologously expressed in E. coli BL21, and successfully used to convert free PCA to 2-OH-PHZ confirming and complementing the reaction mechanism of 2-OH-PHZ discovered by Delaney et al. [14]. We showed that this conversion was dependent on the presence of sufficient NADP(H) and Fe3+. However, the hydroxylation of PCA was inefficient, as there was PCA remaining even after 72 h incubation. A number of studies have shown that 2-hydroxyphenazines have stronger antibiotic activity than PCA toward some pathogens [14], [28], but the low yield of 2-OH-PHZ has limited its applications. The low yield of 2-OH-PHZ is attributed to the low conversion rate of PCA to 2-OH-PHZ (10–20%) [11].
One reason for the low hydroxylation efficiency is the low PhzO yield. We analyzed the soluble and insoluble fractions of PhzO, and found that the expressed PhzO protein was largely obtained in the insoluble inclusion bodies, thus reduced the overall yield of active enzyme (Fig. 1), and reduced the transformation of PCA.
Another reason for the low 2-OH-PHZ yield is the low solubility of 2-OH-PCA. In this study, the intermediate 2-OH-PCA was observed to accumulate as a red pigmented precipitate in the fermentation broth, and could be easily isolated and purified. The fact that 2-OH-PCA can be completely converted to 2-OH-PHZ coupled with the observation that solid 2-OH-PCA was stable over a long period supports the hypothesis that the low conversion of PCA to 2-OH-PHZ is partly due to the low solubility of 2-OH-PCA in the fermentation broth. The precipitation of 2-OH-PCA slowed the conversion from 2-OH-PCA to 2-OH-PHZ and thus, reduced the yield of 2-OH-PHZ.
Iron is an important element that regulates some secondary metabolisms of Pseudomonas [29], [30]. In this work, Fe3+ enhances PhzO enzyme activity,indicating that the ratio of conversion from PCA to 2-OH-PHZ could be enhanced by adding Fe3+, and the latter experiment in vivo confirmed that assumption based on the result that the yield of 2-OH-PHZ doubled by adding 1 mM Fe3+. One hypothesis may be proposed to explain this result. PCA has been reported that can reduce Fe3+ reduction [31]. Thus, the addition of Fe3+ may oxidize PCA and accelerated the reaction that PCA converts to 2-OH-PCA. Work on the regulatory mechanism of Fe3+ on 2-hydroxyphenazines biosynthesis is now in progress.
The quantity of 2-OH-PHZ would be expected to increase when PCA accumulated as it was derived from PCA. No more 2-OH-PHZ was produced in the cultures with the addition of PCA of 80–120 mg/L, indicating that saturation of enzyme PhzO might be occurring under the current culture conditions and fermentation process. Above that amount, for example, at 280 mg/L PCA, the production of 2-OH-PHZ was less than that of 80 mg/L, indicating that substrate inhibition of enzyme PhzO might occurred above 120 mg/L PCA.
The results of our study confirmed the reaction mechanism proposed by Delaney et al. that 2-OH-PCA is spontaneously converted to 2-OH-PHZ in a pH dependent manner [14]. Here it is determined that the conversion of 2-OH-PCA to 2-OH-PHZ followed first order kinetics at different pH values. Based on these results, we assumed that the ratio of conversion from 2-OH-PCA to 2-OH-PHZ is dependent on the different ionic forms of 2-OH-PCA which vary depending on the pH environment.
The ratio of the conversion from 2-OH-PCA to 2-OH-PHZ increased with the reaction temperature when it was below 55°C, but decreased at temperatures above 70°C. Increasing temperature causes 2-OH-PCA degradation along with a decrease in 2-OH-PHZ.
In this study, we discovered that PhzO was sufficient to hydroxylate PCA to produce 2-OH-PHZparticularly in the presence of sufficient NADP(H) and Fe3+, advancing the current knowledge of the biosynthesis pathway of 2-OH-PHZ (Fig. 7). Further we find out that Fe3+ enhanced the conversion from PCA to 2-OH-PHZ and the optimum temperature for the conversion was 28°C. Significantly, the substrate inhibition of enzyme PhzO was investigated. Then a kinetic model was developed to study the conversion kinetics of 2-OH-PHZ. The results showed that temperature and pH values of solutions were the factors that had a strong influence on the conversion of 2-OH-PCA to 2-OH-PHZ. Degradation of 2-OH-PCA followed first-order reaction kinetics, and the rate constant increased with increased temperature but decreased when pH below or above 7.0. In addition, we confirmed that the intermediate product 2-OH-PCA underwent spontaneous decarboxylation to form 2-OH-PHZ and this reaction was not accelerated by any enzyme in Pseudomonas extracts such like PhzC, PhzD and PhzE. This study advances the current understanding of the biosynthetic pathway responsible for the production of 2-OH-PHZ by providing a detailed description of the reaction kinetics for the biocatalytic conversion of PCA to 2-OH-PHZ. This information should provide for improvements in 2-OH-PHZ yield and wide application of 2-OH-PHZ as biopesticiede.
Figure 7. Complemented mechanism of the conversion of PCA to 2-OH-PHZ: PhzO hydroxylates PCA to 2-OH-PCA in the presence of NADP(H), Fe3+ enhanced the activity of PhzO.
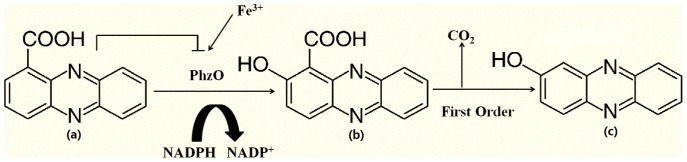
Subsequently, the intermediate product 2-OH-PCA spontaneously decarboxylates to form 2-OH-PHZ followed first-order reaction kinetics. The chemical structure of phenazine-1-carboxylic acid (PCA) (a), 2-hydroxy phenazine-1-carboxylic acid (2-OH-PCA) (b) and 2-hydroxyphenazine (2-OH-PHZ) (c). Arrows indicate positive direction of reaction, lines with flat ends indicate negative regulation.
Supporting Information
The expression profile of protein PhzO in E. coli BL21. (A) The expression of PhzO in E. coli BL21. Lane 1: Whole cell lysate of BL21 harboring pET28a; M: Premixed Protein Marker (Low); Lane 2: Whole cell lysate of BL21 harboring pET28a-phzO. (B) The purification of PhzO. M: Premixed Protein Marker (High); Lane1: Whole cell lysate of BL21 harboring pET28a; Lane 2: PhzO purified by Ni2+-nitrilotriacetic acid chromatography. The gel was loaded with 5 ng of purified PhzO (lane 2).
(TIF)
(A): HPLC analysis of the conversion of PCA to 2-OH-PHZ in E. coli BL21 in a PCA transformation assay. (a) Only PCA was detected from cultures of BL21 harboring pET28a after incubation for 72 h; (b) PCA, 2-OH-PCA and 2-OH-PHZ were detected from cultures of BL21 harboring pET28a-phzO after 12 h; (c) PCA and 2-OH-PHZ were detected from cultures of BL21 harboring pET28a-phzO after 72 h; (d) BL21 harboring pET28a without the addition of PCA (B): production of pigments in LB medium after 72 h incubation: (a) E. coli BL21 harboring pET28a; (b) E. coli BL21 harboring pET28a-phzO.
(TIF)
Acknowledgments
We thank Dr Xuping for generously providing the plasmids used in the present study.
Funding Statement
This research was supported by the National Key Basic Research Program of China (No. 2012CB721005), the National Natural Science Foundation of China (No. 31270084), and the National High Technology Research and Development Program of China (No. 2012AA022107). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Mahajan-Miklos S, Tan MW, Rahme LG, Ausubel FM (1999) Molecular mechanisms of bacterial virulence elucidated using a Pseudomonas aeruginosa-Caenorhabditis elegans pathogenesis model. Cell 96: 47–56. [DOI] [PubMed] [Google Scholar]
- 2. Mazzola M, Cook RJ, Thomashow LS, Weller DM, Pierson LS 3rd (1992) Contribution of phenazine antibiotic biosynthesis to the ecological competence of fluorescent pseudomonads in soil habitats. Appl Environ Microbiol 58: 2616–2624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bloemberg GV, Lugtenberg BJ (2003) Phenazines and their role in biocontrol by Pseudomonas bacteria. New phytologist 157: 503–523. [DOI] [PubMed] [Google Scholar]
- 4. Budzikiewicz H (1993) Secondary metabolites from fluorescent pseudomonads . FEMS Microbiol Rev 10: 209–228. [DOI] [PubMed] [Google Scholar]
- 5. Park JY, Oh SA, Anderson AJ, Neiswender J, Kim JC, et al. (2011) Production of the antifungal compounds phenazine and pyrrolnitrin from Pseudomonas chlororaphis O6 is differentially regulated by glucose. Lett Appl Microbiol 52: 532–537. [DOI] [PubMed] [Google Scholar]
- 6.Selin C, Fernando DW, de Kievit TR (2012) The PhzI/PhzR Quorum-Sensing System is required for Pyrrolnitrin and Phenazine Production and Exhibits Cross Regulation with RpoS in Pseudomonas chlororaphis PA23. Microbiology. [DOI] [PubMed]
- 7. Khan SR, Herman J, Krank J, Serkova NJ, Churchill ME, et al. (2007) N-(3-hydroxyhexanoyl)-l-homoserine lactone is the biologically relevant quormone that regulates the phz operon of Pseudomonas chlororaphis strain 30–84. Appl Environ Microbiol 73: 7443–7455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Turner JM, Messenger AJ (1986) Occurrence, biochemistry and physiology of phenazine pigment production. Adv Microb Physiol 27: 211–275. [DOI] [PubMed] [Google Scholar]
- 9. Liu H, He Y, Jiang H, Peng H, Huang X, et al. (2007) Characterization of a phenazine-producing strain Pseudomonas chlororaphis GP72 with broad-spectrum antifungal activity from green pepper rhizosphere. Curr Microbiol 54: 302–306. [DOI] [PubMed] [Google Scholar]
- 10. Shen X, Chen M, Hu H, Wang W, Peng H, et al. (2011) Genome sequence of Pseudomonas chlororaphis GP72, a root-colonizing biocontrol strain. J Bacteriol 194: 1269–1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Huang L, Chen MM, Wang W, Hu HB, Peng HS, et al. (2010) Enhanced production of 2-hydroxyphenazine in Pseudomonas chlororaphis GP72. Appl Microbiol Biotechnol 89: 169–177. [DOI] [PubMed] [Google Scholar]
- 12. Levitch ME, Rietz P (1966) The isolation and characterization of 2-hydroxyphenazine from Pseudomonas aureofaciens. Biochemistry 5: 689–692. [DOI] [PubMed] [Google Scholar]
- 13. Park GK, Lim JH, Kim SD, Shim SH (2012) Elucidation of Antifungal Metabolites Produced by Pseudomonas aurantiaca IB5-10 with Broad-Spectrum Antifungal Activity. J Microbiol Biotechnol 22: 326–330. [DOI] [PubMed] [Google Scholar]
- 14. Delaney SM, Mavrodi DV, Bonsall RF, Thomashow LS (2001) phzO, a gene for biosynthesis of 2-hydroxylated phenazine compounds in Pseudomonas aureofaciens 30–84. J Bacteriol 183: 318–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Maddula VS, Pierson EA, Pierson LS 3rd (2008) Altering the ratio of phenazines in Pseudomonas chlororaphis (aureofaciens) strain 30–84: effects on biofilm formation and pathogen inhibition. J Bacteriol 190: 2759–2766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Tietze M, Iglesias A, Merisor E, Conrad J, Klaiber I, et al. (2005) Efficient methods for the synthesis of 2-hydroxyphenazine based on the Pd-catalyzed N-arylation of aryl bromides. Org Lett 7: 1549–1552. [DOI] [PubMed] [Google Scholar]
- 17. Mehnaz S, Baig DN, Jamil F, Weselowski B, Lazarovits G (2009) Characterization of a phenazine and hexanoyl homoserine lactone producing Pseudomonas aurantiaca strain PB-St2, isolated from sugarcane stem. J Microbiol Biotechnol 19: 1688–1694. [DOI] [PubMed] [Google Scholar]
- 18. Pierson LS 3rd, Thomashow LS (1992) Cloning and heterologous expression of the phenazine biosynthetic locus from Pseudomonas aureofaciens 30–84. Mol Plant Microbe Interact 5: 330–339. [DOI] [PubMed] [Google Scholar]
- 19. Pierson LS, Gaffney T, Lam S, Gong F (1995) Molecular analysis of genes encoding phenazine biosynthesis in the biological control bacterium Pseudomonas aureofaciens 30–84. FEMS microbiology letters 134: 299–307. [DOI] [PubMed] [Google Scholar]
- 20. Mavrodi DV, Ksenzenko VN, Bonsall RF, Cook RJ, Boronin AM, et al. (1998) A seven-gene locus for synthesis of phenazine-1-carboxylic acid by Pseudomonas fluorescens 2–79. J Bacteriol 180: 2541–2548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Dwivedi D, Johri B (2003) Antifungals from fluorescent pseudomonads: biosynthesis and regulation. Current Science 85: 1693–1703. [Google Scholar]
- 22.Du X, Li Y, Zhou W, Zhou Q, Liu H, et al. (2013) Phenazine-1-carboxylic acid production in a chromosomally non-scar triple-deleted mutant Pseudomonas aeruginosa using statistical experimental designs to optimize yield. Appl Microbiol Biotechnol. [DOI] [PubMed]
- 23. Zhou Q, Su J, Jiang H, Huang X, Xu Y (2010) Optimization of phenazine-1-carboxylic acid production by a gacA/qscR-inactivated Pseudomonas sp. M18GQ harboring pME6032Phz using response surface methodology. Appl Microbiol Biotechnol 86: 1761–1773. [DOI] [PubMed] [Google Scholar]
- 24. Menezes SP, dos Santos JL, Cardoso TH, Pirovani CP, Micheli F, et al. (2012) Evaluation of the allergenicity potential of TcPR-10 protein from Theobroma cacao. PLoS One 7: e37969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Zhang J, Wang W, Lu X, Xu Y, Zhang X (2009) The stability and degradation of a new biological pesticide, pyoluteorin. Pest Manag Sci 66: 248–252. [DOI] [PubMed] [Google Scholar]
- 26. Chin AWTF, Thomas-Oates JE, Lugtenberg BJ, Bloemberg GV (2001) Introduction of the phzH gene of Pseudomonas chlororaphis PCL1391 extends the range of biocontrol ability of phenazine-1-carboxylic acid-producing Pseudomonas spp. strains. Mol Plant Microbe Interact 14: 1006–1015. [DOI] [PubMed] [Google Scholar]
- 27. Mavrodi DV, Bonsall RF, Delaney SM, Soule MJ, Phillips G, et al. (2001) Functional analysis of genes for biosynthesis of pyocyanin and phenazine-1-carboxamide from Pseudomonas aeruginosa PAO1. J Bacteriol 183: 6454–6465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Toohey J, Nelson C, Krotkov G (1965) Toxicity of phenazine carboxylic acids to some bacteria, algae, higher plants, and animals. Canadian Journal of Botany 43: 1151–1155. [Google Scholar]
- 29. Keel C, Voisard C, Berling C, Kahr G, Défago G (1989) Iron sufficiency, a prerequisite for the suppression of tobacco black root rot by Pseudomonas fluorescens strain CHA0 under gnotobiotic conditions. Phytopathology 79: 584–589. [Google Scholar]
- 30. Blumer C, Haas D (2000) Iron regulation of the hcnABC genes encoding hydrogen cyanide synthase depends on the anaerobic regulator ANR rather than on the global activator GacA in Pseudomonas fluorescens CHA0. Microbiology 146: 2417–2424. [DOI] [PubMed] [Google Scholar]
- 31.Hunter RC, Asfour F, Dingemans J, Osuna BL, Samad T, et al. (2013) Ferrous iron is a significant component of bioavailable iron in cystic fibrosis airways. MBio 4.. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The expression profile of protein PhzO in E. coli BL21. (A) The expression of PhzO in E. coli BL21. Lane 1: Whole cell lysate of BL21 harboring pET28a; M: Premixed Protein Marker (Low); Lane 2: Whole cell lysate of BL21 harboring pET28a-phzO. (B) The purification of PhzO. M: Premixed Protein Marker (High); Lane1: Whole cell lysate of BL21 harboring pET28a; Lane 2: PhzO purified by Ni2+-nitrilotriacetic acid chromatography. The gel was loaded with 5 ng of purified PhzO (lane 2).
(TIF)
(A): HPLC analysis of the conversion of PCA to 2-OH-PHZ in E. coli BL21 in a PCA transformation assay. (a) Only PCA was detected from cultures of BL21 harboring pET28a after incubation for 72 h; (b) PCA, 2-OH-PCA and 2-OH-PHZ were detected from cultures of BL21 harboring pET28a-phzO after 12 h; (c) PCA and 2-OH-PHZ were detected from cultures of BL21 harboring pET28a-phzO after 72 h; (d) BL21 harboring pET28a without the addition of PCA (B): production of pigments in LB medium after 72 h incubation: (a) E. coli BL21 harboring pET28a; (b) E. coli BL21 harboring pET28a-phzO.
(TIF)