Abstract
Liriomyza sativae and L. trifolii (Diptera: Agromyzidae) are two highly invasive species of leafmining flies, which have become established as pests of horticultural crops throughout the world. In certain regions where both species have been introduced, L. sativae has displaced L. trifolii, whereas the opposite has occurred in other regions. These opposing outcomes suggest that neither species is an inherently superior competitor. The regions where these displacements have been observed (southern China, Japan and western USA) are climatically different. We determined whether temperature differentially affects the reproductive success of these species and therefore if climatic differences could affect the outcome of interspecific interactions where these species are sympatric. The results of life table parameters indicate that both species can develop successfully at all tested temperatures (20, 25, 31, 33°C). L. sativae had consistently higher fecundities at all temperatures, but L. trifolii developed to reproductive age faster. Age-stage specific survival rates were higher for L. sativae at low temperatures, but these were higher for L. trifolii at higher temperatures. We then compared the net reproductive rates (R 0) for both species in pure and mixed cultures maintained at the same four constant temperatures. Both species had significantly lower net reproductive rates in mixed species cultures compared with their respective pure species cultures, indicating that both species are subject to intense interspecific competition. Net reproductive rates were significantly greater for L. sativae than for L. trifolii in mixed species groups at the lower temperatures, whereas the opposite occurred at the higher temperature. Therefore, interactions between the species are temperature dependent and small differences could shift the competitive balance between the species. These temperature mediated effects may contribute to the current ongoing displacement of L. sativae by the more recent invader L. trifolii in warm climatic areas of China.
Introduction
Species displacement, the elimination of a formerly established species from a habitat as a result of direct or indirect competitive interactions with another species, is a potentially widespread phenomenon [1], [2]. Evidence indicates that in most cases where two species interact in multiple environments, one species maintains its competitive superiority over the other [2]. An exception to this pattern has been observed in interactions between two leafminer species in the genus Liriomyza (Diptera: Agromyzidae). L. sativae Blanchard and L. trifolii (Burgess) have both displaced and been displaced by the other in different locations [2], [3].
These closely related species have similar life histories [4]. Females oviposit directly into foliar tissue, and the larvae forage by mining through the palisade mesophyll of leaves. Mature larvae cut an exit hole, from which they crawl out and drop to the ground to pupate. Both species are highly polyphagous and can be severe pests of numerous horticultural crops [5].
The two species are endemic to the Americas [6], but they have rapidly spread throughout the world through human mediated transport [4]. In China, L. sativae was first found in October 1993 on Hainan Island, and spread northwardly throughout China within a few years [7], [8]. L. trifolii was first recorded in Guangdong province and Hainan Island in 2005 and 2006, respectively [9]. Since then L. sativae has been displaced by L. trifolii on Hainan Island, but it has persisted as an important pest in inland areas of China. In the USA, L. trifolii rapidly replaced L. sativae as the predominant leafminer pest of vegetables and ornamentals in the Mediterranean climates of California and in other regions of the western USA, following its introduction from Florida in the 1970s [10], [11]. In Japan, L. trifolii and L. sativae were first found in 1990 and 1999, respectively [12], [13], and each species rapidly expanded its range soon after their introductions [14]. In Kyoto Prefecture, L. trifolii and L. sativae were found to coexist on the same host plants in 1999. However since 2000, L. sativae has become the dominant species, while L. trifolii is now rarely found [15].
Where the displacement of one of these species by the other has been studied, no unique mechanisms have been identified as causing the displacements. The main reason for the displacement of L. sativae by L. trifolii in Hainan Island, China and California, USA, has been thought to be the lower susceptibility of L. trifolii populations to many commonly used insecticides [2], [3]. In contrast, Abe and Tokumaru [13] concluded that the reason for the displacement of L. trifolii by L. sativae in Kyoto Prefecture of Japan is the higher fecundity of L. sativae and differential effects of the local parasitoid complex on the two Liriomyza species. These mechanisms are likely to be mediated by other local environmental conditions, including temperature.
Temperature is one of the most important factors affecting the distribution and abundance of poikilothermic animals, including insects [16], [17]. The latitudinal and altitudinal distributions of Liriomyza species are directly dependent on environmental temperature [18], [19]. When reared individually, femalesL. Sativae tend to produce more eggs than do females of L. trifolii throughout the temperature ranges [20]. However when coexisting in nature, L. trifolii tends to outperform L. sativae in higher-temperature regions, such as in Hainan, China and California, USA, where as L. sativae tends to outperform L. trifolii in lower-temperature regions, such as in Kyoto, Japan [13]. Reports from other organisms, such as fishes, show that temperature drives differential competitive interaction among species [21]–[23], and temperature also differentially affects the fitness of insecticide resistant and susceptible aphids, Myzus persicae, and house flies, Musca domestica [24].
Mean air temperatures during the growing season in agricultural areas of Hainan, China and California, USA, tend to be higher than in Kyoto, Japan. For example, the average daily mean and maximum temperaturesare greater for 11 months of the year in Sanya, Hainan Island, China and in Fresno, California, USA than in Kyoto, Japan (Western Regional Climate Center, http://www.wrcc.dri.edu/; China Meteorological Administration, http://www.cma.gov.cn/; and World Weather Online, http://www.worldweatheronline.com/). Mean daily maximum temperatures per month are up to 17°C greater in Sanya than in Kyoto, and up to 6°C great in Fresno than in Kyoto. Consequently, differential effects of temperature on the reproductive success and competitive interactions of these species may contribute to displacements occurring in different directions in these localities.
To determine if temperature-mediated variation in reproductive success could account for species displacements occurring in different directions, we conducted a series of studies comparing the reproductive success and life table parameters of L. sativae and L. trifolii under different temperature regimes.
Materials and Methods
Ethics statement
No specific permissions were required for these locations or activities. The location is not privately-owned or protected in any way. The field studies did not involve endangered or protected species.
Insect Strains
Populations of both L. sativae and L. trifolii were collected and maintained according to previously described methods [3]. Briefly, individuals of both species were collected from a cowpea, Vigna unguiculata L. Walp., field in Sanya, Hainan Province (18.25°N, 109.50°E), in 2007. Both populations were subsequently reared separately on cowpea plants at the Sanya Experiment Station under controlled conditions (25±1°C, 70±10% relative humidity [RH], 16∶8 light∶dark [L∶D] photoperiod). Additional wild collected flies were added regularly to these colonies to reduce potential effects of inbreeding. Thirty individuals from each population were identified to species by microscopic observation and/or mitochondrial sequence analysis [25], [26] before initiation of the experiments in 2012, and the results showed that the colonies only contained individuals of the appropriate species.
Population dynamics in response to temperature
To assess the potential of L. sativae and L. trifolii populations to increase under low density at different temperatures, the demographics of each species were determined on cowpea plants, according to the method described by Lanzoni et al. [27]. We selected four experimental temperatures (20, 25, 31, 33°C) that are within the range of temperatures at which each species can develop and at which they are routinely exposed to in the environment [20]. For each temperature and each species, ten plastic pots (11 cm in top diameter, 9 cm in bottom diameter, and 13 cm in height) each containing four bean plants (first true leaves fully expanded) were exposed to leafminer adults for 3–5 h. To minimize adverse effects of intraspecific competition among larvae [28], [29], exposure time was regulated according to the number of adults (shorter exposure time if flies density was high and vice versa) to limit the number of larvae ≤8 per leaf. After exposure, bean plants were checked for the presence of flies, and transferred to environmental chambers at constant temperatures of 20, 25, 31 or 33±1°C, 75±15% RH, and a photoperiod of 16∶8 (L∶D). Progress in immature development and mortality of egg and larval stages were assessedevery 8 h (0600, 1400 and 2200 hours). Eggs were located using transmitted light at 25–50× magnification. If the number of eggs within a leaf exceeded 8, the leaf was not used in the experiment. The initial number of eggs used in each temperature ranged from 85 to 120 and 90 to 118 per replicate for L. sativae and L. trifolii, respectively.
The period during which a larva was outside the leaf before pupariation was included as part of the pupal development time. After pupariation, offspring were transferred individually to small glass scintillation vials, and checked daily for adult eclosion. These individuals were held at their respective larval rearing temperatures.
Newly emerged adults were sexed and subsequently paired in rearing containers with bean plants and maintained at their respective larval rearing temperatures. Males from rearing colonies were added in cases where the number of males was less than the number of females, or if males in the mating pairs died before the female. Bean plants were replaced with new ones daily. Longevity of both sexes and female fecundity, as determined by the number of eggs laid, were checked daily.
Data on survivorship, longevity, and female daily fecundity of L. sativae and L. trifolii were analyzed according to age-stage, two-sex life tables [30]–[32], using the computer program TWOSEX-MSChart [33]. Total preovipositionperiod (TPOP) was calculated as the duration between the birth and the first oviposition day for each individual female. The population parameters calculated were intrinsic rate of increase (r), gross reproductive rate (GRR), net reproductive rate (R0) and the mean generation time (T). The intrinsic rate of increase was estimated by using the iterative bisection method and the Euler-Lotka equation:
| (1) |
with the age indexed from 0[31], in which lx is the age-specific survival rate and mx is the age-specific fecundity of the total population. The mean generation time was defined as the length of time that a population would need to increase to R0-fold of its size (i.e., erT = R0) at the stable age-stage distribution. The mean generation time was calculated as T = (lnR0)/r. Before analysis, development time and fecundity were square root transformed. As the assumptionsof normal distribution for parametric analysis were not fulfilled, the data were analysed using Mann-Whitney tests (U tests) (Sigmaplot12.0, Systat Software Inc., Chicago, IL) to evaluate the differences between the two leafminer species. A bootstrap technique [34] was used to estimate the means and standard errors of population parameters. The differences in the population parameters were compared by using the Tukey-Kramer technique.
Comparison of reproductive success in pure and mixed species cultures
To assess interspecific competition between L. trifolii and L. sativae under different temperatures, a series of laboratory experiments was undertaken, with methods similar to those used by Tantowijoyo and Hoffmann [19]. Temperature settings for the experiments were 20, 25, 31, and 33°C, with ±0.5°C fluctuation. At each temperature, three experimental treatments were established: L. trifolii and L. sativae in mixed species cultures, L. sativae in pure culture, and L. trifolii in pure culture. For each experimental replicate, twenty newly-emerged (<24 h) adults were released into a mesh cage. In the mixed species cultures, there was a 1∶1 ratio of L. sativae to L. trifolii. Equal numbers of females and males were used for each species in all replicates. Cages were 30×50×47 cm and held one pot containing four bean plants. Bean plants were 3–4 weeks post-sowing and had two fully-developed true leaves and three cotyledons. Experiments for both species under each temperature were run simultaneously. There were three replicate cages for each temperature and species composition treatment.
Every 1 day (for 31 and 33°C), 2 days (for 25°C), or 4 days (for 20°C), bean plants were replaced by new ones. By doing so, comparable numbers of larvae per leaf (range 19 to 28 individuals) were achieved across the different temperature conditions. Infested plants were kept in separate boxes without adult flies.
When it became apparent that larvae were ready to exit their mines, infested leaves were harvested and stored in plastic containers to collect puparia. Puparia were collected and placed into plastic vials for adult eclosion. Plants and insects were maintained at their respective experimental temperature throughout the trial. Adults were counted upon eclosion and identified to species based on microscopic observation and/or mitochondrial sequence analysis [6], [35].
Comparisons of net reproductive rates (R 0) were used to assess competitive performance between the two leafminer species [20], [36]. Briefly, R 0 was defined as the number of offspring produced by a leafminer species divided by the initial number of that species. R 0 values were log-transformed (lnR 0) before analysis. Analysis of variance (ANOVA) was used to determine if reproductive success was influenced by species, culture type (single or mixed species), temperature, and the interactions of these factors. The full model included the fixed effects of species (1 df), culture type (pure or mixed species; 1 df), temperature treatment (3 df) and their interactions. To determine responses to temperature within each species and culture type, the temperature effect was partitioned into single-degree-of freedom contrasts to test for linear and quadratic trendsin the response of reproductive success [37].
Intraspecific competition has been observed in both species [38], [39]. If interspecific competition for a species was more intense than intraspecific competition for that species, we would expect that its R 0 in mixed culture to be lower than the R 0 in pure culture. Interspecific differences in R 0 in mixed populations would indicate where one species has a competitive advantage over the other. If the intensity of interspecific competition varied with temperature, then temperature could mediate the outcome of interspecific interactions.
Results
Comparison of population dynamics in response to temperature
The developmental periods for each stage, adult longevity, preoviposition period, and female fecundity of the two leafminer species under different temperatures are given in Table 1. Significant interspecific differences in some of these life history traits were observed. Where there were differences between the species at the same temperature, rates were faster for L. trifolii than for L. sativae. Most importantly from a population dynamics perspective, the mean total preoviposition period (TPOP), which is the age at which a female begins oviposition, was significantly less for L. trifolii than for L. sativae at all temperatures (Table 1). This difference would allow L. trifolii females to begin reproducing at an earlier point in their life than L. sativae could.
Table 1. Developmental duration of various life stages and female fecundity of L. trifolii and L. sativae when reared at different constant temperatures.
| Statistic | Species | Temperature (°C) | |||
| 20 | 25 | 31 | 33 | ||
| Egg stage (d) | L. sativae | 4.77±0.05 b## | 2.30±0.04 a | 1.89±0.03 b | 1.76±0.05 b |
| L. trifolii | 4.64±0.05 a | 2.21±0.04 a | 1.52±0.05 a | 1.58±0.05 a | |
| Larval stage (d) | L. sativae | 8.79±0.06 b | 4.64±0.07 a | 3.57±0.06 a | 3.29±0.06 a |
| L. trifolii | 8.39±0.92 a | 4.45±0.06 a | 3.63±0.07 a | 3.06±0.03 a | |
| Pupal stage (d) | L. sativae | 14.58±0.05 a | 9.49±0.05 a | 7.63±0.09 a | 7.25±0.13 a |
| L. trifolii | 14.39±0.09 a | 9.23±0.07 a | 7.42±0.09 a | 6.91±0.06 a | |
| Total preoviposition period (TPOP) (d)# | L. sativae | 28.16±0.12 b | 16.36±0.15 b | 13.05±0.14 b | 12.5±0.23 b |
| L. trifolii | 27.46±0.21 a | 15.83±0.11 a | 12.51±0.13 a | 11.35±0.09 a | |
| Fecundity (eggs/female) | L. sativae | 77.67±1.85 b | 146.70±3.71 b | 111.68±3.82 b | 72.86±2.79 b |
| L. trifolii | 52.37±1.47 a | 108.52±3.11 a | 101.37±2.90 a | 68.14±2.59 a | |
TPOP, total preoviposition period, the duration from birth to first reproduction.
Means within each statistical category and temperature followed by the same letters are not significantly between the two species (P>0.05).
The mean fecundity for individual females also varied with temperature (Table 1), but regardless of temperature, the mean number of eggs laid by L. sativae females was greater than that for L. trifolii females. However, the differences in fecundity between the species became less pronounced with increasing temperature. For example, at 20°C, L. sativae females laid >48% more eggs than did L. trifolii females, but the difference was <7% at 33°C.
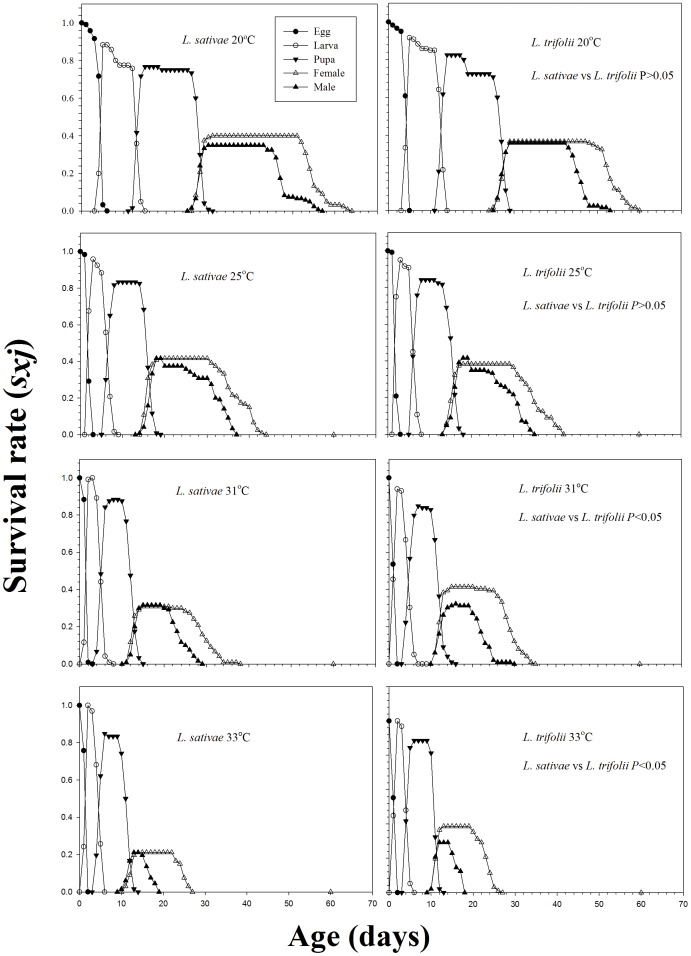
The curves for the age-stage survival rate (sxj) show the probability that an egg will survive to age x and stage j (Fig. 1). At lower temperatures, the probabilities that a newly laid egg of L. trifolii would survive to the adult stage (0.73 and 0.80 at 20, and 25°C, respectively) were comparable with those of L. sativae (0.75 (Mann-Whitney tests (U tests) P>0.05) and 0.83 (Mann-Whitney tests (U tests) P>0.05) at 20 and 25°C, respectively). However, at higher temperatures, the probabilities that L. trifolii progeny would survive to the adult stage (0.75 and 0.73 at 31 and 33°C, respectively) were significantly higher than those for L. sativae (0.67 (Mann-Whitney tests (U tests) P<0.05) and 0.48 (Mann-Whitney tests (U tests) P<0.05) at 31 and 33°C, respectively).
Figure 1. Age-stage specific survival rate (sxj) of Liriomyza sativae and L. trifolii under different constant temperatures.
These biological differences were subsequently expressed in the differences in the intrinsic rate of increase and other life table parameters between the species (Table 2). Because of its faster development time to reproductive age, L. trifolii had a significantly shorter generation time than L. sativae at all temperatures. At lower temperatures (20 and 25°C), the intrinsic rate of increase (r) was greater for L. sativae than for L. trifolii. However, at the higher temperatures (31 and 33°C), these parameters were greater for L. trifolii than for L. sativae. These differences resulted primarily from the greater preadult mortality of L. sativae at higher temperatures (Fig. 1) and the longer time it took L. sativae to begin reproduction (Table 1). Greater fecundity coupled with similar survival rates meant that at lower temperatures (20 and 25°C), the gross reproductive rates (GRR) and the net reproductive rates (R0) for L. sativae were higher than those for L. trifolii (Table 2). However, at higher temperature (31 and 33°C), these two reproductive rates were lower for L. sativae than for L. trifolii. In terms of numbers of eggs laid, the fecundity of L. sativae was higher than that of L. trifolii (Table 1). However, its survival rates declined with temperature, whereas survival rates of L. trifolii were relatively constant across the temperature range (Figure 1).This difference would allow L. trifolii to have greater reproductive success at higher temperatures despite laying fewer eggs.
Table 2. Estimates of life table population parameters (x ± SE) for Liriomyza sativae and L. trifolii reared under different constant temperatures.
| Population parameter | Species | Temperature (°C) | |||
| 20 | 25 | 31 | 33 | ||
| r(d−1) | L. sativae | 0.096±0.004 a# | 0.186±0.005 a | 0.194±0.082 b | 0.171±0.01 b |
| L. trifolii | 0.095±0.004 b | 0.178±0.006 b | 0.224±0.008 a | 0.229±0.010 a | |
| GRR | L. sativae | 41.65±4.23 a | 77.28±7.74 a | 61.63±6.74 a | 43.51±6.80 a |
| L. trifolii | 30.82±2.91 b | 55.55±5.88 b | 58.51±6.12 b | 40.38±4.36 b | |
| R0 | L. sativae | 31.17±3.58 a | 61.10±6.80 a | 34.56±4.83 b | 15.41±3.69 b |
| L. trifolii | 21.82±2.93 b | 41.62±4.95 b | 41.95±5.17 a | 26.24±3.49 a | |
| T | L. sativae | 35.68±0.19 a | 22.10±0.17 a | 18.18±0.20 a | 15.86±0.25 a |
| L. trifolii | 32.13±0.17 b | 20.19±0.19 b | 16.63±0.18 b | 14.23±0.12 b | |
Means of parameter estimates within each temperature with the same lowercase letters are not significantly different (P>0.05).
Comparison of the potential for interspecific competition between two leafminer species under different temperatures
The net reproductive rates for pure cultures of L. sativae showed a pronounced curvilinear response with temperature (Table 3; contrast for linear effect of temperature on R0: F = 24.63; df = 1, 32; P<0.0001; contrast for quadratic effect: F = 74.40; df = 1, 32; P<0.0001). Reproductive success for L. sativae peaked at 25and 31°C; its reproductive success was significantly lower at the extreme lower and upper temperatures of 20 and 33°C than at 25 or 31°C (Table 4; P<0.005). There was no significant difference in net reproductive rates at these two extreme temperatures (P = 0.35).
Table 3. Analysis of variance (ANOVA) results for the net reproductive rate (R0) of Liriomyza sativae and L. trifolii in pure and mixed cultures maintained at four different temperatures#.
| Source | df | F | P |
| Species | 1 | 13.80 | 0.0008 |
| Culture Type | 1 | 265.85 | <0.0001 |
| Temperature | 3 | 89.64 | <0.0001 |
| Species×Culture Type | 1 | 0.13 | 0.72 |
| Species×Temperature | 3 | 32.24 | <0.0001 |
| Culture Type×Temperature | 3 | 0.47 | 0.71 |
| Species×Culture Type×Temperature | 3 | 5.00 | 0.006 |
| Error | 32 |
Values for R0 were logarithmically transformed [ln(R0)] before analysis.
Table 4. Net reproductive rates (R0) for Liriomyza sativae and L. trifolii in pure and mixed cultures maintained at four different temperatures#.
| Temperature (°C) | Pure culture | Mixed culture | Pure culture | |
| L. sativae | L. sativae | L. trifolii | L. trifolii | |
| 20 | 33.17 (26.61–41.34) A##b### | 17.14 (13.75–21.37) Bc | 5.19 (4.17–6.47) Be | 14.91 (11.96–18.59) Ac |
| 25 | 70.54 (56.59–87.93) Aa | 35.49 (28.47–44.23) Ba | 22.36 (17.94–27.86) Bbc | 59.51 (47.75–74.18) Aa |
| 31 | 53.77 (43.15–67.03) Aa | 21.08 (16.91–26.27) Bbc | 24.76 (19.87–30.86) Bb | 52.54 (42.15–65.48) Aa |
| 33 | 28.69 (23.02–35.77) Aa | 7.66 (6.15–9.55) Bd | 16.54 (13.27–20.61) Bc | 32.09 (25.75–40.00) Ab |
Values for R0 were logarithmically transformed [ln (R0)] before analysis. Back transformed means and their 95% confidence limits (in brackets) are presented.
For each species, means within rows followed by the same uppercase letter are not significantly different (P>0.05).
For each culture type, means followed by the same lower case letter are not significantly different (P>0.05).
Reproductive success of pure cultures of L. trifolii also showed a curvilinear increase with temperature; there was a consistent linear increase in R 0 with increasing temperature in addition to the significant quadratic effect of temperature (Table 3; contrast for linear effect of temperature on R 0 : F = 69.59; df = 1, 32; P<0.0001; contrast for quadratic effect: F = 62.42; df = 1, 32; P<0.0001). As with L. sativae, the net reproductive rate for L. trifolii also peaked at 25–31°C (Table 4).
Net reproductive rates for both species in mixed cultures also showed curvilinear relationships with temperature (Tables 3 and 4; L. sativae contrast for linear effect of temperature on R 0: F = 0.42; df = 1, 32; P = 0.52; contrast for quadratic effect: F = 43.24; df = 1, 32; P<0.0001; L. trifolii contrast for linear effect of temperature on R 0: F = 29.55; df = 1, 32; P<0.0001; contrast for quadratic effect: F = 71.54; df = 1, 32; P<0.0001). When in mixed cultures both L. sativae and L. trifolii had lower net reproductive rates than when in pure cultures; this difference occurred at all of the tested temperatures (Table 4). These results indicate that both species suffered from interspecific competition and that interspecific competition was more intense than was any intraspecific competition that may have been present.
Although both species were subject to interspecific competition, the pattern of the effect changed with temperature. At the lower temperatures of 20 and 25°C, L. sativae had significantly higher net reproductive rates in mixed cultures than did L. trifolii. At 31°C, there was no significant difference between the species, but at 33°C, L. trifolii had a significantly higher R0 than L. sativae.
The relative difference in net reproductive rates between mixed and pure cultures for each species also was temperature dependent (Table 4). At the lower temperatures, net reproductive rates for L. sativae in mixed cultures were approximately one half of the corresponding rates for pure cultures. At 31 and 33°C, the net reproductive rates for L. sativae in mixed cultures were only 27–40% of the corresponding rates in pure cultures. However, L. trifolii showed an opposite response. At the higher temperatures, its net reproductive rates in mixed species cultures were approximately half of its corresponding pure culture rates. At the lower temperatures, the effect of L. sativae was much greater, with the net reproductive rates for L. trifolii in mixed species cultures being 35–38% of the rates for L. trifolii in pure cultures.
Discussion
Liriomyza sativae and L. trifolii have become cosmopolitan pest species through human mediated movement over the past 40 years [4]–[6]. Both species have successfully colonized numerous novel habitats with differing climatic and environmental conditions. The two species have also come into contact with each other in these new locations, which has led to cases of displacement occurring. These cases have been bidirectional with the initial colonizing species being displaced by the subsequent invading species. Because of the historic nature of species displacements, cases where a species fails to become established because of the presence of a superior competitor (i.e., the second invader is repulsed) are not likely to be evident.
Our results show that both L. sativae and L. trifolii can reproduce successfully over a similar range of temperatures. In isolation, both species had net reproductive rates far greater than 1 and consequently, their intrinsic rates of increase were greater than 0 at all temperatures. These results indicate that both species would be able to successfully colonize the same habitats and that in isolation their populations would increase and become established.
However, our results demonstrate that the reproductive success of both L. sativae and L. trifolii is severely constrained by the presence of the other species, suggesting that a significant degree of interspecific competition can occur between these species. Although our study was not designed to determine specific mechanisms of interspecific competition, previous studies have identified different forms of intraspecific competition that can occur within these species. Because of the similar life histories of these species, interspecific competition may be expected to operate in a similar manner as intraspecific competition. Larvae are generally confined to their natal leaves, which are spatially isolated from each other. Therefore, intraspecific and/or interspecific competition is likely to occur among larvae within a particular leaf. Interference competition occurs among first and second instars when they may cannibalize one another [39]. Although third instars are not cannibalistic, they consume ∼80% of the total leaf tissue consumed during the entire larval stage [40]. Therefore, this stage would be most responsible for exploitative competition resulting from the depletion of host leaf resources. Size and survival to the adult stage decrease significantly as resources are depleted [38], [39].
L. trifolii would be expected to have a competitive advantage over L. sativae in these types of interactions because of its faster development through the egg and larval stages at most temperatures. This advantage would apply to both interference and exploitative competition. Faster development through the egg and larval stages at most temperatures would allow L. trifolii larvae to escape attacks by L. sativae larvae. In addition, its larvae could obtain necessary resources more rapidly than L. sativae, and at high densities, L. sativae may not be able to acquire sufficient resources to complete development. Such advantages of L. trifolii could increase over multiple generations given the greater speed with which females reach reproductive age. These factors may allow it to outcompete L. sativae, especially at higher temperatures where it had higher survivorship than L. sativae and differences in fecundity are less pronounced. However, these advantages would be counteracted by the greater fecundity and probability of survivorship of L. sativae at lower temperatures, which would change the competitive advantage in favor of L. sativae under those conditions.
Our results provide evidence that significant differences in interspecific interactions can occur within a relatively narrow temperature range. Slight differences in temperature are known to alter the outcome of competitive interactions of species with similar life histories to Liriomyza spp [25], [41]. Other factors, such as differential effects of natural enemies, host plants or insecticides also are likely to influence the populations of these pests, but it is clear that temperature can be a significant factor in altering competitive interactions between these species. Changes in population dynamics and demographics are likely to take place within a growing season as seasonal weather conditions change, but these may also translate over time into long term shifts in the demographics within a region, and ultimately drive the displacement of one species of Liriomyza by another. More favorable climatic conditions therefore may be facilitating the ongoing displacement of L. sativae by L. trifolii in southern China.
Funding Statement
This work was supported in part by the China Agriculture Research System (CARS-25-B-07), the National Natural Science Foundation of China (31201526), and Beijing Municipal Natural Science Foundation (6122026). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. DeBach P (1966) The competitive displacement and coexistence principles. Annual Review of Entomology 11: 183–212. [Google Scholar]
- 2. Reitz SR, Trumble JT (2002) Competitive displacement among insects and arachnids. Annual Review of Entomology 47: 435–465. [DOI] [PubMed] [Google Scholar]
- 3. Gao Y, Reitz SR, Wei Q, Yu W, Lei Z (2012) Insecticide-mediated apparent displacement between two invasive species of leafminer fiy. PLoS ONE 7: e36622 doi:36610.31371/journal.pone.0036622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Parrella MP (1987) Biology of Liriomyza . Annual Review of Entomology 32: 201–224. [Google Scholar]
- 5.Reitz SR, Gao Y, Lei Z (2013) Insecticide use and the ecology of invasive Liriomyza leafminer management. In: Trdan S, editor. Insecticides-Development of Safer and More Effective Technologies. Rijeka, Croatia: InTech. [Google Scholar]
- 6.Spencer KA(1973)Agromyzidae (Diptera) of Economic Importance. Series Entomologica 9: , I-XI. The Hague: Dr.W.Junk B.V. 405 p. [Google Scholar]
- 7.Lei ZR, Wang Y (2005) Liriomyza sativae. In: Wan FH, Zheng XB, Guo JY, editors. Biology and management of invasive alien species in agriculture and forestry. Beijing: Science Press. pp.177–205. [Google Scholar]
- 8.Lei ZR, Wen JZ, Wang Y (1997) Research progress of the vegetable leafminer in China and suggestion in the future control. Annals of Agricultural Science of China Youth Beijing: China Agricultural Press 495–499.
- 9. Xiang J, Lei Z, Wang H, Gao Y (2012) Interspecific competition among three invasive Liriomyza species. Acta Ecologica Sinica 32: 1616–1622. [Google Scholar]
- 10. Reitz SR, Trumble JT (2002) Interspecific and intraspecific differences in two Liriomyza leafminer species in California. Entomologia Experimentalis et Applicata 102: 101–113. [Google Scholar]
- 11. Trumble JT, Nakakihara H (1983) Occurrence, parasitization, and sampling of Liriomyza sativae species (Diptera: Agromyzidae) infesting celery in California. Environmental Entomology 12: 810–814. [Google Scholar]
- 12. Abe Y, Kawahara T (2001) Coexistence of the vegetable leafminer, Liriomyza sativae (Diptera: Agromyzidae), with L. trifolii and L. bryoniae on commercially grown tomato plants. Applied Entomology and Zoology 36: 277–281. [Google Scholar]
- 13. Abe Y, Tokumaru S (2008) Displacement in two invasive species of leafminer fly in different localities. Biological Invasions 10: 951–995. [Google Scholar]
- 14. Tokumaru S, Abe Y (2005) Interspecific hybridization between Liriomyza sativae Blanchard and L. trifolii (Burgess) (Diptera: Agromyzidae). Applied Entomology and Zoology 40: 551–555. [Google Scholar]
- 15. Tokumaru S, Ando Y, Kurita H, Hayashida Y, Ishiyama M, et al. (2007) Seasonal prevalence and species composition of Liriomyza sativae Blanchard, L. trifolii (Burgess), and L. bryomiae (Kaltenbach) (Diptera: Agromyzidae) in Kyoto Prefecture. Applied Entomology and Zoology 42: 317–327. [Google Scholar]
- 16. Bale JS, Masters GJ, Hodkinson ID, Awmack C, Bezemer TM, et al. (2002) Herbivory in global climate change research: Direct effects of rising temperature on insect herbivores. Global Change Biology 8: 1–16. [Google Scholar]
- 17. Hoffmann AA, Sørensen JG, Loeschcke V (2003) Adaptation of Drosophila to temperature extremes: bringing together quantitative and molecular approaches. Journal of Thermal Biology 28: 175–216. [Google Scholar]
- 18. Kang L, Chen B, Wei JN, Liu TX (2009) Roles of thermal adaptation and chemical ecology in Liriomyza distribution and control. Annual Review of Entomology 54: 127–145. [DOI] [PubMed] [Google Scholar]
- 19. Tantowijoyo W, Hoffmann AA (2010) Identifying factors determining the altitudinal distribution of the invasive pest leafminers Liriomyza huidobrensis and Liriomyza sativae . Entomologia Experimentalis et Applicata 135: 141–153. [Google Scholar]
- 20. Tokumaru S, Abe Y (2003) Effects of temperature and photoperiod on development and reproductive potential of Liriomyza sativae, L. trifolii, and L. bryoniae (Diptera: Agromyzidae). Japanese Journal of Applied Entomology and Zoology 47: 143–152. [Google Scholar]
- 21. De Staso J, Rahel FJ (1994) Influence of water temperature on interactions between juvenile Colorado River cutthroat trout and brook trout in a laboratory stream. Transactions of the American Fisheries Society 123: 289–297. [Google Scholar]
- 22. Taniguchi Y, Rahel FJ, Novinger DC, Gerow KG (1998) Temperature mediation of competitive interactions among three fish species that replace each other along longitudinal stream gradients. Canadian Journal of Fisheries and Aquatic Sciences 55: 1894–1901. [Google Scholar]
- 23. Wenger SJ, Isaak DJ, Luce CH, Neville HM, Fausch KD, et al. (2011) Flow regime, temperature, and biotic interactions drive differential declines of trout species under climate change. Proceedings of the National Academy of Sciences 108: 14175–14180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Foster S, Young S, Williamson M, Duce I, Denholm I, et al. (2003) Analogous pleiotropic effects of insecticide resistance genotypes in peach-potato aphids and houseflies. Heredity 91: 98–106. [DOI] [PubMed] [Google Scholar]
- 25. Orengo DJ, Prevosti A (1994) Preadult competition between Drosophila subobscura and Drosophila pseudoobscura . Zeitschrift fuer Zoologische Systematik und Evolutionsforschung 32: 44–50. [Google Scholar]
- 26. Wang S, Lei Z, Wang H, Dong B, Ren B (2011) The complete mitochondrial genome of the leafminer Liriomyza trifolii (Diptera: Agromyzidae). Molecular Biology Reports 38: 687–692. [DOI] [PubMed] [Google Scholar]
- 27. Lanzoni A, Bazzocchi GG, Burgio G, Fiacconi MR (2002) Comparative life history of Liriomyza trifolii and Liriomyza huidobrensis (Diptera:Agromyzidae) on beans: Effect of temperature on development. Environmental Entomology 31: 797–803. [Google Scholar]
- 28. Minkenberg OPJM (1998) Dispersal of Liriomyza trifolii . Bulletin OEPP 18: 173–182. [Google Scholar]
- 29. Scheffer SJ, Lewis Ml (2005) Mitochondrial phylogepgraphy of vegetable pest Liriomyza sativae (Diptera: Agromyzidae): Divergent clades and invasive populations. Annals of the Entomological Society of America 98: 181–186. [Google Scholar]
- 30. Chi H (1988) Life-table analysis incorporating both sexes and variable development rates among individuals. Environmental Entomology 17: 26–34. [Google Scholar]
- 31. Chi H, Liu H (1985) Two new methods for the study of insect population ecology. Bulletin of the Institute of Zoology, Academia Sinica 24: 225–240. [Google Scholar]
- 32. Huang YB, Chi H (2013) Life tables of Bactrocera cucurbitae (Diptera: Tephritidae): with an invalidation of the jackknife technique. Journal of Applied Entomology 137: 327–339. [Google Scholar]
- 33.Chi H (2009) TWOSEX-MSChart: a computer program for the age-stage, two-sex life table analysis. (http://140.120.197.173/Ecology/Download/TwoSex-MSChart.zip).
- 34.Efron B, Tibshirani RJ (1994) An introduction to the bootstrap. Florida: CRC press. 437 p. [Google Scholar]
- 35. Wang S, Lei Z, Wen J, Wang H, Li X, et al. (2013) The complete mitochondrial genome of Liriomyza huidobrensis and comparison with L. trifolii and L. sativae (Diptera: Agromyzidae). Mitochondrial DNA doi:10.3109/19401736.2013.786706 [DOI] [PubMed] [Google Scholar]
- 36. Goodman D (1982) Optimal life histories, optimal notation, and the value of reproductive value. American Naturalist 119: 803–823. [Google Scholar]
- 37.Sokal RR, Rohlf FJ (1995) Biometry: the principles and practice of statistics in biological research. New York: Freeman. 887 p. [Google Scholar]
- 38. Parrella MP (1983) Intraspecific competition among larvae of Liriomyza trifolii (Diptera: Agromyzidae): effects on colony production. Environmental Entomology 12: 1412–1414. [Google Scholar]
- 39. Petitt FL, Wietlisbach DO (1992) Intraspecific competition among same-aged larvae of Liriomyza sativae (Diptera: Agromyzidae) in lima bean primary leaves. Environmental Entomology 21: 136–140. [Google Scholar]
- 40. Parrella MP, Bethke JA (1988) Larval development and leafmining activity of Liriomyza trifoli (Burgess) (Diptera: Agromyzidae). Pan-Pacific Entomologist 64: 17–22. [Google Scholar]
- 41. Carracedo M, Casares P (1986) The influence of species frequency, temperature regime and previous development condition on relative competitive ability between Drosophila melanogaster and Drosophila simulans . Genetica 69: 97–106. [Google Scholar]