Abstract
Sex hormones have diverse immunomodulatory effects that may be involved in the pathogenesis of sepsis. However, the roles of serum sex hormones in predicting outcomes and the severity of organ dysfunction, especially acute kidney injury (AKI), in septic shock patients remains controversial. We prospectively enrolled 107 clinically diagnosed pneumonia-related septic shock patients and serum sex hormone levels were measured on the day of shock onset. The aim of the present study was to investigate the predictive values of serum sex hormones levels for 28-day mortality and organs dysfunction, especially AKI. Compared with survivors, serum levels of progesterone (p<0.001) and estradiol (p<0.001) were significantly elevated in non-survivors. In multivariate Cox regression analysis, serum level of estradiol >40 pg/mL (p = 0.047) and APACHE II score ≥25 (p = <0.001) were found to be independent predictors of day 28 mortality. Inclusion of estradiol levels further enhanced the ability of APACHE II scores to predict survival in patients with high mortality risk. A serum level of estradiol >40 pg/mL was also an independent predictor of concomitant AKI (p = 0.002) and correlated well with severity of renal dysfunction using RIFLE classification. Elevated serum estradiol levels also predicted the development of new AKI within 28 days of shock onset (p = 0.013). In conclusion, serum estradiol levels appear to have value in predicting 28-day mortality in septic shock patients. Increased serum estradiol levels are associated with higher severity of concomitant AKI and predict development of new AKI.
Introduction
Sepsis and septic shock involve dysregulated inflammatory responses caused by interaction between the host immune system and microorganisms. Despite recent progress in care, sepsis and septic shock remain associated with high morbidity and mortality [1], as well as diminished organ function or failure, including the kidneys, lungs, and bone marrow [2]. Among septic shock patients, 60–70% develop acute kidney injury (AKI), which is associated with elevated in-hospital mortality rates that approach 50% [3]–[5]. The RIFLE (Risk, Injury, Failure, Loss, and End-stage renal disease) classification has been proposed to define and classify AKI based on the degree of decrease in urine output and/or increase in the serum creatinine level [6]. In septic patients, RIFLE classifications were found to correlate well with disease severity and clinical outcomes, including mortality [4], [7].
Sex hormones have been reported to have regulatory effects on immune responses. Estradiol can induce the production of pro-inflammatory cytokines and macrophage activation [8], and testosterone was found to have suppressive effects on immune responses and increased susceptibility to infection [9]. Furthermore, epidemiologic studies showing that men are more likely to develop sepsis than women suggest that sex specific hormone levels may affect susceptibility to critical illness [10]. Gender disparities in outcomes following trauma or severe infection have been reported [11], [12]. Limited clinical studies also demonstrated the potential association between serum sex hormones levels and the occurrence and treatment outcomes of septic shock [13], [14]. Information concerning the association between sex hormones and sepsis-related multi-organs dysfunctions are also lacking.
Sepsis-related AKI is associated with increased mortality and morbidity in critically ill patients [4]. Recently, the beneficial effects of estradiol on ischemic AKI were demonstrated in several animal studies [15]–[17]. Therefore, the potential role of serum sex hormones on sepsis-induce AKI, as well as other organs dysfunctions, deserves further investigation. The primary aim of the present study was to investigate the predictive value of serum sex hormone levels when shock onset on outcomes in septic shock patients, and particularly on 28-day mortality. The association between serum sex hormone levels and concomitant organ dysfunction, including AKI, acute respiratory distress syndrome (ARDS), hematologic dysfunction, and metabolic acidosis, were also evaluated.
Materials and Methods
Ethics
The study protocol was approved by the Taipei Veterans General Hospital Institutional Review Board, and the study was conducted in accordance with the Declaration of Helsinki. Written informed consent was obtained from all participants or their authorized representatives before enrollment.
Patients and Settings
This was a prospective, observational study conducted in a referral medical center in Taipei, Taiwan. From January 2008 to December 2011, patients admitted to the medical intensive care unit (ICU) and respiratory ICU were screened for the presence of septic shock associated with pneumonia. Specifically, patients with a diagnosis of pneumonia complicated by septic shock that fulfilled the Surviving Sepsis Campaign criteria for septic shock were included [18]. All patients had hypotension (arterial systolic blood pressure <90 mmHg or mean arterial pressure <65 mmHg despite adequate fluid resuscitation) that required treatment with vasopressor support at the time of enrollment and were recruited within 24 hours after shock onset. The diagnosis of pneumonia was defined by the presence of fever (≥38°C), leukocytosis (≥12,000/mm3) or leukopenia (<4000/mm3), increased sputum production, and new infiltrates on plain chest films [19], [20]. Patients who had underlying malignancy, autoimmune disorders, were taking sex hormone supplements, were less than 18 years old, were premenopausal women, had ARDS caused by non-pulmonary infection, and presented to ICUs with hypotension for more than 24 hours were excluded from enrollment. Demographic characteristics, underlying comorbidities, disease severity, the presence of concomitant organ dysfunction, and the Acute Physiology and Chronic Health Evaluation II (APACHE II) score were determined on the first day of enrollment. The presence of concomitant organ dysfunctions, including AKI, ARDS, hematologic dysfunction, and metabolic acidosis, were defined as previously described [7], [21]; the details of the definitions for organ dysfunction are provided in Table S1. The severity of multi-organ dysfunction was determined by the Sequential Organ Failure Assessment (SOFA) score [22]. The severity of AKI was evaluated according to the RIFLE classification system [7] in which patients were stratified into normal, risk, injury, and failure groups.
Treatment and Outcome Evaluation
Broad-spectrum antibiotics were administered within 1 hour of the onset of septic shock, which is the standard of care in the ICUs where the current study was conducted. The antibiotics were adjusted based on clinical responses and drug susceptibility profiles of bacterial cultures. In addition, all patients were given a physiological dose of corticosteroid (hydrocortisone 200 mg/day, divided in four doses) for refractory hypotension [23]. Survival status at 28 days was set as the measure of treatment outcome.
The development of new AKI within 28 days of enrollment was recorded. New AKI was defined as increased serum creatinine >1.5 times baseline, or GFR decrease >25% of the baseline value, or decreased urine output <0.5 ml/kg/h for 6 hours in patients without concomitant AKI on enrollment.
Blood Sampling and Sex Hormone Measurement
Peripheral blood samples were collected within 24 hours after shock onset. Total serum estradiol and progesterone were measured using radioimmunoassay (RIA) kits (Access estradiol reagent and Access progesterone reagent; Beckman Coulter Inc., Chaska, MN, USA). Serum testosterone was measured using a RIA kit (TEST-CT2, Cis Bio International, Gif sur Yvette, France).
Statistical Analysis
Comparisons of demographic characteristics were carried out using chi-squared or Fisher’s exact tests for categorical variables and two-tailed independent t-test or Mann-Whitney U test for continuous variables. A multivariate Cox proportional hazards regression model with forward stepwise selection procedures was used to identify the risk factors for 28-day mortality. Binary logistic regression analysis was performed to determine independent variables associated with the development of concomitant AKI. Age, gender, comorbitities, and serum sex hormones levels were included in univariate analysis. A p value of less than 0.1 in the univariate analysis was required for a variable to be entered into the multivariate analysis model.
For survival analysis, patients were stratified into subgroups according to serum sex hormone levels. The Kaplan-Meier method was used to estimate survival time and the development of new AKI, and the log-rank test was used to compare mortality between subgroups of patients. Censored analysis was used because observation stopped after a patient was dead or was discharged from the hospital. Receiver operating characteristic (ROC) curves were constructed to determine the predictive abilities of sex hormone levels for survival and the presence of AKI. A p value of less than 0.05 was considered statistically significant for all tests. Statistical analysis was performed using a statistical software package (SPSS version 17.0; SPSS Inc., Chicago, IL, USA).
Results
Patient Characteristics
From January 2008 to December 2011, a total of 151 pneumonia-related septic shock patients were eligible for inclusion. The study profiles and reasons for exclusion are shown in Figure 1. Finally, 107 patients were included for analysis. Among these patients, 50 died within 28 days (28-day mortality 46.7%). The demographic characteristics of the patients are shown in Table 1. The majority of the patients were male (92/107, 86%) and the mean age of the patients included in this study was 79.1±11.1 years. The mean APACHE II score was 27.8±7.5 and more than half of the patients had AKI (58/107, 54.2%) on enrollment. There were no differences in age, gender, or underlying comorbidities between survivors and non-survivors at day 28. When compared with survivors, non-survivors of pneumonia-related septic shock had higher APACHE II score (p<0.001), higher SOFA score (p<0.001), lower PaO2/FiO2 ratio (p<0.001), and a greater number of organ dysfunctions.
Figure 1. Study profile demonstrating the number of cases and reasons for exclusion.
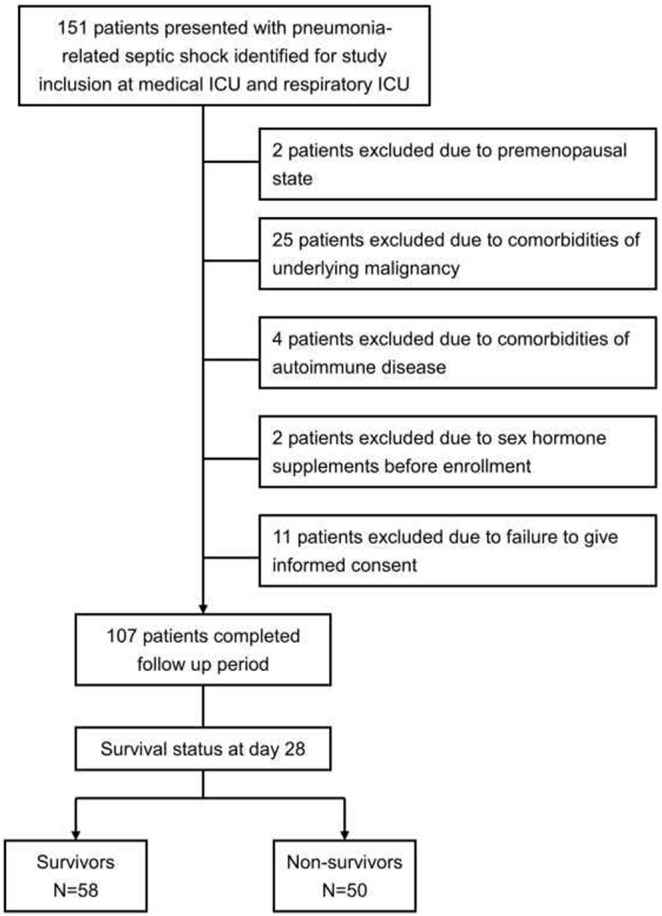
Table 1. Demographic characteristics of patients with pneumonia-related septic shocka.
| Overall | 28-day mortalityb | |||
| Survivors | Non-survivors | p valuec | ||
| Patient numbers | 107 | 57 | 50 | |
| Age, years | 79.1 (11.1) | 78.2 (10.4) | 80 (11.8) | 0.41 |
| Male gender | 92 (86%) | 48 (84.2%) | 44 (88%) | 0.57 |
| Comorbidity | ||||
| Obstructive airway disease | 25 (23.4%) | 13 (22.8%) | 12 (24%) | 0.88 |
| Interstitial lung disease | 11 (10.3%) | 6 (10.5%) | 5 (10%) | 0.93 |
| Congestive heart failure | 13 (12.1%) | 8 (14%) | 5 (10%) | 0.52 |
| Diabetes mellitus | 29 (27.1%) | 14 (24.6%) | 15 (30%) | 0.53 |
| Chronic renal insufficiency | 18 (16.8%) | 8 (14%) | 10 (20%) | 0.41 |
| Pathogens in sputum culture | ||||
| Gram-positive bacteria | 26 (24.3%) | 15 (26.3%) | 11 (22%) | 0.60 |
| Gram-negative bacteria | 85 (79.4%) | 49 (86%) | 36 (72%) | 0.08 |
| Disease severity | ||||
| APACHE II score | 27.8 (7.5) | 23.5 (4.6) | 32.8 (7.1) | <0.001 |
| PaO2/FiO2 ratio | 190.7 (101.3) | 222.6 (95.5) | 156.2 (96.8) | 0.001 |
| Organ dysfunctiond | ||||
| AKI | 58 (54.2%) | 25 (43.9%) | 33 (66%) | 0.022 |
| Hematologic dysfunction | 44 (41.1%) | 19 (33.3%) | 25 (50%) | 0.08 |
| Metabolic acidosis | 29 (27.1%) | 5 (8.8%) | 24 (48%) | <0.001 |
| ARDS | 60 (56.1%) | 23 (40%) | 37 (74%) | <0.001 |
| No. of organ dysfunctions | ||||
| ≥2 organ failure (including shock) | 90 (84.1%) | 43 (75.4%) | 47 (94%) | 0.009 |
| ≥3 organ failure (including shock) | 51 (47.7%) | 15 (26.3%) | 36 (72%) | <0.001 |
| ≥4 organ failure (including shock) | 29 (27.1%) | 4 (7%) | 25 (50%) | <0.001 |
| SOFA score | 11.4 (2.7) | 10.5 (2.3) | 12.5 (2.8) | <0.001 |
| Patients with second source of infection | 19 (17.8%) | 13 (22.8%) | 6 (12%) | 0.14 |
Data are presented as n (%), except for age, APACHE II score, PaO2/FiO2 ratio, SOFA score, hospital LOS and ICU LOS, which are presented as mean (standard deviation).
Pneumonia with septic shock patients were divided according to survival status at day 28.
p value represents differences between survivors and non-survivors of pneumonia-related septic shock.
Organ dysfunction was determined on the day of enrollment.
AKI, acute kidney injury; ARDS, adult respiratory distress syndrome; APACHE II, Acute Physiology and Chronic Health Evaluation II; CAP, community-acquired pneumonia; HAP, hospital-acquired pneumonia; SOFA, Sequential Organ Failure Assessment.
Associations between Serum Sex Hormones and 28-day Mortality
Serum sex hormone levels were comparable between male and female patients (data not shown). When compared with survivors, non-survivors of pneumonia-related septic shock patients had higher progesterone levels (p<0.001), higher estradiol levels (p<0.001), but similar testosterone levels (p = 0.74) (Figure 2).
Figure 2. Serum sex hormone levels in patients with pneumonia-related septic shock.
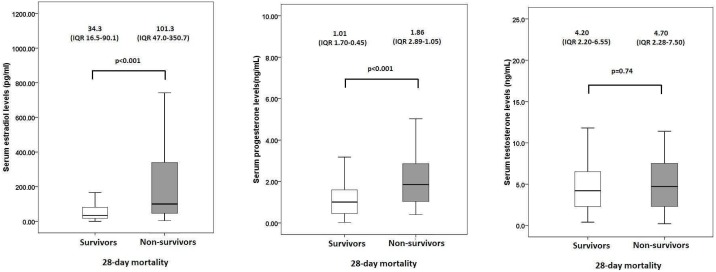
(A) Estradiol and (B) progesterone and (C) testosterone levels in patients with pneumonia-associated septic shock, with survivors at day 28 identified by open bars, and non-survivors at day 28 by shaded bars. Medians and interquartile ranges (IQR) are shown above each plot. Statistical significance was determined with the two-sided Mann-Whitney U test. Extreme values are not shown.
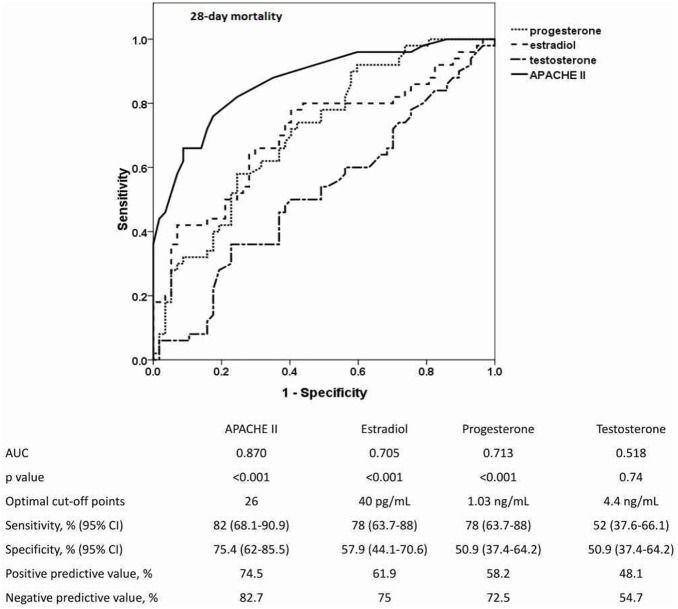
Regarding mortality, ROC curves of day-1 serum sex hormone levels and APACHE II scores in predicting 28-day mortality were constructed and are shown in Figure 3. The APACHE II score was a good predictor of 28-day mortality (AUC, 0.870), and the predictive value of serum levels of progesterone (AUC, 0.713) and estradiol (AUC, 0.705) were acceptable. The AUC for serum testosterone level in predicting 28-day mortality was 0.518. The optimal cutoff points of progesterone and estradiol were 1.03 ng/ml (sensitivity 78%, specificity 50.9%) and 40 pg/ml (sensitivity 78%, specificity 57.9%), respectively.
Figure 3. ROC curves of serum sex hormone levels and APACHE II scores for predicting 28-day mortality.
The areas under the ROC curves (AUCs) for APACHE II scores, estradiol, and progesterone were significantly greater than 0.5. The optimal cutoff points for each sex hormone level and APACHE II score are listed in the attached table, along with their predictive values for the 28-day mortality of patients with pneumonia-related septic shock. ROC, Receiver operator characteristic; APACHE II, Acute Physiology and Chronic Health Evaluation II.
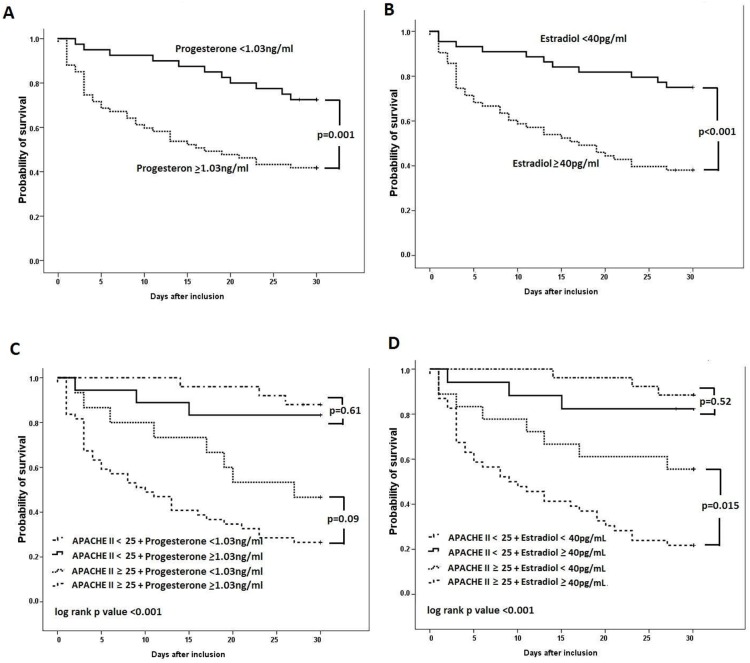
Kaplan-Meier survival curves categorized by the optimal cut-off points of serum sex hormone levels from the ROC curves are shown in Figure 4. Patients with higher serum levels of progesterone and estradiol had significantly higher mortality (progesterone, p = 0.001; estradiol, p<0.001). The survival curves overlapped for patients with different serum testosterone levels (p = 0.87, not shown). In order to investigate the additive value to sex hormones levels on present scoring system, we merged sex hormone levels and APACHE II scores. Kaplan-Meier analyses of survival of the subgroups of patients divided by APACHE II scores and sex hormones levels are also shown in Figure 4. In patients with an APACHE II score ≥25, combining with elevated serum estradiol levels (≥40 pg/mL) had significantly higher mortality than with lower serum estradiol levels (<40 pg/mL). (p = 0.015) (Figure 4D). In patients with an APACHE II score <25, a trend toward higher mortality was found in those with higher estradiol levels (≥40 pg/mL), but without achieving statistical significance (p = 0.52). For the survival analysis of progesterone combining with APACHE II score (Figure 4C), the survival curves separated between subgroups of patients but were all without statistical significance.
Figure 4. Kaplan-Meier survival curves of pneumonia-related septic shock patients, stratified by day-1 serum sex hormone levels.
Patients were categorized into two groups based on the optimal cut-off points of (A) progesterone and (B) estradiol from the ROC curves. Patients were categorized into four groups based on combining (C) progesterone and (D) estradiol optimal cut-off points and high or low Acute Physiology and Chronic Health Evaluation II (APACHE II) scores. Statistical significance was tested with the log-rank test.
Cox regression analyses including clinical factors, serum sex hormone levels and disease severities, which were represented by APACHE II scores, associated with mortality are shown in Table 2. In multivariate analysis, serum estradiol levels ≥40 pg/mL (HR 2.04, CI 1.01–4.10, p = 0.047) and APACHE II scores ≥25 (HR 5.94, CI 2.49–14.20, p<0.001) were independent risk factors of 28-day mortality.
Table 2. Cox proportional hazard models for 28-day mortality prediction by comorbidities and sex hormones in patients with pneumonia-associated septic shocka.
| Univariate Cox Model | Multivariate Cox Model | |||
| Variables | HR (95% CI) | p value | HR (95% CI) | p value |
| Age | 1.01 (0.99–1.04) | 0.34 | ||
| Male gender | 1.20 (0.51–2.83) | 0.67 | ||
| Comorbidity | ||||
| Diabetes mellitus | 1.14 (0.62–2.08) | 0.68 | ||
| Obstructive airway disease | 1.03 (0.54–1.98) | 0.92 | ||
| Interstitial lung disease | 0.89 (0.35–2.24) | 0.80 | ||
| Chronic renal insufficiency | 1.19 (0.59–2.38) | 0.63 | ||
| Congestive heart failure | 0.71 (0.28–1.79) | 0.47 | ||
| Estradiol (E2) >40 pg/mL | 3.41 (1.74–6.67) | <0.001 | 2.04 (1.01–4.10) | 0.047 |
| Progesterone >1.13 ng/mL | 2.83 (1.50–5.33) | 0.001 | 1.65 (0.85–3.18) | 0.14 |
| Testosterone >4.4 ng/mL | 1.10 (0.63–1.91) | 0.74 | ||
| APACHE II ≥25 | 7.73 (3.28–18.22) | <0.001 | 5.94 (2.49–14.20) | <0.001 |
Relative risk and 95% confidence interval were derived from the Cox proportional-hazards regression model.
HR, hazard ratio; CI, confidence interval.
The Value of Serum Hormones in Predicting Organs Dysfunction and AKI
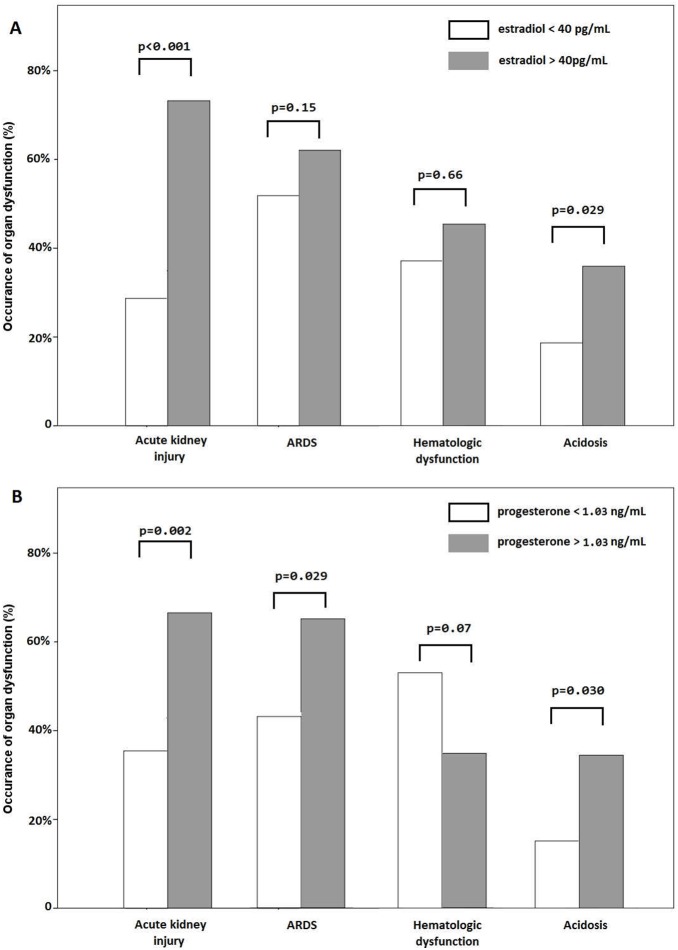
Associations between the presence of organ dysfunction, including AKI, ARDS, hematologic dysfunction and metabolic acidosis, and serum sex hormone levels are shown in Figure 5. Patients with higher estradiol levels showed increased incidence of AKI (p<0.001) and metabolic acidosis (p = 0.029). Patients with higher progesterone levels showed higher incidence of AKI (P = 0.002), ARDS (p = 0.029), and metabolic acidosis (p = 0.030).
Figure 5. Organ dysfunctions in patients with pneumonia-related septic shock.
The occurrence of organ dysfunction at the onset of septic shock was compared based on higher and lower serum (A) estradiol and (B) progesterone levels. Statistical significance was examined using the Pearson’s chi-square test. ARDS, acute respiratory distress syndrome.
Because of the observed close correlation between serum sex hormones and AKI, we further analyzed their predictive values for concomitant AKI. ROC curves of serum sex hormones in predicting the presence of concomitant AKI are shown in Figure 6A. Serum level of estradiol was a good predictor of AKI, with AUCs of 0.788. As shown in Table 3, serum estradiol level was found to be the only independent predictor of the presence of concomitant AKI in the multivariate analysis (OR 4.73, CI 1.80–12.44, p = 0.002). APACHE II scores were not included in our multivariate analysis of concomitant AKI because serum concentration of creatinine was already in the scoring system. The mean serum estradiol and progesterone levels of patients with various levels of AKI severity on the first day of enrollment (as stratified by the RIFLE classification system) are shown in Figure 6B. Patients with more severe AKI had significantly higher estradiol levels. In contrast, there was no correlation between serum progesterone levels and severity of AKI.
Figure 6. Association between serum sex hormone levels and the presence of acute kidney injury.
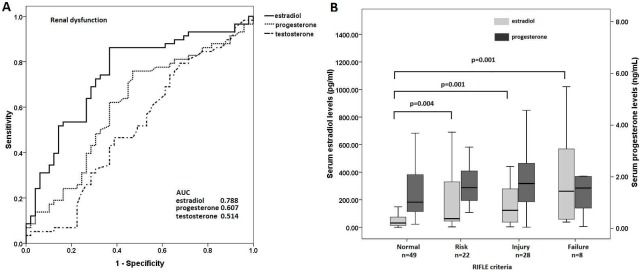
The serum sex hormone levels are determined at the time of study enrollment, i.e. within 24 hours of the development of septic shock. (A) Receiver operator characteristic (ROC) curves of serum sex hormone levels and Acute Physiology and Chronic Health Evaluation II (APACHE II) scores for predicting AKI on enrollment. The areas under the ROC curves (AUCs) for progesterone, estradiol, and APACHE II scores were all greater than 0.5. (B) Serum estradiol and progesterone levels in pneumonia-related septic shock patients with severity of acute kidney injury stratified by RIFLE classification. Medians and interquartile ranges (IQR) of the estradiol (shaded bars) and progesterone levels (solid bars) are shown. Statistical significance was evaluated with the two-sided Mann-Whitney U test. Extreme values are not shown.
Table 3. Univariate and multivariate analysis of predictive factors for the presence of concomitant acute kidney injurya.
| Univariate Analysis | Multivariate Analysis | |||
| Variables | OR (95% CI) | p value | OR (95% CI) | p value |
| Age | 1.05 (1.003–1.09) | 0.034 | 1.03 (0.98–1.08) | 0.26 |
| Male gender | 2.72 (0.86–8.59) | 0.09 | 2.72 (0.74–10.03) | 0.13 |
| Comorbidity | ||||
| Diabetes mellitus | 1.28 (0.54–3.03) | 0.58 | ||
| Obstructive airway disease | 0.89 (0.36–2.19) | 0.80 | ||
| Interstitial lung disease | 0.28 (0.07–1.12) | 0.07 | 0.26 (0.06–1.21) | 0.09 |
| Chronic renal insufficiency | 1.40 (0.50–3.95) | 0.52 | ||
| Congestive heart failure | 1.41 (0.43–4.62) | 0.57 | ||
| Estradiol (E2) >40 pg/mL | 5.96 (2.56–13.91) | <0.001 | 4.73 (1.80–12.44) | 0.002 |
| Progesterone >1.13 ng/mL | 2.73 (1.24–6.02) | 0.013 | 1.33 (0.50–3.50) | 0.57 |
| Testosterone >4.4 ng/mL | 1.04 (0.49–2.23) | 0.92 | ||
Pneumonia-related septic shock patients enrolled for analysis.
OR, odds ratio; CI, confidence interval.
As our analyses demonstrated the strong association between concomitant AKI and serum estradiol levels, but not progesterone, we further evaluated the predictive value of serum estradiol for the development of new AKI. Among 49 patients who had no concomitant AKI on enrollment, AKI subsequently developed in 23 (46.9%) within 28 days after shock onset. As shown in Figure 7A, patients with serum estradiol levels ≥40 pg/mL were more likely to develop AKI (p = 0.035). Kaplan-Meier curves constructed using serum estradiol levels also showed an increased likelihood of developing AKI within 28 days of enrollment in patients with higher initial estradiol levels (p = 0.013) (Figure 7B).
Figure 7. New onset of acute kidney injury within 28 days after septic shock onset.
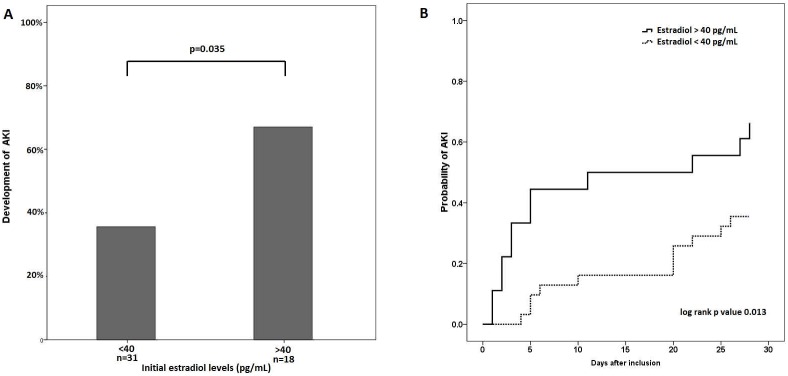
The development of new AKI between patients with higher and lower estradiol levels was compared by (A) proportion, and (B) Kaplan-Meier analysis. Statistical significance was examined with the chi-squared test and log-rank test respectively.
Discussion
In the present study, we found that serum estradiol and progesterone levels significantly increased in non-survivors of pneumonia-related septic shock patients. In multivariate cox regression model, only serum estradiol level was independent predictors for 28-day mortality. We also demonstrated the additive value of estradiol in combination with APACHE II scores for 28-day mortality prediction. Meanwhile, escalated serum estradiol levels correlated well with increased severity of sepsis-related AKI. In multivariate analysis, increased serum estradiol level was an independent factor associated with the presence of AKI on the first day of shock onset. Serum estradiol levels also predicted the development of new AKI within 28 days of shock onset. By contrast, there is no significant difference in testosterone levels regarding survival status and the presence of AKI.
The diverse effects of sex hormones on immune reaction and inflammation have been described extensively in cellular and animal studies. Estrogen appears to exert immunomodulatory effects through increased secretion of cytokines and chemokines from inflammatory cells [24], [25]. Estrogen can also enhance the phagocytic function of macrophages [8]. Exogenous estradiol can upregulate Th17-related inflammation and increase the severity of pneumonia in mice [26]. Both pro-inflammatory and anti-inflammatory effects for progesterone have been reported in autoimmune diseases [27]. Testosterone has been reported to have suppressive effect on immune responses, causing susceptibility to many infections [9]. One study also showed that testosterone-treated macrophages demonstrated increased susceptibility to Leishmania infection [28].
There are only a few reports evaluating serum sex hormone levels in patients with septic shock. One study demonstrated that higher levels of estradiol and estrone, but not of testosterone, were found in critically ill patients with sepsis and septic shock [13]. Another study described a trend of serially increasing serum estrogen levels in non-survivors of septic shock [14]. Consistent with these previous reports, the present study demonstrated the independent predictive value of serum estradiol, but not of progesterone and testosterone, for 28-day mortality in septic shock patients. Moreover, this study had larger sample sizes than previous studies and focused on pneumonia-related septic shock patients. Furthermore, we found that combining estradiol levels with APACHE II scores allows identification of the population at highest risk for mortality among patients with septic shock.
The source of increased estradiol concentration in patients with concomitant AKI remains uncertain. Peripheral aromatase has the ability to convert androgens into estrogens, and could be stimulated by stress [29]. Cellular study demonstrated that aromatase activity could be upregulated by inflammatory cytokines in the presence of glucocorticoid [30]. Another clinical study also reported that aromatase mRNA expression and activities were increased with elevated estrogen levels in patients underwent elective surgery [31]. The main pathway of estrogens metabolism takes place in liver and gastrointestinal tissues. Estradiol will be metabolized in liver and their conjugated form and are excreted via bile, feces and urine [32]. Estrogen conjugates can also be hydrolyzed by intestinal bacteria and excreted in the bile [33]. Therefore, the close correlation between increased serum estradiol and AKI is not simply a consequence of decreased excretion from kidney. More importantly, we identified the predictive value of estradiol level on shock onset in the development of new AKI within 28 days, which was the first report to our knowledge. Therefore, the specific role of estrogen in the pathogenesis of sepsis-related AKI deserves further investigation. Estrogen receptors are presented in the kidney, including mesangial cells, endothelium and vascular smooth muscle cells [34]. Previous experimental animal model demonstrated estrogen can activate inducible nitric oxide synthase (iNOS), leading to increased nitric oxide (NO) production that may protect the kidney from ischemic injury [15]–[17], [35], [36]. However, we did find that elevated serum estradiol levels were associated with an increased likelihood of developing AKI and a greater severity of AKI in septic shock patients. In our speculation, the possible mechanisms may lie on the complicated role of NO in septic shock. NO production is increased in endotoxemia and sepsis, and its related compounds have direct cell toxicity and contribute to profound hypotension in septic shock [37]–[39]. Therefore, despite the renoprotective effect of estrogen in ischemic renal injury, the systemic overproduction of NO in septic shock remains detrimental in sepsis-related AKI. Additionally, a recent study demonstrated a marginal association between C-reactive protein (CRP) and serum estradiol detected by immunoassay, but not in serum estradiol detected by mass spectrometry, in middle-aged and old male population [40]. Although whether the elevated estradiol levels is related to the increased CRP levels remains uncertain, we measured serum estradiol levels by RIA kit because it is still the standard method in clinical practice.
Based on our findings, what’s the practical value of serum estradiol levels in the management of septic shock patients? Although inferior to APACHE II scores, we found that estradiol has the additive value in predicting mortality when combining with APACHE II scores. Moreover, we demonstrated that estradiol could be a novel marker to predict the development of new AKI in septic shock patients. For patients with high estradiol levels, clinicians should keep adequate fluid status, avoid nephrotoxic agents, close monitor renal function and consider early dialysis if the renal function starts to deteriorate. Meanwhile, the specific role of estradiol in the severity stratification of sepsis-related AKI also deserves further investigation.
This study has several limitations worth noting. Only patients with pneumonia-related septic shock were enrolled, and most of them were relatively older with higher disease severity. The homogeneity of the patients reflects the characteristics of a tertiary medical center. Most of the enrolled patients were male, so the results might not hold true for female patients. Although middle-aged individuals were included, we did not enroll premenopausal women in order to avoid the confounding effects from menstruation. Meanwhile, since no differences in serum sex hormone levels were found between male and female patients in the present study, gender disparities in the older population studied might not be an issue. Finally, blood samples for sex hormone measurements were collected only on the first day of shock onset, limiting further evaluation of the changing patterns of sex hormone levels during the course of septic shock in predicting outcomes.
In conclusion, serum estradiol levels determined within 24 hours after the onset of pneumonia-related septic shock are predictive of 28-day mortality in this patient population. Serum estradiol levels are associated with concomitant AKI and correlates well with its severity. Initial serum levels of estradiol, but not of progesterone or testosterone, also predict the development of new AKI within 28 days of shock onset. Further studies are warranted to dissect the specific role of estradiol in sepsis-related AKI and the potential of sex hormone specific therapies in the treatment of septic shock.
Supporting Information
Criteria for organ dysfunction.
(DOC)
Acknowledgments
We greatly appreciated Dr. Shiang-Fen Huang as an independent statistician to review the statistics and assumptions.
Funding Statement
This work was supported by Taiwan National Science Council Research Grant NSC 100-2314-B-075-047-MY3 and Taipei Veterans General Hospital Grants V99A-024, V99C1-167, V99A-023, V100A-002, V100C-159, V101B-027, V101C-065, V102C-025, and V103C-078. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. American College of Chest Physicians/Society of Critical Care Medicine Consensus Conference: definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. Crit Care Med 20: 864–874. [PubMed] [Google Scholar]
- 2. Joannidis M (2006) Acute kidney injury in septic shock–do not under-treat! Intensive Care Med. 32: 18–20. [DOI] [PubMed] [Google Scholar]
- 3. Bagshaw SM, Lapinsky S, Dial S, Arabi Y, Dodek P, et al. (2009) Acute kidney injury in septic shock: clinical outcomes and impact of duration of hypotension prior to initiation of antimicrobial therapy. Intensive Care Med 35: 871–881. [DOI] [PubMed] [Google Scholar]
- 4.Kim WY, Huh JW, Lim CM, Koh Y, Hong SB (2012) Analysis of progression in risk, injury, failure, loss, and end-stage renal disease classification on outcome in patients with severe sepsis and septic shock. J Crit Care 27: 104 e101–107. [DOI] [PubMed]
- 5. Plataki M, Kashani K, Cabello-Garza J, Maldonado F, Kashyap R, et al. (2011) Predictors of acute kidney injury in septic shock patients: an observational cohort study. Clin J Am Soc Nephrol 6: 1744–1751. [DOI] [PubMed] [Google Scholar]
- 6. Bellomo R, Ronco C, Kellum JA, Mehta RL, Palevsky P (2004) Acute renal failure - definition, outcome measures, animal models, fluid therapy and information technology needs: the Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit Care 8: R204–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chen YC, Jenq CC, Tian YC, Chang MY, Lin CY, et al. (2009) Rifle classification for predicting in-hospital mortality in critically ill sepsis patients. Shock 31: 139–145. [DOI] [PubMed] [Google Scholar]
- 8. Liarte S, Chaves-Pozo E, Abellan E, Meseguer J, Mulero V, et al. (2011) 17beta-Estradiol regulates gilthead seabream professional phagocyte responses through macrophage activation. Dev Comp Immunol 35: 19–27. [DOI] [PubMed] [Google Scholar]
- 9. Rahman F, Christian HC (2007) Non-classical actions of testosterone: an update. Trends Endocrinol Metab 18: 371–378. [DOI] [PubMed] [Google Scholar]
- 10. Moss M (2005) Epidemiology of sepsis: race, sex, and chronic alcohol abuse. Clin Infect Dis 41 Suppl 7S490–497. [DOI] [PubMed] [Google Scholar]
- 11. George RL, McGwin G Jr, Windham ST, Melton SM, Metzger J, et al. (2003) Age-related gender differential in outcome after blunt or penetrating trauma. Shock 19: 28–32. [DOI] [PubMed] [Google Scholar]
- 12. Angstwurm MW, Gaertner R, Schopohl J (2005) Outcome in elderly patients with severe infection is influenced by sex hormones but not gender. Crit Care Med 33: 2786–2793. [DOI] [PubMed] [Google Scholar]
- 13. Fourrier F, Jallot A, Leclerc L, Jourdain M, Racadot A, et al. (1994) Sex steroid hormones in circulatory shock, sepsis syndrome, and septic shock. Circ Shock 43: 171–178. [PubMed] [Google Scholar]
- 14. Christeff N, Carli A, Benassayag C, Bleichner G, Vaxelaire JF, et al. (1992) Relationship between changes in serum estrone levels and outcome in human males with septic shock. Circ Shock 36: 249–255. [PubMed] [Google Scholar]
- 15. Satake A, Takaoka M, Nishikawa M, Yuba M, Shibata Y, et al. (2008) Protective effect of 17beta-estradiol on ischemic acute renal failure through the PI3K/Akt/eNOS pathway. Kidney Int 73: 308–317. [DOI] [PubMed] [Google Scholar]
- 16. Shibata Y, Takaoka M, Maekawa D, Kuwahara C, Matsumura Y (2004) Involvement of nitric oxide in the suppressive effect of 17beta-estradiol on endothelin-1 overproduction in ischemic acute renal failure. J Cardiovasc Pharmacol 44 Suppl 1S459–461. [DOI] [PubMed] [Google Scholar]
- 17. Tanaka R, Tsutsui H, Kobuchi S, Sugiura T, Yamagata M, et al. (2012) Protective effect of 17beta-estradiol on ischemic acute kidney injury through the renal sympathetic nervous system. Eur J Pharmacol 683: 270–275. [DOI] [PubMed] [Google Scholar]
- 18. Dellinger RP, Levy MM, Carlet JM, Bion J, Parker MM, et al. (2008) Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock: 2008. Crit Care Med 36: 296–327. [DOI] [PubMed] [Google Scholar]
- 19. Guidelines for the management of adults with hospital-acquired, ventilator-associated, and healthcare-associated pneumonia. Am J Respir Crit Care Med 171: 388–416. [DOI] [PubMed] [Google Scholar]
- 20. Mandell LA, Wunderink RG, Anzueto A, Bartlett JG, Campbell GD, et al. (2007) Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clin Infect Dis 44 Suppl 2S27–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bernard GR, Vincent JL, Laterre PF, LaRosa SP, Dhainaut JF, et al. (2001) Efficacy and safety of recombinant human activated protein C for severe sepsis. N Engl J Med 344: 699–709. [DOI] [PubMed] [Google Scholar]
- 22. Vincent JL, Moreno R, Takala J, Willatts S, De Mendonca A, et al. (1996) The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. Intensive Care Med 22: 707–710. [DOI] [PubMed] [Google Scholar]
- 23. Dellinger RP, Levy MM, Rhodes A, Annane D, Gerlach H, et al. (2013) Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock, 2012. Intensive Care Med 39: 165–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Bengtsson AK, Ryan EJ, Giordano D, Magaletti DM, Clark EA (2004) 17beta-estradiol (E2) modulates cytokine and chemokine expression in human monocyte-derived dendritic cells. Blood 104: 1404–1410. [DOI] [PubMed] [Google Scholar]
- 25. Asai K, Hiki N, Mimura Y, Ogawa T, Unou K, et al. (2001) Gender differences in cytokine secretion by human peripheral blood mononuclear cells: role of estrogen in modulating LPS-induced cytokine secretion in an ex vivo septic model. Shock 16: 340–343. [DOI] [PubMed] [Google Scholar]
- 26. Wang Y, Cela E, Gagnon S, Sweezey NB (2010) Estrogen aggravates inflammation in Pseudomonas aeruginosa pneumonia in cystic fibrosis mice. Respir Res 11: 166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hughes GC (2012) Progesterone and autoimmune disease. Autoimmun Rev 11: A502–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Liu L, Benten WP, Wang L, Hao X, Li Q, et al. (2005) Modulation of Leishmania donovani infection and cell viability by testosterone in bone marrow-derived macrophages: signaling via surface binding sites. Steroids 70: 604–614. [DOI] [PubMed] [Google Scholar]
- 29. May AK, Dossett LA, Norris PR, Hansen EN, Dorsett RC, et al. (2008) Estradiol is associated with mortality in critically ill trauma and surgical patients. Crit Care Med 36: 62–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Zhao Y, Mendelson CR, Simpson ER (1995) Characterization of the sequences of the human CYP19 (aromatase) gene that mediate regulation by glucocorticoids in adipose stromal cells and fetal hepatocytes. Mol Endocrinol 9: 340–349. [DOI] [PubMed] [Google Scholar]
- 31. Spratt DI, Morton JR, Kramer RS, Mayo SW, Longcope C, et al. (2006) Increases in serum estrogen levels during major illness are caused by increased peripheral aromatization. Am J Physiol Endocrinol Metab 291: E631–638. [DOI] [PubMed] [Google Scholar]
- 32. Beleh MA, Lin YC, Brueggemeier RW (1995) Estrogen metabolism in microsomal, cell, and tissue preparations of kidney and liver from Syrian hamsters. J Steroid Biochem Mol Biol 52: 479–489. [DOI] [PubMed] [Google Scholar]
- 33. Adlercreutz H, Martin F, Pulkkinen M, Dencker H, Rimer U, et al. (1976) Intestinal metabolism of estrogens. J Clin Endocrinol Metab 43: 497–505. [DOI] [PubMed] [Google Scholar]
- 34. Potier M, Elliot SJ, Tack I, Lenz O, Striker GE, et al. (2001) Expression and regulation of estrogen receptors in mesangial cells: influence on matrix metalloproteinase-9. J Am Soc Nephrol 12: 241–251. [DOI] [PubMed] [Google Scholar]
- 35. Hutchens MP, Fujiyoshi T, Komers R, Herson PS, Anderson S (2012) Estrogen protects renal endothelial barrier function from ischemia-reperfusion in vitro and in vivo. Am J Physiol Renal Physiol 303: F377–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Hutchens MP, Nakano T, Kosaka Y, Dunlap J, Zhang W, et al. (2010) Estrogen is renoprotective via a nonreceptor-dependent mechanism after cardiac arrest in vivo. Anesthesiology 112: 395–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Petros A, Lamb G, Leone A, Moncada S, Bennett D, et al. (1994) Effects of a nitric oxide synthase inhibitor in humans with septic shock. Cardiovasc Res 28: 34–39. [DOI] [PubMed] [Google Scholar]
- 38. Rudkowski JC, Barreiro E, Harfouche R, Goldberg P, Kishta O, et al. (2004) Roles of iNOS and nNOS in sepsis-induced pulmonary apoptosis. Am J Physiol Lung Cell Mol Physiol 286: L793–800. [DOI] [PubMed] [Google Scholar]
- 39. Hattori Y, Kasai K, Gross SS (2004) NO suppresses while peroxynitrite sustains NF-kappaB: a paradigm to rationalize cytoprotective and cytotoxic actions attributed to NO. Cardiovasc Res. 63: 31–40. [DOI] [PubMed] [Google Scholar]
- 40. Ohlsson C, Nilsson ME, Tivesten A, Ryberg H, Mellstrom D, et al. (2013) Comparisons of immunoassay and mass spectrometry measurements of serum estradiol levels and their influence on clinical association studies in men. J Clin Endocrinol Metab 98: E1097–1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Criteria for organ dysfunction.
(DOC)