Abstract
Infection, cancer and cardiovascular diseases are the major causes for morbidity and mortality in the United States according to the Center for Disease Control. The underlying etiology that contributes to the severity of these diseases is either hypoxia induced inflammation or inflammation resulting in hypoxia. Therefore, molecular mechanisms that regulate hypoxia-induced adaptive responses in cells are important areas of investigation. Oxygen availability is sensed by molecular switches which regulate synthesis and secretion of growth factors and inflammatory mediators. As a consequence, tissue microenvironment is altered by reprogramming metabolic pathways, angiogenesis, vascular permeability, pH homeostasis to facilitate tissue remodeling. Hypoxia inducible factor (HIF) is the central mediator of hypoxic response. HIF regulates several hundred genes and vascular endothelial growth factor (VEGF) is one of the primary target genes. Understanding the regulation of HIF and its influence on inflammatory response offers unique opportunities for drug development to modulate inflammation and ischemia in pathological conditions.
Introduction
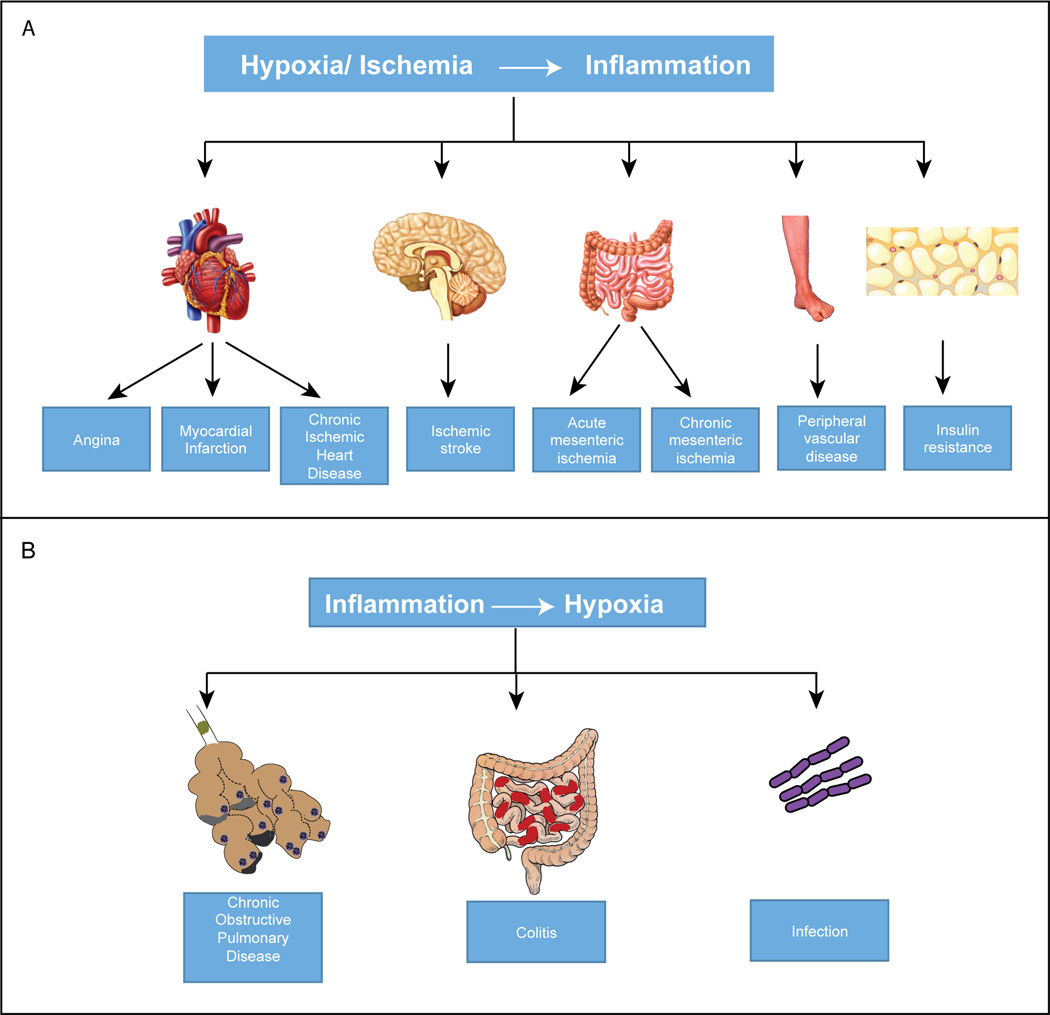
Ischemic diseases are the major cause of morbidity and mortality worldwide. The spectrum of ischemic diseases is wide and includes both acute and chronic conditions. Ischemia can manifest as angina, acute coronary syndrome and chronic ischemic heart disease; as stroke in brain; as acute or chronic mesenteric ischemia in gastrointestinal system and as peripheral vascular diseases in limbs. Ischemic diseases share common risk factors, pathophysiology and etiology (1, 2). It is well known that ischemia initiates an intense inflammatory response that is clinically relevant and worsens the ischemic injury (3, 4). In stroke, cerebral ischemia is accompanied by infiltration of inflammatory cells, which is initiated by ischemia-induced expression of cytokines, adhesion molecules and other inflammatory mediators. Infiltration of inflammatory cells can lead to activation of microglia and astrocytes and persists for hours to days. Inflammation in ischemic tissues exacerbates tissue damage leading to worse neurological outcomes (5–8). The inflammatory changes also occur in ischemic myocardium and peripheral vascular diseases. Ischemic myocardium triggers activation of complement system leading to monocyte and neutrophil recruitment (9, 10). Atherogenesis, an important factor in the pathogenesis of cardiovascular and limb ischemia, is also linked to inflammation (11, 12). Furthermore, obesity is another clinical condition, in which hypoxia is associated with inflammation. The enlarging adipocytes experience an imbalance between demand and supply of oxygen leading to an increase in the secretion of inflammatory adipokines in the fat tissues. Chronic, low-grade inflammation in adipose tissues results in insulin resistance (13). As hypoxia induces inflammation, the reverse is also true in certain other diseases, wherein, inflammation causes hypoxia. Colitis (14), acute lung injury, trauma and infection are some of the examples. Inflammation impairs tissue perfusion because of thrombosis. Vascular leak leads to edema, increased interstitial fluid pressure and compression of vessels (colitis), atelectasis of airways (acute lung injury, chronic obstructive pulmonary disease) and trauma (15). Thus, cellular responses to hypoxia is central to the pathophysiology of major diseases (Fig.1). Hypoxia inducible factor (HIF) is a pivotal transcription factor induced under hypoxia which transactivates target genes such as vascular endothelial growth factor (VEGF). In this review we will focus on the molecular regulation of HIF and VEGF signaling in inflammation and ischemia.
Figure 1.
Hypoxia and inflammation associated diseases. A: Summary of major pathological conditions arising from hypoxia/ischemia-mediated inflammation. B: Shows how inflammation results in hypoxic conditions leading to pulmonary and gastrointestinal diseases.
Hypoxia and ischemia
Evolutionary adaptation to oxygen availability is well documented. However, dramatic variations in the distribution of oxygen concentration has been observed in different tissues and organs of higher mammals. The air we breathe has an oxygen concentration of about 21% (pO2 = 160 mmHg) at sea level. Inhaled air passing through the trachea show a reduction in pO2 to 150 mmHg. At the alveoli the oxygen levels are about 110 mmHg. Oxygenated blood in arteries has pO2 range from 75–100 mmHg. Venous blood has a pO2 of 30–40 mmHg. Oxygen levels differ a lot depending on tissues and tolerance to changing levels of pO2. For example, brain pO2 levels are at 33.8 +/−2.6 mmHg. In contrast, pO2 of kidney is 72 +/− 20 mmHg. Skeletal muscle pO2 is around 29.2 +/− 1.8. Brain tissues cannot tolerate lower pO2 whereas muscle can withstand changes in pO2 levels lower than the resting state. Interestingly, pO2 in developing fetus is much lower than the levels seen in maternal circulation. For example the umbilical vein blood has a pO2 of 20 – 30 mmHg and umbilical artery around 10 – 15 mm Hg. These observations have changed the way mammalian cells are grown in vitro. Induced pluripotent stem cells (iPC) and embryonic stem cells maintain their pluripotency when cultured under hypoxic conditions. Relative pO2 again changes under pathological conditions such as inflammation, cancer, wound healing and tissue remodeling. Inflammation at skin will encounter a different oxygen level than inflammation in the gastrointestinal tract. Dermis and sub dermal tissues have a physiological pO2 of 24 – 35 mmHg whereas the intestine is exposed to higher levels of pO2, 57.6 +/−2.3 mmHg. Hypoxia is defined as a condition in which oxygen supply to tissues or the whole organism is reduced. Ischemia refers to reduced blood flow or perfusion to a region of the body thereby lowering the relative oxygen levels. Each anatomical site in our body has different set point for oxygen requirements.
Inflammation creates hypoxic conditions at the local site due to increased metabolic activity outpacing the availability of oxygen. Furthermore, inflamed tissues often exhibit higher regional temperature, which can affect pO2 at the local site. Thus the hypoxic status of inflammation could be much severe than observed. Hypoxia at the inflammatory site can result in vascular breakdown, coagulation and blockage of blood flow. Tissue necrosis will ensue following prolonged loss of oxygen delivery. There is a strong cross talk between hypoxia and inflammation. Gene expression profile changes significantly when cells are exposed to hypoxia. Transcriptome of monocytes exposed to hypoxia (3 – 5 % O2) was much different than monocytes grown under normoxia (19.95 % O2). Hypoxia alters the repertoire of genes expressed by modulating transcription factors. Hypoxia induced inflammatory response and inflammation can cause hypoxic stress. HIF-1α has a direct effect on NFκB pathway and thereby links innate immunity, inflammation to ischemia. In IKKb knock out mice, Rius et al found that NFκB is a transcriptional activator of HIF-1α (16). Deficiency of IKK beta resulted in abnormal expression of HIF-1 targets including VEGF. IkB alpha interacts with FIH. FIH is a negative regulator of HIF-1α by Asn hydroxylation. IkBa therefore can positively influence HIF-1 activity (17).
Cellular responses to hypoxia - Oxygen sensing molecular switch
Cells adapt to hypoxia by recalibrating their metabolic needs and activating survival pathways. Availability of oxygen is directly linked to energy homeostasis. Lower oxygen levels compromises the function of mitochondria in generating cellular energy currency, ATP, through oxidative phosphorylation, which is the most efficient way of producing ATP from glucose. Cells activate three major pathways to survive the energy crisis created by hypoxia. The first priority for cells under hypoxic stress is to minimize energy demand by conservation. Low-priority, energy consuming processes such as endocytosis is shut down under hypoxia (18). Then, energy production by glycolysis is increased. In order to maintain energy balance, alternate sources of energy are also utilized. Finally, hypoxia forces cells to recycle macromolecules (macroautophagy) and organelles such as mitochondria (mitophagy) to make raw materials available for biosynthetic machinery (19, 20).
Adaptation to nutrients and oxygen availability is an evolutionarily conserved process. From unicellular organisms to mammals, oxygen levels are continuously monitored by oxygen sensing machinery, which reprograms expression of several genes to adapt to changing conditions. Failure to do so will be catastrophic. In this review, we will focus on how mammalian cells respond to hypoxia with a complex regulatory system involving epigenetic and genetic pathways. Semenza and his colleagues discovered hypoxia inducible factor, HIF-1, while studying the regulation of erythropoietin (EPO) gene expression during hypoxic stress. Their pioneering studies identified cis-acting enhancer element in the promoter of EPO (21). Subsequent investigations revealed that HIF-1 binds to a consensus sequence, NCGTG, termed as hypoxia response element (HRE). HRE sites are present in the promoter of hundreds of genes, which are functionally related to hypoxic adaptation. For example, HIF induces expression of GLUT-1, glucose transporter that increases the intracellular levels of glycolytic substrate. At the same time, many of the enzymes needed for increased breakdown of sugar, hexokinase-1, glucose 6-phosphate isomerase, phophofructokinase, PFK1, the rate limiting enzyme of glycolysis, phosphoglycerate mutase-1 (PGAM1), pyruvate kinase type M-2 (PKM2), lactate dehydrogenase (LDHA) are increased by HIF-1-mediated transactivation. While increasing glycolysis, HIF-1 also blocks mitochondrial function and redirects the utilization of glutamine to citrate by upregulating isocitrate dehydrogenase-1 (IDH1) and aconitase1. This pathway is necessary to maintain fatty acid synthesis during hypoxia since, the production of acetyl-coA from pyruvate is prevented during hypoxia by the inhibition of pyruvate dehydrogenase enzyme by pyruvate dehydrogenase kinase1 (PDK1). Details of metabolic pathways regulated by HIF-1 under hypoxia is elegantly summarized in a recent review by Semenza (22). Two recent studies have further confirmed the critical role of HIF-1 in glutamine utilization under hypoxia (23, 24). Reductive carboxylation is a preferred pathway to generate citrate and acetyl-coA from glutamine metabolism. Generally, cells use glutamate dehydrogenase 1 (GLUD1) to produce a-ketoglutarate. However, some malignant cells use a non-canonical to convert glutamine derived aspartate to oxaloacetate/a-ketoglutarate. This pathway was mediated by aspartate transaminase (GOT1) in a HIF-1 dependent manner. HIF-1 seems to be necessary and sufficient for reductive carboxylation, an important metabolic adaptation for the synthesis of fatty acids under hypoxia. Fatty acid precursors are needed for the synthesis of inflammatory mediators. In addition to maintaining energy homeostasis, HIF is also directly responsible for the increased production of EPO, vascular endothelial growth factor (VEGF), necessary for angiogenesis and nitric oxide synthase generating nitric oxide that is responsible for vasodilation and increased blood flow to ischemic tissues. In addition to metabolic and angiogenic regulation, HIF-1 is also responsible for the expression of inflammatory cytokines such as IL-33 in RA synovial fibroblasts. IL-33 binds to IL1Rl1 (ST2) and induce the production of Th-2 cytokines (25). IL-33 has dual role, intracellular and extracellular activation. NH-2 terminus has NLS motif and the full length IL-33 localizes to nucleus and regulates transcriptional activity (26). Proteolysis allows extracellular presence of IL-33 and stimulates IL1RL1 receptors on Th-2 cells (27). It may function as an ‘alarmin’ in directing immune/inflammatory response to the sites of tissue damage, similar to IL-1α and HMGB1 (28). IL-33 is highly expressed in the heavy endothelial venule endothelial cells (HEV EC). HEV EC is directly linked to extravasation of lymphocytes from circulation into lymph nodes by diapedesis (29). IL-33 may have an important role in sustained inflammation associated with diseases such as RA and Crohn’s disease. IL-33 plays a significant role in innate immunity as well (30). Thus HIF is a master regulator of hypoxic adaptation. The repertoire of genes modulated by HIF is being unraveled only recently.
Hypoxia inducible factor (HIF)
There are three members in the family of HIF, HIF-1, HIF-2 and HIF-3. HIF-1 is the founding member. HIF-2 was discovered later (31, 32). HIFs are heterodimeric proteins consisting of an alpha subunit and a beta subunit. Alpha subunit is induced during hypoxia while the beta subunit is constitutively expressed. Beta subunit is called aryl hydrocarbon receptor nuclear translocator (ARNT). HIF belongs to the PER-ARNT-SIM (PAS) subfamily of basic helix-loop-helix (bHLH) family of transcription factors. Both alpha and beta subnits have similar structural domains. NH-2 domain contains a bHLH domain that mediates DNA binding. Central region has two PAS domains, PAS-A and PAS-B, which facilitate heterodimerization and a Cterminal region that regulates recruitment of co-activators and co-repressors (TAD, transactivation domain). TAD binds to co-activators such as CBP/p300, histone acetyltransferases/deacetylases and target gene specific factors such as Smad3 and C/EBP alpha. Most important functional domain of HIF-1, 2alpha is the oxygen dependent degradation domain (ODDD), which determines the stability of the protein under different oxygen concentrations. HIF-3a is less studied and splice variants of HIF-3a have been suggested to play a dominant negative role in regulating HIF-1-mediated transactivation. HIFs are vital for developmental angiogenesis. Deletion of HIF-1 or HIF-2α leads to embryonic lethality at E10.5 stage due to defects in cardiovascular development (33–35). Conditional knock out mice were then generated to investigate the critical role of HIFs in developmental and pathological angiogenesis.
Regulation of HIF-1
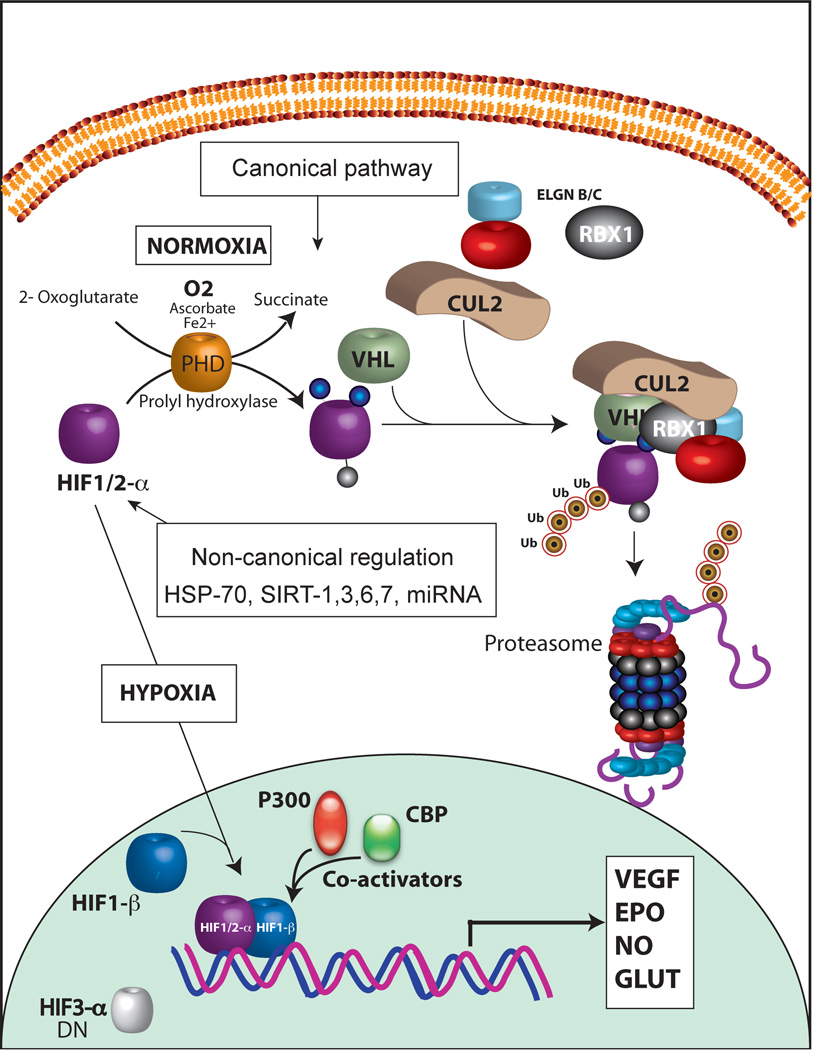
Intracellular HIF levels and its localization are dependent on oxygen levels. HIF-1α will be considered as a prototypical example in this review. In normoxia, HIF-1α undergoes posttranscriptional modifications in the ODDD region. Prolyl hydroxylases (PHD1,2,3) are key oxygen sensing molecular switches. PHDs are a family of 2-alphaketoglutarate-dependent dioxygenases. PHDs use ascorbic acid, iron and oxygen as cofactors in modifying prolyl residues in the presence of 2-alphaketoglutarate (oxoglutarate). PHDs hydroxylate two proline residues located at position 402 and 564. Hydroxylated HIF-1α is then recognized by von Hippel Lindau (VHL) protein and assembled into E3 ligase complex consisting of VHL, Cullin-2, Elongin B/C and ring box protein 1 (RBX1), VCBCR complex. Subsequent polyubiquitation of HIF-1α targets it to 26S proteasome for degradation (canonical pathway, Fig. 2). This process is very efficient and as a result very low levels of HIF-1α are maintained under normoxia. Under hypoxia however, PHDs cannot function efficiently due to lack of oxygen, a cofactor, and HIF-1α hydroxylation is attenuated. Thus, HIF-1α degradation is prevented in low oxygen concentration. HIF-1α then heterodimerize with HIF-1β and translocate to the nucleus. Though PHD-dependent hydroxylation is the predominant mechanism of HIF-1α regulation, another posttranslational modification may also play a role in HIF-1α stability. A lysine residue at position 532 of ODD domain is acetylated by acetyl-transferase arrest defective-1 (ARD1) enzyme in an oxygen dependent manner. Acetylated HIF-1α binds to VCBCR complex efficiently and thereby results in enhanced degradation of HIF-1α. Unlike PHD enzymes, oxygen levels do not directly affect the functional activity of ARD1 but ARD1 levels change under hypoxia by transcriptional regulation. Role of ARD1 in HIF-1α regulation however remains controversial. Another important regulatory pathway that impinges upon the functional activity of HIF-1α is, hydroxylation of an asparagine residue at position 803. Factor inhibiting HIF-1, FIH, mediates the hydroxylation of Asn803 and prevents HIF-1 complex interaction with co-activators, CBP/P300. Inability to interact with co-activators attenuates the ability of HIF-1 to transactivate target genes. FIH activity is directly affected by oxygen, iron and oxoglutarate, which is very similar to the requirements for PHD enzymes. Additionally, HIF-1α is also phosphorylated by mitogen-activated protein kinase (MAPK) p42/p44 and p38, which increases stability and translocation into the nucleus. Other post-translational modifications such as S-nitrosylation increases the transactivating function of HIF-1 and SUMOylation on the other hand represses HIF-1 function. Furthermore, chaperone proteins HSP70 and HSP90, which are stress-induced protective factors, can sequester HIF-1α and prevent its degradation (non-canonical regulation). Chaperone-mediated autophagy seems to affect HIF-1 stability whereas macroautophagy did not influence HIF-1 stability (36). In addition, sirtuins, NAD+-dependent histone deacetylases, influence stability of HIF. Sirt1 was found to differentially deacetylate HIF-2α and not HIF-1α (37, 38). However, another study found that Sirt1 could stabilize HIF-1α (39). Thus role of Sirt1 in HIF-1 activity remains inconclusive and contextual. A recent study by Semenza’s group showed that Sirt7 physically interacts with HIF-1α and HIF-2α and negatively regulates their levels. Sirt7 mediated effects on HIF were found to be independent of the enzymatic activity (40). Thus, complex regulatory pathways fine-tune the stability and function of HIF-1 under hypoxic stress.
Figure 2.
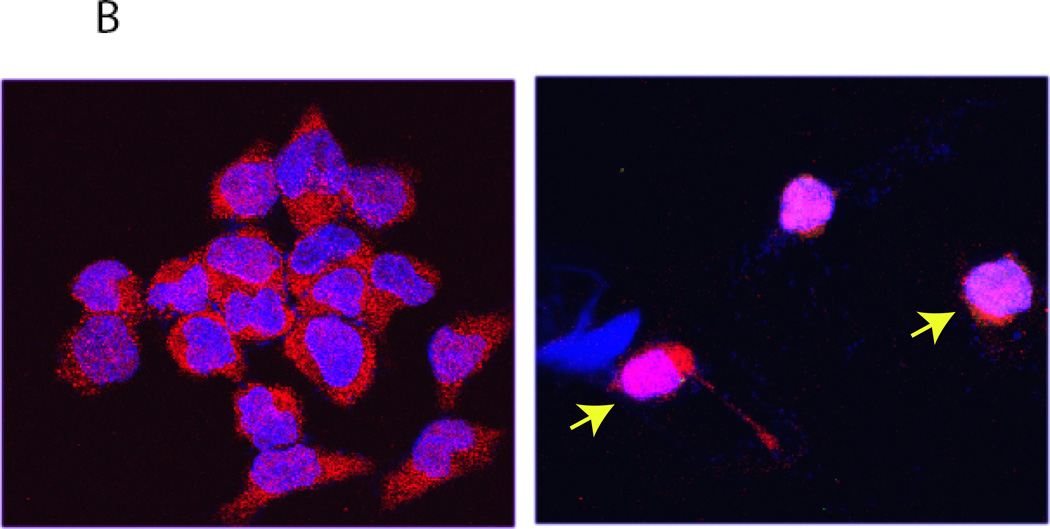
A: HIF-1 regulation. Schematic diagram shows canonical regulation of HIF-1α. In normoxia, HIF-1α is modified by prolyl-4-hydroxylases (PHD). PHD enzymes (PHD1, PHD2, PHD3) are Fe2+ and oxoglutarate dependent dioxygenases that use oxygen as a cofactor. PHD2 is involved in the regulation of developmental angiogenesis. PHD2 Gene deletion increases angiogenesis and erythropoiesis. Hydroxylation of proline residues in HIF-1α is recognized by VHL, tumor suppressor protein. Subsequent assembly of ubiquitin ligases results in polyubiquitination of HIF-1α and proteasomal degradation. HIF-2α is also regulated by a similar mechanism. Hypoxia and iron chelation can inhibit PHD activity and thereby prevents proteasomal degradation of HIF-1/2α. In addition, HIF-1α - mediated transactivation is modulated by hydroxylation of an Asn residue and its ability to recruit co-activators. Noncanonical regulation of HIF-1α involves chaperon-mediated sequestration, NFκB-depdendent signaling and microRNA network. B: A representative confocal image showing HIF-2 nuclear translocation in hypoxia. A2780 ovarian cancer cells were exposed to either normoxia or hypoxia for 24 hours. Indirect immunofluorescence using an antibody to HIF-2α shows nuclear accumulation of HIF-2α (red). Nuclei are stained with DAPI (blue).
HIF-2α and HIF-3α
HIF-2α is a structurally and functionally related transcription factor. HIF-2α is also referred as EPAS1 and contains structural domain organizations similar to HIF-1α, Basic HLH, PAS and TAD domains. It shares about 48 % homology in primary sequence with HIF-1α. HIF-2α is highly expressed in some tissues and cells such as lung vasculature and carotid body. HIF-1α and HIF-2α transactivate genes that are common to both as well as distinct groups of genes (41, 42). For example expression of IL-8 and Myc in endothelial cells are dependent on HIF-2α and not HIF-1α (43). HIF-2α is a major transcription factor expressed in endothelial cells and seems to play a major role in pathological angiogenesis (44). HIF-2α is also the primary driver for erythropoietin expression. In some experimental conditions, loss of HIF-1α is compensated by an increase in HIF-2α. Current evidence suggest tissue specific role for HIF-1α and HIF-2α in hypoxic responses. Unlike HIF-1α and HIF2-α, HIF-3α has not been extensively investigated. HIF-3α has multiple splice variants and expressed in tissue specific manner. Splice variants, HIF-3α2 and HIF-3α4 are shown to act as dominant-negative regulator of HIF-1α function (45, 46). As HIF-3α suppresses transcriptional activity of HIF, it is also called Inhibitory PAS domain protein, IPAS. HIF-3α has been found to interact with Bcl-xL, and may induce proapoptotic signaling (47). IPAS is transcriptionally upregulated by HIF-1 suggesting a negative feedback loop regulating HIF-1-mediated target gene expression (48).
HIF-1 regulation by microRNA
MicroRNAs (miR) are short non-coding RNA 21–23 nucleotide long arising from either introns or exons. There are around one thousand miR characterized in human genome. MicroRNAs are transcribed as a longer (about 70 nt long) strand of RNA with unique stem loop structure (primary miR). Pri-mRNA is transported to the cytoplasm by exportin 5. Inside the cytoplasm they mature into pre-miR and then processed into a double stranded short segment of about 21–23 nucleotide by Dicer. Ago2 binding later selects either one of strand (3p or 5p) to base pair with target complementary sequence in 3’ untranslated region (3’UTR) of mRNA. The first 7 or 8 nucleotides of mature miR are termed ‘seed’ sequence. Binding of seed sequence at target sites in mRNA is necessary for regulating the function of transcripts. A complete match with target sequence results in degradation of target transcript while, an incomplete match affects translational initiation complex by interfering with Eukaryotic translation initiation factor 4G (eIF4G). Net result is inhibition of target protein expression. Each miR can target hundreds of targets and each target mRNA can have target sequence for multiple miR. Hypoxia in inflammatory sites can upregulate or down regulate classes of miR which in turn will have a reciprocal effect on target protein expression. Sequestering miR by competing transcripts can also modulate effects of miR. Our studies have established that under hypoxia, miR-424 is over expressed in endothelial cells. Increase in miR-424 was mediated by PU.1 transcription factor, which was regulated by C/EBP and RUNX2. miR-424 targeted CUL-2, the scaffolding protein which destabilized the E3 ubiquitin complex assembly and prevented degradation of HIF-1/HIF-2α (49, 50). Hypoxia also down regulates the levels of miR-199a-5P, which targets HIF-1α (51, 52). Reduction in miR-199a-5P contributes to increase in HIF-1α transcripts and protein. Thus, a web of miR network fine-tunes the stability of HIF-1 under hypoxia. Furthermore, increased HIF-1α under hypoxia transactivates the expression of miR-210 by binding to HRE elements present in the promoter of miR-210 (53–55). miR-210 is involved in suppressing mitochondrial function by targeting the iron sulfur complex assembly (ISCU), which constitutes the core of electron transport complex I–IV (56, 57). miR-210 further helps in the stabilization of HIF-1 by suppressing the expression of glyceroldehyde 3-phosphate dehydrogenase like 1 enzyme (58, 59). Functional activity of HIF-1 is strengthened by down regulation of FIH by miR-31 (59). Similarly, a recent study has established that miR-183 helps in stabilizing HIF-1 by targeting isocitrate dehydrogenase 2 (IDH2) (60). Thus, HIF-1 levels and its function are regulated by a group of microRNAs which fine-tunes hypoxic adaptation ranging from inflammation (61), angiogenesis (62, 63) and tissue remodeling (64, 65). Biological consequence of altering miR in diseased tissues has become a reality recently. Locked nucleic acid (LNA) strategy has been developed to bind and neutralize activity of specific miR under clinical settings. LNA-miR-92a (66) exerted cell-protective, proangiogenic, and anti-inflammatory effects in pig models. Similarly, miR-15 was found to protect against cardiac ischemic injury (67). These preclinical studies are encouraging and in fact LNA-based miR knock down methods are currently under Phase I/II trials. Clinical success of these methods however depends on effective delivery of LNA-reagents into affected target tissues. Pharmacological stability, tissue penetration, transport across cell membranes, escaping from nucleases and finally specific neutralization of target miR are some of the issues currently being investigated (68–70). Recent advances in the development of nanocarriers and functionalized nanopartlcles have helped in improving microRNA-mediated targeted therapies. These strategies are currently evaluated to knock down miRs (antagomiRs) targeting HIF-1α and VEGF to induce collateral vessel growth in ischemic tissues and to control inflammatory response.
VEGF and VEGF receptors
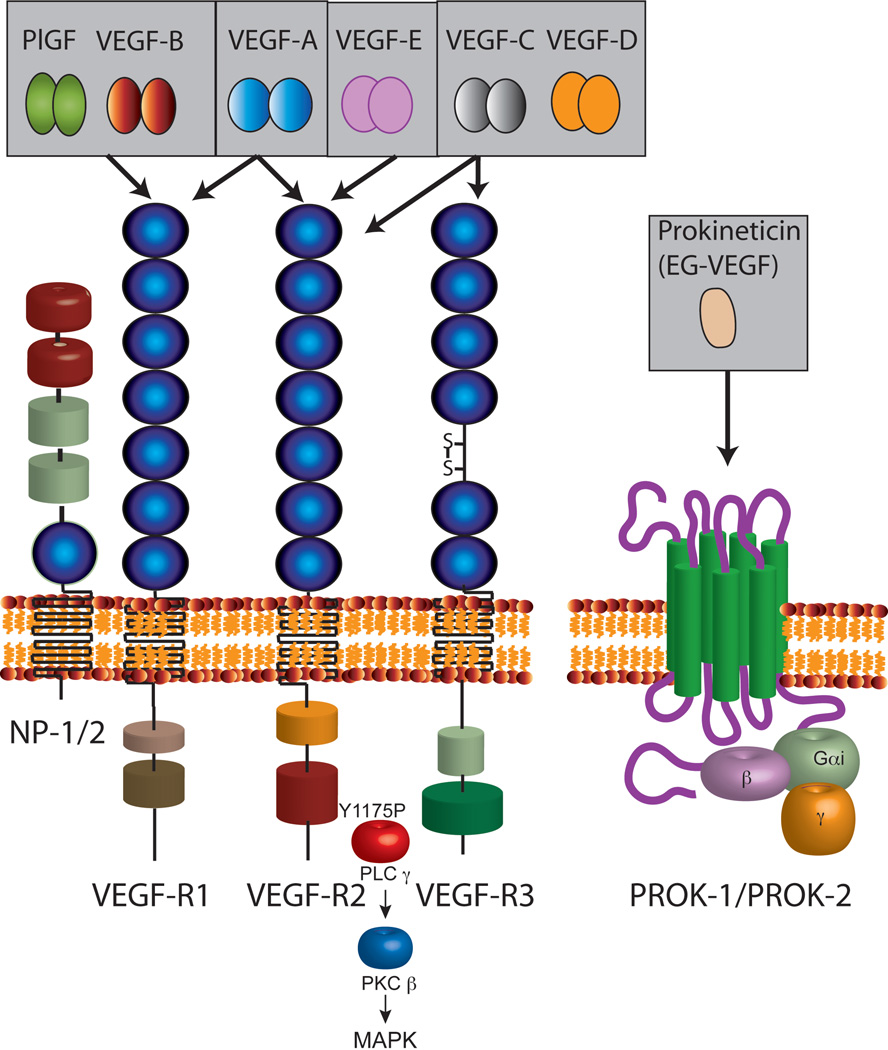
VEGF is a major transcriptional target for HIF-1. Signaling through VEGF receptors have been recently reviewed (71, 72). Three major ligand-receptor systems are upregulated under hypoxic stress, VEGF-VEGFR, Angiopoietins-Tie-1/Tie-2 and Delta-Notch. These signaling pathways help in the recovery from hypoxia-induced tissue damage and inflammation. Coordinated output from these signaling systems determines angiogenesis, blood flow, tissue perfusion, extravasation of inflammatory cells, tissue remodeling and repair. VEGF family of genes contains four members from mammals, one virus-encoded protein (VEGF-E) and a VEGF-like protein found in snake venom, svVEGF. The founding member of this family of proangiogenic growth factor is VEGF-A. Historically, Senger et al., in 1983 identified a protein which was ten thousand-times more potent than histamine to induce vascular permeability and named it as vascular permeability factor, VPF (73). VEGF was described by Leung et al from Genentech in 1989 (74). Sequencing studies confirmed that VPF and VEGF are the same protein. Placenta derived growth factor, PlGF, VEGF-A, VEGF-B, VEGF-C and VEGF-D are induced under hypoxic stress in mammalian cells. VEGF-A is a homodimeric protein. Several splice variants of VEGF-A (121,145,165, 183, 189 and 206 amino acid residues) are generated by alternate splicing. VEGF-A121 lacks a heparin-binding domain (75). Additional splicing of exon 8 at a proximal or distal site results in an anti-angiogenic variant, VEGF (xxx) family of molecules, which can compete with proangiogenic VEGF-A isoforms (76). VEGF-A165 and other longer isoforms have varying ability to interact with heparin sulfate proteoglycans (HSPG) and components of the extracellular matrix. VEGF-A189 and VEGF-A206 in particular contain a stretch of basic amino acid residues, thereby exhibiting stronger affinity to negatively charged HSPG. VEGF-A165 has a lower affinity to HSPGs. VEGF-sequestrated at the extracellular milieu can be conditionally released by matrix metalloproteinases which can be activated under different physiological conditions (77, 78). VEGF-A is a critical mediator of vasculogenesis during development. Deletion of even a single copy (VEGF-A +/−) is lethal and embryos died at E10–E11 stage indicating the importance of VEGF-A gene dosage during development (79, 80). Conditional deletion of VEGF-A in adult mice affects motor neuron development and ophthalmic complications including blindness (81, 82). Consequently, VEGF-A has been found to have acute neuropotective effects in ischemic brain (83). Intraventricular delivery of VEGF stimulated and protected nascent neurons led to a reduction in infarct size. VEGF has a positive influence on Th2 inflammatory response in the lung. VEGF treatment decreased miR-1 levels in the lung. miR-1 targets Mpl. VEGF treatment therefore increased the levels of Mpl and P-selectin in lung endothelium. Attenuation of this pathway by intranasal delivery of miR-1 reduced lung inflammation in experimental models (84). Therefore, VEGF is not only a versatile proangiogenic growth factor, but also limits disease progression in the brain and contributes to inflammation in the lung. Tissue specific role of VEGF can therefore be modulated for therapeutic purposes.
VEGF-B arises from a distinct gene located in chromosome 11. PlGF is encoded by PFG gene which is located in chromosome 14. Both PlGF and VEGF-B bind to VEGFR-1 and coreceptor, Neuropilin-1 (NRP-1) in promoting angiogenesis (85). Neuropilin is a transmembrane glycoprotein originally identified as a coreceptor for members of the semaphorin family of secreted polypeptides (86). Neuropilins have a very short cytoplasmic domain and no kinase activity is associated with NRP. NRP functions as co-receptors to semaphorin receptors, plexins, and regulates axonal guidance during development. Recent studies suggest that NRP locks semaphorins to plexins in forming a ternary signaling complex (87). The isoforms of different members of the VEGF family bind to NRP1 and NRP2, with distinct affinities. Exon 7 of VEGF encodes the key domain essential for the NRP binding. The VEGFR2 forms a complex with NRP1, which is important for VEGFR2 signaling and function in endothelial cells. The VEGF-A121 isoform lacks exon 7 and is therefore unable to bind to NRP1. NRP2 has been found to complex with the receptor for VEGF-C, VEGF-D and VEGF receptor 3, also called fms-related tyrosine kinase receptor 4. Recent studies suggest that VEGF-B has a major role in regulating fatty acid transport across endothelium to tissues (88–90). VEGF-B expression pattern tightly correlated with nuclear encoded mitochondrial proteins constituting electron transport complex. VEGF-B knockout mice showed reduced fatty acid transport across endothelial cells to heart, muscle and brown adipose tissue but increased transport of fatty acids into white adipose tissues. These studies suggest a link between VEGF-B expression and mitochondrial lipid utilization and could have causal relationship with many pathological conditions. For example, peripheral tissue fat deposition contributes to pathobiology of Type 2 diabetes. When VEGF-B null mice were crossed into db/db diabetic mice, the offsprings showed marked improvement in diabetic phenotype. The double transgenic mice showed reduced fat deposition in peripheral tissues, increased glucose uptake in muscles, restored insulin sensitivity and glucose tolerance. These provocative findings suggest that VEGF-B is a novel pharmacological target for therapeutic development against type 2 diabetes and associated cardiovascular problems. Furthermore, absence and neutralization of VEGF-B may aggravate distal neuropathy observed in cancer patients during chemotherapy. VEGF-B is found to protect sensory neurons (91). VEGF-C and VEGF-D bind and activate VEGFR-3 receptor expressed in lymphatic vascular endothelium. VEGF-C is expressed during fetal development and in adult tissues while VEGF-D is not detectable in embryonic tissues but found during postnatal development. Physiological importance of VEGF-C is exemplified by the observation that VEGF-C knockout is embryonically lethal while VEGF-D knockout showed no lethality. Loss of VEGF-C leads to fluid accumulation due to impairment of lymphatic drainage (92, 93).
VEGF receptor signaling
Three major classes of VEGF receptors (VEGFR) are expressed in vascular and extravascular tissues. VEGFR are receptor tyrosine kinases containing an extracellular ligand binding domain, singly membrane spanning and an intracellular domain with a split kinase domain containing a long (60–70 amino acid residues) kinase insert. They are structurally related to PDGFR but contain seven immunoglobulin like (Ig) domains. Another distinction between PDGFR and VEGFR is that the latter lack the YxxM motif of autophosphorylation found in the former. Thus the signaling pathways of VEGFR differ from PDGFR albeit they are structurally related. VEGFR-1 is a high affinity (1–10 pM) receptor for VEGF-A but the binding does not result in strong kinase activation. VEGFR-1 has 10-fold weaker kinase activity when compared to VEGFR-2 (94). Thus VEGFR-1 is generally considered as a non-signaling receptor but has an important biological role in regulating VEGF-mediated signaling through VEGFR-2. VEGF-A binding to VEGFR-2 results in autophosphorylation of Y1175 and recruits PLCg to the phosphorylated site. Binding of SH2 domain of PLCg then activates PKCb leading to stimulation of MAPK pathway (95–97). This signaling pathway is essential for development since transgenic knock in mice having a mutation of Y1175 to F1175 led to embryonic lethality at E9.0 lacking blood vessel development. Similar phenotype was observed in total knockout of VEGFR-2. In addition to Y1175, another tyrosine residue at position 951 is also phosphorylated upon ligand binding. Y951 phosphorylation seems to regulate cell migration induced by VEGF-A (98). A general scheme of VEGF-VEGFR signaling system is shown in Fig. 3.
Figure 3.
Schematic diagram shows VEGF family of growth factors and their receptors. Alternate splicing results in multiple splice variants of VEGF (not shown). Larger variants of VEGF have a heparin binding domain and are sequestered by heparin sulfate proteoglycans (HSPG). Sufatases and proteases secreted into the tissue microenvironment can release HSPG-bound VEGF. Shorter forms of VEGF are secreted and act on distant targets. VEGF binds to multiple VEGFRs. Neuropilin-1 and neuropilin-2 function as co-receptors for VEGF. Protease-mediated release of soluble VEGFR1 (sVEGFR or sFlt-1) can regulate VEGF-mediated signaling by sequestering the ligand. Furthermore, sFlt can heterodimerize with VEGFR2 and inhibit signaling by blocking autophosphorylation.
VEGFA binding to VEGFR-1 does not lead to autophosphorylation of Y1169 site, which is analogous to Y1175 of VEGFR-2 (95, 99). VEGFR-1 stimulation does not generate proangiogenic response in endothelial cells. However, VEGFR-1 has other important physiological role. VEGFR-1 is highly expressed in macrophages implicating its unique role in inflammatory response. VEGFR-1 activation in macrophages activates PI3-AKT pathway in a RACK1 dependent manner and stimulates macrophage cell migration. Thus VEGFA secreted at local inflammatory site can facilitate macrophage migration into target tissues and may help in tissue remodeling. Importance of VEGFR-1 in vascular development is demonstrated in knockout mouse models. VEGFR-1 deletion is embryonically lethal at E8.5 with over production of endothelial cells and complete lack of organized vasculature (100). This phenotype again reiterates the potential negative regulation of vasculogenesis by VEGFR-1. In a subsequent study, the kinase domain of VEGFR1 was deleted. Interestingly, kinase deleted VEGFR1 knockout mice developed normally (101). These studies suggest that ligand-binding domain of VEGFR-1 is important for orderly development of vasculature but not its kinase dependent signaling. However, VEGFR-1 mediated PI3-AKT activation in extravascular cells such as macrophages may still is important in postnatal development and inflammation mediated pathological angiogenesis (101, 102). For example, kinase deleted VEGFR-1 mice show reduced tumor angiogenesis due to impaired functions of tumor-associated macrophages.
VEGFR-3 is preferentially expressed in lymphatic endothelium, discontinuous or fenestrated endothelium of choroid plexus and high endothelial venules indicating an important role in lymphocyte trafficking (103). VEGF-C binding activates PKC and Ras signaling pathways leading to lymphatic endothelial cell proliferation and migration. VEGFR-3 gene deletion leads to embryonic death at E9.5 (104). However, deletion of its ligands VEGF-C and VEGF-D are viable (105). It is likely therefore that VEGFR-3 is stimulated by alternate ligands present in the extracellular matrix. One such candidate has been identified to be Collagen and calcium-binding EGF domains protein-1 (CCBE1). Thus VEGFR-mediated signaling differs during development and depends on cellular and regional context (106, 107).
VEGF in pathological conditions
Inflammatory cells
Inflammation refers to physical and physiological changes observed at the site of injury or infection. The route word for inflammation in Latin is ‘inflammo’ meaning ‘ignite’. Increased redness, heat, pain, and swelling are associated with inflamed tissues. Redness is caused by vasodilation accompanied by vascular stasis causing reduced movement of blood cells. Vasodialation is casued by bradykinin, histamine and VEGF released at the site of tissue damage and nitric oxide generated by vascular endothelium. Stasis is a culmination of changes occurring at the vasculature. Histamine and VEGF induce vascular leak at the site of inflammation leading to exudation of plasma. Vascular permeability increases cell density inside the vessels and interstitial pressure outside the blood vessels. Interstitial pressure can collapse capillaries and increase thrombotic events. Resulting sluggish blood flow causes stasis, which inadvertently helps the innate immune cells to extravasate into tissues. Endothelial cells respond to inflammatory signal by upregulating cell adhesion molecules such as ICAM, P-selectin and E-selectin. E-selectin, CD62, is a cell adhesion molecule selectively upregulated in endothelial cells upon cytokine stimulation. E-selectin has a lectin binding domains at the N and C termini, EGF like domain in the middle along with six-suchi domain repeats (CCR, complement control protein). E-selectin binds to sialyl-Lewisx carbohydrate antigen present on neutrophils, monocytes, eosinophils, T-cells and NK-cells. ESL-1 is described as a counter-receptor for E-selectin and is expressed on inflammatory cells. Expression of E-selectin is induced by P-selectin, IL-1β and TNF-α by transcriptional upregulation (108–111). P-selectin, CD62P, another cell adhesion molecule that is expressed on endothelial cells and platelets is an early responder to inflammatory stimuli (112). P-selectin is stored in vesicles and are quickly expressed on cell surface by vesicle fusion (degranulation). Platelets and endothelial cells respond to thrombin, type II collagen, ATP and respond by expressing P-selectin on cell surface. Similar to E-selectin, P-selectin binds to PSGL-1 ligand expressed on inflammatory cells (113–115). Expression of E and P-selectins are modulated by sheer stress of blood flow and cytokines. Hypoxia alone is not sufficient for E-selctin upregulation but it is superinduced in the presence of cytokines such as TNF-α or LPS.
Intercellular adhesion molecule, ICAM1, and VCAM are other important mediators of inflammatory cell attachment to endothelial cell under hypoxic conditions. ICAM1 (CD54) is a glycoprotein expressed on endothelial cells, macrophages and lymphocytes when stimulated with TNF-α or IL-1α. ICAM binds to LFA-1, an integrin (αL, CD11α; β2, CD18) expressed on lymphocytes and macrophage adhesion ligand-1, MAC-1, (116, 117). ICAM1 can also be induced by RANTES secreted by macrophages and granulocytes. ICAM1 is highjacked by rhinovirus (118) and malaria parasite infected erythrocytes for transendothelial cell migration (119). Recent studies suggest that ICAM1 upregulation during hypoxic stress is independent of HIF-1 but dependent on NFκB-mediated transactivation (120). Even though ICAM1 expression both on macrophages and endothelial cells were independent of HIF-1 but mediated by a novel pathway regulated by prolyl-hydroxylases (PHD) during hypoxia. In a recent study, the role of PHD isoforms on inflammatory response was elucidated in gene knockout models (121). These studies showed that PHD3 is very important for controlled innate immune response during sepsis. PHD3 deficiency (PHD3−/−) aggravated innate immune response leading to early organ failure and death in a HIF-1 and NFκB dependent manner. VCAM-1 (CD106) is an another endothelial cell adhesion molecule upregulated during inflammation and hypoxia (122). VCAM-1 binds to VLA-1 antigen (alpha4 beta1 and alpha4 beta7 integrins) expressed on lymphocytes. VCAM-1 also interact with erzin and Moesin, which act as intermediates of cell membrane (ICAM-2) to actin bundles thereby regulating cell attachment and migration.
Blood borne monocytes and lymphocytes are recruited to inflammatory sites by a complex signaling cascade that involves a plethora of cytokine and chemokines. Circulating monocytes for example attach to cell adhesion molecules over expressed on endothelial cells under hypoxic/inflammatory stimuli (rolling model). Subsequently, monocytes extravasate to reach tissues by a processes called diapesis, squeezing between endothelial cells without affecting barrier functions. Outside the vasculature monocytes differentiate into tissue macrophage or dendritic cells in mediating innate or adaptive immune response respectively. VEGF, CXCL12, endothelial monocyte activating peptide-II (EMAPII) and Angiopoietin-2 (Ang2) mediate extravasation of monocytes under hypoxia. Upon extravasation, human monocyte derived macrophages (hMDM) adapt to hypoxic environment quickly by differentially expressing genes such as FGF, VEGFR and hypoxia-inducible proinflammatory cytokines such as IL-1beta, TNF-alpha, and acute phase protein, IL-6 (123). A comprehensive list of chemokines elaborated under hypoxia is summarized in Table 1 and 2. In addition to chemokines, chemokine receptors are also modulated in monocyte-derived macrophages at hypoxic environment. Of particular interest is that hypoxia inhibits migration of macrophages by inhibiting cell motility promoting factor, and inducing the expression of GRO family of chemokines such as CXCL2 and CXCL3 which are negative regulators of macrophage migration (124). Thus, hypoxia traps monocyte-derived macrophages after their extravasation without affecting their ability to differentiate into immature dendritic cells (iDC). Immature dendritic cells are highly migratory and reach the draining lymph nodes. iDC differentiate to mature CD (mDC), which are retained at the site of inflammation. Hypoxia at the site of inflammation therefore modulates chemokine and chemokine receptor expression in inflammatory cells. One of the final outcomes of these changes is recruitment of monocytes to local site of tissue damage, differentiation of monocytes to tissue macrophage, retention of macrophages at hypoxic site and generation of highly motile immature dendritic cells and less motile mature dendritic cells. These changes are achieved by altering transciptome of monocyte/macrophage by hypoxia. For example, nuclear factor-kappa b (NFκB), the central transcription factor mediating inflammatory response is intricately involved in hypoxia-induced expression of monocyte chemoattractant protein-1 (MCP-1). VEGF induces MCP-1 through the activation of NFκB pathway (125). MCP-1, in turn, can induce VEGF thereby establishing a feed-forward loop in human aortic endothelial cells (126). Inflammatory cytokine, IL-1β, induced NFκB-cyclooxygenase-2 axis was found to stabilize HIF-1 and as a consequence induced VEGF (127). Similarly, TNF-α-induced TLR4-NFκB pathway intersects with HIF-1α mediated inflammatory response as well (128). These studies reiterate cross talk between inflammatory cytokines and hypoxia-induced responses in recruiting and regulating innate immune cells.
Table 1.
*. Hypoxia induced changes in chemokine at inflammatory sites
| Cell source | Chemokine produced | Target cells | Ref |
|---|---|---|---|
| Monocyte | CXCL2, CXCL3, CXCL5, CXCL6 and CXCL8 | Neutrophil | (158, 159) |
| CCL2,CCL8,CCL15, CCL18,CCL19,CCL23,CXCL11 | Macrophages, T-cells, NK-cells, Basophils, DC | (159) | |
| Macrophages | CXCL8 | Neutrophil | (160–162) |
| CCL2 | Macrophages, T-cells, NK-cells, Basophils, DC | ||
| Dendritic cells | CXCL8 | Neutrophil | (163) |
| CCL18, CCL23 | Macrophages, T-cells, NK-cells, Basophils, DC | (163) | |
| Monocytes/Macrophages | CCL20 | iDC, effector and memory T-cells, B-cells | (159, 164) |
| Fibroblasts | SDF-1(Stromal Factor1) or CXCL 12 cell-Derived | Lymphocytes | (165) |
Data based on an excellent review by Bosco et al. (123)
Table 2.
Effect of Hypoxia on Inflammatory cytokines and growth factors
| Cytokines/Chemokines | Levels | Reference | Growth factors |
Levels | Reference |
|---|---|---|---|---|---|
| IL-6 | (166) | VEGF (Vascular endothelial growth factor) | |||
| TNF-α | (167, 168) | FGF (Fibroblast growth factor) | (169, 170) | ||
| IL-1 α, β | (167) | PDGF (Platelet-derived growth factor) | (171) | ||
| IL-33 | (172) | TGF (Transforming growth factor) | (173) | ||
| IL-8 | (174, 175) | EGF* (Epidermal growth factor) | (176) | ||
| IL-18 | (177, 178) | HGF** (Hepatocyte growth factor) | (179) | ||
| IL-20 | (180) | Pleiotrophin/ NEGF1 (neurite growth-promoting factor 1) | (181) | ||
| MIF (Macrophage migration inhibitory factor | ((182, 183) | Angiopoetin-1, 2 (Ang-1,2) | (184) |
Hypoxia promotes ligand-independent EGF receptor signaling
Hypoxia enhances c-Met/HGF receptor expression and signaling
VEGF is effective in treating ischemia-induced damage of neuronal tissues (129). VEGF administration induced not only angiogenesis but also protected neurons from cell death and neurogenesis in preclinical models of focal cerebral ischemia (83). VEGF gene therapy using AAV has a significant impact on reducing neuronal damage following stroke (129). VEGF reduced inflammatory cytokine levels in the brain following ischemic stroke and attenuated immune cell infiltration (130). An alternate approach is to transplant CNS stem cells, which secrete significant amounts of VEGF. Cell-derived VEGF was necessary to recover from ischemic injury. VEGF was necessary to establish BBB and suppress inflammation in addition to neovascularization. Such an approach has been successful in promoting angiogenesis and tissue remodeling at the damaged site (131). VEGF therapy increases pericyte covering, vessel normalization and improved blood flow in experimental animals (132). Same group of researchers have again showed that VEGF mediated signaling and recovery following stroke was attenuated in hyperlipidemia or in ApoE−/− genetic background (133). This raises the potential limitation of VEGF centric therapeutic strategies to improve angiogenesis and blood flow to limit stroke-induced tissue damage.
Inflammation associated with solid tumors
Various in-situ hybridization studies have shown increased VEGF mRNA levels in various solid tumors including carcinomas of lung, gastrointestinal tract, breast, endometrium, urogenital tract and intracranial tumors (134). The expression is particularly found to be correlated to hypoxia in the tumor cells. Although, tumor cells are the major source of VEGF, stromal cells have been identified to be an important source as well (135). Tumor stroma is made of fibroblasts, inflammatory cells, adipocytes and endothelial cells. This unique microenvironment resembles tissues under persistent inflammation in many respects. Tumor hypoxia and inflammation are prognostic in several types of cancers. Higher levels of VEGF secreted by tumor cells and stromal components helps in further mobilization of mesenchymal stem cells and precursors of inflammatory cells from the bone marrow. VEGF induces tumor angiogenesis. Consequently, VEGF inhibitors are effective in inhibiting tumor growth and metastasis. Blocking VEGF and its receptors has been much more effective than monotherapy. Gerber et al showed that chimeric receptor containing first three Ig-like domains of VEGFR-1, common to both human and mouse, resulted in nearly complete suppression of tumor growth in a mouse model of human rhabdomyosacroma (136). VEGF inhibitors combined with chemotherapy and radiotherapy resulted in better tumor suppression compared to either therapy alone (137) (138). Klement et al found that combining a monoclonal neutralizing antibody (DC101) targeting the flk-1/KDR (VEGFR-2) with low dose vinblastine chemotherapy enhanced the anti-vascular effects (139). Currently, many VEGF inhibitors are in various phases of clinical development (antibodies to VEGF, VEGF-R, VEGF-neutralizing aptamers and inhibitors of receptor tyrosine kinases). One of the strategies to inhibit VEGF-VEGFR signaling is a humanized antibody against VEGF, Avastin (Bevacizumab) which has been approved by FDA for clinical use.
Hematological malignancies
Similar to the solid tumors, VEGF is also expressed in a variety of hematological malignancies like T and B-cell lymphomas, acute and chronic myeloid leukemia, multiple myeloma and Burkitt's lymphoma (140). VEGFR-1 and VEGFR-2 are also detected in some leukemia cell lines, however VEGFR-2 is found more frequently compared to VEGFR-1 (141). Additionally, a neutralizing monoclonal antibody IMC-1C11, specific to human VEGFR-2 inhibited proliferation of xenotransplanted human leukemia cells and increased survival in the mouse models (142). Based on the promising results in pre-clinical trials, many of the VEGF inhibitors are currently being tested in the clinical trials of hematological malignancies.
Female reproductive tract
Polycystic ovarian syndrome (PCOS) is an important cause of female infertility characterized by hirsutism, obesity, polycystic ovaries and menstrual irregularities. Hyperplasia of stroma and theca contributes to the excessive androgen production responsible for the symptoms of PCOS. Stromal angiogenesis is an important feature of the polycystic ovaries. VEGF levels are elevated in PCOS patients, compared to healthy females (143). VEGF mRNA levels were also elevated in the cyst walls. An increased expression of VEGF is associated with increased ovarian stromal blood flow, which is believed to disrupt the ovarian autoregulatory mechanisms, thereby leading to uninhibited growth of all cohort follicles. These findings reflect that VEGF may be an important player in the pathogenesis of PCOS. Another important condition characterized by pathologic angiogenesis is endometriosis. Endometriosis is a condition, in which endometrial implants are found outside the uterine cavity. These ectopic implants develop vessels that enable them to survive and grow. Increased VEGF levels are observed in the peritoneal cavity of endometriosis patients (144). Two VEGF inhibitors, a soluble truncated receptor (decoy) and an antibody to VEGF were recently studied in the mouse models and were found to result in significant reduction in the endometrial implant size (145, 146). VEGF is implicated in preeclampsia, a serious obstetrics complication leading to hypertension, proteinuria, and glomerular endotheliosis. Increased circulating levels of soluble VEGFR1 (sFlt1) is observed in pre-eclampsia patients (147). This study demonstrated that endothelial dysfunction was a result of decreased VEGF and PlGF as a consequence of increased levels of sFlt1. Exogenous administration of VEGF and PlGF reversed pre-eclampsia in model systems. Current studies are in progress to evaluate the use of VEGF therapy in pre-eclampsia patients.
Intraocular neovascularization
The conditions associated with intraocular ischemia include diabetes, central retinal vein occlusion, prematurity and wet-type of age-related macular degeneration. These conditions subsequently lead to intraocular neovascularization which is associated with grave consequences including vitreous hemorrhages, retinal detachment and blindness (148), (149). Earlier studies have shown increase in VEGF in aqueous and vitreous humor associated with intraocular ischemia, contributing to the neovascular disorders. Various pre-clinical and clinical studies are currently investigating the effects of VEGF inhibitors in these conditions (150). The VISION trial first showed that pegaptanib, an anti-VEGF agent was able to prevent vision loss in neovascular age related macular degeneration. Fab fragment of a humanized anti-VEGF antibody, Lucentis (Ranibizumab) is now clinically used to treat wet, age-related macular degeneration.
VEGF therapies to rescue ischemic tissues
The ability of VEGF to regulate pathologic angiogenesis in ischemic conditions, led to prospects in vascular occlusive diseases, in which restoration of blood flow is life saving. VEGF targeted therapies have been tried in myocardial infarction, peripheral limb ischemia and stroke (151). An increase in vascularity was observed with adenoviral and plasmid liposome mediated delivery of VEGF165 in limb ischemia patients (152). A double blind randomized controlled trial including diabetic patients with peripheral arterial disease also showed a significant improvement in symptoms in patients treated with intramuscular VEGF165 (153). Patients treated with VEGF had fewer amputations and more skin ulcer healing compared to the placebo group. RAVE, a double-blind trial assessing the intramuscular adenoviral gene transfer of VEGF121 however showed no improvement in the symptoms, in patients with limb ischemia (154). This raises important concerns regarding the difference in efficacy of the various isoforms of VEGF and the importance of consistent method to measure primary endpoint of improved perfusion in different trials.
A phase II KAT trial, assessing the efficacy of VEGF165 gene therapy in patients with coronary artery disease also found improvement in myocardial perfusion, but without any change in rate of restenosis (155). Another trial, Euroinject One, showed significant improvement in the ventricular function in patients treated by intramyocardial VEGF165 plasmid gene transfer (156). However, the results in myocardial infarction have been disappointing in terms of improvement in myocardial stress perfusion and angina class. In an effort to improve these outcomes, Ripa et al conducted a pilot study of combined VEGF165 gene therapy and stem cell mobilization (157). There was still no improvement in primary end point of myocardial stress perfusion probably because of inadequate homing. Future trials should include co-transfer of plasmid encoding SDF-1, a homing factor, timely administration of G-CSF and a consistent method for perfusion assessment. Overall, current evidence suggests numerous factors that could result in lack of benefit in some efficacy end points. Several laboratories are currently investigating these factors and the effects of targeted VEGF therapies in various ischemic diseases.
Concluding remarks
Hypoxia-induced changes at the transcriptome and inflammation-induced cytokine/chemokine responses modulate endothelial cells and innate immune cells. As cancer, cardiovascular and infectious diseases are modulated by hypoxia and inflammation, it provides an opportunity to develop novel drugs to intervene this co-dependent signaling pathways. Hypoxia inducible factor, HIF, is an evolutionarily conserved key transcription factor that regulates vascular biology, angiogenesis, metabolic reprogamming and inflammation. HIF is regulated at the cellular level by canonical pathway involving proteasomal degradation and noncanonical regulation involving HSP-70, NFκB and microRNA. HIF regulates recruitment of inflammatory cells, erythropoiesis, tissue remodeling, and pH homeostasis. Furthermore, HIF-mediated metabolic adaptation creates a niche for stem cells to thrive in a hypoxic microenvironment. One of the primary target genes regulated by HIF is VEGF-A. VEGF-family of growth factors is necessary for developmental angiogenesis, collateral vessel growth in peripheral arterial disease, stroke, and myocardial infraction. Inducing local production of VEGF is therapeutically useful in limiting hypoxia/ischemia-induced tissue damage. Conversely, pathological angiogenesis can be inhibited by strategies to neutralize VEGF or inhibit its signaling pathways. VEGF family of growth factors bind to three distinct receptors. Pleotropism in receptor binding and differences in tissue distribution of receptor/co-receptor offers therapeutic strategies to modulate VEGF signaling. VEGF-induced changes in inflammatory signals and its positive influence on neuroprotection provide a road map for the future development of VEGF-based therapies.
Table 3.
Therapeutic approaches targeting VEGF
| Drug | Mechanism of action | Clinical Use |
|---|---|---|
| Antibodies | ||
| Bevacizumab | Humanized anti-VEGF antibody | Combination chemotherapy for various malignancies including metastatic colorectal cancer, advanced nonsquamous non–small cell lung cancer (NSCLC), renal cell carcinoma and glioblastoma |
| Ranibizumab | Fab fragment of the humanized anti-VEGF antibody | Wet type Age-related macular degeneration, macular edema |
| Other VEGF inhibitors | ||
| Neovastat/ AE-941 | Inhibits binding of VEGF to VEGFR, inhibitor of activity of matrix metalloproteinase-2 and 9 (MMP-2 and MMP-9 | Non-small cell lung cancer, advanced breast and colorectal cancer and psoriasis |
| Pegaptanib | VEGF-neutralizing aptamer; inhibits binding of VEGF to its receptor | Wet type Age-related macular degeneration |
| Small molecular VEGFR kinase inhibitors | ||
| Sorafenib/ BAY 43-9006 | VEGFR2, PDGFRβ, FLT3, c-Kit multitargeted kinase inhibitor | Renal cell carcinoma, hepatocellular carcinoma, Gastrointestinal stromal tumor, angiosarcoma and other malignancies |
| Sunitinib/ SU11248 | multitargeted kinase Inhibitor including VEGFR-1, 2 and 3 | Pancreatic neuroendocrine tumors, gastro-intestinal stromal tumor, renal cell carcinoma and other malignancies |
| Axitinib/ AG013736 | VEGFR-1, VEGFR-2, VEGFR-3 tyrosine kinase inhibitor | Breast cancer and renal cell carcinoma |
| Vatalanib/ PTK787 | VEGFR1, VEGFR2, VEGFR3, PDGFR and Kit tyrosine kinase inhibitor | Advanced solid tumors |
| Cediranib/ AZD2171 | VEGF and PDGF receptor tyrosine kinase inhibitor | Glioblastoma multiforme, sarcoma and other solid tumors |
| Pazopanib | Multi kinase inhibitor including VEGFR-1,2,3, PDGFR-alpha and –beta, fibroblast growth factor receptor, cKIT. | Renal cell carcinoma, soft tissue sarcoma and Gastrointestinal stromal tumor |
| Vandetanib | VEGFR, the epidermal growth factor receptor (EGFR), and the RET-tyrosine kinase inhibitor | Metastatic medullary thyroid cancer |
| VEGF targeted therapies | ||
| VEGF gene therapy | Intramyocardial injection of plasmid VEGF-A165/121, Zinc Finger Protein (ZFP) targeting the VEGF-A promoter | Ischemic cardiovascular disorders, peripheral artery disease and stroke. |
Acknowledgement
This work was supported in part by the following grants, DA012104, DA033881, DA034582, DA031202 and Sparboe Endowment for Women’s Cancer Research.
Footnotes
Conflict of interest There is no conflict of interest.
References
- 1.Weber C, Noels H. Atherosclerosis: current pathogenesis and therapeutic options. Nature medicine. 2011;17(11):1410–1422. doi: 10.1038/nm.2538. [DOI] [PubMed] [Google Scholar]
- 2.Noels H, Weber C. Catching up with important players in atherosclerosis: type I interferons and neutrophils. Current opinion in lipidology. 2011;22(2):144–145. doi: 10.1097/MOL.0b013e328344780b. [DOI] [PubMed] [Google Scholar]
- 3.Braunwald E. Cardiovascular science: opportunities for translating research into improved care. The Journal of clinical investigation. 2013;123(1):6–10. doi: 10.1172/JCI67541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Takahashi M. Role of Innate Immune System in Inflammation and Cardiac Remodeling After Myocardial Infarction. Current vascular pharmacology. 2013 [PubMed] [Google Scholar]
- 5.Tuttolomondo A, et al. Inflammation in ischemic stroke subtypes. Current pharmaceutical design. 2012;18(28):4289–4310. doi: 10.2174/138161212802481200. [DOI] [PubMed] [Google Scholar]
- 6.Iadecola C, Alexander M. Cerebral ischemia and inflammation. Current opinion in neurology. 2001;14(1):89–94. doi: 10.1097/00019052-200102000-00014. [DOI] [PubMed] [Google Scholar]
- 7.Zheng Z, Yenari MA. Post-ischemic inflammation: molecular mechanisms and therapeutic implications. Neurological research. 2004;26(8):884–892. doi: 10.1179/016164104X2357. [DOI] [PubMed] [Google Scholar]
- 8.Kim JY, Kim N, Zheng Z, Lee JE, Yenari MA. The 70 kDa heat shock protein protects against experimental traumatic brain injury. Neurobiology of disease. 2013;58:289–295. doi: 10.1016/j.nbd.2013.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Frangogiannis NG, Smith CW, Entman ML. The inflammatory response in myocardial infarction. Cardiovascular research. 2002;53(1):31–47. doi: 10.1016/s0008-6363(01)00434-5. [DOI] [PubMed] [Google Scholar]
- 10.Marchant DJ, et al. Inflammation in myocardial diseases. Circulation research. 2012;110(1):126–144. doi: 10.1161/CIRCRESAHA.111.243170. [DOI] [PubMed] [Google Scholar]
- 11.Arnaud C, Poulain L, Levy P, Dematteis M. Inflammation contributes to the atherogenic role of intermittent hypoxia in apolipoprotein-E knock out mice. Atherosclerosis. 2011;219(2):425–431. doi: 10.1016/j.atherosclerosis.2011.07.122. [DOI] [PubMed] [Google Scholar]
- 12.Marsch E, Sluimer JC, Daemen MJ. Hypoxia in atherosclerosis and inflammation. Current opinion in lipidology. 2013;24(5):393–400. doi: 10.1097/MOL.0b013e32836484a4. [DOI] [PubMed] [Google Scholar]
- 13.Trayhurn P. Hypoxia and adipose tissue function and dysfunction in obesity. Physiological reviews. 2013;93(1):1–21. doi: 10.1152/physrev.00017.2012. [DOI] [PubMed] [Google Scholar]
- 14.Colgan SP, Taylor CT. Hypoxia: an alarm signal during intestinal inflammation. Nature reviews. Gastroenterology & hepatology. 2010;7(5):281–287. doi: 10.1038/nrgastro.2010.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eltzschig HK, Carmeliet P. Hypoxia and inflammation. The New England journal of medicine. 2011;364(7):656–665. doi: 10.1056/NEJMra0910283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rius J, et al. NF-kappaB links innate immunity to the hypoxic response through transcriptional regulation of HIF-1alpha. Nature. 2008;453(7196):807–811. doi: 10.1038/nature06905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shin DH, et al. Inhibitor of nuclear factor-kappaB alpha derepresses hypoxia-inducible factor-1 during moderate hypoxia by sequestering factor inhibiting hypoxia-inducible factor from hypoxia-inducible factor 1alpha. The FEBS journal. 2009;276(13):3470–3480. doi: 10.1111/j.1742-4658.2009.07069.x. [DOI] [PubMed] [Google Scholar]
- 18.Wang Y, et al. Regulation of endocytosis via the oxygen-sensing pathway. Nature medicine. 2009;15(3):319–324. doi: 10.1038/nm.1922. [DOI] [PubMed] [Google Scholar]
- 19.Bellot G, et al. Hypoxia-induced autophagy is mediated through hypoxia-inducible factor induction of BNIP3 and BNIP3L via their BH3 domains. Molecular and cellular biology. 2009;29(10):2570–2581. doi: 10.1128/MCB.00166-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang H, et al. Mitochondrial autophagy is an HIF-1-dependent adaptive metabolic response to hypoxia. The Journal of biological chemistry. 2008;283(16):10892–10903. doi: 10.1074/jbc.M800102200. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 21.Semenza GL, Wang GL. A nuclear factor induced by hypoxia via de novo protein synthesis binds to the human erythropoietin gene enhancer at a site required for transcriptional activation. Molecular and cellular biology. 1992;12(12):5447–5454. doi: 10.1128/mcb.12.12.5447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Semenza GL. HIF-1 mediates metabolic responses to intratumoral hypoxia and oncogenic mutations. The Journal of clinical investigation. 2013;123(9):3664–3671. doi: 10.1172/JCI67230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Son J, et al. Glutamine supports pancreatic cancer growth through a KRAS-regulated metabolic pathway. Nature. 2013;496(7443):101–105. doi: 10.1038/nature12040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gameiro PA, et al. In vivo HIF-mediated reductive carboxylation is regulated by citrate levels and sensitizes VHL-deficient cells to glutamine deprivation. Cell metabolism. 2013;17(3):372–385. doi: 10.1016/j.cmet.2013.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hu F, et al. Hypoxia-inducible factor-1alpha and interleukin 33 form a regulatory circuit to perpetuate the inflammation in rheumatoid arthritis. PloS one. 2013;8(8):e72650. doi: 10.1371/journal.pone.0072650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Carriere V, et al. IL-33, the IL-1-like cytokine ligand for ST2 receptor, is a chromatin-associated nuclear factor in vivo. Proceedings of the National Academy of Sciences of the United States of America. 2007;104(1):282–287. doi: 10.1073/pnas.0606854104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kurokawa M, et al. Interleukin-33-activated dendritic cells induce the production of thymus and activation-regulated chemokine and macrophage-derived chemokine. International archives of allergy and immunology. 2013;161(Suppl 2):52–57. doi: 10.1159/000350363. [DOI] [PubMed] [Google Scholar]
- 28.Wahamaa H, et al. High mobility group box protein 1 in complex with lipopolysaccharide or IL-1 promotes an increased inflammatory phenotype in synovial fibroblasts. Arthritis research & therapy. 2011;13(4):R136. doi: 10.1186/ar3450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moussion C, Ortega N, Girard JP. The IL-1-like cytokine IL-33 is constitutively expressed in the nucleus of endothelial cells and epithelial cells in vivo: a novel 'alarmin'? PloS one. 2008;3(10):e3331. doi: 10.1371/journal.pone.0003331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yasuda K, et al. Contribution of IL-33-activated type II innate lymphoid cells to pulmonary eosinophilia in intestinal nematode-infected mice. Proceedings of the National Academy of Sciences of the United States of America. 2012;109(9):3451–3456. doi: 10.1073/pnas.1201042109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ema M, et al. A novel bHLH-PAS factor with close sequence similarity to hypoxia-inducible factor 1alpha regulates the VEGF expression and is potentially involved in lung and vascular development. Proceedings of the National Academy of Sciences of the United States of America. 1997;94(9):4273–4278. doi: 10.1073/pnas.94.9.4273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tian H, McKnight SL, Russell DW. Endothelial PAS domain protein 1 (EPAS1), a transcription factor selectively expressed in endothelial cells. Genes & development. 1997;11(1):72–82. doi: 10.1101/gad.11.1.72. [DOI] [PubMed] [Google Scholar]
- 33.Iyer NV, et al. Cellular and developmental control of O2 homeostasis by hypoxia-inducible factor 1 alpha. Genes & development. 1998;12(2):149–162. doi: 10.1101/gad.12.2.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kotch LE, Iyer NV, Laughner E, Semenza GL. Defective vascularization of HIF-1alpha-null embryos is not associated with VEGF deficiency but with mesenchymal cell death. Developmental biology. 1999;209(2):254–267. doi: 10.1006/dbio.1999.9253. [DOI] [PubMed] [Google Scholar]
- 35.Tian H, Hammer RE, Matsumoto AM, Russell DW, McKnight SL. The hypoxia-responsive transcription factor EPAS1 is essential for catecholamine homeostasis and protection against heart failure during embryonic development. Genes & development. 1998;12(21):3320–3324. doi: 10.1101/gad.12.21.3320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hubbi ME, et al. Chaperone-mediated autophagy targets hypoxia-inducible factor-1alpha (HIF-1alpha) for lysosomal degradation. The Journal of biological chemistry. 2013;288(15):10703–10714. doi: 10.1074/jbc.M112.414771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dioum EM, et al. Regulation of hypoxia-inducible factor 2alpha signaling by the stress-responsive deacetylase sirtuin 1. Science. 2009;324(5932):1289–1293. doi: 10.1126/science.1169956. [DOI] [PubMed] [Google Scholar]
- 38.Chen R, et al. The acetylase/deacetylase couple CREB-binding protein/Sirtuin 1 controls hypoxia-inducible factor 2 signaling. The Journal of biological chemistry. 2012;287(36):30800–30811. doi: 10.1074/jbc.M111.244780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lim JH, et al. Sirtuin 1 modulates cellular responses to hypoxia by deacetylating hypoxia-inducible factor 1alpha. Molecular cell. 2010;38(6):864–878. doi: 10.1016/j.molcel.2010.05.023. [DOI] [PubMed] [Google Scholar]
- 40.Hubbi ME, Hu H, Kshitiz, Gilkes DM, Semenza GL. Sirtuin-7 inhibits the activity of hypoxia-inducible factors. The Journal of biological chemistry. 2013;288(29):20768–20775. doi: 10.1074/jbc.M113.476903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Carroll VA, Ashcroft M. Role of hypoxia-inducible factor (HIF)-1alpha versus HIF-2alpha in the regulation of HIF target genes in response to hypoxia, insulin-like growth factor-I, or loss of von Hippel-Lindau function: implications for targeting the HIF pathway. Cancer research. 2006;66(12):6264–6270. doi: 10.1158/0008-5472.CAN-05-2519. [DOI] [PubMed] [Google Scholar]
- 42.Sowter HM, Raval RR, Moore JW, Ratcliffe PJ, Harris AL. Predominant role of hypoxia-inducible transcription factor (Hif)-1alpha versus Hif-2alpha in regulation of the transcriptional response to hypoxia. Cancer research. 2003;63(19):6130–6134. [PubMed] [Google Scholar]
- 43.Florczyk U, et al. Opposite effects of HIF-1alpha and HIF-2alpha on the regulation of IL-8 expression in endothelial cells. Free radical biology & medicine. 2011;51(10):1882–1892. doi: 10.1016/j.freeradbiomed.2011.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Skuli N, et al. Endothelial HIF-2alpha regulates murine pathological angiogenesis and revascularization processes. The Journal of clinical investigation. 2012;122(4):1427–1443. doi: 10.1172/JCI57322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Maynard MA, et al. Human HIF-3alpha4 is a dominant-negative regulator of HIF-1 and is down-regulated in renal cell carcinoma. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2005;19(11):1396–1406. doi: 10.1096/fj.05-3788com. [DOI] [PubMed] [Google Scholar]
- 46.Ando H, et al. A hypoxia-inducible factor (HIF)-3alpha splicing variant, HIF-3alpha4 impairs angiogenesis in hypervascular malignant meningiomas with epigenetically silenced HIF-3alpha4. Biochemical and biophysical research communications. 2013;433(1):139–144. doi: 10.1016/j.bbrc.2013.02.044. [DOI] [PubMed] [Google Scholar]
- 47.Torii S, et al. Pro-apoptotic activity of inhibitory PAS domain protein (IPAS), a negative regulator of HIF-1, through binding to pro-survival Bcl-2 family proteins. Cell death and differentiation. 2011;18(11):1711–1725. doi: 10.1038/cdd.2011.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Makino Y, et al. Transcriptional up-regulation of inhibitory PAS domain protein gene expression by hypoxia-inducible factor 1 (HIF-1): a negative feedback regulatory circuit in HIF-1-mediated signaling in hypoxic cells. The Journal of biological chemistry. 2007;282(19):14073–14082. doi: 10.1074/jbc.M700732200. [DOI] [PubMed] [Google Scholar]
- 49.Ghosh G, et al. Hypoxia-induced microRNA-424 expression in human endothelial cells regulates HIF-alpha isoforms and promotes angiogenesis. J Clin Invest. 2010;120(11):4141–4154. doi: 10.1172/JCI42980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Loscalzo J. The cellular response to hypoxia: tuning the system with microRNAs. J Clin Invest. 2010;120(11):3815–3817. doi: 10.1172/JCI45105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rane S, et al. Downregulation of miR-199a derepresses hypoxia-inducible factor-1alpha and Sirtuin 1 and recapitulates hypoxia preconditioning in cardiac myocytes. Circ Res. 2009;104(7):879–886. doi: 10.1161/CIRCRESAHA.108.193102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang C, et al. Underexpressed microRNA-199b-5p targets hypoxia-inducible factor-1alpha in hepatocellular carcinoma and predicts prognosis of hepatocellular carcinoma patients. J Gastroenterol Hepatol. 2011;26(11):1630–1637. doi: 10.1111/j.1440-1746.2011.06758.x. [DOI] [PubMed] [Google Scholar]
- 53.Cicchillitti L, et al. Hypoxia-inducible factor 1-alpha induces miR-210 in normoxic differentiating myoblasts. J Biol Chem. 2012;287(53):44761–44771. doi: 10.1074/jbc.M112.421255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kulshreshtha R, Davuluri RV, Calin GA, Ivan M. A microRNA component of the hypoxic response. Cell Death Differ. 2008;15(4):667–671. doi: 10.1038/sj.cdd.4402310. [DOI] [PubMed] [Google Scholar]
- 55.McCormick RI, et al. miR-210 is a target of hypoxia-inducible factors 1 and 2 in renal cancer, regulates ISCU and correlates with good prognosis. Br J Cancer. 2013;108(5):1133–1142. doi: 10.1038/bjc.2013.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chan SY, et al. MicroRNA-210 controls mitochondrial metabolism during hypoxia by repressing the iron-sulfur cluster assembly proteins ISCU1/2. Cell metabolism. 2009;10(4):273–284. doi: 10.1016/j.cmet.2009.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Favaro E, et al. MicroRNA-210 regulates mitochondrial free radical response to hypoxia and krebs cycle in cancer cells by targeting iron sulfur cluster protein ISCU. PloS one. 2010;5(4):e10345. doi: 10.1371/journal.pone.0010345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kelly TJ, Souza AL, Clish CB, Puigserver P. A hypoxia-induced positive feedback loop promotes hypoxia-inducible factor 1alpha stability through miR-210 suppression of glycerol-3-phosphate dehydrogenase 1-like. Mol Cell Biol. 2011;31(13):2696–2706. doi: 10.1128/MCB.01242-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Peng H, et al. MicroRNA-31 targets FIH-1 to positively regulate corneal epithelial glycogen metabolism. FASEB J. 2012;26(8):3140–3147. doi: 10.1096/fj.11-198515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tanaka H, et al. MicroRNA-183 upregulates HIF-1alpha by targeting isocitrate dehydrogenase 2 (IDH2) in glioma cells. J Neurooncol. 2013;111(3):273–283. doi: 10.1007/s11060-012-1027-9. [DOI] [PubMed] [Google Scholar]
- 61.Gonsalves CS, Kalra VK. Hypoxia-mediated expression of 5-lipoxygenase-activating protein involves HIF-1alpha and NF-kappaB and microRNAs 135a and 199a-5p. J Immunol. 2010;184(7):3878–3888. doi: 10.4049/jimmunol.0902594. [DOI] [PubMed] [Google Scholar]
- 62.Pulkkinen K, Malm T, Turunen M, Koistinaho J, Yla-Herttuala S. Hypoxia induces microRNA miR-210 in vitro and in vivo ephrin-A3 and neuronal pentraxin 1 are potentially regulated by miR-210. FEBS Lett. 2008;582(16):2397–2401. doi: 10.1016/j.febslet.2008.05.048. [DOI] [PubMed] [Google Scholar]
- 63.Taguchi A, et al. Identification of hypoxia-inducible factor-1 alpha as a novel target for miR-17–92 microRNA cluster. Cancer Res. 2008;68(14):5540–5545. doi: 10.1158/0008-5472.CAN-07-6460. [DOI] [PubMed] [Google Scholar]
- 64.Wei Y, Schober A, Weber C. Pathogenic arterial remodeling: the good and bad of microRNAs. American journal of physiology. Heart and circulatory physiology. 2013;304(8):H1050–H1059. doi: 10.1152/ajpheart.00267.2012. [DOI] [PubMed] [Google Scholar]
- 65.Zampetaki A, Dudek K, Mayr M. Oxidative stress in atherosclerosis: the role of microRNAs in arterial remodeling. Free radical biology & medicine. 2013;64:69–77. doi: 10.1016/j.freeradbiomed.2013.06.025. [DOI] [PubMed] [Google Scholar]
- 66.Hinkel R, et al. Inhibition of MicroRNA-92a Protects Against Ischemia/Reperfusion Injury in a Large-Animal Model. Circulation. 2013;128(10):1066–1075. doi: 10.1161/CIRCULATIONAHA.113.001904. [DOI] [PubMed] [Google Scholar]
- 67.Hullinger TG, et al. Inhibition of miR-15 protects against cardiac ischemic injury. Circulation research. 2012;110(1):71–81. doi: 10.1161/CIRCRESAHA.111.244442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Chistiakov DA, Sobenin IA, Orekhov AN. Strategies to deliver microRNAs as potential therapeutics in the treatment of cardiovascular pathology. Drug delivery. 2012;19(8):392–405. doi: 10.3109/10717544.2012.738436. [DOI] [PubMed] [Google Scholar]
- 69.van den Boorn JG, Dassler J, Coch C, Schlee M, Hartmann G. Exosomes as nucleic acid nanocarriers. Advanced drug delivery reviews. 2013;65(3):331–335. doi: 10.1016/j.addr.2012.06.011. [DOI] [PubMed] [Google Scholar]
- 70.Ramakrishnan S. Hydrogel-siRNA for cancer therapy. Cancer biology & therapy. 2011;11(9):849–851. doi: 10.4161/cbt.11.9.15465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Jeltsch M, Leppanen VM, Saharinen P, Alitalo K. Receptor tyrosine kinase-mediated angiogenesis. Cold Spring Harbor perspectives in biology. 2013;5(9) doi: 10.1101/cshperspect.a009183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Shibuya M. Vascular Endothelial Growth Factor (VEGF) and Its Receptor (VEGFR) Signaling in Angiogenesis: A Crucial Target for Anti- and Pro-Angiogenic Therapies. Genes & cancer. 2011;2(12):1097–1105. doi: 10.1177/1947601911423031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Senger DR, et al. Tumor cells secrete a vascular permeability factor that promotes accumulation of ascites fluid. Science. 1983;219(4587):983–985. doi: 10.1126/science.6823562. [DOI] [PubMed] [Google Scholar]
- 74.Leung DW, Cachianes G, Kuang WJ, Goeddel DV, Ferrara N. Vascular endothelial growth factor is a secreted angiogenic mitogen. Science. 1989;246(4935):1306–1309. doi: 10.1126/science.2479986. [DOI] [PubMed] [Google Scholar]
- 75.Cohen T, et al. VEGF121, a vascular endothelial growth factor (VEGF) isoform lacking heparin binding ability, requires cell-surface heparan sulfates for efficient binding to the VEGF receptors of human melanoma cells. The Journal of biological chemistry. 1995;270(19):11322–11326. doi: 10.1074/jbc.270.19.11322. [DOI] [PubMed] [Google Scholar]
- 76.Pritchard-Jones RO, et al. Expression of VEGF(xxx)b, the inhibitory isoforms of VEGF, in malignant melanoma. British journal of cancer. 2007;97(2):223–230. doi: 10.1038/sj.bjc.6603839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ferrara N. Binding to the extracellular matrix and proteolytic processing: two key mechanisms regulating vascular endothelial growth factor action. Molecular biology of the cell. 2010;21(5):687–690. doi: 10.1091/mbc.E09-07-0590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Miller JW, Le Couter J, Strauss EC, Ferrara N. Vascular endothelial growth factor a in intraocular vascular disease. Ophthalmology. 2013;120(1):106–114. doi: 10.1016/j.ophtha.2012.07.038. [DOI] [PubMed] [Google Scholar]
- 79.Carmeliet P, et al. Abnormal blood vessel development and lethality in embryos lacking a single VEGF allele. Nature. 1996;380(6573):435–439. doi: 10.1038/380435a0. [DOI] [PubMed] [Google Scholar]
- 80.Ferrara N, et al. Heterozygous embryonic lethality induced by targeted inactivation of the VEGF gene. Nature. 1996;380(6573):439–442. doi: 10.1038/380439a0. [DOI] [PubMed] [Google Scholar]
- 81.Kurihara T, Westenskow PD, Bravo S, Aguilar E, Friedlander M. Targeted deletion of Vegfa in adult mice induces vision loss. The Journal of clinical investigation. 2012;122(11):4213–4217. doi: 10.1172/JCI65157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Oosthuyse B, et al. Deletion of the hypoxia-response element in the vascular endothelial growth factor promoter causes motor neuron degeneration. Nature genetics. 2001;28(2):131–138. doi: 10.1038/88842. [DOI] [PubMed] [Google Scholar]
- 83.Sun Y, et al. VEGF-induced neuroprotection, neurogenesis, and angiogenesis after focal cerebral ischemia. The Journal of clinical investigation. 2003;111(12):1843–1851. doi: 10.1172/JCI17977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Takyar S, et al. VEGF controls lung Th2 inflammation via the miR-1-Mpl (myeloproliferative leukemia virus oncogene)-P-selectin axis. The Journal of experimental medicine. 2013;210(10):1993–2010. doi: 10.1084/jem.20121200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Carmeliet P, et al. Synergism between vascular endothelial growth factor and placental growth factor contributes to angiogenesis and plasma extravasation in pathological conditions. Nature medicine. 2001;7(5):575–583. doi: 10.1038/87904. [DOI] [PubMed] [Google Scholar]
- 86.Pellet-Many C, Frankel P, Jia H, Zachary I. Neuropilins: structure, function and role in disease. The Biochemical journal. 2008;411(2):211–226. doi: 10.1042/BJ20071639. [DOI] [PubMed] [Google Scholar]
- 87.Janssen BJ, et al. Neuropilins lock secreted semaphorins onto plexins in a ternary signaling complex. Nature structural & molecular biology. 2012;19(12):1293–1299. doi: 10.1038/nsmb.2416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hagberg CE, et al. Vascular endothelial growth factor B controls endothelial fatty acid uptake. Nature. 2010;464(7290):917–921. doi: 10.1038/nature08945. [DOI] [PubMed] [Google Scholar]
- 89.Hagberg CE, et al. Targeting VEGF-B as a novel treatment for insulin resistance and type 2 diabetes. Nature. 2012;490(7420):426–430. doi: 10.1038/nature11464. [DOI] [PubMed] [Google Scholar]
- 90.Hagberg C, Mehlem A, Falkevall A, Muhl L, Eriksson U. Endothelial fatty acid transport: role of vascular endothelial growth factor B. Physiology. 2013;28(2):125–134. doi: 10.1152/physiol.00042.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Dhondt J, et al. Neuronal FLT1 receptor and its selective ligand VEGF-B protect against retrograde degeneration of sensory neurons. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2011;25(5):1461–1473. doi: 10.1096/fj.10-170944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Karkkainen MJ, et al. Vascular endothelial growth factor C is required for sprouting of the first lymphatic vessels from embryonic veins. Nature immunology. 2004;5(1):74–80. doi: 10.1038/ni1013. [DOI] [PubMed] [Google Scholar]
- 93.Alitalo K, Carmeliet P. Molecular mechanisms of lymphangiogenesis in health and disease. Cancer cell. 2002;1(3):219–227. doi: 10.1016/s1535-6108(02)00051-x. [DOI] [PubMed] [Google Scholar]
- 94.Sawano A, Takahashi T, Yamaguchi S, Aonuma M, Shibuya M. Flt-1 but not KDR/Flk-1 tyrosine kinase is a receptor for placenta growth factor, which is related to vascular endothelial growth factor. Cell growth & differentiation : the molecular biology journal of the American Association for Cancer Research. 1996;7(2):213–221. [PubMed] [Google Scholar]
- 95.Sawano A, Takahashi T, Yamaguchi S, Shibuya M. The phosphorylated 1169-tyrosine containing region of flt-1 kinase (VEGFR-1) is a major binding site for PLCgamma. Biochemical and biophysical research communications. 1997;238(2):487–491. doi: 10.1006/bbrc.1997.7327. [DOI] [PubMed] [Google Scholar]
- 96.Takahashi T, Ueno H, Shibuya M. VEGF activates protein kinase C-dependent, but Ras-independent Raf-MEK-MAP kinase pathway for DNA synthesis in primary endothelial cells. Oncogene. 1999;18(13):2221–2230. doi: 10.1038/sj.onc.1202527. [DOI] [PubMed] [Google Scholar]
- 97.Takahashi T, Yamaguchi S, Chida K, Shibuya M. A single autophosphorylation site on KDR/Flk-1 is essential for VEGF-A-dependent activation of PLC-gamma and DNA synthesis in vascular endothelial cells. The EMBO journal. 2001;20(11):2768–2778. doi: 10.1093/emboj/20.11.2768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Sakurai Y, Ohgimoto K, Kataoka Y, Yoshida N, Shibuya M. Essential role of Flk-1 (VEGF receptor 2) tyrosine residue 1173 in vasculogenesis in mice. Proceedings of the National Academy of Sciences of the United States of America. 2005;102(4):1076–1081. doi: 10.1073/pnas.0404984102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Wang F, et al. RACK1 regulates VEGF/Flt1-mediated cell migration via activation of a PI3K/Akt pathway. The Journal of biological chemistry. 2011;286(11):9097–9106. doi: 10.1074/jbc.M110.165605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Fong GH, Rossant J, Gertsenstein M, Breitman ML. Role of the Flt-1 receptor tyrosine kinase in regulating the assembly of vascular endothelium. Nature. 1995;376(6535):66–70. doi: 10.1038/376066a0. [DOI] [PubMed] [Google Scholar]
- 101.Hiratsuka S, Minowa O, Kuno J, Noda T, Shibuya M. Flt-1 lacking the tyrosine kinase domain is sufficient for normal development and angiogenesis in mice. Proceedings of the National Academy of Sciences of the United States of America. 1998;95(16):9349–9354. doi: 10.1073/pnas.95.16.9349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Murakami M, et al. Signaling of vascular endothelial growth factor receptor-1 tyrosine kinase promotes rheumatoid arthritis through activation of monocytes/macrophages. Blood. 2006;108(6):1849–1856. doi: 10.1182/blood-2006-04-016030. [DOI] [PubMed] [Google Scholar]
- 103.Kaipainen A, et al. Expression of the fms-like tyrosine kinase 4 gene becomes restricted to lymphatic endothelium during development. Proceedings of the National Academy of Sciences of the United States of America. 1995;92(8):3566–3570. doi: 10.1073/pnas.92.8.3566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Dumont DJ, et al. Cardiovascular failure in mouse embryos deficient in VEGF receptor-3. Science. 1998;282(5390):946–949. doi: 10.1126/science.282.5390.946. [DOI] [PubMed] [Google Scholar]
- 105.Haiko P, et al. Deletion of vascular endothelial growth factor C (VEGF-C) and VEGF-D is not equivalent to VEGF receptor 3 deletion in mouse embryos. Molecular and cellular biology. 2008;28(15):4843–4850. doi: 10.1128/MCB.02214-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Hogan BM, et al. Ccbe1 is required for embryonic lymphangiogenesis and venous sprouting. Nature genetics. 2009;41(4):396–398. doi: 10.1038/ng.321. [DOI] [PubMed] [Google Scholar]
- 107.Hagerling R, et al. A novel multistep mechanism for initial lymphangiogenesis in mouse embryos based on ultramicroscopy. The EMBO journal. 2013;32(5):629–644. doi: 10.1038/emboj.2012.340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Somers WS, Tang J, Shaw GD, Camphausen RT. Insights into the molecular basis of leukocyte tethering and rolling revealed by structures of P- and E-selectin bound to SLe(X) and PSGL-1. Cell. 2000;103(3):467–479. doi: 10.1016/s0092-8674(00)00138-0. [DOI] [PubMed] [Google Scholar]
- 109.Huang RB, Eniola-Adefeso O. Shear stress modulation of IL-1beta-induced E-selectin expression in human endothelial cells. PloS one. 2012;7(2):e31874. doi: 10.1371/journal.pone.0031874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Zou X, et al. PSGL-1 derived from human neutrophils is a high-efficiency ligand for endothelium-expressed E-selectin under flow. American journal of physiology. Cell physiology. 2005;289(2):C415–C424. doi: 10.1152/ajpcell.00289.2004. [DOI] [PubMed] [Google Scholar]
- 111.Nimrichter L, et al. E-selectin receptors on human leukocytes. Blood. 2008;112(9):3744–3752. doi: 10.1182/blood-2008-04-149641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.McEver RP, Beckstead JH, Moore KL, Marshall-Carlson L, Bainton DF. GMP-140, a platelet alpha-granule membrane protein, is also synthesized by vascular endothelial cells and is localized in Weibel-Palade bodies. The Journal of clinical investigation. 1989;84(1):92–99. doi: 10.1172/JCI114175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Cleator JH, Zhu WQ, Vaughan DE, Hamm HE. Differential regulation of endothelial exocytosis of P-selectin and von Willebrand factor by protease-activated receptors and cAMP. Blood. 2006;107(7):2736–2744. doi: 10.1182/blood-2004-07-2698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Chen M, Geng JG. P-selectin mediates adhesion of leukocytes, platelets, and cancer cells in inflammation, thrombosis, and cancer growth and metastasis. Archivum immunologiae et therapiae experimentalis. 2006;54(2):75–84. doi: 10.1007/s00005-006-0010-6. [DOI] [PubMed] [Google Scholar]
- 115.Woltmann G, McNulty CA, Dewson G, Symon FA, Wardlaw AJ. Interleukin-13 induces PSGL-1/P-selectin-dependent adhesion of eosinophils, but not neutrophils, to human umbilical vein endothelial cells under flow. Blood. 2000;95(10):3146–3152. [PubMed] [Google Scholar]
- 116.Yang L, et al. ICAM-1 regulates neutrophil adhesion and transcellular migration of TNF-alpha-activated vascular endothelium under flow. Blood. 2005;106(2):584–592. doi: 10.1182/blood-2004-12-4942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Heiska L, et al. Association of ezrin with intercellular adhesion molecule-1 and-2 (ICAM-1 and ICAM-2). Regulation by phosphatidylinositol 4, 5-bisphosphate. The Journal of biological chemistry. 1998;273(34):21893–21900. doi: 10.1074/jbc.273.34.21893. [DOI] [PubMed] [Google Scholar]
- 118.Bella J, Kolatkar PR, Marlor CW, Greve JM, Rossmann MG. The structure of the two amino-terminal domains of human ICAM-1 suggests how it functions as a rhinovirus receptor and as an LFA-1 integrin ligand. Proceedings of the National Academy of Sciences of the United States of America. 1998;95(8):4140–4145. doi: 10.1073/pnas.95.8.4140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Chakravorty SJ, Craig A. The role of ICAM-1 in Plasmodium falciparum cytoadherence. European journal of cell biology. 2005;84(1):15–27. doi: 10.1016/j.ejcb.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 120.Winning S, Splettstoesser F, Fandrey J, Frede S. Acute hypoxia induces HIF-independent monocyte adhesion to endothelial cells through increased intercellular adhesion molecule-1 expression: the role of hypoxic inhibition of prolyl hydroxylase activity for the induction of NF-kappa B. Journal of immunology. 2010;185(3):1786–1793. doi: 10.4049/jimmunol.0903244. [DOI] [PubMed] [Google Scholar]
- 121.Kiss J, et al. Loss of the oxygen sensor PHD3 enhances the innate immune response to abdominal sepsis. Journal of immunology. 2012;189(4):1955–1965. doi: 10.4049/jimmunol.1103471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Barreiro O, et al. Dynamic interaction of VCAM-1 and ICAM-1 with moesin and ezrin in a novel endothelial docking structure for adherent leukocytes. The Journal of cell biology. 2002;157(7):1233–1245. doi: 10.1083/jcb.200112126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Bosco MC, et al. Monocytes and dendritic cells in a hypoxic environment: Spotlights on chemotaxis and migration. Immunobiology. 2008;213(9–10):733–749. doi: 10.1016/j.imbio.2008.07.031. [DOI] [PubMed] [Google Scholar]
- 124.Smith DF, Galkina E, Ley K, Huo Y. GRO family chemokines are specialized for monocyte arrest from flow. American journal of physiology. Heart and circulatory physiology. 2005;289(5):H1976–H1984. doi: 10.1152/ajpheart.00153.2005. [DOI] [PubMed] [Google Scholar]
- 125.Marumo T, Schini-Kerth VB, Busse R. Vascular endothelial growth factor activates nuclear factor-kappaB and induces monocyte chemoattractant protein-1 in bovine retinal endothelial cells. Diabetes. 1999;48(5):1131–1137. doi: 10.2337/diabetes.48.5.1131. [DOI] [PubMed] [Google Scholar]
- 126.Hong KH, Ryu J, Han KH. Monocyte chemoattractant protein-1-induced angiogenesis is mediated by vascular endothelial growth factor-A. Blood. 2005;105(4):1405–1407. doi: 10.1182/blood-2004-08-3178. [DOI] [PubMed] [Google Scholar]
- 127.Jung YJ, Isaacs JS, Lee S, Trepel J, Neckers L. IL-1beta-mediated upregulation of HIF-1alpha via an NFkappaB/COX-2 pathway identifies HIF-1 as a critical link between inflammation and oncogenesis. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2003;17(14):2115–2117. doi: 10.1096/fj.03-0329fje. [DOI] [PubMed] [Google Scholar]
- 128.Tewari R, Choudhury SR, Ghosh S, Mehta VS, Sen E. Involvement of TNF-alpha-induced TLR4-NF-kappaB and TLR4-HIF-1alpha feed-forward loops in the regulation of inflammatory responses in glioma. Journal of molecular medicine. 2012;90(1):67–80. doi: 10.1007/s00109-011-0807-6. [DOI] [PubMed] [Google Scholar]
- 129.Greenberg DA, Jin K. Vascular endothelial growth factors (VEGFs) and stroke. Cellular and molecular life sciences : CMLS. 2013;70(10):1753–1761. doi: 10.1007/s00018-013-1282-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Herz J, et al. Intracerebroventricularly delivered VEGF promotes contralesional corticorubral plasticity after focal cerebral ischemia via mechanisms involving antiinflammatory actions. Neurobiology of disease. 2012;45(3):1077–1085. doi: 10.1016/j.nbd.2011.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Horie N, et al. Transplanted Stem Cell-Secreted VEGF Effects Post-Stroke Recovery, Inflammation, and Vascular Repair. Stem cells. 2011 doi: 10.1002/stem.584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Zechariah A, et al. Vascular endothelial growth factor promotes pericyte coverage of brain capillaries, improves cerebral blood flow during subsequent focal cerebral ischemia, and preserves the metabolic penumbra. Stroke; a journal of cerebral circulation. 2013;44(6):1690–1697. doi: 10.1161/STROKEAHA.111.000240. [DOI] [PubMed] [Google Scholar]
- 133.Zechariah A, et al. Hyperlipidemia attenuates vascular endothelial growth factor-induced angiogenesis, impairs cerebral blood flow, and disturbs stroke recovery via decreased pericyte coverage of brain endothelial cells. Arteriosclerosis, thrombosis, and vascular biology. 2013;33(7):1561–1567. doi: 10.1161/ATVBAHA.112.300749. [DOI] [PubMed] [Google Scholar]
- 134.Ferrara N. Vascular endothelial growth factor: basic science and clinical progress. Endocrine reviews. 2004;25(4):581–611. doi: 10.1210/er.2003-0027. [DOI] [PubMed] [Google Scholar]
- 135.Fukumura D, et al. Tumor induction of VEGF promoter activity in stromal cells. Cell. 1998;94(6):715–725. doi: 10.1016/s0092-8674(00)81731-6. [DOI] [PubMed] [Google Scholar]
- 136.Gerber HP, Kowalski J, Sherman D, Eberhard DA, Ferrara N. Complete inhibition of rhabdomyosarcoma xenograft growth and neovascularization requires blockade of both tumor and host vascular endothelial growth factor. Cancer research. 2000;60(22):6253–6258. [PubMed] [Google Scholar]
- 137.Lee CG, et al. Anti-Vascular endothelial growth factor treatment augments tumor radiation response under normoxic or hypoxic conditions. Cancer research. 2000;60(19):5565–5570. [PubMed] [Google Scholar]
- 138.Jain RK. Normalizing tumor vasculature with anti-angiogenic therapy: a new paradigm for combination therapy. Nature medicine. 2001;7(9):987–989. doi: 10.1038/nm0901-987. [DOI] [PubMed] [Google Scholar]
- 139.Klement G, et al. Continuous low-dose therapy with vinblastine and VEGF receptor-2 antibody induces sustained tumor regression without overt toxicity. The Journal of clinical investigation. 2000;105(8):R15–R24. doi: 10.1172/JCI8829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Gerber HP, Ferrara N. The role of VEGF in normal and neoplastic hematopoiesis. Journal of molecular medicine (Berlin, Germany) 2003;81(1):20–31. doi: 10.1007/s00109-002-0397-4. [DOI] [PubMed] [Google Scholar]
- 141.Dias S, et al. Autocrine stimulation of VEGFR-2 activates human leukemic cell growth and migration. The Journal of clinical investigation. 2000;106(4):511–521. doi: 10.1172/JCI8978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Smolich BD, et al. The antiangiogenic protein kinase inhibitors SU5416 and SU6668 inhibit the SCF receptor (c-kit) in a human myeloid leukemia cell line and in acute myeloid leukemia blasts. Blood. 2001;97(5):1413–1421. doi: 10.1182/blood.v97.5.1413. [DOI] [PubMed] [Google Scholar]
- 143.Peitsidis P, Agrawal R. Role of vascular endothelial growth factor in women with PCO and PCOS: a systematic review. Reproductive biomedicine online. 2010;20(4):444–452. doi: 10.1016/j.rbmo.2010.01.007. [DOI] [PubMed] [Google Scholar]
- 144.McLaren J, et al. Vascular endothelial growth factor is produced by peritoneal fluid macrophages in endometriosis and is regulated by ovarian steroids. The Journal of clinical investigation. 1996;98(2):482–489. doi: 10.1172/JCI118815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Kerbel R, Folkman J. Clinical translation of angiogenesis inhibitors. Nature reviews. Cancer. 2002;2(10):727–739. doi: 10.1038/nrc905. [DOI] [PubMed] [Google Scholar]
- 146.Hull ML, et al. Antiangiogenic agents are effective inhibitors of endometriosis. The Journal of clinical endocrinology and metabolism. 2003;88(6):2889–2899. doi: 10.1210/jc.2002-021912. [DOI] [PubMed] [Google Scholar]
- 147.Maynard SE, et al. Excess placental soluble fms-like tyrosine kinase 1 (sFlt1) may contribute to endothelial dysfunction, hypertension, and proteinuria in preeclampsia. The Journal of clinical investigation. 2003;111(5):649–658. doi: 10.1172/JCI17189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Chang JH, et al. Corneal neovascularization: an anti-VEGF therapy review. Survey of ophthalmology. 2012;57(5):415–429. doi: 10.1016/j.survophthal.2012.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Ciulla TA, Rosenfeld PJ. Anti-vascular endothelial growth factor therapy for neovascular ocular diseases other than age-related macular degeneration. Current opinion in ophthalmology. 2009;20(3):166–174. doi: 10.1097/ICU.0b013e328329d173. [DOI] [PubMed] [Google Scholar]
- 150.Andreoli CM, Miller JW. Anti-vascular endothelial growth factor therapy for ocular neovascular disease. Current opinion in ophthalmology. 2007;18(6):502–508. doi: 10.1097/ICU.0b013e3282f0ca54. [DOI] [PubMed] [Google Scholar]
- 151.Gupta R, Tongers J, Losordo DW. Human studies of angiogenic gene therapy. Circulation research. 2009;105(8):724–736. doi: 10.1161/CIRCRESAHA.109.200386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Makinen K, et al. Increased vascularity detected by digital subtraction angiography after VEGF gene transfer to human lower limb artery: a randomized, placebo-controlled, double-blinded phase II study. Molecular therapy : the journal of the American Society of Gene Therapy. 2002;6(1):127–133. doi: 10.1006/mthe.2002.0638. [DOI] [PubMed] [Google Scholar]
- 153.Kusumanto YH, et al. Treatment with intramuscular vascular endothelial growth factor gene compared with placebo for patients with diabetes mellitus and critical limb ischemia: a double-blind randomized trial. Human gene therapy. 2006;17(6):683–691. doi: 10.1089/hum.2006.17.683. [DOI] [PubMed] [Google Scholar]
- 154.Rajagopalan S, et al. Regional angiogenesis with vascular endothelial growth factor in peripheral arterial disease: a phase II randomized, double-blind, controlled study of adenoviral delivery of vascular endothelial growth factor 121 in patients with disabling intermittent claudication. Circulation. 2003;108(16):1933–1938. doi: 10.1161/01.CIR.0000093398.16124.29. [DOI] [PubMed] [Google Scholar]
- 155.Hedman M, et al. Safety and feasibility of catheter-based local intracoronary vascular endothelial growth factor gene transfer in the prevention of postangioplasty and in-stent restenosis and in the treatment of chronic myocardial ischemia: phase II results of the Kuopio Angiogenesis Trial (KAT) Circulation. 2003;107(21):2677–2683. doi: 10.1161/01.CIR.0000070540.80780.92. [DOI] [PubMed] [Google Scholar]
- 156.Kastrup J, et al. Direct intramyocardial plasmid vascular endothelial growth factor-A165 gene therapy in patients with stable severe angina pectoris A randomized double-blind placebo-controlled study: the Euroinject One trial. Journal of the American College of Cardiology. 2005;45(7):982–988. doi: 10.1016/j.jacc.2004.12.068. [DOI] [PubMed] [Google Scholar]
- 157.Ripa RS, et al. Intramyocardial injection of vascular endothelial growth factor-A165 plasmid followed by granulocyte-colony stimulating factor to induce angiogenesis in patients with severe chronic ischaemic heart disease. European heart journal. 2006;27(15):1785–1792. doi: 10.1093/eurheartj/ehl117. [DOI] [PubMed] [Google Scholar]
- 158.Metinko AP, Kunkel SL, Standiford TJ, Strieter RM. Anoxia-hyperoxia induces monocyte-derived Interleukin-8. The Journal of clinical investigation. 1992;90(3):791–798. doi: 10.1172/JCI115953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Bosco MC, et al. Hypoxia modifies the transcriptome of primary human monocytes: modulation of novel immune-related genes and identification of CCchemokine ligand 20 as a new hypoxia-inducible gene. Journal of immunology. 2006;177(3):1941–1955. doi: 10.4049/jimmunol.177.3.1941. [DOI] [PubMed] [Google Scholar]
- 160.White JR, et al. Genetic amplification of the transcriptional response to hypoxia as a novel means of identifying regulators of angiogenesis. Genomics. 2004;83(1):1–8. doi: 10.1016/s0888-7543(03)00215-5. [DOI] [PubMed] [Google Scholar]
- 161.Bosco MC, et al. Hypoxia selectively inhibits monocyte chemoattractant protein-1 production by macrophages. Journal of immunology. 2004;172(3):1681–1690. doi: 10.4049/jimmunol.172.3.1681. [DOI] [PubMed] [Google Scholar]
- 162.Murdoch C, Muthana M, Lewis CE. Hypoxia regulates macrophage functions in inflammation. Journal of immunology. 2005;175(10):6257–6263. doi: 10.4049/jimmunol.175.10.6257. [DOI] [PubMed] [Google Scholar]
- 163.Ricciardi A, et al. Transcriptome of hypoxic immature dendritic cells: modulation of chemokine/receptor expression. Molecular cancer research : MCR. 2008;6(2):175–185. doi: 10.1158/1541-7786.MCR-07-0391. [DOI] [PubMed] [Google Scholar]
- 164.Battaglia F, et al. Hypoxia transcriptionally induces macrophage-inflammatory protein-3alpha/CCL-20 in primary human mononuclear phagocytes through nuclear factor (NF)-kappaB. Journal of leukocyte biology. 2008;83(3):648–662. doi: 10.1189/jlb.0607349. [DOI] [PubMed] [Google Scholar]
- 165.Hitchon C, et al. Hypoxia-induced production of stromal cell-derived factor 1 (CXCL12) and vascular endothelial growth factor by synovial fibroblasts. Arthritis Rheum. 2002;46(10):2587–2597. doi: 10.1002/art.10520. [DOI] [PubMed] [Google Scholar]
- 166.Jeong HJ, et al. Hypoxia-induced IL-6 production is associated with activation of MAP kinase, HIF-1, and NF-kappaB on HEI-OC1 cells. Hear Res. 2005;207(1-2):59–67. doi: 10.1016/j.heares.2005.04.003. [DOI] [PubMed] [Google Scholar]
- 167.Ghezzi P, et al. Hypoxia increases production of Interleukin-1 and tumor necrosis factor by human mononuclear cells. Cytokine. 1991;3(3):189–194. doi: 10.1016/1043-4666(91)90015-6. [DOI] [PubMed] [Google Scholar]
- 168.Scannell G, et al. Hypoxia induces a human macrophage cell line to release tumor necrosis factor-alpha and its soluble receptors in vitro. J Surg Res. 1993;54(4):281–285. doi: 10.1006/jsre.1993.1044. [DOI] [PubMed] [Google Scholar]
- 169.Ganat Y, Soni S, Chacon M, Schwartz ML, Vaccarino FM. Chronic hypoxia upregulates fibroblast growth factor ligands in the perinatal brain and induces fibroblast growth factor-responsive radial glial cells in the sub-ependymal zone. Neuroscience. 2002;112(4):977–991. doi: 10.1016/s0306-4522(02)00060-x. [DOI] [PubMed] [Google Scholar]
- 170.Egger M, et al. Hypoxia up-regulates the angiogenic cytokine secretoneurin via an HIF-1alpha- and basic FGF-dependent pathway in muscle cells. Faseb j. 2007;21(11):2906–2917. doi: 10.1096/fj.06-7440com. [DOI] [PubMed] [Google Scholar]
- 171.ten Freyhaus H, et al. Hypoxia enhances platelet-derived growth factor signaling in the pulmonary vasculature by down-regulation of protein tyrosine phosphatases. Am J Respir Crit Care Med. 2011;183(8):1092–1102. doi: 10.1164/rccm.200911-1663OC. [DOI] [PubMed] [Google Scholar]
- 172.Hu F, et al. Hypoxia-inducible factor-1alpha and interleukin 33 form a regulatory circuit to perpetuate the inflammation in rheumatoid arthritis. PLoS One. 2013;8(8):e72650. doi: 10.1371/journal.pone.0072650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Falanga V, et al. Hypoxia upregulates the synthesis of TGF-beta 1 by human dermal fibroblasts. J Invest Dermatol. 1991;97(4):634–637. doi: 10.1111/1523-1747.ep12483126. [DOI] [PubMed] [Google Scholar]
- 174.Hirani N, et al. The regulation of Interleukin-8 by hypoxia in human macrophages--α potential role in the pathogenesis of the acute respiratory distress syndrome (ARDS) Mol Med. 2001;7(10):685–697. [PMC free article] [PubMed] [Google Scholar]
- 175.Karakurum M, et al. Hypoxic induction of Interleukin-8 gene expression in human endothelial cells. J Clin Invest. 1994;93(4):1564–1570. doi: 10.1172/JCI117135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Wang Y, et al. Hypoxia promotes ligand-independent EGF receptor signaling via hypoxia-inducible factor-mediated upregulation of caveolin-1. Proc Natl Acad Sci U S A. 2012;109(13):4892–4897. doi: 10.1073/pnas.1112129109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Hedtjarn M, et al. Interleukin-18 involvement in hypoxic-ischemic brain injury. J Neurosci. 2002;22(14):5910–5919. doi: 10.1523/JNEUROSCI.22-14-05910.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Kim J, et al. Hypoxia-induced IL-18 increases hypoxia-inducible factor-1alpha expression through a Rac1-dependent NF-kappaB pathway. Mol Biol Cell. 2008;19(2):433–444. doi: 10.1091/mbc.E07-02-0182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Hara S, et al. Hypoxia enhances c-Met/HGF receptor expression and signaling by activating HIF-1alpha in human salivary gland cancer cells. Oral Oncol. 2006;42(6):593–598. doi: 10.1016/j.oraloncology.2005.10.016. [DOI] [PubMed] [Google Scholar]
- 180.Chen WY, Chang MS. IL-20 is regulated by hypoxia-inducible factor and upregulated after experimental ischemic stroke. J Immunol. 2009;182(8):5003–5012. doi: 10.4049/jimmunol.0803653. [DOI] [PubMed] [Google Scholar]
- 181.Antoine M, et al. Upregulation of pleiotrophin expression in rat hepatic stellate cells by PDGF and hypoxia: implications for its role in experimental biliary liver fibrogenesis. Biochem Biophys Res Commun. 2005;337(4):1153–1164. doi: 10.1016/j.bbrc.2005.09.173. [DOI] [PubMed] [Google Scholar]
- 182.Fu H, Luo F, Yang L, Wu W, Liu X. Hypoxia stimulates the expression of macrophage migration inhibitory factor in human vascular smooth muscle cells via HIF-1alpha dependent pathway. BMC Cell Biol. 2010;11:66. doi: 10.1186/1471-2121-11-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Simons D, et al. Hypoxia-induced endothelial secretion of macrophage migration inhibitory factor and role in endothelial progenitor cell recruitment. J Cell Mol Med. 2011;15(3):668–678. doi: 10.1111/j.1582-4934.2010.01041.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Simon MP, Tournaire R, Pouyssegur J. The angiopoietin-2 gene of endothelial cells is up-regulated in hypoxia by a HIF binding site located in its first intron and by the central factors GATA-2 and Ets-1. J Cell Physiol. 2008;217(3):809–818. doi: 10.1002/jcp.21558. [DOI] [PubMed] [Google Scholar]