Abstract
Epiplexus cells are a population of innate immune cells in the choroid plexus of the brain ventricles. They are thought to contribute to the immune component of the blood-cerebrospinal-fluid-barrier (BCSFB). Here we have developed a novel technique for studying epiplexus cells in acutely isolated, live and intact choroid plexus. We show that epiplexus cells are potently activated by exogenous ATP, increasing their motility within the tissue. This ATP-induced chemokinesis required activation of pannexin-1 channels, which are expressed by the epithelial cells of the choroid plexus and not the epiplexus cells themselves. Furthermore, ATP acts at least in part through the P2X4 ionotropic purinergic receptor. Thus, the resident immune cells of the choroid plexus appear to be in communication with the epithelial cells through pannexin-1 channels.
Keywords: pannexin-1, ATP, choroid plexus, epiplexus, macrophage, P2X4, P2X7, calcium, epithelial, chemotaxis
Introduction
The choroid plexus (CP) is located in the brain ventricles and forms important structural and immune barriers between the cerebrospinal fluid (CSF) and the blood. The blood-cerebrospinal fluid barrier (BCSFB) is found on the apical face of the CP and contacts the CSF. The BCSFB is comprised of a single-layer of polarized cuboidal epithelial cells with tight junctions that restrict the flow of substances between the blood and the CSF.1,2 In addition to this physical barrier, there are also resident native immune cells on the CP surface that are called epiplexus or Kolmer cells.3 Together, the BCSFB and the epiplexus cells constitute the local immune system of the CP, which is likely important during infection, or autoimmune and inflammatory diseases.3,4 The mechanisms by which epiplexus cells communicate with the epithelial cells of the choroid plexus are poorly understood.
Epiplexus cells were first described in 1921 by Kolmer3 and have been extensively imaged with electron microscopy and immunohistochemistry.3,5,6 They express markers of macrophages, dendritic cells, and microglia, but have enough unique features to be classified as resident immune cells of the CP.3,7-10 Like most immune cells, epiplexus cells have been proposed to function in phagocytosis of foreign bodies,11 antigen presentation,12,13 iron accumulation,14 and NO production,12,15 suggesting they act as a ready pool of immune cells in the brain ventricles.12-17
Cells of innate immunity detect pathogen associated patterns (PAMPs) of foreign microorganism, and damage associated molecular patterns (DAMPs),18,19 such as the purine nucleotide, adenosine-5′-triphosphate (ATP).20 Purinergic receptors are expressed on most immune cell types,20-27 but this has not been investigated for epiplexus cells. Inflammatory responses mediated by P2X4 and P2X7 ionotropic purinergic receptors can be augmented by ATP released by pannexin-1 (Panx1) channels.28-30 This suggests that a central component of Panx1’s role in immune responses is potentiation of purinergic receptor currents and possibly ATP-induced release of signaling molecules (reviewed in ref. 31). For example in microglia of the leech, innexins, orthologs of pannexins, appear permissive for directed movements in response to injury.32,33
The CP is a unique environment compared with most tissues because it ‘floats’ in the ventricles and is entirely bathed in CSF. This suggests that immune or damage signals during infection or injury could arise from all directions simultaneously. To investigate the responses of epiplexus cells to DAMPS, we developed a new approach for imaging epiplexus cell behavior in live and intact CP. Here we show that exogenous ATP activates motility of epiplexus cells and that this requires epithelial Panx1, and possibly P2X4 receptors.
Results
To understand the physiology of epiplexus cells, we developed an acutely isolated, intact CP preparation (Fig. 1A-C). The CP was rapidly removed from rat brain and submerged in artificial CSF (aCSF). The CP was then labeled with Alexa Fluor 488 isolectin B4 conjugate (IB4), a fluorescent marker for microglia and macrophages.21,34-36 IB4 labeled a population of cells that were evenly distributed across the apical surface (Fig. 1D and E). The IB4-positive cells had large visible processes. To identify these IB4-positive cells as putative epiplexus cells, we fixed and co-labeled the CP with antibodies against Iba1 (ionized calcium binding adaptor molecule 1), a specific marker for cells of the monocytic lineage.37,38 All IB4 positive cells in the CP co-labeled with Iba1 (Fig. 1E), confirming their identity as epiplexus cells. We then took advantage of the IB4-fluorescence to investigate epiplexus cell responsiveness to exogenous purines by imaging their motility in response to purinergic receptor agonists and antagonists.
Figure 1. Identification of epiplexus cells as immune cells. (A) Scheme of the experimental approach. (B) CPs are located in all four brain ventricles. (C) An acutely isolated and intact CP from the lateral ventricle. Scale bar is approximately 1 mm. (D) A living epiplexus cell resting on the choroidal epithelium, visualized with transmitted light (left) or by labeling with Alexa Fluor 488 isolectin B4 conjugate from Griffonia simplicifolia (center); EC – epiplexus cell, EP – choroidal epithelium, BV – blood vessel, LV – lumen of the lateral ventricle. Scale bar is 20 μm. (E) Immunofluorescent staining for immune cell markers, Iba1 and IB4 in the CP. Note the significant co-localization (yellow cells in the merge image). Scale bar is 20 μm.
Epiplexus cells are activated by ATP
To test the responsiveness of epiplexus cells to purinergic signaling, we bath applied ATP. We reasoned that unlike focal applications, bath exposure to ATP would more closely mimic an infection or injury. The movement of epiplexus cells was first determined under baseline conditions (without exogenously applied ATP) by manually tracking the movement of somas at 5 min intervals (Fig. 2A and B; Video 1). Over a 95 min imaging period epiplexus cells were largely quiescent (Figs. 2 and 3; Video 1) with a mean normalized (i.e., baseline subtracted) movement of 0.05 ± 0.15 μm/5 min (n = 124 cells from 5 CPs). Note that in Figure 2C and F the summative distance (i.e., running sum of distance traveled at 5 min intervals) could appear negative if the cells were active during the early baseline but became subsequently quiescent (see Materials and Methods).
Figure 2. Extracellular ATP triggers chemokinesis of epiplexus cells. (A and D) Representation of the tracked paths superimposed on the original image under control conditions and in the presence of exogenous 100 µM ATP. Labels in the top right of the images represent the time relative to the start of the experiment (0 min). Note the 25 min was the end of the baseline and 120 min was the end of the experiment. Scale bar is 50 µm. Raw data showing the distance traveled by individual epiplexus cells in control (B) and in the presence of ATP (E). Each colored line represents an individual epiplexus cell. (B and E) Raw distance traveled during 5 min intervals; (C and F) normalized summative distance traveled.
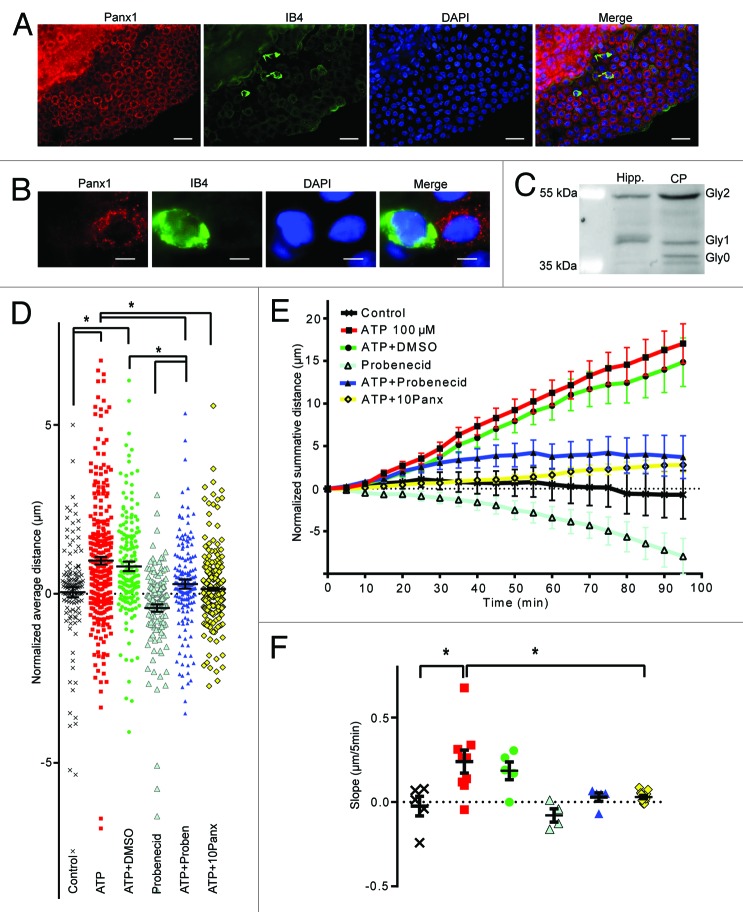
Figure 3. Panx1 channels are involved in epiplexus cells activation by exogenous 100 µM ATP. Panx1 channels are robustly expressed on choroidal epithelium, but were rarely detected in epiplexus cells. Western blotting analysis confirmed presence of Panx1 in the CP. The Panx1 blockers, 500 µM probenecid and 100 µM 10panx significantly decreased ATP-triggered chemokinesis. (A and B) Immunofluorescent staining for Panx1 in the CP and on an individual IB4-positive epiplexus cell. (C) Detection of Panx1 protein by western blotting. (D) Normalized average distance and statistical analysis, each symbol represents a single cell and all cells from the experiments are shown; (E) normalized summative distance; (F) slope of normalized summative distance where each symbol represents a single isolated CP. Scale bars are 50 μm (A) and 5 μm (B).
Exogenous 100 µM ATP, a concentration known to trigger a maximal rise in intracellular Ca2+ in human alveolar macrophages,23 induced “crawling” of the epiplexus cells over the surface of the CP (Fig. 2D, E, and F; Video 2). ATP significantly (P < 0.0001) increased epiplexus cell movement to 0.93 ± 0.12 μm/5 min (n = 293 cells from 9 CPs). Cells traveled varying distances, ranging from tens to more than 100 μm in an hour (Figs. 2 and 3). Enhanced movement of these cells mimicked that of classical chemokinesis, which is an undirected movement in response to a chemical stimulus.39
Pannexin-1 is required for epiplexus cell activation
Panx1 channels are important for ATP-induced-ATP-release from multiple cell types,40,41 ATP-mediated activation of macrophages and T cells,28-30 and release of “find-me” signals from dying cells to attract phagocytes.25,42 We first evaluated the expression of Panx1 in the CP by immunohistochemistry and western blotting. Panx1 labeling was clearly visible in the epithelial cells that comprise the CP (Fig. 3A). However, there was almost no detectable Panx1 in the IB4 positive epiplexus cells (Fig. 3A and B). Epithelial Panx1 appeared punctate, similar to that observed in neural precursor cells.43
Panx1 is known to have several glycosylated isoforms (Gly0, Gly1, and Gly2). Even though all three of them can potentially traffic to the plasma membrane, the fully mature glycosylated species, Gly2, is readily found at the cell surface while the Gly0 and Gly1 forms are primarily intracellular.44 The punctate labeling of the CP epithelia could suggest that Gly0 and Gly1 are predominantly expressed and that Panx1 is largely retained within the cell. We evaluated this by western blot and detected all 3 glycosylated forms of Panx1 (Fig. 3C). Interestingly, the plasma membrane localized Gly2 was the predominant species of Panx1 in the CP (Fig. 3C). For comparative purposes, we investigated the distribution of Panx1 isoforms in the hippocampus, where it plays important roles in pyramidal cells.45-47 Interestingly, the hippocampus did not show significant expression of the Gly0 isoform (Fig. 3C).
Two different Panx1 antagonists, probenecid and 10panx were tested for their ability to alter the activation of epiplexus cells by ATP. Both probenecid (500 μM) (0.29 ± 0.14 μm/5 min, P = 0.0001, n = 165 cells from 5 CPs) and 10panx (100 μM) (0.14 ± 0.04 μm/5 min, P < 0.0001, n = 382 cells from 11 CPs) prevented activation of epiplexus cells by exogenous 100 μM ATP (Fig. 3D–F). Motility of the epiplexus cells in the presence of probenecid only (500 μM) was not significantly different from control (-0.42 ± 0.11 μm/5 min, P = 0.19, n = 151 cells from 4 CPs). This suggests that ATP may act upon purinergic receptors in epithelial cells, which then recruit Panx1 to signal to epiplexus cells.
P2X4 receptors are involved in chemokinesis of epiplexus cells
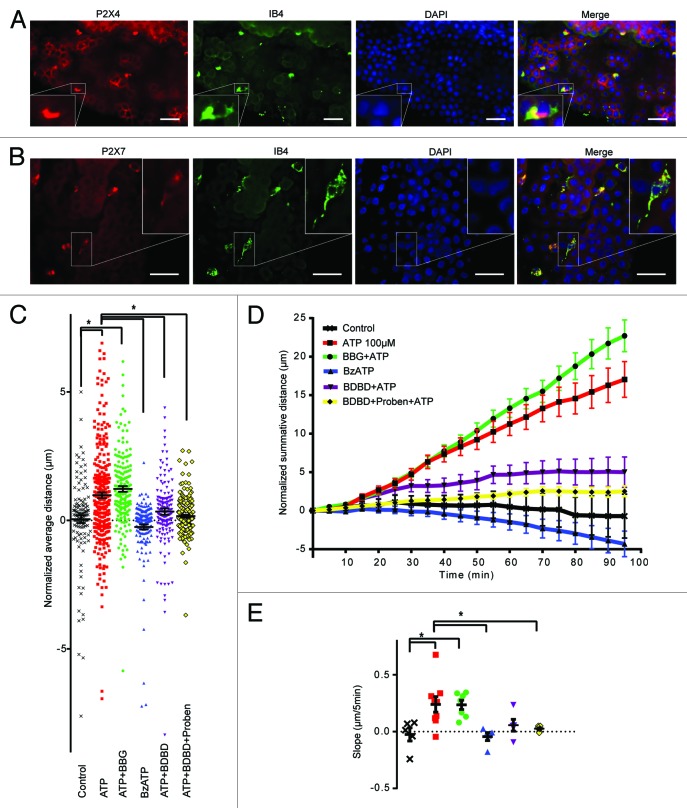
Block of chemokinesis by Panx1 antagonists suggests that purinergic receptors on the epithelial cells may be involved, because this is the identified site of Panx1 expression in the CP. Several ionotropic purinergic receptors have been reported to be linked to Panx1, including P2X7 and P2X4.29,30 Furthermore, both P2X7 and P2X4 are expressed in monocytic cells and can facilitate their activation.24,48 We first examined the expression of P2X7 and P2X4 in the CP by immunohistochemistry. P2X7 strongly co-labeled with IB4, but did not convincingly label the epithelial cells above background (Fig. 4B). In contrast, P2X4 was detected in both the epithelial cells and in IB4 positive epiplexus cells (Fig. 4A). Based on this pattern of labeling we predicted that P2X4, and not P2X7, is important for activation of epiplexus cells by ATP.
Figure 4. P2X7 and P2X4 receptors are both expressed on epiplexus cells, but only P2X4 is involved in chemokinesis. (A) Immunofluorescent staining for P2X4 receptors in epiplexus cells and CP epithelium. Note that both the epithelial and the epiplexus cells are positive for P2X4. (B) Immunofluorescent staining for P2X7 receptors is evident only in epiplexus cells. (C, D, and E) The P2X7 receptor blocker 1 µM BBG did not prevent activation of epiplexus cell by ATP, and the P2X7 receptor agonist BzATP did not trigger an increase in epiplexus cell activity. Thirty µM 5-BDBD, a P2X4 receptor antagonist, alone or in combination with probenecid significantly decreased ATP-triggered chemokinesis. (C) Normalized average distance and statistical analysis where each symbol represents an individual cell; (D) normalized summative distance; (E) slope of normalized summative distance where each symbol represents the average of all cells in a given CP. Scale bars are 50 μm.
As a first step to explore the roles of P2X receptor involvement, we modulated P2X7 receptor activity by bath applying the antagonist, BBG, or the agonist, BzATP. Application of 1 µM BBG (Fig. 4C–E) with ATP did not significantly alter (P > 0.99) epiplexus cell motility (1.22 ± 0.11 μm/5 min; n = 177 cells from 7 CPs) compared with ATP alone (0.93 ± 0.12 μm/frame; n = 293 cells from 9 CPs). Directly activating P2X7 receptors with 100 µM BzATP did not initiate chemokinesis (mean movement was -0.26 ± 0.10μm/5 min; n = 150 cells from 5 CPs; P > 0.99 compared with control). Thus, P2X7 receptors do not appear to be involved in chemokinesis of epiplexus cells induced by ATP.
The P2X4 receptor-specific antagonist, 5-BDBD (30 μM), was used to test whether P2X4 receptors contribute to chemokinesis (Fig. 4C–E). When 5-BDBD was co-applied with ATP, epiplexus cell chemokinesis decreased to 0.35 ± 0.13 μm/5 min (n = 154 cells from 5 CPs), which was significantly (P = 0.0002) different than ATP alone, and not significantly different (P > 0.99) from the control rate. This suggests that P2X4 receptors could be involved in ATP-mediated epiplexus cell activation. Co-application of 5-BDBD and probenecid decreased ATP induced chemokinesis to 0.17 ± 0.04 μm/5 min (n = 275 cells from 9 CPs). Comparison of 5-BDBD alone, probenecid alone and 5-BDBD + probenecid indicated that their effects were not summative. DMSO with ATP was used as the vehicle control for 5-BDBD and probenecid. Importantly, application of ATP plus DMSO (0.1%) did not significantly (P > 0.99) affect activation of epiplexus cells (0.81 ± 0.14 μm/5 min; n = 162 cells from 5 CPs), compared with ATP alone (Fig. 3D–F).
Involvement of Ca2+ in ATP activation of epiplexus cells
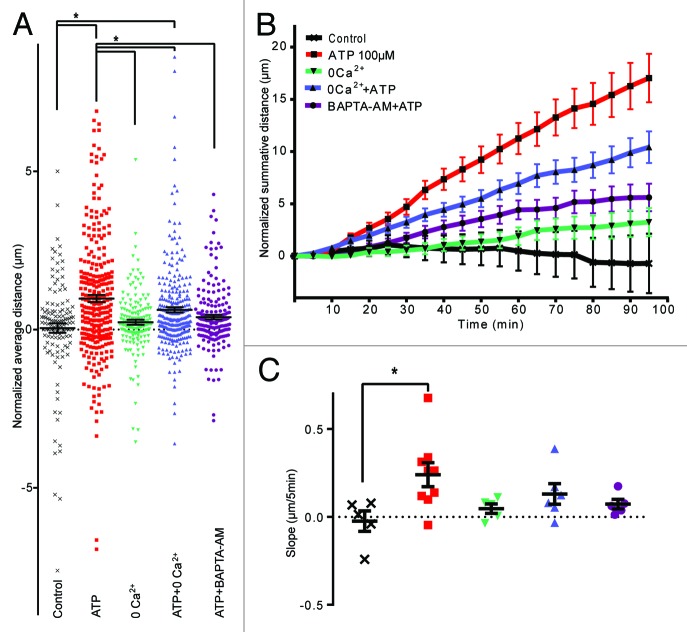
Ionotropic purinergic receptors signal via the influx of Ca2+.23 To investigate if ATP is mediating the chemokinesis of epiplexus cells via Ca2+ influx through P2X receptors, we used a Ca2+-free aCSF. Removal of extracellular Ca2+ did not change baseline motility (0.23 ± 0.08 μm/5 min without Ca2+; P > 0.99 compared with control, n = 161 cells from 5 CPs). However, the absence of Ca2+ significantly altered ATP-induced chemokinesis of epiplexus cells to 0.62 ± 0.08 μm/5 min (n = 276 cells from 6 CPs), which was significantly different from ATP in regular aCSF (P = 0.04), but also from the rate in control conditions (P = 0.004) (Fig. 5).
Figure 5. Ca2+ is involved in mediating chemokinesis. Absence of extracellular Ca2+ and chelation of intracellular Ca2+ significantly decreased ATP-triggered chemokinesis. (A) Normalized average distance and statistical analysis, each symbol represents an individual cell; (B) normalized summative distance; (C) slope of normalized summative distance where each symbol represents the average of all cells in a given CP.
An alternative strategy to investigate the role of intracellular Ca2+ is the use of BAPTA-AM, a membrane permeable Ca2+ chelator. Pre-incubation of the CP with 10 μM BAPTA-AM decreased ATP-induced chemokinesis to 0.39 ± 0.07 μm/5min (n = 182 cells from 5 CPs), which was significantly smaller than ATP (without BAPTA; P = 0.0003) and not different from control (P = 0.46).
Discussion
The choroid plexus plays important roles in brain homeostasis. It produces CSF and distributes neuropeptides, growth factors and cytokines.1 The CP functions as a critical component of the blood-CSF-barrier (BCSFB) that regulates movement of molecules in and out of the brain.1,2 The CP is thought to be an important site for immune defense and resident immune cells are likely critical for this role. Here we developed a novel method for studying the CP’s resident immune cells, the epiplexus cells, in acutely isolated and intact live CP. We report that similar to other immune cells of the monocyte lineage, epiplexus cells are potently activated by exogenous ATP. The presence of Panx1 channels in the epithelial cells of the CP appears critical for this activation. Epiplexus cell activation required P2X4, but not P2X7, ionotropic purinergic receptors. Finally, ATP-induced chemokinesis of epiplexus cells required increased intracellular Ca2+ (Fig. 6).

Figure 6. A model of a proposed cascade for ATP-triggered chemokinesis of epiplexus cells. P2X4 receptors on the epithelial cells may be functionally coupled to Panx1 channels. In this way, activation of P2X4 receptors by ATP would cause a release of an unidentified signaling molecule though Panx1 channels to activate chemokinesis of epiplexus cells. Our current data cannot exclude the possibility that P2X4 receptors on the epiplexus cells may contribute to the activation process.
Chemokinesis of epiplexus cells in intact isolated CP
Without exogenously applied ATP epiplexus cells were mainly quiescent. To investigate if purines could activate epiplexus cells we bath applied 100 μM ATP, which initiated active movements that were characteristic of chemokinesis: the undirected movements of cells in response to a chemical stimulus. While the undirected nature likely reflects an inability of the cells to determine a focal source of ATP, we chose this paradigm because it would closely mimic conditions of CSF infection or brain injury. Chemotaxis toward focally applied ATP is possible and a concept that we will investigate in the future. In some instances, several epiplexus cells would cluster together and move through the CP tissue as a single group, but the significance of this to CP function and defense of the BCSFB is not yet known.
How does the chemokinesis of epiplexus cells fit with our current knowledge of central nervous system immune cells? The characteristic feature of brain microglial activation is that microglial cells very rapidly send fine processes into the site of injury or ATP application to create a barrier between damaged and healthy tissue,49 and then exhibit features of whole-cell migration.32,33 In contrast, macrophages move their cell bodies in response to the signal.22 Our results show that epiplexus cells rapidly respond to ATP with movement of their cell bodies. However, IB4 is a poor label of fine cellular processes. Thus, we are not able to rule out the possibility that there is also increased activity in the fine processes of epiplexus cells upon ATP exposure. Interestingly, formation of a protective network of epiplexus cell’s sheet-like membranes on the ventricular surface of epithelial cells in the presence of a toxic agent has been reported.17 When all of our data are taken together, this suggests that epiplexus cells are indeed resident immune cells with functional features of both microglia and macrophages.
Interestingly, even though all IB4-labeled cells also expressed Iba1, the detected IB4 positive cells were only about two-thirds of the Iba1-positive population. The difference in the IB4 labeling pattern is likely because IB4 was applied to live tissue and anti-Iba1 after fixation and permeabilization. Thus, the live and intact CP likely had a functioning BCSFB that prevented IB4 from reaching the deeper cells. This notion is supported by our observation (not shown) that the endothelial cells lining the blood vessels of the CP also did not label with IB4.50
Pannexin-1 and P2X4 receptors are important for chemokinesis
ATP is a ligand for purinergic receptors and it has been suggested that ionotropic purinergic receptors are linked to activation of immune cells.20-26 In several cases, the Panx1 ion channel is thought to mediate this activation either directly, or through release of signaling molecules.25,28,29,32,33,42 We found Panx1 expression in the epithelial cells that comprise the bulk of the CP, and almost no detectable Panx1 in epiplexus cells. Two blockers of Panx1 potently inhibited ATP-induced chemokinesis of the epiplexus cells. This suggests that ATP acts on the epithelial cells, which release an as yet unidentified molecule to activate the epiplexus cells (Fig. 6). Interesting candidates for this molecule include arachidonic acid, which can direct movement of leech microglia by acting on innexins (orthologs of Panx1),33 and ATP-induced-ATP release.40 Since Panx1 is not likely activated directly by ATP, and may actually be blocked by it,51 it suggests that a purinergic receptor is present on epithelial cells that is functionally coupled to Panx1. We tested if either P2X4 or P2X7 were involved because they have both been linked to Panx1.29,30
The ionotropic P2X4 receptors are expressed on most immune cells where they are functional (mediate ionic current) and participate in cellular activation.24,29 Application of the P2X4R antagonist, 5-BDBD, together with 100 μM ATP decreased epiplexus cell activation. Immunohistochemistry confirmed expression of P2X4 receptors on both epithelial and epiplexus cells in the choroid plexus. This demonstrates that P2X4 receptors may be involved in epiplexus cell activation and suggests that epithelial P2X4 are important. While we cannot exclude a role for P2X4 on the epiplexus cells, our observation that epithelial cell Panx1 is required for chemokinesis supports a role for epithelial purinergic receptors (Fig. 6).
P2X7 receptors participate in activation of macrophages, microglia and other immune cells.30,52,53 The P2X7 receptor antagonist, BBG did not prevent ATP-induced chemokinesis of epiplexus cells. Application of the P2X7 receptor agonist, BzATP, did not result in increased motility of the cells. This suggests that P2X7 receptors are not involved in activation of epiplexus cell chemokinesis, even though they are expressed (Fig. 6). It is possible that P2X7 receptor activation acts as a “stop signal” to migrating immune cells, since these receptors are activated by very high concentrations of extracellular ATP which is usually present in the center of inflammation or injury. Another possibility is that these receptors participate in activation of epiplexus cells in some alternative way that is not related to chemokinesis, such as increasing projections of fine processes in a manner similar to that reported for microglia.49
Calcium influx is required for chemokinesis of epiplexus cells
The absence of extracellular Ca2+ reduced ATP-triggered chemokinesis of epiplexus cells. Chelation of intracellular Ca2+ with BAPTA-AM completely inhibited ATP-mediated activation of epiplexus cells. These experiments indicate that influx of extracellular Ca2+ and/or release of Ca2+ from intracellular stores is responsible, at least in part, for the activation of epiplexus cells (Fig. 6). This may occur at several points in the activation mechanism. Increase in intracellular Ca2+ could directly activate Panx1.54 Alternatively, Ca2+ increases in the epiplexus cells themselves may be critical for activation of cell motility.
Conclusions
The current knowledge of the physiology of epiplexus cells is very limited. To our knowledge, this is the first live cell imaging of motility of these cells, as well as the first report of the action of ATP. While it was not surprising that ATP could activate the epiplexus cells, the critical involvement of Panx1 despite its absence in epiplexus cells was. In the future, we will test for roles of metabotropic purinergic receptors, probe for an endogenous source of ATP and focus on identifying the signal between the epithelial and epiplexus cells.
Materials and Methods
All chemicals were obtained from Sigma Aldrich unless otherwise noted. Sprague-Dawley rats were housed on a 12 h light / dark cycle and given food and water ad libitum according to the guidelines of the Canadian Council for Animal Care.
The isolated and intact CP preparation
Postnatal (P) 21–40 d old Spague-Dawley rats were anaesthetized by inhalation of isofluorane in air. Animals were killed by decapitation and brains were quickly removed and placed in ice-cold aCSF consisting of (in mM) NaCl (120), NaHCO3 (26), KCl (2.5), NaH2PO4 (1.25), MgSO4 (1.3), CaCl2 (2), and glucose (10). Osmolarity was carefully maintained at 291 ± 3 milliosmole. The frontal and medial cortices were resected to expose the corpus callosum and 2 additional incisions along the hippocampus were used to remove the remaining cortex and expose the lateral ventricles. The CP was gently extracted with forceps and a suction pipette, and was placed into a chamber filled with aCSF at 30–33 °C to recover for 30 min (Fig. 1A–C). The CP from the third and fourth ventricles could also be easily obtained, but experiments were performed on CP from the lateral ventricles because it tended to be flat and rested on the cover glass making it suitable for live cell imaging.
Live cell fluorescent imaging
Alexa Fluor 488 isolectin B4 conjugate (IB4) from Griffonia simplicifolia (Invitrogen) was used to label live epiplexus cells. IB4 has been shown to selectively label immune cells, such as macrophages and brain microglia.21,34-36 A 1 mg/ml stock solution of IB4 was prepared in phosphate buffered saline (PBS) (pH 7.4) and 0.5 mM CaCl2. The isolated CP was placed into a 10 µg/ml solution of IB4 in modified Hank’s Balanced Salt Solution (HBSS, 4 mM MgCl2, 1 mM CaCl2, 1 mM pyruvic acid, 1 mM kynurenic acid, 0.005 mM glutathione, pH 7.4)46 for 15 min at room temperature.
The IB4 labeled CP was placed onto cover glass type 0 (Fisher Scientific) and imaged with a Zeiss Axioimager inverted microscope equipped with fluorescence. IB4 was excited by a 470 nm light emitting diode (LED; Zeiss Colibri) and emission was filtered through a high efficiency GFP filter set with single band pass 550/25 nm and collected by CCD camera. Baseline fluorescence was collected with a 40x air objective (NA = 0.6) for 25 min while the CP was perfused with oxygenated (95% O2 / 5% CO2) aCSF and then switched to the experimental solution for up to 95 min. The experimental solutions contained agonists adenosine-5′-triphosphate (ATP), 3′-benzoylbenzoyl adenosine 5′-triphosphate (BzATP) and antagonists of Panx1 (probenecid, 10panx (WRQAAFVDSY, custom synthesized by AnaSpec and New England Peptide)) and of purinergic receptors (brilliant blue G (BBG) and 5-(3-bromophenyl)-1,3-dihydro-2H-benzofuro[3,2-e]-1,4-diazepin-2-one (5-BDBD, from Tocris)), dissolved at the final concentration in aCSF. For experiments determining a role of Ca2+, CPs were pre-incubated in 1,2-bis(2-aminophenoxy)ethane-N,N,N',N'-tetraacetic acid tetrakis (acetoxymethyl ester) (BAPTA-AM, from Molecular Probes) at 30–33 °C for 30 min, or perfused with Ca2+-free aCSF containing ethylene glycol-bis(2-aminoethylether)-N,N,N',N'-tetraacetic acid (10 µM EGTA), with or without ATP. When co-applied, antagonists or Ca2+-free aCSF were introduced 10 min prior to agonist application. If necessary, 0.1% solution of dimethyl sulfoxide (DMSO) (from VWR) was used as a vehicle. The temperature of all solutions was maintained at 30–33 °C with an in-line heater (Warner) placed close to the microscope chamber.
AxioVision Multidimensional Acquisition software (Zeiss) was used to acquire four adjacent images in the xy plane (using the MosaiX module; Zeiss) and each xy image was comprised of 4 to 6 z-stacks collected in 0.5–0.7 µm steps. Images were taken every 5 min. Adjacent images were stitched using the Convert Tile Images parameter of AxioVision and image stacks were collapsed into a single plane using the maximum projection method. This allowed for time series analysis of cellular motility.
Images were processed and analyzed with ImageJ v.1.46 software (National Institutes of Health). The StackReg plug-in was used to align the images to the original position if the xy axes drifted during the course of the experiment. The Manual Tracking plug-in for ImageJ was used to measure the distance traveled by cells for each frame, and generated representations of the tracked path superimposed on the original image.
Cells had 3–5 measurements in the baseline (25 min total) or they were excluded from further analysis. This typically occurred when a cell moved out of or in the field of view during the baseline period. To obtain normalized distance data, the 5 baseline values (in μm) were averaged and then subtracted from the distance at each subsequent time. The mean ± standard error of the mean (SEM) of the normalized difference was taken as the average distance traveled by an individual cell over the course of an experiment, typically 95 min. Population averages were calculated and reported as “normalized average distance.”
To compare the rates of movement of epiplexus cells, we first determined the summative distance for each cell by adding the normalized distance traveled in a cumulative way throughout the course of the experiment. This was plotted as the mean ± SEM for all cells under a given experimental treatment. Slopes (change in distance traveled in 5 min intervals) were determined by linear regression of normalized summative distance. The summative distance of all cells in a given CP were averaged and used to calculate a slope for each CP. These slopes were then averaged to determine population means for a given experiment.
Data were organized and normalized in Microsoft Office Excel 2007, and GraphPad Prism 6 software was used for all statistical analysis. One-way analysis of variance (ANOVA) and Bonferroni’s test for post-hoc analysis were used to compare means. Means were considered significantly different when probability (P) was lower than 0.05. Error bars in all plots represent the standard error of the mean (SEM). Both GraphPad Prism 6 and Microsoft Office Excel 2007 software were used for graphic representations of the data.
Immunohistochemistry
The CP was isolated and labeled with IB4 (20 µg/ml for 30 min at room temperature) as described above and subsequently fixed in 1 or 4% paraformaldehyde (PFA), or 95% ethanol/5% acetic acid. PFA-fixed samples were stored in 30% sucrose in PBS at 4 °C. Alcohol-fixed samples were immediately used for immunolabeling. In all cases, the CP was washed in PBS 3 times for 10 min. Nonspecific binding of the antibodies was blocked by a 2-h exposure to a BSA-based blocking solution (1% BSA, 0.2% Triton X-100, 0.5% sodium azide, 0.4% sodium ethylenediaminetetraacetate (EDTA) in PBS) at room temperature.
Primary antibodies from rabbit were dissolved in blocking solution and applied to the fixed CP tissue for 17–24 h at room temperature. The CP was washed with 0.2% Triton X-100 in PBS 3 times for 10 min. Subsequent incubation in secondary antibody (secondary antibody AF donkey anti-rabbit 555 IgG (A-31572) from Invitrogen at 1:100 and 100 ng/ml 4',6-diamidino-2-phenylindole (DAPI) dissolved in blocking solution) was for 2 h. Secondary antibodies were removed by washing with 0.2% Triton X-100 in PBS 3 times for 10 min, mounted onto chrome alum (chromium (III) potassium sulfate)-coated superfrost white slides (from VWR) in Vectashield (from Vector Lab), and covered with a coverslip type 0 (from Fisher Scientific). Appropriate autofluorescence and negative controls for secondary antibody specificity were performed.
There were some modifications of the procedure for each primary antibody to achieve the best labeling. The CP used for anti–Iba1 labeling (1:500 dilution, 019–19741 from Wako) was fixed overnight in 4% PFA. For anti-Panx1 labeling (1:100, C-Term, 488100 antibody from Invitrogen), the CP was fixed in 1% PFA for 2 h at room temperature. Antigen retrieval was performed by submerging whole CP into a heated (80 °C) sodium citrate buffer containing 10 mM trisodium citrate dihydrate (Fisher Scientific) and 0.05% polyoxyethylenesorbitanmonolaurate (Tween 20, from BioRad, pH 6.0) for 30 min. The CPs were then cooled for 30 min and washed with 0.2% Triton X-100 in PBS 3 times for 10 min and blocked in a BSA-based blocking solution with 10% donkey serum. Primary and secondary antibodies solutions contained 2.5% of donkey serum. For anti-P2X7R (APR-004) and anti-P2X4R (APR-002 from Alomone at 1:200) labeling, CPs were fixed in 95% ethanol/5% glacial acetic acid (from BDH) at -20 °C for 17 min, and blocked in a BSA-based blocking solution with 5% donkey serum. Z-stack images were acquired with AxioVision Multidimensional Acquisition software (Zeiss), deconvolved, and the image stacks collapsed using Extended Focus parameter to create a single image. Brightness and contrast were manually adjusted. Co-expression of markers was quantified manually in the Cell Counter plug-in for ImageJ on the collapsed stack images encompassing a depth of 20 μm from the CP surface.
Western blotting
Western blot analysis of Panx1 expression in CP and hippocampus was performed on rats anesthetized by IP injection of pentobarbital and intracardial perfusion with ice-cold PBS. CPs from the lateral ventricles and/or hippocampi were then dissected and lysed in cold lysis buffer (97% NP-40, 1% phenylmethylsulfonyl fluoride (PMSF), 1% Proteinase Inhibitor Cocktail, and 1% Phosphatase Inhibitor Cocktail from Thermo Scientific) and centrifuged (at 4 °C for 10 min at 12817 rcf). The supernatant was collected and stored at −20 °C until use. Total protein concentration was determined by Bradford assay. Samples containing 25 µg of total protein were mixed in a 1:1 ratio with 2X Laemmli buffer (125 mM tris-HCl, 20% glycerol, 4% SDS, 10% 2-mercaptoethanol, 0.004% bromophenol blue) and boiled for 5 min. Samples were resolved by PAGE in an 8% resolving gel and a 5% stacking gel. The proteins were then transferred to polyvinylidenedifluoride (PVDF) membrane in transfer buffer (25 mM tris-HCl, 0.35 mM SDS, 190 mM glycine, 20% methanol) and run at 0.25 A for 1 h. To block nonspecific binding, the PVDF membrane was agitated in 5% non-fat dry milk in 1X tris-buffered saline and 0.1% Tween 20 (TBST, 10 mM Tris pH 7.5, 100 mM NaCl, 0.1% Tween 20) at 4 °C overnight. Rabbit anti-Panx1 (C-Term, 488100) primary antibody from Invitrogen at 1:1000 was added with the blocking agent and incubated for 1.5 h with gentle agitation. The membranes were washed 3 times for 5 min in TBST and incubated with goat anti-rabbit, horseradish peroxidase secondary antibody at 1:10000 for 2 h. The membrane was washed again 3 times for 5 min with TBST. The bands were detected using Amersham electrochemiluminescence kit (GE Healthcare) and visualized with an UVP BioSpectrum imaging system.
Supplementary Material
Acknowledgments
This work was supported by grants to Thompson RJ from the Natural Sciences and Engineering Research Council of Canada (NSERC), Canadian Institutes of Health Research (CIHR) and Alberta Innovates–Health Solutions (AIHS). We thank Dr Shalina S Ousman and Dr Keith A Sharkey for providing antibodies.
Glossary
Abbreviations:
- 5-BDBD
5-(3-Bromophenyl)-1,3-dihydro-2H-benzofuro[3,2-e]-1,4-diazepin-2-one
- aCSF
artificial cerebrospinal fluid
- ATP
adenosine-5′-triphosphate
- BAPTA-AM
1,2-bis(2-aminophenoxy)ethane-N,N,N',N'-tetraacetic acid tetrakis (acetoxymethyl ester)
- BBG
brilliant blue G
- BCSFB
blood-cerebrospinal fluid barrier
- BzATP
3′-Benzoylbenzoyl adenosine 5′-triphosphate
- CP
choroid plexus
- DMSO
dimethyl sulfoxide
- IB4
Alexa Fluor 488 isolectin B4 conjugate from Griffonia simplicifolia
- Iba1
ionized calcium binding adaptor molecule 1
- Panx1
pannexin-1
References
- 1.Strazielle N, Ghersi-Egea JF. Choroid plexus in the central nervous system: biology and physiopathology. J Neuropathol Exp Neurol. 2000;59:561–74. doi: 10.1093/jnen/59.7.561. [DOI] [PubMed] [Google Scholar]
- 2.Johanson CE, Stopa EG, McMillan PN. The blood-cerebrospinal fluid barrier: structure and functional significance. Methods Mol Biol. 2011;686:101–31. doi: 10.1007/978-1-60761-938-3_4. [DOI] [PubMed] [Google Scholar]
- 3.Ling EA, Kaur C, Lu J. Origin, nature, and some functional considerations of intraventricular macrophages, with special reference to the epiplexus cells. Microsc Res Tech. 1998;41:43–56. doi: 10.1002/(SICI)1097-0029(19980401)41:1<43::AID-JEMT5>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 4.Vercellino M, Votta B, Condello C, Piacentino C, Romagnolo A, Merola A, Capello E, Mancardi GL, Mutani R, Giordana MT, et al. Involvement of the choroid plexus in multiple sclerosis autoimmune inflammation: a neuropathological study. J Neuroimmunol. 2008;199:133–41. doi: 10.1016/j.jneuroim.2008.04.035. [DOI] [PubMed] [Google Scholar]
- 5.Hosoya Y, Fujita T. Scanning electron microscope observation of intraventricular macrophages (Kolmer cells) in the rat brain. Arch Histol Jpn. 1973;35:133–40. doi: 10.1679/aohc1950.35.133. [DOI] [PubMed] [Google Scholar]
- 6.Ling EA. Ultrastruct and origin of epiplexus cells in the telencephalic choroid plexus of postnatal rats studied by intravenous injection of carbon particles. J Anat. 1979;129:479–92. [PMC free article] [PubMed] [Google Scholar]
- 7.Guillemin GJ, Brew BJ. Microglia, macrophages, perivascular macrophages, and pericytes: a review of function and identification. J Leukoc Biol. 2004;75:388–97. doi: 10.1189/jlb.0303114. [DOI] [PubMed] [Google Scholar]
- 8.Schwarze EW. The origin of (Kolmer’s) epiplexus cells. A combined histomorphological and histochemical study. Histochemistry. 1975;44:103–4. doi: 10.1007/BF00490425. [DOI] [PubMed] [Google Scholar]
- 9.McMenamin PG, Wealthall RJ, Deverall M, Cooper SJ, Griffin B. Macrophages and dendritic cells in the rat meninges and choroid plexus: three-dimensional localisation by environmental scanning electron microscopy and confocal microscopy. Cell Tissue Res. 2003;313:259–69. doi: 10.1007/s00441-003-0779-0. [DOI] [PubMed] [Google Scholar]
- 10.Melief J, Koning N, Schuurman KG, Van De Garde MDB, Smolders J, Hoek RM, Van Eijk M, Hamann J, Huitinga I. Phenotyping primary human microglia: tight regulation of LPS responsiveness. Glia. 2012;60:1506–17. doi: 10.1002/glia.22370. [DOI] [PubMed] [Google Scholar]
- 11.Lu J, Kaur C, Ling EA. Uptake of tracer by the epiplexus cells via the choroid plexus epithelium following an intravenous or intraperitoneal injection of horseradish peroxidase in rats. J Anat. 1993;183:609–17. [PMC free article] [PubMed] [Google Scholar]
- 12.Sivakumar V, Lu J, Ling EA, Kaur C. Vascular endothelial growth factor and nitric oxide production in response to hypoxia in the choroid plexus in neonatal brain. Brain Pathol. 2008;18:71–85. doi: 10.1111/j.1750-3639.2007.00104.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lu J, Kaur C, Ling EA. Up-regulation of surface antigens on epiplexus cells in postnatal rats following intraperitoneal injections of lipopolysaccharide. Neuroscience. 1994;63:1169–78. doi: 10.1016/0306-4522(94)90581-9. [DOI] [PubMed] [Google Scholar]
- 14.Lu J, Kaur C, Ling EA. Expression and upregulation of transferrin receptors and iron uptake in the epiplexus cells of different aged rats injected with lipopolysaccharide and interferon-gamma. J Anat. 1995;187:603–11. [PMC free article] [PubMed] [Google Scholar]
- 15.Lu J, Kaur C, Ling EA. Histochemical demonstration of nitric oxide synthase-like immunoreactivity in epiplexus cells and choroid epithelia in the lateral ventricles of postnatal rat brain induced by an intracerebral injection of lipopolysaccharide. Brain Res. 1995;699:275–85. doi: 10.1016/0006-8993(95)00919-H. [DOI] [PubMed] [Google Scholar]
- 16.Maxwell WL, Hardy IG, Watt C, McGadey J, Graham DI, Adams JH, Gennarelli TA. Changes in the choroid plexus, responses by intrinsic epiplexus cells and recruitment from monocytes after experimental head acceleration injury in the non-human primate. Acta Neuropathol. 1992;84:78–84. doi: 10.1007/BF00427218. [DOI] [PubMed] [Google Scholar]
- 17.Ling EA, Gopalakrishnakone P, Tan CK. Electron-microscopical study of the choroid plexus and epiplexus cells in cats following a cisternal injection of crotoxin complex. Acta Anat (Basel) 1988;131:241–8. doi: 10.1159/000146523. [DOI] [PubMed] [Google Scholar]
- 18.Janeway CA, Jr., Medzhitov R. Innate immune recognition. Annu Rev Immunol. 2002;20:197–216. doi: 10.1146/annurev.immunol.20.083001.084359. [DOI] [PubMed] [Google Scholar]
- 19.Rubartelli A, Lotze MT. Inside, outside, upside down: damage-associated molecular-pattern molecules (DAMPs) and redox. Trends Immunol. 2007;28:429–36. doi: 10.1016/j.it.2007.08.004. [DOI] [PubMed] [Google Scholar]
- 20.Vitiello L, Gorini S, Rosano G, la Sala A. Immunoregulation through extracellular nucleotides. Blood. 2012;120:511–8. doi: 10.1182/blood-2012-01-406496. [DOI] [PubMed] [Google Scholar]
- 21.Franke H, Schepper C, Illes P, Krügel U. Involvement of P2X and P2Y receptors in microglial activation in vivo. Purinergic Signal. 2007;3:435–45. doi: 10.1007/s11302-007-9082-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kronlage M, Song J, Sorokin L, Isfort K, Schwerdtle T, Leipziger J, Robaye B, Conley PB, Kim H-C, Sargin S, et al. Autocrine purinergic receptor signaling is essential for macrophage chemotaxis. Sci Signal. 2010;3:ra55. doi: 10.1126/scisignal.2000588. [DOI] [PubMed] [Google Scholar]
- 23.Myrtek D, Müller T, Geyer V, Derr N, Ferrari D, Zissel G, Dürk T, Sorichter S, Luttmann W, Kuepper M, et al. Activation of human alveolar macrophages via P2 receptors: coupling to intracellular Ca2+ increases and cytokine secretion. J Immunol. 2008;181:2181–8. doi: 10.4049/jimmunol.181.3.2181. [DOI] [PubMed] [Google Scholar]
- 24.Sim JA, Park CK, Oh SB, Evans RJ, North RA. P2X1 and P2X4 receptor currents in mouse macrophages. Br J Pharmacol. 2007;152:1283–90. doi: 10.1038/sj.bjp.0707504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Elliott MR, Chekeni FB, Trampont PC, Lazarowski ER, Kadl A, Walk SF, Park D, Woodson RI, Ostankovich M, Sharma P, et al. Nucleotides released by apoptotic cells act as a find-me signal to promote phagocytic clearance. Nature. 2009;461:282–6. doi: 10.1038/nature08296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Idzko M, Dichmann S, Ferrari D, Di Virgilio F, la Sala A, Girolomoni G, Panther E, Norgauer J. Nucleotides induce chemotaxis and actin polymerization in immature but not mature human dendritic cells via activation of pertussis toxin-sensitive P2y receptors. Blood. 2002;100:925–32. doi: 10.1182/blood.V100.3.925. [DOI] [PubMed] [Google Scholar]
- 27.Junger WG. Immune cell regulation by autocrine purinergic signalling. Nat Rev Immunol. 2011;11:201–12. doi: 10.1038/nri2938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schenk U, Westendorf AM, Radaelli E, Casati A, Ferro M, Fumagalli M, Verderio C, Buer J, Scanziani E, Grassi F. Purinergic control of T cell activation by ATP released through pannexin-1 hemichannels. Sci Signal. 2008;1:ra6. doi: 10.1126/scisignal.1160583. [DOI] [PubMed] [Google Scholar]
- 29.Woehrle T, Yip L, Elkhal A, Sumi Y, Chen Y, Yao Y, Insel PA, Junger WG. Pannexin-1 hemichannel-mediated ATP release together with P2X1 and P2X4 receptors regulate T-cell activation at the immune synapse. Blood. 2010;116:3475–84. doi: 10.1182/blood-2010-04-277707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pelegrin P, Surprenant A. Pannexin-1 mediates large pore formation and interleukin-1beta release by the ATP-gated P2X7 receptor. EMBO J. 2006;25:5071–82. doi: 10.1038/sj.emboj.7601378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Baroja-Mazo A, Barberà-Cremades M, Pelegrín P, Pelegrín P. The participation of plasma membrane hemichannels to purinergic signaling. Biochim Biophys Acta. 2013;1828:79–93. doi: 10.1016/j.bbamem.2012.01.002. [DOI] [PubMed] [Google Scholar]
- 32.Samuels SE, Lipitz JB, Dahl G, Muller KJ. Neuroglial ATP release through innexin channels controls microglial cell movement to a nerve injury. J Gen Physiol. 2010;136:425–42. doi: 10.1085/jgp.201010476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Samuels SE, Lipitz JB, Wang J, Dahl G, Muller KJ. Arachidonic acid closes innexin/pannexin channels and thereby inhibits microglia cell movement to a nerve injury. Dev Neurobiol. 2013;73:621–31. doi: 10.1002/dneu.22088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sorokin SP, Hoyt RF., Jr. Macrophage development: I. Rationale for using Griffonia simplicifolia isolectin B4 as a marker for the line. Anat Rec. 1992;232:520–6. doi: 10.1002/ar.1092320409. [DOI] [PubMed] [Google Scholar]
- 35.Dailey ME, Waite M. Confocal imaging of microglial cell dynamics in hippocampal slice cultures. Methods. 1999;18:222–30, 177. doi: 10.1006/meth.1999.0775. [DOI] [PubMed] [Google Scholar]
- 36.Streit WJ. An improved staining method for rat microglial cells using the lectin from Griffonia simplicifolia (GSA I-B4) J Histochem Cytochem. 1990;38:1683–6. doi: 10.1177/38.11.2212623. [DOI] [PubMed] [Google Scholar]
- 37.Ohsawa K, Imai Y, Kanazawa H, Sasaki Y, Kohsaka S. Involvement of Iba1 in membrane ruffling and phagocytosis of macrophages/microglia. J Cell Sci. 2000;113:3073–84. doi: 10.1242/jcs.113.17.3073. [DOI] [PubMed] [Google Scholar]
- 38.Imai Y, Ibata I, Ito D, Ohsawa K, Kohsaka S. A novel gene iba1 in the major histocompatibility complex class III region encoding an EF hand protein expressed in a monocytic lineage. Biochem Biophys Res Commun. 1996;224:855–62. doi: 10.1006/bbrc.1996.1112. [DOI] [PubMed] [Google Scholar]
- 39.Wilkinson PC. Assays of leukocyte locomotion and chemotaxis. J Immunol Methods. 1998;216:139–53. doi: 10.1016/S0022-1759(98)00075-1. [DOI] [PubMed] [Google Scholar]
- 40.Hayoz S, Jia C, Hegg C. Mechanisms of constitutive and ATP-evoked ATP release in neonatal mouse olfactory epithelium. BMC Neurosci. 2012;13:53. doi: 10.1186/1471-2202-13-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Locovei S, Bao L, Dahl G. Pannexin 1 in erythrocytes: function without a gap. Proc Natl Acad Sci U S A. 2006;103:7655–9. doi: 10.1073/pnas.0601037103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chekeni FB, Elliott MR, Sandilos JK, Walk SF, Kinchen JM, Lazarowski ER, Armstrong AJ, Penuela S, Laird DW, Salvesen GS, et al. Pannexin 1 channels mediate ‘find-me’ signal release and membrane permeability during apoptosis. Nature. 2010;467:863–7. doi: 10.1038/nature09413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wicki-Stordeur LE, Dzugalo AD, Swansburg RM, Suits JM, Swayne LA. Pannexin 1 regulates postnatal neural stem and progenitor cell proliferation. Neural Dev. 2012;7:11. doi: 10.1186/1749-8104-7-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Penuela S, Bhalla R, Nag K, Laird DW. Glycosylation regulates pannexin intermixing and cellular localization. Mol Biol Cell. 2009;20:4313–23. doi: 10.1091/mbc.E09-01-0067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Weilinger NL, Tang PL, Thompson RJ. Anoxia-induced NMDA receptor activation opens pannexin channels via Src family kinases. J Neurosci. 2012;32:12579–88. doi: 10.1523/JNEUROSCI.1267-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Thompson RJ, Zhou N, MacVicar BA. Ischemia opens neuronal gap junction hemichannels. Science. 2006;312:924–7. doi: 10.1126/science.1126241. [DOI] [PubMed] [Google Scholar]
- 47.Thompson RJ, Jackson MF, Olah ME, Rungta RL, Hines DJ, Beazely MA, MacDonald JF, MacVicar BA. Activation of pannexin-1 hemichannels augments aberrant bursting in the hippocampus. Science. 2008;322:1555–9. doi: 10.1126/science.1165209. [DOI] [PubMed] [Google Scholar]
- 48.Monif M, Burnstock G, Williams DA. Microglia: proliferation and activation driven by the P2X7 receptor. Int J Biochem Cell Biol. 2010;42:1753–6. doi: 10.1016/j.biocel.2010.06.021. [DOI] [PubMed] [Google Scholar]
- 49.Davalos D, Grutzendler J, Yang G, Kim JV, Zuo Y, Jung S, Littman DR, Dustin ML, Gan W-B. ATP mediates rapid microglial response to local brain injury in vivo. Nat Neurosci. 2005;8:752–8. doi: 10.1038/nn1472. [DOI] [PubMed] [Google Scholar]
- 50.Xiang Z, Burnstock G. Expression of P2X receptors in rat choroid plexus. Neuroreport. 2005;16:903–7. doi: 10.1097/00001756-200506210-00006. [DOI] [PubMed] [Google Scholar]
- 51.Qiu F, Dahl G. A permeant regulating its permeation pore: inhibition of pannexin 1 channels by ATP. Am J Physiol Cell Physiol. 2009;296:C250–5. doi: 10.1152/ajpcell.00433.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wiley JS, Sluyter R, Gu BJ, Stokes L, Fuller SJ. The human P2X7 receptor and its role in innate immunity. Tissue Antigens. 2011;78:321–32. doi: 10.1111/j.1399-0039.2011.01780.x. [DOI] [PubMed] [Google Scholar]
- 53.Di Virgilio F. Liaisons dangereuses: P2X(7) and the inflammasome. Trends Pharmacol Sci. 2007;28:465–72. doi: 10.1016/j.tips.2007.07.002. [DOI] [PubMed] [Google Scholar]
- 54.Locovei S, Wang J, Dahl G. Activation of pannexin 1 channels by ATP through P2Y receptors and by cytoplasmic calcium. FEBS Lett. 2006;580:239–44. doi: 10.1016/j.febslet.2005.12.004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.