Abstract
Background and Aims
The Houston Intra-Arterial Therapy score predicts poor functional outcome following endovascular treatment for acute ischemic stroke based on clinical variables. The present study sought to (a) create a predictive scoring system that included a neuroimaging variable and (b) determine if the scoring systems predict the clinical response to reperfusion.
Methods
Separate datasets were used to derive (n = 110 from the Diffusion and Perfusion Imaging Evaluation for Understanding Stroke Evolution 2 study) and validate (n = 125 from Massachusetts General Hospital) scoring systems that predict poor functional outcome, defined as a modified Rankin Scale score of 4–6 at 90 days.
Results
Age (P < 0·001; β = 0·087) and diffusion-weighted imaging volume (P = 0·023; β = 0·025) were the independent predictors of poor functional outcome. The Stanford Age and Diffusion-Weighted Imaging score was created based on the patient’s age (0–3 points) and diffusion-weighted imaging lesion volume (0–1 points). The percentage of patients with a poor functional outcome increased significantly with the number of points on the Stanford Age and Diffusion-Weighted Imaging score (P < 0·01 for trend). The area under the receiver operating characteristic curve for the Stanford Age and Diffusion-Weighted Imaging score was 0·82 in the derivation dataset. In the validation cohort, the area under the receiver operating characteristic curve was 0·69 for the Stanford Age and Diffusion-Weighted Imaging score and 0·66 for the Houston Intra-Arterial Therapy score (P = 0·45 for the difference). Reperfusion, but not the interactions between the prediction scores and reperfusion, were predictors of outcome (P > 0·5).
Conclusions
The Stanford Age and Diffusion-Weighted Imaging and Houston Intra-Arterial Therapy scores can be used to predict poor functional outcome following endovascular therapy with good accuracy. However, these scores do not predict the clinical response to reperfusion. This limits their utility as tools to select patients for acute stroke interventions.
Keywords: acute care, acute stroke, interventional neuroradiology, thrombolysis
Introduction
Despite high rates of arterial recanalization, a large proportion of acute stroke patients who undergo endovascular therapy have poor clinical outcomes (1–3). The ability to predict a poor outcome from information available prior to intervention could be informative for patients and their family members. To this end, the Houston Intra-Arterial Therapy (HIAT) score was developed as a prognostic scoring system to predict poor outcome (4). It awards points for three baseline clinical variables: age, National Institutes of Health Stroke Scale (NIHSS) score, and blood glucose level. Initial studies have shown that the HIAT score can predict poor functional outcome, defined as a modified Rankin Scale (mRS) score of 4–6, with good accuracy. The aims of this study were twofold. First, we sought to determine if a clinical prediction tool that includes baseline neuroimaging variables as well as clinical variables has better predictive accuracy. Accordingly, we derived a new scoring system that included magnetic resonance imaging (MRI) diffusion-weighted imaging (DWI) lesion volume based on the Diffusion and Perfusion Imaging Evaluation for Understanding Stroke Evolution 2 (DEFUSE 2) dataset. We validated the new scoring system and compared it with the HIAT score in an independent cohort. Second, to determine the suitability of the scoring systems as tools to select patients for acute stroke interventions, we assessed if the scoring systems predicted patients’ clinical response to reperfusion.
Methods
The derivation cohort consisted of patients from the DEFUSE 2 multicenter prospective cohort study, which examined clinical and neuroimaging outcomes in acute ischemic stroke patients treated with endovascular therapies (5). Briefly, patients were eligible to participate in the study if endovascular treatment occurred within 12 hours of symptom onset, they had an NIHSS score of ≥5, were ≥18 years old, and had a baseline MRI prior to treatment. Patients with persistent large vessel occlusions following treatment with intravenous tissue plasminogen activator (IV tPA) were eligible to participate in the study. Baseline MRI scans were performed within 90 minutes prior to the start of the endovascular procedure. DWI lesion volumes were calculated with image reconstruction software (RAPID) (6). Reperfusion was defined according to the criteria used in the primary DEFUSE 2 analysis (5). Functional outcome was assessed by the mRS at days 30 and 90. Poor functional outcome was defined as a mRS score of 4–6 at day 90 in order to be consistent with the HIAT score. Clinical outcomes were determined blinded to the patients’ baseline clinical and neuroimaging data. Comorbidities (hypertension, atrial fibrillation, myocardial infarction, diabetes, history of stroke or transient ischemic attacks, and smoking history) were determined from patient interview and/or assessment of each patient’s medical record. Laboratory values, including blood glucose levels, were obtained as part of a standard work-up.
The validation cohort consisted of consecutive anterior circulation ischemic stroke patients who underwent pretreatment MRI with DWI and subsequent endovascular therapy at the Massachusetts General Hospital (MGH). Thirteen out of 138 patients who satisfied these criteria were excluded; 11 patients had artifacts that precluded DWI lesion volume measurements, in one patient, the 90-day mRS score was unavailable, and one patient also had a recent basilar stroke requiring endovascular therapy. In general, endovascular treatment at MGH is offered to anterior circulation stroke patients who have a proximal occlusion (i.e. internal carotid artery (ICA) terminus, middle cerebral artery (MCA) M1, or M2 segment) causing a significant neurologic deficit (NIHSS score ≥8), who do not have extensive infarction (i.e. DWI lesion volume >70–100 ml), and who can be treated within eight hours of onset.
A systematic review of the literature was conducted to identify scoring systems that predicted poor outcome following endovascular therapy for acute ischemic stroke. The HIAT score was the only clinical prediction scoring system that fulfilled these criteria.
Statistical analysis
Statistical analysis was performed with IBM SPSS Statistics (version 19.0, IBM, Armonk, NY, USA). Differences in baseline demographic, clinical and neuroimaging variables were assessed between the group with poor functional outcome (mRS 4–6) and the group without (mRS 0–3). Mann–Whitney U-tests were used for continuous variables and Fisher exact or χ2 tests for categorical variables. Variables with a P < 0·2 in the univariate comparisons were entered into a step-wise multivariate logistic regression analysis. In a secondary multivariate analysis, reperfusion was added to the model to adjust the beta values of the independent predictors for reperfusion. Variables that were independent predictors of poor outcome (P < 0·05) in the multivariate model were used to create a scoring system. Beta values for each of the significant independent predictors were used to determine the relative weight of each predictor in the clinical scoring system, the Stanford Age and DWI (SAD) score. To determine if there was a differential effect of reperfusion according to SAD score, the interaction between SAD score and reperfusion was tested in a logistic regression model with poor outcome as the dependent variable. The SAD score was derived using data from the DEFUSE 2 cohort. It was validated and compared with the HIAT score in the MGH cohort. Scoring systems were evaluated using area under the receiver operator characteristic curve (AUC).
Results
Derivation dataset
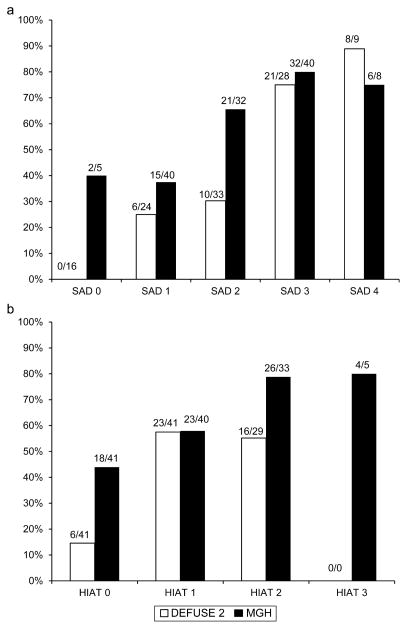
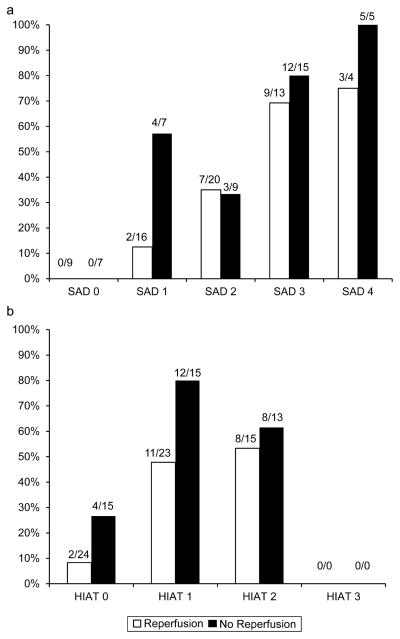
Differences in baseline demographic, clinical and neuroimaging variables between the group with poor functional outcome and the group without poor functional outcome are shown in Table 1. Age (P < 0·001; β = 0·087) and DWI volume (P = 0·023; β = 0·025) were the independent predictors of poor functional outcome at 90 days in multivariate analyses. They remained significant predictors after controlling for reperfusion (age, P = 0·882; β = 0·094; DWI volume, P = 0·415; β = 0·025). Based on these beta values, the median for DWI volume and quartiles for age were used as cut points for the new scoring system. This yielded the SAD scale, a 5-point scale with a maximum of three points awarded for the patient’s age and one point for DWI lesion volume (see text box). The number of patients in each score category is listed in Fig. 1a. The percentage of patients with a poor functional outcome (defined as mRS 4–6) increased significantly with the number of points awarded on the SAD score (P < 0·01 for trend; Fig. 1a). Sensitivity, specificity, positive predictive value, and negative predictive value for poor outcome for each score on the SAD scale are listed in Table 2. The AUC for the SAD score was 0·82. The percentage of patients who reperfused did not differ according to SAD score (χ2 = , P > 0·05). The interaction between SAD score and reperfusion for predicting poor functional outcome was not significant (P = 0·81; Fig. 2a). Likewise, the interaction between HIAT score and reperfusion for predicting poor outcome was not significant (P = 0·51; Fig. 2b).
Table 1.
Predictors of Poor Outcome in the DEFUSE 2 Cohort
| Poor outcome Day 90 (mRS 4–6) n = 45 |
Not poor outcome Day 90 (mRS 0–3) n = 65 |
P value | |
|---|---|---|---|
| Median age (years; IQR) | 78·0 (67·5–82·0) | 60·0 (50·0–73·5) | <0·001 |
| Women (%) | 29/45 (64%) | 24/65 (37%) | 0·005 |
| History of stroke or TIA (%) | 14/44 (32%) | 12/65 (18%) | 0·108 |
| Hypertension (%) | 38/44 (86%) | 37/65 (57%) | 0·001 |
| Atrial fibrillation (%) | 18/44 (41%) | 20/65 (31%) | 0·276 |
| Diabetes (%) | 11/44 (25%) | 11/65 (17%) | 0·303 |
| Hyperlipidemia (%) | 26/44 (59%) | 29/65 (45%) | 0·138 |
| Median baseline NIHSS score (IQR) | 18·0 (13·0–20·5) | 14·0 (9·0–19·0) | 0·049 |
| Median baseline DWI volume (IQR) | 22·5 (8·0–43·0) | 12·0 (5·0–27·5) | 0·007 |
| Median volumetric mismatch (IQR) | 49·5 (22·5–80·3) | 54·7 (17·1–79·4) | 0·698 |
| Median glucose value (IQR) | 131·0 (105·0–150·0) | 115·0 (102·5–141·0) | 0·121 |
| Target mismatch (%) | 34/43 (79%) | 44/61 (72%) | 0·421 |
| Median symptom onset to catheterization time (hours; IQR) | 5·7 (3·5) | 5·8 (3·8) | 0·874 |
| Reperfusion (%) | 21/45 (47%) | 41/60 (68%) | 0·025 |
Poor outcome was defined as mRS 4–6 at 90 days. Univariate comparisons were made with Mann-Whitney U tests for continuous variables and Chi-Square tests for categorical variables. DWI = Diffusion-Weighted Imaging; IQR = inter-quartile range.
Text box. Stanford Age and DWI (SAD) Score.
| DWI Volume |
| ≤15 cc = 0 points |
| > 15 cc = 1 point |
| Age |
| ≤ 55 years = 0 points |
| 56–69 years = 1 point |
| 70–79 years = 2 points |
| ≥ 80 years = 3 points |
Fig. 1.
Percentage of patients with poor outcome, defined as mRS score of 4–6 at 90 days as a function of (a) SAD score and (b) HIAT score. White bars correspond to the DEFUSE 2 dataset and black bars correspond to the MGH validation dataset. The proportion of patients with poor outcome is shown above each bar. SAD Score = Stanford Age and DWI score; HIAT score, Houston IAT score.
Table 2.
Test Characteristics of the SAD Score for Predicting Poor Outcome in the DEFUSE 2 cohort
| SAD score | Sensitivity | Specificity | Positive predictive value | Negative predictive value |
|---|---|---|---|---|
| 0 | 100·0 | 0·0 | 40·9 | 0·0 |
| 1 | 100·0 | 24·6 | 47·9 | 100·0 |
| 2 | 86·7 | 52·3 | 55·7 | 85·0 |
| 3 | 64·4 | 87·7 | 78·4 | 78·1 |
| 4 | 17·8 | 98·5 | 88·9 | 63·4 |
Poor outcome was defined as mRS 4–6 at 90 days. SAD Score = Stanford Age and DWI Score.
Fig. 2.
Percentage of patients with poor outcome, defined as mRS score of 4–6 at 90 days as a function of (a) SAD score and (b) HIAT score. The proportion of patients with poor outcome is shown above each bar. SAD score, Stanford Age and DWI score; HIAT score, Houston IAT score.
Validation dataset
The SAD score was validated and compared with the HIAT score in the MGH cohort. The AUC for the SAD score was 0·69 compared with an AUC of 0·66 for the HIAT score. The difference between these values was not significant (P = 0·45).
Discussion
This study demonstrates that a simple scoring system based on age and DWI volume performs well in predicting poor functional outcomes following endovascular treatment for acute ischemic stroke. Age and DWI lesion volume remained as significant predictors of poor functional outcome even after controlling for the effect of reperfusion. When the new scoring system was compared with the HIAT score, a scoring system based entirely on clinical variables, its predictive accuracy was, however, comparable.
Previous studies have shown that infarct volume is highly associated with clinical outcome following endovascular treatment of acute ischemic stroke (7,8). Not surprisingly, larger DWI volumes are associated with poorer outcomes. For example, a final infarct volume of 90 ml has been shown to be a highly specific cutoff value for identifying patients with a poor outcome (mRS 3–6) (8). In the present study, the goal was to derive a tool that predicts the chance of poor outcome over a range from low to high risk. Age was a stronger predictor of poor outcome than DWI lesion volume. In order to distribute patients evenly among the risk categories, age quartiles and the median DWI volume (15 ml) were considered the optimal cutoffs for the prediction tool. A higher threshold for the DWI lesion volume would have made the prediction tool more specific at its upper range but would have reduced predictive accuracy over the full range of potential scores. Sub-dividing DWI lesion volume into multiple subcategories did not add significantly to the predictive accuracy of the SAD score.
Reperfusion was a significant predictor of clinical outcome in the dataset. This is consistent with previously reported studies (9,10). There was no interaction between the SAD score and reperfusion or between the HIAT score and reperfusion in predicting outcome. This suggests that these scores do not discriminate patients who are likely to benefit from reperfusion from those who are unlikely to benefit or may be harmed by reperfusion. The main utility of these scores is therefore as a prognostic marker and not as a selection criterion for interventions aimed at restoring perfusion.
In contrast to the HIAT score (4), which found that age, NIHSS score, and blood glucose level were significant predictors of poor outcome, the present study identified age as the single clinical predictor of poor functional outcome. This difference is likely explained by the correlation between DWI lesion volume and NIHSS. In univariate analysis, NIHSS score was a significant predictor of poor outcome and serum glucose level was borderline significant. However, in the multivariate model that also included DWI lesion volume, NIHSS and glucose did not remain significant.
The results of the present study are in contrast to the NAV (NIHSS, age, and decreased blood volume) scale (11), which included NIHSS score as an independent predictor of good outcome. Differences in the definition of clinical outcome might have contributed to discrepancies in predictor variables between the studies. The NAV score identified predictors of good clinical outcome defined as mRS 0–2, whereas the present study identified predictors of poor functional outcome, defined as mRS 4–6. It is notable that both the SAD score and NAV scale found evidence that neuroimaging variables indicative of ischemic brain injury are predictors of clinical outcome.
The new scoring system had only a slightly better predictive accuracy than the HIAT score in the validation cohort, as evidenced by a comparison of the areas under the ROC curves (AUC for SAD = 0·69; HIAT = 0·66). At best, this result suggests that addition of neuroimaging variables to a clinical prediction scoring system marginally enhances the ability to predict poor functional outcome following endovascular treatment. However, neither model seems suitable for making individual patient decisions given their modest AUC values.
Strengths of this study include the validation of the scoring systems in an independent dataset, comparison of the SAD score to an established scoring system (the HIAT score) in the validation cohort, and the assessment of the effect of reperfusion according to SAD and HIAT scores. However, there are also some limitations. First, the number of patients in the derivation and validation cohorts was moderate. Consequently, there was limited power to identify predictor variables with smaller effect sizes. Also, it precludes our ability to draw a firm conclusion regarding the interactions between reperfusion and the prediction scores on outcome. Second, the derivation and validation cohorts were skewed toward smaller lesions. The added value of DWI to outcome prediction may be greater among patients with a wider range of infarct volumes.
In summary, a prediction model based on age and DWI predicted poor outcome following endovascular therapy with good accuracy. However, similar accuracy was achieved with a prediction tool based on clinical and demographic variables alone. Thus, in a population selected for relatively smaller infarcts, it might be sufficient to predict poor functional outcome with a clinical scoring system comprised entirely of baseline demographic and clinical variables. Because neither scoring system predicts the response to reperfusion, they do not appear to be effective tools for patient selection in acute stroke trials.
Acknowledgments
Sources of funding
The study was funded by grants from the National Institute for Neurological Disorders and Stroke (R01 NS03932505 to GWA and K23 NS051372 to MGL) and the Stanford Medical Scholars Fellowship Program.
Footnotes
Conflicts of interest:
JTPL – none declared.
AJY reports a research grant from Penumbra, Inc. and Remedy Pharmaceuticals for core imaging laboratory activities (significant).
NKM – none declared.
HMW – none declared.
MS – none declared.
TMLM – none declared.
ZAC – none declared.
SK – none declared.
MM – none declared.
RB has equity interest in iSchemaView.
GWA has equity interest in iSchemaView.
MGL – none declared.
References
- 1.Ciccone A, Valvassori L, Nichelatti M, et al. Endovascular treatment for acute ischemic stroke. N Engl J Med. 2013;368:904–13. doi: 10.1056/NEJMoa1213701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Broderick JP, Palesch YY, Demchuk AM, et al. Endovascular therapy after intravenous t-PA versus t-PA alone for stroke. N Engl J Med. 2013;368:893–903. doi: 10.1056/NEJMoa1214300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Saver JL, Jahan R, Levy EI, et al. Solitaire flow restoration device versus the merci retriever in patients with acute ischaemic stroke (SWIFT): a randomised, parallel-group, non-inferiority trial. Lancet. 2012;380:1241–9. doi: 10.1016/S0140-6736(12)61384-1. [DOI] [PubMed] [Google Scholar]
- 4.Hallevi H, Barreto AD, Liebeskind DS, et al. Identifying patients at high risk for poor outcome after intra-arterial therapy for acute ischemic stroke. Stroke. 2009;40:1780–5. doi: 10.1161/STROKEAHA.108.535146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lansberg MG, Straka M, Kemp S, et al. MRI profile and response to endovascular reperfusion after stroke (DEFUSE 2): a prospective cohort study. Lancet Neurol. 2012;11:860–7. doi: 10.1016/S1474-4422(12)70203-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Straka M, Albers GW, Bammer R. Real-time diffusion-perfusion mismatch analysis in acute stroke. J Magn Reson Imaging. 2010;32:1024–37. doi: 10.1002/jmri.22338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zaidi SF, Aghaebrahim A, Urra X, et al. Final infarct volume is a stronger predictor of outcome than recanalization in patients with proximal middle cerebral artery occlusion treated with endovascular therapy. Stroke. 2012;43:3238–44. doi: 10.1161/STROKEAHA.112.671594. [DOI] [PubMed] [Google Scholar]
- 8.Yoo AJ, Chaudhry ZA, Nogueira RG, et al. Infarct volume is a pivotal biomarker after intra-arterial stroke therapy. Stroke. 2012;43:1323–30. doi: 10.1161/STROKEAHA.111.639401. [DOI] [PubMed] [Google Scholar]
- 9.Rha JH, Saver JL. The impact of recanalization on ischemic stroke outcome: a meta-analysis. Stroke. 2007;38:967–73. doi: 10.1161/01.STR.0000258112.14918.24. [DOI] [PubMed] [Google Scholar]
- 10.Lansberg MG, Lee J, Christensen S, et al. RAPID automated patient selection for reperfusion therapy: a pooled analysis of the echoplanar imaging thrombolytic evaluation trial (EPITHET) and the diffusion and perfusion imaging evaluation for understanding stroke evolution (DEFUSE) study. Stroke. 2011;42:1608–14. doi: 10.1161/STROKEAHA.110.609008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fargen KM, Chaudry I, Turner RD, Bennett JA, Turk A, Mocco J. A novel clinical and imaging based score for predicting outcome prior to endovascular treatment of acute ischemic stroke. J Neurointerv Surg. 2013;5(Suppl 1):i38–43. doi: 10.1136/neurintsurg-2012-010513. [DOI] [PubMed] [Google Scholar]