Abstract
Pancreatic adenosquamous carcinoma (ASC) is an enigmatic and aggressive tumor that has a worse prognosis and higher metastatic potential than its adenocarcinoma counterpart. Here we report that ASC tumors frequently harbor somatically acquired mutations in the UPF1 gene, which encodes the core component of the nonsense-mediated RNA decay (NMD) pathway. These tumor-specific mutations alter UPF1 RNA splicing and perturb NMD, leading to upregulated levels of NMD substrate mRNAs. UPF1 mutations are the first known unique molecular signatures of ASC.
There has been little progress in understanding pancreatic ASC since these aggressive tumors were first described over a century ago1,2. One problem is that no mutations unique to this class of pancreatic tumors have been identified. Thus, while oncogenes and tumor suppressor genes have been shown to be altered in ASC—including K-RAS and p53—these same genes are also abnormal in other forms of pancreatic cancer3–5. In this communication, we report that ASC pancreatic tumors have somatic mutations in Up-frameshift 1 (UPF1), which encodes an RNA helicase essential for a highly conserved RNA degradation pathway called nonsense-mediated RNA decay (NMD)6. We were initially led to investigate the possibility that NMD had a role in ASC because reverse transcription-polymerase chain reaction (RT-PCR) analysis showed that an ASC tumor (TU) with wild-type p53 alleles expressed an abnormally large band that was not detectable in the adjacent normal pancreas (NP) (Supplementary Fig. 1a). Sequence analysis of this large band demonstrated that it was generated by alternatively splicing using a non-canonical splice donor in intron 6 and a non-canonical splice acceptor in intron 10, which both share 14 nt of sequence identity with the splice donor (Supplementary Fig. 1b). Quantitative PCR (qPCR) analysis demonstrated that the alternatively spliced p53 mRNA was only detectably expressed in tumor tissue, not adjacent normal tissue, while the normally spliced p53 mRNA was expressed at similar levels in the tumor and adjacent normal tissue (Supplementary Fig. 1c).
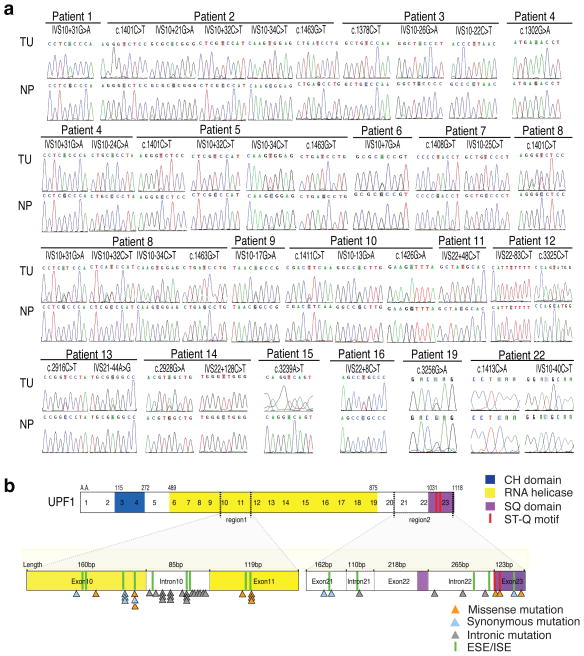
The alternative splicing event creating this p53 isoform generates an in-frame premature termination codon (PTC) in intron 6 (Supplementary Fig. 1b). Because NMD degrades mRNAs with PTCs6,7, the selective accumulation of this “alt-PTC-IVS6-p53” mRNA in the tumor tissue but not the adjacent normal tissue, raised the possibility that the tumor tissue had deficient NMD. To test this, we screened ASC tumor samples for mutations in core NMD genes. We found genomic mutations in the UPF1 gene in ASC tumors from 18 of 23 patients (see TU in Fig. 1a), whereas the 3 other NMD genes we tested—UPF2, UPF3A, and UPF3B—did not have detectable mutations (Supplementary Table 1). The UPF1 mutations were somatic in origin, since they were not present in matched normal pancreatic tissues from these 18 patients (see NP in Fig. 1a). UPF1 mutations were also not present in 50 non-ASC pancreatic and SCC lung tumors that we tested (Supplementary Table 2). Together, these results suggest that UPF1 mutations are a unique signature of ASC pancreatic tumors.
Figure 1.
Somatic UPF1 mutations in ASC tumors. (a) Chromatograms of UPF1 DNA sequences from tumor (TU) and normal adjacent pancreatic tissue (NP). A total of 36 single-base substitutions in genomic UPF1 DNA were found in the tumors; each patient had between one to five point mutations. All NP samples had wild-type UPF1 sequences, indicating mutations were somatic in origin. (b) Top: schematic of UPF1 protein domains. Bottom: the type and location of mutations found in the two regions of UPF1 that harbored mutations. ESEs and ISEs are exonic and intronic splicing enhancers, respectively (sequences are provided on Supplementary Figure 2).
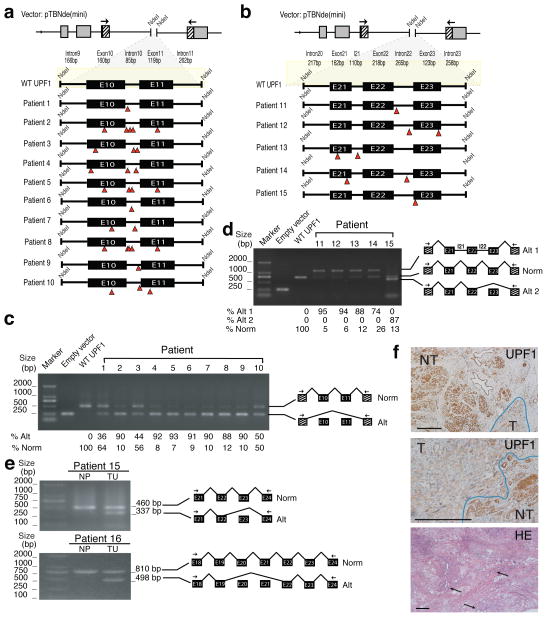
The point mutations in the ASC tumors clustered in two regions of the UPF1 gene (Fig. 1b). Surprisingly, these mutations were nearly equally distributed in the exons and introns in these two regions. This raised the possibility that they alter UPF1 splicing by disrupting intronic splicing enhancers (ISEs) and exonic splicing enhancers (ESEs), which are essential for the inclusion of a subset of exons during splicing8,9. In support of this, a subset of the mutations in the ASC tumors disrupted predicted ESEs/ISEs (Fig. 1b and Supplementary Fig. 2). To directly test whether the ASC mutations disrupt UPF1 splicing, we generated UPF1 mini-gene constructs corresponding to the two regions mutated in ASC tumors (Fig. 2a,b). We then introduced, by site-specific mutagenesis, the mutations found in 15 of the patients. Transfection analysis showed that the wild-type versions of the two mini-gene constructs expressed normally spliced mRNA that included all exons, as determined by direct sequencing of the bands generated by RT-PCR (Fig. 2c,d). No other bands were detected, indicating that most of the mature mRNA generated by these mini-gene constructs was normally spliced. In contrast, all 15 mutant mini-genes expressed alternatively spliced transcripts (Fig. 2,d). While none of the patient’s mutations completely eliminated normal splicing, the ratio of alternatively spliced-to-normally spliced mRNA was 10 to 1 or higher in many cases. As confirmation, analysis of endogenous UPF1 splicing in 2 of 2 frozen tumor samples revealed an alternatively spliced UPF1 mRNA that was not present in the adjacent normal tissue (Fig. 2e). We conclude that ASC-specific mutations in the UPF1 gene trigger alternative splicing of UPF1 pre-mRNA.
Figure 2.
ASC-specific UPF1 mutations trigger alternative UPF1 RNA splicing. (a) The indicated region of human UPF1 (nt 22,779-23,570, RefSeq accession number NC_000019.9) was cloned into the NdeI site of the pTBNde mini-gene construct. All mutations were generated by site-directed mutagenesis to match those in the indicated patient’s tumors. (b) The indicated region of human UPF1 (nt 33,138-34,490) was cloned and mutated as described in panel a. (c) RT-PCR analysis of HEK293 cells transfected with the constructs shown in panel a (primer locations are indicated by the arrows). Direct sequencing of the large (792 nt) and small (239 nt) bands indicated that they correspond to normally spliced and exon-skipped transcripts, respectively. The numbers below the gel are the average values from five independent transfections. (d) RT-PCR analysis performed as in panel c. Direct sequencing of the bands in the gel indicated they were derived from mRNA spliced in the manner shown in the schematic. The bands corresponding to normally spliced, alt 1, and alt 2 mRNA had lengths of 742, 1117, and 619 nt, respectively. The numbers below the gel are the average values from five independent transfections. (e) RT-PCR analysis of normal pancreas (NP) and ASC frozen samples (TU) from patients 15 and 16. RT-PCR sequencing results are indicated as schematics next to the gels. (f) Immunohistochemical analysis of UPF1 staining in ASC tumor samples from patient 15. Tumor (T) and normal tissue (NT) are indicated. Bottom panel shows H & E staining, with arrows pointing to the adenocarinoma component in this ASC tumor. The scale bar represents 200 micrometers on each picture.
The UPF1 alternative splicing events caused by mutations in the exon 10/11 region eliminate a portion of UPF1’s RNA helicase domain essential for UPF1 function6, while mutations in the exon 21/23 region truncate the carboxy-terminal region of UPF1, which contains [S/T]Q motifs phosphorylated by SMG1, a serine/threonine kinase required for NMD10 (Figs. 1b and 2c). Given that essential UPF1 domains are lost, these mutant forms of UPF1 are likely to have lost their function or have dominant-negative activity. In the case of the latter, inhibited NMD would be predicted to occur when only one UPF1 allele is mutated. In support of this possibility, mutations in various regions of UPF1 have previously been shown to confer dominant-negative activity11–13. With regard to the former, we found that at least one ASC tumor (from patient 22) had mutations in both UPF1 alleles. Several other ASC tumors have multiple UPF1 mutations (Supplementary Table 1), consistent with some of them also having mutations in both UPF1 alleles. In further support of this notion, we observed little or no UPF1 expression in many ASC tumors compared to adjacent normal tissue, as demonstrated by immunohistochemical (IHC) analysis (Fig. 2f and Supplementary Table 1). As direct evidence that NMD is inhibited in ASC tumors, we found that the NMD substrates, ATF3 and MAP3K14 mRNA14, were dramatically upregulated in tumor tissue relative to adjacent normal tissue (Supplementary Fig. 3).
Since perturbation of NMD is known to dysregulate ~3 to 10% of mRNAs in a variety of cell lines and organisms6,14,15, it is likely this will also be the case of ASC tumors, which could shift the balance towards a more malignant phenotype16,17. One candidate NMD substrate that could serve in this capacity is the alternatively spliced p53 transcript—alt-PTC-IVS6-p53—that we identified above (Supplementary Fig. 1), as we found it encoded a protein with dominant-negative activity (Supplementary Fig. 4).
The discovery of mutations in the UPF1 gene in ASC tumors represents the first known example of genetic alterations in a NMD gene in human tumors. To our knowledge, UPF1 is also the first gene known to be selectively mutated in pancreatic ASC tumors. Other genes mutated in these tumors, including p53 and K-RAS (see Supplementary Fig. 5 and Supplementary Table 1) are also mutated in other pancreatic tumor types4,5. We note that it is possible that UPF1 mutations in ASC tumors have broad disruptive effects that that extend beyond NMD, including Staufen-mediated RNA decay18. Our finding that UPF1 is selectively mutated in pancreatic ASC suggests that this feature will be useful for diagnosis of this type of tumor and also raises the possibility that ASC patients can benefit from therapy designed to target NMD substrates19,20.
METHODS
Methods are available in the Supplementary Information section.
Supplementary Material
Acknowledgments
This work was supported by The National Key Basic Research Program of China grants 2013CB967500 and 2011DFB30010 (to GX), 2011CB965102 (to LL), the National Natural Science Foundation of China grant 81071740 (to YJ), and NIH grant GM58595 (to MFW).
Footnotes
AUTHOR CONTRIBUTIONS
C.L., R.K., Y.Z., F.S., C.W., M.S., Y.W., G.X., L.L., J.Z., and Y.M. performed the experiments. Y.Z., Y.J., G.L., W.F., M.Z., M.A.V. and M.J. provided clinical samples and expertise on pancreatic cancer. R.K. and C.L. prepared the figures and tables and wrote the manuscript. Y.L. and M.F.W. equally contributed to this study by designing and supervised all experiments and assisting in writing the manuscript.
Includes Supplementary Figures 1 to 5, Supplementary Tables 1 to 7, Methods, and Supplementary References.
COMPETING FINANACIAL INTERESTS
The authors declare no competing financial interests.
References
- 1.Regi P, et al. Clinicopathological features of adenosquamous pancreatic cancer. Langenbecks Arch Surg. 2011;396:217–22. doi: 10.1007/s00423-010-0677-3. [DOI] [PubMed] [Google Scholar]
- 2.Madura JA, Jarman BT, Doherty MG, Yum MN, Howard TJ. Adenosquamous carcinoma of the pancreas. Arch Surg. 1999;134:599–603. doi: 10.1001/archsurg.134.6.599. [DOI] [PubMed] [Google Scholar]
- 3.Kardon DE, Thompson LD, Przygodzki RM, Heffess CS. Adenosquamous carcinoma of the pancreas: a clinicopathologic series of 25 cases. Mod Pathol. 2001;14:443–51. doi: 10.1038/modpathol.3880332. [DOI] [PubMed] [Google Scholar]
- 4.Murakami Y, et al. Adenosquamous carcinoma of the pancreas: preoperative diagnosis and molecular alterations. J Gastroenterol. 2003;38:1171–5. doi: 10.1007/s00535-003-1226-4. [DOI] [PubMed] [Google Scholar]
- 5.Brody JR, et al. Adenosquamous carcinoma of the pancreas harbors KRAS2, DPC4 and TP53 molecular alterations similar to pancreatic ductal adenocarcinoma. Mod Pathol. 2009;22:651–9. doi: 10.1038/modpathol.2009.15. [DOI] [PubMed] [Google Scholar]
- 6.Chang YF, Imam JS, Wilkinson MF. The nonsense-mediated decay RNA surveillance pathway. Annu Rev Biochem. 2007;76:51–74. doi: 10.1146/annurev.biochem.76.050106.093909. [DOI] [PubMed] [Google Scholar]
- 7.Karam R, et al. The NMD mRNA surveillance pathway downregulates aberrant E-cadherin transcripts in gastric cancer cells and in CDH1 mutation carriers. Oncogene. 2008;27:4255–60. doi: 10.1038/onc.2008.62. [DOI] [PubMed] [Google Scholar]
- 8.Wang Y, Ma M, Xiao X, Wang Z. Intronic splicing enhancers, cognate splicing factors and context-dependent regulation rules. Nat Struct Mol Biol. 2012;19:1044–52. doi: 10.1038/nsmb.2377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chasin LA. Searching for splicing motifs. Adv Exp Med Biol. 2007;623:85–106. doi: 10.1007/978-0-387-77374-2_6. [DOI] [PubMed] [Google Scholar]
- 10.Isken O, et al. Upf1 phosphorylation triggers translational repression during nonsense-mediated mRNA decay. Cell. 2008;133:314–27. doi: 10.1016/j.cell.2008.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Leeds P, et al. Gene products that promote mRNA turnover in Gene Products That Promote mRNA Turnover in Saccharomyces cerevisiaet. 1992;12 doi: 10.1128/mcb.12.5.2165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Frischmeyer-Guerrerio Pa, et al. Perturbation of thymocyte development in nonsense-mediated decay (NMD)-deficient mice. Proc Natl Acad Sci U S A. 2011;108:10638–43. doi: 10.1073/pnas.1019352108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sun X, Perlick HA, Dietz HC, Maquat LE. A mutated human homologue to yeast Upf1 protein has a dominant-negative effect on the decay of nonsense-containing mRNAs in mammalian cells. Proc Natl Acad Sci U S A. 1998;95:10009–10014. doi: 10.1073/pnas.95.17.10009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mendell JT, Sharifi NA, Meyers JL, Martinez-Murillo F, Dietz HC. Nonsense surveillance regulates expression of diverse classes of mammalian transcripts and mutes genomic noise. Nat Genet. 2004;36:1073–1078. doi: 10.1038/ng1429. [DOI] [PubMed] [Google Scholar]
- 15.Conti E, Izaurralde E. Nonsense-mediated mRNA decay: molecular insights and mechanistic variations across species. Curr Opin Cell Biol. 2005;17:316–25. doi: 10.1016/j.ceb.2005.04.005. [DOI] [PubMed] [Google Scholar]
- 16.Karam R, Wengrod J, Gardner LB, Wilkinson MF. Regulation of nonsense-mediated mRNA decay: Implications for physiology and disease. Biochim Biophys Acta. 2013;1829:624–33. doi: 10.1016/j.bbagrm.2013.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gardner LB. Nonsense-mediated RNA decay regulation by cellular stress: implications for tumorigenesis. Mol Cancer Res. 2010;8:295–308. doi: 10.1158/1541-7786.MCR-09-0502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Isken O, Maquat LE. The multiple lives of NMD factors: balancing roles in gene and genome regulation. Nat Rev Genet. 2008;9:699–712. doi: 10.1038/nrg2402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bordeira-Carriço R, Pêgo AP, Santos M, Oliveira C. Cancer syndromes and therapy by stop-codon readthrough. Trends Mol Med. 2012;18:667–78. doi: 10.1016/j.molmed.2012.09.004. [DOI] [PubMed] [Google Scholar]
- 20.Pastor F, Kolonias D, Giangrande PH, Gilboa E. Induction of tumour immunity by targeted inhibition of nonsense-mediated mRNA decay. Nature. 2010;465:227–30. doi: 10.1038/nature08999. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.