Abstract
Alterations in cerebrovascular structure and function may underlie the most common age-associated cognitive, psychiatric, and neurological conditions presented by older adults. Although much remains to understand, existing research suggests several age-associated detrimental conditions may be mediated through sometimes subtle small vessel-induced damage to the cerebral white matter. Here we review a selected portion of the vast work that demonstrates links between changes in vascular and neural health as a function of advancing age, and how even changes in low-to-moderate risk individuals, potentially beginning early in the adult age-span, may have an important impact on functional status in late life.
Introduction
It has been long understood that severe disruption of the cerebral vasculature is a major cause of functional decline in older adults resulting in an appreciable scourge on society. Proceedings from a panel discussion on ‘Problems of Aging’ at The New York Academy of Medicine in 1955 highlight the urgency at the time to better understand overt forms of cerebrovascular disease (Adams et al., 1956). The Moderator, Irving S. Wright (Figure 1), founder of the American Federation for Aging Research and the first physician to use heparin to treat blood clots (Wright, 1959, Mueller, 1995), opened the session as follows:
“This major problem has been sadly neglected in the past but in the last year or two has come more to the forefront of medicine. In 1952 approximately I70,000 people died with cerebralvascular diseases in this country. It is true that many of them were elderly. However, more than 44,000 of them were in the productive age group under 65 years of age. This represents an enormous loss of productivity to the country. In addition it is estimated that approximately 1,8oo,ooo people alive today in the United States have suffered from some manifestations of cerebral vascular disease. Many of them are not only incapacitated themselves but in addition they require the aid, often full time, of from one to three or four additional people, which again represents an enormous loss to our community life in this country. There are many thousands confined for long periods in various private, municipal, state and veterans hospitals unable to care for themselves as the result of these diseases. Many more thousands are in nursing homes or the homes of their families where they constitute a heavy burden. It is not necessary for me to go into this in greater detail since I am sure that this audience fully recognizes the gravity of the problem. The question is, what are we going to do about it?”
Figure 1.
Irvine H. Page's group to discuss the formation of a National Academy of Medicine met for the first time on January 17, 1967, at the Cleveland Clinic Foundation. Although Page never realized his ambition to create such on academy, this group supplied the impetus that eventually led to the founding of the institute of Medicine. Top, left to right: Fay H. Lefevre, M.D.; J. Englebert Dunphy, M.D.; Carleton B. Chapman, M.D.; Francis D. Moore, M.D.; William B. Bean, M.D.; John B. Hickam, M.D.; E. Cowles Andrus, M.D.; Robert A. Aldrich, M.D.; Ivan L. Bennett, Jr., M.D.; and Stuart M. Sessoms, M.D.; Bottom, left to right: James A. Shannon, M.D.; Frederick C. Robbins, M.D. (who would later become the institute's fourth president, in 1980); Irving S. Wright, M.D.; Irvine H. Page, M.D.; Douglas D. Bond, M.D.; and Robert H. Williams. Photograph courtesy of The Cleveland Clinic Foundation. Reprinted from To Improve Human Health: A History of the Institute of Medicine (1998).
Although it is clear that major cerebrovascular injury has a profoundly destructive influence on brain tissue and subsequently on society, the full extent of vascular influences is still unknown. This lack of knowledge is particularly evident with regard to the subtle variation in microvascular health that begins to decline almost ubiquitously as part of the aging process and may begin to exert influence on the brain and clinical course much earlier in adulthood than previously suspected (Swan et al., 1998, Launer et al., 2000, Kivipelto et al., 2001a, Kivipelto et al., 2001b, Korf et al., 2004, Debette et al., 2011, Gorelick et al., 2011, Tolppanen et al., 2012). A range of systemic vascular conditions elevate with increasing age. For example, hypertension alone has an age-adjusted prevalence of approximately 40% in Americans aged 45-64 and this pervasiveness jumps to approximately 70% in individuals >65 years of age (Keenan et al., 2011)(see also ranging estimates of hypertension prevalence across varying samples and assessment methods, e.g. (Egan et al., 2010, Kaplan et al., 2010, Mujahid et al., 2011, Guo et al., 2012, Joffres et al., 2013)). Hypertension contributes to detrimental remodeling of large and small cerebral and peripheral vessels (Lammie, 2000, Feihl et al., 2008, Lemarie et al., 2010, Schiffrin, 2012), and these deviations from optimal health diminish routine cerebrovascular function (Paulson et al., 1990, Iadecola and Davisson, 2008, Toth et al., 2013). In more advanced stages vascular alterations are hypothesized to create a state of chronic hypoperfusion to brain tissue (Fernando et al., 2006, Iadecola and Davisson, 2008). With similar timing in the age-span as these vascular changes, cerebral white matter exhibits several forms of deterioration including substantial volume loss (Guttmann et al., 1998, Salat et al., 1999, Bartzokis et al., 2001, Ge et al., 2002a, Raz et al., 2005, Walhovd et al., 2005, Salat et al., 2009, Westlye et al., 2010), increased lesion volume (Fazekas, 1989, Jernigan et al., 1991a, DeCarli et al., 1995), and accelerated alterations in tissue microstructure (Ge et al., 2002b, Bartzokis et al., 2003, Westlye et al., 2010).
There is an immense literature describing the broad topic of cerebrovascular contributions to neural aging. We review here neuroimaging studies linking vascular and white matter health with a focus on how subtle variation in small vessel vascular function, even within a relatively low risk range, may have cognitive, behavioral and psychiatric consequences. We focus selectively on a small portion of this work contributing to the idea that subtle changes may in fact be a primary mechanism of decline with advancing age; contributing to reduction in quality of life, and in more extreme forms, heralding significant disability. A full review of vascular contributions to psychiatric symptoms is beyond the scope of this summary however readers are referred to several existing resources on this topic (O'Brien and Ames, 1996, Thomas et al., 2002, Fields, 2008, Herrmann et al., 2008).
We refer here to ‘white matter lesions’ generically as either signal abnormalities within the white matter on neuroimaging or tissue changes measured histologically without a reference to a specific pathophysiology. It is important to recognize that a range of pathologies contribute to lesioned tissue, and the type of damage may differ depending on various factors associate with the population under study (e.g. (Tomimoto et al., 1996, Gouw et al., 2008, Gouw et al., 2011, Schmidt et al., 2011)). It is also essential to consider that much of the literature reviewed here is cross-sectional and associational. Although substantial data have accumulated to support the notions presented, and while certain concepts seem intuitive (e.g. white matter damage is a result of poor vascular health), any assumed directionality from association is inferential and should be considered cautiously. In fact, little is known about the precise mechanisms by which vascular dysfunction contributes to observed changes in neural tissue (Pantoni, 2010). Aside from a small number of existing interventional studies, much work remains to demonstrate a direct causal mechanistic link between small vessel pathology and the common white matter changes associated with vascular risk. Finally, much of the literature reviewed used classical imaging markers of white matter damage (e.g. ‘white matter hyperintensities’) as a proxy for vascular associated tissue damage. However, the volume of overt white matter lesions is strongly correlated with the microstructural integrity of normal appearing white matter (NAWM)(Vernooij et al., 2008, Leritz et al., 2013) and the integrity of the NAWM is reduced in individuals with white matter lesions (O'Sullivan et al., 2001). These findings are not surprising given the fact that there is a global decrease in vascular density throughout the brain in individuals with vascular-associated white matter damage as opposed to vessel changes being limited to lesioned tissue (although not necessarily accompanied by parenchymal damage)(Brown et al., 2007). Vascular-associated influence to the white matter therefore expands beyond the classically examined ‘hyperintensities’ to the microstructural properties of NAWM. In fact, statistical control for the degree of white matter lesions removes a substantial portion of the variance shared between age and microstructural changes in NAWM (Vernooij et al., 2008, Leritz et al., 2013). Thus, although overt lesions seem to intuitively be an appropriate target for investigation and therapeutics, this damage is strongly linked to the overall connective integrity of the brain. Selected studies demonstrating associations between white matter microstructural properties in NAWM and vascular parameters are therefore additionally reviewed here. The current data suggest that subtle inter-individual variations in health parameters associated with vascular structure and function contribute to degenerative changes in cerebral white matter. These degenerative changes in the tissue supporting neural connectivity in turn contribute to functional decline in older adults. This influence potentially begins in midlife and occurs even within the range of variation considered normal to moderate risk for cerebrovascular disease; yet has an appreciable effect on cognition and other functional parameters and may progress substantially across time. These data highlight the need to consider an additional category of vascular health which is characterized by subtle deviation from the optimal state for maintenance of brain tissue and cognition, and may provide a target for therapeutics to maintain peak neural and functional health in late life (Gorelick et al., 2011).
Imaging white matter integrity and damage
Early imaging work using computed tomography (CT) noted the prevalence and extent of incidental white matter damage in older adults. It was through this work that the term ‘leukoaraiosis’, referring to a hypodensity on CT, was coined as an image-associated occurrence without claim to a specific pathology (Hachinski et al., 1986, 1987, Pantoni and Garcia, 1997, O'Sullivan, 2008). Routine access to MRI provided the ability to better visualize the morphology of the lesions and subsequent procedures for the quantitative measurement of lesion in the form of hyperintense signal within white matter, typically measured on T2 or fluid attenuated inversion recovery (FLAIR) imaging. Initially, white matter lesions were assessed by semi-quantitative rating scales of the degree of total damage (e.g. (Fazekas et al., 1987, Scheltens et al., 1993)), and such scales continue to provide a reasonable and simple means for clinical and research assessment (King et al., 2013). Subsequent development of computer-based image segmentation routines (Jernigan et al., 1991a, Jernigan et al., 1991b) led to the more routine quantitative volumetric measurement and regional mapping of white matter lesions in patient populations (Tanabe et al., 1997). These procedures also facilitated the volumetric measurement of the ‘normal appearing white matter’ (NAWM), typically referring to tissue that is not hyperintense on T2 or FLAIR MRI. In addition to the macrostuctural procedures for calculating total lesion volume, current assessment often additionally includes procedures for measurement of tissue ‘microstructure’, referring to certain biophysical properties of the tissue within the unit of resolution (e.g. within an MRI voxel; Figure 2). Such procedures produce varying measures thought to be linked to the ‘integrity’ of tissue (measured within and outside of the lesion). For example, measures extracted from diffusion tensor imaging (DTI) show strong associations with age (Pfefferbaum et al., 2000, Abe et al., 2002, Moseley, 2002, Salat et al., 2005a, Salat et al., 2005b, Sullivan and Pfefferbaum, 2006, Davis et al., 2009), are related to cognitive and behavioral performance (Madden et al., 2004, O'Sullivan et al., 2004, Charlton et al., 2006, Grieve et al., 2007, Madden et al., 2009), are predictive of subsequent white matter atrophy (Ly et al., 2013), and can provide information about the degree of signal abnormality within a lesion (e.g. see (Jones et al., 1999))(see also review in (Salat, 2011)). Diffusion measures loosely reflect a mix of the degree of myelination, fiber density, and fiber organization within tissue (Beaulieu, 2002); however, it's important to emphasize that imaging provides an indirect marker of histological phenomena and the use of these procedures to robustly quantitate specific pathologies remains to be developed and validated. With these potential caveats in mind, neuroimaging procedures provide several means by which the white matter anatomy and integrity can be understood in the context of healthy and diseased individuals as well as monitored for progressive changes across time.
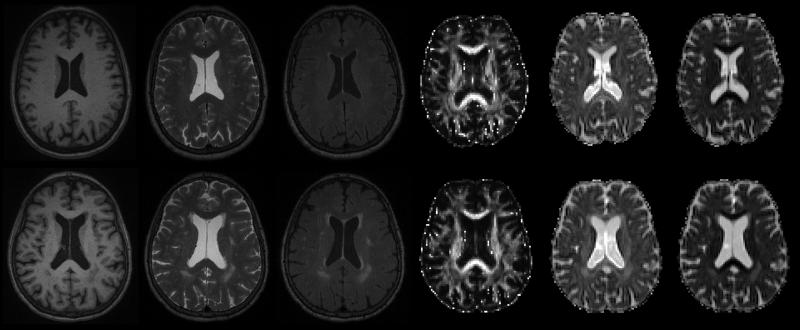
Figure 2.
Multiple image types in an older adult without (top) and with (bottom) white matter lesions. Variation in periventricular lesion contrast is obvious across the different image modalities, and this variation may provide important quantitative information allowing the specification of lesion pathology. In order from left to right: T1, T2, FLAIR, diffusion tensor imaging (DTI) fractional anisotropy, DTI axial diffusivity, DTI radial diffusivity.
Systemic, demographic, and disease factors associated with white matter damage in older adults
Several factors related to systemic physiology have been associated with severity of white matter damage. It was recognized early on that lesioned tissue was more often found in older individuals with vascular disease, however, tissue damage is also apparent incidentally in older adults without symptomatic disease (Roman, 1987). An early imaging study by Fazekas and colleagues examined the relationship between white matter lesions and a range of parameters linked to vascular health in individuals 51-70 years of age with no evidence of cerebrovascular, neurologic, or psychiatric disease on history or neurologic examination. Individuals with white matter lesions (T2 hyperintensities) had greater extracranial carotid artery disease, lower mean gray matter blood flow, a significant reduction in blood flow of the ‘slow-flowing ‘ compartment (an estimate of white matter flow), hypertension, and diabetes (Fazekas et al., 1988). The lesions were attributed to general microcirculatory disturbance. The link between white matter lesions and metrics of vascular health is now fairly well established in diseased and non-diseased populations. Findings include elevated lesion volume in individuals with hypertension (Breteler et al., 1994, Longstreth et al., 1996, Dufouil et al., 2001, de Leeuw et al., 2002, Raz et al., 2007), individuals with elevated plasma homocysteine (Hogervorst et al., 2002, Wright et al., 2005), smokers (Hogervorst et al., 2002), and individuals with elevated composite risk scores such as the Framingham Stroke Risk Profile (Jeerakathil et al., 2004). The association between multiple cerebrovascular risk factors and white matter lesions may be greater than additive. Lechner and colleagues demonstrated that, in older adults without cerebrovascular symptoms, 92% had white matter lesions when two risk factors were present, and 100% when three risk factors were present (Lechner et al., 1988). It is not clear exactly when in the lifespan adverse vascular health may influence brain tissue. Adults with high midlife systolic blood pressure have greater white matter lesion volumes in later life compared to individuals with lower blood pressure suggesting a long-term impact (Swan et al., 1998). White matter lesions are progressive, and can approximately double in volume across a five year period in a vascular risk population (Raz et al., 2007). Additionally, white matter lesions have > 70% heritability in twin studies (Carmelli et al., 1998, Atwood et al., 2004), however, it is not clear whether this heritability is directly related to vascular risk per se, or some other aspect of lesion formation. Although often found incidentally, in contrast to normative populations, the prevalence of white matter lesions may be low and show reduced longitudinal increase in older adult populations selected for good vascular health (Kozachuk et al., 1990, Raz et al., 2007). These studies and supporting work are suggestive of a multifactorial condition by which changes in vascular health, potentially across a span of decades, subtly erodes the integrity of the tissue that functions to provide optimized information transmission throughout the central nervous system.
Imaging/pathologic correlations
Studies linking imaging findings to tissue pathology add support to the connection between microvascular changes and lesion formation. Awald and colleagues used MRI to identify lesions for pathologic analysis in older adults dying of non-neurologic causes and found that subcortical lesions were associated with arteriosclerosis, dilated perivascular spaces, and dilation of vessels accompanied occasionally by gliosis and infarction (Awad et al., 1986). The authors noted the nonspecific nature of the MRI signal and attributed signal changes to aging, hypertension, and vascular factors. Related studies found lesions to be associated with wide-ranging phenomena including perivascular demyelination, small vascular malformations, deformations of the lateral ventricle extending into the white matter, infarction, fiber loss, cavitation, and arteriolosclerosis (Kirkpatrick and Hayman, 1987, Yamanouchi et al., 1989, van Swieten et al., 1991, Fazekas et al., 1993, Brown et al., 2000). Although much of the imaging/pathologic correlations suggest that age-associated white matter lesions have a vascular etiology, it is important to note that these should not be equated in all cases as other disease processes can result in similar appearing findings. Detailed characterization of damage with regard to location, size, morphology, and clinical presentation is necessary to distinguish vascular contributions from lesions due to non-vascular origins. For example, the more common periventricular ‘caps’ (typically observed as MR signal abnormality in the frontal horns of the ventricles) often do not reflect ischemic damage but occur with subependymal gliotic demyelination with alterations in the ependymal lining (Fazekas et al., 1993, Takao et al., 1999). It is also important to consider that certain prior concepts of differentiating lesions based on localization, such as deep versus periventricular, may not actually reflect distinct categories of damage given their high correlation across a broad clinical sample (DeCarli et al., 2005), although certain categorical differentiation may provide important etiological and prognostic information (Kim et al., 2008, Schmidt et al., 2011).
Brain changes associated with small vessel damage such as white matter lesions are often linked to other types of neural damage. For example, in a sample of 51 healthy adults across a broad age-span (ages 19-91), DeCarli and colleagues demonstrated that white matter lesion volume was associated with increased ventricular volume, reduced total brain volume, and reduced cerebral metabolism measured by flurodeoxyglucose (FDG) positron emission tomography (PET) (DeCarli et al., 1995). Given these findings, and the greater degree of hypertension and reduced cognition in individuals with white matter lesions, this work provided an important demonstration of a potentially broad syndrome accompanying this type of damage and the authors concluded that such lesions be considered pathologic (DeCarli et al., 1995). It is currently unclear, however, whether such neural changes represent a coherent cascade of events, or if each form of neural deterioration is an independent manifestation of microvascular dysfunction. Associations between measures of cortical integrity and vascular risk profile exist even when limiting the analysis to individuals in the low-to-moderate risk range of inter-individual variation (Leritz et al., 2011). It is therefore uncertain at present the degree to which white matter damage mediates cognitive, behavioral, and neurological decline relative to other types of tissue damage that may also be present in individuals with poor vascular health. Future work should aim to employ multimodal neural assessment to determine the specific contributions of white matter damage to cognitive and behavioral profiles while controlling for the influence of other neural markers vulnerable to small vessel changes.
Cognitive, behavioral, and psychiatric consequences of vascular associated white matter damage
The clinical significance of white matter lesions was unclear for some time (Pantoni and Garcia, 1995, O'Sullivan, 2008) and remains somewhat unresolved. In part, this based on the somewhat anecdotal and qualitative fact that certain individuals have appreciable lesion volume yet demonstrate general function relatively on par with others in their demographic, likely due to heterogeneity in the pathology contributing to the imaging result. Multiple large-sample studies have accumulated demonstrating that these lesions, or conditions tied to lesion formation, are in fact associated with detrimental conditions and phenomena that are of great importance to better characterize. Impairment across several major functional domains is related to the existence of microvascular associated white matter damage (Pantoni, 2008, Brickman et al., 2009a). Cognitively, individuals with greater white matter lesion volumes perform worse on tests of frontal and executive and speeded function with lesions having more minimal impact on domains supported by medial temporal lobe (de Groot et al., 2000, Gunning-Dixon and Raz, 2000, Au et al., 2006, Jacobs et al., 2013). The partial cognitive selectivity is potentially due to a preferential disruption of frontal circuitry (Nordahl et al., 2006) and/or to disruption of cholinergic pathways (Swartz et al., 2003). Although lesions tend to be similarly spatially distributed in specific hemodynamic risk zones across various clinical populations (Holland et al., 2008), lesion location is to some degree related to the cognitive deficits observed (Smith et al., 2011), and lesions are independently associated with cognition relative to other measures of brain structure such as prefrontal volume (Gunning-Dixon and Raz, 2003). Associations between executive cognition and white matter microstructure may be substantially mediated by the degree of white matter lesions (Jacobs et al., 2013), potentially suggesting that vulnerability to vascular-associated tissue damage accounts for much of age-associated variation in cognition. Behaviorally, altered gait (Whitman et al., 2001, Baloh et al., 2003, Baezner et al., 2008) and balance (Starr et al., 2003) are conditions often reported in individuals with white matter damage. Psychiatrically, hyperintensities on MRI have been associated with geriatric depression (Hickie et al., 1997, Gunning-Dixon and Raz, 2003, Sheline et al., 2010) and an association between blood pressure and white matter microstructure has been demonstrated in individuals with geriatric depression (Hoptman et al., 2009). White matter lesions are associated with other clinical manifestations that impact quality of life including urinary incontinence and urgency (Poggesi et al., 2008, Kuchel et al., 2009). These findings alone demonstrate that although once considered ‘silent’, white matter lesions may in fact influence a range of cognitive, behavioral, and neurological domains and have a cumulative effect on functional independence.
Prognosis for disability
Microvascular changes in white matter may indicate future conditions with severe consequences including disability and death. The population based Leukoariosis and Disability (LADIS) study found that the severity of white matter damage was strongly related to time to convert to a dependent state measured by the Instrumental Activities of Daily Living (IADL), a scale of an individuals’ independent functioning in a community (Inzitari et al., 2007, Inzitari et al., 2009). The investigators found that the risk of transition to disability or death was more than twice as high in older adults with severe compared to mild white matter lesions across an approximate period of 2.5-3 years with decline apparent in some in as early as one year (Inzitari et al., 2007, Inzitari et al., 2009). Findings from The Rotterdam Scan Study and The Framingham Offspring Study and others demonstrate the independent predictive nature of white matter lesions for risk of stroke (Vermeer et al., 2003, Kuller et al., 2004), cognitive impairment, and death relative to vascular risk factors (Debette et al., 2010). Thus, neuroimaging measures of white matter lesion index vascular risk that is not detectable by standard clinical measures.
A recent meta-analysis on prognosis of individuals with white matter lesions measured on MRI supported these conclusions finding that this tissue damage was associated with increased stroke risk (hazard ratio 3.3, 95% confidence interval 2.6 to 4.4), dementia (1.9, 1.3 to 2.8), and death (2.0, 1.6 to 2.7) (Debette and Markus, 2010). The authors suggested that, given the independent association of white matter hyperintensities with stroke after adjustment for vascular risk factors in prior work, white matter lesions reflect an important marker of uncontrolled vascular risk that may be more valuable than any individual risk factor. These studies demonstrate the critical need for novel sensitive markers of cerebral vascular sufficiency, as well as quantitative metrics of cumulative burden to brain tissue that may be important to consider with regard to clinical intervention.
Cerebral amyloid angiopathy (CAA; (Vanley et al., 1981, Gilbert and Vinters, 1983, Vinters and Gilbert, 1983, Cosgrove et al., 1985, Biffi and Greenberg, 2011) (Cosgrove et al., 1985, Itoh et al., 1993, Biffi and Greenberg, 2011)), is condition in which excessive aggregations of beta-amyloid protein fibrils accumulate in the walls of small to medium-sized cerebral vessels, initially disrupting overall vascular function and ultimately resulting in lobar hemorrhage in a portion of affected individuals. The sporadic prevalence of this condition is high in adults over 60 years of age and even greater in patients with AD (Attems et al., 2008). As might be expected, individuals with probable CAA have impaired vascular reactivity (Dumas et al., 2012), increased prevalence of white matter lesions (Greenberg et al., 2004, Holland et al., 2008), and accelerated progression of lesions over time (Chen et al., 2006). Individuals with CAA additionally have reduced tissue integrity in the normal appearing white matter (Salat et al., 2006). Therefore, it is possible the substantial changes in white matter microstructure consistently observed in older adults may, in certain cases, reflect subclinical CAA and may indicate risk for future CAA-associated hemorrhage. Procedures for identifying probable CAA based in part on imaging findings have been described (Knudsen et al., 2001, Smith and Greenberg, 2003) and more information about early subtle effects of CAA to white matter damage may improve this in vivo detection in future work.
Vascular damage and white matter lesions are elevated in patients with AD (Steingart et al., 1987, Hogervorst et al., 2002, Makedonov et al., 2013)(for review see (Brickman et al., 2009a)). Whether this finding is related to a simple comorbid condition with an additive influence on clinical presentation, reflects an interaction between an independent classical AD and vascular pathology, or is related to a primary mechanism of AD is yet to be determined. Although there is a high prevalence of CAA in AD, at least a portion of the tissue changes in AD reflect similar types of microvascular pathology that accompanies non-amyloid based vascular conditions (Brun and Englund, 1986). White matter lesions seem to play a more prominent role modifying clinical presentation in the early stages of AD, or with mild cognitive impairment, but are less predictive of clinical status with greater disease severity (Chui et al., 2006, Debette and Markus, 2010). Regionally, individuals with AD and mild cognitive impairment are more likely to have lesions in posterior periventricular and posterior callosal regions compared to cognitively healthy older adults (Yoshita et al., 2006). Cognitive abilities may be less associated with the severity of white matter lesions in patients with AD without cerebrovascular risk factors (Kozachuk et al., 1990) suggesting that vascular risk may contribute to lesion formation with unique variance when vascular health is poor and when degenerative changes of later stage AD do not dominate clinical presentation. Recent data from the Alzheimer's Disease Neuroimaging Initiative (ADNI) highlight the fact that even in a sample selected to mirror a clinical trial for AD and screening and statistically controlling for several factors, white matter disease at baseline still remained an important predictor of short-term (1 year) changes in global cognition (Carmichael et al., 2010) highlighting the necessity to consider vascular risk and white matter lesions in clinical trials of age-associated disease, even in relatively low-risk populations. Additional data from ADNI suggests that white matter damage may be a ‘second hit’ necessary for the clinical expression of AD (Provenzano et al., 2013). Evidence has been presented suggesting that cerebrovascular dysfunction, deterioration of the neurovascular unit, and homeostatic responses to myelin deterioration may contribute to pathologic cascades that promote or exacerbate classically recognized pathologies of AD (Wardlaw et al., 2003, de la Torre, 2004, Bell and Zlokovic, 2009, de la Torre, 2010, Bartzokis, 2011). These ideas provide valuable mechanisms to explore with regard to AD therapeutics in addition to the more commonly considered pathologic targets.
Vascular health
The literature reviewed suggests that in addition to the common consideration of vascular-linked clinical categories such as ‘cerebrovascular disease’ and ‘vascular dementia’, a more subtle class of cerebral phenomena is governed by variation in the degree of overall ‘vascular health’ and that there are functional consequences to this variation. That is, in addition to the focus on extreme deviation that contributes to explicit disease and disability, a secondary goal is to understand the optimal physiological state for peak neural health and the variations from this optimal state that occur yet are not deviant enough to be classified as overt disease. Age alone, independent of vascular risk is considered one of the primary factors contributing to the increase in white matter lesions (Longstreth et al., 1996, Brickman et al., 2008, Debette and Markus, 2010). Chronological age is an arbitrary marker of health that may instead represent, in part, a proxy for deviation in the optimal vascular state beyond what is detected with standardized values for vascular disease (e.g. hypertensive/normotensive). In fact, it has been suggested that any individual category of vascular risk is not particularly informative in the context of treatment (Jackson et al., 2005). In support of this, prior work has demonstrated that white matter lesions are elevated in individuals with higher blood pressure within the normal range (DeCarli et al., 1995). We and others have found cross-sectional associations between blood pressure (Kennedy and Raz, 2009, Leritz et al., 2010, Salat et al., 2012) as well as serum lipids (Williams et al., 2012) and integrity of NAWM in relatively low-risk populations of older adults. Systemic measures are indirect markers of cerebral vascular risk but do not provide direct information about cerebral vascular function. A limited set of studies have examined the association between white matter lesions and more direct measures of vascular activity in brain tissue as opposed to systemic measures. Individuals with white matter lesions have reduced cerebral blood flow (CBF) (Fazekas et al., 1988) as well as reduced flow and reactivity within the lesions (Marstrand et al., 2002, Brickman et al., 2009b, Makedonov et al., 2013). O'Sullivan demonstrated reduced CBF in the NAWM in individuals with ischemic white matter lesions which was interpreted to potentially demonstrate hypoperfusion as a precursor to periventricular lesions (O'Sullivan et al., 2002). We have recently demonstrated that cortical blood flow, which is reduced with advancing age (Chen et al., 2011), is strongly associated with white matter integrity measured by DTI in a sample of generally healthy adults with some mild vascular risk (Chen et al., 2013). This association was only minimally mediated by age and vascular risk. These cross-sectional associations may suggest that subtle variation in vascular parameters have neural consequences in even low-risk populations. However, alternative interpretations of these data, namely a reduction in metabolic demands resulting in a reduction in CBF are also valid and therefore these relationships must be explored more directly. The data at least argue for the need to perform additional work in this domain and may suggest that normative considerations of vascular contributions to white matter damage are different from those for overt cardio/cerebrovascular disease. These studies also highlight the importance of direct measures of vascular compliance and regulation in monitoring parameters that contribute to common neural changes in older adults as a range of systemic conditions likely combine to determine an individual's unique vascular-functional state. Development of sensitive procedures for the measurement of specific aspects of vascular structure (e.g. vessel density and caliber) and function (e.g. endothelial activity and white matter perfusion regulation) could provide optimal screening and endpoint targets to advance clinical trials evaluating therapies to reduce vascular-associated disability in older adults.
Potential therapeutic routes for the reduction of microvascular associated white matter damage
It is an unfortunate fact that the microvascular system is detrimentally susceptible to a range of biological and environmental influences. However, the malleability of this system also provides a entry into therapeutic interventions that may either attenuate or reverse associated neural damage. Frameworks based in existing medicine have been presented providing valuable guidance for clinical trials (e.g. see discussions in (Zieman et al., 2005, Alagiakrishnan et al., 2006, Gorelick et al., 2011)). The effective application of such therapeutics specific to microvascular-associated white matter damage in the ‘preclinical’ stages is however yet to be demonstrated. A primary purported mechanism of white matter damage is due to hypoperfusion and ischemic tissue damage that results from a number of factors including age-associated increase in systemic vascular risk, alterations in cardiovascular function, and damage to or loss of deep penetrating vessels. Novel neuroimaging procedures for hemodynamic monitoring could provide sensitive information about the degree and quality of the blood supply throughout the brain and therefore can be used as a screening tool to determine individuals who are most likely to develop future damage. Once identified, several procedures exist for enhancing vascular health, which could be tailored to the individual. These include dietary (Esposito et al., 2004, Barcelo et al., 2009, Esposito et al., 2011, Scarmeas et al., 2011, Anton and Leeuwenburgh, 2013), lifestyle (Higashi et al., 1999, DeSouza et al., 2000, Kozakova et al., 2007, Seals et al., 2008, Erickson et al., 2013), and pharmaceutical (Takami and Shigemasa, 2003, Jackson et al., 2005, Rizos et al., 2010) intervention, which all may mechanistically ameliorate limitations in cerebral blood flow and enhance blood flow regulation. In the more distant future, experimental procedures for angiogenesis therapy (e.g. (Kusaka et al., 2005, Xiong et al., 2010, Ergul et al., 2012)) may one day be applicable to chronic cerebral hypoperfusion.
In addition to the plasticity of the vascular system, there is a natural myelin restoration process within white matter (Franklin and Ffrench-Constant, 2008). White matter lesions in demyelinating conditions such as multiple sclerosis demonstrate dynamic patterns of deterioration and some repair (Meier et al., 2007), and experimental therapeutics targeting myelin signaling pathways may enhance this repair ability (Taveggia et al., 2010). It is therefore possible that future therapies may promote repair of damaged tissue on top of enhancing the vascular environment promoting neural health. Monitoring for vulnerability and therapeutic intervention would likely need to begin early in life, at least by midlife, for greatest potential preventative efficacy.
Given the range of conditions contributing to vascular dysfunction, it has been suggested that a therapeutic focus on small vessel cognitive impairment would be a beneficial first step in understanding therapeutic possibilities because of the high prevalence and relatively homogenous, and moderately progressive nature of this condition (Pantoni, 2010). As discussed in prior sections, such trials would greatly benefit from advances in the noninvasive measurement of tissue pathology and vascular structure and function. Thus, these concepts provide an initial framework to address the most ubiquitous forms of cognitive-altering conditions in seniors.
Future directions
Our understanding of cerebrovascular contributions to white matter damage and resultant clinical course has been greatly enhanced over decades of study via neuroimaging, however, much remains to elucidate. Specifically, understanding the optimal physiological state for neural health, the causes of deviations from this optimal state, and procedures for re-attaining this state could result in significant advances towards preservation of functional independence and improved quality of life of seniors. Advances in technology since initial studies using computed tomography and visual rating scales have opened several novel doors. Neuroimaging has provided us with tools to explore vascular phenomena across multiple populations, matched to in vivo physiology and simultaneous cognitive and clinical scales in large population samples. More work is necessary to better describe the full biological milieu that ultimately determines the vascular influence of neural health. Multispectral imaging procedures must be developed to better differentiate among pathologies given the heterogeneous nature of vascular-associated tissue damage (Gouw et al., 2008, Schmidt et al., 2011, Wardlaw et al., 2013). It is promising that vascular targeted therapies may reduce white matter lesion progression across time (Dufouil et al., 2005, Richard et al., 2010) advancing possibilities for early and aggressive targeted interventions in the future. Such work to date is limited but is effective at least in individuals with clinically expressed cerebrovascular disease (Dufouil et al., 2005). Given the modifiable nature of the cerebral vasculature across dietary, lifestyle, pharmacological and surgical manipulation, it is hopeful that a combined therapeutic approach would be effective in the reduction in the highly prevalent condition of vascular-associated deterioration and impairment in the foreseeable future.
Conclusions
The cerebral white matter is vulnerable to damage associated with suboptimal vascular health. It is possible that even subtle variation in normal vascular function beginning in middle age may contribute to the degradation of the connectivity of the brain and in turn promote a range of detrimental conditions. More extreme cases of dysfunction contribute to appreciable tissue damage as well as the clinical expression of dementing illness including AD, and alterations in vascular integrity and white matter deterioration may even contribute to the initiation of AD pathologic cascades. Given the high prevalence of vascular risk and disease in older adults, a focus on therapeutics to optimize vascular health across the lifespan may have considerable implications in ameliorating late-life disability.
Acknowledgements
This work supported by National Institutes of Health/National Institute of Nursing Research R01NR010827. We thank Dr. Jean Augustinack for providing helpful comments on this manuscript and Jean-Philippe Coutu for assistance with figure preparation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abe O, Aoki S, Hayashi N, Yamada H, Kunimatsu A, Mori H, Yoshikawa T, Okubo T, Ohtomo K. Normal aging in the central nervous system: quantitative MR diffusion-tensor analysis. Neurobiol Aging. 2002;23:433–441. doi: 10.1016/s0197-4580(01)00318-9. [DOI] [PubMed] [Google Scholar]
- Adams RD, Covalt DA, Fazekas JF, Merritt HH, Wright IS. Cerebrovascular disease with aging; transcription of a panel meeting. Bull N Y Acad Med. 1956;32:657–684. [PMC free article] [PubMed] [Google Scholar]
- Alagiakrishnan K, McCracken P, Feldman H. Treating vascular risk factors and maintaining vascular health: is this the way towards successful cognitive ageing and preventing cognitive decline? Postgrad Med J. 2006;82:101–105. doi: 10.1136/pgmj.2005.035030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anton S, Leeuwenburgh C. Fasting or caloric restriction for healthy aging. Exp Gerontol. 2013;48:1003–1005. doi: 10.1016/j.exger.2013.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attems J, Lauda F, Jellinger KA. Unexpectedly low prevalence of intracerebral hemorrhages in sporadic cerebral amyloid angiopathy: an autopsy study. J Neurol. 2008;255:70–76. doi: 10.1007/s00415-008-0674-4. [DOI] [PubMed] [Google Scholar]
- Atwood LD, Wolf PA, Heard-Costa NL, Massaro JM, Beiser A, D'Agostino RB, DeCarli C. Genetic variation in white matter hyperintensity volume in the Framingham Study. Stroke. 2004;35:1609–1613. doi: 10.1161/01.STR.0000129643.77045.10. [DOI] [PubMed] [Google Scholar]
- Au R, Massaro JM, Wolf PA, Young ME, Beiser A, Seshadri S, D'Agostino RB, DeCarli C. Association of white matter hyperintensity volume with decreased cognitive functioning: the Framingham Heart Study. Arch Neurol. 2006;63:246–250. doi: 10.1001/archneur.63.2.246. [DOI] [PubMed] [Google Scholar]
- Awad IA, Johnson PC, Spetzler RF, Hodak JA. Incidental subcortical lesions identified on magnetic resonance imaging in the elderly. II. Postmortem pathological correlations. Stroke. 1986;17:1090–1097. doi: 10.1161/01.str.17.6.1090. [DOI] [PubMed] [Google Scholar]
- Baezner H, Blahak C, Poggesi A, Pantoni L, Inzitari D, Chabriat H, Erkinjuntti T, Fazekas F, Ferro JM, Langhorne P, O'Brien J, Scheltens P, Visser MC, Wahlund LO, Waldemar G, Wallin A, Hennerici MG, Group LS Association of gait and balance disorders with age-related white matter changes: the LADIS study. Neurology. 2008;70:935–942. doi: 10.1212/01.wnl.0000305959.46197.e6. [DOI] [PubMed] [Google Scholar]
- Baloh RW, Ying SH, Jacobson KM. A longitudinal study of gait and balance dysfunction in normal older people. Arch Neurol. 2003;60:835–839. doi: 10.1001/archneur.60.6.835. [DOI] [PubMed] [Google Scholar]
- Barcelo F, Perona JS, Prades J, Funari SS, Gomez-Gracia E, Conde M, Estruch R, Ruiz-Gutierrez V. Mediterranean-style diet effect on the structural properties of the erythrocyte cell membrane of hypertensive patients: the Prevencion con Dieta Mediterranea Study. Hypertension. 2009;54:1143–1150. doi: 10.1161/HYPERTENSIONAHA.109.137471. [DOI] [PubMed] [Google Scholar]
- Bartzokis G. Alzheimer's disease as homeostatic responses to age-related myelin breakdown. Neurobiol Aging. 2011;32:1341–1371. doi: 10.1016/j.neurobiolaging.2009.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartzokis G, Beckson M, Lu PH, Nuechterlein KH, Edwards N, Mintz J. Age-related changes in frontal and temporal lobe volumes in men: a magnetic resonance imaging study. Arch Gen Psychiatry. 2001;58:461–465. doi: 10.1001/archpsyc.58.5.461. [DOI] [PubMed] [Google Scholar]
- Bartzokis G, Cummings JL, Sultzer D, Henderson VW, Nuechterlein KH, Mintz J. White matter structural integrity in healthy aging adults and patients with Alzheimer disease: a magnetic resonance imaging study. Arch Neurol. 2003;60:393–398. doi: 10.1001/archneur.60.3.393. [DOI] [PubMed] [Google Scholar]
- Beaulieu C. The basis of anisotropic water diffusion in the nervous system - a technical review. NMR Biomed. 2002;15:435–455. doi: 10.1002/nbm.782. [DOI] [PubMed] [Google Scholar]
- Bell RD, Zlokovic BV. Neurovascular mechanisms and blood-brain barrier disorder in Alzheimer's disease. Acta Neuropathol. 2009;118:103–113. doi: 10.1007/s00401-009-0522-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biffi A, Greenberg SM. Cerebral amyloid angiopathy: a systematic review. J Clin Neurol. 2011;7:1–9. doi: 10.3988/jcn.2011.7.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breteler MM, van Swieten JC, Bots ML, Grobbee DE, Claus JJ, van den Hout JH, van Harskamp F, Tanghe HL, de Jong PT, van Gijn J, et al. Cerebral white matter lesions, vascular risk factors, and cognitive function in a population-based study: the Rotterdam Study. Neurology. 1994;44:1246–1252. doi: 10.1212/wnl.44.7.1246. [DOI] [PubMed] [Google Scholar]
- Brickman AM, Muraskin J, Zimmerman ME. Structural neuroimaging in Altheimer's disease: do white matter hyperintensities matter? Dialogues Clin Neurosci. 2009a;11:181–190. doi: 10.31887/DCNS.2009.11.2/ambrickman. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brickman AM, Schupf N, Manly JJ, Luchsinger JA, Andrews H, Tang MX, Reitz C, Small SA, Mayeux R, DeCarli C, Brown TR. Brain morphology in older African Americans, Caribbean Hispanics, and whites from northern Manhattan. Arch Neurol. 2008;65:1053–1061. doi: 10.1001/archneur.65.8.1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brickman AM, Zahra A, Muraskin J, Steffener J, Holland CM, Habeck C, Borogovac A, Ramos MA, Brown TR, Asllani I, Stern Y. Reduction in cerebral blood flow in areas appearing as white matter hyperintensities on magnetic resonance imaging. Psychiatry Res. 2009b;172:117–120. doi: 10.1016/j.pscychresns.2008.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown WR, Moody DM, Thore CR, Challa VR. Cerebrovascular pathology in Alzheimer's disease and leukoaraiosis. Ann N Y Acad Sci. 2000;903:39–45. doi: 10.1111/j.1749-6632.2000.tb06348.x. [DOI] [PubMed] [Google Scholar]
- Brown WR, Moody DM, Thore CR, Challa VR, Anstrom JA. Vascular dementia in leukoaraiosis may be a consequence of capillary loss not only in the lesions, but in normal-appearing white matter and cortex as well. J Neurol Sci. 2007;257:62–66. doi: 10.1016/j.jns.2007.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brun A, Englund E. A white matter disorder in dementia of the Alzheimer type: a pathoanatomical study. Ann Neurol. 1986;19:253–262. doi: 10.1002/ana.410190306. [DOI] [PubMed] [Google Scholar]
- Carmelli D, DeCarli C, Swan GE, Jack LM, Reed T, Wolf PA, Miller BL. Evidence for genetic variance in white matter hyperintensity volume in normal elderly male twins. Stroke. 1998;29:1177–1181. doi: 10.1161/01.str.29.6.1177. [DOI] [PubMed] [Google Scholar]
- Carmichael O, Schwarz C, Drucker D, Fletcher E, Harvey D, Beckett L, Jack CR, Jr., Weiner M, DeCarli C, Alzheimer's Disease Neuroimaging I Longitudinal changes in white matter disease and cognition in the first year of the Alzheimer disease neuroimaging initiative. Arch Neurol. 2010;67:1370–1378. doi: 10.1001/archneurol.2010.284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charlton RA, Barrick TR, McIntyre DJ, Shen Y, O'Sullivan M, Howe FA, Clark CA, Morris RG, Markus HS. White matter damage on diffusion tensor imaging correlates with age-related cognitive decline. Neurology. 2006;66:217–222. doi: 10.1212/01.wnl.0000194256.15247.83. [DOI] [PubMed] [Google Scholar]
- Chen JJ, Rosas HD, Salat DH. Age-associated reductions in cerebral blood flow are independent from regional atrophy. Neuroimage. 2011;55:468–478. doi: 10.1016/j.neuroimage.2010.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen JJ, Rosas HD, Salat DH. The Relationship between Cortical Blood Flow and Sub-Cortical White-Matter Health across the Adult Age Span. PLoS One. 2013;8:e56733. doi: 10.1371/journal.pone.0056733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen YW, Gurol ME, Rosand J, Viswanathan A, Rakich SM, Groover TR, Greenberg SM, Smith EE. Progression of white matter lesions and hemorrhages in cerebral amyloid angiopathy. Neurology. 2006;67:83–87. doi: 10.1212/01.wnl.0000223613.57229.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chui HC, Zarow C, Mack WJ, Ellis WG, Zheng L, Jagust WJ, Mungas D, Reed BR, Kramer JH, Decarli CC, Weiner MW, Vinters HV. Cognitive impact of subcortical vascular and Alzheimer's disease pathology. Ann Neurol. 2006;60:677–687. doi: 10.1002/ana.21009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosgrove GR, Leblanc R, Meagher-Villemure K, Ethier R. Cerebral amyloid angiopathy. Neurology. 1985;35:625–631. doi: 10.1212/wnl.35.5.625. [DOI] [PubMed] [Google Scholar]
- Davis SW, Dennis NA, Buchler NG, White LE, Madden DJ, Cabeza R. Assessing the effects of age on long white matter tracts using diffusion tensor tractography. Neuroimage. 2009;46:530–541. doi: 10.1016/j.neuroimage.2009.01.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Groot JC, de Leeuw FE, Oudkerk M, van Gijn J, Hofman A, Jolles J, Breteler MM. Cerebral white matter lesions and cognitive function: the Rotterdam Scan Study. Ann Neurol. 2000;47:145–151. doi: 10.1002/1531-8249(200002)47:2<145::aid-ana3>3.3.co;2-g. [DOI] [PubMed] [Google Scholar]
- de la Torre JC. Is Alzheimer's disease a neurodegenerative or a vascular disorder? Data, dogma, and dialectics. Lancet Neurol. 2004;3:184–190. doi: 10.1016/S1474-4422(04)00683-0. [DOI] [PubMed] [Google Scholar]
- de la Torre JC. Vascular risk factor detection and control may prevent Alzheimer's disease. Ageing Res Rev. 2010;9:218–225. doi: 10.1016/j.arr.2010.04.002. [DOI] [PubMed] [Google Scholar]
- de Leeuw FE, de Groot JC, Oudkerk M, Witteman JC, Hofman A, van Gijn J, Breteler MM. Hypertension and cerebral white matter lesions in a prospective cohort study. Brain. 2002;125:765–772. doi: 10.1093/brain/awf077. [DOI] [PubMed] [Google Scholar]
- Debette S, Beiser A, DeCarli C, Au R, Himali JJ, Kelly-Hayes M, Romero JR, Kase CS, Wolf PA, Seshadri S. Association of MRI markers of vascular brain injury with incident stroke, mild cognitive impairment, dementia, and mortality: the Framingham Offspring Study. Stroke. 2010;41:600–606. doi: 10.1161/STROKEAHA.109.570044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debette S, Markus HS. The clinical importance of white matter hyperintensities on brain magnetic resonance imaging: systematic review and meta-analysis. BMJ. 2010;341:c3666. doi: 10.1136/bmj.c3666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debette S, Seshadri S, Beiser A, Au R, Himali JJ, Palumbo C, Wolf PA, DeCarli C. Midlife vascular risk factor exposure accelerates structural brain aging and cognitive decline. Neurology. 2011;77:461–468. doi: 10.1212/WNL.0b013e318227b227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeCarli C, Fletcher E, Ramey V, Harvey D, Jagust WJ. Anatomical mapping of white matter hyperintensities (WMH): exploring the relationships between periventricular WMH, deep WMH, and total WMH burden. Stroke. 2005;36:50–55. doi: 10.1161/01.STR.0000150668.58689.f2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeCarli C, Murphy DG, Tranh M, Grady CL, Haxby JV, Gillette JA, Salerno JA, Gonzales-Aviles A, Horwitz B, Rapoport SI, et al. The effect of white matter hyperintensity volume on brain structure, cognitive performance, and cerebral metabolism of glucose in 51 healthy adults. Neurology. 1995;45:2077–2084. doi: 10.1212/wnl.45.11.2077. [DOI] [PubMed] [Google Scholar]
- DeSouza CA, Shapiro LF, Clevenger CM, Dinenno FA, Monahan KD, Tanaka H, Seals DR. Regular aerobic exercise prevents and restores age-related declines in endothelium-dependent vasodilation in healthy men. Circulation. 2000;102:1351–1357. doi: 10.1161/01.cir.102.12.1351. [DOI] [PubMed] [Google Scholar]
- Dufouil C, Chalmers J, Coskun O, Besancon V, Bousser MG, Guillon P, MacMahon S, Mazoyer B, Neal B, Woodward M, Tzourio-Mazoyer N, Tzourio C, Investigators PMS. Effects of blood pressure lowering on cerebral white matter hyperintensities in patients with stroke: the PROGRESS (Perindopril Protection Against Recurrent Stroke Study) Magnetic Resonance Imaging Substudy. Circulation. 2005;112:1644–1650. doi: 10.1161/CIRCULATIONAHA.104.501163. [DOI] [PubMed] [Google Scholar]
- Dufouil C, de Kersaint-Gilly A, Besancon V, Levy C, Auffray E, Brunnereau L, Alperovitch A, Tzourio C. Longitudinal study of blood pressure and white matter hyperintensities: the EVA MRI Cohort. Neurology. 2001;56:921–926. doi: 10.1212/wnl.56.7.921. [DOI] [PubMed] [Google Scholar]
- Dumas A, Dierksen GA, Gurol ME, Halpin A, Martinez-Ramirez S, Schwab K, Rosand J, Viswanathan A, Salat DH, Polimeni JR, Greenberg SM. Functional magnetic resonance imaging detection of vascular reactivity in cerebral amyloid angiopathy. Ann Neurol. 2012;72:76–81. doi: 10.1002/ana.23566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egan BM, Zhao Y, Axon RN. US trends in prevalence, awareness, treatment, and control of hypertension, 1988-2008. JAMA. 2010;303:2043–2050. doi: 10.1001/jama.2010.650. [DOI] [PubMed] [Google Scholar]
- Ergul A, Alhusban A, Fagan SC. Angiogenesis: a harmonized target for recovery after stroke. Stroke. 2012;43:2270–2274. doi: 10.1161/STROKEAHA.111.642710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson KI, Gildengers AG, Butters MA. Physical activity and brain plasticity in late adulthood. Dialogues Clin Neurosci. 2013;15:99–108. doi: 10.31887/DCNS.2013.15.1/kerickson. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esposito K, Di Palo C, Maiorino MI, Petrizzo M, Bellastella G, Siniscalchi I, Giugliano D. Long-term effect of mediterranean-style diet and calorie restriction on biomarkers of longevity and oxidative stress in overweight men. Cardiol Res Pract. 2011;2011:293916. doi: 10.4061/2011/293916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esposito K, Marfella R, Ciotola M, Di Palo C, Giugliano F, Giugliano G, D'Armiento M, D'Andrea F, Giugliano D. Effect of a mediterranean-style diet on endothelial dysfunction and markers of vascular inflammation in the metabolic syndrome: a randomized trial. JAMA. 2004;292:1440–1446. doi: 10.1001/jama.292.12.1440. [DOI] [PubMed] [Google Scholar]
- Fazekas F. Magnetic resonance signal abnormalities in asymptomatic individuals: their incidence and functional correlates. Eur Neurol. 1989;29:164–168. doi: 10.1159/000116401. [DOI] [PubMed] [Google Scholar]
- Fazekas F, Chawluk JB, Alavi A, Hurtig HI, Zimmerman RA. MR signal abnormalities at 1.5 T in Alzheimer's dementia and normal aging. Ajr. 1987;149:351–356. doi: 10.2214/ajr.149.2.351. [DOI] [PubMed] [Google Scholar]
- Fazekas F, Kleinert R, Offenbacher H, Schmidt R, Kleinert G, Payer F, Radner H, Lechner H. Pathologic correlates of incidental MRI white matter signal hyperintensities. Neurology. 1993;43:1683–1689. doi: 10.1212/wnl.43.9.1683. [DOI] [PubMed] [Google Scholar]
- Fazekas F, Niederkorn K, Schmidt R, Offenbacher H, Horner S, Bertha G, Lechner H. White matter signal abnormalities in normal individuals: correlation with carotid ultrasonography, cerebral blood flow measurements, and cerebrovascular risk factors. Stroke. 1988;19:1285–1288. doi: 10.1161/01.str.19.10.1285. [DOI] [PubMed] [Google Scholar]
- Feihl F, Liaudet L, Levy BI, Waeber B. Hypertension and microvascular remodelling. Cardiovasc Res. 2008;78:274–285. doi: 10.1093/cvr/cvn022. [DOI] [PubMed] [Google Scholar]
- Fernando MS, Simpson JE, Matthews F, Brayne C, Lewis CE, Barber R, Kalaria RN, Forster G, Esteves F, Wharton SB, Shaw PJ, O'Brien JT, Ince PG, Function MRCC, Ageing Neuropathology Study G White matter lesions in an unselected cohort of the elderly: molecular pathology suggests origin from chronic hypoperfusion injury. Stroke. 2006;37:1391–1398. doi: 10.1161/01.STR.0000221308.94473.14. [DOI] [PubMed] [Google Scholar]
- Fields RD. White matter in learning, cognition and psychiatric disorders. Trends Neurosci. 2008;31:361–370. doi: 10.1016/j.tins.2008.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin RJ, Ffrench-Constant C. Remyelination in the CNS: from biology to therapy. Nature reviews. 2008;9:839–855. doi: 10.1038/nrn2480. [DOI] [PubMed] [Google Scholar]
- Ge Y, Grossman RI, Babb JS, Rabin ML, Mannon LJ, Kolson DL. Age-related total gray matter and white matter changes in normal adult brain. Part I: volumetric MR imaging analysis. AJNR Am J Neuroradiol. 2002a;23:1327–1333. [PMC free article] [PubMed] [Google Scholar]
- Ge Y, Grossman RI, Babb JS, Rabin ML, Mannon LJ, Kolson DL. Age-related total gray matter and white matter changes in normal adult brain. Part II: quantitative magnetization transfer ratio histogram analysis. AJNR Am J Neuroradiol. 2002b;23:1334–1341. [PMC free article] [PubMed] [Google Scholar]
- Gilbert JJ, Vinters HV. Cerebral amyloid angiopathy: incidence and complications in the aging brain. I. Cerebral hemorrhage. Stroke. 1983;14:915–923. doi: 10.1161/01.str.14.6.915. [DOI] [PubMed] [Google Scholar]
- Gorelick PB, Scuteri A, Black SE, Decarli C, Greenberg SM, Iadecola C, Launer LJ, Laurent S, Lopez OL, Nyenhuis D, Petersen RC, Schneider JA, Tzourio C, Arnett DK, Bennett DA, Chui HC, Higashida RT, Lindquist R, Nilsson PM, Roman GC, Sellke FW, Seshadri S, American Heart Association Stroke Council CoE, Prevention CoCNCoCR, Intervention, Council on Cardiovascular S, Anesthesia Vascular contributions to cognitive impairment and dementia: a statement for healthcare professionals from the american heart association/american stroke association. Stroke. 2011;42:2672–2713. doi: 10.1161/STR.0b013e3182299496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gouw AA, Seewann A, van der Flier WM, Barkhof F, Rozemuller AM, Scheltens P, Geurts JJ. Heterogeneity of small vessel disease: a systematic review of MRI and histopathology correlations. J Neurol Neurosurg Psychiatry. 2011;82:126–135. doi: 10.1136/jnnp.2009.204685. [DOI] [PubMed] [Google Scholar]
- Gouw AA, Seewann A, Vrenken H, van der Flier WM, Rozemuller JM, Barkhof F, Scheltens P, Geurts JJ. Heterogeneity of white matter hyperintensities in Alzheimer's disease: post-mortem quantitative MRI and neuropathology. Brain. 2008;131:3286–3298. doi: 10.1093/brain/awn265. [DOI] [PubMed] [Google Scholar]
- Greenberg SM, Gurol ME, Rosand J, Smith EE. Amyloid angiopathy-related vascular cognitive impairment. Stroke. 2004;35:2616–2619. doi: 10.1161/01.STR.0000143224.36527.44. [DOI] [PubMed] [Google Scholar]
- Grieve SM, Williams LM, Paul RH, Clark CR, Gordon E. Cognitive aging, executive function, and fractional anisotropy: a diffusion tensor MR imaging study. AJNR Am J Neuroradiol. 2007;28:226–235. [PMC free article] [PubMed] [Google Scholar]
- Gunning-Dixon FM, Raz N. The cognitive correlates of white matter abnormalities in normal aging: a quantitative review. Neuropsychology. 2000;14:224–232. doi: 10.1037//0894-4105.14.2.224. [DOI] [PubMed] [Google Scholar]
- Gunning-Dixon FM, Raz N. Neuroanatomical correlates of selected executive functions in middle-aged and older adults: a prospective MRI study. Neuropsychologia. 2003;41:1929–1941. doi: 10.1016/s0028-3932(03)00129-5. [DOI] [PubMed] [Google Scholar]
- Guo F, He D, Zhang W, Walton RG. Trends in prevalence, awareness, management, and control of hypertension among United States adults, 1999 to 2010. J Am Coll Cardiol. 2012;60:599–606. doi: 10.1016/j.jacc.2012.04.026. [DOI] [PubMed] [Google Scholar]
- Guttmann CR, Jolesz FA, Kikinis R, Killiany RJ, Moss MB, Sandor T, Albert MS. White matter changes with normal aging. Neurology. 1998;50:972–978. doi: 10.1212/wnl.50.4.972. [DOI] [PubMed] [Google Scholar]
- Hachinski VC, Potter P, Merskey H. Leuko-araiosis: an ancient term for a new problem. Can J Neurol Sci. 1986;13:533–534. doi: 10.1017/s0317167100037264. [DOI] [PubMed] [Google Scholar]
- Hachinski VC, Potter P, Merskey H. Leuko-araiosis. Arch Neurol. 1987;44:21–23. doi: 10.1001/archneur.1987.00520130013009. [DOI] [PubMed] [Google Scholar]
- Herrmann LL, Le Masurier M, Ebmeier KP. White matter hyperintensities in late life depression: a systematic review. J Neurol Neurosurg Psychiatry. 2008;79:619–624. doi: 10.1136/jnnp.2007.124651. [DOI] [PubMed] [Google Scholar]
- Hickie I, Scott E, Wilhelm K, Brodaty H. Subcortical hyperintensities on magnetic resonance imaging in patients with severe depression--a longitudinal evaluation. Biol Psychiatry. 1997;42:367–374. doi: 10.1016/S0006-3223(96)00363-0. [DOI] [PubMed] [Google Scholar]
- Higashi Y, Sasaki S, Kurisu S, Yoshimizu A, Sasaki N, Matsuura H, Kajiyama G, Oshima T. Regular aerobic exercise augments endothelium-dependent vascular relaxation in normotensive as well as hypertensive subjects: role of endothelium-derived nitric oxide. Circulation. 1999;100:1194–1202. doi: 10.1161/01.cir.100.11.1194. [DOI] [PubMed] [Google Scholar]
- Hogervorst E, Ribeiro HM, Molyneux A, Budge M, Smith AD. Plasma homocysteine levels, cerebrovascular risk factors, and cerebral white matter changes (leukoaraiosis) in patients with Alzheimer disease. Arch Neurol. 2002;59:787–793. doi: 10.1001/archneur.59.5.787. [DOI] [PubMed] [Google Scholar]
- Holland CM, Smith EE, Csapo I, Gurol ME, Brylka DA, Killiany RJ, Blacker D, Albert MS, Guttmann CR, Greenberg SM. Spatial distribution of white-matter hyperintensities in Alzheimer disease, cerebral amyloid angiopathy, and healthy aging. Stroke. 2008;39:1127–1133. doi: 10.1161/STROKEAHA.107.497438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoptman MJ, Gunning-Dixon FM, Murphy CF, Ardekani BA, Hrabe J, Lim KO, Etwaroo GR, Kanellopoulos D, Alexopoulos GS. Blood pressure and white matter integrity in geriatric depression. J Affect Disord. 2009;115:171–176. doi: 10.1016/j.jad.2008.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iadecola C, Davisson RL. Hypertension and cerebrovascular dysfunction. Cell Metab. 2008;7:476–484. doi: 10.1016/j.cmet.2008.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inzitari D, Pracucci G, Poggesi A, Carlucci G, Barkhof F, Chabriat H, Erkinjuntti T, Fazekas F, Ferro JM, Hennerici M, Langhorne P, O'Brien J, Scheltens P, Visser MC, Wahlund LO, Waldemar G, Wallin A, Pantoni L, Group LS Changes in white matter as determinant of global functional decline in older independent outpatients: three year follow-up of LADIS (leukoaraiosis and disability) study cohort. BMJ. 2009;339:b2477. doi: 10.1136/bmj.b2477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inzitari D, Simoni M, Pracucci G, Poggesi A, Basile AM, Chabriat H, Erkinjuntti T, Fazekas F, Ferro JM, Hennerici M, Langhorne P, O'Brien J, Barkhof F, Visser MC, Wahlund LO, Waldemar G, Wallin A, Pantoni L, Group LS Risk of rapid global functional decline in elderly patients with severe cerebral age-related white matter changes: the LADIS study. Arch Intern Med. 2007;167:81–88. doi: 10.1001/archinte.167.1.81. [DOI] [PubMed] [Google Scholar]
- Itoh Y, Yamada M, Hayakawa M, Otomo E, Miyatake T. Cerebral amyloid angiopathy: a significant cause of cerebellar as well as lobar cerebral hemorrhage in the elderly. J Neurol Sci. 1993;116:135–141. doi: 10.1016/0022-510x(93)90317-r. [DOI] [PubMed] [Google Scholar]
- Jackson R, Lawes CM, Bennett DA, Milne RJ, Rodgers A. Treatment with drugs to lower blood pressure and blood cholesterol based on an individual's absolute cardiovascular risk. Lancet. 2005;365:434–441. doi: 10.1016/S0140-6736(05)17833-7. [DOI] [PubMed] [Google Scholar]
- Jacobs HI, Leritz EC, Williams VJ, Van Boxtel MP, Elst W, Jolles J, Verhey FR, McGlinchey RE, Milberg WP, Salat DH. Association between white matter microstructure, executive functions, and processing speed in older adults: The impact of vascular health. Human brain mapping. 2013;34:77–95. doi: 10.1002/hbm.21412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeerakathil T, Wolf PA, Beiser A, Massaro J, Seshadri S, D'Agostino RB, DeCarli C. Stroke risk profile predicts white matter hyperintensity volume: the Framingham Study. Stroke. 2004;35:1857–1861. doi: 10.1161/01.STR.0000135226.53499.85. [DOI] [PubMed] [Google Scholar]
- Jernigan TL, Archibald SL, Berhow MT, Sowell ER, Foster DS, Hesselink JR. Cerebral structure on MRI, Part I: Localization of age-related changes. Biol Psychiatry. 1991a;29:55–67. doi: 10.1016/0006-3223(91)90210-d. [DOI] [PubMed] [Google Scholar]
- Jernigan TL, Salmon DP, Butters N, Hesselink JR. Cerebral structure on MRI, Part II: Specific changes in Alzheimer's and Huntington's diseases. Biol Psychiatry. 1991b;29:68–81. doi: 10.1016/0006-3223(91)90211-4. [DOI] [PubMed] [Google Scholar]
- Joffres M, Falaschetti E, Gillespie C, Robitaille C, Loustalot F, Poulter N, McAlister FA, Johansen H, Baclic O, Campbell N. Hypertension prevalence, awareness, treatment and control in national surveys from England, the USA and Canada, and correlation with stroke and ischaemic heart disease mortality: a cross-sectional study. BMJ Open. 2013;3:e003423. doi: 10.1136/bmjopen-2013-003423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones DK, Lythgoe D, Horsfield MA, Simmons A, Williams SC, Markus HS. Characterization of white matter damage in ischemic leukoaraiosis with diffusion tensor MRI. Stroke. 1999;30:393–397. doi: 10.1161/01.str.30.2.393. [DOI] [PubMed] [Google Scholar]
- Kaplan MS, Huguet N, Feeny DH, McFarland BH. Self-reported hypertension prevalence and income among older adults in Canada and the United States. Soc Sci Med. 2010;70:844–849. doi: 10.1016/j.socscimed.2009.11.019. [DOI] [PubMed] [Google Scholar]
- Keenan NL, Rosendorf KA, Centers for Disease C, Prevention Prevalence of hypertension and controlled hypertension - United States, 2005-2008. MMWR Surveill Summ. 2011;60(Suppl):94–97. [PubMed] [Google Scholar]
- Kennedy KM, Raz N. Pattern of normal age-related regional differences in white matter microstructure is modified by vascular risk. Brain Res. 2009;1297:41–56. doi: 10.1016/j.brainres.2009.08.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim KW, MacFall JR, Payne ME. Classification of white matter lesions on magnetic resonance imaging in elderly persons. Biol Psychiatry. 2008;64:273–280. doi: 10.1016/j.biopsych.2008.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King KS, Peshock RM, Warren MW, Alhilali L, Hulsey K, McColl R, Weiner MF, Ayers C, Whittemore A. Evaluation of a practical visual MRI rating scale of brain white matter hyperintensities for clinicians based on largest lesion size regardless of location. AJNR Am J Neuroradiol. 2013;34:797–801. doi: 10.3174/ajnr.A3283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkpatrick JB, Hayman LA. White-matter lesions in MR imaging of clinically healthy brains of elderly subjects: possible pathologic basis. Radiology. 1987;162:509–511. doi: 10.1148/radiology.162.2.3797666. [DOI] [PubMed] [Google Scholar]
- Kivipelto M, Helkala EL, Hanninen T, Laakso MP, Hallikainen M, Alhainen K, Soininen H, Tuomilehto J, Nissinen A. Midlife vascular risk factors and late-life mild cognitive impairment: A population-based study. Neurology. 2001a;56:1683–1689. doi: 10.1212/wnl.56.12.1683. [DOI] [PubMed] [Google Scholar]
- Kivipelto M, Helkala EL, Laakso MP, Hanninen T, Hallikainen M, Alhainen K, Soininen H, Tuomilehto J, Nissinen A. Midlife vascular risk factors and Alzheimer's disease in later life: longitudinal, population based study. BMJ. 2001b;322:1447–1451. doi: 10.1136/bmj.322.7300.1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knudsen KA, Rosand J, Karluk D, Greenberg SM. Clinical diagnosis of cerebral amyloid angiopathy: validation of the Boston criteria. Neurology. 2001;56:537–539. doi: 10.1212/wnl.56.4.537. [DOI] [PubMed] [Google Scholar]
- Korf ES, White LR, Scheltens P, Launer LJ. Midlife blood pressure and the risk of hippocampal atrophy: the Honolulu Asia Aging Study. Hypertension. 2004;44:29–34. doi: 10.1161/01.HYP.0000132475.32317.bb. [DOI] [PubMed] [Google Scholar]
- Kozachuk WE, DeCarli C, Schapiro MB, Wagner EE, Rapoport SI, Horwitz B. White matter hyperintensities in dementia of Alzheimer's type and in healthy subjects without cerebrovascular risk factors. A magnetic resonance imaging study. Arch Neurol. 1990;47:1306–1310. doi: 10.1001/archneur.1990.00530120050009. [DOI] [PubMed] [Google Scholar]
- Kozakova M, Palombo C, Mhamdi L, Konrad T, Nilsson P, Staehr PB, Paterni M, Balkau B, Investigators R. Habitual physical activity and vascular aging in a young to middle-age population at low cardiovascular risk. Stroke. 2007;38:2549–2555. doi: 10.1161/STROKEAHA.107.484949. [DOI] [PubMed] [Google Scholar]
- Kuchel GA, Moscufo N, Guttmann CR, Zeevi N, Wakefield D, Schmidt J, Dubeau CE, Wolfson L. Localization of brain white matter hyperintensities and urinary incontinence in community-dwelling older adults. J Gerontol A Biol Sci Med Sci. 2009;64:902–909. doi: 10.1093/gerona/glp037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuller LH, Longstreth WT, Jr., Arnold AM, Bernick C, Bryan RN, Beauchamp NJ, Jr., Cardiovascular Health Study Collaborative Research G White matter hyperintensity on cranial magnetic resonance imaging: a predictor of stroke. Stroke. 2004;35:1821–1825. doi: 10.1161/01.STR.0000132193.35955.69. [DOI] [PubMed] [Google Scholar]
- Kusaka N, Sugiu K, Tokunaga K, Katsumata A, Nishida A, Namba K, Hamada H, Nakashima H, Date I. Enhanced brain angiogenesis in chronic cerebral hypoperfusion after administration of plasmid human vascular endothelial growth factor in combination with indirect vasoreconstructive surgery. J Neurosurg. 2005;103:882–890. doi: 10.3171/jns.2005.103.5.0882. [DOI] [PubMed] [Google Scholar]
- Lammie GA. Pathology of small vessel stroke. Br Med Bull. 2000;56:296–306. doi: 10.1258/0007142001903229. [DOI] [PubMed] [Google Scholar]
- Launer LJ, Ross GW, Petrovitch H, Masaki K, Foley D, White LR, Havlik RJ. Midlife blood pressure and dementia: the Honolulu-Asia aging study. Neurobiol Aging. 2000;21:49–55. doi: 10.1016/s0197-4580(00)00096-8. [DOI] [PubMed] [Google Scholar]
- Lechner H, Schmidt R, Bertha G, Justich E, Offenbacher H, Schneider G. Nuclear magnetic resonance image white matter lesions and risk factors for stroke in normal individuals. Stroke. 1988;19:263–265. doi: 10.1161/01.str.19.2.263. [DOI] [PubMed] [Google Scholar]
- Lemarie CA, Tharaux PL, Lehoux S. Extracellular matrix alterations in hypertensive vascular remodeling. J Mol Cell Cardiol. 2010;48:433–439. doi: 10.1016/j.yjmcc.2009.09.018. [DOI] [PubMed] [Google Scholar]
- Leritz EC, Salat DH, Milberg WP, Williams VJ, Chapman CE, Grande LJ, Rudolph JL, Schnyer DM, Barber CE, Lipsitz LA, McGlinchey RE. Variation in blood pressure is associated with white matter microstructure but not cognition in African Americans. Neuropsychology. 2010;24:199–208. doi: 10.1037/a0018108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leritz EC, Salat DH, Williams VJ, Schnyer DM, Rudolph JL, Lipsitz L, Fischl B, McGlinchey RE, Milberg WP. Thickness of the human cerebral cortex is associated with metrics of cerebrovascular health in a normative sample of community dwelling older adults. Neuroimage. 2011;54:2659–2671. doi: 10.1016/j.neuroimage.2010.10.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leritz EC, Shepel J, Williams VJ, Lipsitz LA, McGlinchey RE, Milberg WP, Salat DH. Associations between T(1) white matter lesion volume and regional white matter microstructure in aging. Human brain mapping. 2013 doi: 10.1002/hbm.22236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longstreth WT, Jr., Manolio TA, Arnold A, Burke GL, Bryan N, Jungreis CA, Enright PL, O'Leary D, Fried L. Clinical correlates of white matter findings on cranial magnetic resonance imaging of 3301 elderly people. The Cardiovascular Health Study. Stroke. 1996;27:1274–1282. doi: 10.1161/01.str.27.8.1274. [DOI] [PubMed] [Google Scholar]
- Ly M, Canu E, Xu G, Oh J, McLaren DG, Dowling NM, Alexander AL, Sager MA, Johnson SC, Bendlin BB. Midlife measurements of white matter microstructure predict subsequent regional white matter atrophy in healthy adults. Human brain mapping. 2013 doi: 10.1002/hbm.22311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madden DJ, Bennett IJ, Song AW. Cerebral white matter integrity and cognitive aging: contributions from diffusion tensor imaging. Neuropsychol Rev. 2009;19:415–435. doi: 10.1007/s11065-009-9113-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madden DJ, Whiting WL, Huettel SA, White LE, MacFall JR, Provenzale JM. Diffusion tensor imaging of adult age differences in cerebral white matter: relation to response time. Neuroimage. 2004;21:1174–1181. doi: 10.1016/j.neuroimage.2003.11.004. [DOI] [PubMed] [Google Scholar]
- Makedonov I, Black SE, MacIntosh BJ. Cerebral small vessel disease in aging and Alzheimer's disease: a comparative study using MRI and SPECT. Eur J Neurol. 2013;20:243–250. doi: 10.1111/j.1468-1331.2012.03785.x. [DOI] [PubMed] [Google Scholar]
- Marstrand JR, Garde E, Rostrup E, Ring P, Rosenbaum S, Mortensen EL, Larsson HB. Cerebral perfusion and cerebrovascular reactivity are reduced in white matter hyperintensities. Stroke. 2002;33:972–976. doi: 10.1161/01.str.0000012808.81667.4b. [DOI] [PubMed] [Google Scholar]
- Meier DS, Weiner HL, Guttmann CR. MR imaging intensity modeling of damage and repair in multiple sclerosis: relationship of short-term lesion recovery to progression and disability. AJNR Am J Neuroradiol. 2007;28:1956–1963. doi: 10.3174/ajnr.A0701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moseley M. Diffusion tensor imaging and aging - a review. NMR Biomed. 2002;15:553–560. doi: 10.1002/nbm.785. [DOI] [PubMed] [Google Scholar]
- Mueller RL, Irving S. Wright--innovator in cardiovascular medicine. Clin Cardiol. 1995;18:181–183. doi: 10.1002/clc.4960180315. [DOI] [PubMed] [Google Scholar]
- Mujahid MS, Diez Roux AV, Cooper RC, Shea S, Williams DR. Neighborhood stressors and race/ethnic differences in hypertension prevalence (the Multi-Ethnic Study of Atherosclerosis). Am J Hypertens. 2011;24:187–193. doi: 10.1038/ajh.2010.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordahl CW, Ranganath C, Yonelinas AP, Decarli C, Fletcher E, Jagust WJ. White matter changes compromise prefrontal cortex function in healthy elderly individuals. J Cogn Neurosci. 2006;18:418–429. doi: 10.1162/089892906775990552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien JT, Ames D. White matter lesions in depression and Alzheimer's disease. Br J Psychiatry. 1996;169:671. doi: 10.1192/bjp.169.5.671a. [DOI] [PubMed] [Google Scholar]
- O'Sullivan M. Leukoaraiosis. Pract Neurol. 2008;8:26–38. doi: 10.1136/jnnp.2007.139428. [DOI] [PubMed] [Google Scholar]
- O'Sullivan M, Lythgoe DJ, Pereira AC, Summers PE, Jarosz JM, Williams SC, Markus HS. Patterns of cerebral blood flow reduction in patients with ischemic leukoaraiosis. Neurology. 2002;59:321–326. doi: 10.1212/wnl.59.3.321. [DOI] [PubMed] [Google Scholar]
- O'Sullivan M, Morris RG, Huckstep B, Jones DK, Williams SC, Markus HS. Diffusion tensor MRI correlates with executive dysfunction in patients with ischaemic leukoaraiosis. J Neurol Neurosurg Psychiatry. 2004;75:441–447. doi: 10.1136/jnnp.2003.014910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Sullivan M, Summers PE, Jones DK, Jarosz JM, Williams SC, Markus HS. Normal-appearing white matter in ischemic leukoaraiosis: a diffusion tensor MRI study. Neurology. 2001;57:2307–2310. doi: 10.1212/wnl.57.12.2307. [DOI] [PubMed] [Google Scholar]
- Pantoni L. Leukoaraiosis: from an ancient term to an actual marker of poor prognosis. Stroke. 2008;39:1401–1403. doi: 10.1161/STROKEAHA.107.505602. [DOI] [PubMed] [Google Scholar]
- Pantoni L. Cerebral small vessel disease: from pathogenesis and clinical characteristics to therapeutic challenges. Lancet Neurol. 2010;9:689–701. doi: 10.1016/S1474-4422(10)70104-6. [DOI] [PubMed] [Google Scholar]
- Pantoni L, Garcia JH. The significance of cerebral white matter abnormalities 100 years after Binswanger's report. A review. Stroke. 1995;26:1293–1301. doi: 10.1161/01.str.26.7.1293. [DOI] [PubMed] [Google Scholar]
- Pantoni L, Garcia JH. Pathogenesis of leukoaraiosis: a review. Stroke. 1997;28:652–659. doi: 10.1161/01.str.28.3.652. [DOI] [PubMed] [Google Scholar]
- Paulson OB, Strandgaard S, Edvinsson L. Cerebral autoregulation. Cerebrovasc Brain Metab Rev. 1990;2:161–192. [PubMed] [Google Scholar]
- Pfefferbaum A, Sullivan EV, Hedehus M, Lim KO, Adalsteinsson E, Moseley M. Age-related decline in brain white matter anisotropy measured with spatially corrected echo-planar diffusion tensor imaging. Magn Reson Med. 2000;44:259–268. doi: 10.1002/1522-2594(200008)44:2<259::aid-mrm13>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Poggesi A, Pracucci G, Chabriat H, Erkinjuntti T, Fazekas F, Verdelho A, Hennerici M, Langhorne P, O'Brien J, Scheltens P, Visser MC, Crisby M, Waldemar G, Wallin A, Inzitari D, Pantoni L, Leukoaraiosis, Group DIS Urinary complaints in nondisabled elderly people with age-related white matter changes: the Leukoaraiosis And DISability (LADIS) Study. J Am Geriatr Soc. 2008;56:1638–1643. doi: 10.1111/j.1532-5415.2008.01832.x. [DOI] [PubMed] [Google Scholar]
- Provenzano FA, Muraskin J, Tosto G, Narkhede A, Wasserman BT, Griffith EY, Guzman VA, Meier IB, Zimmerman ME, Brickman AM, Alzheimer's Disease Neuroimaging I White matter hyperintensities and cerebral amyloidosis: necessary and sufficient for clinical expression of Alzheimer disease? JAMA Neurol. 2013;70:455–461. doi: 10.1001/jamaneurol.2013.1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raz N, Lindenberger U, Rodrigue KM, Kennedy KM, Head D, Williamson A, Dahle C, Gerstorf D, Acker JD. Regional brain changes in aging healthy adults: general trends, individual differences and modifiers. Cereb Cortex. 2005;15:1676–1689. doi: 10.1093/cercor/bhi044. [DOI] [PubMed] [Google Scholar]
- Raz N, Rodrigue KM, Kennedy KM, Acker JD. Vascular health and longitudinal changes in brain and cognition in middle-aged and older adults. Neuropsychology. 2007;21:149–157. doi: 10.1037/0894-4105.21.2.149. [DOI] [PubMed] [Google Scholar]
- Richard E, Gouw AA, Scheltens P, van Gool WA. Vascular care in patients with Alzheimer disease with cerebrovascular lesions slows progression of white matter lesions on MRI: the evaluation of vascular care in Alzheimer's disease (EVA) study. Stroke. 2010;41:554–556. doi: 10.1161/STROKEAHA.109.571281. [DOI] [PubMed] [Google Scholar]
- Rizos EC, Agouridis AP, Elisaf MS. The effect of statin therapy on arterial stiffness by measuring pulse wave velocity: a systematic review. Curr Vasc Pharmacol. 2010;8:638–644. doi: 10.2174/157016110792006950. [DOI] [PubMed] [Google Scholar]
- Roman GC. Senile dementia of the Binswanger type. A vascular form of dementia in the elderly. JAMA. 1987;258:1782–1788. doi: 10.1001/jama.1987.03400130096040. [DOI] [PubMed] [Google Scholar]
- Salat DH. The declining infrastructure of the aging brain. Brain Connect. 2011;1:279–293. doi: 10.1089/brain.2011.0056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salat DH, Greve DN, Pacheco JL, Quinn BT, Helmer KG, Buckner RL, Fischl B. Regional white matter volume differences in nondemented aging and Alzheimer's disease. Neuroimage. 2009;44:1247–1258. doi: 10.1016/j.neuroimage.2008.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salat DH, Kaye JA, Janowsky JS. Prefrontal gray and white matter volumes in healthy aging and Alzheimer disease. Arch Neurol. 1999;56:338–344. doi: 10.1001/archneur.56.3.338. [DOI] [PubMed] [Google Scholar]
- Salat DH, Smith EE, Tuch DS, Benner T, Pappu V, Schwab KM, Gurol ME, Rosas HD, Rosand J, Greenberg SM. White matter alterations in cerebral amyloid angiopathy measured by diffusion tensor imaging. Stroke. 2006;37:1759–1764. doi: 10.1161/01.STR.0000227328.86353.a7. [DOI] [PubMed] [Google Scholar]
- Salat DH, Tuch DS, Greve DN, van der Kouwe AJ, Hevelone ND, Zaleta AK, Rosen BR, Fischl B, Corkin S, Rosas HD, Dale AM. Age-related alterations in white matter microstructure measured by diffusion tensor imaging. Neurobiol Aging. 2005a;26:1215–1227. doi: 10.1016/j.neurobiolaging.2004.09.017. [DOI] [PubMed] [Google Scholar]
- Salat DH, Tuch DS, Hevelone ND, Fischl B, Corkin S, Rosas HD, Dale AM. Age-related changes in prefrontal white matter measured by diffusion tensor imaging. Ann N Y Acad Sci. 2005b;1064:37–49. doi: 10.1196/annals.1340.009. [DOI] [PubMed] [Google Scholar]
- Salat DH, Williams VJ, Leritz EC, Schnyer DM, Rudolph JL, Lipsitz LA, McGlinchey RE, Milberg WP. Inter-individual variation in blood pressure is associated with regional white matter integrity in generally healthy older adults. Neuroimage. 2012;59:181–192. doi: 10.1016/j.neuroimage.2011.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scarmeas N, Luchsinger JA, Stern Y, Gu Y, He J, DeCarli C, Brown T, Brickman AM. Mediterranean diet and magnetic resonance imaging-assessed cerebrovascular disease. Ann Neurol. 2011;69:257–268. doi: 10.1002/ana.22317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheltens P, Barkhof F, Leys D, Pruvo JP, Nauta JJ, Vermersch P, Steinling M, Valk J. A semiquantative rating scale for the assessment of signal hyperintensities on magnetic resonance imaging. J Neurol Sci. 1993;114:7–12. doi: 10.1016/0022-510x(93)90041-v. [DOI] [PubMed] [Google Scholar]
- Schiffrin EL. Vascular remodeling in hypertension: mechanisms and treatment. Hypertension. 2012;59:367–374. doi: 10.1161/HYPERTENSIONAHA.111.187021. [DOI] [PubMed] [Google Scholar]
- Schmidt R, Schmidt H, Haybaeck J, Loitfelder M, Weis S, Cavalieri M, Seiler S, Enzinger C, Ropele S, Erkinjuntti T, Pantoni L, Scheltens P, Fazekas F, Jellinger K. Heterogeneity in age-related white matter changes. Acta Neuropathol. 2011;122:171–185. doi: 10.1007/s00401-011-0851-x. [DOI] [PubMed] [Google Scholar]
- Seals DR, Desouza CA, Donato AJ, Tanaka H. Habitual exercise and arterial aging. J Appl Physiol. 2008;105:1323–1332. doi: 10.1152/japplphysiol.90553.2008. 1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheline YI, Pieper CF, Barch DM, Welsh-Bohmer K, McKinstry RC, MacFall JR, D'Angelo G, Garcia KS, Gersing K, Wilkins C, Taylor W, Steffens DC, Krishnan RR, Doraiswamy PM. Support for the vascular depression hypothesis in late-life depression: results of a 2-site, prospective, antidepressant treatment trial. Arch Gen Psychiatry. 2010;67:277–285. doi: 10.1001/archgenpsychiatry.2009.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith EE, Greenberg SM. Clinical diagnosis of cerebral amyloid angiopathy: validation of the Boston criteria. Curr Atheroscler Rep. 2003;5:260–266. doi: 10.1007/s11883-003-0048-4. [DOI] [PubMed] [Google Scholar]
- Smith EE, Salat DH, Jeng J, McCreary CR, Fischl B, Schmahmann JD, Dickerson BC, Viswanathan A, Albert MS, Blacker D, Greenberg SM. Correlations between MRI white matter lesion location and executive function and episodic memory. Neurology. 2011;76:1492–1499. doi: 10.1212/WNL.0b013e318217e7c8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starr JM, Leaper SA, Murray AD, Lemmon HA, Staff RT, Deary IJ, Whalley LJ. Brain white matter lesions detected by magnetic resonance [correction of resosnance] imaging are associated with balance and gait speed. J Neurol Neurosurg Psychiatry. 2003;74:94–98. doi: 10.1136/jnnp.74.1.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steingart A, Hachinski VC, Lau C, Fox AJ, Fox H, Lee D, Inzitari D, Merskey H. Cognitive and neurologic findings in demented patients with diffuse white matter lucencies on computed tomographic scan (leuko-araiosis). Arch Neurol. 1987;44:36–39. doi: 10.1001/archneur.1987.00520130028013. [DOI] [PubMed] [Google Scholar]
- Sullivan EV, Pfefferbaum A. Diffusion tensor imaging and aging. Neurosci Biobehav Rev. 2006;30:749–761. doi: 10.1016/j.neubiorev.2006.06.002. [DOI] [PubMed] [Google Scholar]
- Swan GE, DeCarli C, Miller BL, Reed T, Wolf PA, Jack LM, Carmelli D. Association of midlife blood pressure to late-life cognitive decline and brain morphology. Neurology. 1998;51:986–993. doi: 10.1212/wnl.51.4.986. [DOI] [PubMed] [Google Scholar]
- Swartz RH, Sahlas DJ, Black SE. Strategic involvement of cholinergic pathways and executive dysfunction: Does location of white matter signal hyperintensities matter? J Stroke Cerebrovasc Dis. 2003;12:29–36. doi: 10.1053/jscd.2003.5. [DOI] [PubMed] [Google Scholar]
- Takami T, Shigemasa M. Efficacy of various antihypertensive agents as evaluated by indices of vascular stiffness in elderly hypertensive patients. Hypertens Res. 2003;26:609–614. doi: 10.1291/hypres.26.609. [DOI] [PubMed] [Google Scholar]
- Takao M, Koto A, Tanahashi N, Fukuuchi Y, Takagi M, Morinaga S. Pathologic findings of silent hyperintense white matter lesions on MRI. J Neurol Sci. 1999;167:127–131. doi: 10.1016/s0022-510x(99)00158-6. [DOI] [PubMed] [Google Scholar]
- Tanabe JL, Amend D, Schuff N, DiSclafani V, Ezekiel F, Norman D, Fein G, Weiner MW. Tissue segmentation of the brain in Alzheimer disease. AJNR Am J Neuroradiol. 1997;18:115–123. [PMC free article] [PubMed] [Google Scholar]
- Taveggia C, Feltri ML, Wrabetz L. Signals to promote myelin formation and repair. Nat Rev Neurol. 2010;6:276–287. doi: 10.1038/nrneurol.2010.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas AJ, O'Brien JT, Davis S, Ballard C, Barber R, Kalaria RN, Perry RH. Ischemic basis for deep white matter hyperintensities in major depression: a neuropathological study. Arch Gen Psychiatry. 2002;59:785–792. doi: 10.1001/archpsyc.59.9.785. [DOI] [PubMed] [Google Scholar]
- Tolppanen AM, Solomon A, Soininen H, Kivipelto M. Midlife vascular risk factors and Alzheimer's disease: evidence from epidemiological studies. J Alzheimers Dis. 2012;32:531–540. doi: 10.3233/JAD-2012-120802. [DOI] [PubMed] [Google Scholar]
- Tomimoto H, Akiguchi I, Suenaga T, Nishimura M, Wakita H, Nakamura S, Kimura J. Alterations of the blood-brain barrier and glial cells in white-matter lesions in cerebrovascular and Alzheimer's disease patients. Stroke. 1996;27:2069–2074. doi: 10.1161/01.str.27.11.2069. [DOI] [PubMed] [Google Scholar]
- Toth P, Tucsek Z, Sosnowska D, Gautam T, Mitschelen M, Tarantini S, Deak F, Koller A, Sonntag WE, Csiszar A, Ungvari Z. Age-related autoregulatory dysfunction and cerebromicrovascular injury in mice with angiotensin II-induced hypertension. J Cereb Blood Flow Metab. 2013;33:1732–1742. doi: 10.1038/jcbfm.2013.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Swieten JC, van den Hout JH, van Ketel BA, Hijdra A, Wokke JH, van Gijn J. Periventricular lesions in the white matter on magnetic resonance imaging in the elderly. A morphometric correlation with arteriolosclerosis and dilated perivascular spaces. Brain. 1991;114(Pt 2):761–774. doi: 10.1093/brain/114.2.761. [DOI] [PubMed] [Google Scholar]
- Vanley CT, Aguilar MJ, Kleinhenz RJ, Lagios MD. Cerebral amyloid angiopathy. Hum Pathol. 1981;12:609–616. doi: 10.1016/s0046-8177(81)80044-5. [DOI] [PubMed] [Google Scholar]
- Vermeer SE, Hollander M, van Dijk EJ, Hofman A, Koudstaal PJ, Breteler MM, Rotterdam Scan S. Silent brain infarcts and white matter lesions increase stroke risk in the general population: the Rotterdam Scan Study. Stroke. 2003;34:1126–1129. doi: 10.1161/01.STR.0000068408.82115.D2. [DOI] [PubMed] [Google Scholar]
- Vernooij MW, de Groot M, van der Lugt A, Ikram MA, Krestin GP, Hofman A, Niessen WJ, Breteler MM. White matter atrophy and lesion formation explain the loss of structural integrity of white matter in aging. Neuroimage. 2008;43:470–477. doi: 10.1016/j.neuroimage.2008.07.052. [DOI] [PubMed] [Google Scholar]
- Vinters HV, Gilbert JJ. Cerebral amyloid angiopathy: incidence and complications in the aging brain. II. The distribution of amyloid vascular changes. Stroke. 1983;14:924–928. doi: 10.1161/01.str.14.6.924. [DOI] [PubMed] [Google Scholar]
- Walhovd KB, Fjell AM, Reinvang I, Lundervold A, Dale AM, Eilertsen DE, Quinn BT, Salat D, Makris N, Fischl B. Effects of age on volumes of cortex, white matter and subcortical structures. Neurobiol Aging. 2005;26:1261–1270. doi: 10.1016/j.neurobiolaging.2005.05.020. discussion 1275-1268. [DOI] [PubMed] [Google Scholar]
- Wardlaw JM, Sandercock PA, Dennis MS, Starr J. Is breakdown of the blood-brain barrier responsible for lacunar stroke, leukoaraiosis, and dementia? Stroke. 2003;34:806–812. doi: 10.1161/01.STR.0000058480.77236.B3. [DOI] [PubMed] [Google Scholar]
- Wardlaw JM, Smith EE, Biessels GJ, Cordonnier C, Fazekas F, Frayne R, Lindley RI, O'Brien JT, Barkhof F, Benavente OR, Black SE, Brayne C, Breteler M, Chabriat H, Decarli C, de Leeuw FE, Doubal F, Duering M, Fox NC, Greenberg S, Hachinski V, Kilimann I, Mok V, Oostenbrugge R, Pantoni L, Speck O, Stephan BC, Teipel S, Viswanathan A, Werring D, Chen C, Smith C, van Buchem M, Norrving B, Gorelick PB, Dichgans M, nEuroimaging STfRVco Neuroimaging standards for research into small vessel disease and its contribution to ageing and neurodegeneration. Lancet Neurol. 2013;12:822–838. doi: 10.1016/S1474-4422(13)70124-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westlye LT, Walhovd KB, Dale AM, Bjornerud A, Due-Tonnessen P, Engvig A, Grydeland H, Tamnes CK, Ostby Y, Fjell AM. Life-span changes of the human brain white matter: diffusion tensor imaging (DTI) and volumetry. Cereb Cortex. 2010;20:2055–2068. doi: 10.1093/cercor/bhp280. [DOI] [PubMed] [Google Scholar]
- Whitman GT, Tang Y, Lin A, Baloh RW. A prospective study of cerebral white matter abnormalities in older people with gait dysfunction. Neurology. 2001;57:990–994. doi: 10.1212/wnl.57.6.990. [DOI] [PubMed] [Google Scholar]
- Williams VJ, Leritz EC, Shepel J, McGlinchey RE, Milberg WP, Rudolph JL, Lipsitz LA, Salat DH. Interindividual variation in serum cholesterol is associated with regional white matter tissue integrity in older adults. Human brain mapping. 2012 doi: 10.1002/hbm.22030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright CB, Paik MC, Brown TR, Stabler SP, Allen RH, Sacco RL, DeCarli C. Total homocysteine is associated with white matter hyperintensity volume: the Northern Manhattan Study. Stroke. 2005;36:1207–1211. doi: 10.1161/01.STR.0000165923.02318.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright IS. Experience with anticoagulants. Circulation. 1959;19:110–113. doi: 10.1161/01.cir.19.1.110. [DOI] [PubMed] [Google Scholar]
- Xiong Y, Mahmood A, Chopp M. Angiogenesis, neurogenesis and brain recovery of function following injury. Curr Opin Investig Drugs. 2010;11:298–308. [PMC free article] [PubMed] [Google Scholar]
- Yamanouchi H, Sugiura S, Tomonaga M. Decrease in nerve fibres in cerebral white matter in progressive subcortical vascular encephalopathy of Binswanger type. An electron microscopic study. J Neurol. 1989;236:382–387. doi: 10.1007/BF00314894. [DOI] [PubMed] [Google Scholar]
- Yoshita M, Fletcher E, Harvey D, Ortega M, Martinez O, Mungas DM, Reed BR, DeCarli CS. Extent and distribution of white matter hyperintensities in normal aging, MCI, and AD. Neurology. 2006;67:2192–2198. doi: 10.1212/01.wnl.0000249119.95747.1f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zieman SJ, Melenovsky V, Kass DA. Mechanisms, pathophysiology, and therapy of arterial stiffness. Arterioscler Thromb Vasc Biol. 2005;25:932–943. doi: 10.1161/01.ATV.0000160548.78317.29. [DOI] [PubMed] [Google Scholar]